Abstract
The ability of bilateral medial temporal lobe amnesic patients (MT; n = 8) and normal participants (NC; n = 8) to acquire a conditional discrimination in trace and delay eyeblink conditioning paradigms was investigated. Experiment 1 assessed trace conditional discrimination learning by using a light conditional stimulus (S+/S−) and tone conditioned stimulus (CS) separated by a 1-s trace. NCs responded differentially on S+ trials (mean percent conditioned responses = 66) versus S− trials (30), whereas MTs were impaired in their acquisition of the conditional discrimination (S+ = 51, S− = 43). In Experiment 2, the temporal separation was eliminated. NCs acquired the conditional discrimination (S+ = 70, S− = 29). MTs were unable to respond differentially (S+ = 42, S− = 37). The findings indicate that the hippocampal system is essential in acquiring a conditional discrimination, even in a delay paradigm.
The hippocampus and related structures play a crucial role in learning. One type of learning in which the hippocampus has been implicated is the acquisition of a conditional discrimination, a form of complex associative learning involving two conditions of differential reinforcement (Daum, Channon, & Canavan, 1989; Daum Channon, Polkey, & Gray, 1991).
Investigations of associative learning in memory-disordered individuals using the eyeblink classical conditioning paradigm have ranged from relatively simple learning tasks, such as simple discrimination learning, to more complex and demanding paradigms, including trace discrimination learning and conditional discrimination learning. Daum, Channon, and Gray (1992) investigated simple two-tone discrimination learning in 16 patients who had undergone right or left unilateral temporal lobe resections as compared with a control group. Temporal lobectomy patients, regardless of laterality, were able to learn a simple two-tone discrimination and then extinguish the response to the same extent as the normal control participants.
Daum, Breitenstein, Ackermann, and Schugens (1997) later investigated discrimination reversal learning in amnesic patients. Nine amnesic patients (including both hippocampal and diencephalic damage) and 9 matched control participants were tested in both a simple discrimination and a discrimination reversal task. Similar to the control participants, amnesic patients produced a significantly greater number of conditioned responses (CRs) on reinforced trials (conditioned stimulus [CS]+) than on nonrein-forced trials (CS−) during discrimination learning, replicating Daum et al.'s (1992) earlier study. During reversal learning, however, the amnesic patients produced a similar number of CRs during both trial types, whereas the control participants were able to reverse the discrimination by extinguishing CRs to the old CS+ and producing CRs to the old CS−. Because the authors did not analyze the data on the basis of etiology, it is difficult to draw conclusions about the role of specific neural substrates (hippocampal vs. diencephalic structures) in these tasks. These initial studies by Daum's laboratory support the notion that simple delay discrimination is likely spared in bilateral medial temporal lobe as well as diencephalic damage.
To further define the role of the hippocampal system in particular, Carrillo and colleagues (Carrillo et al., 2001) conducted an investigation of discrimination reversal learning in the context of a delay paradigm in a group of 8 bilateral medial temporal lobe amnesic patients and matched controls. The medial temporal amnesic patients were able to learn the initial discrimination but were unable to reverse the previously acquired discrimination during the reversal learning phase, producing a similar number of CRs on both the CS+ and CS− trials. These results further support the idea that the hippocampal system is not essential in initial acquisition of a delay discrimination paradigm but becomes more critical as task complexity increases, such as during discrimination reversal.
Clark and Squire (1998) also investigated simple delay and trace discrimination in 4 severely amnesic patients and 48 normal control participants. Results from this comprehensive study indicated that amnesic patients were able to acquire CRs differentially, depending on trial type, in both delay discrimination tasks but were unable to acquire differential conditioning in either of the trace discrimination tasks. On a post-testing questionnaire of awareness (true–false format), none of the amnesic patients displayed awareness of the stimulus contingencies. Normal control participants who were aware of the stimulus contingencies demonstrated differential conditioning in the trace tasks, whereas the unaware normal control participants performed similarly to the amnesic patients. On the basis of this analysis of awareness, Clark and Squire (1998, 1999) suggested that hippocampal damage in humans selectively impairs declarative memory but spares procedural memory function and proposed that the important factor for learning in trace conditioning is declarative knowledge of the task demands, that is, awareness of the stimulus contingencies.
Daum et al. (1989) were the first to investigate conditional discrimination learning in humans. This small group (n = 3) of memory-disordered patients of mixed etiology demonstrated an increase in CR acquisition across learning blocks. However, results indicated that patients produced CRs on approximately the same number of reinforced trials as they did during nonreinforced trials, indicating that the patients were not discriminating between the two trial types. The normalcy of the patients' acquisition and extinction were difficult to assess from this study because of the small size of the patient group and the lack of a control group.
On the basis of the inconclusive results described above, Daum et al. (1991) further investigated conditional discrimination learning in unilateral temporal lobe patients (right, n = 8; left, n = 9), frontal lobe patients (n = 6), and normal control participants (n = 17). Conditional learning was manipulated with the use of two conditional stimuli according to a 2:1 reinforcement schedule. Learning trials consisted of a 4-s signal light (S+); a 1-s trace period; an 800-ms, 1000-Hz, 65-dB tone CS; and an 80-ms airpuff unconditioned stimulus (US) that coterminated with the tone. During nonreinforced trials, a different color light signal was presented and there was no US. Unilateral temporal lobe patients, regardless of lateralization of lesion site, were unable to differentially respond to the S+ and S− trials. Further analysis demonstrated that the temporal lobe patients responded similarly to the control group during S+ trials but differed significantly during S− trials, such that they produced a significantly higher number of CRs. Normal control participants and all but 1 frontal patient were aware of the stimulus contingencies, whereas only about half of the temporal lobe patients demonstrated awareness. When the data from the temporal lobe patients were analyzed as a function of awareness, the unaware patients produced a greater number of CRs on S− trials than S+ trials as compared with the aware group. The aware group did not show a significant difference between S+ and S− CR production, indicating that awareness was not the crucial factor in acquisition.
Daum et al. (1991) argued that the impairment in discrimination learning was due to deficits in configural cue learning (Sutherland & Rudy, 1989), impaired if–then rules (Hirsch, 1974), or impaired response inhibition as observed in some studies of animals with hippocampal lesions (Gray & McNaughton, 1983). These conclusions may be premature, however, because of a number of limitations of Daum et al.'s study. First, the study investigated only unilateral patients and lacked specification of the unilateral temporal lobe patients' memory functioning (no neuropsychological test data were presented). Further, it was unclear whether the 1-s trace period between the light (S+) and the tone (CS) or the conditional discrimination itself (binding the two pieces of information together) led to the observed impairment.
The present study extends this line of research to further investigate the underlying cause of impairment in amnesic patients' ability to learn a conditional discrimination. Daum et al. (1991) noted that their data allowed them to eliminate a “trace” account of the patients' impaired performance, suggesting that if such were the case it would have led to reduced responding on S+ trials. We suggest, however, that their data cannot rule this possibility out completely and that the impairment might have been due to a temporal processing deficit, given that there was a 1-s gap between the S+/S− and the tone CS. If amnesic patients are impaired in generating and/or maintaining a stimulus trace, as demonstrated in McGlinchey-Berroth et al.'s trace conditioning study (McGlinchey-Berroth, Carrillo, Gabrieli, Brawn, & Disterhoft, 1997), the patients would have been unable to associate the S+/S− to the tone/airpuff configuration in order for the delay conditioning of the tone to be conditional. In other words, being unable to represent the light through the 1-s gap may have essentially eliminated the light's effect on conditioning. Functionally, it would be as if the light were not present, which would lead to equal responding to both the S+ and S−. Therefore, we investigated whether the conditional discrimination deficit observed by Daum et al. was attributable to a deficit in temporal processing.
Experiment 1 assessed trace conditional discrimination learning using a light conditional stimulus (S+/S−) and tone CS separated by a 1-s trace period. It was predicted that this study would extend findings of a conditional discrimination deficit observed by Daum and colleagues (Daum et al., 1991) from unilateral temporal lobectomy patients to severely amnesic patients with bilateral temporal lobe pathology. In Experiment 2, the temporal separation between the light and tone was removed, with the goal of eliminating the need for the amnesic patients to form a memory trace of the S+, which might permit successful acquisition. Thus, in Ex- periment 2, the tone and light overlapped temporally. This stimulus arrangement automatically bound the S+/S− with the tone. By eliminating the temporal processing requirement (trace period), it was hypothesized that conditioning similar to that of the delay-discrimination learning task might be accomplished.
Method
Participants
There were 8 bilateral medial temporal lobe (MT) patients and 8 control participants. Fourteen of the 16 participants had participated in previous eyeblink conditioning studies (Capozzi, Fortier, McGlinchey-Berroth, & Disterhoft, 2002; Carrillo et al., 2001; Gabrieli et al., 1995; McGlinchey-Berroth, Brawn, & Disterhoft, 1999; McGlinchey-Berroth et al., 1997). Because 1 MT amnesic patient had not been tested in any previous eyeblink experiments, a matched untrained control participant was also recruited to control for possible transfer of learning across eyeblink paradigms. Approximately half of the amnesic patients have had extensive experience with conditioning studies (see Table 1). Amnesic and control participants were matched for prior experience with eyeblink conditioning studies. Possible effects of prior training are addressed in the Discussion.
Table 1.
Patient Participation in Previous Eyeblink Classical Conditioning Studies
| Study type and stimuli |
|||||
|---|---|---|---|---|---|
| Patient | Delay (tone CS) Gabrieli et al., 1995 | Trace (tone CS) McGlinchey-Berroth et al., 1997 | Temporal discrimination (2 tone CSs) McGlinchey-Berroth et al., 1999 | Discrimination reversal (2 tone CSs) Carrillo et al., 2001 | Trace interval (tone CS) Capozzi et al., 2002 |
| K. D. | |||||
| P. D. | |||||
| R. G. | |||||
| R. L. | |||||
| J. M. | |||||
| P. S. | |||||
| W. S. | |||||
| S. S. | |||||
Note. Check mark indicates that patient participated in this study prior to participation in the conditional discrimination task. CS = conditioned stimulus.
Amnesic participants
The amnesic patients in this study were recruited from the Memory Disorders Research Center at the Veterans Affairs Boston Healthcare System. Amnesic patients were recruited from area hospitals and referred to the Center by a neurologist. Etiologies included anoxia (n = 6), encephalitis (n = 1), and status epilepticus (n = 1). Amnesia was defined based on the presence of two factors: (1) an impairment in new learning in the context of preserved intellect, and (2) presumed or confirmed bilateral damage to the hippocampal system. Confirmation of bilateral damage to the hippocampal formation was obtained by CT or MRI in 6 of the 8 cases. Of the remaining 2 cases, 1 had enlarged ventricles and diffuse cortical atrophy, and the other had moderate white matter and cortical atrophy (both were amnesic as a result of an anoxic episode).
The impairment in new learning over time was determined with neuro-psychological testing. Demographic and neuropsychological characteristics of the amnesic participants are presented in Table 2 and include age (M = 53, SEM = 6.08), years of education (M = 15, SEM = 1.20), etiology, performance on the Wechsler Memory Scale—Third Edition, the Warrington Recognition Test, and the Wechsler Adult Intelligence Scale—Third Edition (WAIS–III).
Table 2.
Patient Demographic and Neuropsychological Characteristics
| WMS—III |
Warrington |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Age | Education | Etiology | WAIS—III Verbal IQ | General memory | Visual delay | Auditory delay | Working memory | Words | Faces |
| K. D. | 23 | 10 | epilepsy | 92 | 45 | 50 | 58 | 81 | 36 | 36 |
| P. D. | 65 | 20 | anoxia | 111 | 52 | 56 | 64 | 83 | 26 | 26 |
| R. G. | 47 | 14 | anoxia | 92 | 45 | 56 | 55 | 85 | 24 | 34 |
| R. L. | 73 | 18 | anoxia | 113 | 75 | 72 | 80 | 102 | 35 | 33 |
| J. M. | 52 | 12 | anoxia | 83 | 52 | 56 | 55 | 91 | 31 | 33 |
| P. S. | 43 | 14 | anoxia | 90 | 45 | 53 | 52 | 93 | 33 | 29 |
| W. S. | 46 | 14 | anoxia | 111 | 59 | 72 | 52 | 96 | 41 | 43 |
| S. S. | 74 | 18 | encephalitis | 135 | 45 | 53 | 58 | 141 | 35 | 32 |
| M | 53 | 15 | 103 | 52 | 59 | 59 | 97 | 33 | 33 | |
| SD | 17.0 | 3.4 | 17.0 | 11.0 | 8.6 | 9.2 | 19.0 | 5.5 | 5.0 | |
Note. The Wechsler Adult Intelligence Scale—Third Edition (WAIS—III) and the Wechsler Memory Scale—Third Edition (WMS—III) scaled scores yield a normalized, age-adjusted mean of 100. On the Warrington Recognition test, one point is scored for each of 50 items. Age and education are expressed in years.
Control participants
The participants in this study were recruited from the Memory Disorders Research Center in Boston by means of distribution of flyers at local institutions, advertisements in local newspapers, and from the Harvard Cooperative Program on Aging. Control participants were recruited from a pool of volunteers and screened to be free of any neurological disease or illness. The control group was matched to the amnesic patients with regard to age (M = 52, SEM = 5.65), years of education (M = 16.5, SEM = 0.73), and verbal intelligence as measured by the WAIS–III (M = 111, SD = 4.01). t tests indicated that the medial temporal amnesic patients and control participants were equivalent on each of these measures (ps > .16).
Procedure
Apparatus
The apparatus used was a modified version of that used for eyeblink conditioning in the rabbit (Akase, Thompson, & Disterhoft, 1994; Knuttinen, Power, Preston, & Disterhoft, 2001; Thompson, Moyer, Akase, & Disterhoft, 1994). Eyeblink responses were measured with surface electromyography (EMG) electrodes (Nicolet Biomedical, Madison, WI) placed over the orbicularis oculi muscle of the right eye. An adjustable headband was worn to support the airpuff delivery nozzle.
Data were acquired by a custom data acquisition system developed with National Instruments LabVIEW (National Instruments, Austin, TX). Data were acquired at 5 kHz and filtered at 2 kHz with a low-pass Bessel filter. Stimulus presentation and data acquisition were controlled by custom software written in LabVIEW. EMG activity was digitized at 2−5 kHz. The digitized EMG signal was rectified (absolute value of the amplitude) and integrated, with a decay time constant of 10 ms. The integrated–rectified signal is well correlated with eyelid closure measured with reflectance eyelid detectors (Knuttinen et al., 2001).
Stimuli and design
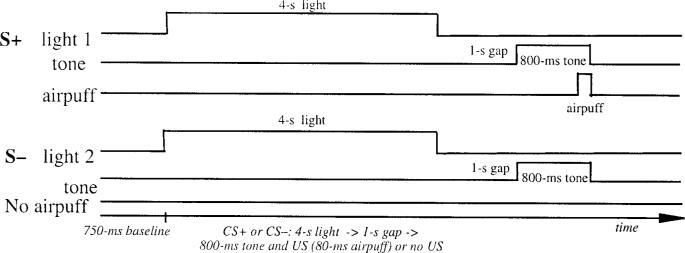
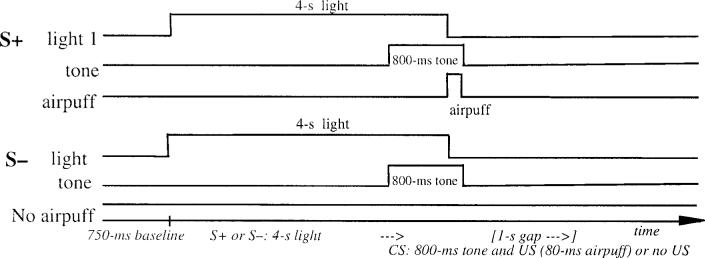
The two experiments consisted of a serial light–tone compound stimulus. The light conditional stimulus (S+ or S−) was either green or red and signaled the onset of a reinforced or a nonreinforced trial. The assignment of the light to these two conditions was counterbalanced across subjects. The light was illuminated for a period of 4 s. The CS was a 1000-Hz, 85-dB tone that was presented for a period of 800 ms. In Experiment 1 (see Figure 1), the tone followed the light after a 1-s silent trace period. In Experiment 2 (Figure 2), the tone was presented 3,300 ms following light onset (the light offset immediately prior to US onset), creating a temporally contiguous light–tone stimulus arrangement. In both experiments, the tone terminated simultaneously with the 100-ms airpuff US. Prior to the onset of each trial, there was a 750-ms baseline recording period. The intertrial interval during conditioning and extinction averaged 8 s but varied randomly from 4 to 12 s. Presentation of trial type was determined by computer-generated pseudorandomized series such that no more than three reinforced or nonreinforced trials could occur in succession. Seventy-two mixed acquisition trials were given, half reinforced (S+), half nonreinforced (S−). A series of 12 extinction trials followed acquisition during both trace and delay experiments. During extinction trials, the S+/S− (light) and the CS (tone) were presented without the airpuff.
Figure 1.
Trace conditional discrimination learning. S+ = reinforced conditional stimulus; S− = nonrein-forced conditional stimulus; CS = conditioned stimulus; US = unconditioned stimulus.
Figure 2.
Delay conditional discrimination learning. S+ = reinforced conditional stimulus; S− = nonreinforced conditional stimulus; CS = conditioned stimulus; US = unconditioned stimulus.
All subjects participated in Experiment 1, trace conditional discrimination learning, followed by Experiment 2, delay conditional discrimination learning. This order of participation was adhered to in an effort to eliminate possible carry-over learning effects that could boost participants' performance from delay to trace conditional discrimination learning if delay conditioning had been administered prior to trace conditioning. The interval between Experiments 1 and 2 averaged approximately 1−2 months for each participant.
Each participant underwent an audiology screening with a portable audiometer (Model 119, Beltone Electronics Corp., Chicago, IL). The criterion of Solomon (Solomon, Pomerleau, Bennet, Jams, & Morse, 1989) was used, which demanded that participants whose threshold in either ear was greater than 15 dB above normal (40 dB) be excluded. However, all participants' thresholds fell within the normal range, and thus none of the participants recruited for this study were excluded on the basis of the results of the audiology screening. Participants were then seated in an upright chair in a dimly lit room and fitted with the eyeblink apparatus by the examiner. Throughout the session, the experimenter sat in the same room, out of the direct view of the participant, and answered questions as they arose. Prior to the acquisition phase, the experimenter read the following instructions:
Please listen carefully to the following instructions. Remain seated comfortably and look straight ahead, avoiding all eye movements such as looking around the room. Please do not touch the headband or earphones at any time during the experiment, yet if you feel uncomfortable or feel you need to adjust anything, please let me know and I will stop the experiment to make any adjustments.
You will see, hear and feel a series of stimuli during the session. These stimuli will consist of some lights, beeps and a light puff of air. Please feel free to blink whenever you want. All you are asked to do is to concentrate on what is going on and let your natural reactions take over.
Definitions
Eyeblink responses that reached 4 SD above the mean baseline amplitude for a minimum duration of 15 ms were classified as CRs if they occurred more than 100 ms after CS onset, to correct for voluntary responses (Gormezano, 1966). Alpha, or short-latency, responses were classified as those eyeblinks that occurred during the first 100 ms of the CS (Gormezano, 1966) and were not counted as CRs. Blinks occurring during the 750-ms baseline period that reached 4 SD above the mean baseline amplitude for a minimum duration of 15 ms were classified as spontaneous blinks. The unconditioned response (UR) amplitude was used to confirm that participants were adequately stimulated to permit conditioning to occur and to ensure that the unconditioned reflex was intact.
Awareness
Immediately following each experiment, participants were given post-session questionnaires in which they were asked a series of five questions concerning stimulus contingencies:
What do you think was going on in the experiment?
Do you think there was any relationship between the lights and the airpuff?
Do you think there was any relationship between the lights and the tone?
Do you think there was any relationship between the tone and the airpuff?
Did you notice anything different happening during various parts of the experiment?
Participants were given an awareness rating from 0−5 on the basis of their explicit awareness or recall of stimulus contingencies as assessed with this open-ended postsession questionnaire.
Results
The primary dependent measure used to determine the extent to which participants acquired the conditional discrimination was the overall level of acquisition as measured by the mean percentage of trials on which a participant produced a CR. Other dependent variables examined included characteristics of both the CRs and URs: CR onset latency, peak latency, amplitude, and duration, and UR onset latency, amplitude, and duration. CR onset latency refers to the time at which the CR amplitude first reached 4 SD above baseline. CR peak latency represents the time at which the given CR reached its highest amplitude. CR peak latency likely captures the level of adaptiveness of a CR (optimally, a CR will peak just before the onset of the airpuff). CR amplitude is measured as peak amplitude and refers to the amount of EMG muscle activity during a CR. CR duration refers to the length of time a CR remains 4 SD above baseline. UR onset latency refers to the time at which the UR amplitude first reaches 4 SD above baseline. UR amplitude is measured as peak amplitude and refers to the amount of EMG muscle activity during the UR period, and reflects the unconditioned reflex in response to the airpuff. UR duration refers to the length of time a UR remains 4 SD above baseline.
Each of the dependent measures was analyzed with a repeated measures analysis of variance (ANOVA) with group (MT, NC) as a between-subjects factor and trial type (S+ vs. S−) as a within-subjects factor. Analyses of covariance (ANCOVAs) were performed to investigate the role of UR amplitude on dependent measures of learning. Regression analyses were performed on the percentage of trials on which a CR occurred as a function of six-trial blocks to examine the rate of learning or acquisition. Last, possible group differences in the number of spontaneous eyeblinks were assessed with one-way ANOVAs.
Experiment 1: Trace Conditional Discrimination Learning
CR acquisition
The ANOVA indicated that the main effect of group was not significant, F(1, 14) = 0.01, p = .92, suggesting that the percentage of CRs acquired, collapsed across trial type, was roughly equivalent in the amnesic patients and control participants (MT: M = 47, SE = 9.9; NC: M = 48, SE = 5.2). The ANOVA revealed a main effect of trial type, F (1, 14) = 22.95, p < .01, indicating that, overall, more CRs occurred during reinforced versus nonreinforced trials (S+ trials: M = 58, SE = 5.4; S− trials: M = 36, SE = 6.0). This main effect, however, was qualified by the presence of a significant interaction of Group × Trial Type, F(1, 14) = 9.72, p < .01. Analysis of this interaction using means comparisons revealed that control participants were able to respond differentially on S+ trials (mean percentage CRs = 66, SE = 5.89) versus S− trials (M = 30, SE = 4.54), F(1, 14) = 31.27, p < .01. Amnesic patients, in contrast, were impaired in their ability to acquire the conditional discrimination (S+: M = 51, SE = 8.67; S−: M = 43, SE = 11.09). As shown in Figure 3, the amnesic patients showed similar acquisition during reinforced and nonreinforced trials, F(1, 14) = 1.40, p = .26. In addition, the control participants produced significantly more CRs during S+ trials (M = 66, SE = 5.89) than the amnesic patients (M = 51, SE = 8.67), F(1, 14) = 5.60, p = .03, and there was a noticeable trend toward production of fewer CRs during S− trials for control participants (M = 30, SE = 4.54) as compared with amnesic patients (M = 43, SE = 11.09), F(1, 14) = 4.18, p = .06.
Figure 3.
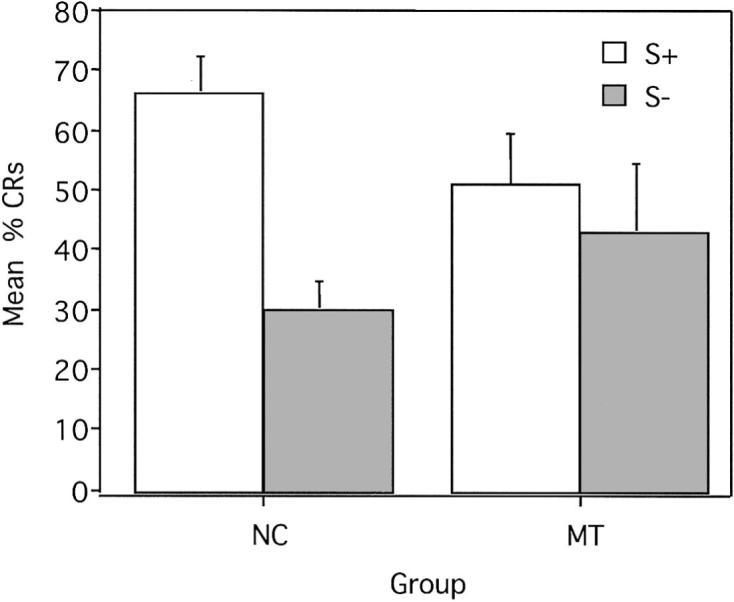
Mean (± SEM) percent conditioned responses (CRs) for reinforced and nonreinforced trials during trace conditional discrimination learning. S+ = reinforced conditional stimulus; S− = nonreinforced conditional stimulus; NC = normal control participants; MT = medial temporal lobe amnesic patients.
Trace conditional discrimination learning curves
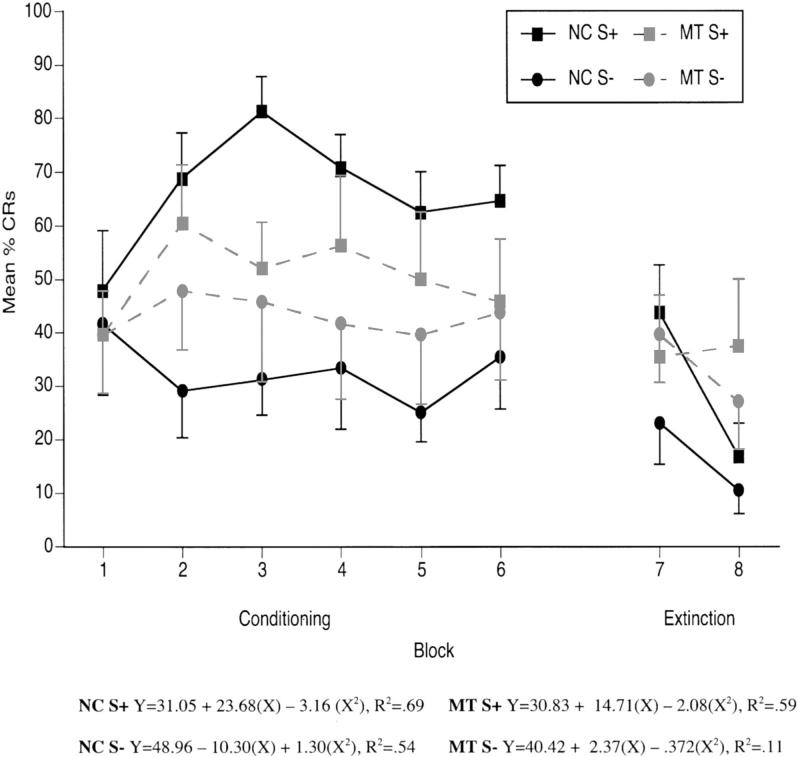
The percentage of trials on which a CR occurs was expected to increase during reinforced trials during the initial phases of learning. Conversely, the percentage of CRs produced for nonreinforced trials was expected to remain stable or decrease across learning trials. As can be seen in Figure 4, when conditioning trials were collapsed into six blocks of six trials each, the control participants demonstrated an overall increase in the percentage of CRs across the six learning blocks of reinforced trials, peaking at Block 3. The percentage of CRs on nonreinforced trials remained stable and, in fact, decreased somewhat across learning blocks in control participants. Amnesic patients, on the other hand, showed similar response rates across learning blocks for both reinforced and non-reinforced trial types.
Figure 4.
Trace conditional discrimination learning curves showing mean (± SEM) percent conditioned responses (CRs) from six blocks of six reinforced trials, six blocks of six nonreinforced trials, and two blocks of six extinction trials. Quadratic regression analyses were performed for the mean percentage of CRs for each group separately for the six blocks of acquisition trials. NC = normal control participants; MT = medial temporal lobe amnesic patients; S+ = reinforced conditional stimulus; S− = nonreinforced conditional stimulus.
To examine the rate of learning or acquisition, quadratic regression analyses were performed for the mean percentage of CRs for each group separately for the six blocks of acquisition trials. Quadratic regression analyses provided the best fit for the data in this as well as past studies, as a leveling off of acquisition during the later learning blocks is typically seen. As shown in Figure 4, the quadratic analysis neared significance for the control participants on reinforced trials during trace conditioning. The function was characterized by an intercept of 31.05; a linear term of 23.68 (p = .08); and a quadratic term of −3.16, −2.08 (p = .09), R2 = .59. The quadratic analysis was not, however, significant for the amnesic patients on S+ trials. The MT quadratic function was characterized by an intercept of 30.83; a linear term of 14.71 (p = .13); and a quadratic term of −2.08 (p = .13), R2 = .59. As also shown in Figure 4, the quadratic functions were not significant for nonreinforced trials for either group during trace conditioning (ps > .17).
Extinction
The mean percentage of CRs was assessed with a Group × Block ANOVA comparing the last learning block (Conditioning Block 6) to the two extinction blocks of six trials each. A significant interaction of Group × Block, F(1, 14) = 4.28, p = .02, indicated that control participants significantly decreased the number of CRs produced during reinforced trials from the last conditioning block (M = 65, SE = 6.63) to the first extinction block (M = 44, SE = 8.87), F(1, 14) = 4.41, p = .04; from the last conditioning block to the second extinction block (M = 17, SE = 6.30), F(1, 14) = 23.34, p < .01; and from the first extinction block to the second extinction block, F(1, 14) = 7.46, p = .01 (see Figure 5). In other words, there was a significant stepwise reduction in the percentage of CRs across the extinction blocks. In contrast, the amnesic patients were unable to extinguish their learned response as rapidly and produced a similar number of CRs during reinforced trials from the last conditioning block (M = 46, SE = 11.68) to the first extinction block (M = 35, SE = 11.55), F(1, 14) = 1.10, p = .30; from the last conditioning block to the second extinction block (M = 38, SE = 12.50), F(1, 14) = 0.71, p = .41; and from the first extinction block to the second extinction block, F(1, 14) = 0.04, p = .84. Normal control participants produced somewhat fewer CRs during the first extinction block than amnesic patients, F(1, 14) = 3.57, p = .07, and significantly fewer CRs during the second extinction block than MTs, F(1, 14) = 4.41, p = .04.
Figure 5.
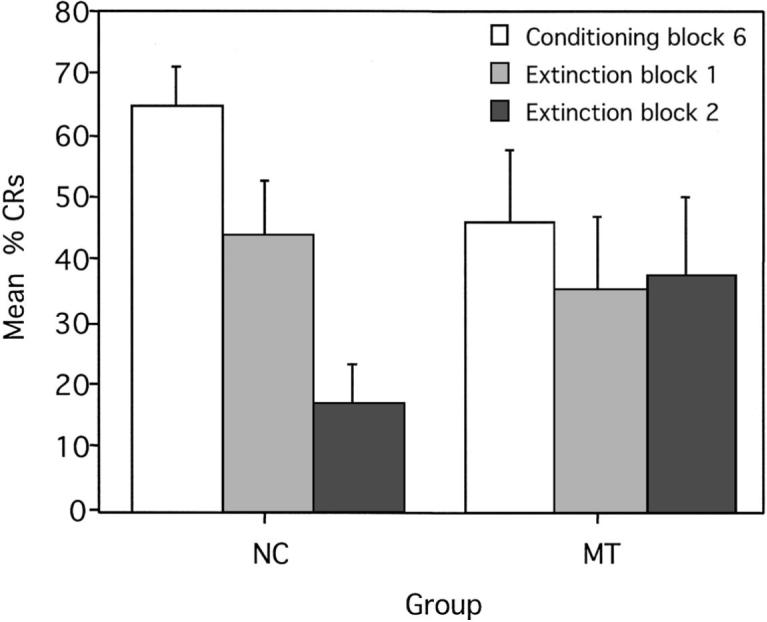
Mean (± SEM) percent conditioned responses (CRs) for the last block of conditioning trials (Block 6) and two extinction blocks for reinforced trials during trace conditional discrimination learning. NC = normal control participants; MT = medial temporal lobe amnesic patients.
The mean percentage of CRs during nonreinforced extinction trials was also assessed with a Group × Block ANOVA comparing the last learning block (Conditioning Block 6) to the two extinction blocks of six trials each. There was a main effect of block, F(1, 14) = 3.72, p = .03, and no significant interaction, indicating that both groups similarly decreased their production of CRs during S− trials. Normal participants decreased their mean percentage of CRs from 35% (SE = 9.68) during the last conditioning block to 23% (SE = 7.67) during the first extinction block and 10% (SE = 4.38) during the last extinction block. Amnesic patients also gradually decreased their mean percentage of CRs across blocks, going from 44% (SE = 12.57) during the last conditioning block to 40% (SE = 8.87) during the first block of extinction trials and 27% (SE = 8.87) during the last block of extinction trials.
CR onset latency and peak latency. An ANOVA on CR onset latency revealed no main effect of group, F(1, 14) = 0.18, p = .68, or trial type, F(1, 14) = 0.21, p = .66, and no interaction, F(1, 14) = 2.08, p = .17, suggesting that when producing a CR, both control participants and amnesic patients timed their responses similarly during both reinforced and nonreinforced trials (see Table 3). Analysis of CR peak latency indicated that there was no significant difference in the ability of NC participants and MT patients to adaptively time their responses, F(1, 14) = 0.06, p = .81.
Table 3.
Mean (± SEM) Conditioned Response (CR) Measures During Trace Conditional Discrimination Learning
| CR onset latency (ms) |
CR peak latency (ms) |
CR amplitude (mV) |
CR duration (ms) |
|||||
|---|---|---|---|---|---|---|---|---|
| Group | S+ | S- | S+ | S- | S+ | S- | S+ | S- |
| NC | 290 ± 38 | 351 ± 49 | 464 ± 27 | 430 ± 38 | 21.0 ± 4.5 | 13.0 ± 2.3 | 269 ± 45 | 121 ± 30 |
| MT | 354 ± 35 | 322 ± 23 | 453 ± 34 | 421 ± 31 | 13.0 ± 2.4 | 16.0 ± 2.8 | 146 ± 31 | 149 ± 31 |
Note. S+ = reinforced stimulus; S- = nonreinforced stimulus; NC = normal control participants; MT = medial temporal lobe amnesic patients.
CR amplitude and duration
A Group × Trial Type repeated measures ANOVA examining CR amplitude revealed only a significant interaction, F(1, 14) = 5.89, p = .03. A series of means comparisons indicated that the CR amplitude measure differed as a function of trial type in the control participants, F(1, 14) = 6.90, p = .02. Control participants produced CRs of greater magnitude during reinforced versus nonreinforced trials (see Table 3). CR amplitude did not differ across trial type for the amnesic patients, F(1, 14) = 0.65, p = .44. CR amplitude also varied by group during reinforced trials. Control participants' CR amplitude was significantly greater than that of the amnesic patients during S+ trials, F(1, 14) = 6.75, p = .02. There were no group differences in CR amplitude during nonreinforced trials, F(1, 14) = 0.69, p = .42.
CR duration varied by trial type, indicating significantly longer CR durations in response to S+ trials as compared with S− trials, F(1, 14) = 9.95, p < .01. However, there was also a significant interaction, F(1, 14) = 11.00, p < .01. NC participants demonstrated longer CR duration (M = 269 ms) on reinforced trials than amnesic patients (M = 146 ms), F(1, 14) = 14.57, p < .01 (see Table 3). There was no group difference in CR duration during nonreinforced trials, F(1, 14) = 0.76, p = .40. CR duration also varied as a function of trial type in the control group. Control participants produced significantly longer CRs during reinforced versus nonreinforced trials, F(1, 14) = 20.94, p < .01. Amnesic patients' CR duration did not differ on reinforced and nonrein-forced trials, F(1, 14) = 0.01, p = .91.
UR amplitude, onset latency, peak latency, and duration
Control participants' UR amplitude averaged 49 mV (SE = 3.4) on S+ trials, whereas amnesic patients' UR amplitude averaged 36 mV (SE = 3.7) on S+ trials. UR amplitude varied by group, F(1, 14) = 6.41, p = .03. Control participants' UR amplitude was of a greater magnitude on reinforced trials than that of the amnesic patients on reinforced trials, F(1, 14) = 13.53, p < .01. As reflected in Table 4, there were no differences between groups in timing of the UR: UR onset latency, F(1, 14) = 0.11, p = .74; UR peak latency, F(1, 14) = 0.90, p = .36; or UR duration, F(1, 14) = 1.98, p = .18.
Table 4.
Mean (± SEM) Unconditioned Response (UR) Measures During Trace Conditional Discrimination Learning
| Group | UR onset latency (ms) S+ | UR peak latency (ms) S- | UR amplitude (mV) S+ | UR duration (ms) S- |
|---|---|---|---|---|
| NC | 727 ± 4.98 | 778 ± 3.3 | 49 ± 3.4 | 71 ± 5.0 |
| MT | 738 ± 3.6 | 780 ± 2.5 | 36 ± 3.7 | 58 ± 3.7 |
Note. S+ = reinforced stimulus; S- = nonreinforced stimulus; NC = normal control participants; MT = medial temporal lobe amnesic patients.
UR amplitude as a covariate in mean percentage of CRs
As shown in Table 4, mean UR amplitude during reinforced trials was greater in the control participants as compared with the amnesic patients. Consequently, to ensure that the differences observed in acquisition were not confounded by a difference in unconditioned reflex to the airpuff, UR amplitude was entered as a covariate in an analysis of the mean percentage of CRs. A Group × Trial Type repeated measures analysis of covariance (ANCOVA) examining mean percentage of CRs produced during reinforced trials revealed that when covarying for UR amplitude, the interaction remained significant, F(1, 14) = 5.90, p < .05.
Alpha responses
There were no systematic differences observed in the number of alpha (short-latency) responses during reinforced or nonreinforced trials, F(1, 14) = 3.02, p = .10. Amnesic patients produced an average of 7 (SE = 1.8) alpha responses during reinforced trials and 4 (SE = 1.3) alpha responses during nonreinforced trials. Control participants produced 14 (SE = 3.8) alpha responses during reinforced trials and 6 (SE = 1.9) during nonreinforced trials.
Spontaneous blinks
There were no systematic differences observed in the number of spontaneous blinks during trace conditional discrimination (p = .52). Control participants produced an average of 4 (SE = 1.09) spontaneous blinks, and amnesic patients produced an average of 3 (SE = 1.28) spontaneous blinks.
Experiment 2: Delay Conditional Discrimination Learning
CR acquisition
The pattern of results for the percentage of trials in which a CR occurred during the delay conditional discrimination task was similar to that found in the trace conditional discrimination task. That is, the main effect of group was not significant, F(1, 14) = 1.34, p = .27, again suggesting that the percentage of CRs acquired, collapsed across trial type, was roughly equivalent in the amnesic patients and control participants (MT: M = 42, SE = 6.9; NC: M = 51, SE = 4.7). Also, the ANOVA revealed a main effect of trial type, F(1, 14) = 29.78, p < .01, indicating that, collapsed across group, there were more CRs during reinforced versus nonreinforced trials. This main effect, however, was qualified by the presence of a significant interaction of Group × Trial Type (S+ vs. S−), F(1, 14) = 19.59, p < .01. Analysis of this interaction using means comparisons revealed that control participants were able to respond differentially on reinforced trials (mean percentage CRs = 71, SE = 3.93) as compared to nonreinforced trials (M = 31, SE = 5.52), F(1, 14) = 48.84, p < .01. Amnesic patients, in contrast, were impaired in their ability to acquire the conditional discrimination (S+: M = 44, SE = 5.87; S−: M = 40, SE = 7.77). As shown in Figure 6, the amnesic patients' difference in CR acquisition during reinforced and nonreinforced trials did not differ significantly, F(1, 14) = 0.53, p = .48. The control participants demonstrated greater acquisition during S+ (M = 71, SE = 3.93) trials than the amnesic patients (M = 44, SE = 5.87), F(1, 14) = 21.33, p < .01. There were no group differences in the production of CRs during S− trials, F(1, 14) = 2.69, p = .12.
Figure 6.
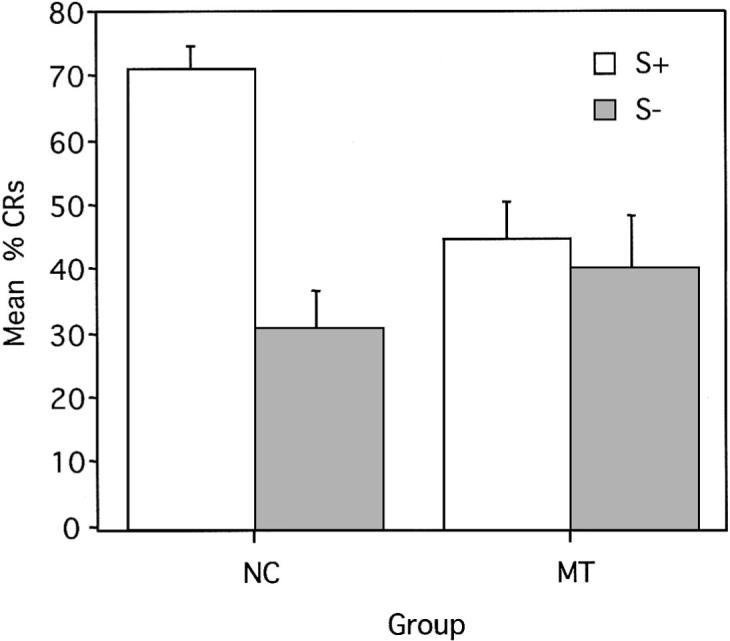
Mean (± SEM) percent conditioned responses (CRs) for reinforced and nonreinforced trials during delay conditional discrimination learning. S+ = reinforced conditional stimulus; S− = nonreinforced conditional stimulus; NC = normal control participants; MT = medial temporal lobe amnesic patients.
Learning curves
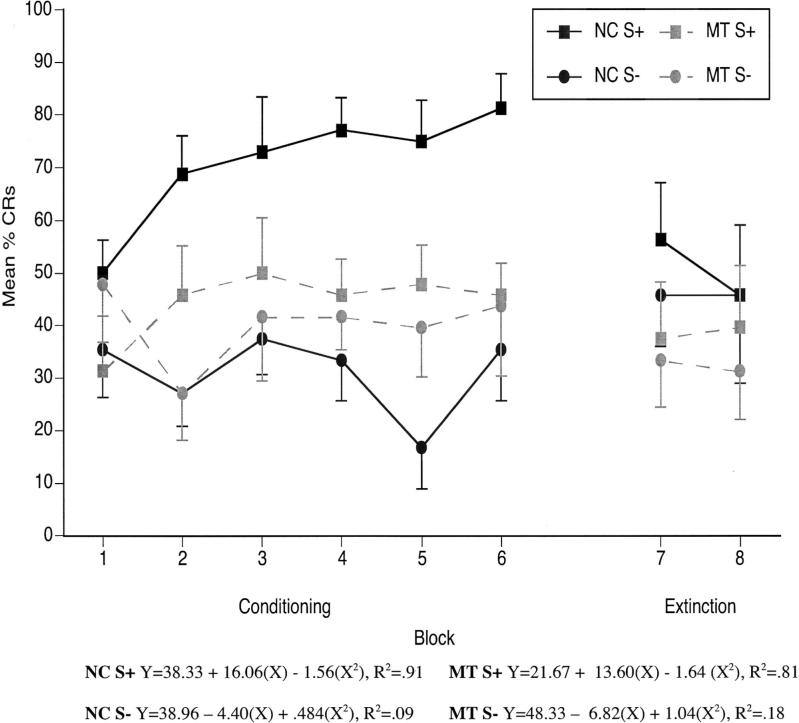
As can be seen in Figure 7, the control participants demonstrated a consistent increase in the percentage of CRs across six learning blocks of reinforced trials. The percentage of CRs on nonreinforced trials remained stable and, in fact, decreased somewhat across learning blocks. In contrast, the amnesic patients showed similar response rates across learning blocks for both reinforced and nonreinforced trial types.
Figure 7.
Delay conditional discrimination learning curves showing mean (± SEM) percent conditioned responses (CRs) from six blocks of six reinforced trials, six blocks of six nonreinforced trials, and two blocks of six extinction trials. Quadratic regression analyses were performed for the mean percentage of CRs for each group separately for the six blocks of acquisition trials. NC = normal control participants; MT = medial temporal lobe amnesic patients; S+ = reinforced stimulus; S− = nonreinforced stimulus.
To examine the rate of learning or acquisition, quadratic regression analyses were performed for the mean percentage of CRs for each group separately for the six blocks of acquisition trials. As shown in Figure 7, the quadratic analysis was significant for the control participants on reinforced trials during delay conditioning. The NC function was characterized by an intercept of 38.33; a linear term of 16.06 (p = .05); and a quadratic term of −1.56 (p = .11), R2 = .91. The quadratic analysis was also significant for the amnesic patients on S+ trials during delay conditioning. The MT quadratic function was characterized by an intercept of 21.67; a linear term of 13.60 (p = .05); and a quadratic term of −1.64 (p = .08), R2 = .81. As also shown in Figure 7, the quadratic functions were not significant for nonreinforced trials for either group during delay conditioning (ps > .50).
Extinction
The mean percentage of CRs was assessed with a Group × Block ANOVA comparing the last learning block (Conditioning Block 6) to the two extinction blocks (each block contains six trials). As can be seen in Figure 8, although the interaction of Group × Block was not significant, F(1, 14) = 2.09, p = .14, control participants significantly decreased the mean number of trials in which a CR occurred during reinforced trials from the last conditioning block (M = 81, SE = 6.63) to the first extinction block (M = 56, SE = 10.88), F(1, 14) = 6.12, p = .02, and from the last conditioning block to the second extinction block (M = 46, SE = 13.27), F(1, 14) = 12.29, p < .01. The difference between the first extinction block and the second extinction block was not significant, F(1, 14) = 1.06, p = .31. In contrast, the amnesic patients were unable to extinguish their learned response as rapidly and produced a similar number of CRs during reinforced trials from the last conditioning block (M = 46, SE = 6.10) to the first extinction block (M = 38, SE = 10.80), F(1, 14) = 0.68, p = .42; from the last conditioning block to the second extinction block (M = 40, SE = 11.76), F(1, 14) = 0.38, p = .54; and from the first extinction block to the second extinction block, F(1, 14) = 0.04, p = .84. Normal control participants produced a somewhat greater number of CRs during the first extinction block than amnesic patients, F(1, 14) = 3.44, p = .07. There were no group differences in the number of CRs produced during the second extinction block, F(1, 14) = 0.38, p = .54.
Figure 8.
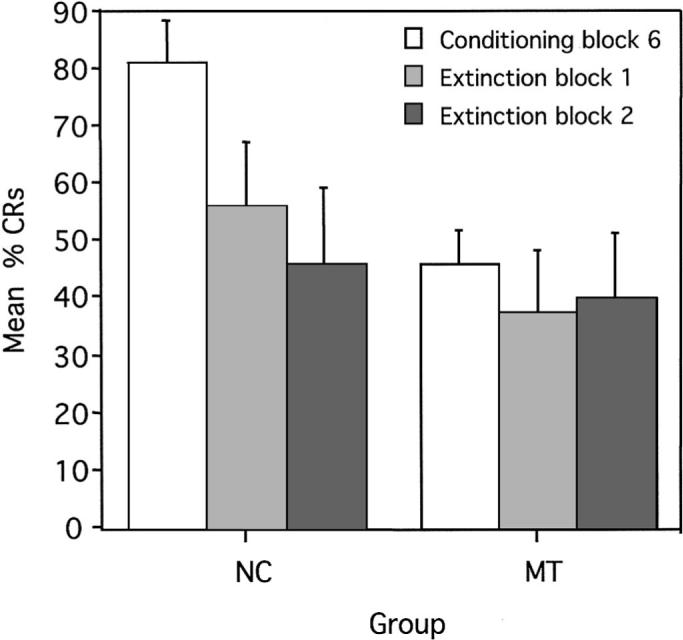
Mean (± SEM) percent conditioned responses (CRs) for the last block of conditioning trials (Block 6) and two extinction blocks for reinforced trials during delay conditional discrimination learning. NC = normal control participants; MT = medial temporal lobe amnesic patients.
The mean percentage of CRs during nonreinforced extinction trials was also assessed with a Group × Block ANOVA comparing the last learning block (Conditioning Block 6) to the two extinction blocks of six trials each. There was no main effect of group or block and no significant interaction. Normal control participants produced a CR on 35% (SE = 9.68) of S− trials during the last block of conditioning trials, 46% (SE = 9.83) during the first extinction block and 46% (SE = 16.89) during the last extinction block. Amnesic patients produced a CR on 44% (SE = 13.34) of S− trials during the last conditioning block, 33% (SE = 8.91) during the first block of extinction trials and 31% (SE = 9.15) during the last block of extinction trials.
CR onset latency and peak latency
An ANOVA on CR onset latency revealed no main effect of group, F(1, 14) = 0.25, p = .62, or trial type, F(1, 14) = 0.39, p = .54; and no interaction, F(1, 14) = 0.04, p = .84, suggesting that when producing a CR, both control participants and patients timed their responses similarly during both reinforced and nonreinforced trials (see Table 5). Similarly, analysis of CR peak latency indicated that there was no significant difference in the ability of control participants and amnesic patients to adaptively time their responses, F(1, 14) = 0.40, p = .54.
Table 5.
Mean (± SEM) Conditioned Response (CR) Measures During Delay Conditional Discrimination Learning
| CR onset latency (ms) |
CR peak latency (ms) |
CR amplitude (mV) |
CR duration (ms) |
|||||
|---|---|---|---|---|---|---|---|---|
| Group | S+ | S- | S+ | S- | S+ | S- | S+ | S- |
| NC | 281 ± 35 | 304 ± 63 | 519 ± 22 | 434 ± 41 | 18.0 ± 2.5 | 12.0 ± 1.2 | 315 ± 52 | 209 ± 67 |
| MT | 318 ± 51 | 330 ± 40 | 470 ± 34 | 434 ± 37 | 10.0 ± 1.7 | 10.0 ± 2.0 | 203 ± 53 | 184 ± 57 |
Note. S+ = reinforced stimulus; S- = nonreinforced stimulus; NC = normal control participants; MT = medial temporal lobe amnesic patients.
CR amplitude and duration
In examining the mean amplitude of CRs during the delay conditional discrimination task, a repeated measures ANOVA revealed a main effect of trial type, F(1, 14) = 14.37, p < .01, indicating that, collapsed across group, CRs were of a greater amplitude during reinforced versus nonreinforced trials. This main effect, however, was qualified by the presence of a significant interaction, F(1, 14) = 13.14, p < .01. Control participants' CR amplitude averaged 18 mV (SE = 2.5) for S+ trials and 12 mV (SE = 1.2) for S− trials, F(1, 14) = 27.49, p < .01, whereas the amnesic patients' CR amplitude averaged 10 mV (SE = 1.7) on S+ trials and 10 mV (SE = 2.0) on S− trials, F(1, 14) = 0.01, p = .91. NCs demonstrated significantly greater CR amplitude during S+ trials than MTs, F(1, 14) = 39.03, p < .01. NCs and MTs CR amplitude did not differ during nonreinforced trials, F(1, 14) = 1.26, p = .28.
CR duration varied by trial type, indicating that both groups demonstrated longer CR durations in response to S+ trials as compared with S− trials, F(1, 14) = 8.78, p = .01. However, there was also a significant interaction, F(1, 14) = 4.29, p = .06. Control participants demonstrated significantly longer CR duration (M = 315 ms) on reinforced trials than amnesic patients (M = 203 ms), F(1, 14) = 13.99, p < .01 (see Table 4). CR duration also varied in the NC group as a function of trial type, F(1, 14) = 12.67, p < .01. Amnesic patients' CR duration did not differ on reinforced and nonreinforced trials, F(1, 14) = 0.40, p = .54.
UR amplitude, onset latency, peak latency, and duration
Control participants' UR amplitude averaged 40 mV (SE = 3.90) on S+ trials, whereas amnesic patients' UR amplitude averaged 33.5 mV (SE = 3.86) on S+ trials. As presented in Table 6, there were no differences between groups in timing of the UR: UR onset latency, F(1, 14) = 1.28, p = .28; UR peak latency, F(1, 14) = 0.20, p = .66; UR duration, F(1, 14) = 1.46, p = .25.
Table 6.
Mean (± SEM) Unconditioned Response (UR) Measures During Delay Conditional Discrimination Learning
| Group | UR onset latency (ms) S+ | UR peak latency (ms) S- | UR amplitude (mV) S+ | UR duration (ms) S- |
|---|---|---|---|---|
| NC | 720 ± 3.5 | 773 ± 2.1 | 40 ± 3.9 | 78 ± 3.7 |
| MT | 735 ± 4.4 | 780 ± 2.3 | 34 ± 3.9 | 63 ± 3.8 |
Note. S+ = reinforced stimulus; S- = nonreinforced stimulus; NC = normal control participants; MT = medial temporal lobe amnesic patients.
Alpha responses
There were no systematic differences observed in the number of alpha responses during reinforced or nonreinforced trials, F(1, 14) = 1.88, p = .19. Amnesic patients produced an average of 8 (SE = 3.1) alpha responses during reinforced trials and 6 (SE = 2.8) alpha responses during nonreinforced trials. Control participants produced an average of 17 (SE = 5.1) alpha responses during reinforced trials and 12 (SE = 4.5) during nonreinforced trials.
Spontaneous blinks
There were no differences observed in the number of spontaneous blinks. Control participants produced an average of 3 (SE = 1.030) spontaneous blinks during delay conditional discrimination, and amnesic patients also produced an average of 3 (SE = 0.854) spontaneous blinks.
Trace versus delay conditional discrimination learning
Last, CR acquisition was examined during trace as compared to delay conditional discrimination learning. A repeated measures Group × Trial Type (S+ vs. S−) × Paradigm (trace vs. delay) ANOVA revealed a main effect of trial type, F(1, 28) = 59.10, p < .01, indicating an overall greater CR acquisition rate on reinforced as compared with nonreinforced trials (percentage CRs: S+ trials, M = 58, SE = 3.57; S− trials, M = 36, SE = 3.78). This main effect, however, was qualified by the presence of a significant three-way interaction, F(1, 28) = 9.37, p < .01. Analysis of this interaction using means comparisons demonstrated that acquisition differed as a function of trial type (S+ versus S−) in the normal control group in both trace, F(1, 28) = 35.08, p < .01, and delay, F(1, 28) = 42.89, p < .01, conditioning. However, CR acquisition did not vary as a function of trial type in the amnesic patients in trace, F(1, 28) = 1.57, p = .22, or delay conditioning, F(1, 28) = 0.47, p = .50; see Figure 9. When comparing performance during the trace and delay paradigms directly, control participants demonstrated similar levels of CR acquisition for reinforced trials during both paradigms, F(1, 14) = .636, p = .43. Similarly, there was no difference in the control participants' acquisition during S− trials in trace versus delay conditioning, F(1, 14) = 0.03, p = .87. The amnesic patients also demonstrated consistent levels of CR acquisition for S+, F(1, 14) = 1.05, p = .31, and S−, F(1, 14) = 0.21, p = .65, trials during both trace and delay conditioning.
Figure 9.
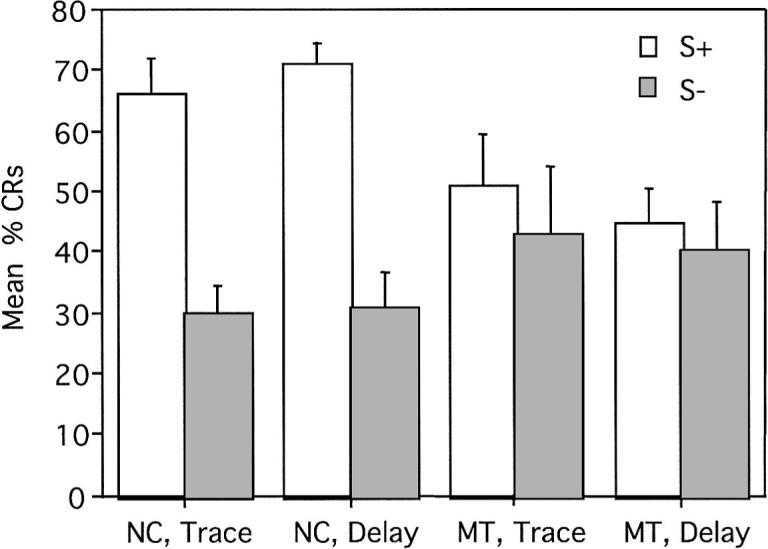
Mean (± SEM) percent conditioned responses (CRs) during reinforced and nonreinforced trials during both trace and delay conditional discrimination learning. S+ = reinforced stimulus; S− = nonreinforced stimulus; NC = normal control participants; MT = medial temporal lobe amnesic patients.
Awareness
Participants were given an awareness rating from 0−5 based on their explicit awareness of stimulus contingencies as assessed with the five-question postsession questionnaire. Amnesic patients' mean awareness rating was 0.875 (SD = 1.130) following trace conditioning and 0.500 (SD = 0.756) following delay conditioning. In contrast, normal control participants' mean awareness score was 4.500 (SD = 0.535) following trace conditioning and 4.750 (SD = 0.463) following delay conditioning. Overall, awareness was significantly correlated to performance during trace conditioning (mean percentage CRs during S+ trials minus mean percentage CRs during S− trials, p < .01). However, this correlation was based on low awareness scores in the MT group and the intact awareness in the NC participants. When analyzed by group, there were no correlations between awareness and performance (ps > .40). The pattern was similar during delay conditioning. Overall, awareness during delay conditioning was significantly correlated to performance (p < .01). When analyzed by group, there were no correlations between awareness and performance (ps > .10).
Discussion
Using eyeblink classical conditioning, we found that bilateral medial temporal lobe amnesic patients were impaired in acquiring a conditional discrimination in both trace and delay paradigms. As predicted, the mean percentage of trials on which amnesic patients produced a CR was similar for both reinforced and nonreinforced trials during trace conditional discrimination learning. This inability to respond differentially to S+ versus S− trials indicated an impairment in the amnesic patients' ability to acquire a conditional discrimination when the light conditional stimulus (S+/S−) and the tone CS were temporally separated, as was predicted by the findings of Daum et al. (1991) in unilateral temporal lobectomy patients. Similarly, the amnesic patients were also impaired in their ability to acquire a conditional discrimination in the context of a delay paradigm in which the light and tone overlapped and co-terminated. Control participants were able to respond differentially to reinforced trials and nonreinforced trials when the S+/S− and CS were temporally distinct (trace paradigm) as well as when the S+/S− and CS were temporally contiguous (delay paradigm). The amnesic patients were also impaired in their ability to extinguish the CR in the context of a conditioning discrimination paradigm.
As evidenced in the learning curves in Figures 4 and 7, normal control participants demonstrated clear acquisition of differential responding on reinforced and nonreinforced trials over time in both trace and delay paradigms. In the early stages of acquisition (Block 1), control participants produced a similar number of CRs on S+ and S− trials. However, from the second trial block forward, they began to consistently produce CRs to S+ trials and to decrease their CR production during S− trials, providing clear verification of differential responding. Regression analyses confirmed significant acquisition across blocks on S+ trials in the control participants during both trace and delay conditional discrimination learning.
In contrast, MT amnesic patients showed both impaired acquisition on S+ trials, and to a lesser extent, nonadaptive, high levels of responding on S− trials. MTs produced a similar number of CRs to both S+ and S− trials throughout all six learning blocks during both trace and delay learning. When the first block of learning was examined on a trial-by-trial basis for reinforced trials, MTs demonstrated some acquisition and an intact, although depressed, learning curve during both trace and delay conditioning. Regression analyses revealed that amnesic patients demonstrated systematic acquisition during delay (primarily during the initial three learning blocks), but not trace, conditional discrimination learning. Thus, although amnesic patients' overall acquisition during reinforced trials was impaired during both trace and delay paradigms, and there was no significant difference in their overall mean percentage of CRs during reinforced trials between paradigms, the patients demonstrated some systematic learning occurring across blocks during delay conditioning, but not during trace conditioning. This evidence of acquisition in the MTs during delay conditioning, albeit small, is likely due to the decreased temporal processing demands in this task as compared with the trace conditional discrimination paradigm.
The overall inability of the MT amnesic patients to respond differentially to S+ versus S− trials during both trace and delay paradigms indicates that the medial temporal lobe system is essential for the acquisition of a conditional discrimination. The previous findings of Daum and colleagues (1991) left open the possibility that the amnesic patients' impaired performance was due to a temporal processing requirement rather than the inability to acquire the conditional discrimination itself. The current study's demonstration of a deficit in the acquisition of a conditional discrimination during both trace and, more important, delay learning paradigms observed in a group of well-characterized bilateral MT amnesic patients clearly indicates that the hippocampal system plays a critical role in the acquisition of a conditional discrimination.
The preceding discussion presumes that the amnesic patients' impairment in differential responding was due to an inability to acquire the conditional discrimination itself. However, Cohen and Eichenbaum (1993) offer an alternative explanation that is also consistent with the data. These authors suggest that the hippocampal-dependent declarative memory system supports a relational form of representation exhibiting the critical property of flexibility, capable of being accessed and expressed in novel contexts; whereas procedural memory, operating independently of the hippocampal system, supports a fundamentally inflexible form of representation that can be expressed only in virtual repetitions of the initial learning situation. (p. 49)
In both trace and delay conditional discrimination learning, the tone CS coterminated with the US. Thus, both paradigms have a temporally contiguous tone CS−US arrangement, whereas the light conditional stimulus and the US did not overlap in time (essentially creating a 0-ms trace condition, see Figure 1).
The hippocampal system is believed to be critically involved in associative learning when there is a temporal separation between the CS and the US (McGlinchey-Berroth et al., 1997; Moyer, Deyo, & Disterhoft, 1990). A recent study in our laboratory further suggests that the hippocampal system may be critical even at a 0-ms trace interval (Capozzi, Fortier, Disterhoft, & McGlinchey, 2003). Unexpectedly, the amnesic patients did not show an overall impairment in acquisition during the trace paradigm. Rather, their level of acquisition was similar to that of the control participants when data were collapsed across reinforced and nonreinforced trials. Therefore, it is possible that the light conditional stimulus was inconsequential and the amnesic patients were in fact responding only to the tone CS, the only stimulus that was temporally contiguous with the US. If this interpretation is true, it may shed light on the inflexibility inherent in the memory system of medial temporal amnesic patients. Perhaps, whenever two stimuli overlap in time, this inflexible system seizes that temporal pairing and it becomes the only basis for learning, regardless of its adaptive benefit. The result is an inflexible system that can bind temporally contiguous stimuli but cannot flexibly adjust to more complex task demands (such as conditional or reversal learning), as was initially described by Cohen and Eichenbaum (1993). Alternatively, the normal control participants were able to approach the task more flexibly given their intact hippocampal system. If this account of the data is valid, the inflexibility of the amnesic patients' memory system may have far-reaching effects on the ability of the patients to function in an ever-changing, unpredictable world.
There was no difference between the amnesic and control groups for URs other than UR amplitude during the trace paradigm. This group difference in UR amplitude observed during trace conditioning was examined with regard to acquisition using ANCOVA to ensure that the differences observed in acquisition were not confounded by a difference in unconditioned reflex to the airpuff. As the group difference in acquisition was maintained, it is clear that the observed impairment was not due to sensory–motor factors such as the unconditioned eyeblink reflex. While not typical, we have observed a similar group difference in UR amplitude in one previous study investigating trace eyeblink conditioning (McGlinchey-Berroth et al., 1997). In this study, the amnesic patients' URs tended to be smaller in amplitude than the control participants' URs. It is possible that the amnesic patients did not find the airpuff aversive enough to produce consistent URs to support acquisition during trace conditional discrimination learning. On the basis of the ANCOVA findings, however, this possibility does not appear to be substantiated. Another possibility is that normal control participants' UR amplitude was a direct extension of their CR amplitude. Control participants tended to produce high-amplitude adaptive CRs that bled into their URs. In contrast, amnesic patients tended to produce “unadaptive,” mis-timed, and low-amplitude CRs that often returned to baseline before UR onset. This may have contributed to the observed group difference in UR amplitude.
When trace and delay conditional discrimination paradigms were compared directly, no significant differences in acquisition were observed within groups, indicating that there were no differences in the overall production of CRs for either NCs or MTs based on paradigm. Because of the greater temporal processing demands involved in the trace conditional discrimination paradigm, it was expected that amnesic patients might show lower levels of acquisition in this task as compared with delay conditioning. However, overall acquisition in the two paradigms was equivalent. The complex processing demands involved in acquisition of the conditional discrimination itself may therefore have driven the observed impairment, leaving little room for an added temporal processing deficit.
Building on the findings of Carrillo and colleagues (2001) with human subjects and Berger and Orr (Berger & Orr, 1983; Orr & Berger, 1985) with animals (i.e., rabbits), these results support the notion that the hippocampal system is not necessary for encoding of a CS−US association during a simple delay discrimination but becomes more critical as task complexity increases (e.g., reversal or conditional discrimination learning).
Reminiscent of the compositionality theory of Cohen and Eichenbaum (Cohen & Eichenbaum, 1993; Eichenbaum, Otto, & Cohen, 1992) as well as hippocampally mediated conditional operations described by Hirsch (1974, 1980), Orr and Berger (1985) further suggested that the hippocampus becomes vital for the modification of behavior when environmental constraints change. The hippocampus permits simultaneous access to an event's constituent elements and their conjunctions. As Daum et al. (1991) pointed out, the conditional discrimination eyeblink task involves the use of conditional operations. On the basis of these theories, and in particular Hirsch's conditional operations theory of hippocampal action, damage to the hippocampal system might impair the function of conditional operations such as the “if–then” rule.
The hippocampal system's involvement in complex forms of associative learning (such as conditions of differential reinforcement) may be related to its neuroanatomical location. The hippocampal system is anatomically situated in a position to bind discrete pieces of information, in that it receives a convergence of inputs from many higher order association cortices in the brain (e.g., Cohen & Eichenbaum, 1993; Eichenbaum, 1992; Sutherland & Rudy, 1989; Wickelgren, 1979). The impairment of bilateral medial temporal lobe amnesic patients in acquiring a conditional discrimination in a delay paradigm indicates more specifically that the hippocampal system may be critical not only in binding temporally discrete pieces of information (as is demonstrated by hippocampal involvement in simple trace conditioning tasks), but also in binding temporally contiguous pieces of information. In other words, the hippocampus may be necessary to bind two discrete pieces of information together under circumstances of greater processing demands, regardless of temporal processing requirements.
Impaired acquisition of a conditional discrimination is consistent with previous human studies with unilateral temporal lobe patients (Daum et al., 1991) and memory disordered patients of mixed etiology (Daum et al., 1989). The current findings are largely consistent with the animal literature to date on conditional discrimination learning. Studies in animals have also implicated the hippocampus in conditional discrimination learning including lesion studies (Good, 1987 [in the pigeon]; Murray & Ridley, 1999 [in rats]; Ross, Orr, Holland, & Berger, 1984 [in rats]) and a recent ischemia hippocampal occlusion study in rats (Modo, Sowinski, & Hodges, 2000). In a review of the animal literature, Gray and McNaughton (1983) concluded that the most critical structure in conditional discrimination learning was the hippocampus. A study by Davidson and Jarred (1989) on excitotoxic lesions of the hippocampus and retention of previously acquired conditional discriminations indicated that although the hippocampus is critical in the acquisition of a conditional discrimination, it is not essential in the retention of the learned response. This finding would indicate that the memory trace associated with conditional discrimination learning that is laid down during acquisition is stored elsewhere in the brain. There is, however, one animal study in rats that has documented spared conditional discrimination after hippocampal lesions (Winocur, 1991).
Awareness was assessed with an open-ended postconditioning questionnaire. Overall, awareness was significantly correlated to performance during trace and delay conditioning. However, this correlation is based on low awareness scores in the MT group and intact awareness in the NC participants. There was very little variability in awareness in the normal control participants (all NCs achieved scores of 4 or 5 out of 5), whereas the amnesic patients' scores indicated they were largely unaware of the stimulus contingencies (MTs scores were primarily 0 and 1). Despite the amnesic patients' lack of awareness, they were able to acquire the S+/US association, albeit to an impaired level, indicating that awareness may not be crucial in simple associative learning. Amnesic patients also demonstrated a high rate of responding during nonreinforced trials. It could be argued that awareness therefore may play a role in inhibiting responses or in acquisition of complex associations. The data from this study are not conclusive given the unsystematic assessment of awareness and the lack of variability in the normal control group. Further complicating the issue, the assessment of awareness by means of an explicit post-session questionnaire in the amnesic group is confounded by the patients' memory deficits. Future analysis of awareness in memory-disordered groups should include an online measure of awareness to control for short-term memory deficits.
Approximately half of the amnesic patients examined in trace and delay conditional discrimination learning had extensive prior training in eyeblink classical conditioning studies (see Table 1). This methodology is not ideal; however, given the rarity of human amnesia, it is necessary. It is important that past experience in conditioning studies and possible carry-over learning effects did not substantiate acquisition in the conditional discrimination paradigm. The amnesic patients were, in fact, impaired despite some patients' prior training in various conditioning tasks. This is underscored by the patients' varied performance across previous conditioning paradigms. Specifically, the amnesic patients performed normally in some past paradigms (e.g., delay conditioning, simple discrimination) but were impaired in other paradigms (e.g., trace conditioning, reversal learning), findings that are consistent with the animal literature. Therefore prior training does not mediate the effects of hippocampal system damage in humans.
There was no observed impairment in the timing of responses in this study. There were no differences in CR onset latency or CR peak latency between groups. This is surprising given the consistent timing deficits in medial temporal lobe amnesic patients in previous studies in our laboratory (McGlinchey-Berroth et al., 1997, 1999). As indicated by McGlinchey-Berroth et al. (1999), it may be that lesions to the cerebellar cortex may eliminate the appropriate expression of a learned temporal discrimination, whereas lesions to the hippocampal system may produce a more subtle alteration in the adaptive timing of CRs.
A final point of discussion is the lack of a significant difference between conditioning trials and extinction trials for the amnesic patients in both trace and delay paradigms. This finding indicates an impairment in the amnesic patients' ability to extinguish the CR in the context of a conditional discrimination paradigm. This impairment is not likely to be due entirely to the fact that the amnesic patients' acquisition rate was lower during learning trials, because they also generated more CRs during the extinction trials than control participants. This is the second demonstration of impaired extinction following hippocampal system damage in humans (McGlinchey-Berroth et al., 1999). However, this finding is somewhat unexpected in light of the fact that 4 of the current group of 8 amnesic patients showed normal extinction in previous eye-blink conditioning studies involving delay and trace simple associative conditioning paradigms (Gabrieli et al., 1995; McGlinchey-Berroth et al., 1997). Impaired extinction following hippocampal system damage is not without precedent, however. Moyer et al. (1990) reported a profound impairment in extinction in hippocampectomized rabbits in a 300-ms trace conditioning study. Thus it appears that, under certain conditions, hippocampal damage or removal may interfere with the extinction of CRs. Perhaps the combined requirement of discriminating a sensory characteristic like various visual stimuli (lights), associating it with an auditory stimulus (tone CS), and the differential occurrence of the US added a level of processing demand that was cognitively complex enough to require hippocampal system involvement for extinction to occur. Future studies are needed to directly address the role of the hippocampal system during extinction in more complex forms of associative learning in humans.
In conclusion, the current data support the idea that the hippocampus plays an important role in the acquisition of a conditional discrimination in both trace and delay paradigms. The impairment in acquisition observed in this group of patients with bilateral medial temporal lobe amnesia was due to an inability to respond differentially to reinforced versus nonreinforced trials. Amnesic patients showed both impaired acquisition on S+ trials, and to a lesser extent, nonadaptive, high levels of responding on S− trials. There were no differences in the production of CRs for either NCs or MTs based on trace versus delay paradigm. These findings indicate that the hippocampal system may be critical not only in binding temporally discrete pieces of information, but also in binding temporally contiguous pieces of information under conditions that are highly complex and demanding.
Acknowledgments
This research was supported by National Institute of Neurological Disorders and Stroke Grants 1P50NS26985 and MH47340. This work represents the doctoral dissertation of Catherine Brawn Fortier. As such she would also like to extend gratitude to Michael Lyons and Martha Tompson for their support and valuable insights into this research. We thank John Power for his assistance in designing the LabVIEW software used to acquire and analyze the eyeblink conditioning data, and the Harvard Cooperative Program on Aging for referring normal control participants.
Footnotes
Editor's Note. Raymond Kesner served as the action editor for this article.—JFD
Contributor Information
Alice Cronin-Golomb, Department of Psychology, Boston University..
John F. Disterhoft, Department of Physiology, Northwestern University Feinberg Medical School
References
- Akase E, Thompson LT, Disterhoft JF. A system for quantitative analysis of associative learning. Journal of Neuroscience Methods. 1994;54:119–130. doi: 10.1016/0165-0270(94)90166-x. [DOI] [PubMed] [Google Scholar]
- Berger T, Orr WB. Hippocampectomy selectively disrupts discrimination reversal conditioning of the rabbit nictitating membrane response. Behavioural Brain Research. 1983;8:49–68. doi: 10.1016/0166-4328(83)90171-7. [DOI] [PubMed] [Google Scholar]
- Capozzi S, Fortier CB, Disterhoft JF, McGlinchey R. Varying trace interval reveals underlying deficit in trace eyeblink acquisition in bitemporal human amnesics. 2003 Manuscript submitted for publication. [Google Scholar]
- Capozzi S, Fortier CB, McGlinchey-Berroth R, Disterhoft JF. Abstract viewer and itinerary planner [CD-ROM]. Society for Neuroscience; Washington, DC: 2002. Delay and trace eyeblink conditioning in patients with frontal lesions (Program No. 872.18). [Google Scholar]
- Carrillo MC, Gabrieli JDE, Hopkins RO, McGlinchey-Berroth R, Fortier CB, Kesner RP, Disterhoft JF. Spared discrimination and impaired reversal eyeblink conditioning in patients with temporal lobe amnesia. Behavioral Neuroscience. 2001;115:1171–1179. doi: 10.1037//0735-7044.115.6.1171. [DOI] [PubMed] [Google Scholar]
- Clark RE, Squire LR. Classical conditioning and brain systems: The role of awareness. Science. 1998 April 3;280:77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- Clark RE, Squire LR. Human eyeblink classical conditioning: Effects of manipulating awareness of the stimulus contingencies. Psychological Science. 1999;10(1):14–18. [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, amnesia, and the hippocampal system. MIT Press; Cambridge, MA: 1993. [Google Scholar]
- Daum I, Breitenstein C, Ackermann H, Schugens MM. Impairment of eyeblink discrimination reversal learning in amnesia. Society for Neuroscience Abstracts. 1997;23:207. [Google Scholar]
- Daum I, Channon S, Canavan AGM. Classical conditioning in patients with severe memory problems. Journal of Neurology, Neurosurgery and Psychiatry. 1989;52:47–51. doi: 10.1136/jnnp.52.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum I, Channon S, Gray JA. Classical conditioning after temporal lobe lesions in man: Sparing of simple discrimination and extinction. Behavioural Brain Research. 1992;52:159–165. doi: 10.1016/s0166-4328(05)80226-8. [DOI] [PubMed] [Google Scholar]
- Daum I, Channon S, Polkey CE, Gray JA. Classical conditioning after temporal lobe lesions in man: Impairment in conditional discrimination. Behavioral Neuroscience. 1991;105:396–408. doi: 10.1037//0735-7044.105.3.396. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Jarred LE. Retention of concurrent conditional discriminations in rats with ibotenate lesions of the hippocampus. Psychobiology. 1989;17:49–60. [Google Scholar]
- Eichenbaum H. The hippocampal system and declarative memory in animals. Journal of Cognitive Neuroscience. 1992;4:217–231. doi: 10.1162/jocn.1992.4.3.217. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T, Cohen NJ. The hippocampus—What does it do? Behavioral and Neural Biology. 1992;57:2–36. doi: 10.1016/0163-1047(92)90724-i. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE, McGlinchey-Berroth R, Carrillo MC, Gluck MA, Cermak LS, Disterhoft JF. Intact delay-eyeblink classical conditioning in amnesia. Behavioral Neuroscience. 1995;109:819–827. doi: 10.1037//0735-7044.109.5.819. [DOI] [PubMed] [Google Scholar]
- Good M. The effects of hippocampal-area parahippocampus lesions on discrimination learning in the pigeon. Behavioural Brain Research. 1987;26:171–184. doi: 10.1016/0166-4328(87)90165-3. [DOI] [PubMed] [Google Scholar]
- Gormezano I. Classical conditioning. In: Sidowski JB, editor. Experimental methods and instrumentation in psychology. McGraw-Hill; New York: 1966. pp. 385–420. [Google Scholar]
- Gray JA, McNaughton N. Comparison between the behavioural effects of septal and hippocampal lesions: A review. Neuroscience and Biobehavioral Reviews. 1983;7:119–188. doi: 10.1016/0149-7634(83)90014-3. [DOI] [PubMed] [Google Scholar]
- Hirsch R. The hippocampus and contextual retrieval of information from memory: A theory. Behavioral Biology. 1974;12:421–444. doi: 10.1016/s0091-6773(74)92231-7. [DOI] [PubMed] [Google Scholar]
- Hirsch R. The hippocampus, conditional operations, and cognition. Physiological Psychology. 1980;8:175–182. [Google Scholar]
- Knuttinen MG, Power JM, Preston AR, Disterhoft JF. Awareness in classical differential eyeblink conditioning in young and aging humans. Behavioral Neuroscience. 2001;115:747–757. doi: 10.1037//0735-7044.115.4.747. [DOI] [PubMed] [Google Scholar]
- McGlinchey-Berroth R, Brawn CM, Disterhoft JF. Temporal discrimination learning in severe amnesics reveals an alteration in the timing of eyeblink conditioned responses. Behavioral Neuroscience. 1999;113:10–18. doi: 10.1037//0735-7044.113.1.10. [DOI] [PubMed] [Google Scholar]
- McGlinchey-Berroth R, Carrillo MC, Gabrieli JDE, Brawn CM, Disterhoft JF. Impaired trace eyeblink conditioning in bilateral medial temporal lobe amnesia. Behavioral Neuroscience. 1997;111:873–882. doi: 10.1037//0735-7044.111.5.873. [DOI] [PubMed] [Google Scholar]
- Modo M, Sowinski P, Hodges H. Conditional discrimination learning in rats with global ischemic brain damage. Behavioural Brain Research. 2000;111:213–221. doi: 10.1016/s0166-4328(00)00160-1. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behavioral Neuroscience. 1990;104:243–252. doi: 10.1037//0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- Murray TK, Ridley RM. The effect of excitotoxic hippocampal lesions on simple and conditional discrimination learning in the rat. Brain Research. 1999;99:103–113. doi: 10.1016/s0166-4328(98)00077-1. [DOI] [PubMed] [Google Scholar]
- Orr WB, Berger TW. Hippocampectomy disrupts the topography of conditioned nictitating membrane responses during reversal learning. Behavioral Neuroscience. 1985;99:35–45. doi: 10.1037//0735-7044.99.1.35. [DOI] [PubMed] [Google Scholar]
- Ross RT, Orr WB, Holland PC, Berger TW. Hippocampectomy disrupts acquisition and retention of learned conditioning and responding. Behavioral Neuroscience. 1984;98:211–225. doi: 10.1037//0735-7044.98.2.211. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Pomerleau D, Bennet L, James J, Morse DL. Acquisition of the classically conditioned eyeblink response in humans over the life span. Psychology and Aging. 1989;4:34–41. doi: 10.1037//0882-7974.4.1.34. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Rudy JW. Configural association theory: The role of the hippocampal formation in learning, memory, and amnesia. Psychobiology. 1989;17:129–144. [Google Scholar]
- Thompson LT, Moyer JR, Akase E, Disterhoft JF. A system for quantitative analysis of associative learning: Hardware interfaces with cross-species applications. Journal of Neuroscience Methods. 1994;54:109–117. doi: 10.1016/0165-0270(94)90165-1. [DOI] [PubMed] [Google Scholar]
- Wickelgren WA. Chunking and consolidation: A theoretical synthesis of semantic networks, configuring in conditioning, S-R versus cognitive learning, normal forgetting, the amnesic syndrome and the hippocampus arousal system. Psychological Review. 1979;86:44–60. [PubMed] [Google Scholar]
- Winocur G. Functional dissociation of the hippocampus and prefrontal cortex in learning and memory. Psychobiology. 1991;19:11–20. [Google Scholar]