Abstract
Background & Aims
With advancing age there is a gradual decline in muscle mass, strength and function. The aim of this study was to determine if regular intake of a nutritional supplement containing essential amino acids (EAA) + arginine could reverse these responses in elderly subjects.
Methods
Twelve glucose intolerant subjects (67.0 ± 5.6 (SD) years, 7 females, 5 males) ingested 11 g of EAA + arginine two times a day, between meals for 16 weeks. Diet and activity were not otherwise modified. Lean body mass (DEXA) was measured every 4th week. Maximal leg strength was tested and functional tests were performed at week 0, 8, 12, and 16.
Results
Lean body mass (LBM) increased during the study (p = 0.038). At week 12, the average increase in LBM was 1.14 ± 0.36 kg (p < 0.05 vs baseline), whereas at week 16, the increase was 0.60 ± 0.38 kg (NS vs baseline). The lower extremity strength measure score (sum of individual knee flexors and extensors 1-repetition maximum, n = 10) was 127.5 ± 21.8 kg at baseline, and average increase during the study was 22.2 ± 6.1% (p < 0.001). Improvements were also observed in usual gait speed (p = 0.002), timed 5-step test (p = 0.007), and timed floor-transfer test (p = 0.022).
Conclusion
Supplementation of the diet with EAA + arginine improves lean body mass, strength and physical function compared to baseline values in glucose intolerant elderly individuals.
Keywords: elderly, sarcopenia, amino acids
Introduction
The decline in muscle mass, strength and function that occurs with aging, as well as the impact of those changes on health and quality of life, is well documented.1 Whereas the effectiveness of resistance exercise training in improving strength, is also clear,2 less than 30% of all adult North Americans exercise regularly, and 50% of those who start an exercise program drop out within the first six months.3 Additional approaches are necessary to effectively ameliorate the loss of muscle mass and strength that occurs with aging.
Amino acids are potent stimulators of muscle protein synthesis in both the young and elderly.4,5 However, the anabolic response to a mixed meal containing both amino acids and carbohydrates is diminished in elderly individuals.6 It is therefore possible that the loss of muscle mass and strength that occurs with aging may be due, in part, to an intake of protein that is less than optimal. If this is the case, ingestion of a nutritional supplement containing amino acids may be a practical approach to improving muscle mass and strength in the elderly.
Previous studies in elderly subjects have shown that provision of dietary supplements have not been effective in improving lean body mass.7-9 However, ingestion of nutritionally balanced supplements have often been found to reduce the caloric intake of the rest of the food eaten in the day by an amount equivalent to the calories supplied in the supplement.8 Therefore a dietary supplement in the elderly would be more appropriately considered as a dietary substitute. It is therefore optimal to provide the most nutritionally-effective supplement possible. In that light, we have previously determined that ingestion of only essential amino acids (EAA) is necessary to stimulate muscle protein synthesis,10 and that the stimulatory effect of EAA on muscle protein synthesis was twice or more than that of an equal amount of a high quality protein.11 Further, a unique acute anabolic effect of arginine has been suggested (Zhang et al, unpublished data).12 The purpose of the current study was to extend those acute observations to determine if chronic ingestion of a dietary supplement of EAA + arginine can increase lean body mass, strength, and functional capacity in free-living elderly.
Materials and Methods
Study Design
Elderly individuals (n = 12) ingested 11 g of EAA + arginine two times a day between meals for 16 weeks. Diet and activity were not otherwise modified. Every 4th week, the subjects were admitted to the General Clinical Research Center (GCRC) at UTMB, Galveston for an overnight stay. Tests of maximal leg strength and muscle function were performed on the day of admission (not week 4). The following morning, body composition was measured by DEXA, and blood was drawn for determination of plasma glucose and insulin concentrations. A blood sample for determination of venous plasma amino acid concentrations was drawn at baseline and after 16 weeks of supplementation.
The protocol was approved by the Institutional Review Board and the General Advisory Committee of the GCRC at UTMB.
Subjects
Twelve elderly individuals (7 females, 5 males, 67.0 ± 5.6 (SD) years, 74.3 ± 19.7 kg at baseline) participated in the study. The subjects were fully informed about the purpose and procedures of the study before written consent was obtained. Prior to participation, each subject had a complete medical screening, including vital signs, blood tests, urine tests, and a 12-lead electrocardiogram. Subjects were excluded from the study if there was evidence of heart disease, hyperlipidemia, kidney or liver disease, or any other disease that might influence the results of the study.
Undiagnosed insulin resistance is extremely common in the elderly population. In order to control for this to some extent, a standard oral glucose tolerance test (OGTT) was performed to assess insulin sensitivity at the outset. Subjects were chosen on the basis of impaired glucose tolerance. We reasoned that if there was an anabolic effect of the amino acids in insulin resistant subjects, then individuals with normal insulin sensitivity would be expected to benefit to an even greater extent because of the normal positive interaction between insulin and amino acids.13 Thus, only subjects with impaired glucose tolerance defined as a plasma glucose concentration >180 mg/dl at 1 hr or >140 mg/dl at 2 hr after oral intake of 75 g glucose, were included. Diabetic subjects (plasma glucose concentration >200 mg/dl at 1 hr or 2 hr after glucose intake) with a reduced insulin production, and subjects taking any medication to treat abnormal blood lipid levels were excluded.
Nutritional Supplement
The nutritional supplement consisted of essential amino acids (EAA) plus arginine (Table 1). The supplement was taken between meals with water in two daily doses of 11 g in the form of capsules. Each capsule contained 0.5 g of the amino acid supplement. The first dose was taken between breakfast and lunch, and the second dose was ingested between lunch and dinner. The subjects recorded the time of intake of every dose. The subjects visited the hospital every two weeks to pick up a new supply of supplements. The supplement was packaged in small labeled plastic bags, with each bag containing one dose of supplement. In the weeks without hospital visits, the subjects were given follow-up calls to verify intake of the supplements, as well as to document possible changes in diet, activity and anything else (sickness, etc.) that might influence the results of the study.
Table 1.
Composition of amino acid supplement
| Amino acid | g | % of total |
|---|---|---|
| Histidine | 0.36 | 3.26 |
| Isoleucine | 0.94 | 8.57 |
| Leucine | 3.95 | 35.88 |
| Lysine•HCl | 1.88 | 17.08 |
| Methionine | 0.39 | 3.59 |
| Phenylalanine | 0.51 | 4.65 |
| Threonine | 1.05 | 9.57 |
| Valine | 0.82 | 7.44 |
| Arginine | 1.10 | 9.97 |
|
| ||
| Total | 11 | 100 |
The supplement was taken in two daily doses of 11 g, i.e., in total 22 g/day was ingested.
Diet and Physical Activity
Before starting, subjects were counseled to maintain their usual dietary intake and physical activity. There was close follow-up during their visits to the hospital and by telephone calls between visits. Before the start of the study (week -1) and every 4th week thereafter (week 3, 7, 11, and 15), the participants recorded their diet for 3 days (2 week-days and 1 weekend-day). The Physical Activity Scale for Elderly (PASE) was also used to estimate their physical activity during the study period.
Muscular Strength
One repetition maximum (1RM) for leg extension and leg flexion for each leg respectively, was determined at weeks 0, 8, 12, and 16. Following 15 min of pre-test stretching and warm-up, the maximum amount of weight that the subject could lift just once (i.e., 1RM) was determined. The weight was progressively increased until the subject could not, on at least two attempts, move the lever arm through the full range of motion. To minimize the effects of fatigue, 1 min of rest was allowed between attempts, and 3-5 min between each movement. Subjects were closely monitored to avoid compensation movement. The two values for each leg were added and expressed as the lower extremity score or sum of 1RM.
Usual and Fast Walking Speed and Endurance
The subjects were instructed to walk on a pressure sensitive mat (Gaitrite®, CIR Systems, Inc. Havertown, PA, USA), first at self-selected normal walking pace and thereafter at self-selected fastest speed.14,15 In order to exclude the first and last steps (to avoid acceleration and deceleration effects), each subject started walking from a point two m in front of the mat and did not stop until two m beyond the mat. The test was repeated twice for each condition and the data for the two trials (expressed in m/s) were thereafter averaged. A 5-min walking test combined with a portable gas analyzer (VO2000 Portable Metabolic System, Medical Graphics Corporation, St. Paul, MN, USA) was used to assess gait endurance and cost.16
Functional Tests
The subjects also underwent a series of functional tests consisting of a timed step test,17 a timed floor transfer test,17 and the short physical performance battery test (SPPB).18 Briefly, in the timed 5-step test, the subject was asked to step up on and down from a 4 inches bench as fast as possible five times. In the timed floor transfer test, the subject was asked to go from a standing position to a supine position on the floor and thereafter up to a standing position again, as fast as possible for one repetition. Finally, the short physical performance battery test (SPPB) consisted of a static balance test, a chair test and a walking test. Each test was scored from 0 to 4, inversely corresponding to the quartile of performance. The data were thereafter added together, and the maximum score that could be achieved for the SPPB was 12.
Duel-Energy X-ray Absorptiometry (DEXA)
The subjects underwent a full body DEXA at week 0, 4, 8, 12, and 16 to determine body composition. All DEXA scans were performed on a Hologic QDR4500A system (Hologic, Inc., Bedford, MA, USA).
Sample Analyses
For determination of plasma amino acid concentrations, 50 μl of plasma was mixed with 100 μl internal standard and 100 μl acetonitrile (HPLC grade). This was thoroughly mixed, before incubated for 30 min on ice. Thereafter 750 μl ddH2O was added before the sample was centrifuged at 1500 g for 10 min. Finally, 100 μl of the supernatant was transferred to a 0.2 cm Ultrafree-MC Centrifugal Filter (Millipore, Billerica, MA, USA) and spun at 3000 g for 4 hours at 4°C. Plasma amino acid concentrations were thereafter determined by high-performance liquid chromatography (Waters Alliance® HPLC System 2690, Milford, MA).
Plasma glucose concentration was determined enzymatically (YSI 1500, Yellowspring Instruments, Yellowspring, OH, USA). Plasma insulin concentration was determined by a radioimmunoassay method (Diagnostic Products Corporation, Los Angeles, CA, USA).
Statistical Methods
Overall significance of differences in response with time was tested by one-way repeated measures analysis of variance followed by Dunnett's test with week 0 as control (SigmaStat 2.03, SPSS Inc, Chicago, IL). Results were considered significant if p < 0.05. The results are presented as means ± SE unless otherwise noted.
Results
Amino Acid Supplementation, Diet and Physical Activity
The amino acid supplementation was well tolerated by the subjects. All except one subject ingested all the doses. The one subject forgot to ingest an average of one dose per week.
No overall changes in physical activity were observed during the study period. Similarly, dietary records indicated that there were no changes in diet (n = 9). The dietary intake was 1733 ± 226 kcal/day when no supplement was taken vs an average of 1735 ± 176 kcal/day during the study period. Corresponding values for protein intake were 1.03 ± 0.19 vs 0.99 ± 0.21 g · kg-1 · day-1, fat intake was 0.86 ± 0.15 vs 0.86 ± 0.09 g · kg-1 · day-1, and carbohydrate intake was 3.05 ± 0.70 vs 3.11 ± 0.70 g · kg-1 · day-1.
No changes in fasting plasma amino acid concentrations were found between week 0 and 16, except a slight increase in methionine (39.4 ± 1.2 vs 43.2 ± 1.9 μmol/l, t-test: p = 0.05) and an increase in threonine (195.3 ± 8.4 vs 227.6 ± 9.7 μmol/l, p = 0.004) concentrations.
There was an increase in fasting plasma glucose concentration during the study period (ANOVA: p < 0.001; week 0: 4.7 ± 0.1 mmol/l; week 16: 5.5 ± 0.1 mmol/l), whereas changes in insulin concentration did not reach significance (week 0: 8.64 ± 1.57 mU/l; week 16: 6.97 ± 1.21 mU/l).
Body Composition
There was a significant overall increase in lean body mass during the supplementation period (p = 0.038; Table 2). There was a gradual increase in lean body mass to a value of 1.14 ± 0.36 kg above the basal value at 12 weeks (p < 0.05 vs baseline). After that, there was a drop, and as a result, lean body mass was 0.60 ± 0.38 kg higher than baseline at the end of the study (NS vs. baseline).
Table 2.
Body composition at baseline, and after 4, 8, 12 and 16 weeks of amino acid supplementation.
| Week 0 | Week 4 | Week 8 | Week 12 | Week 16 | |
|---|---|---|---|---|---|
| Lean body mass (kg)* | 47.97 ± 3.42 | 48.49 ± 3.44 | 48.89 ± 3.47 | 49.11 ± 3.36# | 48.57 ± 3.30 |
| Leg lean mass (kg) | 14.98 ± 1.13 | 15.28 ± 1.19 | 15.18 ± 1.15 | 15.23 ± 1.11 | 15.31 ± 1.08 |
| Total body mass (kg) | 74.31 ± 5.67 | 74.71 ± 5.75 | 75.09 ± 5.77 | 74.99 ± 5.70 | 74.60 ± 5.62 |
| Total body fat (kg) | 24.19 ± 3.59 | 24.02 ± 3.68 | 24.00 ± 3.67 | 23.67 ± 3.62 | 23.90 ± 3.70 |
| Bone mineral content (kg) | 2.21 ± 0.12 | 2.20 ± 0.12 | 2.21 ± 0.12 | 2.21 ± 0.12 | 2.21 ± 0.12 |
Values are mean ± SE; n = 12.
ANOVA; p = 0.038.
p < 0.05 vs baseline.
Leg lean mass was 14.98 ± 1.13 kg at baseline (Table 2). It increased about 0.30 kg during the study, but this did not reach significance.
There were no changes in total body mass from the start of the study to the end of the 16 week supplementation period (Table 2). Total body fat mass also did not change significantly during the study (Table 2).
Leg Strength
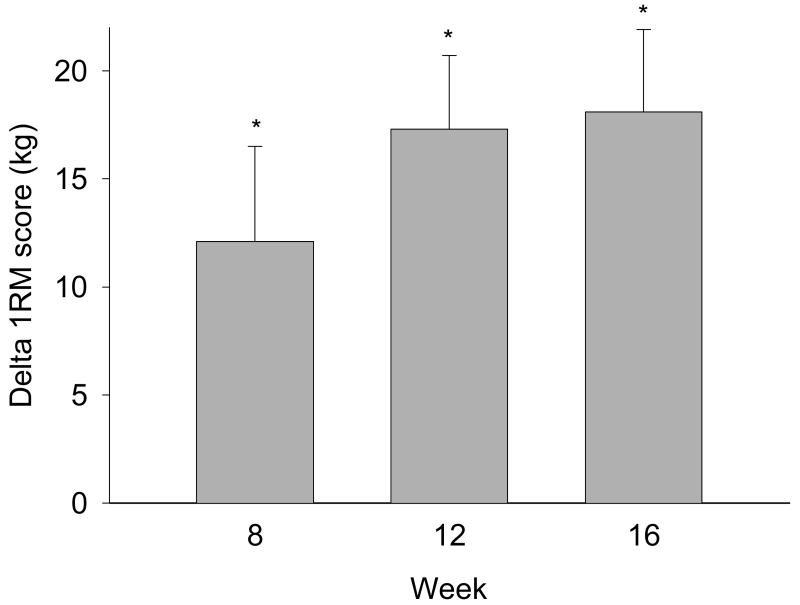
The summed 1RM (n = 10) score increased from the initial value of 127.5 ± 21.8 kg at baseline to 145.6 ± 19.2 at the end of the supplementation period (p < 0.001; Figure 1). The average of the individual percent gains in the sum scores was 22.2 ± 6.1% at week 16. The changes vs baseline were significant at all time points.
Figure 1.
Changes in 1 repetition maximum score (sum of the individual knee flexors and knee extensors' 1 repetition maximum) vs basal value (134.3 ± 23.1 kg) after 8, 12 and 16 weeks of amino acid supplementation (mean ± SE; n = 10); * p < 0.05 vs baseline.
Two women were also tested during the screening before the start of the study. We could not identify any learning effect since there were no differences in the 1RM scores during screening vs week 0 (66.90 vs 68.04 kg and 65.77 vs 63.50 kg, respectively).
Walking Tests
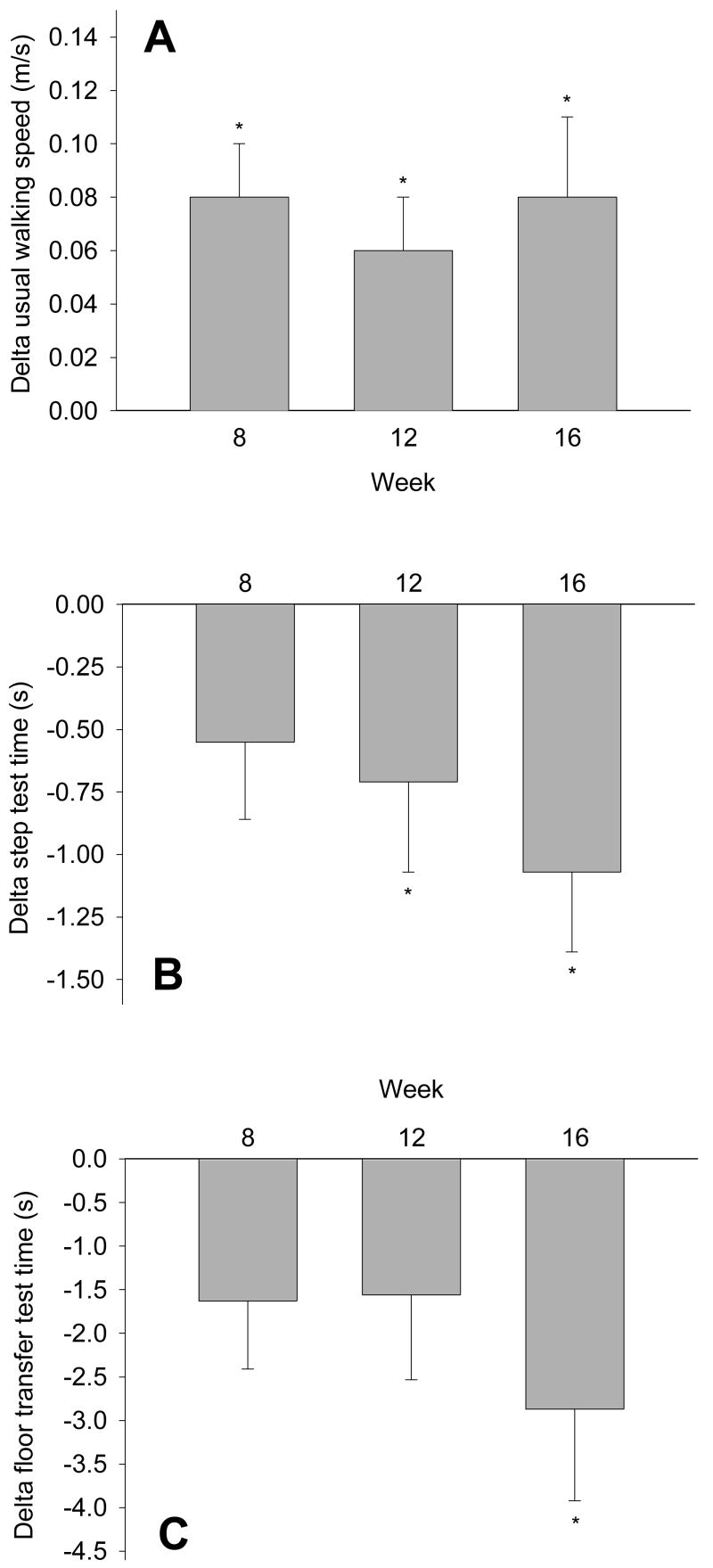
The usual walking speed (n = 11) increased from 1.26 ± 0.05 m/s at basal to 1.34 ± 0.05 m/s at week 16 (p = 0.002; Figure 2, panel A), whereas no changes could be seen in the fastest walking speed (week 0 vs week 16: 1.94 ± 0.08 vs 2.00 ± 0.09 m/s). No changes were seen during the study period in the distance walked.
Figure 2.
Changes in normal walking speed (panel A; n = 11; One-way RM ANOVA: p = 0.002), time to perform a 5-step test (B; n = 12; p = 0.007), and time to perform a floor transfer test (C; n = 12; p = 0.022) vs baseline after 8, 12 and 16 weeks of amino acid supplementation (mean ± SE). * p < 0.05 vs baseline. See text for values at week 0.
Functional Tests
Significant improvements were seen in the time to perform the 5-step test (week 0 vs 16: 8.77 ± 0.78 vs 7.71 ± 0.60 s; p = 0.007; Figure 2, panel B) and the floor transfer test (week 0 vs 16: 9.57 ± 1.74 vs 6.82 ± 1.49 s; p = 0.022; Figure 2, panel C).
No changes were seen in the Short Physical Performance Battery (SPPB) Score. The highest possible score is 12 (4 for each test), and the scores at week 0, 8, 12 and 16 were 11.6 ± 0.3, 11.6 ± 0.3, 11.3 ± 0.3, 11.7 ± 0.2 and 11.6 ± 0.3, respectively, indicating highly functional volunteers by SPPB at all time points.
Discussion
The results of this study showed that supplementation of the diet with EAA + arginine between meals increased lean body mass, muscle strength and physical function compared to baseline values in glucose intolerant elderly subjects. These changes occurred without significant changes in other dietary intake or physical activity.
A dietary supplement must stimulate muscle protein synthesis more than the same amount of normal food intake to be effective in this age group. Thus, the EAA mixture is an innovative approach to dietary supplementation of the elderly. Our previous results have showed that ingestion of a bolus of formulated EAA stimulated muscle protein synthesis in elderly individuals, and that the efficiency of utilization of EAA alone for protein synthesis is more than twice that of whey protein on a g/g basis.11 Thus, even if ingestion of EAA causes a corresponding decrease in a calorically-equivalent amount of the daily food intake, a beneficial effect of the EAA would be expected because of (a) the low caloric value of the EAA mixture (84 kcal), (b) the far greater efficiency of EAA in stimulating muscle protein synthesis than normal food intake, and (c) the absence of the satiating capacity of whole protein. Finally, in healthy young subjects it has been shown that intake of 15 g EAA (plus carbohydrates) did not interfere with the anabolic response to a mixed meal 2.5 hours later.19
The composition of the mixture of EAA was designed to be maximally effective in stimulating muscle protein synthesis in the elderly. It was based on the composition we have previously used in many of our acute studies and also in a study of 28 days of bed rest.20,21 In the bed rest study,21 EAA + carbohydrate supplementation was shown to preserve muscle mass and decrease the loss of muscle function.
Leucine comprised the highest percentage of the mixture (Table 1). The rationale for this high percentage was that leucine can potentially activate initiation factors involved in the acceleration of protein synthesis,22 which was particularly important in the elderly due to the diminished responsiveness of those factors.23,24 The percentage of leucine we used was based on our findings that the acute stimulation of muscle protein synthesis by EAA was enhanced in elderly (but not young) subjects by the addition of a high (40%) proportion of leucine in a mixture of EAA.25
We also included arginine in the supplement. In healthy young adults sufficient arginine can be synthesized to meet demands, but during rapid growth26 or in response to stress27 the demand for arginine may not be fully met by de novo synthesis or diet intake. Further, we have recently shown that arginine has a unique stimulatory effect on muscle protein synthesis that is not mediated via nitric oxide production (Zhang et al, unpublished data).12 As in the case of leucine, the mechanism of arginine-mediated stimulation of muscle protein synthesis may be by activation of eucaryotic initiation factors.28
Despite the modest response of muscle mass to the EAA supplement, the improvement in both strength and function points towards a change in muscle quality. Improvement in 5-min walking distance was not found, but this may be explained by the fact that our volunteers were a healthy study population (confirmed by results of SPPB and walking speed). Three months of amino acid supplementation (12 g/day) was found to improve 6-min walking distance, in addition to self-perceived ambulatory dysfunction and maximal isometric muscle strength of the hand in more sedentary and frail elderly.29,30 A possible explanation for the principal effect of EAA supplementation to be on the quality, rather than quantity, is that the fractional turnover of muscle (i.e., both synthesis and breakdown) was stimulated. The rate of muscle protein synthesis has been found to be related to strength in the elderly, irrespective of muscle size.31
Generally, inclusion of a placebo group is preferred. However, we assumed that no improvement in lean body mass, muscle strength and physical function would have occurred over 16 weeks without intervention. Thus, the individual pre-intervention data were used as control. The recently-reported results of the Health, Aging and Body Composition Study support the suggestion that in weight-stable elderly subjects no significant changes in lean body mass can be expected over that time interval.32 Consistent with this expectation, no changes were found in maximal isometric muscle strength in free-living elderly subjects over a three month period.33 In a study performed in our own lab, free living elderly men had a decline in lean body mass, and decreases in several parameters of strength over a six-month period without treatment.34 The lack of changes, and in some cases reduction, in strength over time without intervention in previous studies also supports the validity of the test-retest model for strength that we used. Similarly, no changes in physical function is expected in elderly without any intervention.30,35-37) Consequently, we feel confident that the changes we observed in this study were due to the ingestion of the amino acids.
It may also be argued that more familiarization sessions should have been performed. However, the functional tests we used have been shown to have excellent test-retest reliabilities even without previous familiarization,15-17 with the exception of the floor-transfer test which has moderately high test-retest reliability (ICC2,1 = 0.79, p = 0.000117). In addition, the tests were always performed by the same tester, using the same procedures and oral feedback each time. Further, the changes in strength were much greater than what has been reported for learning effects.38,39 In fact, it was recently shown that for relatively healthy elderly men, familiarization sessions for 1-RM testing is not necessary.39 Finally, there was an initial 8 week period (and thereafter 4 weeks) between testing, which seems too long for a learning effect, and still improvements were observed.
Results from acute studies have shown that exercise and amino acid intake have additive effects on muscle protein synthesis.40 We propose that over a more prolonged time, exercise will amplify the beneficial effects of EAA supplementation on lean body mass, strength, and muscle function in both healthy and insulin-resistant elderly. This remains to be studied.
In summary, the results of the present study showed improvements of lean body mass, muscle strength and physical function in response to supplementation of the diet with EAA + arginine in glucose intolerant elderly subjects.
Acknowledgments
The authors thank the subjects who participated in the study for their time and dedication. We thank Sue Minello R.N., Roxana Hirst M.S., and Nancy Poore at the Pepper Center for help in recruiting the volunteers, and the nurses, dieticians and the staff at the General Clinical Research Center (GCRC) at the University of Texas Medical Branch (UTMB) at Galveston, TX. We thank Kendrick Armstrong, Melissa Bailey, Donovan Randolph, Stephaine J. Blase, Tara Cocke, Daniel L. Creson, Christopher P. Danesi, Melanie C. Green, Ola Rønsen, and Scott Schutzler for skillful technical assistance. We acknowledge Elizabeth Protas PhD for support of Sandrine Tissier.
The study was supported by PO1 AG23591, M01 RR 00073, P30 AG024832 (NIH/NIA), NPFR-0043 National Space Biological Research Institute (NSBRI) and Ajinomoto Co, Inc (provided the amino acids).
RRW, AAF, HK, and EB designed the study. Q-YTB, ST and EB organized the study and carried out the experiments. Q-YTB provided medical coverage. EB performed the data handling and statistical analyses, and also wrote the manuscript with the assistance of the others.
Non-Standard Abbreviation
- EAA
essential amino acids
Footnotes
Conference presentation: 53rd annual meeting of the American College of Sports Medicine. Denver, CO, USA, 2006.
Financial disclosure: Hisamine Kobayashi is working for AminoScience Laboratories that is part of Ajinomoto Co which is the company that provided the free amino acids in this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Evans WJ. What is sarcopenia. J Gerontol A Biol Sci Med Sci. 1995;50 doi: 10.1093/gerona/50a.special_issue.5. Spec No:5-8. [DOI] [PubMed] [Google Scholar]
- 2.Evans WJ. Protein nutrition, exercise and aging. J Am Coll Nutr. 2004;23:601S–609S. doi: 10.1080/07315724.2004.10719430. [DOI] [PubMed] [Google Scholar]
- 3.Estabrooks P. Sustaining exercise participation through group cohesion. Exerc Sport Sci Rev. 2000;28:63–67. [PubMed] [Google Scholar]
- 4.Bohe J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. J Physiol. 2003;552:315–324. doi: 10.1113/jphysiol.2003.050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol. 1999;277:E513–E520. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- 6.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85:4481–4490. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell WW, Crim MC, Young VR, Joseph LJ, Evans WJ. Effects of resistance training and dietary protein intake on protein metabolism in older adults. Am J Physiol. 1995;268:E1143–E1153. doi: 10.1152/ajpendo.1995.268.6.E1143. [DOI] [PubMed] [Google Scholar]
- 8.Fiatarone MA, O'Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA, Evans WJ. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 9.Welle S, Thornton CA. High-protein meals do not enhance myofibrillar synthesis after resistance exercise in 62- to 75-yr-old men and women. Am J Physiol. 1998;274:E677–E683. doi: 10.1152/ajpendo.1998.274.4.E677. [DOI] [PubMed] [Google Scholar]
- 10.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78:250–258. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang XJ, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp Gerontol. 2006;41:215–219. doi: 10.1016/j.exger.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Paddon-Jones D, Børsheim E, Wolfe RR. Potential ergogenic effects of arginine and creatine supplementation. J Nutr. 2004;134:2888S–2894S. doi: 10.1093/jn/134.10.2888s. [DOI] [PubMed] [Google Scholar]
- 13.Wolfe RR, Volpi E. Insulin and protein metabolism. In: Jefferson LS, Cherrington AD, editors. Handbook of Physiology. Vol. 2001. New York: Oxford; pp. 735–757. [Google Scholar]
- 14.Cutlip RG, Mancinelli C, Huber F, DiPasquale J. Evaluation of an instrumented walkway for measurement of the kinematic parameters of gait. Gait Posture. 2000;12:134–138. doi: 10.1016/s0966-6362(00)00062-x. [DOI] [PubMed] [Google Scholar]
- 15.Webster KE, Wittwer JE, Feller JA. Validity of the GAITRite walkway system for the measurement of averaged and individual step parameters of gait. Gait Posture. 2005;22:317–321. doi: 10.1016/j.gaitpost.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Cunha-Filho IT, Henson H, Protas EJ. Reliability of a portable gas analyzer during a 5-min walk test. Am J Phys Med Rehabil. 2007;86:469–473. doi: 10.1097/PHM.0b013e31805b9505. [DOI] [PubMed] [Google Scholar]
- 17.Murphy MA, Olson SL, Protas EJ, Overby AR. Screening for falls in community-dwelling elderly. J Aging Phys Act. 2003;11:64–78. [Google Scholar]
- 18.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S, Berkman LF, Wallace RB. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 19.Paddon-Jones D, Sheffield-Moore M, Aarsland A, Wolfe RR, Ferrando AA. Exogenous amino acids stimulate human muscle anabolism without interfering with the response to mixed meal ingestion. Am J Physiol Endocrinol Metab. 2005;288:E761–E767. doi: 10.1152/ajpendo.00291.2004. [DOI] [PubMed] [Google Scholar]
- 20.Børsheim E, Tipton KD, Wolf SE, Wolfe RR. Essential amino acids and muscle protein recovery from resistance exercise. Am J Physiol Endocrinol Metab. 2002;283:E648–E657. doi: 10.1152/ajpendo.00466.2001. [DOI] [PubMed] [Google Scholar]
- 21.Paddon-Jones D, Sheffield-Moore M, Urban RJ, Sanford AP, Aarsland A, Wolfe RR, Ferrando AA. Essential amino acid and carbohydrate supplementation ameliorates muscle protein loss in humans during 28 days bedrest. J Clin Endocrinol Metab. 2004;89:4351–4358. doi: 10.1210/jc.2003-032159. [DOI] [PubMed] [Google Scholar]
- 22.Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr. 2000;130:139–145. doi: 10.1093/jn/130.2.139. [DOI] [PubMed] [Google Scholar]
- 23.Guillet C, Prod'homme M, Balage M, Gachon P, Giraudet C, Morin L, Grizard J, Boirie Y. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J. 2004;18:1586–1587. doi: 10.1096/fj.03-1341fje. [DOI] [PubMed] [Google Scholar]
- 24.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 25.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291:E381–E387. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 26.Kim SW, McPherson RL, Wu G. Dietary arginine supplementation enhances the growth of milk-fed young pigs. J Nutr. 2004;134:625–630. doi: 10.1093/jn/134.3.625. [DOI] [PubMed] [Google Scholar]
- 27.Saito H, Trocki O, Wang SL, Gonce SJ, Joffe SN, Alexander JW. Metabolic and immune effects of dietary arginine supplementation after burn. Arch Surg. 1987;122:784–789. doi: 10.1001/archsurg.1987.01400190050010. [DOI] [PubMed] [Google Scholar]
- 28.Ban H, Shigemitsu K, Yamatsuji T, Haisa M, Nakajo T, Takaoka M, Nobuhisa T, Gunduz M, Tanaka N, Naomoto Y. Arginine and Leucine regulate p70 S6 kinase and 4E-BP1 in intestinal epithelial cells. Int J Mol Med. 2004;13:537–543. [PubMed] [Google Scholar]
- 29.Scognamiglio R, Avogaro A, Negut C, Piccolotto R, de Kreutzenberg SV, Tiengo A. The effects of oral amino acid intake on ambulatory capacity in elderly subjects. Aging Clin Exp Res. 2004;16:443–447. doi: 10.1007/BF03327399. [DOI] [PubMed] [Google Scholar]
- 30.Scognamiglio R, Piccolotto R, Negut C, Tiengo A, Avogaro A. Oral amino acids in elderly subjects: effect on myocardial function and walking capacity. Gerontology. 2005;51:302–308. doi: 10.1159/000086366. [DOI] [PubMed] [Google Scholar]
- 31.Balagopal P, Rooyackers OE, Adey DB, Ades PA, Nair KS. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am J Physiol. 1997;273:E790–E800. doi: 10.1152/ajpendo.1997.273.4.E790. [DOI] [PubMed] [Google Scholar]
- 32.Newman AB, Lee JS, Visser M, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Nevitt M, Harris TB. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr. 2005;82:872–878. doi: 10.1093/ajcn/82.4.872. [DOI] [PubMed] [Google Scholar]
- 33.Scognamiglio R, Negut C, Piccolotto R, Dioguardi FS, Tiengo A, Avogaro A. Effects of oral amino acid supplementation on myocardial function in patients with type 2 diabetes mellitus. Am Heart J. 2004;147:1106–1112. doi: 10.1016/j.ahj.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, Lieberman SA, Tipton K, Wolfe RR, Urban RJ. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002;282:E601–E607. doi: 10.1152/ajpendo.00362.2001. [DOI] [PubMed] [Google Scholar]
- 35.Kenny AM, Kleppinger A, Wang Y, Prestwood KM. Effects of ultra-low-dose estrogen therapy on muscle and physical function in older women. J Am Geriatr Soc. 2005;53:1973–1977. doi: 10.1111/j.1532-5415.2005.53567.x. [DOI] [PubMed] [Google Scholar]
- 36.Sallinen J, Fogelholm M, Volek JS, Kraemer WJ, Alen M, Hakkinen K. Effects of strength training and reduced training on functional performance and metabolic health indicators in middle-aged men. Int J Sports Med. 2007;28:815–822. doi: 10.1055/s-2007-964901. [DOI] [PubMed] [Google Scholar]
- 37.Rooks DS, Kiel DP, Parsons C, Hayes WC. Self-paced resistance training and walking exercise in community-dwelling older adults: effects on neuromotor performance. J Gerontol A Biol Sci Med Sci. 1997;52:M161–M168. doi: 10.1093/gerona/52a.3.m161. [DOI] [PubMed] [Google Scholar]
- 38.Phillips WT, Batterham AM, Valenzuela JE, Burkett LN. Reliability of maximal strength testing in older adults. Arch Phys Med Rehabil. 2004;85:329–334. doi: 10.1016/j.apmr.2003.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Schroeder ET, Wang Y, Castaneda-Sceppa C, Cloutier G, Vallejo AF, Kawakubo M, Jensky NE, Coomber S, Azen SP, Sattler FR. Reliability of maximal voluntary muscle strength and power testing in older men. J Gerontol A Biol Sci Med Sci. 2007;62:543–549. doi: 10.1093/gerona/62.5.543. [DOI] [PubMed] [Google Scholar]
- 40.Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol. 1997;273:E122–E129. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]