Abstract
Background
Pathogens have been implicated in the pathogenesis of inflammatory atherosclerosis. Given the pleiotropic role of Interleukin-6 in the regulation of cytokines, lipid homeostasis, vascular remodeling, and apoptosis we hypothesized that IL-6 plays an important role in development and progression to inflammatory atherosclerosis.
Methods and Results
To explore the role of IL-6 in inflammation- and infection-associated atherosclerosis, 10 week-old ApoE+/−-IL-6+/− and ApoE+/−-IL-6−/− mice fed either high fat diet or regular chow diet were inoculated intravenously, once per week for 14 or 24 consecutive weeks with 50µl live Porphyromonas gingivalis (P.g.) (107CFU) or vehicle (normal saline). Animals were euthanized at 24 weeks of age (14 weeks injection) or 34 weeks of age (24 weeks injection). Histomorphometric analysis of atheromatous lesions, en face analysis over the aortic tree, immunohistochemistry for macrophages and smooth muscle cell, TUNEL staining for apoptotic cells, serum amyloid A (SAA) levels, serum lipids and glucose level, serum cytokines were obtained. ApoE+/−-IL-6−/− mice showed a significant increase in atheromatous lesions in proximal aorta and aortic tree compared to ApoE+/−-IL-6+/− mice for all conditions (chow diet and P.g-inoculated, high fat diet and P.g-inoculated, high fat diet and vehicle-inoculated) at 14 weeks and greater at 24 weeks. SAA levels from ApoE+/−-IL-6−/− mice were significantly higher than ApoE+/−-IL-6+/− mice. IL-6 deficiency led to profound changes in plaque composition evidenced by increased macrophage infiltration, apoptosis, lipid content and decreased smooth muscle cell mass reflecting an unstable plaque phenotype. Array analysis revealed increased levels of proinflammatory cytokines in ApoE+/−-IL-6−/− mice compared to ApoE+/−-IL-6+/− mice, irrespective of diet or inoculation.
Conclusion
The genetic deficiency of IL-6 was found to enhance the formation of diet- and/or pathogen associated atherosclerotic plaques and suggests that IL-6 may play an atheroprotective role.
Keywords: Atherosclerosis, Cytokine, Infection, Inflammation, Porphyromonas gingivalis
INTRODUCTION
Atherosclerosis, which causes ischemic cardiopathy and stroke, is the most common cause of mortality and morbidity in developed countries. High levels of LDL-cholesterol or oxidized-LDL, free radicals (often caused by smoking), hyperglycemia, as well as infectious agents (e.g. Chlamydia pneumonia, Porphyromonas gingivalis (P.g.), herpes viruses) have been suggested to be implicated in the pathogenesis of atherosclerosis 1–3. Atherosclerosis results from multiple interactions between injurious stimuli and the healing responses of the arterial wall. The starting point is functional endothelial damage, secondary to mechanical or vascular insult, which is followed by an inflammatory cascade that involves humoral (cytokines, growth factors) and cellular (increased chemotaxis, adherence and infiltration of inflammatory cells) mechanisms. These factors combine in an unbalanced and progressive manner that leads to the final fibroproliferative response 4. After endothelial injury, direct cell-cell interaction and secretion of chemotactic and growth factors occur, resulting in recruitment of monocytes to subintimal regions, smooth muscle cell proliferation, and increased synthesis of matrix proteins. The recruited monocytes become macrophages, accumulate lipid, and ultimately become foam cells 5, 6. Although all of the potential triggers of inflammation are not fully known, cytokines, oxidized lipoproteins, and local (arterial) and distant infections such as chronic periodontitis and pneumonia have been implicated in atherogenesis. Vascular cells, including smooth muscle, endothelium, and leukocytes which constitute various atherosclerotic lesions, can produce as well as respond to cytokines such as IL-6, TNF-α, IL-1α, and MCP-17. Among them, IL-6 (a 26 kDa cytokine), produced by lymphocytes, monocytes, fibroblasts, vascular smooth muscle cells, and endothelial cells has been identified as a local and circulating marker of coronary plaque inflammation8. It is expressed by lipid-laden macrophages, known as foam cells, and smooth muscle cells in fatty streaks. It is also expressed in the cap and shoulder regions of atheromatous plaques, suggesting an important role in atherogenesis9. Furthermore, IL-6 is an important stimulator of the acute phase reaction, acting on both the liver and hypothalamus 10, 11 leading to release of X-reactive protein, serum amyloid A (SAA), and fibrinogen, all known to have proinflammatory roles 12. However, IL-6 is a pleiotropic cytokine and its role in the modulation of inflammation-related processes, particularly cytokine responses and tissue inflammatory cell infiltration, remains equivocal. Indeed, recently, IL-6 deficiency was reported to result in an enhanced formation of atherosclerotic lesions, reduced collagen metabolism, and elevated levels of serum cholesterol in an ApoE−/− model of atherosclerosis 13. However weekly, super-physiological injections of recombinant IL-6 promoted a ~2-fold increase in lesion development in ApoE−/− mice, known to rapidly develop extensive atherosclerotic lesions throughout their vascular tree 14. To our knowledge, the role of IL-6 in bacteria enhanced atherogenic process has never been addressed and warrants further investigation Recent studies in animal models from our group and others have shown that chronic oral infections with microorganisms such as P.g., a pathogen involved in chronic periodontal diseases, increase the risk for coronary vascular disease15–17. The present study was conducted to establish the role of IL-6 in diet- and/or bacteria-enhanced atherosclerosis using a IL-6- deficient mice rendered susceptible to atherosclerosis (ApoE+/−) where inflammation is a major component.
METHODS
Mice and Diets
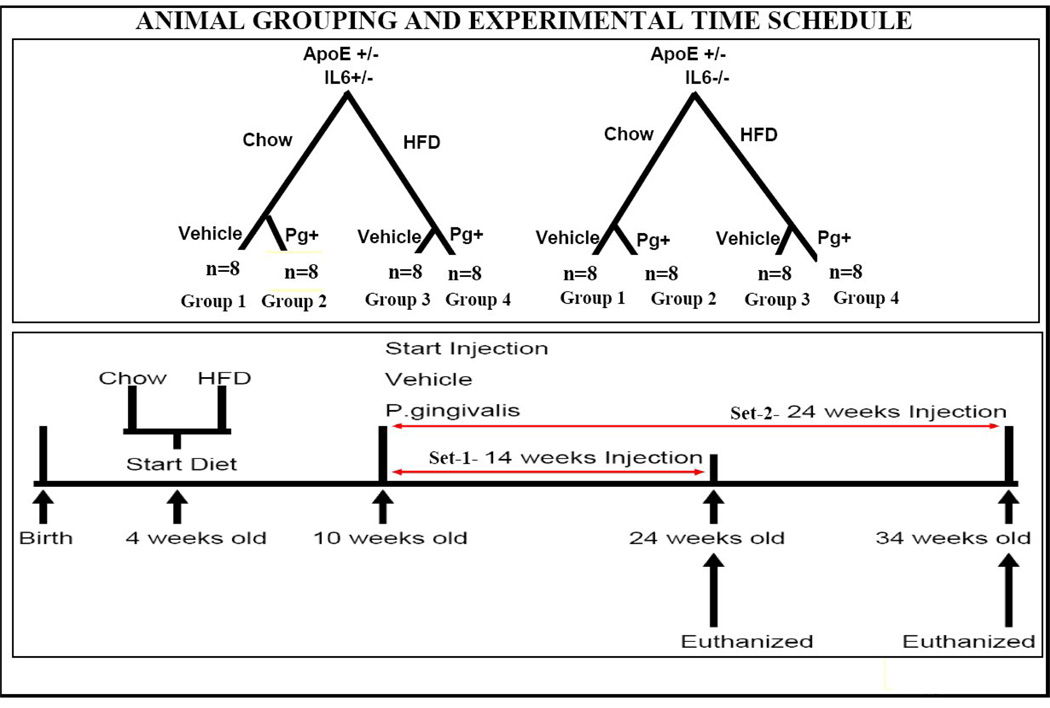
The Institutional Animal Care and Use Committee of Boston University approved all animal protocols. In this study, three strains of mice were obtained from Jackson Laboratories (Bar Harbor, ME) as breeding mice. These were apolipoprotein E deficient homozygote (ApoE−/−), wild type C57BL/6J hybrids, and IL-6 knock-out (IL-6−/−) mice. Female ApoE−/− and male wild-type C57BL/6J hybrids were crossed to generate apolipoprotein E deficient heterozygotes (ApoE+/−). ApoE−/−-IL-6−/− mice were generated by crossing male ApoE−/− with female IL-6−/− mice. ApoE−/−- IL-6−/− were bred with wild-type C57BL/6J to generate ApoE+/−-IL-6+/− mice. After an 8-week growing period, male ApoE−/−-IL-6−/− mice were backcrossed with female IL-6−/− mice to generate ApoE+/−-IL-6−/−. The nature of all the strains was verified by polymerase chain reaction method (genotyping protocols from Jackson Laboratory). The experimental protocols are shown in Fig. 1. Two strains of experimental mice, including ApoE+/−-IL-6+/− and ApoE+/−-IL-6−/− were weaned at 4 weeks of age, and randomly assigned to either a high fat diet (test diet) containing 1.25% cholesterol, 15% fat (No. 21539, Test Diet) or regular mouse chow (lab diet) containing 0.02% cholesterol and 4.5% fat (rodent diet 5001).
Figure 1.
Four week old ApoE+/-IL-6−/− and ApoE+/-IL-6+/− mice fed either HFD or regular chow diet for 6 weeks were divided into 4 groups {Group 1 was fed a standard chow diet and inoculated with 50µl vehicle (CS) (normal saline); Group 2 was fed a standard chow diet and inoculated with 50µl (107CFU) P.g.(CP); Group 3 was fed high fat diet and inoculated with 50µl vehicle(HS) (normal saline); Group 4 was fed high fat diet and inoculated with 50µl (107CFU) P.g.(HP)}. All groups were divided in two sets for testing two time points of 14 weeks (set-1) and 24 weeks (set-2) post inoculation in this study. In summary mice from set-1 received 14 tail vein injections of either vehicle or P.g. once weekly and set-2 received 24 tail vein injections of either vehicle or P.g. once weekly.
Bacterial strain, dose and route of inoculation
P. g A7436 human isolate was grown on anaerobic agar plates as described previously 18. We used live bacterial inoculation (107 CFU) because in preliminary experiments heat killed P. g. failed to produce atherosclerosis. The dose (107 CFU) and delivery route (intravenous) of the pathogen compares well with bacteremia encountered in human after dental infection, periodontal surgery, scaling, tooth extraction and flossing. 19–22
Animal Grouping and Experimental Time Schedule
Mice heterozygous for Apolipoprotein E (ApoE+/−) with susceptibility to atherosclerosis were generated as they do not develop spontaneous atherosclerosis 15, 18. Four week old ApoE+/−-IL-6+/− and ApoE +/−-IL-6−/− mice fed either HFD or regular chow diet for 6 weeks (n=8) and divided into 4 groups (Group 1 was fed a standard chow diet and inoculated with 50µl vehicle saline (CS)); Group 2 was fed a standard chow diet and inoculated with 50µl (107CFU) P.g.(CP); Group 3 was fed high fat diet and inoculated with 50µl vehicle saline (HS); Group 4 was fed high fat diet and inoculated with 50µl (107CFU) P.g. (HP). All groups divided in two sets for testing two time points of 14 weeks (set-1) and 24 weeks (set-2) post inoculation in this study (Fig.1). In summary mice from set-1 received 14 tail vein injections of either vehicle or P.g. once weekly and set-2 received 24 tail vein injections of either vehicle or P.g. once weekly.
Tissue Harvesting and Preparation
After overnight fasting, mice were heavily sedated with inhaled isoflurane (SOLVAY) and exsanguinated from the femoral arteries. The heart and aorta were perfused, separated close to the heart and processed as described previously 18 23
Lipids and glucose analysis
Serum samples from 14 weeks and 24 weeks mice from all the groups were evaluated for total cholesterol (TC)(Cayman chemicals), HDL, LDL (Wako Chemicals) and Glucose levels (BioAssay Systems) according to manufacturer’s instructions.
Morphometric Analysis
a. En face morphometric analysis of the aortic tree: The extent of atherosclerosis in the aortic tree was determined by en face quantification as described previously 23 24. The data is presented as the percentage of the aorta occupied by the lesions; b. Histomorphometric and histopathological analysis of atheroma lesions in the proximal aorta: Proximal aortic cross-sections for quantitative and histopathological evaluation of atherosclerotic lesions were prepared as described previously 25. Briefly stated five sections per animal, each separated by 80 µm, were stained (Sudan IV), counterstained (hematoxylin), and assessed with a computer-assisted image analysis. The total cross-sectional area of the aortic lumen and lesion from the five images were measured using Image ProPlus 4.0. The value was averaged and then the percentage lesion was expressed as a ratio of total lesion size / total sinus area.
Immunohistochemistry
Briefly, five sections of the proximal aorta per animal, each separated by 80 µm were fixed with ice cold acetone for 10 minutes and incubated for an hour at room temperature with primary antibody to MOMA-2 (1:25) (Chemicon) or alpha smooth muscle actin (1:50) (Σ). Samples were then developed using the ABC elite kit (vector labs) and staining revealed by texas red conjugated avidin (vector labs). Nonimmune IgG from appropriate species were used as negative controls. Stained Macrophages or smooth muscle cells within atherosclerotic plaques in the aortic sinus were quantified using Image ProPlus 4.0. The ratio of the macrophage or smooth muscle stained area / total plaque area was calculated and plotted as described previously 26. Apoptosis was detected by TUNEL staining of five frozen section per animal, each separated by 80 µm using DeadEnd flourimetric kit (Promega) according to manufacturer’s instructions and expressed as a percentage of positive cells relative to the total number of cells in the field of lesion examined 26. Samples in absence of the TdT enzyme were used as negative controls.
Cytokine Antibody Array
Serum samples from mice euthanized after 24 weeks were analyzed two times with a cytokine antibody array using RayBio Mouse Cytokine Antibody Array III (RayBiotech, Inc., Norcross, GA). These arrays detected 62 mouse cytokines. For each spot, the net optical density level was determined by subtracting the background optical level from the total raw optical density. The level of each cytokine is represented as a percentage of the positive control and presented as expression of cytokines in the ApoE+/−-IL-6−/− group compared to the ApoE+/−-IL-6+/− group (table-2).
Table-2.
Cytokine profile
| Cytokine | Group 2 (CP) | Group 3 (HS) | Group 4 (HP) | Cytokine | Group 2 (CP) | Group 3 (HS) | Group 4 (HP) | Cytokine | Group 2 (CP) | Group 3 (HS) | Group 4 (HP) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AXL | + | + | ++ | IL-1β | ++ | ++ | +++ | MCP-1 | +++ | +++ | ++++ |
| BLC | +++ | +++ | ++++ | IL-2 | + | + | + | MCP-5 | ++++ | +++ | ++++ |
| CD-30L | ++++ | +++ | ++ | IL-3 | ++ | + | + | M-CSF | ++ | +++ | +++ |
| CD-40 | ++++ | +++ | +++ | IL-3Rb | +++ | ++ | ++ | MIG | + | + | + |
| CRG-2 | +++ | ++ | ++++ | IL-4 | − | − | − | MIP-1α | ++ | ++ | +++ |
| CTACK | +++ | ++ | +++ | IL-5 | − | − | − | MIP-1β | ++ | ++ | ++ |
| CXL-16 | +++ | + | ++ | IL-6 | − | − | − | MIP-1γ | ++ | + | + |
| EOTAXIN | +++ | +++ | ++++ | IL-9 | + | + | − | MIP-2 | ++ | + | ++ |
| EOTAXIN-2 | +++ | +++ | ++++ | IL-10 | − | − | − | MIP-3β | +++ | +++ | +++ |
| FAS LIGAND | + | + | + | IL-12p70 | ++ | +++ | ++++ | MIP-3α | − | − | − |
| FRACTALKINE | + | ++ | +++ | IL-13 | − | − | − | P-SELECTIN | ++ | +++ | ++++ |
| GCSF | +++ | + | − | IL-17 | + | − | + | RANTES | ++ | +++ | ++ |
| GM-CSF | + | ++ | +++ | KC | − | − | + | SCE | +++ | +++ | +++ |
| IFNγ | + | + | + | LEPTIN-R | ++ | − | +++ | SDF-1α | ++++ | +++ | +++ |
| IGFBP-3 | + | + | +++ | LEPTIN | ++ | − | +++ | TARC | +++ | +++ | +++ |
| IGFBP-5 | +++ | ++ | ++++ | LIX | + | + | + | TCA-3 | ++++ | ++++ | ++++ |
| IGFBP-6 | ++ | + | ++++ | L-SELECTIN | ++ | ++ | +++ | TECK | ++ | ++ | + |
| IL-1α | +++ | ++++ | ++++ | LYMPHOTACTIN | ++ | ++ | + | TNF-α | +++ | +++ | ++++ |
| VCAM-1 | +++ | +++ | ++++ | TNFR-II | ++ | +++ | +++ | TBFR-I | +++ | ++ | +++ |
| VEGF | ++++ | +++ | ++++ | TPO | +++ | ++++ | ++ |
Expression of cytokines in the ApoE+/-IL-6−/− group compared to the ApoE+/-IL-6+/− group. Results are presented for subgroups receiving a standard chow diet and inoculated with Pg (CP); a high fat diet and inoculations with vehicle (HS); and a high fat diet and inoculated with Pg (HP)
− Unchanged
< 20% INCREASE: +
21–40% INCREASE: ++
41–60% INCREASE: +++
≥61% INCREASE: ++++
ELISA
Serum samples were used to determine levels of SAA (Biosource International), VCAM-1, L-Selectin, MCP-1, IL-10, mouse MMP-9, and mouse TIMP-1 (R&D Systems), and VEGF (Biosource International) in 24 week serum samples. Cytokine protein levels were assessed using ELISA kits for mouse MMP-9, and mouse TIMP-1(Quantikine; R&D Systems). Heart tissue fragments were proceeded for protein extraction as previously described27. 100µg of protein were used for cytokine, MMP-9 and TIMP-1 detection in the myocardium 28. Assays were done in triplicates and repeated three times with similar results.
Statistical Analysis
All histomorphometric measurements were blinded to the examiner. All quantitative measurements were confirmed by random analysis of one fourth of the specimens by the same examiner (R>0.92) and by another independent examiner (pathologist) to ensure consistency. The intra-examiner and inter-examiner variation was <10%. A level of p<0.05 was considered significant. Extent of atherosclerosis was analyzed by Student’s paired 2-tailed t-test.
RESULTS
Body weight, glucose level, total serum cholesterol, LDL, and HDL levels
No significant difference in body weight was observed between all groups. Total serum cholesterol, LDL and glucose levels were higher in ApoE+/−-IL-6−/− mice when compared to ApoE+/−-IL-6+/− mice only in diet- and or bacterial-challenged animal; Furthermore, HDL levels were also decreased in the in ApoE+/−-IL-6−/− mice versus ApoE+/−-IL-6+/− only in diet- and or bacterial-challenged animal; no significant difference was observed in the blood chemistry in chow saline groups (table-1)
TABLE-1.
Metabolic profile of animals
| ApoE+/−-IL-6+/− | ApoE+/−-IL-6+/− | ApoE+/−-IL-6+/− | ApoE+/−-IL-6+/− | |||
|---|---|---|---|---|---|---|
| 1. Serum lipids and glucose level in mice fed with chow diet and receiving vehicle (normal saline) | ||||||
| Duration (weeks) | 14 | 14 | P value* | 24 | 24 | P values* |
| Number of mice (n) | 8 | 8 | 8 | 8 | ||
| Weight (g) | 28.1±4 | 29.3±5 | 30.3±4 | 31.3±2 | ||
| Total cholesterol (mg/dl) | 180.2±12 | 198.2±11 | >0.05 | 195.8±10 | 205±15 | >0.05 |
| HDL (mg/dl) | 34±12 | 28±5 | >0.05 | 35.3±5 | 30±8 | >0.05 |
| LDL (mg/dl) | 41±7 | 46±4 | >0.05 | 48.5±11 | 54.1±9 | >0.05 |
| Glucose (mg/dl) | 78.5±12 | 87.9±14 | >0.05 | 91.4±16 | 93.3±3 | >0.05 |
| 2. Serum lipids and glucose level in mice fed with chow diet and receiving P. gingivalis | ||||||
| Duration (weeks) | 14 | 14 | P value* | 24 | 24 | P value* |
| Number of mice (n) | 8 | 8 | 8 | 8 | ||
| Weight (g) | 30.1±4 | 31.1±2 | 31.7±3 | 31.0±3 | ||
| Total cholesterol (mg/dl) | 201.1±13 | 258.9±18 | <0.05 | 264.6±10 | 331.6±11 | <0.05 |
| HDL (mg/dl) | 67±9 | 48.9±5 | <0.05 | 56±4 | 40.6±9 | <0.05 |
| LDL (mg/dl) | 52±6 | 69±3 | <0.05 | 59.9±8 | 84.5±11 | <0.05 |
| Glucose (mg/dl) | 93.3±5 | 115.9±12 | >0.05 | 119.3±16 | 133±10 | >0.05 |
| 3. Serum lipids and glucose level in mice fed with high fat diet and receiving vehicle (normal saline) | ||||||
| Duration (weeks) | 14 | 14 | P valve* | 24 | 24 | P value* |
| Number of mice (n) | 8 | 8 | 8 | 8 | ||
| Weight (g) | 30.1±4 | 29.1±5 | 30.1±6 | 31.1±4 | ||
| Total cholesterol (mg/dl) | 609±11 | 811.3±10 | <0.05 | 806.13±14 | 1069.3±13 | <0.05 |
| HDL (mg/dl) | 106.1±11 | 88±12 | <0.05 | 99.6±4 | 70±8 | <0.05 |
| LDL (mg/dl) | 114.5±6 | 151±6 | <0.05 | 143.9±5 | 208.5±9 | <0.05 |
| Glucose (mg/dl) | 125±7 | 166.6±9 | <0.05 | 134±10 | 179±15 | <0.05 |
| 4. Serum lipids and glucose level in mice fed with high fat diet and receiving P. giugivalis | ||||||
| Duration (weeks) | 14 | 14 | P value* | 24 | 24 | P value* |
| Number of mice (n) | 8 | 8 | 8 | 8 | ||
| Weight (g) | 29.1±3 | 30.1±2 | 31.1±2 | 32.1±1.2 | ||
| Total cholesterol (mg/dl) | 645.67±16 | 909±11 | <0.05 | 819.13±14 | 1135±18 | <0.05 |
| HDL (mg/dl) | 154±6 | 80.8±11 | <0.05 | 117±10 | 76±9 | <0.05 |
| LPL (mg/dl) | 142.8±6 | 197.2±10 | <0.05 | 168.6±11 | 249.6±9 | <0.05 |
| Glucose (mg/dl) | 140.3±13 | 182.9±14 | <0.05 | 149±21 | 195±15 | <0.05 |
Significance between ApoE+/−-IL-6+/− and ApoE+/−-IL-6+/− for respective groups. Abbreviattions are as defined in the text.
En face and histomorphometric analysis of atheroma lesions
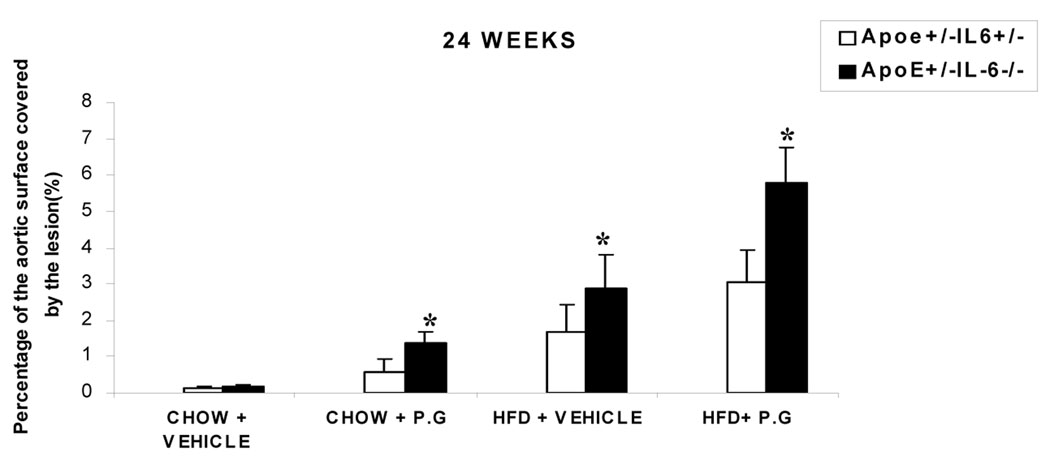
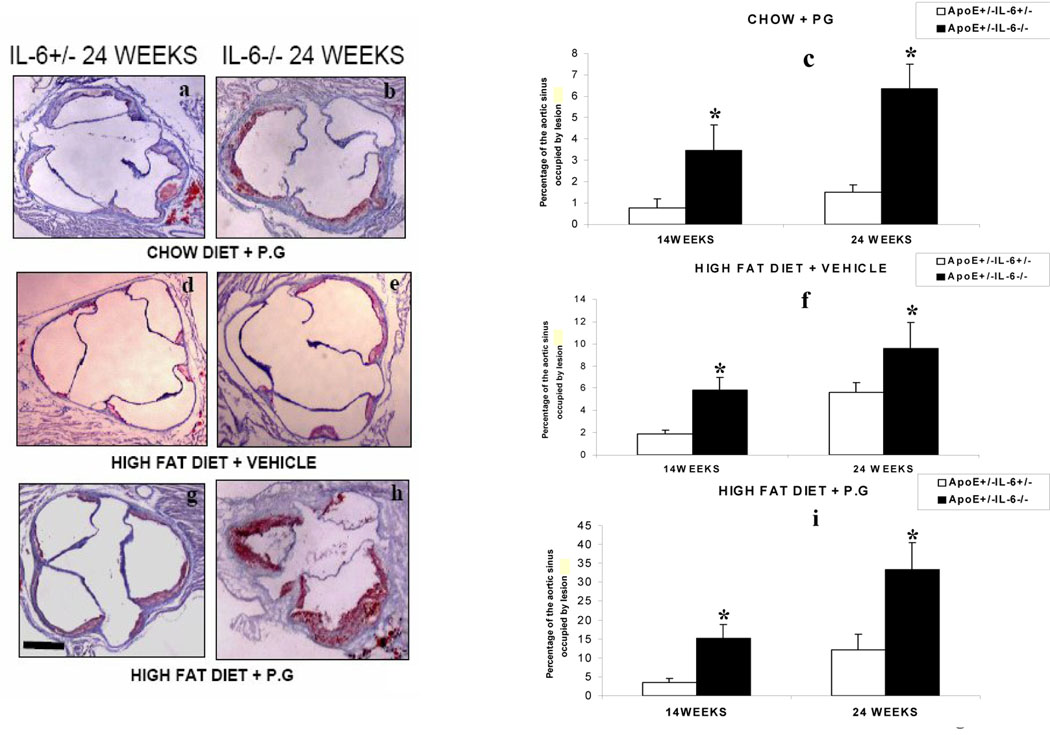
Quantitative en face analysis revealed a statistically significant increase in lesion size in ApoE+/−-IL-6−/− mice compared to ApoE+/−-IL-6+/− mice (HP: 3.0±1.0% to 5.8 ±1.1%; HS: 1.6±0.7% to 2.9±1.0%; CP: 0.59±0.3% to 1.38±0.3%) respectively at 24 weeks (p<0.05) (Fig. 2). A similar pattern was observed at 14 week (data not shown). The histomorphological analysis of Sudan red-stained lesions was used to evaluate the percentage of the aortic lumen occupied by the lesion. We did not observe any lesions in the chow diet with vehicle mice (CS) irrespective of the IL-6 genotype. However, we observed an increase in the percentage of aortic sinus occupied by the lesions in mice fed with chow diet and inoculated with P.g (CP) in ApoE+/−-IL-6−/− versus ApoE+/−-IL-6+/− the percentage rose from 0.74±0.4% to 3.57±1.2% (p<0.05) at 14 weeks (Fig. 3c) and from 1.52±0.34% to 6.3±1.9% (p<0.05) at 24 weeks (Figs 3 a, b & c). There was an increase in the percentage of the aortic sinus occupied by the lesion in mice fed with high fat diet and receiving vehicle(HS) in ApoE+/−-IL-6+/− mice: 1.9±0.29% compared to 5.8±1.17% (p<0.05) in ApoE+/−-IL-6−/− mice at 14 weeks (Fig 3f) and 5.6±1.1% compared to 9.6±2.3% (p<0.05) at 24 weeks (Figs 3 d, e & f). A progression of lesion development was observed in mice from 14 weeks to 24 weeks, with significant increases in mice fed with high fat diet and inoculated with P.g. (HP). As anticipated, there was an increase in percentage lesion from ApoE+/−-IL-6+/− to ApoE+/-IL-6−/− in mice fed with high fat diet and inoculated with P.g (HP) :3.5±1.1% vs 15.34±3.7 % ( p<0.05) at 14 weeks (Fig.3i). The largest and the most significant increase observed was from 12.2±4.01% to 33.3±7.1% (p<0.05), seen after 24 weeks (Fig3 g, h & i).
Figure 2.
En face analysis: percentage of aortic surface area covered by lesions in chow-fed or HFD groups for 2 genetic background mouse groups (ApoE+/−-IL-6−/− and ApoE+/−-IL-6+/−) inoculated with P gingivalis or vehicle for 24 weeks. Values represent mean±SD, *p <0.05 for ApoE+/−-IL-6−/−mice as compared to ApoE+/−-IL-6+/− mice in same condition on same diet. Abbreviations are as defined in text.
Figure 3.
Microscopic cross sections (10 µm) of the proximal aortic root were stained with sudan and counterstained with hematoxylin to reveal lipid deposition and quantified by digital morphometry for both ApoE+/−- IL-6−/− mice and ApoE+/−-IL-6+/− mice maintained on standard chow diet and inoculated weekly with Pg (CP)(fig-3c), on high fat diet and inoculated weekly with vehicle (HS)( (normal saline) fig-3f) and on high fat diet and inoculated weekly with P.g.(HP)( fig-3i) .The data is presented as percentage of total lumen of the proximal aorta occupied by lesions at 14 weeks and 24 weeks. Values represent mean±SD, *p <0.05 for ApoE+/−-IL-6−/−mice as compared to ApoE+/−-IL-6+/− mice in same condition on same diet. Abbreviations are as defined in text. Photomicrographs shown are representative images used to obtain the data presented in (Fig-3c, 3f & 3i), of proximal aortic lesions in ApoE+/−-IL6+/− and ApoE+/−-IL-6−/− mice maintained on standard chow diet and inoculated weekly Pg (CP)(fig-3a & 3b), on high fat diet and inoculated weekly with vehicle(normal saline) (HS)(fig-3d & 3e) and on high fat diet and inoculated weekly with P.g.(HP) ( fig-3g & 3h) for 24 weeks. Original magnifications 20X. Scale bar represents 0.5mm.
Immunohistochemical Analysis of Proximal Aorta
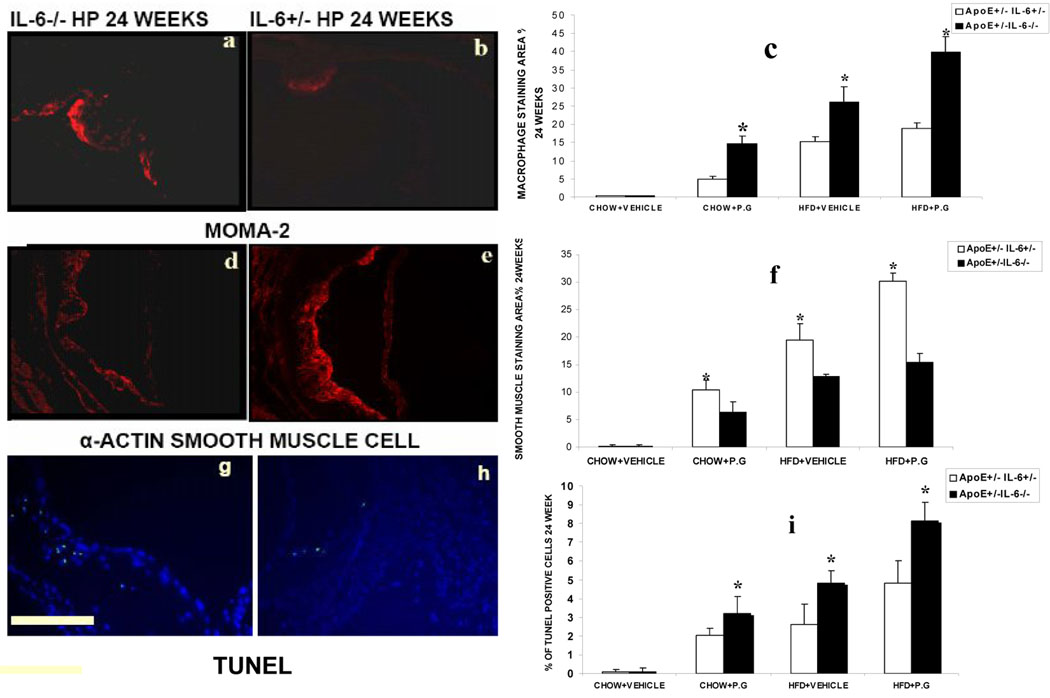
We did not observe any changes in mice fed with chow diet and injected with saline. In all the other experimental groups, atherosclerotic lesions exhibited a significant increase in macrophage infiltration in ApoE+/−-IL-6−/−mice compared to ApoE+/−-IL-6+/− mice (HP: 18.9±1.5% to 39.9±4.1%; HS: 15.2±125% to 26.18±4.09%; CP: 5.1±1.1% to 14.7±2.1% respectively (p <0.05) (Fig 4 a, b & c). There was a decrease in smooth muscle cell accumulation in ApoE+/-IL-6−/−mice versus ApoE+/−-IL-6+/− mice (fig4 d, e & f). The percentage decreased from 30.2±1.4% to 15.4±1.6%, 19.4±3% to 17.9±1.4% and from 10.4±1.7% to 6.3±2% (p<0.05) in HP, HS and CP mice respectively. Furthermore, the marked increase in the inflammatory component of the lesions in ApoE+/-IL-6−/−mice with high fat diet and inoculated with P.g. was associated with a substantial increase in the occurrence of cell death within the plaques in ApoE+/−-IL-6−/−mice (8.1±1.0%) versus (4.8±1.2%) in ApoE+/−-IL-6+/− (p <0.05) (Fig4 g, h & i)
Figure 4.
Representative photomicrographs show immunostained sections of macrophage infiltration (MOMA-2 red staining) (fig-4a & 4b), smooth muscle cell(α-SMA red staining) (fig-4d & 4e) and TUNEL positive cells (green spots coinciding with nuclear stain DAPI)(fig 4g & 4h) in atherosclerotic plaques from the aortic sinus of ApoE+/−-IL6+/− and ApoE+/−-IL-6−/− mice maintained on high fat diet and inoculated weekly with P.g (HP) for 24 weeks. Quantitative computer-assisted image analysis(as described in material and methods) was used to determine the percentage of macrophage-positive areas (fig-4c),smooth muscle cell area(fig-4f) and TUNEL/DAPI positive cells(fig-4i) in proximal aortic lesions in ApoE+/−-IL6+/− and ApoE+/−-IL-6−/− mice of all the groups at 24 weeks. Data represent mean ± SD. *p<0.05. Original magnifications 100X. Abbreviations are defined in the text. Scale bar represents 0.5mm.
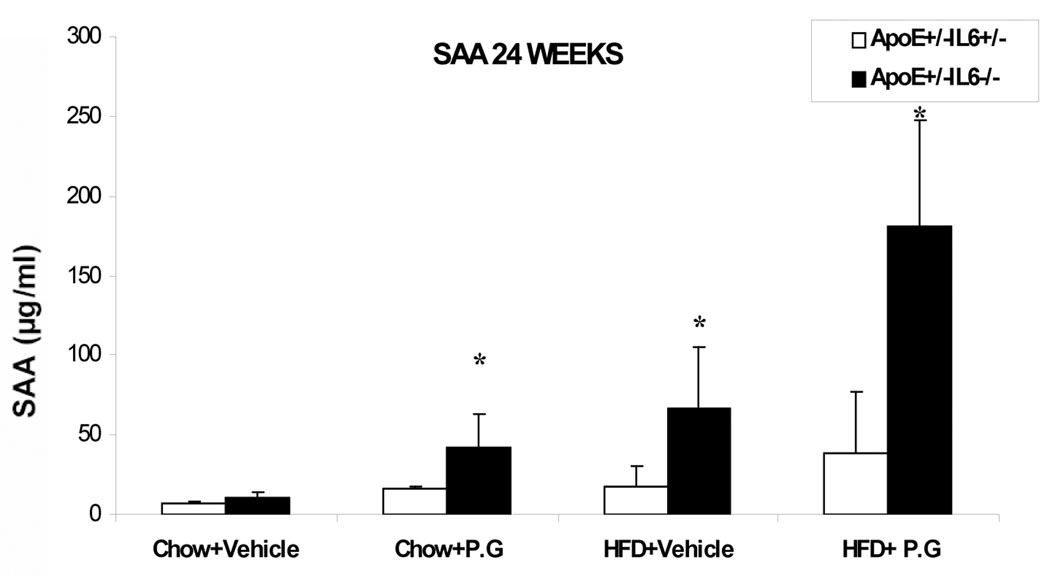
Increase in Serum level of SAA in ApoE+/−-IL-6−/− mice as compared to ApoE+/−-IL-6+/− mice
There was a significant increase in the SAA level in ApoE+/-IL-6−/−mice as compared to ApoE+/−-IL-6+/− mice with high fat diet and inoculated with P.g. (HP): 38.3±5.35µg/ml compared to 181.2±15.47µg/ml; (p <0.05). We also observed increases in SAA levels in mice fed with high fat diet with vehicle (HS) from 17.6±4.2µg/ml in ApoE+/−-IL-6+/−mice to 67±9.33µg/ml in the ApoE+/−-IL-6−/− animals; (p <0.05) and in mice with chow diet and with P.g. (CP), from 16.3±1.4 µg/ml in ApoE+/−-IL-6+/− to 42±8.56µg/ml in ApoE+−/-IL-6−/− animals; (p <0.05) (Fig. 5).
Figure 5.
SAA levels in 24 weeks post inoculation serum samples as determined by ELISA. Data represent mean ± SD. *p<0.05. Abbreviations are as defined in the text.
Serum proinflammatory cytokine level
Twenty-four week serum samples were analyzed for 62 cytokines (Table 2). Cytokine array data were validated independently by ELISA and found congruent. ApoE+/−-IL-6−/− mice displayed profound increases in most proinflammatory cytokines and chemokines, such as IL-1α, IL-1β, TNF- α, MCP-1, MCP-5, VEGF, L-Selectin, Π-Selectin, VCAM-1, TNFR-I, TNFR-II, M-CSF, and CD40 relative to ApoE+/−-IL-6+/− mice only in diet-and or bacterial-challenged animals; no significant difference was observed in the cytokine levels in chow saline groups. The most significant increase was seen in mice with high fat diet and inoculated with P.g. (HP). However, none of the groups demonstrated any changes in the anti-inflammatory cytokines such as IL-10 and IL-4. To validate the cytokine array data, selected cytokines (VCAM-1, L-Selectin, VEGF, MCP-1 and IL-10) were measured using the ELISA. The level of cytokines detected using ELISA was consistent with cytokine array data and representative data is shown in table 3.
Table-3.
Cytokine level detected using ELISA
| ApoE+/−-IL-6+/− | ApoE+/−-IL-6−/− | p-value* | |
|---|---|---|---|
| Number of mice (n) | 8 | 8 | |
| VEGF (pg/ml) | |||
| CHOW DIET + VEHICLE | 30.1±11 | 41.2±18 | >0.05 |
| CHOW DIET + P.G | 47.3±33 | 144.2±49 | <0.05 |
| HFD + VEHICLE | 58.1±54 | 223.4±63 | <0.05 |
| HFD + P.G | 85.0±53 | 310.6±43 | <0.05 |
| VCAM-1 (pg/ml) | |||
| CHOW DIET + VEHICLE | 261.4±23 | 301.3±32 | >0.05 |
| CHOW DIET + P.G | 352.4±43 | 965.2±67 | <0.05 |
| HFD + VEHICLE | 481.3±54 | 1106±72 | <0.05 |
| HFD + P.G | 676.6±65 | 1645±74 | <0.05 |
| L-selectin (pg/ml) | |||
| CHOW DIET + VEHICLE | 643.2±20 | 685.3±23 | >0.05 |
| CHOW DIET + P.G | 718.0±58 | 2151.5±76 | <0.05 |
| HFD + VEHICLE | 920.0±76 | 1909.3±54 | <0.05 |
| HFD + P.G | 1145.0±34 | 2558.1±68 | <0.05 |
| MCP-1 (pg/ml) | |||
| CHOW DIET + VEHICLE | 20.34±11 | 25.2±10 | >0.05 |
| CHOW DIET + P.G | 40.4±21 | 170.5±34 | <0.05 |
| HFD + VEHICLE | 168.9±45 | 356.2±65 | <0.05 |
| HFD + P.G | 242.5±63 | 549.6±78 | <0.05 |
| IL-10 (pg/ml) | |||
| CHOW DIET + VEHICLE | 455.2±8.1 | 464.2±3.4 | >0.05 |
| CHOW DIET + P.G | 462.4±3.9 | 468.5±9.8 | >0.05 |
| HFD + VEHICLE | 469.9±6.5 | 474.3±7.6 | >0.05 |
| HFD + P.G | 484.5±8.3 | 487.3±10.8 | >0.05 |
Significance between ApoE+/−-IL-6−/− and ApoE+/−-IL-6+/− for respective groups. Abbreviattions are as defined in the text.
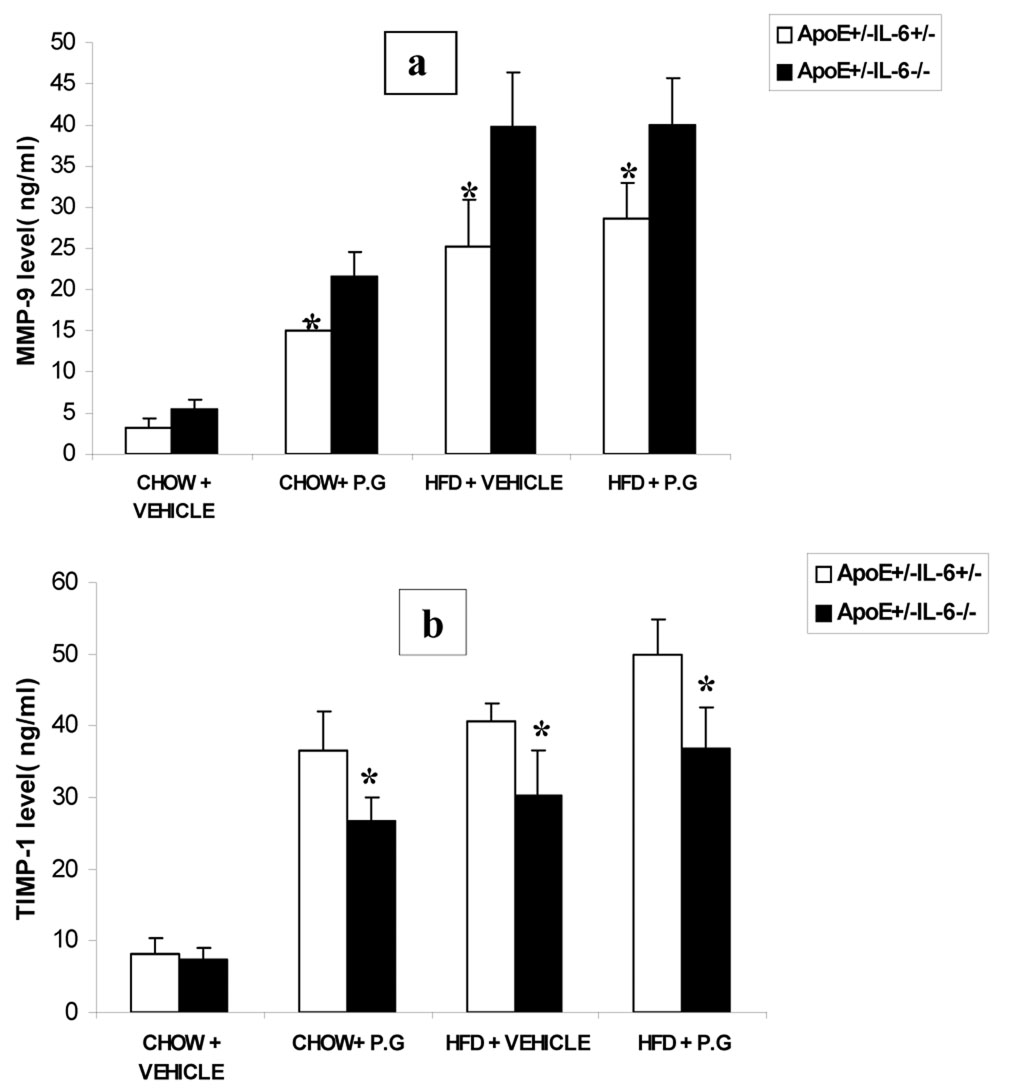
Total MMP-9 and TIMP-1 levels in serum and heart tissues
Total MMP-9 levels increased significantly by 48%, 53% and 43% and TIMP-1 levels decreased significantly by 22%, 24% and 23% in ApoE+/−-IL-6−/− mice when compared to ApoE+/−-IL-6+/−mice in high fat diet inoculated with P.g ,high fat diet injected with vehicle and chow diet inoculated with P.g respectively; no significant difference was observed in the chow saline groups (fig-6). ELISA for protein levels in heart tissue exhibited similar results (data not shown).
Figure 6.
Level of total MMP-9(6a; ng/ml) and TIMP-1(6b; ng/ml) assessed in serum at 24 week by ELISA. Data represent mean ± SD*p< 0.05.
DISCUSSION
The purpose of this investigation was to establish the role of IL-6 in inflammation and to test the hypothesis that blocking IL-6 signaling by genetic deletion alters the atherogenic process that can be enhanced by bacteria (P. gingivalis) or high fat diet in an ApoE+/− murine model. Both enface and histomorphometric data revealed a greater percentage of the aorta and aortic lumen being occupied by the atherosclerotic lesions in ApoE+/−-IL-6−/− mice. IL-6-deficient mice fed with high fat diet and inoculated with P.g. (HP) exhibited greater lesions compared to mice fed with high fat diet and receiving vehicle (HS) and mice fed with chow diet and inoculated with P.g. (CP).
Highly proteolytic organisms like P.g. possess important virulence factors (gingipains, fimbrillin peptides, capsule polysaccharides, lipopolysaccharides, outer membrane vesicles and proteases) which influence the balance between pro- and anti-inflammatory mediators 29 and aggravate atherosclerosis 15. Both the bacterial endotoxins and OxLDL 30, 31 are known activators of cytokine cascades. These cytokines are typically released from stimulated mononuclear phagocytes and augment endothelial cell expression of many vascular cell adhesion molecules implicated in atherogenesis. By augmenting the expression of leukocyte adhesion molecules, bacterial infection and OxLDL could influence the crucial aspects of atherogenesis.
Classical risk factors for atherosclerosis are elevated total cholesterol and LDL and decreased HDL levels 32, 33. In the present study IL-6-deficiency leads to an increase in the serum total cholesterol and LDL and decrease in HDL levels as compared to ApoE+/−- IL-6+/− mice only in diet-and or bacteria-challenged groups. No significant difference in serum total cholesterol, LDL and HDL levels were observed in chow saline groups irrespective of the IL-6 status. Furthermore, it is known that the hyperglycemia can lead to modification of macromolecules, such as forming advanced glycation end products (AGE). By engaging surface receptors such as RAGE (receptor for AGE), AGE-modified proteins can augment the production of proinflammatory cytokines and activate other inflammatory pathways in vascular endothelial cells and lead to increased atherosclerosis 34. Consistent with the current literature, our data demonstrate that high fat diet and/or P.g., stimulated an elevation in serum glucose which in the absence of IL-6 amplified the atherosclerosis phenotype observed while chow saline animals did not exhibit serum glucose elevation irrespective of the IL-6 status. Altogether the present data strongly suggest that diet- and or bacterial exposure are responsible for the blood chemistry changes observed irrespective of the IL-6 status.
It has been shown that pro-atherosclerotic factors such as OxLDL, reactive oxygen species, high glucose, and inflammatory cytokines activate apoptotic pathways in cellular elements of the lesion 35–37. Various studies have demonstrated that vulnerable or unstable plaques are rich in inflammatory cells and exhibit a substantial increase in apoptotic cell death leading to the formation of a highly thrombogenic lipid core 38–40. Histological analysis of the proximal aorta with specific staining for smooth muscle cells, macrophages and tunnel positive apoptotic cells to determine plaque stability, demonstrated that the composition of advanced lesions was profoundly affected by bacteria-challenged IL-6-deficient mice. IL-6-deficient mice fed a high fat diet and/or inoculated with P.g. (HP) resulted in increased macrophage accumulation, decreased smooth muscle cell mass and increased apoptosis in lesion areas as compared to IL-6 heterozygote mice. Thus, we conclude that in wild type animals endogenous levels of IL-6 would contribute to the attenuation of the inflammatory process within the atherosclerotic lesions. This result shows also that high fat diet and/or P.g. may initiate the apoptotic pathway in various cellular elements of the plaque explaining the enhanced and unstable atherosclerotic lesions in the IL-6 deficient mice.
One of the earliest pathophysiological changes manifested after an inflammatory stimulus, is altered concentrations of certain plasma proteins synthesized by the liver. Although IL-6 serves as a major mediator, regulating the synthesis of most acute phase proteins, other cytokines such as TNF-α and IL-1 also regulate these proteins41. Our observation of significant increase in the SAA levels in IL-6 deficient mice may stem from TNF-α and IL-1 stimulation.
The evidence implicating cytokines as inflammatory mediators in atherosclerosis led us to survey cytokines to determine their involvement in atherosclerotic progression in ApoE+/−-IL-6−/− and ApoE+/−-IL-6+/− mice under various conditions. Increase in the serum levels of IL-1 α, IL-1 β, MCP-1, MCP-5, TNF-α, VEGF, VCAM-1, P-Selectin, and L-Selectin in IL-6 deficient mice fed with high fat diet and/or inoculated with Pg cannot be disregarded, as they play a critical role in progression of atherosclerosis 7, 18, 42.
It has been shown that peripheral blood levels of MMP’s are elevated in unstable angina 43. Our observation that IL-6 may be involved in plaque stability, can be further explained by the reduced level of TIMP-1 in ApoE+/−-IL-6−/− compared to ApoE+/−-IL-6+/− mice as evidenced in serum and heart tissue. Indeed it is known that reduced TIMPs stimulate oxidative stress and promote gelatinolytic MMP activity in vivo thereby favoring unstable plaque44. The increased MMP activity observed during atherosclerotic plaque development and instability was shown to stem from an increased cytokine and growth factor-stimulated gene transcription, elevated zymogen activation and an imbalance in the MMP: TIMP ratio 45. Our data demonstrate an increase in MMP-9 and a decrease in TIMP-1 levels in serum and heart tissue of diet and/or P.g inoculated ApoE+/−-IL-6−/− mice compared to the ApoE+/−-IL-6+/− mice and suggest that IL-6 deficiency may shift the equilibrium of TIMP-1 and MMP-9 toward a more unbalanced MMP activation thereby promoting unstable atherosclerotic plaques.
Of most prominence was that, in stark contrast to proinflammatory cytokines, the levels of IL-10 in ApoE+/−-IL-6−/− mice were similar to those in ApoE+/−-IL-6+/− mice. This suggests that a balanced interplay between proinflammatory and anti-inflammatory mediators (IL-6 and IL-10) may be involved in the perpetuation of atherosclerosis within the vessel wall, and may modulate the development of atherosclerotic plaques. The mechanism may involve recruiting inflammatory cells to the plaque which implies that one function of IL-6 is to suppress the level of proinflammatory cytokines without compromising the level of anti-inflammatory cytokines. Therefore the absence of IL-6 results in more pronounced response of proinflammatory cytokines.
Conclusions
Lifetime deficiency of IL-6 in the ApoE+/− murine model results not only in enhanced but also unstable atherosclerotic lesion formation, both in response to a high fat diet and/or weekly challenge with live P.g. IL-6 is suggested to have an atheroprotective role in diet-and/or bacteria-challenged animals.
Acknowledgments
This study was supported by National Institutes of Health grants #RO1 HL 76801 (to S. Amar)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Epstein SE, Zhou YF, Zhu J. Infection and atherosclerosis: emerging mechanistic paradigms. Circulation. 1999;100:e20–e28. doi: 10.1161/01.cir.100.4.e20. [DOI] [PubMed] [Google Scholar]
- 3.Doron Aronson, Rayfield Elliot J. How hyperglycemia promotes atherosclerosis: molecular mechanism. Cardiovascular Diabetology. 2002:1475–2840. doi: 10.1186/1475-2840-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvaro-Gonzalez LC, Freijo-Guerrero MM, Sadaba-Garay F. Inflammatory mechanisms, arteriosclerosis and ischemic stroke: clinical data and perspectives. Rev Neurol. 2002;35:452–462. [PubMed] [Google Scholar]
- 5.Gerrity RG. The role of the monocyte in atherogenesis: II. Migration of foam cells from atherosclerotic lesions. American Journal of Pathology. 1981;103:191–200. [PMC free article] [PubMed] [Google Scholar]
- 6.Ross R. The pathogenesis of atherosclerosis :a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 7.Gosling J, Slaymaker S, Gu L, Tseng S, Zlot CH, Young SG, Rollins BJ, Charo IF. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest. 1999;103:773–778. doi: 10.1172/JCI5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uichi Ikeda MD PhD, Takayuki Ito MD, Kazuyuki Shimada MD PhD Interleukin-6 and Acute Coronary Syndrome. Clin. Cardiol. 2001;24:701–704. doi: 10.1002/clc.4960241103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 10.Fattori E, Cappelletti M, Costa P, Sellitto C, Cantoni L, Carelli M, Faggioni R, Fantuzzi G, Ghezzi P, Poli V. Defective inflammatory response in interleukin 6-deficient mice. J. Exp. Med. 1994;180:1243–1250. doi: 10.1084/jem.180.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruzek MC, Miller AH, Opal SM, Pearce BD, Biron CA. Characterization of Early Cytokine Responses and an Interleukin (IL)-6-dependent Pathway of Endogenous Glucocorticoid Induction during Murine Cytomegalovirus Infection. J. Exp. Med. 1997;185:1185–1192. doi: 10.1084/jem.185.7.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei X-F, Achong MK. IL-6 Is an Antiinflammatory Cytokine Required for Controlling Local or Systemic Acute Inflammatory Responses. J. Clin. Invest. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schieffer B, Selle T, Hilfiker A, Hilfiker-Kleiner D, Grote K, Tietge UJF, Trautwein C, Luchtefeld M, Schmittkamp C, Heeneman S, Daemen MJAP, Drexler H. Impact of Interleukin-6 on Plaque Development and Morphology in Experimental Atherosclerosis. Circulation. 2004;110:3493–3500. doi: 10.1161/01.CIR.0000148135.08582.97. [DOI] [PubMed] [Google Scholar]
- 14.Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R. Interleukin-6 Exacerbates Early Atherosclerosis in Mice. Arterioscler Thromb Vasc Biol. 1999;19:2364–2367. doi: 10.1161/01.atv.19.10.2364. [DOI] [PubMed] [Google Scholar]
- 15.Li L, Messas E, Batista EL, Jr, Levine RA, Amar S. Porphyromonas gingivalis infection accelerates the progression of atherosclerosis in a heterozygous apolipoprotein E-deficient murine model. Circulation. 2002;105:861–867. doi: 10.1161/hc0702.104178. [DOI] [PubMed] [Google Scholar]
- 16.Pussinen PJ, Mattila K. Periodontal infections and atherosclerosis: mere associations? Curr Opin Lipidol. 2004;15:583–588. doi: 10.1097/00041433-200410000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Genco R, Offenbacher S, Beck J. Periodontal disease and cardiovascular disease: epidemiology and possible mechanisms. J Am Dent Assoc. 2002;133 Suppl:14S–22S. doi: 10.14219/jada.archive.2002.0375. [DOI] [PubMed] [Google Scholar]
- 18.Chi H, Messas E, Levine RA, Graves DT, Amar S. Interleukin-1 receptor signaling mediates atherosclerosis associated with bacterial exposure and/or a high-fat diet in a murine apolipoprotein E heterozygote model: pharmacotherapeutic implications. Circulation. 2004;110:1678–1685. doi: 10.1161/01.CIR.0000142085.39015.31. [DOI] [PubMed] [Google Scholar]
- 19.Bhanji SWB, Sheller B, Elwood T, Mancl L. Transient bacteremia induced by toothbrushing a comparison of the Sonicare toothbrush with a conventional toothbrush. Pediatr Dent. 2002 Jul–Aug;24:295–299. [PubMed] [Google Scholar]
- 20.Elhage R, Clamens S, Besnard S, Mallat Z, Tedgui A, Arnal JF, Maret A, Bayard F. Involvement of interleukin-6 in atherosclerosis but not in the prevention of fatty streak formation by 17[beta]-estradiol in apolipoprotein E-deficient mice. Atherosclerosis. 2001;156:315–320. doi: 10.1016/s0021-9150(00)00682-1. [DOI] [PubMed] [Google Scholar]
- 21.Wank HALM, Rose LF, Cohen DW. A quantitative measurement of bacteremia and its relationship to plaque control. J Periodontol. 1976 Dec;47:683–686. doi: 10.1902/jop.1976.47.12.683. [DOI] [PubMed] [Google Scholar]
- 22.Sukovich DA, Kauser K, Shirley FD, DelVecchio V, Halks-Miller M, Rubanyi GM. Expression of Interleukin-6 in Atherosclerotic Lesions of Male ApoE-Knockout Mice : Inhibition by 17β-Estradiol. Arterioscler Thromb Vasc Biol. 1998;18:1498–1505. doi: 10.1161/01.atv.18.9.1498. [DOI] [PubMed] [Google Scholar]
- 23.Palinski W, Ord VA, Plump AS, Breslow JL, Steinberg D, Witztum JL. ApoE-deficient mice are a model of lipoprotein oxidation in atherogenesis. Demonstration of oxidation-specific epitopes in lesions and high titers of autoantibodies to malondialdehyde-lysine in serum. Arterioscler Thromb. 1994;14:605–616. doi: 10.1161/01.atv.14.4.605. [DOI] [PubMed] [Google Scholar]
- 24.Madan M, Bishayi B, Hoge M, Messas E, Amar S. Doxycycline affects diet- and bacteria-associated atherosclerosis in an ApoE heterozygote murine model: Cytokine profiling implications. Atherosclerosis. 2007;190:62–72. doi: 10.1016/j.atherosclerosis.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 25.Plump AS, Smith JD, Hayek T, Aalto-Setala K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 26.Mallat Z, Corbaz A, Scoazec A, Graber P, Alouani S, Esposito B, Humbert Y, Chvatchko Y, Tedgui A. Interleukin-18/Interleukin-18 Binding Protein Signaling Modulates Atherosclerotic Lesion Development and Stability. Circ Res. 2001;89:41e–45e. doi: 10.1161/hh1901.098735. [DOI] [PubMed] [Google Scholar]
- 27.Lu H, Raptis M, Black E, Stan M, Amar S, Graves DT. Influence of Diabetes on the Exacerbation of an Inflammatory Response in Cardiovascular Tissue. Endocrinology. 2004;145:4934–4939. doi: 10.1210/en.2004-0737. [DOI] [PubMed] [Google Scholar]
- 28.Kadokami T, McTiernan CF, Kubota T, Frye CS, Feldman AM. Sex-related survival differences in murine cardiomyopathy are associated with differences in TNF-receptor expression. J. Clin. Invest. 2000;106:589–597. doi: 10.1172/JCI9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kantarci A, Van Dyke TE. Neutrophil-mediated host response to Porphyromonas gingivalis. J Int Acad Periodontol. 2002;4:119–125. [PubMed] [Google Scholar]
- 30.Hulthe J, Fagerberg B. Circulating oxidized LDL is associated with subclinical atherosclerosis development and inflammatory cytokines (AIR Study) Arterioscler Thromb Vasc Biol. 2002;22:1162–1167. doi: 10.1161/01.atv.0000021150.63480.cd. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Q, Desta T, Fenton M, Graves DT, Amar S. Cytokine Profiling of Macrophages Exposed to Porphyromonas gingivalis, Its Lipopolysaccharide, or Its FimA Protein. Infect. Immun. 2005;73:935–943. doi: 10.1128/IAI.73.2.935-943.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross R, Glomset JA. The pathogenesis of atherosclerosis (second of two parts) N Engl J Med. 1976;295:420–425. doi: 10.1056/NEJM197608192950805. [DOI] [PubMed] [Google Scholar]
- 33.Ross R, Glomset JA. The pathogenesis of atherosclerosis (first of two parts) N Engl J Med. 1976;295:369–377. doi: 10.1056/NEJM197608122950707. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt AM, Yan SD, Wautier J-L, Stern D. Activation of Receptor for Advanced Glycation End Products : A Mechanism for Chronic Vascular Dysfunction in Diabetic Vasculopathy and Atherosclerosis. Circ Res. 1999;84:489–497. doi: 10.1161/01.res.84.5.489. [DOI] [PubMed] [Google Scholar]
- 35.Dimmeler S, Hermann C, Zeiher AM. Apoptosis of endothelial cells. Contribution to the pathophysiology of atherosclerosis? Eur Cytokine Netw. 1998;9:697–698. [PubMed] [Google Scholar]
- 36.Hegyi L, Hardwick SJ, Siow RC, Skepper JN. Macrophage death and the role of apoptosis in human atherosclerosis. J Hematother Stem Cell Res. 2001;10:27–42. doi: 10.1089/152581601750098192. [DOI] [PubMed] [Google Scholar]
- 37.Scheidegger KJ, James RW, Delafontaine P. Differential Effects of Low Density Lipoproteins on Insulin-like Growth Factor-1 (IGF-1) and IGF-1 Receptor Expression in Vascular Smooth Muscle Cells. J. Biol. Chem. 2000;275:26864–26869. doi: 10.1074/jbc.M002887200. [DOI] [PubMed] [Google Scholar]
- 38.Davies MJ. The Composition of Coronary-Artery Plaques. N Engl J Med. 1997;336:1312–1314. doi: 10.1056/NEJM199705013361809. [DOI] [PubMed] [Google Scholar]
- 39.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons From Sudden Coronary Death : A Comprehensive Morphological Classification Scheme for Atherosclerotic Lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 40.Mallat Z, Hugel B, Ohan J, Leseche G, Freyssinet J-M, Tedgui A. Shed Membrane Microparticles With Procoagulant Potential in Human Atherosclerotic Plaques : A Role for Apoptosis in Plaque Thrombogenicity. Circulation. 1999;99:348–353. doi: 10.1161/01.cir.99.3.348. [DOI] [PubMed] [Google Scholar]
- 41.Darlington GJ, Wilson DR, Revel M, Kelly JH. Response of liver genes to acute phase mediators. Ann N Y Acad Sci. 1989;557:310–315. doi: 10.1111/j.1749-6632.1989.tb24023.x. discussion 315–316. [DOI] [PubMed] [Google Scholar]
- 42.Johnson-Tidey RR, McGregor JL, Taylor PR, Poston RN. Increase in the adhesion molecule P-selectin in endothelium overlying atherosclerotic plaques. Coexpression with intercellular adhesion molecule-1. Am J Pathol. 1994;144:952–961. [PMC free article] [PubMed] [Google Scholar]
- 43.Hisashi Kai HI, Hideo Yasukawa, Mamiko Kai, Yukihiko Seki, FumitakaKuwahara, Takafumi Ueno, Kenzo Sugi, Tsutomu Imaizumi Peripheral blood levels of matrix metalloproteases-2 and -9 are elevated in patients with acute coronary syndromes. J. Am. Coll. Cardiol. 1998;32:368–372. doi: 10.1016/s0735-1097(98)00250-2. 368–372. [DOI] [PubMed] [Google Scholar]
- 44.Pawlak K, Pawlak D, Mysliwiec M. Extrinsic coagulation pathway activation and metalloproteinase-2/TIMPs system are related to oxidative stress and atherosclerosis in hemodialysis patients. Thromb Haemost. 2004;92:646–653. doi: 10.1160/TH04-02-0128. [DOI] [PubMed] [Google Scholar]
- 45.Xu X-P, Meisel SR, Ong JM, Kaul S, Cercek B, Rajavashisth TB, Sharifi B, Shah PK. Oxidized Low-Density Lipoprotein Regulates Matrix Metalloproteinase-9 and Its Tissue Inhibitor in Human Monocyte-Derived Macrophages. Circulation. 1999;99:993–998. doi: 10.1161/01.cir.99.8.993. [DOI] [PubMed] [Google Scholar]