Abstract
Plasmodium gallinaceum typically causes sub-clinical disease with low mortality in its primary host, the Indian jungle fowl Gallus sonnerati. Domestic chickens of European origin, however, are highly susceptible to this avian malaria parasite. Here we describe the development of P. gallinaceum in young White Leghorn chicks with emphasis on the primary exoerythrocytic phase of the infection. Using various regimens for infection, we found that P. gallinaceum induced a transient primary exoerythrocytic infection followed by a fulminant lethal erythrocytic phase. Prerequisite for the appearance of secondary exoerythrocytic stages was the development of a certain level of parasitemia. Once established, secondary exoerythrocytic stages could be propagated from bird to bird for several generations without causing fatalities. Infected brains contained large secondary exoerythrocytic stages in capillary endothelia, while in the liver primary and secondary erythrocytic stages developed primarily in Kupffer cells and remained smaller. At later stages, livers exhibited focal hepatocyte necrosis, Kupffer cell hyperplasia, stellate cell proliferation, inflammatory cell infiltration and granuloma formation. Because P. gallinaceum selectively infected Kupffer cells in the liver and caused a histopathology strikingly similar to mammalian species, this avian Plasmodium species represents an evolutionarily closely related model for studies on the hepatic phase of mammalian malaria.
Keywords: Avian malaria, Sporozoite, Merozoite, Exoerythrocytic stage, Invasion, Electron microscopy, Confocal microscopy, Organ distribution
1. Introduction
Immediately after transmission by mosquito bite, mammalian Plasmodium sporozoites begin to migrate in the skin, gradually enter dermal capillaries (Sidjanski and Vanderberg, 1997; Vanderberg and Frevert, 2004; Amino et al., 2006), travel to the liver and undergo a first round of multiplication in hepatocytes (Sinden, 1978; Hollingdale, 1985). According to our current model (Frevert, 2004; Frevert et al., 2006a, b), the initial sporozoite arrest in the liver sinusoid is mediated by unique stellate cell-derived extracellular matrix proteoglycans (Gressner and Schäfer, 1989; Cerami et al., 1992; Frevert et al., 1993; Lyon et al., 1994; Robson et al., 1995; Pinzon-Ortiz et al., 2001; Pradel et al., 2002, 2004). To reach hepatocytes, sporozoites must first cross a layer of endothelia and Kupffer cells, the resident macrophages of the liver (Wisse et al., 1985; Bouwens and Wisse, 1992). The route sporozoites take across this sinusoidal cell barrier has been the subject of controversial discussions for many years (for reviews see (Vreden, 1994; Sinnis, 1996; Frevert, 2004; Yuda and Ishino, 2004)), partially because attempts to functionally or physically eliminate Kupffer cells yielded contradictory effects on Plasmodium infection of the liver (Sinden and Smith, 1982; Vreden et al., 1993; Ishino et al., 2004,, 2005a, 2005b). More recent work showed that Plasmodium berghei and Plasmodium yoelii sporozoites actively invade Kupffer cells, enter a non-fusogenic vacuole and safely exit the macrophages towards the space of Disse (Pradel and Frevert, 2001). Intravital observations of P. berghei sporozoites passing through Kupffer cells (Frevert et al., 2005) in combination with P. yoelii infection studies using two different Kupffer cell-deficient murine models (Baer et al., 2007b) confirmed the essential role of Kupffer cells for entry of mammalian Plasmodium sporozoites into the liver (Frevert et al., 2006a, b). Reports documenting the ability of rodent Plasmodium species to manipulate Kupffer cell function support this notion. Plasmodium berghei and P. yoelii sporozoites: i) down-modulate the expression of major histocompatibility complex (MHC) class I molecules and the production of IL-12p40 (Steers et al., 2005); ii) alter cytokine expression to generate an overall anti-inflammatory profile (Klotz and Frevert, unpublished data); and iii) induce a signal transduction cascade that blocks the respiratory burst in Kupffer cells (Usynin et al., 2007). After entry into the liver parenchyma, mammalian Plasmodium sporozoites migrate through several hepatocytes before eventually settling down in a final one for exoerythrocytic form development and differentiation to merozoites (Mota et al., 2001; Frevert et al., 2005). This first generation of merozoites is released into the bloodstream in the form of merosomes, large packets of parasites enveloped in host cell membrane (Sturm et al., 2006; Tarun et al., 2006; Baer et al., 2007a). Because free merozoites are sensitive to phagocytosis (Terzakis et al., 1979), it is thought that the parasites safely bypass the gauntlet of Kupffer cells lining the sinusoids camouflaged as merosomes (Cowman and Kappe, 2006). Once emerged from the liver, mammalian Plasmodium species remain confined to the blood of the host.
Much less is known about the cellular and molecular interactions between the evolutionarily related avian and reptilian Plasmodium species and their hosts. Originally discovered by Émile Brumpt in 1935 (Brumpt, 1935) and isolated from domestic chickens in Sri Lanka, P. gallinaceum was later reported to be endemic in various wild jungle fowl in several South Asian countries (Africa et al., 1940; Williams, 2005b). While typically causing subclinical disease with low mortality in its primary host (Fernando and Dissanaike, 1975), the Indian jungle fowl Gallus sonnerati (Shortt et al., 1941), domestic chickens of European origin (Gallus gallus) are highly susceptible to P. gallinaceum with mortality rates of up to 90% (Soulsby, 1982; Springer, 1996). Extensive work performed several decades ago has defined the exoerythrocytic phase of the malaria life cycle in various avian and reptilian hosts ((James and Tate, 1937; Huff and Coulston, 1944); reviewed in (Bray, 1957; Huff, 1957, 1968, 1969)). In contrast to the mammalian species, P. gallinaceum sporozoites infect local macrophages at the mosquito bite site and differentiate to cryptozoites, the first generation of exoerythrocytic parasites in the avian host. Some of these cryptozoites infect new dermal macrophages, while others develop to metacryptozoites in macrophages of the reticulo-endothelial system, including Kupffer cells of the liver. These primary exoerythrocytic parasites can either differentiate to merozoites or differentiate to phanerozoites, i.e. secondary exoerythrocytic stages, which continue to replicate in the tissues. In addition, merozoites can return from the blood to the tissues and convert back to phanerozoites. Aside from macrophages, phanerozoites can also infect vascular endothelia, in particular those of the brain. In natural infections, the primary and secondary exoerythrocytic development lasts several weeks after which time the infection becomes confined to the blood. Death was reported to occur either during the acute phase of the disease due to severe parasitemia or during the subsequent chronic stage due to paralysis caused by occlusion of infected brain capillaries (Garnham, 1966; Huff, 1968). The pathological changes produced by these endothelial phanerozoites resemble those of severe Plasmodium falciparum malaria in humans, which is caused by occlusion of brain capillaries by sequestered infected erythrocytes and immune cells (Seed and Manwell, 1977; MacPherson et al., 1985; Oo et al., 1987; Rogerson et al., 2004). Thus, while the mammalian Plasmodium life cycle is comprised of a short clinically silent liver phase followed by a symptomatic blood phase, the exoerythrocytic infection of the tissues of avian malaria can occur in parallel with the erythrocytic phase, thus causing the resulting clinical symptoms to overlap (Garnham, 1966; Huff, 1968).
In this study, we focus on P. gallinaceum as a model organism for mammalian malaria. Because this avian parasite is able to develop in Kupffer cells, its molecular mechanisms of communication with macrophages are of great significance for understanding the process of entry of the evolutionarily related mammalian Plasmodium species into the liver. Most of our current knowledge on the exoerythrocytic phase of avian and reptilian malaria life cycles derives from studies conducted before parasite-specific markers, genetic tools and modern instrumentation became available (James and Tate, 1937; Huff and Coulston, 1944; Bray, 1957; Huff, 1957, 1969). Reports on ultrastructure are limited to parasitized embryonic tissues and muscle cell cultures (Meyer and Oliveira Musacchio, 1960, 1965; Aikawa et al., 1968) and virtually nothing is known about the cell biology of the exoerythrocytic phase of P. gallinaceum, the molecular interactions these parasite stages undergo with their various host cells, and the underlying mechanisms of pathogenesis. Here, we describe the development of P. gallinaceum in young White Leghorn chicks, with emphasis on pre-erythrocytic infection of the liver, as a basis for future studies on molecular interactions with Kupffer cells.
2. Materials and methods
2.1. Parasites
The two P. gallinaceum sub-strains used for this study were kindly provided by Dr. Kenneth Vernick, New York University (Barreau et al., 1995) and Dr. Robert Sinden, Imperial College, London (Alavi et al., 2003). Both sub-strains were derived from the 8A strain, which was originally established by passage through chickens by Brumpt (1937) and subsequently maintained in many laboratories worldwide (for reviews of the provenance of the 8A strain see (Garnham, 1966; Williams, 2005b)). Sporozoites were purified from the salivary glands of infected Aedes aegypti mosquitoes (Sinden et al., 1990). The parasite cycle was maintained by infection of chicks via mosquito bite.
2.2. Vector
The Liverpool Black Eye strain of Aedes aegypti mosquitoes was raised and maintained at 25°C and 80% relative humidity (Barreau et al., 1995). Female mosquitoes were fed on chickens with rising parasitemia of 10–40%.
2.3. Animals
White Leghorn chickens (G. gallus) were purchased from Charles River Laboratories (Wilmington, MA) and kept on a commercial feed free of coccidiostats or any other medication. Swiss Webster mice were from Taconic Farms, Inc. (Germantown, NY). The animals were housed in a parasite-free environment on a 12 h light/12 h dark program and maintained and used in accordance with recommendations in the guide for the Care and Use of Laboratory Animals. Ethics approval was provided by the NYU Animal Ethics Committee (IACUC).
2.4. Polyclonal rabbit anti-P. berghei antiserum (anti-Pb)
Swiss Webster mice were inoculated with P. berghei-infected blood, used for feeding of Anopheles stephensi mosquitoes (Sinden et al., 1990), and bled at a parasitemia of approximately 50%. The blood was washed with PBS and the buffy coat discarded. The remaining cell pellet was then resuspended in 50 ml PBS and the erythrocyte membranes were lysed with 0.05% saponin. After several washes, the intact parasites were resuspended in PBS. This parasite suspension was frozen in aliquots in liquid nitrogen and stored at −80°C. Aliquots were diluted in PBS, mixed with an equal volume of FCA and inoculated i.p. into rabbits. After boosting twice with Freund’s incomplete adjuvant, the rabbits were bled for the preparation of serum.
2.5. Immunolabeling
Paraformaldehyde-fixed cell cultures were labeled with mAb N2H6D5 against P. gallinaceum circumsporozoite protein (CSP) (Krettli et al., 1988; Warburg et al., 1992; Rocha et al., 1993; Ramirez et al., 1995) in combination with goat anti-mouse IgG conjugated to Texas Red (GAM-TX). This antibody reacts with sporozoites and the initial pre-erythrocytic stages (cryptozoites), but recognizes neither secondary exoerythrocytic stages (phanerozoites) nor merozoites (Ramirez et al., 1991, 1995). Tissue sections or methanol-fixed blood smears were incubated with anti-Pb in combination with protein A conjugated either to Alexa 488 or Texas Red (Molecular Probes). In addition, we used the fluorescent nucleic acid stains Hoechst 33342, YOYO-1, and SYTO 16 (all from Molecular Probes) for nuclear detection.
2.6. In vitro infection
Purified P. gallinaceum sporozoites were allowed to infect and develop in cultures of chicken SL-29 fibroblasts (ATCC # CRL-1590), HD-11 macrophages (Beug et al., 1979), or primary murine bone marrow macrophages. Cultivation was done in Dulbecco’s modified eagle medium (DMEM) with Eagle’s salts (GIBCO Invitrogen, Carlsbad, CA) containing 10% chicken serum (HyClone Laboratories, Inc., Logan, UT), non-essential amino acids, L-glutamine, penicillin, streptomycin and fungizone (all from Sigma) at 41°C and 5% CO2 using established in vitro systems (Beaudoin et al., 1974; Ramirez et al., 1991, 1995; Rocha et al., 1993). The initial contact between parasites and cell cultures was improved by low speed centrifugation (10 min at 50 g) to increase invasion rates.
2.7. Infection with P. gallinaceum and tissue preservation
To determine the course of the infection, groups of two to three chicks were infected at an age of 2–14 days either by i.p. inoculation of 500 µl blood (parasitemia 10–50%), by bites of 100 mosquitoes into the skin of the chest, or by i.p. inoculation of 500 µl infected brain emulsion (Lewert, 1950a) (see Section 2.9). To generate high numbers of primary exoerythrocytic stages, other chicks were infected by daily bites of 100–150 mosquitoes for 8–9 consecutive days. On the following day, all major organs were removed. Fixation was done either with 4% paraformaldehyde in PBS for subsequent immunofluorescence labeling, confocal microscopy or immunohistochemistry or with 4% paraformaldehyde and 1% glutaraldehyde (GA; grade I, Sigma) in PBS for electron microscopy.
2.8. Anti-malarial treatment
Alkaloid quinine or 4-aminoquinoline chloroquine was used to suppress the P. gallinaceum-induced parasitemia. Both anti-malarials inhibit blood-stage replication by DNA intercalation but lack activity against tissue stages (Seed and Manwell, 1977). Starting 24 h p.i., chicks were treated orally with 70 mg/kg quinine hydrochloride (Sigma, St. Louis, MO) (Haas et al., 1948; Lewert, 1948) for up to 4 weeks. The treatment was then suspended and their blood was monitored daily for another 2–3 weeks by Giemsa staining. Similarly, some chicks were given oral doses of 10 mg/kg chloroquine (diphosphate salt; Sigma) starting from day 1 after infection for 3–4 weeks. Other chicks received chloroquine orally for only 3 days when parasitemia became apparent. After discontinuation of chloroquine treatment, the chicks were monitored for another 2–3 weeks for recurrence of blood infection.
2.9. Plasmodium gallinaceum-infected brain emulsion
Brains were removed from infected chicks, forced repeatedly through a 20 gauge needle, diluted with 4 ml PBS (Lewert, 1950a), and either injected i.p. into naïve chicks or stored in liquid nitrogen after addition of 10% glycerol and 50% chicken serum.
2.10. Cryosectioning
Fixed tissue samples were cryoprotected with 2.3 M and 1 M sucrose and frozen sections were prepared, respectively, with an MT-7 ultramicrotome equipped with a CR-21 cryochamber (RMC, Tucson, AZ) or a 2800 Frigocut-E cryostat (Reichert-Jung, Vienna, Austria).
2.11. Histopathology and immunohistochemistry
Paraffin-embedded liver tissue was processed for H&E and Masson’s trichrome staining. Reticulin fibers were stained black using Snook’s stain for argyrophilic fibers. Immunohistochemistry was performed on deparaffinized mouse liver sections with an automated NexES immunostainer (Ventana Medical Systems, Tucson, AZ). Antibody PC10, which detects proliferating cell nuclear antigen (PCNA), was used as a marker for proliferating cells and antibody HHF35 against alpha-smooth muscle-specific actin (SMA)as a marker specific for dedifferentiating stellate cells. Both antibodies (Ventana Medical Systems) react with all vertebrate species tested. Immunohistochemistry was based on the generation of avidin-biotin horseradish peroxidase complex with diaminobenzidine (DAB) as a substrate.
2.12. Diaminobenzidine staining
Endogenous Kupffer cell peroxidase reactivity was detected in glutaraldehyde-fixed liver specimens by incubation for 60 min with 0.5 mg/ml DAB (Sigma) in 50 mM Tris-HCl, pH 7.6 containing 0.01% H2O2 (Fahimi, 1970; Wisse, 1974; Varndell and Polak, 1987). Post-fixation was done with osmium tetroxide (Electron Microscopy Sciences, Hatfield, PA), followed by dehydration in ethanol and embedding in Epon (Ted Pella, Redding, CA).
2.13. Confocal laser scanning microscopy
Immunofluorescent specimens were analyzed using Zeiss LSM 510 and Leica TCS SP2 AOBS confocal laser scanning microscopes.
2.14. Electron microscopy
Tissues were post-fixed with osmium tetroxide, dehydrated with ethanol and embedded in Epon as previously described (Hügel et al., 1996). Thin sections were cut using an RMC MT-7 ultramicrotome and viewed with a Zeiss EM 910 electron microscope.
2.15. Image processing
Electron microscopy negatives were scanned with an AGFA Horizon Plus flatbed scanner. Digital electron and confocal microscopy images were processed using Image-Pro Plus (Media Cybernetics, Silver Spring, MD), Adobe Photoshop (Adobe, San Jose, CA), and AutoDeBlur software (AutoQuant Imaging, Inc., Troy, NY).
3. Results
3.1. Detection of exoerythrocytic stages
Initially, we developed an in vitro sporozoite invasion assay to optimize parasite detection. Sporozoites and cryptozoites are readily detectable with mAb N2H6D6 against P. gallinaceum CSP (Ramirez et al., 1991; Rocha et al., 1993). After 1 h of exposure to P. gallinaceum sporozoites, SL-29 chicken fibroblast cultures contained extracellular sporozoites (Fig. 1A). After entry into the fibroblasts, sporozoites developed within 24 h to trophozoites (Fig. 1B) and matured within 48 h to multi-nucleated schizonts (C, D). CSP remained detectable until schizogony was complete. The nucleic acid dye YOYO-1 was used to identify parasite and host cell nuclei (Fig. 1A–D).
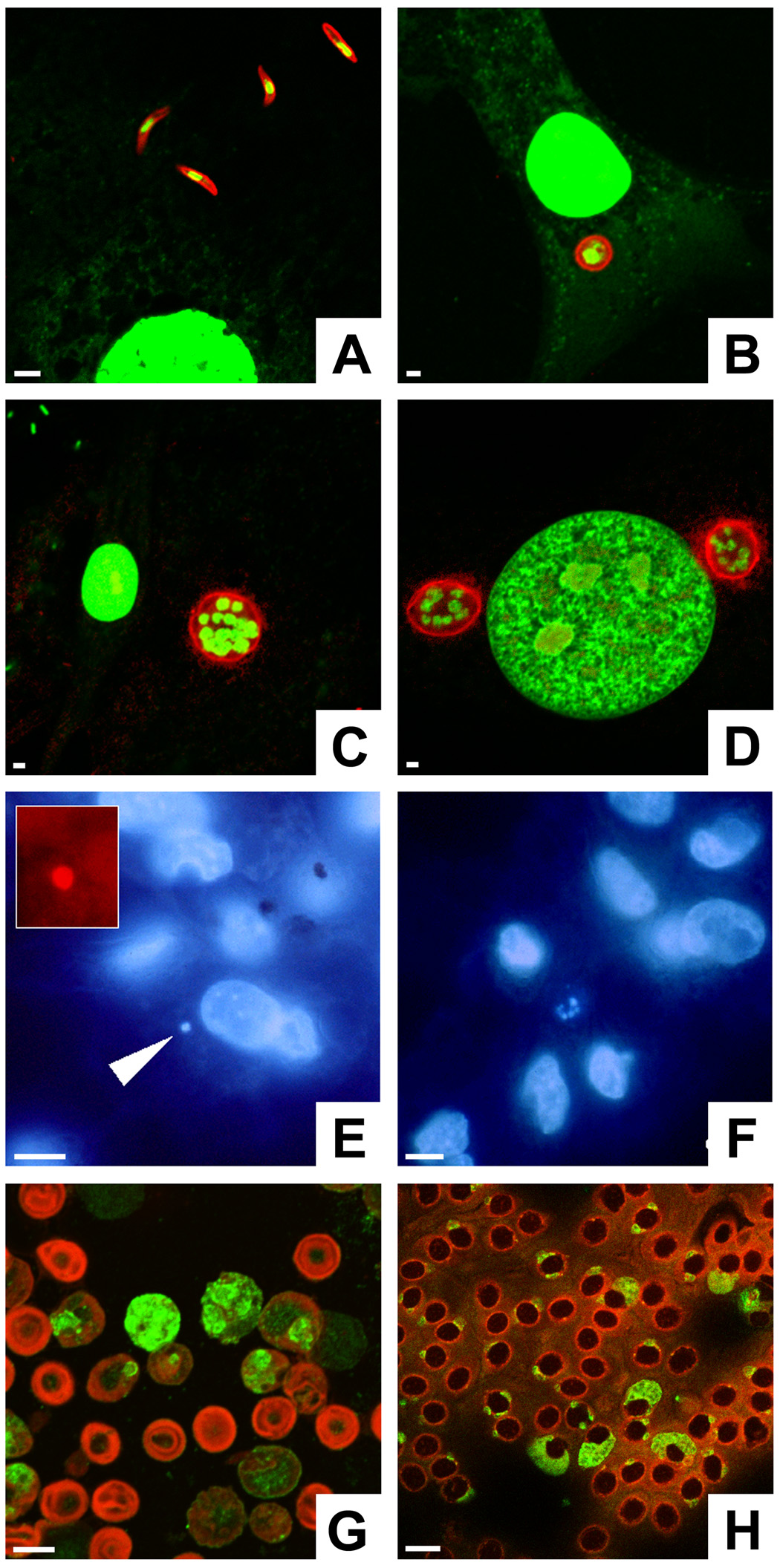
Fig. 1.
Detection methods for various Plasmodium gallinaceum life cycle stages. A–D) Sporozoites and first generation exoerythrocytic stages can be detected by circumsporozoite protein (CSP) labeling. SL-29 chicken fibroblasts were co-cultivated with sporozoites and fixed after different periods of time. The parasites were labeled with mAb N2H6D5 against P. gallinaceum CSP in combination with goat anti-mouse IgG conjugated to Texas Red (red). The nucleic acid stain YOYO-1 was used to visualize parasite and host cell nuclei (green). Fibroblast cultures contained extracellular sporozoites at 1 h (A), immature trophozoites at 24 h (B), and mature schizonts at 48 h (C). At 48 h, two mature schizonts flank the nucleus of the host cell (D). Note the relatively small diameter of the parasites compared with liver stages from mammalian Plasmodium species, which grow larger than the original host hepatocyte. E, F) Bone marrow macrophage cultures were infected with P. gallinaceum sporozoites and stained with the nucleic acid dye Hoechst 33342. E) An infected cell contains a parasite with a single nucleus (arrow). F) Multi-nucleated schizonts, recognizable by their small nuclei (center), were found after 2 days of co-cultivation. G) Anti-Pb, generated against Plasmodium berghei-infected murine erythrocyte ghosts, reacts with all blood stages of this rodent parasite. Anti-Pb was used in combination with goat anti-rabbit IgG conjugated to fluorescein isothiocyanate (green); the blood cells were counter-stained with Evans blue (red). H) Anti-Pb labels conserved blood stage antigens in P. gallinaceum-infected chicken erythrocytes (green). Bars = 5 µm.
In contrast to P. gallinaceum sporozoites and cryptozoites, all subsequent tissue and blood stages no longer express CSP and therefore require other means of detection. Although not parasite-specific, membrane-permeable DNA stains such as Hoechst 33342 represented useful tools for the detection of all P. gallinaceum life cycle stages. For example, P. gallinaceum sporozoites (Ramire et al., 1991), early trophozoite (Fig. 1E) and late schizonts stages (Fig. 1F) could be detected by Hoechst staining 2 h and 2 days after infection, respectively, in cultures of primary bone marrow-derived macrophages.
Detection of exoerythrocytic stages in the avian host has so far relied on Giemsa staining (Permin and Juhl, 2002; Williams, 2005a, b). To overcome the lack of a Plasmodium-specific marker, we immunized rabbits with P. berghei blood stages to generate antibodies against conserved Plasmodium antigens for the detection of second-generation preerythrocytic forms (metacryptozoites) and secondary exoerythrocytic stages (phanerozoites) in infected chicken tissues. The resulting polyclonal antiserum, termed anti-Pb, enabled us to recognize virtually all vertebrate life cycle stages from all Plasmodium species tested, i.e. sporozoites (not shown), exoerythrocytic stages (see below) and blood stages from P. berghei, P. yoelii and P. gallinaceum (Fig. 1G, H). In the following, all pre- and post-erythrocytic parasite forms will be named primary and secondary exoerythrocytic stages, respectively.
3.2. The primary exoerythrocytic infection phase in young chicks
To characterize the initial tissue phase of the P. gallinaceum life cycle, young chicks were infected with sporozoites and their major internal organs were removed for analysis before the onset of a parasitemia. Specifically, starting at an age of 2–4 days, chicks were exposed to the bites of 100–150 P. gallinaceum-infected Ae. aegypti mosquitoes either once or daily for 8 or 9 consecutive days to generate higher infection rates. The major organs were then removed for determination of the distribution of primary exoerythrocytic stages in various tissues. Examination by anti-Pb labeling and confocal microscopy (Fig. 2) documented the presence of pigment-free exoerythrocytic stages in all major organs including liver, brain, spleen, heart, kidney and lung. In the liver, parasites were found primarily in Kupffer cells, identifiable by their typical location in the sinusoidal lumen (Fig. 2A–D). The avian hepatic schizonts had a considerably smaller overall size and contained a lower number of parasite nuclei compared twith their mammalian counterparts developing in hepatocytes (Meis and Verhave, 1988). In frozen brain sections, primary exoerythrocytic stages in endothelia appeared to be similar in size to those found in the liver (Fig. 2E, F). However, analysis of thick impression smears prepared from fresh unfixed brain tissue, which typically contain long sections of capillaries, revealed large extended parasites with frequently over 100 nuclei, filling the entire cytoplasm of the elongated vascular endothelia (data not shown). Electron microscopic examination of the major organs revealed exoerythrocytic forms inside Kupffer cells of the liver, splenic macrophages, vascular endothelia from brain, lung and heart as well as in unidentified cells from kidney (Fig. 3).
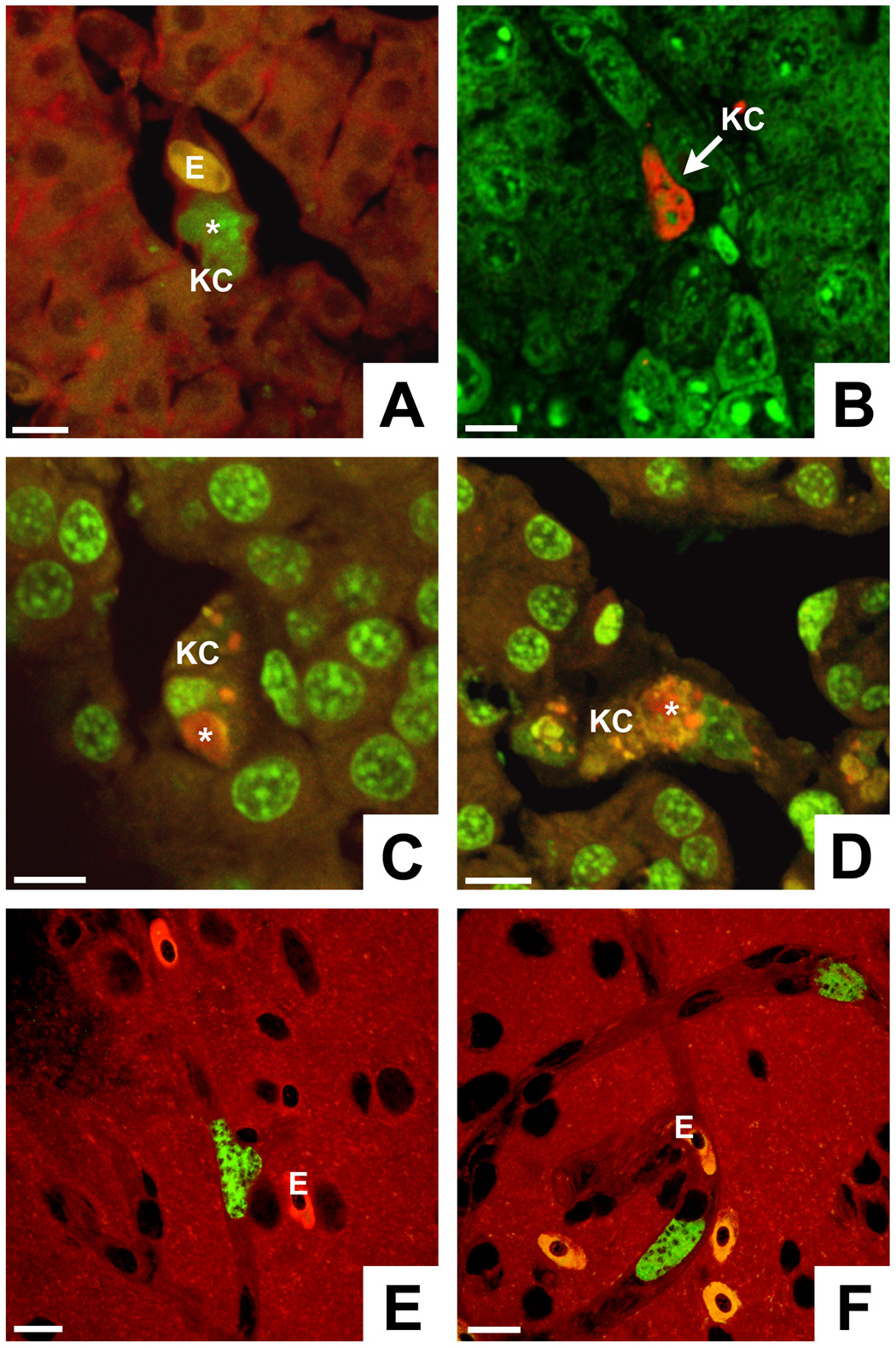
Fig. 2.
Development of Plasmodium gallinaceum in chicken tissues. A–D) Cryostat sections reveal that exoerythrocytic stage development in the liver occurs primarily in Kupffer cells (KC). The phagocytic activity of the Kupffer cell in A is demonstrated by uptake of an erythrocyte (E). Note that the parasites (*) are considerably smaller than mammalian liver stages developing inside hepatocytes. Liver stages were labeled as follows: A) anti-Pb and protein A (PA) conjugated to fluorescein isothiocyanate (FITC) (green) plus Evans blue as a counter stain (red), B) anti-Pb and goat anti-rabbit IgG (GAR) conjugated to Texas Red (TX) (red) plus YOYO-1 (green nucleic acid dye), C, D) anti-Pb and PA-TX (red) plus SYTO 16 (green nucleic acid stain). E, F) Brain sections show exoerythrocytic stages exclusively inside vascular endothelia. The parasites were labeled with anti-Pb and GAR-FITC, the tissue was counterstained with Evans blue. Erythrocytes (E) are nucleated and appear bright red. Host and parasite nuclei are unstained and appear dark. Bars = 10 µm.
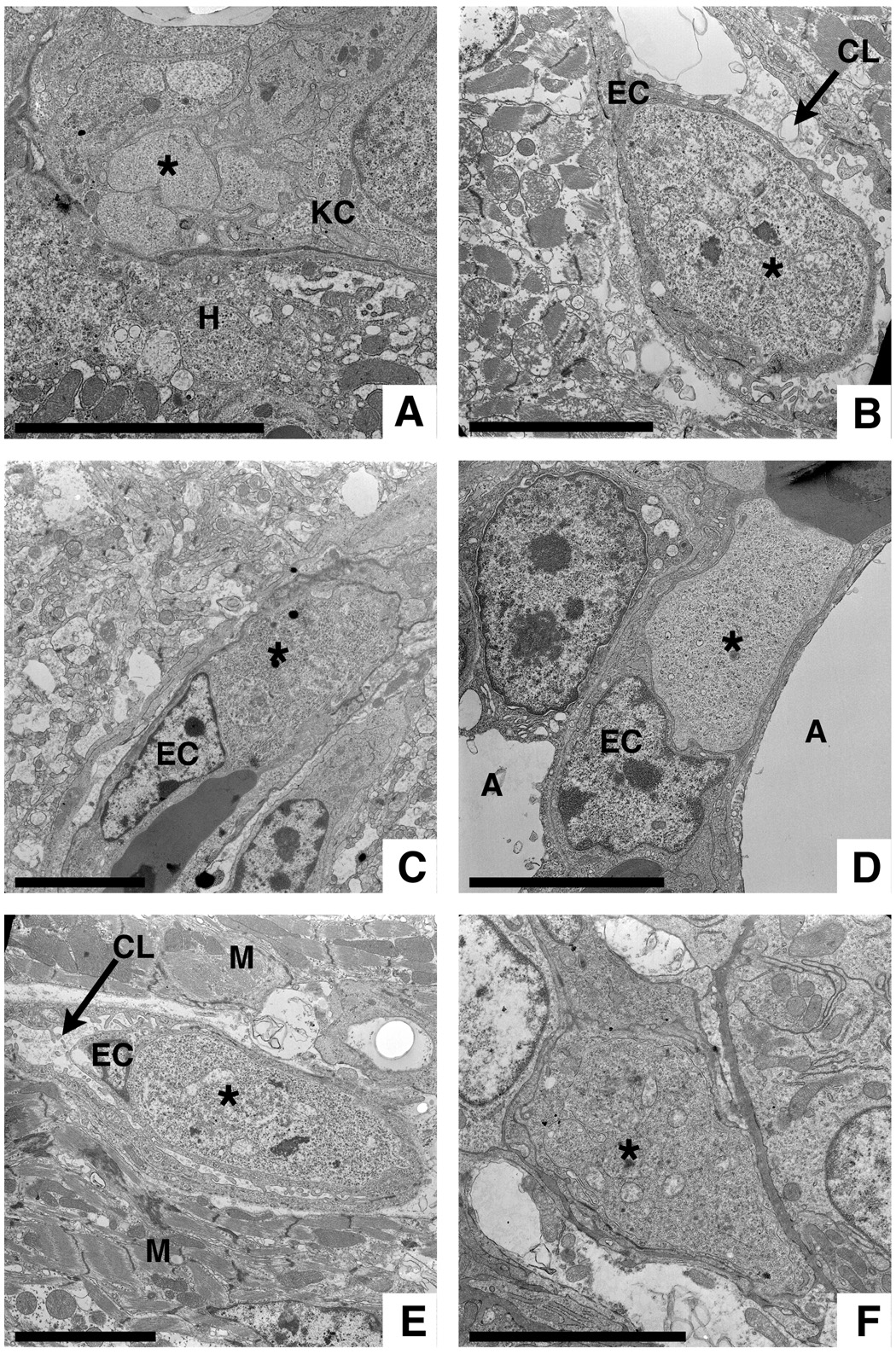
Fig. 3.
Electron microscopic detection of Plasmodium gallinaceum in various chicken organs. Exoerythrocytic stages (*) at different stages of maturation can be found in A) Kupffer cells of the liver, B) endothelial cells (EC) from spleen, C) brain, D) lung, and E) heart as well as in F) an unidentified kidney cell. H = hepatocyte, A = alveolar space, M = myocyte, CL = capillary lumen. Bars = 5 µm.
3.3. Primary exoerythrocytic stages induce liver injury
When fixed prior to the onset of the parasitemia, livers from repeatedly sporozoite-infected chicks (see above) appeared normal in size and color. On the microscopic level, however, compared with normal chick liver tissue (Fig. 4A and Fig. 5A,B), the livers from P. gallinaceum sporozoite-infected birds showed foci of necrotic hepatocytes and marked alterations of the sinusoidal cell layer, the number of which corresponded roughly to the concentration of exoerythrocytic stages found in these livers. Figure 4B–D shows hepatocytes with a swollen cytoplasm, which appears light compared with the normal neighboring parenchymal cells. The extent of the damage ranged from individual hepatocytes (Fig. 4B) via small clusters of necrotic cells (Fig. 4C) to wide-spread areas of parenchymal damage (Fig. 4D). The lumina of the sinusoids lining such damaged or dead hepatocytes were typically filled with cellular masses and lacked erythrocytes. On the ultrastructural level, necrotic hepatocytes exhibited a swollen cytoplasm and mitochondrial disintegration (Fig. 5C, D). The sinusoids in such areas were virtually blocked by masses of cells with a light cytoplasm containing lysosomes, mitochondria and a cell membrane enlarged by numerous pseudopodia (Fig. 5D, E). These features are typically found in proliferating Kupffer cells and infiltrating macrophages (Philips et al., 1987). While mature Kupffer cells can be identified by their peroxidase-positive endoplasmic reticulum, their monocytic precursors and other macrophages are negative (Fahimi, 1970; Wisse, 1974; Pradel and Frevert, 2001). Not unexpectedly, therefore, the proliferating Kupffer cells and macrophages filling the sinusoidal lumina exhibited only small foci of endogenous peroxidase activity, while erythrocytes were strongly DAB-positive (Fig. 5F).
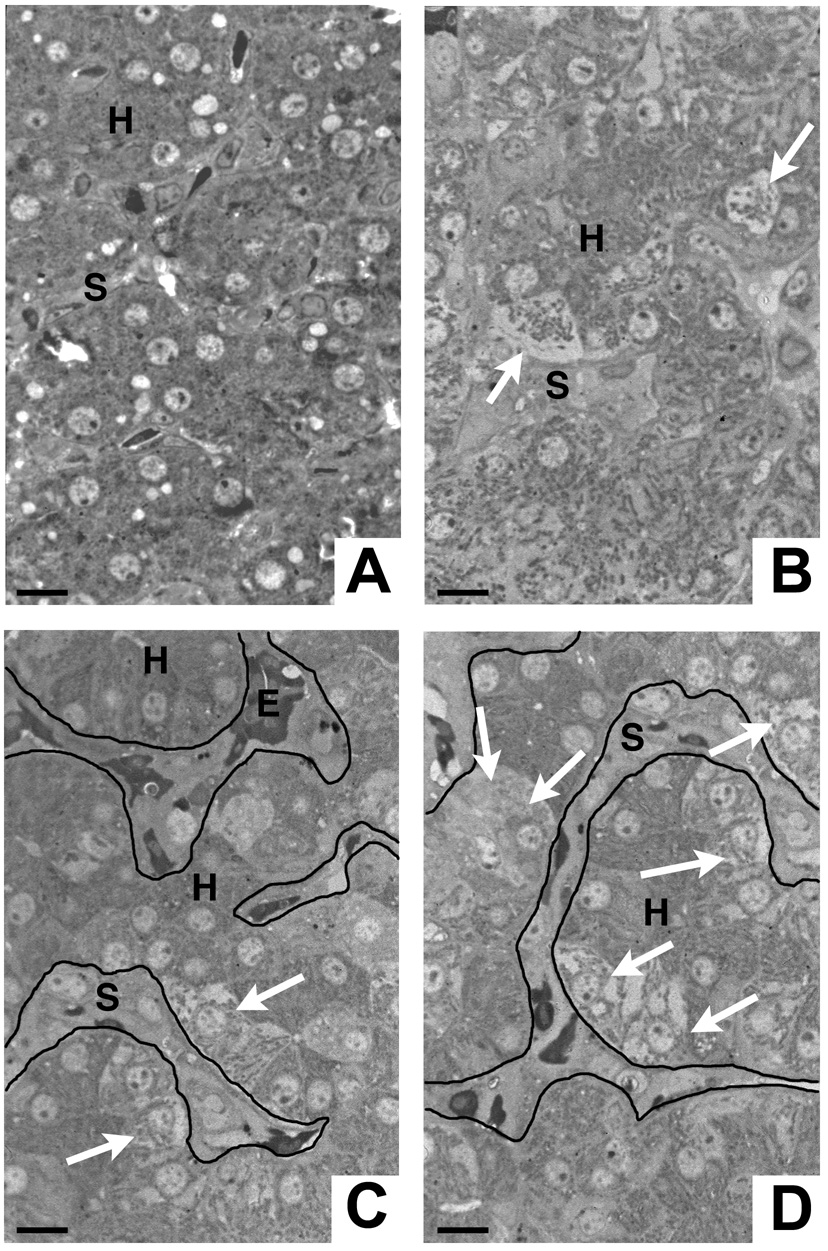
Fig. 4.
Focal hepatocyte necrosis coincides with cellular occlusion of the sinusoidal lumen. A) Uninfected chick liver exhibiting normal tissue architecture with intact hepatocytes and narrow sinusoids. B–D) Livers from chicks infected by mosquito bite on 8 consecutive days. Note the hydropic swelling and necrotic disintegration of individual hepatocytes (B, arrows) or groups of hepatocytes (C, D; arrows). Patches of necrotic hepatocytes are typically found adjacent to widened sinusoids, which are occluded by infiltrating cells. Note the paucity of erythrocytes in the affected areas. C, D) For clarity, the outline of the sinusoids is visualized by lines. H = hepatocyte, S = sinusoid, E = erythrocyte. Bars = 5 µm.
Fig. 5.
Hepatocyte necrosis and associated sinusoidal infiltration. A, B) Normal liver structure with intact hepatocytes (H) and sinusoidal endothelia (EC). The sinusoidal lumen is filled with erythrocytes (E). Note the homogeneous hepatocytic cytoplasm, which is filled with numerous intact mitochondria (M). C) Remnants of swollen necrotic hepatocytes (H) surrounding an occluded sinusoid (arrows). D) A sinusoid (arrows) is occluded with cellular masses. Note that the hepatocytes (H) lining this vessel are swollen. E) A proliferating Kupffer cell fills the entire lumen of a sinusoid. Only few lysosomes (L) are visible. The convoluted phospholipid membranes (✶) represent numerous protrusions of the cell membrane demonstrating the markedly enlarged surface of the macrophage. One hepatocyte (H1) adjacent to this sinusoid is swollen, while another (H2) has a normal ultrastructure. A–E) Bars = 5 µm. F) Diaminobenzidine staining reveals that only a few Kupffer cells (KC) possess endogenous peroxidase activity, while erythrocytes (E) are strongly positive. Other cells infiltrating the sinusoidal lumen are negative. Bar = 20 µm.
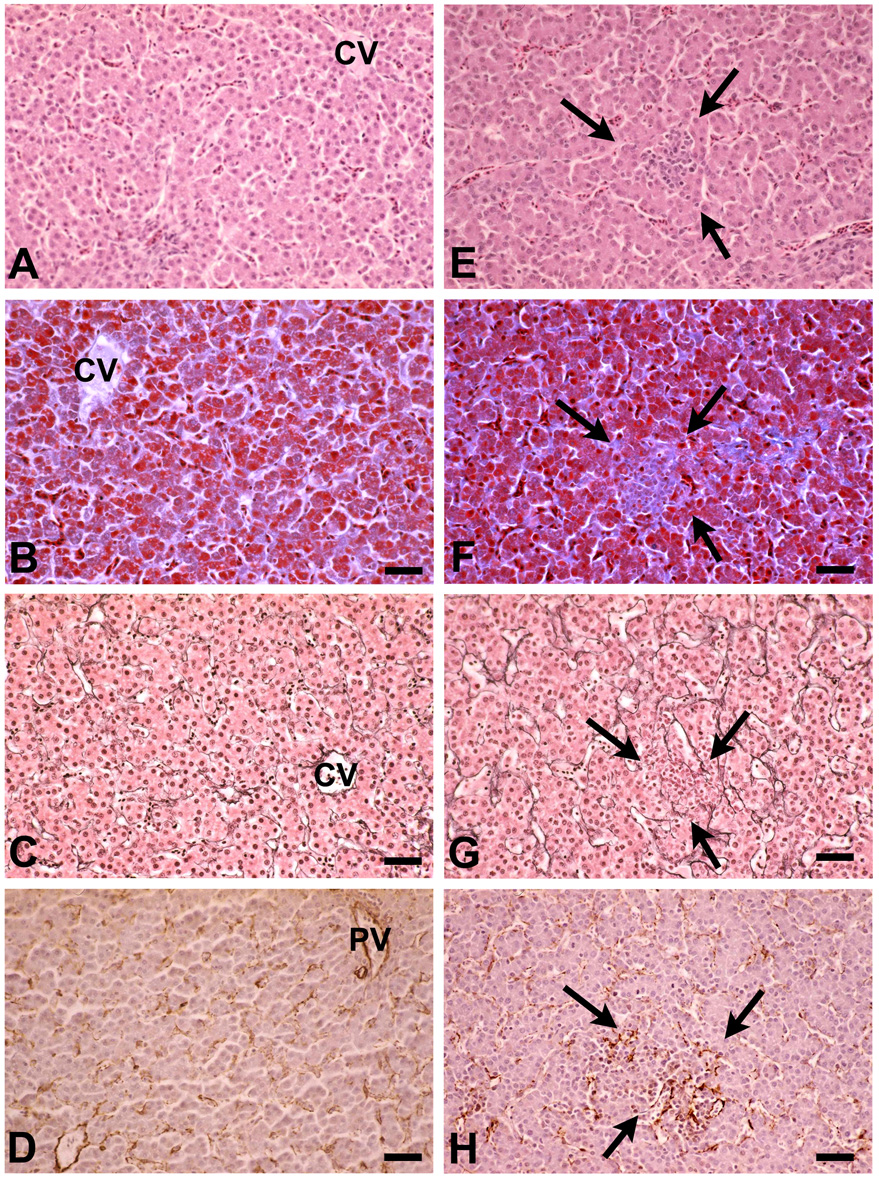
In contrast to normal chicken liver tissue, H&E stained liver sections from chicks infected by daily bites of 100–150 mosquitoes for 8–9 consecutive days showed focal accumulations of non-parenchymal and mononuclear cells (Fig. 6A,E). These foci differed in size and were more frequent in the periportal region of the liver lobule. Collagen, which is normally restricted to the portal areas, was found in increased amounts in the Disse spaces of the focally altered parenchyma by Masson’s trichrome staining (Fig. 6B,F). The fine network of reticulin fibers, which typically lines the sinusoids surrounding the cords of hepatocytes inside the Disse spaces, was disrupted or absent from the altered areas (Fig. 6C,G). Labeling with mAb HHF35 revealed an increased deposition of alpha-smooth muscle actin (SMA) suggesting focal stellate cell activation (Fig. 6D.H). Qualitatively comparable, albeit much less frequent, histopathological alterations were found in livers from chicks fixed 8 days after a single exposure to 100–150 infected mosquitoes (data not shown). Taken together, these results suggest a dose-dependent focal induction of hepatocyte necrosis in P. gallinaceum-infected livers, followed by Kupffer cell hyperplasia and stellate cell proliferation, eventually leading to infiltration of inflammatory cells and formation of granulomata.
Fig. 6.
Histopathological changes in chicken livers infected with primary exoerythrocytic stages. Compared with normal liver tissue (A–D), deviations from the regular architecture were found in the livers from chicks that had been infected by eight to nine consecutive daily mosquito bites and fixed before the onset of the parasitemia (E–H). B) H&E staining reveals non-parenchymal cells focally infiltrating the sinusoids (arrows). These infiltrates (arrows) contain F) an increased deposition of collagen Masson’s trichrome staining, G) a disrupted reticulin distribution, and H) enhanced expression of smooth muscle antigen indicating stellate cell activation. A,E) H&E, B,F) trichrome, and C,G) reticulin staining; D,H) muscle-specific actin immunolabeling. CV = central vein, PV = portal vein. Bars = 20 µM.
3.4. Parasitemia
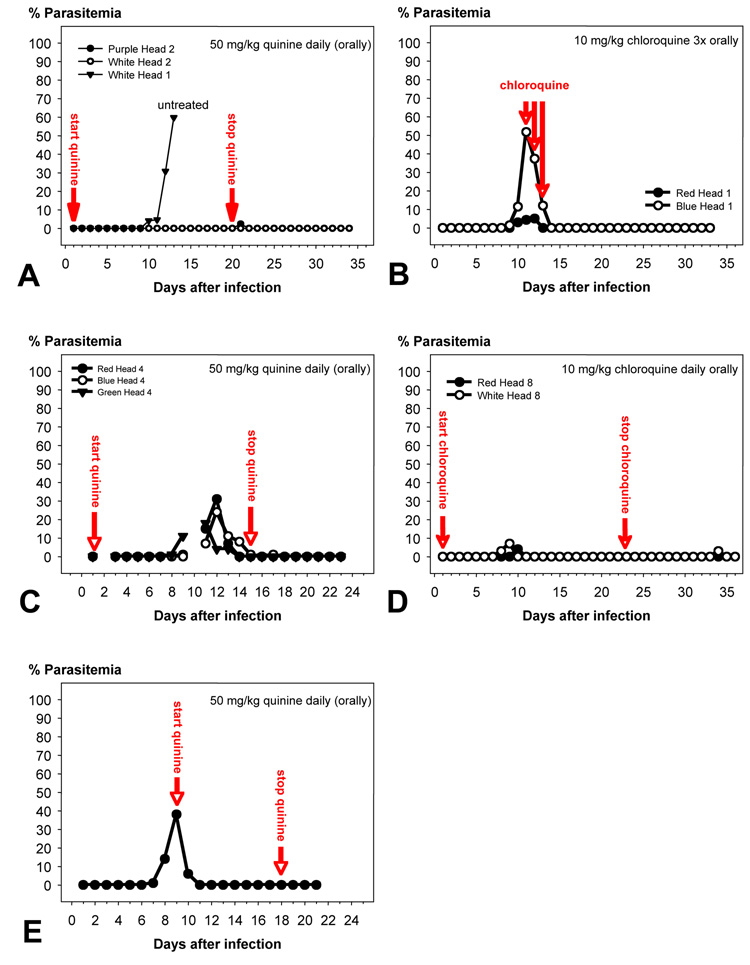
White Leghorn chicks under 1 week of age were infected with P. gallinaceum, either by bites of 100–150 mosquitoes or by i.p. inoculation of 500 µl infected blood (parasitemia 10–50%) or 500 µl infected chicken tissue. The first parasitized erythrocytes were typically found 7.4 ± 1.0 days after infection with sporozoites (n = 12), 8.4 ± 2.1 days after i.p. inoculation of blood (n = 16), and 10.0 ± 2.2 days after i.p. inoculation of brain emulsion (n = 10). If untreated, the chicks succumbed within 3–4 days after the first appearance of infected erythrocytes to the rapidly rising parasitemia (Fig. 7A), which typically exceeded 90% shortly before death.
Fig. 7.
Time course of the parasitemia in Plasmodium gallinaceum-infected chicks. Infection was done by i.p. injection of infected blood (A, B), mosquito bite (C, D), or i.p. inoculation of brain emulsion (E). The animals were given one oral dose per day of either 50 mg/kg quinine (A, C) or 10 mg/kg chloroquine (B, D, E) at the indicated times. Both treatments suppressed the infection to undetectable levels in all cases.
3.5. The secondary exoerythrocytic phase of the infection
Several decades ago, various investigators reported that suppression of the parasitemia by daily treatment with quinine forced P. gallinaceum to multiply exclusively in the tissues (Haas et al., 1948; Lewert, 1950b, a). In these studies, White Leghorn chickens died within 2 weeks from fulminant multiplication of exoerythrocytic parasites. To characterize the secondary exoerythrocytic phase of the P. gallinaceum life cycle, we replicated these studies using the same chicken breed and the same 8A strain of P. gallinaceum. Infection was done by mosquito bite as above and the blood schizonticides quinine or chloroquine were used to suppress intra-erythrocytic parasite development (Fig. 7A–D). Daily oral administration of 70 mg/kg quinine, starting on the day after infection and continued for 16–20 days, suppressed the parasitemia as expected, but none of the animals died and recrudescences or relapses were never observed within 2–3 weeks after termination of the drug treatment (n = 12; see Fig. 7A and C for representative examples). Microscopic examination of the major organs (including liver, spleen, brain, kidney and lungs) from these animals failed to demonstrate tissue stages (data not shown). Re-infection, whether by mosquito bite or blood transfer, was consistently unsuccessful suggesting that the animals had mounted a protective immune response. Similar results were obtained by oral treatment with 10 mg/kg chloroquine (Fig. 7B), with the difference that three consecutive daily doses starting at the onset of the parasitemia were sufficient to abrogate the parasitemia (Fig. 7D). Thus, suppression of the parasitemia appeared to permit clearance of all primary exoerythrocytic stages, prevented proliferation of secondary exoerythrocytic phanerozoites and allowed the birds to develop protection against reinfection.
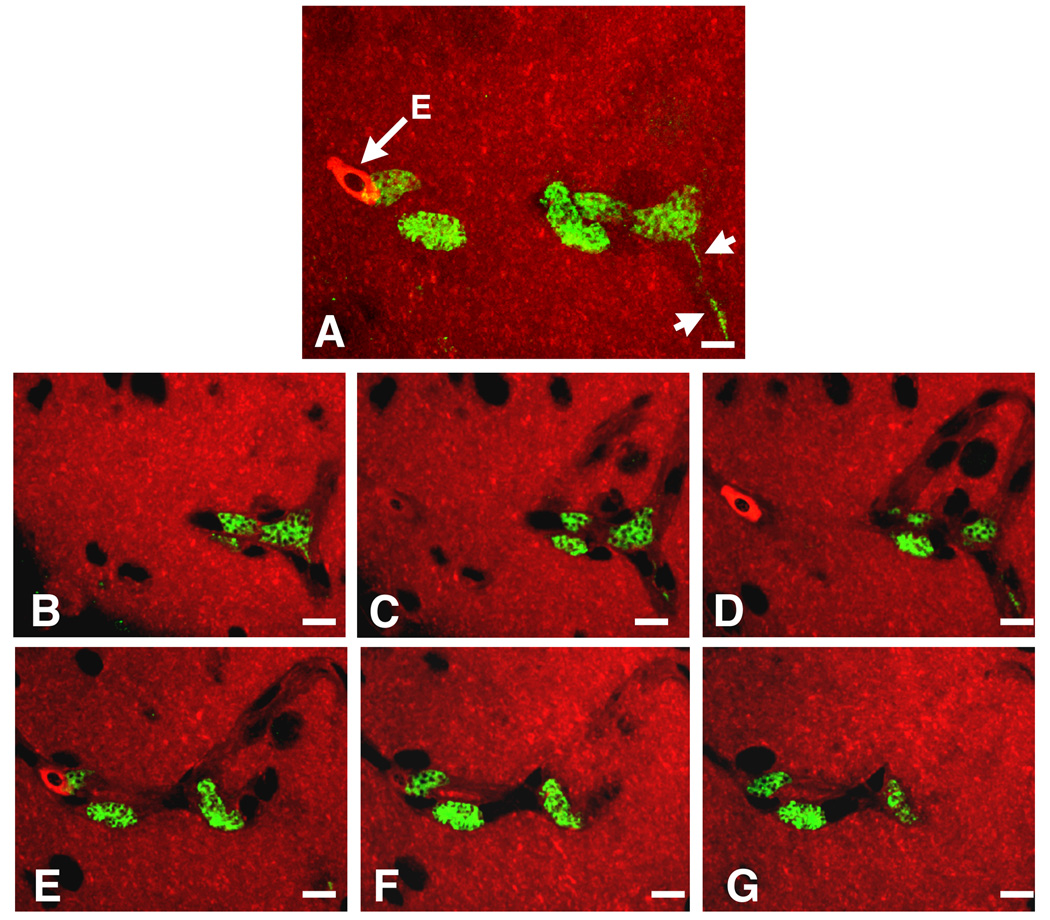
Transfer of infected blood was used to generate secondary exoerythrocytic stages. Without treatment, young White Leghorn chicks succumbed to the fulminant expansion of erythrocytic stages 3–4 days after the onset of the parasitemia (data not shown). Primary exoerythrocytic stages were usually absent in such acute blood infections. However, birds whose survival was prolonged by starting the anti-malarial treatment at a parasitemia of 40–50% harbored secondary exoerythrocytic stages in the brain and other major organs. As observed during the primary exoerythrocytic phase of the infection, these parasites contained a large number of small nuclei and were frequently elongated, filling the entire cytoplasm of capillary endothelia (Fig. 8 and Supplementary Movie 1). Because these parasites originated from infected blood, they clearly represented secondary exoerythrocytic phanerozoites. Thus, an advanced level of parasitemia was required to allow manifestation of phanerozoites in the tissues. Together with the above sporozoite infection/anti-malarial treatment studies, these data indicate that sporozoite infections did not progress to secondary exoerythrocytic stages.
Fig. 8.
Plasmodium gallinaceum-infected brain tissue. A) Focus series through a three-dimensoinal stack from the brain of a P. gallinaceum-infected chicken. The tissue specimen contains several infected vascular endothelia. B) The maximum projection shows that each of the exoerythrocytic forms is multi-nucleated. One of the parasites extends into the cytoplasm of an endothelial cell (arrowheads). The cytoplasm of the parasites appears green due to labeling with anti-Pb in combination with goat anti-rabbit IgG conjugated to fluorescein isothiocyanate; the tissue was counter-stained with Evans blue (red). Note the bright red nucleated erythrocyte (E). B) Six individual frames from the same stack show the infected endothelia in relation to the capillary lumen. Bar = 10 µm. See Supplementary Movie 1.
3.6. Continuous passage of exoerythrocytic stages
Lewert (1950a) conducted serial passages of infected chicken brain tissue from bird to bird to generate P. gallinaceum strains with a selectively exoerythrocytic growth behavior for in vivo and in vitro studies (Greenberg et al., 1950). In an attempt to select such exoerythrocytic P. gallinaceum strains, young chicks were infected by the bites of 100–150 mosquitoes and their brains were removed before the onset of parasitemia. Nuclear staining of brain impression smears revealed large elongated primary exoerythrocytic schizonts inside brain capillary endothelia, frequently containing more than 100 nuclei (data not shown). Emulsions prepared from such infected brains (Lewert, 1950a) were i.p. inoculated into naïve chicks. If untreated, these chicks died from a rapidly rising parasitemia. Immediate treatment with quinine suppressed the parasitemia as expected, but allowed replication of exoerythrocytic stages in the brain. By transferring infected brain emulsion from chick to chick, the exoerythrocytic infection could be maintained for at least six passages, albeit at a low level. This partially reproduced earlier models (Adler and Tchernomoretz, 1941; Haas et al., 1948; Lewert, 1950b, a) in that exoerythrocytic parasite populations could be expanded and transferred from chick to chick, but not to the extent that the expansion of these parasites led to death.
In conclusion, it appears that the currently available P. gallinaceum 8A strain induced in modern White Leghorn chickens a transient primary exoerythrocytic infection followed by a fulminant and, if untreated, lethal erythrocytic phase. Prerequisite for the appearance of phanerozoites was the development of parasitemia. Once established under pressure by blood schizonticides, secondary exoerythrocytic stages could be propagated from bird to bird without, however, multiplying uncontrollably and thus causing the tissue infection to become fatal.
4. Discussion
Infection of young chicks with the 8A strain of P. gallinaceum can be summarized as follows. i) Sporozoite transmission by mosquito bite resulted in the transient appearance of primary exoerythrocytic stages throughout the body followed by a rapidly rising lethal parasitemia. ii) Suppression of the blood infection with quinine or chloroquine prevented disease, led to the clearance of all exoerythrocytic stages and resulted in immunity. iii) Inoculation of infected blood caused a rapidly rising parasitemia which, if untreated, killed the birds before the appearance of secondary exoerythrocytic stages. iv) If treated after the onset of the parasitemia, blood transfer did induce infection of brain endothelia with secondary exoerythrocytic stages. The intensity of the infection appeared to increase with the level and duration of the parasitemia. v) Transfer of infected brain emulsion from chicks, which had been kept under quinine pressure and therefore did not develop a parasitemia, caused blood infections in untreated recipient birds, but remained strictly exoerythrocytic through six passages in birds maintained under quinine pressure. vi) Sporozoites readily infected cultured chicken fibroblast or macrophage cell lines and developed to primary exoerythrocytic stages in vitro, whereas co-cultivation with infected chicken blood or extract from brain or spleen did not result in adaptation of the parasites to these avian cell lines. Likewise, attempts to propagate exoerythrocytic stages by cultivating infected tissues failed. Thus, the 8A strain was capable of performing primary exoerythrocytic to erythrocytic, erythrocytic to secondary exoerythrocytic as well as secondary exoerythrocytic to erythrocytic stage transitions in vivo. The cause of death in young chicks was exclusively the rapidly rising parasitemia; exoerythrocytic stages appeared either transiently or remained limited in number and thus had no lethal consequences.
More than half a century ago, several authors reported that inoculation of tissue or blood infected with the 8A strain of P. gallinaceum into White Leghorn chickens maintained under daily quinine treatment caused the death of the birds within 2 weeks from uncontrolled expansion of exoerythrocytic forms in endothelia of the brain and phagocytes of the reticulo-endothelial system (Adler and Tchernomoretz, 1941; Haas et al., 1948; Lewert, 1950b, a). Using the same parasite strain, chicken breed and therapeutic regimen, we were unable to reproduce this parasite behavior, whether infection was by mosquito bite, blood transfer or inoculation of infected brain or spleen. In our hands, quinine or chloroquine treatment not only prevented the development of a parasitemia, but also led to the elimination of the exoerythrocytic stages, eventually conferring resistance to reinfection. Possible explanations for this outcome include the following: i) The 8A strain may have undergone significant changes in tissue tropism while being propagated for decades under laboratory conditions (Permin and Juhl, 2002; Williams, 2005a). However, because we obtained identical results with two 8A substrains, one of which had been maintained predominantly by serial weekly blood passages (Warburg et al., 1992; Barreau et al., 1995; Korochkina et al., 2006), while the other was propagated by alternating blood and mosquito passages (Alavi et al., 2003), we conclude that at least extensive blood passage may not be responsible for the apparent reduction in virulence of the exoerythrocytic tissue stages. This interpretation concurs with earlier reports (Haas et al., 1948; Greenberg et al., 1950) demonstrating that the mode of passage of the 8A strain, whether by blood transfer or mosquito bite, does not facilitate selection of sub-strains that are preferentially erythrocytic or exoerythrocytic in behavior. In agreement with our data, other recent studies also document that in various commercial chicken breeds, exoerythrocytic stages of the 8A strain could be found only transiently and then disappeared (Permin and Juhl, 2002; Paulman and McAllister, 2005; Williams, 2005a, b). As shown here, the tissue infection in these studies was not lethal and left the birds immune to reinfection. ii) We excluded the possibility that our chicks harbored residual coccidiostats, which are frequently included in chicken feed to prevent devastating outbreaks of the related apicomplexan parasite Eimeria tenella (Takaya et al., 1999; Pines et al., 2000), which could have affected the exoerythrocytic stages of P. gallinaceum. iii) One remaining possibility is that White Leghorns have gained resistance to tissue stages of P. gallinaceum, because modern commercial chicken breeds have been selected for increased resistance to disease, including E. tenella infection (Edgar et al., 1951; Champion, 1954; Rosenberg et al., 1954; Johnson and Edgar, 1982; Caron et al., 1997). 4) Finally, the quinine used in the 1940s may have been of lesser purity thus permitting the generation of secondary exoerythrocytic stages from a low level parasitemia. Further analysis is required to explain the reduced virulence of the exoerythrocytic stages.
The molecular basis for the different patterns of dissemination and host cell preference of avian versus mammalian malaria species is unknown. With minor exceptions, the architecture of the avian liver lobule (Elias and Bengelsdorf, 1952; Hickey and Elias, 1954; Purton, 1969a, b; Hodges, 1972, 1974; Purton, 1976; Yamashiro and Bast, 1978; McLelland, 1979; Bhatnagar et al., 1980; Bhatnagar and Singh, 1982; Bhatnagar et al., 1982; Ohata et al., 1982; Ohata and Ito, 1986; Abdelwahab, 1987; Ghoddusi and Kelly, 2004) is essentially identical to that of humans and most mammals (Jones and Spring-Mills, 1984; Gumucio et al., 1994). Further, birds generally synthesize the same classes of glycosaminoglycans as mammals (Owens and Wagner, 1992), rendering the possibility unlikely that these host factors are responsible for the lack of selective targeting of P. gallinaceum sporozoites to the liver. However, because CSP plays an important role in sporozoite targeting to the mammalian liver (Cerami et al., 1992, 1994; Frevert et al., 1993; Sinnis et al., 1996), it is possible that the binding characteristics of P. gallinaceum CSP are less restrictive and allow recognition of extrahepatic glycosaminoglycans such as those from tissue macrophages or certain vascular endothelia. Notably, P. gallinaceum CSP differs from that of all mammalian species in that it lacks the N-terminal cluster of positively charged amino acids (McCutchan et al., 1996) which cooperate with region II-plus in the binding to liver-specific heparan sulfate proteoglycans (Ying et al., 1997; Rathore et al., 2002; Ancsin and Kisilevsky, 2004). Further, P. gallinaceum CSP lacks the conserved region I which contains a cleavage site for a parasite-derived cysteine protease (Coppi et al., 2005). Although the exact mode and site of CSP processing during sporozoite entry into the liver are unknown, proteolytic removal of the N-terminus is required for sporozoite infectivity of mice. Interestingly, replacement of the native P. berghei CSP by the P. gallinaceum homolog abolished sporozoite infectivity for mice (Tewari et al., 2005), and it is possible that one or more of the steps of the liver infection cascade, i.e. sporozoite arrest in the sinusoid, Kupffer cell passage, and/or hepatocyte entry, was disrupted by the absence of the proteolytic processing and/or proteoglycan recognition domains in the avian CSP. Thus, there is a possibility that CSP contributes to the tissue tropism of avian versus mammalian Plasmodium sporozoites.
The liver typically responds to the death of parenchymal cells with hyperplasia of non-parenchymal cells, in particular Kupffer cells, which then secrete mediators including hepatocyte growth factor (HGF) that stimulate hepatocyte proliferation and eventually allow regeneration of the damaged liver tissue (Matsumoto and Nakamura, 1991). A role for HGF in mammalian liver infection has been pointed out in the past (Carrolo et al., 2003). We found the sinusoidal lumina in the damaged areas to be obstructed with large numbers of cells, whose general ultrastructure, largely extended plasma membrane and lack of peroxidase activity all classified them as proliferating Kupffer cells or their monocytic precursors (Bouwens et al., 1986; Philips, 1987b, a; Yamamoto et al., 1996). This is not surprising because: i) as stated above, Kupffer cell hyperplasia is a typical response of the liver to hepatocyte injury (Matsumoto and Nakamura, 1991); ii) avian malaria parasites are known to disseminate extensively throughout the tissues of the avian host; and iii) very similar histopathological changes are induced in the mouse liver by migrating P. berghei (Khan and Vanderberg, 1991a; Vanderberg et al., 1993; Frevert et al., 2005) and, to a lesser degree, P. yoelii sporozoites (Khan and Vanderberg, 1991b, 1992). The relatively low parasite density we found in the chick livers renders the possibility unlikely that the substantial hepatocyte damage was caused by excessive parasite multiplication. The most likely explanation for our findings is therefore that P. gallinaceum parasites, like mammalian malaria sporozoites, migrated through the liver parenchyma thus causing hepatocyte necrosis. The resulting Kupffer cell hyperplasia and granuloma formation would have cleared the damage and induced liver regeneration had the birds survived and controlled the infection. Conversely, it could be argued that parasite contact with sinusoidal Kupffer cells activated these macrophages leading to proliferation of non-parenchymal cells and infiltration of mononuclear phagocytes. In this scenario, hepatocyte death could have been a consequence of anoxia caused by stagnation of the sinusoidal blood flow. However, because mammalian malaria sporozoites do not activate but, on the contrary, suppress Kupffer cell activation (Steers et al., 2005; Usynin et al., 2007) (Klotz and Frevert, unpublished data), it seems more likely that hepatocyte death was a secondary event which was caused by migrating parasites and triggered sinusoidal occlusion by infiltrating mononuclear cells and hyperplastic Kupffer cells. Future cell wounding assays should clarify this matter.
The exoerythrocytic stages of the related avian and reptilian Plasmodium species lack the restricted tissue tropism of their mammalian siblings and do not infect hepatocytes (Bray, 1957; Huff, 1968). We expect, therefore, that parasite migration occurred in other organs as well; this may be required to prepare exoerythrocytic stages of P. gallinaceum for productive infection (Mota et al., 2001, 2002). Finally, there is a possibility that excessive tissue migration has its origin in host parasite mismatch, since neither P. gallinaceum, P. berghei or P. yoelii have been monitored in real-time in their original hosts. Analyzing P. gallinaceum in the Asian jungle fowl or P. berghei in the African tree rat should reveal if parasite migration and associated histopathological changes are natural phenomena.
The obligatory role of Kupffer cells for P. yoelii sporozoite entry in to the liver was demonstrated by comparison of two different Kupffer cell-deficient rodent models (Frevert et al., 2006a, b; Baer et al., 2007b), homozygous osteopetrotic (op/op) mice deficient in macrophage colony stimulating Factor 1 (CSF-1) (Wiktor-Jedrzejczak and Gordon, 1996) and mice which were macrophage-depleted by injection of liposome-encapsulated dichloromethylene diphosphonate (clodronate) (van Rooijen and van Kesteren-Hendrikx, 2002), thus confirming earlier indicative studies with P. berghei (Meis et al., 1983,, 1985; Pradel and Frevert, 2001; Pradel et al., 2002; Ishino et al., 2004, 2005a; Frevert et al., 2005). Further, electron microscopy revealed that clodronate treatment leads to the temporary appearance of numerous focal disruptions in the sinusoidal cell layer thus providing sporozoites with direct access to the liver parenchyma (Baer et al., 2007b). This explains why liver infection with wild-type P. berghei sporozoites is increased (Vreden et al., 1993) and why sporozoite microneme protein essential for cell traversal (SPECT)-deficient P. berghei mutants, which are incapable of cell passage and therefore not infective for intact mice, can infect clodronate-treated mice (Ishino et al., 2004, 2005a). The finding that primary and secondary exoerythrocytic P. gallinaceum schizonts develop in the liver primarily in Kupffer cells lends additional strength to the hypothesis that mammalian Plasmodium sporozoites specifically recognize and safely traverse these macrophages to reach hepatocytes.
Plasmodium gallinaceum schizonts in liver, spleen, kidney and lungs were typically rare and small in size, while large schizonts were found only in the brain, which was in addition the most heavily infected. This is in agreement with previous systematic studies, which all document large secondary exoerythrocytic stages of P. gallinaceum developing in vascular endothelia of the brain, while the parasites remain much smaller in cells of monocytic origin in organs such as liver, spleen, lung, heart and kidney (James and Tate, 1938; Lewert, 1950a; Huff, 1954; Bray, 1957; Permin and Juhl, 2002; Williams, 2005b). We also show that sporozoite-induced primary exoerythrocytic liver stages of P. gallinaceum mature inside Kupffer cells to a size considerably smaller than their mammalian counterparts developing in hepatocytes. It is tempting to speculate that in the course of evolution, Plasmodium has given up its broad range of host cells in favor of the nutritionally richer environment of the large and metabolically highly active hepatocyte.
Systematic studies on the cell biology of mammalian Plasmodium liver stages have been difficult because of the scarcity of sporozoite infection of the liver and the limited invasion rates currently achievable in vitro (Sinden et al., 1990; Cerami et al., 1992; Sinnis et al., 1994; Frevert et al., 1996). Toxoplasma gondii, a distant cousin of Plasmodium, was frequently used as a model organism to fill this gap (Roos et al., 1999). Although knowledge of this apicomplexan parasite is important for understanding Plasmodium biology, the closely related avian malaria species are more likely to use the same mechanisms for interaction with host macrophages. Since evolution involves adaptation of old processes for new purposes, it appears that the use of Kupffer cells as a portal of mammalian Plasmodium species to the liver reflects phylogeny in that preserved mechanisms for recognition, entry and survival in Kupffer cells are employed. The evolutionary advantage of shifting the primary exoerythrocytic development to the liver can be explained by hepatocytes being a niche with high metabolic activity which can support the enormous growth of mammalian parasites, with scarce expression of histocompatibility antigens which helps avoid an immune response and with a concealed location which protects the parasites from immune surveillance.
Supplementary Material
Supplementary files Supplementary Movie 1. Plasmodium gallinaceum-infected brain tissue. The focus series through a three-dimensional stack from the brain of a P. gallinaceum-infected chicken shows several infected vascular endothelia. Each of the exoerythrocytic forms is multi-nucleated. The parasites were labeled with anti-Pb in combination with GAR-FITC (green); the tissue was counter-stained with Evans blue (red).
Acknowledgements
UF was supported by NIH grants RO1 AI51656 and S10 RR019288 and NSF grant 9977430. We thank Drs. Kenneth Vernick and Robert Sinden for generous gifts of P. gallinaceum blood stabilates, Dr. Robert Gwadz for Aedes aegypti eggs, and Dr. Antoniana Krettli for good advice and the mAb N2H6D5 against P. gallinaceum CSP. We are grateful to Drs. Karen Day and Jane Carlton for valuable discussions and for critically reading the manuscript. Many thanks to Yasin Gregg and Astrid Berg for dedicated technical assistance.
Footnotes
Note: Supplementary data associated with this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelwahab EM. Ultrastructure and arrangement of hepatocyte cords in the duckling's liver. J Anat. 1987;150:181–189. [PMC free article] [PubMed] [Google Scholar]
- Adler S, Tchernomoretz I. Continued passage of extra-erythrocytic forms of Plasmodium gallinaceum in the absence of erythrocytic schizogany. Ann Trop Med Parasit. 1941;35:241–246. [Google Scholar]
- Africa CM, Dy FJ, Soriano LJ. A study on the identity of a Plasmodium in the Philippine domestic fowl (Gallus gallus) Univ Phil App Sci Bull. 1940;7:279–284. [Google Scholar]
- Aikawa M, Huff CG, Sprinz H. Exoerythrocytic stages of Plasmodium gallinaceum in chick-embryo liver as observed electron microscopically. Am J Trop Med Hyg. 1968;17:156–169. doi: 10.4269/ajtmh.1968.17.156. [DOI] [PubMed] [Google Scholar]
- Alavi Y, Arai M, Mendoza J, Tufet-Bayona M, Sinha R, Fowler K, Billker O, Franke-Fayard B, Janse CJ, Waters A, Sinden RE. The dynamics of interactions between Plasmodium and the mosquito: a study of the infectivity of Plasmodium berghei and Plasmodium gallinaceum, and their transmission by Anopheles stephensi, Anopheles gambiae and Aedes aegypti. Int J Parasitol. 2003;33:933–943. doi: 10.1016/s0020-7519(03)00112-7. [DOI] [PubMed] [Google Scholar]
- Amino R, Thiberge S, Martin B, Celli S, Shorte S, Frischknecht F, Menard R. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat Med. 2006;12:220–224. doi: 10.1038/nm1350. [DOI] [PubMed] [Google Scholar]
- Ancsin JB, Kisilevsky R. A binding site for highly sulfated heparan sulfate is identified in the amino-terminus of the circumsporozoite protein: Significance for malarial sporozoite attachment to hepatocytes. J Biol Chem. 2004;279:21824–21832. doi: 10.1074/jbc.M401979200. [DOI] [PubMed] [Google Scholar]
- Baer K, Klotz C, Kappe SHIK, Schnieder T, Frevert U. Release of hepatic Plasmodium yoelii merozoites into the pulmonary microvasculature. PLoS Pathog. 2007a doi: 10.1371/journal.ppat.0030171. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer K, Roosevelt M, Van Rooijen N, Clarkson AB, Jr, Schnieder T, Frevert U. Kupffer cells are obligatory for Plasmodium sporozoite infection of the liver. Cell Microbiol. 2007b;9:397–412. doi: 10.1111/j.1462-5822.2006.00798.x. [DOI] [PubMed] [Google Scholar]
- Barreau C, Touray M, Pimenta PF, Miller LH, Vernick KD. Plasmodium gallinaceum: sporozoite invasion of Aedes aegypti salivary glands is inhibited by anti-gland antibodies and by lectins. Exp Parasitol. 1995;81:332–343. doi: 10.1006/expr.1995.1124. [DOI] [PubMed] [Google Scholar]
- Beaudoin RL, Strome CP, Clutter WG. Cultivation of avian malaria parasites in mammalian liver cells. Exp Parasitol. 1974;36:355–339. doi: 10.1016/0014-4894(74)90075-7. [DOI] [PubMed] [Google Scholar]
- Beug H, Kirchbach Av, Döderlein G, Conscience J-F, Graf T. Chicken hematopoetic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell. 1979;18:375–390. doi: 10.1016/0092-8674(79)90057-6. [DOI] [PubMed] [Google Scholar]
- Bhatnagar MK, Yamashiro S, David LL. Ultrastructural study of liver fibrosis in turkeys fed diets containing rapeseed meal. Res Vet Sci. 1980;29:260–265. [PubMed] [Google Scholar]
- Bhatnagar MK, Singh A. Ultrastructure of turkey hepatocytes. Anat Rec. 1982;202:473–482. doi: 10.1002/ar.1092020406. [DOI] [PubMed] [Google Scholar]
- Bhatnagar MK, Vrablic OE, Yamashiro S. Ultrastructural alterations of the liver of Pekin ducks fed methyl mercury-containing diets. J Toxicol Environ Health. 1982;10:981–1003. doi: 10.1080/15287398209530311. [DOI] [PubMed] [Google Scholar]
- Bouwens L, Baekeland M, De Zanger R, Wisse E. Quantitation, tissue distribution and proliferation kinetics of Kupffer cells in normal rat liver. Hepatology. 1986;6:718–722. doi: 10.1002/hep.1840060430. [DOI] [PubMed] [Google Scholar]
- Bouwens L, Wisse E. The origin of Kupffer cells and their relationship to hepatocytes. In: Billiar TR, Curran RD, editors. Hepatocyte and Kupffer Cell Interactions. Boca Raton: CRC Press; 1992. pp. 3–21. [Google Scholar]
- Bray RS. Studies on the exo-erythrocytic cycle in the genus Plasmodium. Mem London School Hyg Trop Med. 1957;12:1–92. [Google Scholar]
- Brumpt E. Paludisme aviaire: Plasmodium gallinaceum n. sp. de la poule domestique. C R Acad Sci Paris. 1935;200:783–785. [Google Scholar]
- Brumpt E. Schizogonie parfois intense du Plasmodium gallinaceum dans les cellules endotheliales des poules. C R Soc Biol Paris. 1937;125:810–813. [Google Scholar]
- Caron LA, Abplanalp H, Taylor RL., Jr Resistance, susceptibility, and immunity to Eimeria tenella in major histocompatibility (B) complex congenic lines. Poultry Sci. 1997;76:677–682. doi: 10.1093/ps/76.5.677. [DOI] [PubMed] [Google Scholar]
- Carrolo M, Giordano S, Cabrita-Santos L, Corso S, Vigario AM, Silva S, Leiriao P, Carapau D, Armas-Portela R, Comoglio PM, Rodriguez A, Mota MM. Hepatocyte growth factor and its receptor are required for malaria infection. Nature Med. 2003;9:1363–1369. doi: 10.1038/nm947. [DOI] [PubMed] [Google Scholar]
- Cerami C, Frevert U, Sinnis P, Takacs B, Clavijo P, Santos MJ, Nussenzweig V. The basolateral domain of the hepatocyte plasma membrane bears receptors for the circumsporozoite protein of Plasmodium falciparum sporozoites. Cell. 1992;70:1021–1033. doi: 10.1016/0092-8674(92)90251-7. [DOI] [PubMed] [Google Scholar]
- Cerami C, Frevert U, Sinnis P, Takacs B, Nussenzweig V. Rapid clearance of malaria circumsporozoite protein (CS) by hepatocytes. J Exp Med. 1994;179:695–701. doi: 10.1084/jem.179.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion LR. The inheritance of resistance to cecal coccidiosis in the domestic fowl. Poultry Sci. 1954;33:670–681. [Google Scholar]
- Coppi A, Pinzon-Ortiz C, Hutter C, Sinnis P. The Plasmodium circumsporozoite protein is proteolytically processed during cell invasion. J Exp Med. 2005;201:27–33. doi: 10.1084/jem.20040989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman AF, Kappe SH. Malaria's stealth shuttle. Science. 2006;313:1245–1246. doi: 10.1126/science.1132940. [DOI] [PubMed] [Google Scholar]
- Edgar SA, King DF, Johnson LW. Control of avian coccidiosis through breeding or immunization. Poultry Sci. 1951;30:911. [Google Scholar]
- Elias H, Bengelsdorf H. The structure of the liver of vertebrates. Acta Anatomica. 1952;14:297–337. doi: 10.1159/000140715. [DOI] [PubMed] [Google Scholar]
- Fahimi HD. The fine structural localization of endogenous and exogenous peroxidase activity in Kupffer cells of rat liver. J Cell Biol. 1970;47:247–262. doi: 10.1083/jcb.47.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando MA, Dissanaike AS. Studies on Plasmodium gallinaceum and Plasmodium juxtanucleare the Malayan jungle fowl Gallus gallus spadiceus. SE Asian J Trop Med Pub Hlth. 1975;6:25–32. [PubMed] [Google Scholar]
- Frevert U, Sinnis P, Cerami C, Shreffler W, Takacs B, Nussenzweig V. Malaria circumsporozoite protein binds to heparan sulfate proteoglycans associated with the surface membrane of hepatocytes. J Exp Med. 1993;177:1287–1298. doi: 10.1084/jem.177.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frevert U, Sinnis P, Esko JD, Nussenzweig V. Cell surface glycosaminoglycans are not obligatory for Plasmodium berghei invasion in vitro. Mol Biochem Parasitol. 1996;76:257–266. doi: 10.1016/0166-6851(95)02563-4. [DOI] [PubMed] [Google Scholar]
- Frevert U. Sneaking in through the back entrance: The biology of malaria liver stages. Trends Parasitol. 2004;20:417–424. doi: 10.1016/j.pt.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Frevert U, Engelmann S, Zougbédé S, Stange J, Ng B, Matuschewski K, Liebes L, Yee H. Intravital observation of Plasmodium berghei sporozoite infection of the liver. PLoS Biol. 2005;3:e192. doi: 10.1371/journal.pbio.0030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frevert U, Usynin I, Baer K, Klotz C. Nomadic or sessile: can Kupffer cells function as portals for malaria sporozoites to the liver? Cell Microbiol. 2006a;8:1537–1546. doi: 10.1111/j.1462-5822.2006.00777.x. [DOI] [PubMed] [Google Scholar]
- Frevert U, Usynin I, Baer K, Klotz C. Penetrating biological barriers. Liver: Plasmodium Sporozoite Passage across the Sinusoidal Cell Layer. In: Soldati D, Burleigh BA, editors. Molecular Mechanisms of Parasite Invasion. Austin, TX: Landes Bioscience; 2006b. pp. 1–16. [Google Scholar]
- Garnham PCC. Malaria parasites and other Haemosporidia. Oxford: Blackwell; 1966. [Google Scholar]
- Ghoddusi M, Kelly WR. Ultrastructure of in situ perfusion-fixed avian liver, with special reference to structure of the sinusoids. Microsc Res Tech. 2004;65:101–111. doi: 10.1002/jemt.20107. [DOI] [PubMed] [Google Scholar]
- Greenberg J, Trembley HL, Coatney GR. Strain differences in Plasmodium gallinaceum Brumpt. I. Differences in the behavior of the exoerythrocytic forms of a blood-passaged (BI) and sporozoite-passaged (SP) strain of Plasmodium gallinaceum. J Natl Malar Soc. 1950;9:320–326. [PubMed] [Google Scholar]
- Gressner AM, Schäfer S. Comparison of sulphated glycosaminoglycan and hyaluronate synthesis and secretion in cultured hepatocytes, fat storing cells, and Kupffer cells. J Clin Chem Clin Biochem. 1989;27:141–149. doi: 10.1515/cclm.1989.27.3.141. [DOI] [PubMed] [Google Scholar]
- Gumucio JJ, Bilir BM, Moseley RH, Berkowitz CM. The biology of the liver cell plate. In: Arias IM, Boyer JL, Fausto N, Jakoby WB, Schachter DA, Shafritz DA, editors. The Liver: Biology and Pathobiology. New York: Raven Press; 1994. pp. 1143–1163. [Google Scholar]
- Haas VH, Wilcox A, Laird RL, Ewing FM, Coleman H. Symposium on erythrocytic forms of malarial parasites. VI. Response of exoerythrocytic forms to alterations in the life cycle of Plasmodium gallinaceum. J Parasitol. 1948;34:306–320. [PubMed] [Google Scholar]
- Hickey JJ, Elias H. The Structure of the Liver of Birds. Auk. 1954;4:458–462. [Google Scholar]
- Hodges RD. The ultrastructure of the liver parenchyma of the immature fowl (Gallus domesticus) Z Zellforsch Mikrosk Anat. 1972;133:35–46. doi: 10.1007/BF00307066. [DOI] [PubMed] [Google Scholar]
- Hodges RD. The liver, The Histology of the Fowl. London and New York: Academic Press; 1974. pp. 88–112. [Google Scholar]
- Hollingdale MR. Malaria and the liver. Hepatology. 1985;5:327–335. doi: 10.1002/hep.1840050230. [DOI] [PubMed] [Google Scholar]
- Huff CG, Coulston F. The development of Plasmodium gallinaceum from sporozoite to erythrocytic trophozoite. J Infect Dis. 1944;75:231–249. [Google Scholar]
- Huff CG. Merozoite size in exoerythrocytic infections of Plasmodium gallinaceum, P. fallax, P. lophurae, and P. cathemerium. Exp Parasitol. 1954;3:433–444. doi: 10.1016/0014-4894(54)90039-9. [DOI] [PubMed] [Google Scholar]
- Huff CG. Organ and tissue distribution of the exoerythrocytic stages of various avian malarial parasites. Exp Parasitol. 1957;6:143–162. doi: 10.1016/0014-4894(57)90012-7. [DOI] [PubMed] [Google Scholar]
- Huff CG. Recent experimental research on avian malaria. Adv Parasitol. 1968;6:293–311. doi: 10.1016/s0065-308x(08)60476-5. [DOI] [PubMed] [Google Scholar]
- Huff CG. Exoerythrocytic stages of avian and reptilian malarial parasites. Exp Parasitol. 1969;24:383–421. doi: 10.1016/0014-4894(69)90176-3. [DOI] [PubMed] [Google Scholar]
- Hügel F-U, Pradel G, Frevert U. Release of malaria circumsporozoite protein into the host cell cytoplasm and interaction with ribosomes. Mol Biochem Parasitol. 1996;81:151–170. doi: 10.1016/0166-6851(96)02701-6. [DOI] [PubMed] [Google Scholar]
- Ishino T, Yano K, Chinzei Y, Yuda M. Cell-passage activity is required for the malarial parasite to cross the liver sinusoidal cell layer. PLoS Biol. 2004;2:E4. doi: 10.1371/journal.pbio.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino T, Chinzei H, Yuda M. A Plasmodium sporozoite protein with a membrane attack complex domain is required for breaching the liver sinusoidal cell layer prior to hepatocyte infection. Cell Microbiol. 2005a;7:199–208. doi: 10.1111/j.1462-5822.2004.00447.x. [DOI] [PubMed] [Google Scholar]
- Ishino T, Chinzei Y, Yuda M. Two proteins with 6-cys motifs are required for malarial parasites to commit to infection of the hepatocyte. Mol Microbiol. 2005b;58:1264–1275. doi: 10.1111/j.1365-2958.2005.04801.x. [DOI] [PubMed] [Google Scholar]
- James SP, Tate P. New knowledge of the life cycle of malaria parasites. Nature. 1937;139:545. [Google Scholar]
- James SP, Tate P. Exo-erythrocytic schizogony in Plasmodium gallinaceumBrumpt, 1935. Parasitology. 1938;30:128–139. [Google Scholar]
- Johnson LW, Edgar SA. Responses to prolonged selection for resistance and susceptibility to acute cecal coccidiosis in the auburn strain single comb White Leghorn. Poultry Sci. 1982;61:2344–2355. doi: 10.3382/ps.0612344. [DOI] [PubMed] [Google Scholar]
- Jones AL, Spring-Mills E. The liver and the gallbladder. In: Weiss L, editor. Modern Concepts of Gastrointestinal Histology. New York: Elsevier; 1984. pp. 707–748. [Google Scholar]
- Khan ZM, Vanderberg JP. Eosinophil-rich, granulomatous inflammatory response to Plasmodium berghei hepatic schizonts in non-immunized rats is age-related. Am J Trop Med Hyg. 1991a;45:190–201. doi: 10.4269/ajtmh.1991.45.190. [DOI] [PubMed] [Google Scholar]
- Khan ZM, Vanderberg JP. Role of host cellular response in differential susceptibility of nonimmunized BALB/C mice to Plasmodium berghei and Plasmodium yoelii sporozoites. Infect Immun. 1991b;59:2529–2534. doi: 10.1128/iai.59.8.2529-2534.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan ZM, Vanderberg JP. Specific inflammatory cell infiltration of hepatic schizonts in BALB/c mice immunized with attenuated Plasmodium yoelii sporozoites. Int Immunol. 1992;4:711–718. doi: 10.1093/intimm/4.7.711. [DOI] [PubMed] [Google Scholar]
- Korochkina S, Barreau C, Pradel G, Jeffery E, Li J, Natarajan R, Shabanowitz J, Hunt D, Frevert U, Vernick KD. A mosquito-specific protein family includes candidate receptors for malaria sporozoite invasion of salivary glands. Cell Microbiol. 2006;8:163–175. doi: 10.1111/j.1462-5822.2005.00611.x. [DOI] [PubMed] [Google Scholar]
- Krettli AU, Rocha EMM, Lopes JD, Carneiro CRW, Kamboi KK, Cochrane AH, Nussenzweig RS. Circumsporozoite protein of Plasmodium gallinaceum characterized by monoclonal antibodies. Parasite Immunol. 1988;10:523–533. doi: 10.1111/j.1365-3024.1988.tb00240.x. [DOI] [PubMed] [Google Scholar]
- Lewert RM. Exoerythrocytic infection by Plasmodium gallinaceum in blood-infected, quinine-treated chicks. Am J Hyg. 1948;48:158–170. doi: 10.1093/oxfordjournals.aje.a119231. [DOI] [PubMed] [Google Scholar]
- Lewert RM. Alterations in the cycle of Plasmodium gallinaceum following passage through tissue culture. II. The behavior of the strains during multiple passage through chicks. Am J Hyg. 1950a;51:178–193. [Google Scholar]
- Lewert RM. Alterations in the cycle of Plasmodium gallinaceum following passage through tissue culture. I. Tissue culture studies. Am J Hyg. 1950b;51:155–177. doi: 10.1126/science.107.2775.250. [DOI] [PubMed] [Google Scholar]
- Lyon M, Denkin JA, Gallagher JT. Liver heparan sulfate structure. A novel molecular design. J Biol Chem. 1994;269:11208–11215. [PubMed] [Google Scholar]
- MacPherson GG, Warrell MJ, White NJ, Looareesuwan S, Warrell DA. Human cerebral malaria a quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985;119:385–401. [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Nakamura T. Hepatocyte growth factor: molecular structure and implications for a central role in liver regeneration. J Gastroenterol Hepatol. 1991;6:509–519. doi: 10.1111/j.1440-1746.1991.tb00897.x. [DOI] [PubMed] [Google Scholar]
- McCutchan TF, Kissinger JC, Touray MG, Rogers MJ, Li J, Sullivan M, Braga EM, Krettli AU, Miller LH. Comparison of circumsporozoite proteins from avian and mammalian malarias: biological and phylogenetic implications. Proc Natl Acad Sci USA. 1996;93:11889–11894. doi: 10.1073/pnas.93.21.11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLelland J. Digestive System. In: King AS, McLelland J, editors. Form and Function in Birds. London and New York: Academic Press; 1979. pp. 69–181. [Google Scholar]
- Meis JFGM, Verhave JP, Jap PHK, Meuwissen JHET. An ultrastructural study on the role of Kupffer cells in the process of infection by Plasmodium berghei sporozoites in rats. Parasitology. 1983;86:231–242. doi: 10.1017/s003118200005040x. [DOI] [PubMed] [Google Scholar]
- Meis JFGM, Verhave JP, Brouwer A, Meuwissen JHET. Electron microscopic studies on the interaction of rat Kupffer cells and Plasmodium berghei sporozoites. Z Parasitenkd. 1985;71:473–483. doi: 10.1007/BF00928350. [DOI] [PubMed] [Google Scholar]
- Meis JFGM, Verhave JP. Exoerythrocytic development of malaria parasites. Adv Parasitol. 1988;27:1–61. doi: 10.1016/s0065-308x(08)60352-8. [DOI] [PubMed] [Google Scholar]
- Meyer H, Oliveira Musacchio Md. Electon microscope study of the exoerythrocytic form of Plasmodium gallinaceum in thin sections of infected tissue cultures. J Protozool. 1960;7:222–228. doi: 10.1111/j.1550-7408.1965.tb01836.x. [DOI] [PubMed] [Google Scholar]
- Meyer H, Oliveira Musacchio Md. An electron microscopic study of the final and initial forms of Plasmodium gallinaceum in thin sections of infected tissue cultures. J Protozool. 1965;12:193–202. doi: 10.1111/j.1550-7408.1965.tb01836.x. [DOI] [PubMed] [Google Scholar]
- Mota MM, Pradel G, Vanderberg JP, Hafalla JCR, Frevert U, Nussenzweig RS, Nussenzweig V, Rodriguez A. Migration of Plasmodium sporozoites through cells before infection. Science. 2001;291:141–144. doi: 10.1126/science.291.5501.141. [DOI] [PubMed] [Google Scholar]
- Mota MM, Hafalla JC, Rodriguez A. Migration through host cells activates Plasmodium sporozoites for infection. Nat Med. 2002;8:1318–1322. doi: 10.1038/nm785. [DOI] [PubMed] [Google Scholar]
- Ohata M, Tanuma Y, Ito T. Electron microscopic study on avian livers with special remarks on the fine structure of sinusoidal cells. Okajimas Folia Anat Jpn. 1982;58:325–368. doi: 10.2535/ofaj1936.58.4-6_325. [DOI] [PubMed] [Google Scholar]
- Ohata M, Ito T. Experimental study on the fine structure of chicken liver parenchyme with special references to extrasinusoidal macrophages and sinusoidal blood cells. Part 2. Sinusoidal blood cells in normal and India ink perfused livers. Arch Histol Jpn. 1986;49:199–209. doi: 10.1679/aohc.49.199. [DOI] [PubMed] [Google Scholar]
- Oo MM, Aikawa M, Than T, Aye TM, Myint PT, Igarashi I, Schoene WC. Human cerebral malaria: a pathological study. J Neuropathol Exp Neurol. 1987;46:223–231. doi: 10.1097/00005072-198703000-00009. [DOI] [PubMed] [Google Scholar]
- Owens RT, Wagner WD. Chondroitin sulfate proteoglycan and heparan sulfate proteoglycan production by cultured pigeon peritoneal macrophages. J Leukocyte Biol. 1992;51:626–633. doi: 10.1002/jlb.51.6.626. [DOI] [PubMed] [Google Scholar]
- Paulman A, McAllister MM. Plasmodium gallinaceum: clinical progression, recovery, and resistance to disease in chickens infected via mosquito bite. Am J Trop Med Hyg. 2005;73:1104–1107. [PubMed] [Google Scholar]
- Permin A, Juhl J. The development of Plasmodium gallinaceum infections in chickens following single infections with three different dose levels. Vet Parasitol. 2002;105:1–10. doi: 10.1016/s0304-4017(01)00645-8. [DOI] [PubMed] [Google Scholar]
- Philips MJ. Drug and toxic effects. In: Philips MJ, Poucell S, Patterson J, Valencia A, editors. The Liver: An Atlas and Text of Ultrastructural Pathology. New York: Raven Press; 1987a. pp. 159–238. [Google Scholar]
- Philips MJ. Miscellaneous hepatic conditions. In: Philips MJ, Poucell S, Patterson J, Valencia A, editors. The Liver: An Atlas and Text of Ultrastructural Pathology. New York: Raven Press; 1987b. pp. 519–567. [Google Scholar]
- Philips MJ, Poucell S, Patterson J, Valencia P. The Liver: An Atlas and Text of Ultrastructural Pathology. New York: Raven Press; 1987. [Google Scholar]
- Pines M, Vlodavsky I, Nagler A. Halofuginone: from veterinary use to human therapy. Drug Dev Res. 2000;50:371–378. [Google Scholar]
- Pinzon-Ortiz C, Friedman J, Esko J, Sinnis P. The binding of the circumsporozoite protein to cell surface heparan sulfate proteoglycans is required for Plasmodium sporozoite attachment to target cells. J Biol Chem. 2001;276:26784–26791. doi: 10.1074/jbc.M104038200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradel G, Frevert U. Plasmodium sporozoites actively enter and pass through Kupffer cells prior to hepatocyte invasion. Hepatology. 2001;33:1154–1165. doi: 10.1053/jhep.2001.24237. [DOI] [PubMed] [Google Scholar]
- Pradel G, Garapaty S, Frevert U. Proteoglycans mediate malaria sporozoite targeting to the liver. Mol Microbiol. 2002;45:637–651. doi: 10.1046/j.1365-2958.2002.03057.x. [DOI] [PubMed] [Google Scholar]
- Pradel G, Garapaty S, Frevert U. Kupffer and stellate cell proteoglycans mediate malaria sporozoite targeting to the liver. Comp Hepatol. 2004;3 Suppl 1:S47. doi: 10.1186/1476-5926-2-S1-S47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purton MD. Structure and ultrastructure of the liver in the domestic fowl, Gallus gallus. J Zool Lond. 1969a;159:273–282. [Google Scholar]
- Purton MD. The structure and ultrastructure of the liver in Gallus domesticus. J Anat. 1969b;105:212. [PubMed] [Google Scholar]
- Purton MD. Extravascular cells within the perisinusoidal space of the avian liver. Experientia. 1976;32:737–740. doi: 10.1007/BF01919862. [DOI] [PubMed] [Google Scholar]
- Ramirez AD, Rocha EMM, Krettli AU. In vitro development of exoerythrocytic forms of Plasmodium gallinaceum sporozoites in avian macrophages. J Protozool. 1991;38:40–44. doi: 10.1111/j.1550-7408.1991.tb04796.x. [DOI] [PubMed] [Google Scholar]
- Ramirez AD, Rocha EMM, Krettli AU. Antisporozoite antibodies with protective and nonprotective activities: in vitro and in vivo correlations using Plasmodium gallinaceum, an avian model. J Euk Microbiol. 1995;42:705–708. doi: 10.1111/j.1550-7408.1995.tb01620.x. [DOI] [PubMed] [Google Scholar]
- Rathore D, Sacci JB, de la Vega P, McCutchan TF. Binding and invasion of liver cells by Plasmodium falciparum sporozoites. J Biol Chem. 2002;277:7092–7098. doi: 10.1074/jbc.M106862200. [DOI] [PubMed] [Google Scholar]
- Robson KJH, Frevert U, Reckmann I, Cowan G, Beier J, Scragg IG, Takehara K, Bishop DHL, Pradel G, Sinden R, Saccheo S, Müller H-M, Crisanti A. Thrombospondin related adhesive protein (TRAP) of Plasmodium falciparum: expression during sporozoite ontogeny and binding to human hepatocytes. EMBO J. 1995;14:3883–3894. doi: 10.1002/j.1460-2075.1995.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha EMM, Hollingdale MR, Gwadz R, Krettli AU. Exoerythrocytic development of Plasmodium gallinaceum sporozoites in a chicken fibroblast cell line and inhibition of the cell invasion by specific anti-sporozoite monoclonal antibodies. J Euk Microbiol. 1993;40:64–66. doi: 10.1111/j.1550-7408.1993.tb04883.x. [DOI] [PubMed] [Google Scholar]
- Rogerson SJ, Grau GE, Hunt NH. The microcirculation in severe malaria. Microcirculation. 2004;11:559–576. doi: 10.1080/10739680490503311. [DOI] [PubMed] [Google Scholar]
- Roos DS, Crawford MJ, Donald RGK, Fohl LM, Hager KM, Kissinger JC, Reynolds MG, Striepen B, W.J Transport and trafficking: Toxoplasma as a model for Plasmodium. Novartis Found Symp. 1999;226:176–198. doi: 10.1002/9780470515730.ch13. [DOI] [PubMed] [Google Scholar]
- Rosenberg MM, Alicata JE, Palafox AL. Further evidence of hereditary resistance and susceptibility to cecal coccidiosis in chickens. Poultry Sci. 1954;33:972–980. [Google Scholar]
- Seed TM, Manwell RD. Plasmodia of birds. In: Kreier JP, editor. Parasitic Protozoa Vol. III. Gregarines, Haemogregarines, Coccidia, Plasmodia, and Haemoproteids. New York: Academic Press; 1977. pp. 311–357. [Google Scholar]
- Shortt HE, Menon KP, Seetharama Iyer PV. The natural host of Plasmodium gallinaceum (Brumpt, 1935) J Malar Inst India. 1941;4:175. [Google Scholar]
- Sidjanski S, Vanderberg JP. Delayed migration of Plasmodium sporozoites from the mosquito bite site to the blood. Am J Trop Med Hyg. 1997;57:426–429. doi: 10.4269/ajtmh.1997.57.426. [DOI] [PubMed] [Google Scholar]
- Sinden RE. Cell biology. In: Killick-Kendrick R, Peters W, editors. Rodent Malaria. London: Academic Press; 1978. pp. 85–168. [Google Scholar]
- Sinden RE, Smith JE. The role of the Kupffer cell in the infection of rodents by sporozoites of Plasmodium: uptake of sporozoites by perfused liver and the establishment of infection in vivo. Acta Trop. 1982;39:11–27. [PubMed] [Google Scholar]
- Sinden RE, Suhrbier A, Davies CS, Fleck SL, Hodivala K, Nicholas JC. The development and routine application of high-density exoerythrocytic-stage culture of Plasmodium berghei. WHO Bulletin. 1990;68 Suppl.:115–125. [PMC free article] [PubMed] [Google Scholar]
- Sinnis P, Clavijo P, Fenyö D, Chait BT, Cerami C, Nussenzweig V. Structural and functional properties of region-II plus of the malaria circumsporozoite protein. J Exp Med. 1994;180:297–306. doi: 10.1084/jem.180.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnis P. The malaria sporozoite's journey into the liver. Infect Agents Disease. 1996;5:182–189. [PubMed] [Google Scholar]
- Sinnis P, Willnow TE, Briones MRS, Herz J, Nussenzweig V. Remnant lipoproteins inhibit malaria sporozoite invasion of hepatocytes. J Exp Med. 1996;184:945–954. doi: 10.1084/jem.184.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulsby EJL. Avian malaria, Helminths, Arthropods and Protozoa of Domesticated Animals. Philadelphia: Lea & Febiger; 1982. pp. 683–689. [Google Scholar]
- Springer WT. Other blood and tissue protozoa. In: Calnek BW, Beard CW, McDougald LR, Saif YM, editors. Diseases of Poultry. Ames, IA: Iowa State University Press; 1996. pp. 900–911. [Google Scholar]
- Steers N, Schwenk R, Bacon DJ, Berenzon D, Williams J, Krzych U. The immune status of Kupffer cells profoundly influences their responses to infectious Plasmodium berghei sporozoites. Eur J Immunol. 2005;35:2335–2346. doi: 10.1002/eji.200425680. [DOI] [PubMed] [Google Scholar]
- Sturm A, Amino R, van de Sand C, Regen T, Retzlaff S, Rennenberg A, Krueger A, Pollok JM, Menard R, Heussler VT. Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science. 2006;313:1287–1290. doi: 10.1126/science.1129720. [DOI] [PubMed] [Google Scholar]
- Takaya Y, Tasaka H, Chiba T, Uwai K, Tanitsu M, Kim HS, Wataya Y, Miura M, Takeshita M, Oshima Y. New type of febrifugine analogues, bearing a quinolizidine moiety, show potent antimalarial activity against Plasmodium malaria parasite. J Med Chem. 1999;42:3163–3166. doi: 10.1021/jm990131e. [DOI] [PubMed] [Google Scholar]
- Tarun AS, Baer K, Dumpit RF, Gray S, Lejarcegui N, Frevert U, Kappe S. Quantitative isolation and in vivo imaging of malaria parasite liver stages. Int J Parasitol. 2006;36:1283–1293. doi: 10.1016/j.ijpara.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Terzakis JA, Vanderberg JP, Foley D, Shustak S. Exoerythrocytic merozoites of Plasmodium berghei in rat hepatic Kupffer cells. J Protozool. 1979;26:385–389. doi: 10.1111/j.1550-7408.1979.tb04641.x. [DOI] [PubMed] [Google Scholar]
- Tewari R, Rathore D, Crisanti A. Motility and infectivity of Plasmodium berghei sporozoites expressing avian Plasmodium gallinaceum circumsporozoite protein. Cell Microbiol. 2005;7:699–707. doi: 10.1111/j.1462-5822.2005.00503.x. [DOI] [PubMed] [Google Scholar]
- Usynin I, Klotz C, Frevert U. Malaria circumsporozoite protein inhibits the respiratory burst in Kupffer cells. Cell Microbiol. 2007 June 15; doi: 10.1111/j.1462-5822.2007.00982.x. [Epub ahead of press] [DOI] [PubMed] [Google Scholar]
- van Rooijen N, van Kesteren-Hendrikx E. Clodronate liposomes: perspectives in research and therapeutics. J Liposome Res. 2002;12:81–94. doi: 10.1081/lpr-120004780. [DOI] [PubMed] [Google Scholar]
- Vanderberg JP, Khan ZM, Stewart MJ. Induction of hepatic inflammatory response by Plasmodium berghei sporozoites protects BALB/C mice against challenge with Plasmodium yoelii sporozoites. J Parasitol. 1993;79:763–767. [PubMed] [Google Scholar]
- Vanderberg JP, Frevert U. Intravital microscopy demonstrating antibody-mediated immobilization of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int J Parasitol. 2004;34:991–996. doi: 10.1016/j.ijpara.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Varndell IM, Polak JM. EM immunolabelling. In: Sommerville J, Scheer U, editors. Electron Microscopy in Molecular Biology, Practical Approach Series. Oxford: IRL Press; 1987. pp. 179–200. [Google Scholar]
- Vreden SGS, Sauerwein RW, Verhave JP, Van Rooijen N, Meuwissen JHET, Broek MF. Kupffer cell elimination enhances development of liver schizonts of Plasmodium berghei in rats. Infect Immun. 1993;61:1936–1939. doi: 10.1128/iai.61.5.1936-1939.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreden SGS. The role of Kupffer cells in the clearance of malaria sporozoites from the circulation. Parasitol Today. 1994;10:304–308. doi: 10.1016/0169-4758(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Warburg A, Touray M, Krettli AU, Miller LH. Plasmodium gallinaceum: antibodies to circumsporozoite protein prevent sporozoites from invading the salivary glands of Aedes aegypti. Exp Parasitol. 1992;75:303–307. doi: 10.1016/0014-4894(92)90215-v. [DOI] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W, Gordon S. Cytokine regulation of the macrophage (Mθ) system studied using the colony stimulating factor-1-deficient op/op mouse. Physiol Rev. 1996;76:927–947. doi: 10.1152/physrev.1996.76.4.927. [DOI] [PubMed] [Google Scholar]
- Williams RB. The efficacy of a mixture of trimethoprim and sulphaquinoxaline against Plasmodium gallinaceum malaria in the domesticated fowl Gallus gallus. Vet Parasitol. 2005a;129:193–207. doi: 10.1016/j.vetpar.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Williams RB. Avian malaria: clinical and chemical pathology of Plasmodium gallinaceum in the domesticated fowl Gallus gallus. Avian Pathol. 2005b;34:29–47. doi: 10.1080/03079450400025430. [DOI] [PubMed] [Google Scholar]
- Wisse E. Observations on the fine structure and peroxidase cytochemistry of normal rat liver Kupffer cells. J Ultrastruct Res. 1974;46:393–426. doi: 10.1016/s0022-5320(74)90064-1. [DOI] [PubMed] [Google Scholar]
- Wisse E, Zanger RB, Charels K, Smissen P, McCuskey RS. The liver sieve: considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall and the space of Disse. Hepatology. 1985;5:683–692. doi: 10.1002/hep.1840050427. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Naito M, Moriyama H, Umezu H, Matsuo H, Kiwada H, Arakawa M. Repopulation of murine Kupffer cells after intravenous administration of liposome-encapsulated dichloromethylene diphosphonate. Am J Pathol. 1996;149:1271–1286. [PMC free article] [PubMed] [Google Scholar]
- Yamashiro S, Bast T. Ultrastructure of livers of broiler chickens fed diets containing rapeseed meal. Res Vet Sci. 1978;25:21–24. [PubMed] [Google Scholar]
- Ying P, Shakibaei M, Patankar MS, Clavijo P, Beavis RC, Clark GF, Frevert U. The malaria circumsporozoite protein: interaction of the conserved regions I and II-plus with heparin-like oligosaccharides in heparan sulfate. Exp Parasitol. 1997;5:168–182. doi: 10.1006/expr.1996.4134. [DOI] [PubMed] [Google Scholar]
- Yuda M, Ishino T. Liver invasion by malarial parasites - how do malarial parasites break through the host barrier? Cell Microbiol. 2004;6:1119–1125. doi: 10.1111/j.1462-5822.2004.00474.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary files Supplementary Movie 1. Plasmodium gallinaceum-infected brain tissue. The focus series through a three-dimensional stack from the brain of a P. gallinaceum-infected chicken shows several infected vascular endothelia. Each of the exoerythrocytic forms is multi-nucleated. The parasites were labeled with anti-Pb in combination with GAR-FITC (green); the tissue was counter-stained with Evans blue (red).