Abstract
We evaluated the effects of physiologic increases in insulin on hepatic and peripheral glucose metabolism in nonpregnant (NP) and pregnant (P; 3rd trimester) conscious dogs (n = 9 each) using tracer and arteriovenous difference techniques during a hyperinsulinemic euglycemic clamp. Insulin was initially (−150 to 0 min) infused intraportally at a basal rate. During 0–120 min (Low Insulin), the rate was increased by 0.2 mU·kg−1·min−1, and from 120 to 240 min (High Insulin) insulin was infused at 1.5 mU·kg−1·min−1. Insulin concentrations were significantly higher in NP than P during all periods. Matched subsets (n = 5 NP and 6 P) were identified. In the subsets, insulin was 7 ± 1, 9 ± 1, and 28 ± 3 μU/ml (basal, Low Insulin, and High Insulin, respectively) in NP, and 5 ± 1, 7 ± 1, and 27 ± 3 μU/ml in P. Net hepatic glucose output was suppressed similarly in both subsets (≥50% with Low Insulin, 100% with High Insulin), as was endogenous glucose rate of appearance. During High Insulin, NP dogs required more glucose (10.8 ± 1.5 vs. 6.2 ± 1.0 mg·kg−1·min−1, P < 0.05), and hindlimb (primarily skeletal muscle) glucose uptake tended to be greater in NP than P (18.6 ± 2.5 mg/min vs. 13.6 ± 2.0 mg/min, P = 0.06). The normal canine liver remains insulin sensitive during late pregnancy. Differing insulin concentrations in pregnant and nonpregnant women and excessive insulin infusion rates may explain previous findings of hepatic insulin resistance in healthy pregnant women.
Keywords: insulin resistance, skeletal muscle, liver, hyperinsulinemic euglycemic clamp
INSULIN ACTION DURING THE third trimester is 45–70% reduced, compared with that in nonpregnant women (1, 7, 8). Skeletal muscle and adipose tissue are known to participate in the insulin resistance, and the possibility of insulin resistance at the liver has been a subject of much study. In two investigations in rabbits during the third trimester, endogenous glucose production (EGP) fell only 30–32% during a hyperinsulinemic euglycemic clamp, compared with 85–100% reduction in non-pregnant rabbits with similar insulin concentrations (16, 19). Hepatic insulin resistance was also reported in pregnant rats (22). In contrast, during hyperinsulinemic euglycemic clamps in the third trimester, normal pregnant dogs demonstrated complete suppression of EGP (3, 11).
The data regarding hepatic insulin sensitivity in human pregnancy are equally conflicting. While there seems to be little doubt that hepatic insulin resistance is a feature of gestational diabetes (5), at least in obese women, different authors have analyzed the available reports in the literature and concluded that the liver in normal women exhibits insulin resistance in late pregnancy (24) or, conversely, remains insulin sensitive throughout pregnancy (2). Unfortunately, in all of the human and animal studies available, the insulin concentrations achieved [even at the lower step when two steps were examined (5)] were excessive for examining subtle defects in hepatic insulin sensitivity (10).
We hypothesized that the liver remains insulin sensitive in late pregnancy in women and animal models with normal glucose tolerance. This project was undertaken to examine hepatic and peripheral insulin sensitivity and glucose metabolism in normal pregnant (3rd trimester) and nonpregnant dogs during a two-step hyperinsulinemic euglycemic clamp. The first step utilized an insulin infusion rate previously demonstrated to suppress EGP in normal dogs ~50%, and the second step used an infusion rate known to suppress EGP fully and increase whole body glucose utilization approximately sevenfold in normal dogs (10). The goal was to match the insulin concentrations between pregnant and nonpregnant animals in a model in which net hepatic glucose balance could be directly measured. At the same time, we utilized the arteriovenous difference technique to assess hindlimb flux of substrates.
METHODS
Animals and surgical procedures
Experiments were performed in overnight-fasted (18 h), conscious adult female mongrel dogs. Nine of the dogs (P group) were at 7–8 wk gestation (term = 9 wk) at the time of study, and nine were nonpregnant (NP group) with basal estradiol and progesterone concentrations. Weights were 21.3 ± 1.1 and 24.0 ± 1.4 kg in NP and P, respectively, and diet and housing were as previously described (12). The protocol was approved by the Vanderbilt University Animal Care and Use Committee.
Approximately 16 days prior to the experiment, the dogs underwent laparotomy and surgical insertion of splenic and jejunal vein infusion catheters for intraportal infusions, as well as insertion of sampling catheters in the left femoral artery, hepatic portal vein, left common hepatic vein, and right common iliac vein. Ultrasonic flow probes (Transonic Systems, Ithaca, NY) were placed around the hepatic artery, portal vein, and external iliac artery (33). Criteria and preparation for study were as previously described (12).
Experimental design
Each study consisted of three periods: basal insulinemia (referred to as basal), −150 to 0 min; mild hyperinsulinemia (Low Insulin), 0 to 120 min; and marked hyperinsulinemia (High Insulin), 120 to 240 min. Blood samples were collected from the artery and the portal, hepatic, and iliac veins during the last 30 min of each experimental period following techniques previously described (12, 30).
Infusions
Each experiment began at −150 min with initiation of a primed (40 μCi), constant infusion (0.35 μCi/min) of [3-3H]glucose (New England Nuclear, Boston, MA) to allow time for tracer equilibration; this infusion continued throughout the experiment. A continuous infusion of Indocyanine Green dye (0.08 mg/min; Sigma, St. Louis, MO) was begun at the same time (3). Also at −150 min, a continuous infusion of somatostatin (0.8 μg·kg−1·min−1; Bachem, Torrance, CA) was begun via peripheral vein. In addition, intraportal infusion of regular pork insulin (Eli Lilly, Indianapolis, IN) was begun at 0.3 mU·kg−1·min−1 via the splenic and jejunal catheters to allow infusion into the hepatic portal circulation. Glucagon (GlucaGen; Novo Nordisk, Denmark) was also begun and infused intraportally at 0.6 ng·kg−1·min−1 for the remainder of the study to maintain basal concentrations. During the basal period, the arterial plasma glucose concentrations were obtained every 5 min, and the insulin infusion rate was adjusted as necessary to maintain euglycemia. No further changes in insulin infusion were made during the period from −60 min through the end of the basal period. At 0 min, the insulin infusion rate was increased by 0.2 mU·kg−1·min−1 over the basal rate (Low Insulin period) (10). At 120 min, the insulin infusion rate was increased in both groups to 1.5 mU·kg−1·min−1 (High Insulin period)(10). During the Low and High Insulin periods, glucose (D50W; Abbott Laboratories, North Chicago, IL) was infused via peripheral vein as needed to maintain euglycemia.
These studies were begun before it had been established that insulin clearance is altered in pregnancy. Consequently, the same insulin infusion protocol was used in P and NP. However, the insulin concentrations differed significantly between groups in all three periods of study (8 ± 1, 10 ± 1, and 37 ± 4 μU/ml in NP and 4 ± 1, 6 ± 1, and 24 ± 2 μU/ml in P). Since this made interpretation of insulin sensitivity difficult, dogs from each group were selected so that insulin concentrations were matched between groups. Thus, this report includes the data from all 18 dogs but, where appropriate, also includes data from the subsets (6 dogs in the P group and 5 dogs in the NP group, referred to as the “matched subsets”) that had similar insulin concentrations. The conclusions drawn from the data are the same, whether all 18 dogs or only the 11 in the subsets are considered.
Analytical procedures
Analyses have been described in detail previously (12) and included plasma concentrations of glucose, insulin, glucagon, cortisol, estrogen, progesterone, epinephrine, norepinephrine, nonesterified fatty acids, and [3H]glucose content and blood concentrations of lactate, alanine, glycine, theonine, serine, glutamine, glutamate, glycerol, β-hydroxybutyrate, and acetoacetate.
Calculations
Total hepatic blood flow was assessed by hepatic extraction of Indocyanine Green and ultrasonic flow probes (11). Where the ultrasonic probes functioned, balance data were calculated using ultrasonic-derived flows, because this does not require an assumption about the relative proportion of hepatic flow provided by the hepatic artery vs. portal vein. Where both ultrasonic and ICG flows were available, however, calculations performed with the two different sets of flow data do not differ significantly. Net hepatic substrate balance was calculated as LOADout − LOADin. The LOADout = H × FH and LOADin = (FA × A) + (FP × P), where A, P, and H are the arterial, portal vein, and hepatic vein substrate concentrations, respectively, and FA, FP, and FH are the arterial, portal vein, and total hepatic blood or plasma flow, as appropriate for the particular substrate. Hindlimb balance was calculated as the difference between the iliac vein and arterial blood or plasma substrate concentrations multiplied by the iliac blood or plasma flow (as appropriate). Glucose turnover was calculated with a two-compartment model (23) using dog parameters (13).
Statistical comparisons were made using two-way repeated-measures ANOVA with post hoc analysis employing Tukey’s test. All data in the text are expressed as means ± SE of the three sampling times in each period. Balance data expressed on a weight basis refer to total maternal body weight.
RESULTS
Arterial plasma hormone and glucagon concentrations
The basal insulin infusion rates were 321 ± 48 and 197 ± 53 μU·kg−1·min−1 in the full NP and P groups, respectively (P = 0.1), whereas the rates in the matched subsets were 310 ± 52 and 208 ± 89 μU·kg−1·min−1 in NP and P, respectively (P = 0.3). Arterial plasma insulin was significantly higher in each period in the NP group compared with P when all nine dogs in each group are considered (Fig. 1), as previously mentioned in METHODS, but the concentrations in the matched subsets were very similar, as designed. In the subsets, the concentrations rose from 7 ± 1 (basal) to 9 ± 1 and 28 ± 3 μU/ml in NP and from 5 ± 1 to 7 ± 1 and 27 ± 3 μU/ml in P during the Low and High Insulin periods, respectively (Fig. 1).
Fig. 1.
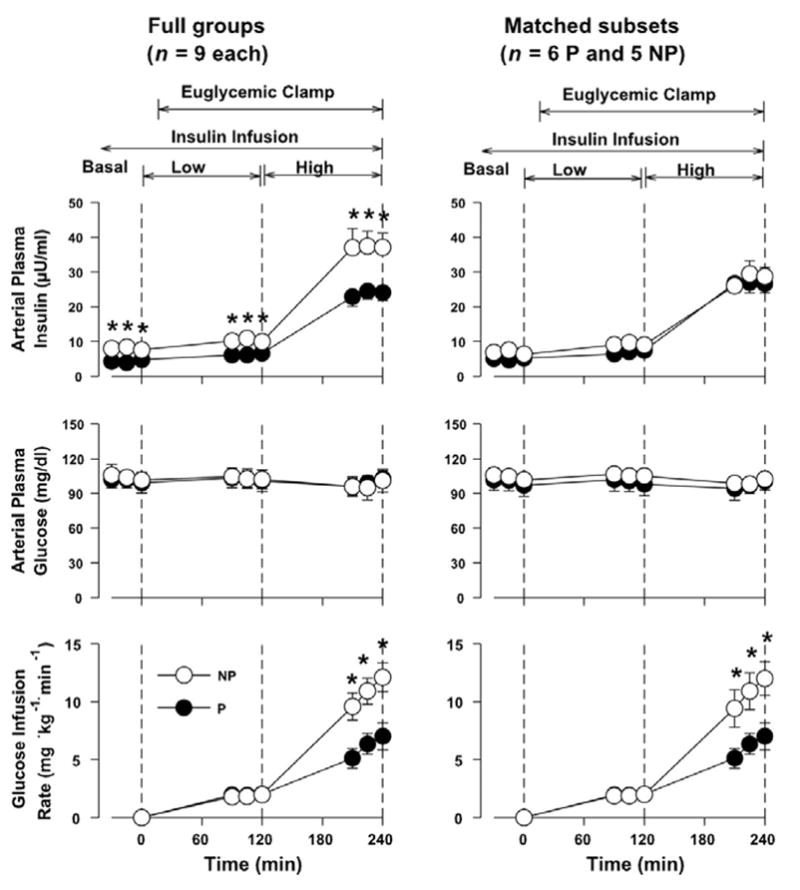
Arterial plasma concentrations of insulin and glucose and glucose infusion rate in nonpreg-nant (NP) female and pregnant (P) chronically catheterized, overnight-fasted conscious dogs during the basal period (−30 to 0 min) and during infusion of insulin at the basal rate plus 0.2 mU·kg−1·min−1 (Low Insulin) and at 1.5 mU·kg−1·min−1 (High Insulin). Data are means ± SE. *P < 0.05 between groups (post hoc analysis).
Arterial plasma glucagon and cortisol concentrations remained basal in both the full groups and the matched subsets throughout the experiments, and they did not differ between groups at any time (Table 1; data shown only for the full groups, which did not differ in these parameters from the matched subsets). Epinephrine and norepinephrine data were also indistinguishable in the two groups, whether the full groups or the matched subsets are considered, with no significant changes over time (data not shown).
Table 1.
Arterial plasma glucagon and cortisol concentrations and total hepatic and hindlimb blood flow
| Experimental Period
|
|||
|---|---|---|---|
| Group | Basal | Low Insulin | High Insulin |
| Glucagon, ng/l | |||
| NP | 38 ± 3 | 36 ± 2 | 32 ± 3 |
| P | 38 ± 4 | 36 ± 6 | 34 ± 6 |
| Cortisol, μg/dl | |||
| NP | 1.9 ± 0.3 | 1.8 ± 0.3 | 2.0 ± 0.2 |
| P | 3.1 ± 0.7 | 2.8 ± 0.5 | 2.6 ± 0.6 |
| Hepatic blood flow, ml·kg−1·min−1 | |||
| NP | 25 ± 3 | 26 ± 3 | 27 ± 3 |
| P | 21 ± 2 | 21 ± 2 | 23 ± 2 |
| Hindlimb blood flow, ml/min | |||
| NP | 225 ± 25 | 227 ± 25 | 250 ± 24* |
| P | 219 ± 19 | 208 ± 17 | 233 ± 25 |
Data are means ± SE for 3 sampling times in each period; n = 9 per group. NP, nonpregnant group; P, pregnant group. There are no differences between groups;
P < 0.05 vs. basal.
Glucose concentrations and blood flow
The plasma glucose concentrations did not change from basal in either group, and they did not differ between groups at any time, whether the full groups or the matched subsets are considered (Fig. 1). Hepatic blood flow was also stable over time in each group and did not differ significantly between groups (Table 1). Hindlimb blood flow increased significantly in NP during High Insulin, and it tended to increase in P (P = 0.1). It was not significantly different between the groups at any time, whether the full groups or the matched subsets were considered.
Glucose kinetics
Net hepatic glucose output (NHGO) in the presence of basal insulinemia was ~70% higher in the P group than in NP (P < 0.05 for comparison of the full groups; P = 0.11 between the matched subsets) (Fig. 2). During Low Insulin, NHGO in the two groups fell ≥50%, and the rates were no different between groups, whether the full groups or the matched subsets are considered (P = 0.3 and 0.4, respectively). Both groups exhibited a low rate of net hepatic glucose uptake during High Insulin. The rate was significantly greater in NP in the matched subsets (0.80 ± 0.17 and 0.11 ± 0.23 mg·kg−1·min−1; P < 0.05) (Fig. 2). Endogenous glucose rate of appearance was suppressed ~50% from basal during Low Insulin, with no differences between groups. During High Insulin, it was suppressed 96 ± 3 and 92 ± 6% in NP and P, respectively (full groups); in the matched subsets, the percent suppression was 92 ± 6 and 88 ± 10% (not different between groups).
Fig. 2.
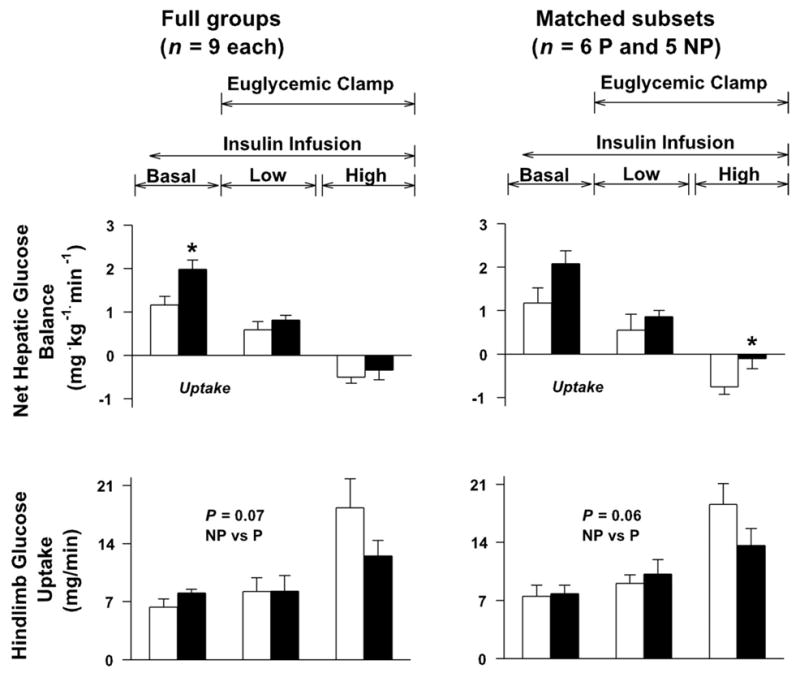
Net hepatic glucose balance and hind-limb glucose uptake in NP and P dogs. Study conditions were as described in the legend to Fig. 1. *P < 0.05 between groups (post hoc analysis).
The glucose infusion rates required to maintain euglycemia during Low and High Insulin, respectively, were 1.9 ± 0.4 and 10.8 ± 1.5 mg·kg−1·min−1 in NP and 2.0 ± 0.3 and 6.2 ± 1.0 in P (P < 0.05 between groups during High Insulin) (Fig. 1). Hindlimb glucose uptake was no different in the two groups under basal conditions and during Low Insulin (Fig. 2). During High Insulin, hindlimb glucose uptake doubled in NP but increased only 50% (in the full P group) or 33% (in the matched subset of P dogs), although there was no statistical difference between groups (Fig. 2).
Gluconeogenic precursor metabolism
Gluconeogenic precursor data are presented only for the full groups, as these data did not differ materially from those in the matched subsets. Arterial blood lactate concentrations did not change significantly over time in either group and did not differ between groups. Both groups exhibited net hepatic lactate uptake throughout all study periods, with no difference in rates between groups. Both groups exhibited net hindlimb uptake of lactate during the basal period and switched to net lactate output during Low and High Insulin, with no significant differences between groups and no significant changes over time. (Lactate data not shown.)
Net hepatic alanine uptake did not change significantly over the course of the experiments in either group, but it was significantly lower in P at all times (Table 2). Both groups switched from net hindlimb output to net uptake of alanine during High Insulin (not significantly different, NP vs. P). There were no significant differences in the arterial concentrations and net hepatic or hindlimb balances of the other gluconeogenic amino acids, glutamine, glutamate, glycerol, serine, and threonine (data not shown).
Table 2.
Arterial blood alanine and glycerol concentrations (μmol/l), net hepatic uptakes (μmol·kg−1·min−1), and hindlimb balances (μmol/min)
| Parameter and Group | Basal | Low Insulin | High Insulin |
|---|---|---|---|
| Alanine | |||
| Arterial | |||
| NP | 324 ± 46 | 295 ± 38 | 254 ± 21 |
| P | 226 ± 42 | 198 ± 32 | 196 ± 23 |
| Net hepatic uptake | |||
| NP | 2.4 ± 0.3 | 2.8 ± 0.3 | 2.7 ± 0.3 |
| P | 1.5 ± 0.3* | 1.7 ± 0.2* | 1.5 ± 0.3* |
| Net hindlimb balance | |||
| NP | 0.0 ± 1.4 | 0.2 ± 0.8 | −1.3 ± 1.2 |
| P | −0.2 ± 0.9 | 0.4 ± 1.4 | −2.4 ± 1.4 |
| Glycerol | |||
| Arterial | |||
| NP | 89 ± 16 | 75 ± 15 | 55 ± 7† |
| P | 87 ± 14 | 63 ± 8† | 49 ± 11† |
| Net hepatic uptake | |||
| NP | 1.6 ± 0.3 | 1.4 ± 0.3 | 1.2 ± 0.2† |
| P | 1.3 ± 0.3 | 0.9 ± 0.2† | 0.7 ± 0.3† |
| Net hindlimb output | |||
| NP | 3.0 ± 1.7 | 2.2 ± 0.8 | 1.9 ± 0.9 |
| P | 1.1 ± 1.3 | 0.4 ± 0.7 | 0.0 ± 0.5† |
Values are means ± SE for the 3 time points during each period; n = 9 per group. Negative values for balance data indicate net uptake.
P < 0.05 vs. NP;
P < 0.05 vs. basal.
Arterial blood glycerol levels fell significantly ~40% from basal in both groups during hyperinsulinemia and did not differ between groups (Table 2). Net hepatic glycerol uptake declined significantly (28% and 50%, respectively) from basal in both groups during High Insulin and did not differ between groups. Net hindlimb glycerol output declined 1.1 μmol·kg−1·min−1 from basal by the end of study in both groups.
Free fatty acid and ketone metabolism
As for the gluconeogenic precursor data, free fatty acid (FFA) and ketone data are given only for the full groups, because the response of the matched subsets did not differ from the full groups. There were no differences in FFA or ketone data between groups at any time, except as noted below. Arterial plasma FFA concentrations fell ~30–35% in the two groups during Low Insulin and declined to 20% of basal during High Insulin (Table 3). Net hepatic FFA uptake fell to ~15% of basal in both groups. Net hindlimb FFA balance shifted from output in the basal period to net uptake in the Low and High Insulin periods. During High Insulin, arterial blood β-hydroxybutyrate concentrations were markedly suppressed in both groups (P < 0.05 vs. basal) (Table 3). Net hepatic β-hydroxybutyrate output during High Insulin was <10% of basal in both groups (P < 0.05 vs. basal). Net hindlimb β-hydroxybutyrate uptake declined significantly in both groups during High Insulin, with the rate in NP being significantly higher than P. Acetoacetate concentrations and balance data followed the same pattern as β-hydroxybutyrate (data not shown).
Table 3.
Arterial free fatty acid and β-hydroxybutyrate concentrations (μmol/l), net hepatic balances (μmol·kg−1·min−1), and hindlimb balances (μmol/min)
| Group and parameter | Basal | Low Insulin | High Insulin |
|---|---|---|---|
| Free Fatty Acids | |||
| Arterial plasma | |||
| NP | 862 ± 74 | 547 ± 90 | 156 ± 32* |
| P | 830 ± 65 | 592 ± 49 | 172 ± 27* |
| Net hepatic uptake | |||
| NP | 2.8 ± 0.6 | 1.9 ± 0.7 | 0.4 ± 0.2* |
| P | 2.6 ± 0.4 | 2.2 ± 0.5 | 0.4 ± 0.1* |
| Net hindlimb balance | |||
| NP | 2.8 ± 4.3 | −0.6 ± 2.2 | −1.2 ± 0.9 |
| P | 0.5 ± 3.6 | −0.7 ± 2.1 | −0.6 ± 1.3 |
| β-Hydroxybutyrate | |||
| Arterial blood | |||
| NP | 39 ± 13 | 29 ± 10 | 7 ± 2* |
| P | 68 ± 19 | 29 ± 7* | 5 ± 1* |
| Net hepatic output | |||
| NP | 1.6 ± 0.7 | 1.2 ± 0.5 | 0.2 ± 0.1* |
| P | 1.5 ± 0.3 | 0.7 ± 0.2* | 0.1 ± 0.1* |
| Net hindlimb uptake | |||
| NP | 2.1 ± 0.7 | 1.9 ± 0.6 | 0.6 ± 0.2* |
| P | 2.7 ± 0.5 | 1.5 ± 0.4 | 0.0 ± 0.2*† |
Values are means ± SE for the 3 time points during each period; n = 9 per group. Negative values for balance data indicate net uptake.
P < 0.05 vs. basal;
P < 0.05 vs. NP.
DISCUSSION
The pregnant dogs in the current report exhibited ~43% reduction in whole body insulin sensitivity, based on the differences in the glucose infusion rate during the High Insulin period. This is very similar to the 45–70% blunting of insulin sensitivity reported in pregnant women during the third trimester (1, 2, 7, 8). The hyperinsulinemic euglycemic clamp technique has been assumed to reflect primarily muscle insulin sensitivity (20). We relied on the hindlimb glucose balance to provide an index of muscle insulin sensitivity, since the hindlimb of the dog is 66% skeletal muscle, with most of the remainder of the mass being composed of bone (30). Consistent with the difference between groups in whole body insulin sensitivity, hindlimb glucose uptake during High Insulin was 33% greater in NP than in P, although this did not reach statistical significance.
Basal NHGO and endogenous glucose Ra were ~70% greater in P vs. NP dogs. An increase in the basal rate of glucose production has previously been observed in normal pregnant women (9), dogs (12), and rats (22). At the lower step of the two-step hyperinsulinemic euglycemic clamp, arterial plasma insulin concentrations increased only 2 μU/ml. This subtle increase in insulin was sufficient to decrease NHGO by 50% or more in both the pregnant and the nonpregnant dogs, as previously demonstrated in normal dogs of both sexes (10). Cumulatively, the decreases in NHGO during the three periods were very similar between the two groups, with the change from basal totaling 1.9 ± 0.3 and 2.2 ± 0.4 mg·kg−1·min−1 in the matched subsets of NP and P, respectively (Δ1.7 ± 0.1 and 2.3 ± 0.4 mg·kg−1·min−1 in the full groups). Similarly, the percentage suppression of glucose rate of appearance was not different between groups at either step. These data are consistent with unimpaired hepatic insulin action in the pregnant animals and in general agreement with the findings of Catalano et al. (9) in normal-weight women in late pregnancy.
Two reports in vivo have suggested that loss of hepatic insulin sensitivity occurs in obese glucose-tolerant women (5, 29). A longitudinal study, utilizing euglycemic clamps with two rates of insulin infusion (resulting in insulin concentrations of ~50 and 100 μU/ml, respectively) was carried out in eight obese women with normal glucose tolerance (5). In that study, there was ~90% suppression of EGP during the 50 μU/ml clamp in the pregravid state and late pregnancy (34–36 wk). In contrast, suppression of EGP during the 100 μU/ml study was modestly but significantly impaired in late pregnancy (93% suppression, compared with 97% in the pregravid state). The investigators concluded that the combination of obesity and pregnancy created sufficient stress to make evident a loss of hepatic insulin sensitivity in the third trimester (5). However, insulin concentrations during the clamps differed (94 and 71 μU/ml in the pregravid and late pregnancy studies, respectively) (5), presumably because of increased insulin clearance in late pregnancy (4). This strongly suggests that if the insulin concentrations in the third trimester study had been equivalent to those in the pregravid study, suppression of EGP in the third trimester would have been as complete as in the study prior to pregnancy. The second investigation in which hepatic insulin resistance was observed in late human pregnancy also involved repeated euglycemic hyperinsulinemic clamps in a small group (n = 6) of obese women, but utilized a single insulin infusion rate. Again, insulin concentrations tended to be lower during the third trimester than in the nonpregnant study in the same subjects (88 vs. 101 μU/ml), although this did not reach statistical significance (29). EGP was suppressed 77–79% within 2 h during the second trimester and 15.6 wk postpartum, but it was only suppressed 39% during the third trimester (29). However, after 4 h of insulin infusion, EGP in all of the studies was suppressed ~86% (29). Hepatic insulin resistance has been reported in both rabbits and rats during the third trimester of pregnancy (19, 25). However, the insulin concentrations achieved were supraphysiologic (10- to 50-fold basal), not optimal for examining hepatic insulin sensitivity.
The molecular basis for the insulin resistance of pregnancy is still poorly understood (32). Most investigations have found insulin receptor binding to be unimpaired in normal pregnancies and in those complicated by gestational diabetes, consistent with a postreceptor defect (26, 32). Yamada et al. (31) reported that GLUT4 protein content was reduced in adipose tissue but not skeletal muscle during pregnancy in normal and streptozotocin-diabetic rats, a finding in general agreement with data from a small number of pregnant women (15). A number of defects suggesting impaired insulin signaling have been noted in pregnancy. These include impaired insulin receptor tyrosine kinase activity (14, 27), decreased insulin receptor substrate-1 (IRS-1) expression and/or altered phosphorylation of IRS-1 (6, 26), redistribution of phosphatidylinositol 3-kinase (PI3-kinase) and/or increased expression of the p85α-subunit of PI3-kinase (21, 28). In the late-pregnant rat, assessment of insulin receptor expression and tyrosine phosphorylation in liver, skeletal muscle, and adipose tissue indicated that muscle and adipose tissue were the tissues with the most significant reduction in insulin action, with liver being less affected (18). Subsequent studies also indicated that the phosphorylation and expression of IRS-1 was less impaired in the liver than in skeletal muscle and adipose tissue during late pregnancy (17). In contrast, Saad et al. (26) found that IRS-1 protein and the association of IRS-1 with PI3-kinase were significantly and similarly reduced in both skeletal muscle and liver in the pregnant rat. An increase in skeletal muscle IRS-1 protein and a decrease of the p85α-subunit of PI3-kinase were observed in nonobese, nondiabetic women postpartum, compared with an assessment carried out in the same women during late pregnancy (21). No assessment of liver protein expression could be made, however.
There was no evidence of a loss of insulin’s ability to suppress proteolysis or lipolysis in the nonhepatic tissues. The hindlimb balances of alanine and the other gluconeogenic amino acids were not different between pregnant and nonpregnant dogs at any time. Glycerol concentrations were similarly suppressed by insulin in the NP and P groups, and net hindlimb glycerol output declined 1.1 μmol·kg−1·min−1 from basal in both groups during High Insulin. Since glycerol is a sensitive index of lipolysis, it appears that the insulin resistance observed was limited to glucose metabolism.
The current studies are unique in being among the first to examine hepatic insulin sensitivity in pregnancy in a model in which a physiologic elevation of insulin could be brought about, with the insulin concentrations closely matched in the pregnant and nonpregnant states. There was no evidence of impaired hepatic insulin sensitivity under these conditions. In agreement with in vitro findings (26) and a previous investigation in lean women (9), it appears that insulin action in the liver is preserved in the third trimester of normal pregnancy. In previous studies where loss of hepatic insulin sensitivity has been observed in the last trimester of human pregnancy, the findings are likely explained by lower insulin concentrations during studies conducted in the third trimester and/or the use of insulin infusion rates too high to isolate the liver’s response.
Acknowledgments
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-58134 and Diabetes Research and Training Center Grant AM20593.
References
- 1.Buchanan TA, Metzger BE, Freinkel N, Bergman RN. Insulin sensitivity and B-cell responsiveness to glucose during late pregnancy in lean and moderately obese women with normal glucose tolerance or mild gestational diabetes. Am J Obstet Gynecol. 1990;162:1008–1014. doi: 10.1016/0002-9378(90)91306-w. [DOI] [PubMed] [Google Scholar]
- 2.Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J Clin Nutr. 2000;71:1256S–1261S. doi: 10.1093/ajcn/71.5.1256s. [DOI] [PubMed] [Google Scholar]
- 3.Canniff KM, Smith MS, Lacy DB, Williams PE, Moore MC. Glucagon secretion and autonomic signaling during hypoglycemia in late pregnancy. Am J Physiol Regul Integr Comp Physiol. 2006;291:R788–R795. doi: 10.1152/ajpregu.00125.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catalano PM, Drago NM, Amini SB. Longitudinal changes in pancreatic β-cell function and metabolic clearance rate of insulin in pregnant women with normal and abnormal glucose tolerance. Diabetes Care. 1998;21:403–408. doi: 10.2337/diacare.21.3.403. [DOI] [PubMed] [Google Scholar]
- 5.Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol. 1999;180:903–916. doi: 10.1016/s0002-9378(99)70662-9. [DOI] [PubMed] [Google Scholar]
- 6.Catalano PM, Nizielski SE, Shao J, Preston L, Qiao L, Friedman JE. Downregulated IRS-1 and PPARγ in obese women with gestational diabetes: relationship to FFA during pregnancy. Am J Physiol Endocrinol Metab. 2002;282:E522–E533. doi: 10.1152/ajpendo.00124.2001. [DOI] [PubMed] [Google Scholar]
- 7.Catalano PM, Tyzbir ED, Roman NM, Amini SB, Sims EA. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am J Obstet Gynecol. 1991;165:1667–1672. doi: 10.1016/0002-9378(91)90012-g. [DOI] [PubMed] [Google Scholar]
- 8.Catalano PM, Tyzbir ED, Wolfe RR, Calles J, Roman NM, Amini SB, Sims EA. Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. Am J Physiol Endocrinol Metab. 1993;264:E60–E67. doi: 10.1152/ajpendo.1993.264.1.E60. [DOI] [PubMed] [Google Scholar]
- 9.Catalano PM, Tyzbir ED, Wolfe RR, Roman NM, Amini SB, Sims EA. Longitudinal changes in basal hepatic glucose production and suppression during insulin infusion in normal pregnant women. Am J Obstet Gynecol. 1992;167:913–919. doi: 10.1016/s0002-9378(12)80011-1. [DOI] [PubMed] [Google Scholar]
- 10.Cherrington AD, Sindelar D, Edgerton D, Steiner K, McGuinness OP. Physiological consequences of phasic insulin release in the normal animal. Diabetes. 2002;51(Suppl 1):S103–S108. doi: 10.2337/diabetes.51.2007.s103. [DOI] [PubMed] [Google Scholar]
- 11.Connolly CC, Aglione LN, Smith MS, Lacy DB, Moore MC. Insulin action during late pregnancy in the conscious dog. Am J Physiol Endocrinol Metab. 2004;286:E909–E915. doi: 10.1152/ajpendo.00143.2003. [DOI] [PubMed] [Google Scholar]
- 12.Connolly CC, Holste LC, Aglione LN, Neal DW, Lacy DB, Smith MS, Diamond MP, Cherrington AD, Chiasson JL. Alterations in basal glucose metabolism during late pregnancy in the conscious dog. Am J Physiol Endocrinol Metab. 2000;279:E1166–E1177. doi: 10.1152/ajpendo.2000.279.5.E1166. [DOI] [PubMed] [Google Scholar]
- 13.Dobbins RL, Davis SN, Neal DW, Cobelli C, Jaspan J, Cherrington AD. Compartmental modeling of glucagon kinetics in the conscious dog. Metabolism. 1995;44:452–459. doi: 10.1016/0026-0495(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 14.Friedman JE, Ishizuka T, Shao J, Huston L, Highman T, Catalano P. Impaired glucose transport and insulin receptor tyrosine phosphorylation in skeletal muscle from obese women with gestational diabetes. Diabetes. 1999;48:1807–1814. doi: 10.2337/diabetes.48.9.1807. [DOI] [PubMed] [Google Scholar]
- 15.Garvey WT, Maianu L, Hancock JA, Golichowski AM, Baron A. Gene expression of GLUT4 in skeletal muscle from insulin-resistant patients with obesity, IGT, GDM, and NIDDM. Diabetes. 1992;41:465–475. doi: 10.2337/diab.41.4.465. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert M, Pere MC, Baudelin A, Battaglia FC. Role of free fatty acids in hepatic insulin resistance during late pregnancy in conscious rabbits. Am J Physiol Endocrinol Metab. 1991;260:E938–E945. doi: 10.1152/ajpendo.1991.260.6.E938. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez C, Alonso A, Fernandez R, Patterson AM. Regulation of insulin receptor substrate-1 in the liver, skeletal muscle and adipose tissue of rats throughout pregnancy. Gynecol Endocrinol. 2003;17:187–197. doi: 10.1080/gye.17.3.187.197. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez CG, Alonso A, Balbin M, Diaz F, Fernandez S, Patterson AM. Effects of pregnancy on insulin receptor in liver, skeletal muscle and adipose tissue of rats. Gynecol Endocrinol. 2002;16:193–205. [PubMed] [Google Scholar]
- 19.Hauguel S, Gilbert M, Girard J. Pregnancy-induced insulin resistance in liver and skeletal muscles of the conscious rabbit. Am J Physiol Endocrinol Metab. 1987;252:E165–E169. doi: 10.1152/ajpendo.1987.252.2.E165. [DOI] [PubMed] [Google Scholar]
- 20.James DE, Jenkins AB, Kraegen EW. Heterogeneity of insulin action in individual muscles in vivo: euglycemic clamp studies in rats. Am J Physiol Endocrinol Metab. 1985;248:E567–E574. doi: 10.1152/ajpendo.1985.248.5.E567. [DOI] [PubMed] [Google Scholar]
- 21.Kirwan JP, Varastehpour A, Jing M, Presley L, Shao J, Friedman JE, Catalano PM. Reversal of insulin resistance postpartum is linked to enhanced skeletal muscle insulin signaling. J Clin Endocrinol Metab. 2004;89:4678–4684. doi: 10.1210/jc.2004-0749. [DOI] [PubMed] [Google Scholar]
- 22.Leturque A, Burnol AF, Ferre P, Girard J. Pregnancy-induced insulin resistance in the rat: assessment by glucose clamp technique. Am J Physiol Endocrinol Metab. 1984;246:E25–E31. doi: 10.1152/ajpendo.1984.246.1.E25. [DOI] [PubMed] [Google Scholar]
- 23.Mari A. On the calculation of glucose rate of disappearance in nonsteady state. Am J Physiol Endocrinol Metab. 1994;266:E825–E826. doi: 10.1152/ajpendo.1994.266.5.E825. [DOI] [PubMed] [Google Scholar]
- 24.Reece EA, Homko CJ. Diabetes mellitus and pregnancy. In: Scott JR, Gibbs RS, Karlan BY, Haney BF, editors. Danforth’s Obstetrics and Gynecology. Philadelphia, PA: Lippincott Williams & Wilkins; 2003. [Google Scholar]
- 25.Rossi G, Sherwin RS, Penzias AS, Lapaczewski P, Jacob RJ, Shulman GI, Diamond MP. Temporal changes in insulin resistance and secretion in 24-h-fasted conscious pregnant rats. Am J Physiol Endocrinol Metab. 1993;265:E845–E851. doi: 10.1152/ajpendo.1993.265.6.E845. [DOI] [PubMed] [Google Scholar]
- 26.Saad MJ, Maeda L, Brenelli SL, Carvalho CR, Paiva RS, Velloso LA. Defects in insulin signal transduction in liver and muscle of pregnant rats. Diabetologia. 1997;40:179–186. doi: 10.1007/s001250050660. [DOI] [PubMed] [Google Scholar]
- 27.Shao J, Catalano PM, Yamashita H, Ruyter I, Smith S, Youngren J, Friedman JE. Decreased insulin receptor tyrosine kinase activity and plasma cell membrane glycoprotein-1 overexpression in skeletal muscle from obese women with gestational diabetes mellitus (GDM): evidence for increased serine/threonine phosphorylation in pregnancy and GDM. Diabetes. 2000;49:603–610. doi: 10.2337/diabetes.49.4.603. [DOI] [PubMed] [Google Scholar]
- 28.Shao J, Yamashita H, Qiao L, Draznin B, Friedman JE. Phosphatidylinositol 3-kinase redistribution is associated with skeletal muscle insulin resistance in gestational diabetes mellitus. Diabetes. 2002;51:19–29. doi: 10.2337/diabetes.51.1.19. [DOI] [PubMed] [Google Scholar]
- 29.Sivan E, Chen X, Homko CJ, Reece EA, Boden G. Longitudinal study of carbohydrate metabolism in healthy obese pregnant women. Diabetes Care. 1997;20:1470–1475. doi: 10.2337/diacare.20.9.1470. [DOI] [PubMed] [Google Scholar]
- 30.Wasserman DH, Lacy DB, Bracy D, Williams PE. Metabolic regulation in peripheral tissues and transition to increased gluconeogenic mode during prolonged exercise. Am J Physiol Endocrinol Metab. 1992;263:E345–E354. doi: 10.1152/ajpendo.1992.263.2.E345. [DOI] [PubMed] [Google Scholar]
- 31.Yamada K, Yamakawa K, Terada Y, Kawaguchi K, Sugaya A, Sugiyama T, Toyoda N. Expression of GLUT4 glucose transporter protein in adipose tissue and skeletal muscle from streptozotocin-induced diabetic pregnant rats. Horm Metab Res. 1999;31:508–513. doi: 10.1055/s-2007-978785. [DOI] [PubMed] [Google Scholar]
- 32.Yamashita H, Shao J, Friedman JE. Physiologic and molecular alterations in carbohydrate metabolism during pregnancy and gestational diabetes mellitus. Clin Obstet Gynecol. 2000;43:87–98. doi: 10.1097/00003081-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Zinker BA, Mohr T, Kelly P, Namdaran K, Bracy DP, Wasserman DH. Exercise-induced fall in insulin: mechanism of action at the liver and effects on muscle glucose metabolism. Am J Physiol Endocrinol Metab. 1994;266:E683–E689. doi: 10.1152/ajpendo.1994.266.5.E683. [DOI] [PubMed] [Google Scholar]


