Abstract
Immunoglobulins (Igs) that bind amyloid β peptide (Aβ) are under clinical trials for immunotherapy of Alzheimer disease (AD). We have identified IgMs and recombinant Ig fragments that hydrolyze Aβ. Hydrolysis of peripheral Aβ by the IgMs may induce increased Aβ release from the brain. The catalytic IgMs are increased in AD patients, presumably reflecting a protective autoimmune response. Reduced Aβ aggregation and neurotoxicity attributable to the catalytic function were evident. These findings provide a foundation for development of catalytic Igs for AD immunotherapy.
Keywords: Catalytic antibodies, proteolytic IgM, amyloid β peptide, Alzheimer’s disease, immunotherapy
Take-home messages
Human IgM autoantibodies hydrolyze Aβ via a serine protease-like mechanism. Brain and peripheral Aβ are in equilibrium with each other. Peripheral Aβ hydrolysis may induce depletion of the brain Aβ stores without IgM passage across the BBB.
Recombinant Ig fragments that hydrolyze Aβ have been isolated from human libraries. Theoretically, catalytic Igs that enter the brain are predicted to clear Aβ deposits without inducing inflammatory and microhemorrhage effects associated with conventional Igs.
Catalytic autoantibodies appear to represent a natural protective mechanism against AD. High activity catalytic Igs isolated from the human repertoire are candidates for for further consideration as immunotherapeutic agents.
1. Immunotherapy of Alzheimer disease (AD)
Approximately 26 million humans have AD worldwide. No truly effective therapy is available for AD treatment. Accumulation of amyloid β (Aβ) peptide aggregates is thought to play a crucial role in the neurodegenerative events underlying AD.1 The Aβ aggregates are composed of 39–43 residue peptides generated by proteolytic processing of amyloid precursor protein (APP) by the β- and γ-secretases. The predominant product of this processing pathway is Aβ1-40 (Aβ40; corresponding to APP residues 597–636) with Aβ1-42 (Aβ42; APP residues 597–638) being the next-most abundant product.2 Aβ42 and Aβ40 form oligomeric aggregates and fibrillar structures.3 Aβ42 aggregates more rapidly, and is the majority species in amyloid plaques characteristic of the AD brain.2 In peripheral blood, the major species is Aβ40.4 Soluble Aβ oligomers impair neuronal function by altering the expression of memory-related receptors,5 inducing aberrant neuronal responses to electrical stimulation6 and inducing neural death.3,7
Aβ has emerged as a promising target for immunotherapeutic intervention in AD. Two issues developed in clinical trials of active immunization of AD patients with Aβ42.8 First, only about 20% of the recipients developed Aβ binding antibodies. Second, the trials were suspended because 5% of the immunized AD patients developed sterile encephalitis. Passive immunotherapy approaches for AD are under development. Transgenic mice expressing mutant human APP genes (APP-Tg mice) develop an age-associated increase in cerebral Aβ as well as cognitive decline.9,10 Peripheral administration of monoclonal and polyclonal IgG class Aβ binding antibodies to APP-Tg mice clears brain Aβ deposits and improves various behavioral indices.11 Aβ binding autoantibodies are present in healthy humans and AD patients.12,13 Pooled human IgG marketed as IVIG formulations (intravenously administered immunoglobulins) contains small amounts of Aβ binding autoantibodies.14 A phase I trial entailing intravenous administration of large IVIG doses to AD patients (1.2 grams/kg over 3 days) was encouraging.14 Humanized monoclonal IgGs are under clinical trials at smaller dose levels (1–5 mg/kg; Wyeth-Elan, Lily). Humanization is the process of substituting human IgG sequences for conserved murine IgG sequences while leaving in place the complementarity determining region sequences necessary for Aβ binding. This reduces infusion reactions, anaphylactic reactions and induction of neutralizing antibodies to the IgG in human recipients.
IgGs are proposed to reduce Aβ deposition in the brain by the following mechanisms: (a) Small amounts of peripherally administered IgGs (~0.1% of injected dose) that cross the blood-brain barrier (BBB) bind Aβ in the brain, and Fcγ-receptor mediated immune complex ingestion by microglial cells clears Aβ15 (Table 1)16; (b) The IgGs can also bind the neonatal Fc receptor (FcRn) located on the abluminal (brain) side of the endothelial cells constituting the blood-brain barrier (BBB), thereby facilitating Aβ efflux into the periphery17; (c) Aβ binding to IgG may constrain the peptide into a non-aggregable conformation18; and (d) according to the ‘peripheral sink’ hypothesis,19 Aβ is cleared from the brain without IgG entry into the brain. In this hypothesis peripheral Aβ-antibody binding perturbs the equilibrium between the peptide pools in the brain and periphery, thereby stimulating Aβ release from the brain. In principle, these mechanisms are not mutually exclusive and may be triggered by the same IgG.
Table 1. Proposed mechanisms for antibody dependent brain Aβ clearance.
| Clearance mechanism | Site of action | Undesired effects |
|---|---|---|
| Fc receptor mediated microglial uptake of immune complexes | Central | Inflammatory mediator release, microhemorrhages |
| Fc receptor (FcRn) mediated Aβ efflux into periphery | ||
| Induction of non-aggregable Aβ conformation | ||
| Catalytic small Ig mediated Aβ clearance | Central | Little or no inflammation and microhemorrhages anticipated |
| Catalytic IgM mediated Aβ clearance | Peripheral |
2. Catalytic autoantibodies to Aβ
Our approach to developing immunotherapeutic reagents for AD is based on the expression of specific proteolytic activity by naturally occurring Igs.20 The antigen combining site of Igs is composed of light and heavy chain variable domains (VL and VH domains) derived from about 50 germline V gene segments each. Proteolytic Igs are present in the preimmune repertoire21 and under certain circumstances, they can be improved by adaptive immunological selection processes.20 Ig proteolytic sites display nucleophilic character and utilize covalent catalysis mechanisms similar to classical serine proteases. Nucleophilic triads have been identified by mutagenesis and crystallography in proteolytic Igs.22,23 Electrophilic phosphonates originally synthesized as probes for enzymatic nucleophiles react covalently with Ig proteolytic sites.24 Proteolytic Igs react specifically and irreversibly with peptide analogs containing electrophilic phosphonates within their antigenic epitopes, indicating that noncovalent binding renders the nucleophilic reactivity specific for the cognate antigen.25
We have reported the proteolytic activity of a panel of 10 monoclonal IgMs from patients with Waldenström’s macroglobulinemia using model peptide substrates.26 Two monoclonal IgMs from this panel also hydrolyzed Aβ40 and Aβ42.27 Neither Aβ40-hydrolyzing IgM displayed binding of biotinylated Aβ40 in an ELISA test. Electrospray ionization-mass spectrometry (ESI-MS) of the product peptides generated by IgMs yielded mass values suggesting that Lys28-Gly29 is the major hydrolysis site and Lys16-Leu17, the minor hydrolysis site (Fig 1A). The catalytic activity of a monoclonal catalytic IgM was titrated using various concentrations of the electrophilic phosphonate diester. This yielded a value of 10.2 catalytic sites/IgM molecule, compared to the theoretical value of 10 antigen combining sites.27 The catalytic activity was retained in the Fab fragments of the IgM and the activity maintained at constant levels following successive purification steps,26 indicating that the activity is attributable to the IgM.
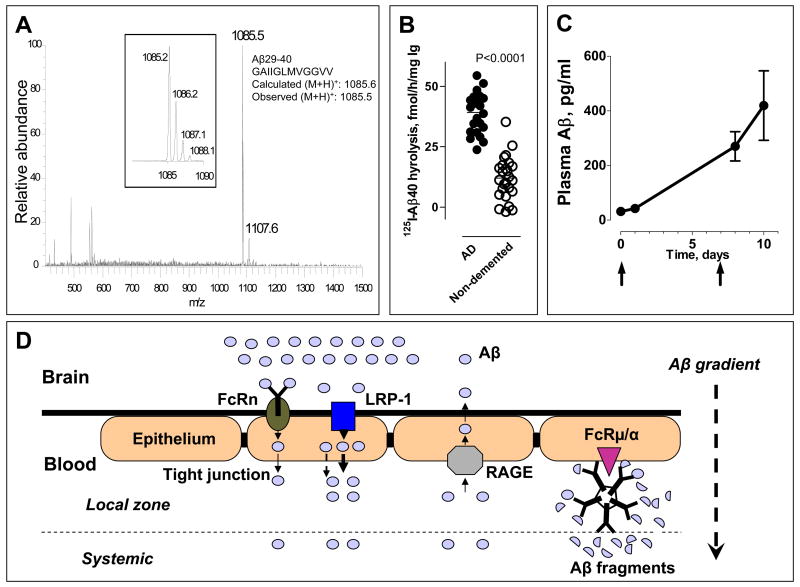
Fig 1.
(A) Aβ40 peptide bonds hydrolyzed by monoclonal IgM Yvo. ESI-MS spectrum of Aβ fragments generated by IgM. Inset, Zoom scan of spectrum region around m/z peak 1085.5, corresponding to the exact theoretical m/z for singly charged (M+H)+ ion of Aβ29-40. The peak-splitting in the zoom scan reflects the natural isotopic distribution of the singly charged peptide ions. (B) Increased Aβ hydrolysis by IgMs from patients with Alzheimer’s disease. Shown are values of 125I-Aβ1-40 (0.1 nM) hydrolysis (means of duplicates) incubated with the IgM preparations (0.023 mg/ml) purified from AD patients (n=23) and elderly, non-demented control subjects (n=25). Each point represents a different human subject. 2-tailed unpaired t-test.(C) Plasma Aβ mobilization induced by peripheral catalytic IgM administration. Pooled polyclonal IgM from human subjects purified by affinity chromatography using immobilized anti-IgM antibody was injected (360 μg) intravenously into APP/PS1-Tg mice (n=3) on day 0 and 7. Plasma Aβ was measured before and after IgM administration by an ELISA kit that detects intact Aβ40. The Aβ hydrolyzing activity of the human IgM is described in ref 27. (D) Hypothesis of catalytic IgM accelerated Aβ efflux from the brain. Hydrolysis of Aβ by peripherally IgM increases the Aβ brain-periphery concentration difference and thereby enhances Aβ efflux from the brain. Catalytic IgM bound to FcRμ/α on the luminal (blood) side of the blood-brain-barrier will accentuate the local concentration difference and may facilitate sustained brain Aβ depletion. FcRμ/α, Fc receptor for IgM. FcRn, neonatal Fc receptor. LRP-1, low-density lipoprotein receptor related protein 1; RAGE, receptor for advanced glycation end products.
To evaluate disease association, we studied the hydrolysis of 125I-Aβ40 by IgMs purified from the sera of AD patients and age-matched elderly subjects without dementia. Twenty two of the 25 IgM preparations from undemented elderly humans studied displayed detectable 125I-Aβ40 hydrolytic activity varying over a 118-fold range. This suggests polymorphic and variable catalytic IgM response in different individuals. IgMs from the AD group displayed superior hydrolytic activity (P<0.0001; Fig. 1B). The IgMs did not hydrolyze irrelevant polypeptides determined by an electrophoresis assay.27 It may be concluded that increased Aβ40 hydrolysis by IgM preparations from AD patients is not due to an increase of non-specific catalytic activity.
In view of the natural development of catalytic Aβ Igs, libraries of human Ig V domains are promising source of homogeneous proteolytic Igs. Previous studies with recombinant Igs have suggsted that the Aβ hydrolyzing activity can be be traced to Ig VL domains. We have described the hydrolysis of Aβ40 recombinant Ig light chains (IgLs).28 One of these IgLs cleaved Aβ40 at a single peptide bond, Lys16-Leu17. Another IgL displayed more complex cleavage patterns with apparent dependence on the aggregation state of Aβ40. Hydrolysis of highly aggregated Aβ40 by this IgL generated several peptide fragments with length differing only by a single residue each, suggesting an exopeptidase-like reaction28 whereas Aβ in a lesser aggregated state was hydrolyzed mainly at the His14-Gln15 bond.29 We searched for Aβ hydrolyzing catalysts present in a human IgV domain (IgV) library by our previously described screening and electrophilic selection procedures.24 This has permitted identification of IgVL domains that hydrolyze Aβ with catalytic efficiency that are 3–4 magnitudes superior to the polylclonal Ig preparations, suggesting the feasibility of identifying Igs with proteolytic activity sufficient for therapeutic application.
3. Functional catalytic Ig effects
Initial evidence indicating the functional utility of the catalytic function is available as follows. Aβ40 is neurotoxic.3 We studied the viability of SH-SY5Y neuronal cells following treatment with Aβ40 alone, Aβ40 pretreated with IgM Yvo and IgM Yvo alone. The toxic effect of Aβ40 was reduced significantly in the presence of IgM Yvo (P<0.0001).27 The IgM alone did not influence the level of cell viability. Sierks and coworkers have reported similar results using a catalytic Ig light chain with Aβ hydrolyzing activity.7 Oligomeric and fibrillar Aβ structures are thought to be responsible for the neurotoxicity. Using atomic force microscopy, we observed reduced formation of Aβ oligomers (spherical particles with diameter 4–20 nm), protofibrils and short fibrils following Aβ40 treatment with a model catalytic IgM compared to the control reaction mixture of the peptide and a noncatalytic IgM (by 72–83%).27 Aβ40 was present at 13-fold excess over the IgM in this experiment. No Aβ40 binding by the IgM was detected by ELISA. Therefore, the observed effects of IgM Yvo can not be attributed Aβ40 binding.
Peripherally injected monoclonal IgGs (150 kD mass) can cross the BBB at low levels in the APP-Tg mouse model.30 A recent mouse study has raised the possibility of selective transport of a monoclonal IgM into the brain.31 However, IgMs are very large molecules (900 vs 150 kDa). IgM concentrations in the cerebrospinal fluid (CSF) of non-demented humans and AD patients are no different, and the CSF IgM concentrations are very low (<0.001 mg/ml, compared to blood IgM levels of ~2 mg/ml).32 If CSF IgM expresses catalytic activity equivalent to blood-borne IgM, only 0.01% of Aβ40 and Aβ42 present in CSF will undergo IgM-catalyzed hydrolysis in 5 days (CSF Aβ40 and Aβ42 concentrations are ~2.7 and ~0.28 ng/ml, respectively4). It is debatable, therefore, whether catalytic IgMs are present in the brain at concentrations sufficient to degrade Aβ appreciably. In contrast, clearance of large amounts of Aβ found in peripheral blood by the IgMs can be anticipated based on the observed rates of hydrolysis. Peripheral blood Aβ concentrations are ~0.25 ng/ml, respectively.4 The IgM catalytic rates are sufficient to hydrolyze 93 % of blood-borne Aβ in 5 days, corresponding to the half-life of IgM in humans.33 Under similar conditions, noncatalytic IgMs with Kd (equilibrium dissociation constant) equivalent to the observed Michaelis constant (Km) will bind only 7.7 % of the Aβ in blood at equilibrium.
Peripheral and brain Aβ exist in a state of equilibrium. Other groups have observed that peripheral Aβ binding reagents induce the release of Aβ from the brain Aβ,11 leading to suggestions that peripheral administration of Aβ binding IgGs can be applied to clear Aβ from the brain. In a preliminary study, a preparation of catalytic human IgM from pooled human serum was administered intravenously on day 0 and day 8 to 6 month old APP-Tg mice that overexpress human Aβ (APPSwe/PS1ΔE9 mouse strain). A sustained increase of intact Aβ concentrations in peripheral blood determined was evident (Fig 1C). As the injected human IgM did not bind Aβ detectably, the evident increase of pepripheral Aβ is not due to peptide stabilization by formation of immune complexes. This suggests the feasibility of depleting brain Aβ as a consequence of peripheral IgM catalyzed Aβ hydrolysis. Receptors for the Fc region of IgG expressed on the abluminal side of the BBB have recently been implicated in enhancing IgG-dependent Aβ efflux from the brain.17 Fcμ/α receptors expressed on the luminal side of the BBB could fulfill a similar function in enhancing catalytic IgM-dependent efflux of the peptide (Fig 1D). These receptors are abundantly distributed on various cells.34,35 Local IgM-catalyzed Aβ hydrolysis at BBB can be predicted to strengthen the trans-BBB Aβ concentration gradient, resulting in enhanced peptide efflux be in the microenvironment, and explaining the sustained increase of peripheral Aβ concentrations noted after peripheral catalytic IgM administration.
An important consideration is whether the catalytic Igs can cause pathogenic effects. If Aβ fulfils a vital physiological function, its removal may be deleterious. The neurotrophic effects of very low Aβ concentrations in tissue culture have been reported.36 However, there appears to be no physiological purpose for accumulation of Aβ in the brains of AD patients. Therefore, it is assumed that removal of excess Aβ will be without negative effects. Active vaccination of humans with Aβ42 resulted in the development of encephalitis in some AD subjects, a finding attributed to undesirable T lymphocyte responses.37 However, there is no formal proof for this, and the potential negative role of Aβ binding IgGs remains an open issue. This is highlighted by observations of undesirable inflammatory and vascular effects of the IgGs in tissue culture. Aβ-IgG immune complexes bind Fcγ receptors expressed by microglial cells and induce the release of inflammatory mediators.16 This could exacerbate the already inflamed state of AD brain. In mouse AD models, clearance of amyloid plaques from the brain parenchyma induced by Aβ-binding IgGs can be accompanied by Aβ deposition in the blood vessels and microhemorrhages.38,39 In human trials of the Wyeth-Elan humanized monoclonal IgG, abnormal magnetic resonance images suggestive of angiogenic edema have been observed.40 In theory, catalytic Igs can be predicted to exert lesser side effects compared to Aβ binding IgGs. If the catalytic rate constant is sufficiently rapid, stable immune complexes will not be formed, and Fc receptor mediated inflammatory release from inflammatory cells should be precluded. Concerning microhemorrhages, provided the Aβ fragments generated by the catalysts are less aggregogenic than intact Aβ, permanent clearance of Aβ from the brain should occur, and no catalytic Ig-induced Aβ deposition in the blood vessel walls in anticipated.
4. Conclusions
Ig-catalyzed Aβ hydrolysis is a novel mechanism that may afford clearance of large amounts Aβ. A single catalyst molecule permanently inactivates thousands of target antigen molecules. Therefore, the biological efficacy of proteolytic Igs is predicted to be superior compared to Igs that bind Aβ reversibly and stoichiometrically. Rapid Aβ hydrolysis precludes long-lived immune complexes. Aβ fragments are less aggregation-prone than intact Aβ. The catalytic function, therefore, also reduces the known negative effects of conventional Aβ binding IgGs that can cross the BBB, that is, Fc receptor-mediated inflammatory reactions, Aβ deposition in blood vessels, and cerebral microhemorrhages.38,39 AD patients produce increased amounts of Aβ hydrolyzing IgMs. IgMs are unlikely to cross the BBB in substantial amounts. However, the peripheral clearance of Aβ can induce release of brain Aβ stores. As Aβ does not fulfill any known function in the periphery, the observed catalytic autoantibodies to Aβ may represent a defensive immune response. Further studies of the relationship between catalytic IgM production and disease progression will be instructive. High activity Aβ hydrolyzing Igs have been identified from recombinant Ig libraries. In view of the advantages of the catalytic function, these are suitable for development as candidate reagents for AD immunotherapy.
Acknowledgments
This work was supported by the US National Institutes of Health (1R01AG025304).
References
- 1.Walsh DM, Selkoe DJ. Abeta Oligomers - a decade of discovery. J Neurochem. 2007;101(5):1172–84. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe MS. Secretase as a target for Alzheimer’s disease. Curr Top Med Chem. 2002;2(4):371–83. doi: 10.2174/1568026024607535. [DOI] [PubMed] [Google Scholar]
- 3.Dahlgren KN, Manelli AM, Stine WB, Jr, Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem. 2002;277(35):32046–53. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- 4.Ida N, Hartmann T, Pantel J, et al. Analysis of heterogeneous A4 peptides in human cerebrospinal fluid and blood by a newly developed sensitive Western blot assay. J Biol Chem. 1996;271(37):22908–14. doi: 10.1074/jbc.271.37.22908. [DOI] [PubMed] [Google Scholar]
- 5.Palop JJ, Chin J, Mucke L. A network dysfunction perspective on neurodegenerative diseases. Nature. 2006;443(7113):768–73. doi: 10.1038/nature05289. [DOI] [PubMed] [Google Scholar]
- 6.Knobloch M, Farinelli M, Konietzko U, Nitsch RM, Mansuy IM. Abeta oligomer-mediated long-term potentiation impairment involves protein phosphatase 1-dependent mechanisms. J Neurosci. 2007;27(29):7648–53. doi: 10.1523/JNEUROSCI.0395-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu R, McAllister C, Lyubchenko Y, Sierks MR. Proteolytic antibody light chains alter beta-amyloid aggregation and prevent cytotoxicity. Biochemistry. 2004;43(31):9999–10007. doi: 10.1021/bi0492354. [DOI] [PubMed] [Google Scholar]
- 8.Gilman S, Koller M, Black RS, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64(9):1553–62. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 9.Games D, Adams D, Alessandrini R, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373(6514):523–7. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 10.Morgan D, Diamond DM, Gottschall PE, et al. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408(6815):982–5. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 11.Wilcock DM, Rojiani A, Rosenthal A, et al. Passive immunotherapy against Abeta in aged APP-transgenic mice reverses cognitive deficits and depletes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage. J Neuroinflammation. 2004;1(1):24. doi: 10.1186/1742-2094-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weksler ME, Relkin N, Turkenich R, LaRusse S, Zhou L, Szabo P. Patients with Alzheimer disease have lower levels of serum anti-amyloid peptide antibodies than healthy elderly individuals. Exp Gerontol. 2002;37(7):943–8. doi: 10.1016/s0531-5565(02)00029-3. [DOI] [PubMed] [Google Scholar]
- 13.Mruthinti S, Buccafusco JJ, Hill WD, et al. Autoimmunity in Alzheimer’s disease: increased levels of circulating IgGs binding Abeta and RAGE peptides. Neurobiol Aging. 2004;25(8):1023–32. doi: 10.1016/j.neurobiolaging.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Dodel RC, Du Y, Depboylu C, et al. Intravenous immunoglobulins containing antibodies against beta-amyloid for the treatment of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2004;75(10):1472–4. doi: 10.1136/jnnp.2003.033399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schenk D, Hagen M, Seubert P. Current progress in beta-amyloid immunotherapy. Curr Opin Immunol. 2004;16(5):599–606. doi: 10.1016/j.coi.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Lue LF, Walker DG. Modeling Alzheimer’s disease immune therapy mechanisms: interactions of human postmortem microglia with antibody-opsonized amyloid beta peptide. J Neurosci Res. 2002;70(4):599–610. doi: 10.1002/jnr.10422. [DOI] [PubMed] [Google Scholar]
- 17.Deane R, Sagare A, Hamm K, et al. IgG-assisted age-dependent clearance of Alzheimer’s amyloid beta peptide by the blood-brain barrier neonatal Fc receptor. J Neurosci. 2005;25(50):11495–503. doi: 10.1523/JNEUROSCI.3697-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solomon B, Koppel R, Frankel D, Hanan-Aharon E. Disaggregation of Alzheimer beta-amyloid by site-directed mAb. Proc Natl Acad Sci U S A. 1997;94(8):4109–12. doi: 10.1073/pnas.94.8.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2001:8850–5. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paul S, Nishiyama Y, Planque S, et al. Antibodies as defensive enzymes. Springer Semin Immunopathol. 2005;26(4):485–503. doi: 10.1007/s00281-004-0191-1. [DOI] [PubMed] [Google Scholar]
- 21.Kalaga R, Li L, O’Dell JR, Paul S. Unexpected presence of polyreactive catalytic antibodies in IgG from unimmunized donors and decreased levels in rheumatoid arthritis. J Immunol. 1995;155(5):2695–702. [PubMed] [Google Scholar]
- 22.Ramsland PA, Terzyan SS, Cloud G, et al. Crystal structure of a glycosylated Fab from an IgM cryoglobulin with properties of a natural proteolytic antibody. Biochem J. 2006;395(3):473–81. doi: 10.1042/BJ20051739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao QS, Sun M, Rees AR, Paul S. Site-directed mutagenesis of proteolytic antibody light chain. J Mol Biol. 1995;253(5):658–64. doi: 10.1006/jmbi.1995.0580. [DOI] [PubMed] [Google Scholar]
- 24.Paul S, Tramontano A, Gololobov G, et al. Phosphonate ester probes for proteolytic antibodies. J Biol Chem. 2001;276(30):28314–20. doi: 10.1074/jbc.M102530200. [DOI] [PubMed] [Google Scholar]
- 25.Taguchi H, Burr G, Karle S, et al. A mechanism-based probe for gp120-Hydrolyzing antibodies. Bioorg Med Chem Lett. 2002;12(21):3167–70. doi: 10.1016/s0960-894x(02)00640-6. [DOI] [PubMed] [Google Scholar]
- 26.Planque S, Bangale Y, Song XT, et al. Ontogeny of proteolytic immunity: IgM serine proteases. J Biol Chem. 2004;279(14):14024–32. doi: 10.1074/jbc.M312152200. [DOI] [PubMed] [Google Scholar]
- 27.Taguchi H, Planque S, Nishiyama Y, et al. Autoantibody-catalyzed Hydrolysis of Amyloid {beta} Peptide. J Biol Chem. 2008;283(8):4714–22. doi: 10.1074/jbc.M707983200. [DOI] [PubMed] [Google Scholar]
- 28.Rangan SK, Liu R, Brune D, Planque S, Paul S, Sierks MR. Degradation of beta-amyloid by proteolytic antibody light chains. Biochemistry. 2003;42(48):14328–34. doi: 10.1021/bi035038d. [DOI] [PubMed] [Google Scholar]
- 29.Taguchi H, Planque S, Nishiyama Y, et al. IgM defense enzymes directed to amyloid β peptide. 9th International Conference on Alzheimer’s Disease and Related Disorders; July 17–22, 2004; Philadelphia, PA. pp. P3–418. [Google Scholar]
- 30.Bard F, Cannon C, Barbour R, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6(8):916–9. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 31.Banks WA, Farr SA, Morley JE, Wolf KM, Geylis V, Steinitz M. Anti-amyloid beta protein antibody passage across the blood-brain barrier in the SAMP8 mouse model of Alzheimer’s disease: an age-related selective uptake with reversal of learning impairment. Exp Neurol. 2007;206(2):248–56. doi: 10.1016/j.expneurol.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elovaara I, Icen A, Palo J, Erkinjuntti T. CSF in Alzheimer’s disease. Studies on blood-brain barrier function and intrathecal protein synthesis. J Neurol Sci. 1985;70(1):73–80. doi: 10.1016/0022-510x(85)90189-3. [DOI] [PubMed] [Google Scholar]
- 33.Saxon A, Stiehm E. The B-lymphocyte system; immunologic Disorders. W. B. Saunders Co.; 1989. [Google Scholar]
- 34.Shibuya A, Sakamoto N, Shimizu Y, et al. Fc alpha/mu receptor mediates endocytosis of IgM-coated microbes. Nat Immunol. 2000;1(5):441–6. doi: 10.1038/80886. [DOI] [PubMed] [Google Scholar]
- 35.Shibuya A, Honda S. Molecular and functional characteristics of the Fcalpha/muR, a novel Fc receptor for IgM and IgA. Springer Semin Immunopathol. 2006;28(4):377–82. doi: 10.1007/s00281-006-0050-3. [DOI] [PubMed] [Google Scholar]
- 36.Koo EH, Park L, Selkoe DJ. Amyloid beta-protein as a substrate interacts with extracellular matrix to promote neurite outgrowth. Proc Natl Acad Sci U S A. 1993;90(10):4748–52. doi: 10.1073/pnas.90.10.4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2003;9(4):448–52. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 38.Morgan D. Mechanisms of A beta plaque clearance following passive A beta immunization. Neurodegener Dis. 2005;2(5):261–6. doi: 10.1159/000090366. [DOI] [PubMed] [Google Scholar]
- 39.Burbach GJ, Vlachos A, Ghebremedhin E, et al. Vessel ultrastructure in APP23 transgenic mice after passive anti-Abeta immunotherapy and subsequent intracerebral hemorrhage. Neurobiol Aging. 2007;28(2):202–12. doi: 10.1016/j.neurobiolaging.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Black RS, Sperling R, Kirby L, Safirstein B, Motter R, Pallay A. A single ascending dose study of bapineuzumab, a humanized monoclonal antibody to Aβ in AD. The 9th International Geneva/Springfield Symposium on Advances in Alzheimer Therapy; April 19–22, 2006; p. 21. [Google Scholar]