Introduction
Human lungs move around 14,000 liters of air every day. Thus significant amounts of organic and inorganic particulates and microbes inhaled from the environment and aspirated from the posterior pharynx can reach the 150 square meters of alveolar surface. The integrity of the thin alveolar membrane is essential to assure oxygen and CO2 gas exchange; therefore the recognition and handling of these particulates without causing excessive inflammation are extremely important. Specialized lung innate immune responses play a key role in this process. Recognition of particulates is broad and based on use of Pattern Recognition Receptors (PRRs); however, the immune responses following recognition in the lung are unique enabling dampening of pro-inflammation and thereby limiting damage to the alveolar surface. Alveolar macrophages (AMs) and dendritic cells (DC) are the first cellular line of defense in the alveoli and their surfaces are rich in PRRs. Evidence is accumulating that soluble and cell-associated C-type (Ca2+-dependent) lectins play a key role in shaping the innate response in the lung. In addition to the established role for Toll-like receptors (TLRs) in this process, recent evidence indicates that NOD-like proteins (NLR) also play an important role as intracellular sensors that regulate inflammatory responses. Here we will discuss these important cellular and soluble determinants of the lung innate immune response, provide examples of their roles in modifying the host response to specific infectious agents during disease pathogenesis and address potential therapeutic applications.
Surfactant proteins A and D
The alveolar space consists of flat lining cells or type I cells important in gas exchange and type II cells that produce and secrete a mixture of proteins and phospholipids that comprise surfactant. Surfactant proteins B and C have important biological properties that result in lowering surface tension and preventing the alveoli from collapsing. A deficiency of these proteins in premature infants leads to infant respiratory distress syndrome, for which administration of exogenous surfactant is one of the treatments (1). Surfactant proteins A and D (SP-A and SP-D) are secreted collectins that belong to the C-type lectin super family. SP-A and SP-D contain specialized domains, assemble as trimers and form oligomers with specific physicochemical properties. They contain an N-terminal collagen-like region important in the formation of trimers and a carbohydrate recognition domain (CRD) at the C-terminus that is important in the recognition of microorganisms and host determinants. CRDs bind to carbohydrates such as mannose and fucose that are prevalent on the surface of microbial species, but not on the surface of mammalian cells (2) and regulate interactions between microbes and host cellular components.
SP-A and SP-D bind to different types of microbes including Gram positive, and Gram negative bacteria, mycobacteria, yeast and viruses as previously reviewed (3). Models using SP-A knockout mice have shown that there is a decreased clearance of H. influenzae (4), P. aeruginosa (5) and Pneumocystis carinii (6, 7) after tracheal instillation with the concomitant increase in microbial dissemination (8). The binding of these lung collectins with microbes promotes opsonization and growth inhibition. Some microbial surface molecules have been identified as ligands of SP-A and SP-D, among them LPS (9, 10) and lipoarabinomannan (LAM) from M. tuberculosis (11, 12).
SP-A enhances the uptake of microbes through Fc receptors and complement receptor 1 on alveolar macrophages (13, 14). In vitro studies have confirmed that lung collectins increase receptor-mediated uptake of different pathogens. SP-A enhances the phagocytosis of S. aureus, K. pneumoniae, S. pneumoniae and H. influenzae by macrophages (15, 16) (17, 18). There is growing evidence that SP-A and SP-D increase the expression of other phagocytic receptors of macrophages, independent of microbial binding. For example, SP-A and SP-D increase the cell surface localization of the mannose receptor (MR) on macrophages, which enhances M. avium and M. tuberculosis phagocytosis (19, 20). Another example is the increased scavenger receptor A (SR-A) cell surface expression on macrophages mediated by SP-A that enhances the uptake of S. pneumoniae (21). The nature of the macrophage-collectin interactions and mechanisms implicated in the up-regulation of phagocytosis continue to be uncovered (22).
Because uncontrolled inflammation within the lung can be lethal, mechanisms to modulate the inflammatory response are necessary. Such mechanisms are in place to alert the host of the presence of pathogens while avoiding excessive inflammation and compromise of gas exchange. One recently proposed mechanism suggests dual functions for SP-A and SP-D. During basal conditions both SP-A and SP-D bind to the signal-inhibitory protein α (SIRP α) through their CRDs blocking pro-inflammatory mediator production. In contrast, when collectins bind microbes or cell debris, the collagenous tails will interact with calreticulin/CD91 on the cell surface stimulating phagocytosis and pro-inflammatory responses (23). The role of collectins in modulation of inflammation has been demonstrated in SP-A -/- mice which produced more pro-inflammatory cytokines and nitric oxide compared to SP-A +/+ mice after endotracheal instillation of LPS (5, 24). SP-A may interact with TLR2 decreasing the inflammatory response elicited by peptidoglycan (PGN) (25). Finally, SP-A has been shown to down-regulate the oxidative response to agonists in macrophages by decreasing the recruitment of p47phox to the phagosomal membrane (26).
The genes encoding SP-A and SP-D contain several polymorphic sites. SP-A is encoded by two highly polymorphic genes. Some of the single nucleotide polymorphisms (SNPs) occur with relative frequency in the general population (27). SP-A gene polymorphisms are associated with neonatal respiratory distress syndrome (27-32). SP-A and SP-D polymorphisms are also associated with tuberculosis and respiratory syncytial virus infection (RSV) (33-35), and with chronic cavitary pulmonary aspergillosis (36), supporting their role in innate immunity. Recently, a SP-A2 gene polymorphism in the CRD of SP-A has been associated with increased susceptibility and risk of death in patients with meningococcal disease (37).
In conclusion SP-A and SP-D are essential players of the lung innate immune system. They participate in the agglutination and opsonization of microbes and enhance phagocytosis through different mechanisms, while modulating the immune response to prevent excessive inflammation. They may also play roles in innate immunity outside of the lung (38)
Alveolar Macrophages
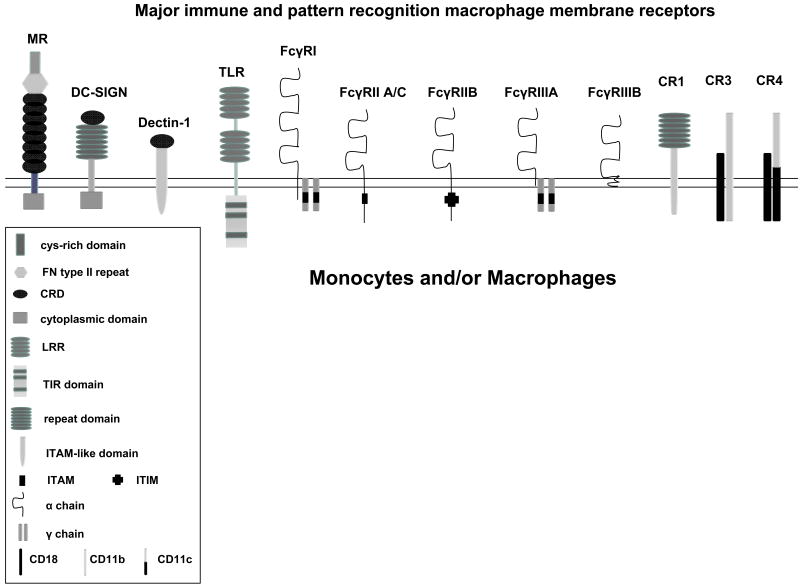
Because of its location at the alveolar air tissue interface, the alveolar macrophage (AM) is the first line of cellular defense against inhaled environmental particles and infectious microorganisms that enter the lungs. These cells express several immune receptors, including Fc-γ receptors and complement receptors (e.g. CR1, CR3 and CR4); and particularly high levels of pattern recognition receptors (PRRs) such as the MR, Dectin-1 (β-glucan receptor), scavenger receptors, Toll-like receptors (TLRs) and NOD like receptors (NLRs) (39-44) (Figure 1). During inflammation, immigrating neutrophils also contribute to the inflammatory response. These cells possess their own array of PRRs and express significant quantities of cationic anti-microbial peptides (45).
Figure 1. Cartoon depiction of major immune and pattern recognition monocyte/macrophage membrane receptors.
These receptors play a major role in the recognition of a variety of pathogenic microbes and have been found to dictate early host responses, such as phagocytosis, trafficking, the oxidative response, pro- and anti-inflammatory cytokines, and antigen presentation. The MR, DC-SIGN and Dectin-1 are calcium-dependent (C-type) lectins. Abbreviations; FN = fibronectin, CRD = carbohydrate recognition domain, cys = cysteine, LRR = Leucine-Rich Repeats, TIR = Toll-IL-1 receptor domain, ITAM = immunoreceptor tyrosine-based activation motif, ITIM = immunoreceptor tyrosine-based inhibitory motif, MR (CD206) = mannose receptor, DC-SIGN (CD209) = dendritic cell-specific ICAM-grabbing non-integrin, TLR = Toll-like receptor, FcγRI (CD64), FcγRIIA,BC (CD32), FcγRIIIA,B (CD16), CR1 (CD35) = complement receptor 1, CR3 (Mac-1, CD11b/CD18) = complement receptor 3, CR4 (CD11c/CD18) = complement receptor 4
Despite constant stimulation by inhaled particles and pathogens, AMs display an anti-inflammatory phenotype described as an “alternative activation” state, which includes altered cytokine responses (e.g. increased IL-10 and TGFβ (40), (46, 47), reduced oxidant production in response to stimuli (48), and reduced microbicidal activity (49). Thus, AMs seem best adapted for removal of small airborne particulates with minimal induction of inflammatory immune responses.
Phagocytosis is considered the primordial function of AMs; the uptake of microorganisms is significantly enhanced by the surface receptors for antibody (IgG subtypes) and complement (most prominently the C3 fragments C3b and C3bi) which serve as opsonins. Once the microbe is engulfed by the AM, the formed phagosome fuses with lysosomes; an important step in the destruction of microorganisms. This process is followed by antigen processing and presentation, and subsequent lymphocyte stimulation with initiation of the acquired immune response. While alternatively activated macrophages may be adequate for the efficient clearance of routinely inhaled extracellular pathogens, they may be inadequate for host-adapted intracellular pathogens. For instance M. tuberculosis is highly adapted to survive in the phagosome, interfering with its normal maturation and avoiding its fusion with the lysosome (50, 51). AM can be stimulated by bacterial products, such as LPS or peptidoglycan, which triggers the translocation of NF-κB with the subsequent transcription of pro-inflammatory cytokines such as TNF-α and IL-8. The importance of TNF-α in lung immunity has been confirmed after episodes of M. tuberculosis reactivation and fungal infections that occur among patients receiving TNF-α blocking agents for certain medical conditions (52).
The Mannose Receptor
The mannose receptor (MR) is a C-type lectin that is expressed on tissue macrophages, AMs and DCs but not monocytes (53-55). The MR is a Type I transmembrane protein with a short cytoplasmic tail and an extracellular domain that shares homology with other C-type lectins. The extracellular domain is a PRR that binds with high affinity to mannose- and fucose-containing glycoconjugates frequently found on the surface of a variety of microbes referred to as pathogen-associated molecular patterns (PAMPs) (56). Macrophages contain large intracellular pools of MR within early endosomes, which undergo continual rapid recycling to the cell surface (57). SP-A increases trafficking of preformed MR to the macrophage cell surface (19). Additionally, the MR can serve as a molecular link between innate and adaptive immune responses (55). For example, the MR mediates loading of mycobacterial LAM onto CD1 molecules for LAM presentation to T cells (58).
The MR also plays a role in the internalization of microbial glycoconjugates by receptor-mediated pinocytosis (57) and different organisms including C. albicans, Pneumocystis carinii and M. tuberculosis by phagocytosis (57, 59-63). MR-mediated phagocytosis can direct the fate of the ingested microorganism. Engagement of the MR on human macrophages by M. tuberculosis mannosylated LAM (ManLAM) and phosphatidyl-myo-inositol mannosides (PIMs) initiates a specific phagocytic pathway that results in limited phagosome-lysosome (P-L) fusion for the bacterium (64, 65). Conversely, although the MR facilitates entry of HIV-1 through gp120, the MR-mediated pathway does not lead to a productive HIV-1 infection possibly by the MR facilitating antigen presentation via CD1b and inducing cell-mediated immunity (66).
DC-SIGN
Dendritic cells are a diverse group of myeloid and lymphoid-origin cells that play a key role in the adaptive immune response. One way these cells can regulate this immune response is through the expression of major pattern recognition receptors (67). The dendritic cell (DC)–specific ICAM-grabbing non-integrin (DC-SIGN, CD209) is a C-type lectin that binds to HIV gp120 (68) (69). Like the MR, DC-SIGN has an extracellular CRD. Its cytoplasmatic domain is important for antigen internalization and signal transduction (70). DC-SIGN is expressed by immature and mature DCs (71) but its presence on macrophages is dependent on the tissue type and state of activation. Like other members of the C-type lectin family, DC-SIGN binds pathogens that express mannose- or fucose-containing glycans such as HIV, M. tuberculosis, C. albicans, H. pylori and Schistosoma mansoni (72, 73). The formation of DC-SIGN tetramers facilitates high-affinity ligand binding (74). DC-SIGN also directs antigens to late endosomes or lysosomes for processing and presentation to T cells (75), thus initiating the adaptive immune response. Some organisms can exploit DC-SIGN internalization to avoid normal pathways of lysosomal degradation. For instance, M. tuberculosis ManLAM binding to DC-SIGN prevents DC maturation and induces the secretion of IL-10, an immunosuppressive cytokine (76). A recent study showed that certain DC-SIGN polymorphisms are associated with a reduced risk of tuberculosis (77).
Dectin-1
This C-type lectin is expressed on macrophages, DCs and neutrophils and is primarily a PRR for fungal β-glucan (74, 78). It contains an extracellular CRD and an intracellular immunoreceptor tyrosine-based activation motif (ITAM) required for interactions with TLR2 (74) and the cytoskeletal changes that occur after Dectin-1 mediated phagocytosis (79). A unique feature of Dectin-1 is that it mediates the production of TNF-α in response to C. albicans and Streptomyces cerevisiae (80). The production of pro-inflammatory cytokines such as IL-12 and TNF-α by macrophages and DCs following stimulation of Dectin-1 requires collaboration with TLR2 (80-82). There is recent evidence that Dectin-1 in cooperation with TLR2 mediates the production of TNF-α by murine macrophages infected with avirulent or attenuated mycobacteria strains but not virulent strains (82).
Toll-like receptors
Toll-like receptors (TLRs) are membrane-associated type I receptors that largely function to recognize PAMPs (83). There are 11 mammalian TLRs which vary in function largely with respect to the ligands that they recognize (84). The externalized amino terminus contains variable arrangements of leucine rich repeats (LRRs) which serve to recognize the PAMPs. The cytosolic carboxy terminus of TLRs is highly homologous to the IL-1 receptor and contains a Toll/IL-1R (TIR) domain that forms the nidus for the assembly of signaling intermediates such as MyD88, IRAK1, IRAK4, IRAKM and Mal/Tiram.
The most extensively studied TLRs are TLR4, which senses the endotoxin of Gram negative organisms (85), and TLR2, which has a particular affinity for Gram positive PAMPs, such as lipoteichoic acid (84, 86). In general, TLR signaling drives NFκB activation via phosphorylation of IκBα.
NOD Proteins
Although surface PRRs such as TLRs are widely recognized regulators of immune responses, 23 cytosolic NOD (nucleotide-binding oligomerization domain) like receptors (NLRs) implicated in the innate recognition of intracellular pathogens have been recently described (87-92). They are composed of a C-terminal series of leucine-rich repeats (LRRs) similar to the extracellular domain of TLRs, a central nucleotide-oligomerization domain (NOD) and an amino-terminal protein-protein interaction domain, such as caspase activation and recruitment domain (CARD), baculovirus inhibitor repeat domains, or pyrin domains. NOD1/CARD4 is a ubiquitous protein that recognizes only microbial components of Gram-negative bacteria (93) while NOD2/CARD15 is restricted to antigen presenting cells and has been implicated in the recognition of Gram-negative and Gram-positive bacterial cell wall products (92, 94, 95). NOD1 and NOD2 have been found to recognize the peptidoglycan (PGN)-derived peptides γ-d-Glu-meso-diaminopimelic acid (iE-DAP) and muramyl dipeptide (MDP), respectively (94, 96). As a result of the NOD-PGN interaction, the NOD protein undergoes oligomerization leading to activation of NF-κB and release of pro-inflammatory cytokines (97, 98). This pro-inflammatory response is enhanced by simultaneous stimulation of TLR3, TLR4 and TLR9 (99-101). Recent articles showed that the NOD protein Ipaf/CARD12 senses bacterial flagellin independent of TLR5, while cryopyrin/NALP3 senses bacterial RNA. Upon recognition of their ligands, Ipaf and cryopyrin mediate caspase-1 activation and IL-1β release (102, 103).
The importance of NLRs in the recognition of pathogens intracellularly has been demonstrated in several microbial models (91, 104-110). The etiology of Crohn's disease (a granulomatous disease of the bowel) has been hotly debated for many years, with some researchers advocating M. paratuberculosis as the etiologic agent (111). In recent years the discovery that NOD2 gene mutations are the most common mutations associated with Crohn's disease (112) has spurred the debate again regarding mycobacteria as potential etiologic agents.
Mannose binding lectin (MBL)
The mannose binding lectin (MBL) is a soluble collectin present in serum (113). Like other collectins, it serves as a PRR for microorganisms. There is increasing evidence that polymorphisms in the MBL are associated with different types of infections such as HIV, cryptosporidiosis, meningococcal disease and tuberculosis (114-117).
Examples of lung innate immune responses to infectious agents
M. tuberculosis
M. tuberculosis is an intracellular pathogen of mononuclear phagocytes and highly adapted to the human host. This bacterium enters macrophages by the phagocytic process using a defined subset of receptors, and subsequently multiplies within a unique phagosomal compartment. SP-A and SP-D regulate the early interaction between M. tuberculosis and macrophages. SP-A increases the phagocytosis of M. tuberculosis through a direct interaction of the protein with macrophages (118), which up-regulates MR activity (19). In contrast, SP-D has been shown to decrease M. tuberculosis phagocytosis by macrophages by binding with high avidity to the mannose caps of ManLAM on the bacilli, thereby reducing the interaction of the bacterium with the MR (12). SP-D-opsonized M. tuberculosis bacilli that are phagocytosed undergo increased phagosome-lysosome (P-L) fusion and have reduced intracellular growth (12, 119). Heterogeneity in the genetic background of patients with tuberculosis can be important in the progression of disease. As one example, different SP-A and SP-D alleles may increase or decrease the host susceptibility to tuberculosis (33).
The presence of mannose on the surface of M. tuberculosis aids in host cell recognition and response. The terminal mannose caps of ManLAM bind to the macrophage MR (120, 121). ManLAMs from different M. tuberculosis strains vary in the degree to which they bind to the MR pointing to a potential relationship between the length and/or presentation of the mannose caps and their avidity for the MR (121). ManLAM caps also bind to DC-SIGN on DCs (122, 123). Recent studies show that the MR and DC-SIGN regulate phagosome trafficking differently. As noted earlier, MR, but not DC-SIGN-mediated phagocytosis of M. tuberculosis, is associated with decreased P-L fusion (64). Since macrophages express high MR and low DC-SIGN, it is speculated that this difference may explain why macrophages serve as the major intracellular niche for M. tuberculosis.
Lipomannan and the 19kDa glycolipoprotein from M. tuberculosis have been shown to induce apoptosis through TLR2 (124, 125). In vivo studies showed that TLR4 deficient mice had reduced bacterial clearance and develop a chronic pneumonia (126). Although there is conflicting evidence about the role of TLR2 in M. tuberculosis-infected mice, TLR2−/− mice had reduced bacterial clearance, a defective granulomatous response and developed chronic pneumonia (127). There is additional evidence that after stimulation with sonicated M. tuberculosis, TLR2 and TLR4 deficient cells produce less TNF-α, however TNF-α production is not completely abolished suggesting the presence of other pathways important in producing pro-inflammatory cytokines (110). Recent evidence supports the importance of NOD2 as an intracellular sensor for M. tuberculosis; a synergistic effect in TNF-α production was observed when TLR2 and NOD2 were simultaneously stimulated by the 19kDa glycoprotein and M. tuberculosis, respectively (110). The effects of the C-type lectins in the function of NODs is unknown, however it is possible that similar to the TLRs, they may act in concert with the NODs to regulate the inflammatory response.
Streptococcus pneumoniae
S. pneumoniae is the principal cause of bacterial pneumonia in the general population. Evidence supports the importance of surfactant in the lung innate immune response to S. pneumoniae. SP-A augments scavenger receptor A (SR-A)-mediated phagocytosis of the bacteria by murine AMs (21). SP-D has been shown to bind to and aggregate three serotypes of S. pneumoniae enhancing their uptake by neutrophils (17). As discussed above, SP-A and SP-D bind carbohydrate structures present on the pneumococcal surface through their CRDs (21, 128). Experiments using SP-D deficient mice showed enhanced colonization and infection of the upper and lower respiratory tract by the pneumococci and an earlier onset and longer persistence of bacteremia (129).
Evidence supports the importance of TLR2 and TLR4 in pneumococcal infection (130-132). The recognition of pneumococci cell wall products by TLRs may cause the up-regulation of NODs, as demonstrated by the increased levels of NOD1 and NOD2 6 hours after infection in mice. In the same study the authors showed that NOD2 but not NOD1 mediated NF-kB activation after exposure to inactivated pneumococci (109).
Pseudomonas aeruginosa
Among immunocompromised patients, patients on mechanical ventilation or those with cystic fibrosis, P. aeruginosa is a major cause of pneumonia. In SP-D -/- mice there is a decrease in bacterial phagocytosis by AMs, that is partially restored after the addition of exogenous recombinant SP-D (133). Studies have shown that SP-A -/- mice have decreased clearance of bacteria after intratracheal instillation (4, 5, 133).
Different cell wall components of P. aeruginosa such as peptidoglycan, LPS, flagellin and CpG DNA, are recognized by TLR2, TLR4, TLR5 and TLR9 (134-137). Although important in P. aeruginosa infection, TLRs are not the only system implicated in the genesis of a pro-inflammatory response. In the absence of TLR2-, TLR4- and TLR5-mediated signaling there is still a significant production of inflammatory cytokines and unimpaired bacterial clearance (137). In this regard, after infection of epithelial cells deficient in TLRs, NOD1 was able to induce NF-kB activation; the same study showed that in mice, the activation of NOD1 was necessary for the production of KC, a CXC chemokine important in the recruitment of neutrophils (138).
Future Directions and Therapeutic Options
For more than 25 years surfactant therapy has been successfully used in neonates with respiratory distress syndrome with the purpose of facilitating alveolar gas interchange. Surfactant therapy has also been used in adults with acute respiratory distress syndrome (ARDS) without benefit (139). However, surfactant replacement therapy has been based on the biomechanical properties of surfactant rather than its biological properties, and neither SP-A nor SP-D are components of artificial surfactant. Since the levels of these collectins are decreased in certain diseases such as ARDS and severe bacterial and viral pneumonias (140, 141), replacement therapy may be useful in these clinical settings. In one study, the use of recombinant human SP-D was associated with the down-regulation of allergic hypersensitivity in mice sensitized to allergens of Aspergillus fumigatus (142). Because of their immunomodulatory and antimicrobial properties, aerosolized SP-A and SP-D may be useful as adjuncts to conventional treatment in patients with selected lung disorders.
Although TLRs play an important role in increasing the pro-inflammatory response to infectious agents, they are not essential as recently demonstrated (143). Thus other surface receptors including C-type lectins may be important in this process. Targeting antigens to C-type lectin surface receptors to enhance the cellular immune response against pathogens deserves some attention. For example, as reviewed above, the MR and DC-SIGN are capable of directing antigen presentation, thus serving as links between innate and adaptive immunity, a property than can be exploited in the development of more effective vaccines. For example, directing mannosylated proteins from Cryptoccocus neoformans into APCs through the MR is necessary for an efficient T cell response (144). The MR can also be used as a target for delivering drugs to macrophages; this approach has been explored to deliver antibiotics into the cytosol (145). On the other hand, since MR-mediated phagocytosis of M. tuberculosis favors the survival of bacilli by inhibition of P-L fusion, therapeutic blockade of this pathway may theoretically decrease the survival of M. tuberculosis in the macrophage. Another potential vaccine strategy is to develop agonists that target NODs, thereby increasing the immunogenicity of vaccine antigens. New antibiotics that target bacterial cell wall PGN would potentially enhance the production of MDP and DAP which would be expected to activate the NODs, thus bolstering the cellular immune response during therapy.
In conclusion, a better understanding of the soluble and cellular determinants underlying lung immune mechanisms will allow for the development of better diagnostic and screening tests, therapies and vaccines. Development of agents to these targets will represent a fundamentally new way to treat lung infections.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mason RJ, Greene K, Voelker DR. Surfactant protein A and surfactant protein D in health and disease. Am J Physiol. 1998;275:L1–13. doi: 10.1152/ajplung.1998.275.1.L1. [DOI] [PubMed] [Google Scholar]
- 2.Kuhlman M, Joiner K, Ezekowitz RA. The human mannose-binding protein functions as an opsonin. J Exp Med. 1989;169:1733–1745. doi: 10.1084/jem.169.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawson PR, Reid KB. The roles of surfactant proteins A and D in innate immunity. Immunol Rev. 2000;173:66–78. doi: 10.1034/j.1600-065x.2000.917308.x. [DOI] [PubMed] [Google Scholar]
- 4.LeVine AM, Whitsett JA, Gwozdz JA, Richardson TR, Fisher JH, Burhans MS, Korfhagen TR. Distinct effects of surfactant protein A or D deficiency during bacterial infection on the lung. J Immunol. 2000;165:3934–3940. doi: 10.4049/jimmunol.165.7.3934. [DOI] [PubMed] [Google Scholar]
- 5.LeVine AM, Kurak KE, Bruno MD, Stark JM, Whitsett JA, Korfhagen TR. Surfactant protein-A-deficient mice are susceptible to Pseudomonas aeruginosa infection. Am J Respir Cell Mol Biol. 1998;19:700–708. doi: 10.1165/ajrcmb.19.4.3254. [DOI] [PubMed] [Google Scholar]
- 6.Linke M, Ashbaugh A, Koch J, Tanaka R, Walzer P. Surfactant protein A limits Pneumocystis murina infection in immunosuppressed C3H/HeN mice and modulates host response during infection. Microbes Infect. 2005;7:748–759. doi: 10.1016/j.micinf.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Linke MJ, Harris CE, Korfhagen TR, McCormack FX, Ashbaugh AD, Steele P, Whitsett JA, Walzer PD. Immunosuppressed surfactant protein A-deficient mice have increased susceptibility to Pneumocystis carinii infection. J Infect Dis. 2001;183:943–952. doi: 10.1086/319252. [DOI] [PubMed] [Google Scholar]
- 8.Sano H, Kuroki Y. The lung collectins, SP-A and SP-D, modulate pulmonary innate immunity. Mol Immunol. 2005;42:279–287. doi: 10.1016/j.molimm.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Van Iwaarden JF, Pikaar JC, Storm J, Brouwer E, Verhoef J, Oosting RS, van Golde LM, van Strijp JA. Binding of surfactant protein A to the lipid A moiety of bacterial lipopolysaccharides. Biochem J. 1994;303(Pt 2):407–411. doi: 10.1042/bj3030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuan SF, Rust K, Crouch E. Interactions of surfactant protein D with bacterial lipopolysaccharides. Surfactant protein D is an Escherichia coli-binding protein in bronchoalveolar lavage. J Clin Invest. 1992;90:97–106. doi: 10.1172/JCI115861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sidobre S, Nigou J, Puzo G, Riviere M. Lipoglycans are putative ligands for the human pulmonary surfactant protein A attachment to mycobacteria. Critical role of the lipids for lectin-carbohydrate recognition. J Biol Chem. 2000;275:2415–2422. doi: 10.1074/jbc.275.4.2415. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson JS, Voelker DR, McCormack FX, Schlesinger LS. Surfactant protein D binds to Mycobacterium tuberculosis bacilli and lipoarabinomannan via carbohydrate-lectin interactions resulting in reduced phagocytosis of the bacteria by macrophages. J Immunol. 1999;163:312–321. [PubMed] [Google Scholar]
- 13.Tenner AJ, Robinson SL, Borchelt J, Wright JR. Human pulmonary surfactant protein (SP-A), a protein structurally homologous to C1q, can enhance FcR- and CR1-mediated phagocytosis. J Biol Chem. 1989;264:13923–13928. [PubMed] [Google Scholar]
- 14.van Iwaarden F, Welmers B, Verhoef J, Haagsman HP, van Golde LM. Pulmonary surfactant protein A enhances the host-defense mechanism of rat alveolar macrophages. Am J Respir Cell Mol Biol. 1990;2:91–98. doi: 10.1165/ajrcmb/2.1.91. [DOI] [PubMed] [Google Scholar]
- 15.Kabha K, Schmegner J, Keisari Y, Parolis H, Schlepper-Schaeffer J, Ofek I. SP-A enhances phagocytosis of Klebsiella by interaction with capsular polysaccharides and alveolar macrophages. Am J Physiol. 1997;272:L344–352. doi: 10.1152/ajplung.1997.272.2.L344. [DOI] [PubMed] [Google Scholar]
- 16.Geertsma MF, Nibbering PH, Haagsman HP, Daha MR, van Furth R. Binding of surfactant protein A to C1q receptors mediates phagocytosis of Staphylococcus aureus by monocytes. Am J Physiol. 1994;267:L578–584. doi: 10.1152/ajplung.1994.267.5.L578. [DOI] [PubMed] [Google Scholar]
- 17.Hartshorn KL, Crouch E, White MR, Colamussi ML, Kakkanatt A, Tauber B, Shepherd V, Sastry KN. Pulmonary surfactant proteins A and D enhance neutrophil uptake of bacteria. Am J Physiol. 1998;274:L958–969. doi: 10.1152/ajplung.1998.274.6.L958. [DOI] [PubMed] [Google Scholar]
- 18.Tino MJ, Wright JR. Surfactant protein A stimulates phagocytosis of specific pulmonary pathogens by alveolar macrophages. Am J Physiol. 1996;270:L677–688. doi: 10.1152/ajplung.1996.270.4.L677. [DOI] [PubMed] [Google Scholar]
- 19.Beharka AA, Gaynor CD, Kang BK, Voelker DR, McCormack FX, Schlesinger LS. Pulmonary surfactant protein A up-regulates activity of the mannose receptor, a pattern recognition receptor expressed on human macrophages. J Immunol. 2002;169:3565–3573. doi: 10.4049/jimmunol.169.7.3565. [DOI] [PubMed] [Google Scholar]
- 20.Kudo K, Sano H, Takahashi H, Kuronuma K, Yokota S, Fujii N, Shimada K, Yano I, Kumazawa Y, Voelker DR, Abe S, Kuroki Y. Pulmonary collectins enhance phagocytosis of Mycobacterium avium through increased activity of mannose receptor. J Immunol. 2004;172:7592–7602. doi: 10.4049/jimmunol.172.12.7592. [DOI] [PubMed] [Google Scholar]
- 21.Kuronuma K, Sano H, Kato K, Kudo K, Hyakushima N, Yokota S, Takahashi H, Fujii N, Suzuki H, Kodama T, Abe S, Kuroki Y. Pulmonary surfactant protein A augments the phagocytosis of Streptococcus pneumoniae by alveolar macrophages through a casein kinase 2-dependent increase of cell surface localization of scavenger receptor A. J Biol Chem. 2004;279:21421–21430. doi: 10.1074/jbc.M312490200. [DOI] [PubMed] [Google Scholar]
- 22.Beharka AA, Crowther JE, McCormack FX, Denning GM, Lees J, Tibesar E, Schlesinger LS. Pulmonary surfactant protein A activates a phosphatidylinositol 3-kinase/calcium signal transduction pathway in human macrophages: participation in the up-regulation of mannose receptor activity. J Immunol. 2005;175:2227–2236. doi: 10.4049/jimmunol.175.4.2227. [DOI] [PubMed] [Google Scholar]
- 23.Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 2003;115:13–23. doi: 10.1016/s0092-8674(03)00758-x. [DOI] [PubMed] [Google Scholar]
- 24.Borron P, McIntosh JC, Korfhagen TR, Whitsett JA, Taylor J, Wright JR. Surfactant-associated protein A inhibits LPS-induced cytokine and nitric oxide production in vivo. Am J Physiol Lung Cell Mol Physiol. 2000;278:L840–847. doi: 10.1152/ajplung.2000.278.4.L840. [DOI] [PubMed] [Google Scholar]
- 25.Murakami S, Iwaki D, Mitsuzawa H, Sano H, Takahashi H, Voelker DR, Akino T, Kuroki Y. Surfactant protein A inhibits peptidoglycan-induced tumor necrosis factor-alpha secretion in U937 cells and alveolar macrophages by direct interaction with toll-like receptor 2. J Biol Chem. 2002;277:6830–6837. doi: 10.1074/jbc.M106671200. [DOI] [PubMed] [Google Scholar]
- 26.Crowther JE, Kutala VK, Kuppusamy P, Ferguson JS, Beharka AA, Zweier JL, McCormack FX, Schlesinger LS. Pulmonary surfactant protein a inhibits macrophage reactive oxygen intermediate production in response to stimuli by reducing NADPH oxidase activity. J Immunol. 2004;172:6866–6874. doi: 10.4049/jimmunol.172.11.6866. [DOI] [PubMed] [Google Scholar]
- 27.Haataja R, Hallman M. Surfactant proteins as genetic determinants of multifactorial pulmonary diseases. Ann Med. 2002;34:324–333. doi: 10.1080/078538902320772089. [DOI] [PubMed] [Google Scholar]
- 28.Floros J, Fan R, Matthews A, DiAngelo S, Luo J, Nielsen H, Dunn M, Gewolb IH, Koppe J, van Sonderen L, Farri-Kostopoulos L, Tzaki M, Ramet M, Merrill J. Family-based transmission disequilibrium test (TDT) and case-control association studies reveal surfactant protein A (SP-A) susceptibility alleles for respiratory distress syndrome (RDS) and possible race differences. Clin Genet. 2001;60:178–187. doi: 10.1034/j.1399-0004.2001.600303.x. [DOI] [PubMed] [Google Scholar]
- 29.Kala P, Ten Have T, Nielsen H, Dunn M, Floros J. Association of pulmonary surfactant protein A (SP-A) gene and respiratory distress syndrome: interaction with SP-B. Pediatr Res. 1998;43:169–177. doi: 10.1203/00006450-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Marttila R, Haataja R, Ramet M, Pokela ML, Tammela O, Hallman M. Surfactant protein A gene locus and respiratory distress syndrome in Finnish premature twin pairs. Ann Med. 2003;35:344–352. doi: 10.1080/07853890310006389. [DOI] [PubMed] [Google Scholar]
- 31.Ramet M, Haataja R, Marttila R, Floros J, Hallman M. Association between the surfactant protein A (SP-A) gene locus and respiratory-distress syndrome in the Finnish population. Am J Hum Genet. 2000;66:1569–1579. doi: 10.1086/302906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramet M, Haataja R, Marttila R, Hamalainen AM, Knip M, Hallman M. Human surfactant protein--A gene locus for genetic studies in the Finnish population. Dis Markers. 2000;16:119–124. doi: 10.1155/2000/914814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Floros J, Lin HM, Garcia A, Salazar MA, Guo X, DiAngelo S, Montano M, Luo J, Pardo A, Selman M. Surfactant protein genetic marker alleles identify a subgroup of tuberculosis in a Mexican population. J Infect Dis. 2000;182:1473–1478. doi: 10.1086/315866. [DOI] [PubMed] [Google Scholar]
- 34.Lahti M, Lofgren J, Marttila R, Renko M, Klaavuniemi T, Haataja R, Ramet M, Hallman M. Surfactant protein D gene polymorphism associated with severe respiratory syncytial virus infection. Pediatr Res. 2002;51:696–699. doi: 10.1203/00006450-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Lofgren J, Ramet M, Renko M, Marttila R, Hallman M. Association between surfactant protein A gene locus and severe respiratory syncytial virus infection in infants. J Infect Dis. 2002;185:283–289. doi: 10.1086/338473. [DOI] [PubMed] [Google Scholar]
- 36.Vaid M, Kaur S, Sambatakou H, Madan T, Denning DW, Sarma PU. Distinct alleles of mannose-binding lectin (MBL) and surfactant proteins A (SP-A) in patients with chronic cavitary pulmonary aspergillosis and allergic bronchopulmonary aspergillosis. Clin Chem Lab Med. 2007;45:183–186. doi: 10.1515/CCLM.2007.033. [DOI] [PubMed] [Google Scholar]
- 37.Jack DL, Cole J, Naylor SC, Borrow R, Kaczmarski EB, Klein NJ, Read RC. Genetic polymorphism of the binding domain of surfactant protein-A2 increases susceptibility to meningococcal disease. Clin Infect Dis. 2006;43:1426–1433. doi: 10.1086/508775. [DOI] [PubMed] [Google Scholar]
- 38.Khubchandani KR, Snyder JM. Surfactant protein A (SP-A): the alveolus and beyond. Faseb J. 2001;15:59–69. doi: 10.1096/fj.00-0318rev. [DOI] [PubMed] [Google Scholar]
- 39.Stephenson JD, Shepherd VL. Purification of the human alveolar macrophage mannose receptor. Biochem Biophys Res Commun. 1987;148:883–889. doi: 10.1016/0006-291x(87)90958-2. [DOI] [PubMed] [Google Scholar]
- 40.Fels AO, Cohn ZA. The alveolar macrophage. J Appl Physiol. 1986;60:353–369. doi: 10.1152/jappl.1986.60.2.353. [DOI] [PubMed] [Google Scholar]
- 41.Taylor PR, Brown GD, Reid DM, Willment JA, Martinez-Pomares L, Gordon S, Wong SY. The beta-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J Immunol. 2002;169:3876–3882. doi: 10.4049/jimmunol.169.7.3876. [DOI] [PubMed] [Google Scholar]
- 42.Palecanda A, Kobzik L. Receptors for unopsonized particles: the role of alveolar macrophage scavenger receptors. Curr Mol Med. 2001;1:589–595. doi: 10.2174/1566524013363384. [DOI] [PubMed] [Google Scholar]
- 43.Means TK, Jones BW, Schromm AB, Shurtleff BA, Smith JA, Keane J, Golenbock DT, Vogel SN, Fenton MJ. Differential effects of a Toll-like receptor antagonist on Mycobacterium tuberculosis-induced macrophage responses. J Immunol. 2001;166:4074–4082. doi: 10.4049/jimmunol.166.6.4074. [DOI] [PubMed] [Google Scholar]
- 44.Srivastava M, Meinders A, Steinwede K, Maus R, Lucke N, Buhling F, Ehlers S, Welte T, Maus UA. Mediator responses of alveolar macrophages and kinetics of mononuclear phagocyte subset recruitment during acute primary and secondary mycobacterial infections in the lungs of mice. Cell Microbiol. 2007;9:738–752. doi: 10.1111/j.1462-5822.2006.00824.x. [DOI] [PubMed] [Google Scholar]
- 45.Pham CT. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol. 2006;6:541–550. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- 46.Lambrecht BN. Alveolar macrophage in the driver's seat. Immunity. 2006;24:366–368. doi: 10.1016/j.immuni.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Takabayshi K, Corr M, Hayashi T, Redecke V, Beck L, Guiney D, Sheppard D, Raz E. Induction of a homeostatic circuit in lung tissue by microbial compounds. Immunity. 2006;24:475–487. doi: 10.1016/j.immuni.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Oren R, Farnham AE, Saito K, Milofsky E, Karnovsky ML. Metabolic patterns in three types of phagocytizing cells. J Cell Biol. 1963;17:487–501. doi: 10.1083/jcb.17.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 50.Armstrong JA, Hart PD. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971;134:713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clemens DL, Horwitz MA. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J Exp Med. 1995;181:257–270. doi: 10.1084/jem.181.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 53.Wileman TE, Lennartz MR, Stahl PD. Identification of the macrophage mannose receptor as a 175-kDa membrane protein. Proc Natl Acad Sci U S A. 1986;83:2501–2505. doi: 10.1073/pnas.83.8.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stahl PD. The macrophage mannose receptor: current status. Am J Respir Cell Mol Biol. 1990;2:317–318. doi: 10.1165/ajrcmb/2.4.317. [DOI] [PubMed] [Google Scholar]
- 55.Stahl PD, Ezekowitz RA. The mannose receptor is a pattern recognition receptor involved in host defense. Curr Opin Immunol. 1998;10:50–55. doi: 10.1016/s0952-7915(98)80031-9. [DOI] [PubMed] [Google Scholar]
- 56.Medzhitov R, Janeway C., Jr Innate immunity. N Engl J Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 57.Stahl P, Schlesinger PH, Sigardson E, Rodman JS, Lee YC. Receptor-mediated pinocytosis of mannose glycoconjugates by macrophages: characterization and evidence for receptor recycling. Cell. 1980;19:207–215. doi: 10.1016/0092-8674(80)90402-x. [DOI] [PubMed] [Google Scholar]
- 58.Prigozy TI, Sieling PA, Clemens D, Stewart PL, Behar SM, Porcelli SA, Brenner MB, Modlin RL, Kronenberg M. The mannose receptor delivers lipoglycan antigens to endosomes for presentation to T cells by CD1b molecules. Immunity. 1997;6:187–197. doi: 10.1016/s1074-7613(00)80425-2. [DOI] [PubMed] [Google Scholar]
- 59.Ezekowitz RA, Sastry K, Bailly P, Warner A. Molecular characterization of the human macrophage mannose receptor: demonstration of multiple carbohydrate recognition-like domains and phagocytosis of yeasts in Cos-1 cells. J Exp Med. 1990;172:1785–1794. doi: 10.1084/jem.172.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ezekowitz RA, Williams DJ, Koziel H, Armstrong MY, Warner A, Richards FF, Rose RM. Uptake of Pneumocystis carinii mediated by the macrophage mannose receptor. Nature. 1991;351:155–158. doi: 10.1038/351155a0. [DOI] [PubMed] [Google Scholar]
- 61.Sung SS, Nelson RS, Silverstein SC. Yeast mannans inhibit binding and phagocytosis of zymosan by mouse peritoneal macrophages. J Cell Biol. 1983;96:160–166. doi: 10.1083/jcb.96.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schlesinger LS. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol. 1993;150:2920–2930. [PubMed] [Google Scholar]
- 63.Kitz DJ, Stahl PD, Little JR. The effect of a mannose binding protein on macrophage interactions with Candida albicans. Cell Mol Biol. 1992;38:407–412. [PubMed] [Google Scholar]
- 64.Kang PB, Azad AK, Torrelles JB, Kaufman TM, Beharka A, Tibesar E, DesJardin LE, Schlesinger LS. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med. 2005;202:987–999. doi: 10.1084/jem.20051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Torrelles JB, Azad AK, Schlesinger LS. Fine discrimination in the recognition of individual species of phosphatidyl-myo-inositol mannosides from Mycobacterium tuberculosis by C-type lectin pattern recognition receptors. J Immunol. 2006;177:1805–1816. doi: 10.4049/jimmunol.177.3.1805. [DOI] [PubMed] [Google Scholar]
- 66.Trujillo JR, Rogers R, Molina RM, Dangond F, McLane MF, Essex M, Brain JD. Noninfectious entry of HIV-1 into peripheral and brain macrophages mediated by the mannose receptor. Proc Natl Acad Sci U S A. 2007;104:5097–5102. doi: 10.1073/pnas.0611263104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu L, KewalRamani VN. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol. 2006;6:859–868. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 69.Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 70.Zhou T, Chen Y, Hao L, Zhang Y. DC-SIGN and immunoregulation. Cell Mol Immunol. 2006;3:279–283. [PubMed] [Google Scholar]
- 71.Bleijs DA, Geijtenbeek TB, Figdor CG, van Kooyk Y. DC-SIGN and LFA-1: a battle for ligand. Trends Immunol. 2001;22:457–463. doi: 10.1016/s1471-4906(01)01974-3. [DOI] [PubMed] [Google Scholar]
- 72.Curtis BM, Scharnowske S, Watson AJ. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc Natl Acad Sci U S A. 1992;89:8356–8360. doi: 10.1073/pnas.89.17.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Appelmelk BJ, van Die I, van Vliet SJ, Vandenbroucke-Grauls CM, Geijtenbeek TB, van Kooyk Y. Cutting edge: carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J Immunol. 2003;170:1635–1639. doi: 10.4049/jimmunol.170.4.1635. [DOI] [PubMed] [Google Scholar]
- 74.McGreal EP, Miller JL, Gordon S. Ligand recognition by antigen-presenting cell C-type lectin receptors. Curr Opin Immunol. 2005;17:18–24. doi: 10.1016/j.coi.2004.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Engering A, Geijtenbeek TB, van Vliet SJ, Wijers M, van Liempt E, Demaurex N, Lanzavecchia A, Fransen J, Figdor CG, Piguet V, van Kooyk Y. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J Immunol. 2002;168:2118–2126. doi: 10.4049/jimmunol.168.5.2118. [DOI] [PubMed] [Google Scholar]
- 76.Geijtenbeek TB, Van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CM, Appelmelk B, Van Kooyk Y. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barreiro LB, Neyrolles O, Babb CL, Tailleux L, Quach H, McElreavey K, Helden PD, Hoal EG, Gicquel B, Quintana-Murci L. Promoter variation in the DC-SIGN-encoding gene CD209 is associated with tuberculosis. PLoS Med. 2006;3:e20. doi: 10.1371/journal.pmed.0030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 79.Herre J, Marshall AS, Caron E, Edwards AD, Williams DL, Schweighoffer E, Tybulewicz V, Reis e Sousa C, Gordon S, Brown GD. Dectin-1 uses novel mechanisms for yeast phagocytosis in macrophages. Blood. 2004;104:4038–4045. doi: 10.1182/blood-2004-03-1140. [DOI] [PubMed] [Google Scholar]
- 80.Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 2003;197:1119–1124. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yadav M, Schorey JS. The beta-glucan receptor dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood. 2006;108:3168–3175. doi: 10.1182/blood-2006-05-024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Basu S, Fenton MJ. Toll-like receptors: function and roles in lung disease. Am J Physiol Lung Cell Mol Physiol. 2004;286:L887–892. doi: 10.1152/ajplung.00323.2003. [DOI] [PubMed] [Google Scholar]
- 84.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 85.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 86.Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 87.Bertin J, Nir WJ, Fischer CM, Tayber OV, Errada PR, Grant JR, Keilty JJ, Gosselin ML, Robison KE, Wong GH, Glucksmann MA, DiStefano PS. Human CARD4 protein is a novel CED-4/Apaf-1 cell death family member that activates NF-kappaB. J Biol Chem. 1999;274:12955–12958. doi: 10.1074/jbc.274.19.12955. [DOI] [PubMed] [Google Scholar]
- 88.Inohara N, Koseki T, del Peso L, Hu Y, Yee C, Chen S, Carrio R, Merino J, Liu D, Ni J, Nunez G. Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-kappaB. J Biol Chem. 1999;274:14560–14567. doi: 10.1074/jbc.274.21.14560. [DOI] [PubMed] [Google Scholar]
- 89.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nunez G, Cho JH. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 90.Inohara N, Ogura Y, Chen FF, Muto A, Nunez G. Human Nod1 confers responsiveness to bacterial lipopolysaccharides. J Biol Chem. 2001;276:2551–2554. doi: 10.1074/jbc.M009728200. [DOI] [PubMed] [Google Scholar]
- 91.Girardin SE, Tournebize R, Mavris M, Page AL, Li X, Stark GR, Bertin J, DiStefano PS, Yaniv M, Sansonetti PJ, Philpott DJ. CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2001;2:736–742. doi: 10.1093/embo-reports/kve155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Inohara, Chamaillard, McDonald C, Nunez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem. 2005;74:355–383. doi: 10.1146/annurev.biochem.74.082803.133347. [DOI] [PubMed] [Google Scholar]
- 93.Girardin SE, Boneca IG, Carneiro LA, Antignac A, Jehanno M, Viala J, Tedin K, Taha MK, Labigne A, Zahringer U, Coyle AJ, DiStefano PS, Bertin J, Sansonetti PJ, Philpott DJ. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science. 2003;300:1584–1587. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 94.Gutierrez O, Pipaon C, Inohara N, Fontalba A, Ogura Y, Prosper F, Nunez G, Fernandez-Luna JL. Induction of Nod2 in myelomonocytic and intestinal epithelial cells via nuclear factor-kappa B activation. J Biol Chem. 2002;277:41701–41705. doi: 10.1074/jbc.M206473200. [DOI] [PubMed] [Google Scholar]
- 95.McDonald C, Inohara N, Nunez G. Peptidoglycan signaling in innate immunity and inflammatory disease. J Biol Chem. 2005;280:20177–20180. doi: 10.1074/jbc.R500001200. [DOI] [PubMed] [Google Scholar]
- 96.Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001;276:4812–4818. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 97.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 98.Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M, Foster SJ, Moran AP, Fernandez-Luna JL, Nunez G. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J Biol Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 99.Fritz JH, Girardin SE, Fitting C, Werts C, Mengin-Lecreulx D, Caroff M, Cavaillon JM, Philpott DJ, Adib-Conquy M. Synergistic stimulation of human monocytes and dendritic cells by Toll-like receptor 4 and NOD1- and NOD2-activating agonists. Eur J Immunol. 2005;35:2459–2470. doi: 10.1002/eji.200526286. [DOI] [PubMed] [Google Scholar]
- 100.Tada H, Aiba S, Shibata K, Ohteki T, Takada H. Synergistic effect of Nod1 and Nod2 agonists with toll-like receptor agonists on human dendritic cells to generate interleukin-12 and T helper type 1 cells. Infect Immun. 2005;73:7967–7976. doi: 10.1128/IAI.73.12.7967-7976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van Heel DA, Ghosh S, Butler M, Hunt K, Foxwell BM, Mengin-Lecreulx D, Playford RJ. Synergistic enhancement of Toll-like receptor responses by NOD1 activation. Eur J Immunol. 2005;35:2471–2476. doi: 10.1002/eji.200526296. [DOI] [PubMed] [Google Scholar]
- 102.Amer A, Franchi L, Kanneganti TD, Body-Malapel M, Ozoren N, Brady G, Meshinchi S, Jagirdar R, Gewirtz A, Akira S, Nunez G. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem. 2006;281:35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- 103.Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, Bertin J, Coyle A, Grant EP, Akira S, Nunez G. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 104.Kim JG, Lee SJ, Kagnoff MF. Nod1 is an essential signal transducer in intestinal epithelial cells infected with bacteria that avoid recognition by toll-like receptors. Infect Immun. 2004;72:1487–1495. doi: 10.1128/IAI.72.3.1487-1495.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marriott I, Rati DM, McCall SH, Tranguch SL. Induction of Nod1 and Nod2 intracellular pattern recognition receptors in murine osteoblasts following bacterial challenge. Infect Immun. 2005;73:2967–2973. doi: 10.1128/IAI.73.5.2967-2973.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Opitz B, Forster S, Hocke AC, Maass M, Schmeck B, Hippenstiel S, Suttorp N, Krull M. Nod1-mediated endothelial cell activation by Chlamydophila pneumoniae. Circ Res. 2005;96:319–326. doi: 10.1161/01.RES.0000155721.83594.2c. [DOI] [PubMed] [Google Scholar]
- 107.Travassos LH, Carneiro LA, Girardin SE, Boneca IG, Lemos R, Bozza MT, Domingues RC, Coyle AJ, Bertin J, Philpott DJ, Plotkowski MC. Nod1 participates in the innate immune response to Pseudomonas aeruginosa. J Biol Chem. 2005 doi: 10.1074/jbc.M501649200. [DOI] [PubMed] [Google Scholar]
- 108.Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE, Moran AP, Athman R, Memet S, Huerre MR, Coyle AJ, DiStefano PS, Sansonetti PJ, Labigne A, Bertin J, Philpott DJ, Ferrero RL. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol. 2004;5:1166–1174. doi: 10.1038/ni1131. [DOI] [PubMed] [Google Scholar]
- 109.Opitz B, Puschel A, Schmeck B, Hocke AC, Rosseau S, Hammerschmidt S, Schumann RR, Suttorp N, Hippenstiel S. Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized Streptococcus pneumoniae. J Biol Chem. 2004;279:36426–36432. doi: 10.1074/jbc.M403861200. [DOI] [PubMed] [Google Scholar]
- 110.Ferwerda G, Girardin SE, Kullberg BJ, Le Bourhis L, de Jong DJ, Langenberg DM, van Crevel R, Adema GJ, Ottenhoff TH, Van der Meer JW, Netea MG. NOD2 and toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog. 2005;1:279–285. doi: 10.1371/journal.ppat.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Prantera C. Mycobacteria and Crohn's disease: The endless story. Dig Liver Dis. 2007;39:452–454. doi: 10.1016/j.dld.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 112.Helio T, Halme L, Lappalainen M, Fodstad H, Paavola-Sakki P, Turunen U, Farkkila M, Krusius T, Kontula K. CARD15/NOD2 gene variants are associated with familially occurring and complicated forms of Crohn's disease. Gut. 2003;52:558–562. doi: 10.1136/gut.52.4.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dommett RM, Klein N, Turner MW. Mannose-binding lectin in innate immunity: past, present and future. Tissue Antigens. 2006;68:193–209. doi: 10.1111/j.1399-0039.2006.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Garred P, Madsen HO, Balslev U, Hofmann B, Pedersen C, Gerstoft J, Svejgaard A. Susceptibility to HIV infection and progression of AIDS in relation to variant alleles of mannose-binding lectin. Lancet. 1997;349:236–240. doi: 10.1016/S0140-6736(96)08440-1. [DOI] [PubMed] [Google Scholar]
- 115.Kirkpatrick BD, Huston CD, Wagner D, Noel F, Rouzier P, Pape JW, Bois G, Larsson CJ, Alston WK, Tenney K, Powden C, O'Neill JP, Sears CL. Serum mannose-binding lectin deficiency is associated with cryptosporidiosis in young Haitian children. Clin Infect Dis. 2006;43:289–294. doi: 10.1086/505396. [DOI] [PubMed] [Google Scholar]
- 116.Bathum L, Hansen H, Teisner B, Koch C, Garred P, Rasmussen K, Wang P. Association between combined properdin and mannose-binding lectin deficiency and infection with Neisseria meningitidis. Mol Immunol. 2006;43:473–479. doi: 10.1016/j.molimm.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 117.Soborg C, Madsen HO, Andersen AB, Lillebaek T, Kok-Jensen A, Garred P. Mannose-binding lectin polymorphisms in clinical tuberculosis. J Infect Dis. 2003;188:777–782. doi: 10.1086/377183. [DOI] [PubMed] [Google Scholar]
- 118.Gaynor CD, McCormack FX, Voelker DR, McGowan SE, Schlesinger LS. Pulmonary surfactant protein A mediates enhanced phagocytosis of Mycobacterium tuberculosis by a direct interaction with human macrophages. J Immunol. 1995;155:5343–5351. [PubMed] [Google Scholar]
- 119.Ferguson JS, Martin JL, Azad AK, McCarthy TR, Kang PB, Voelker DR, Crouch EC, Schlesinger LS. Surfactant protein D increases fusion of Mycobacterium tuberculosis-containing phagosomes with lysosomes in human macrophages. Infect Immun. 2006;74:7005–7009. doi: 10.1128/IAI.01402-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schlesinger LS, Hull SR, Kaufman TM. Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J Immunol. 1994;152:4070–4079. [PubMed] [Google Scholar]
- 121.Schlesinger LS, Kaufman TM, Iyer S, Hull SR, Marchiando LK. Differences in mannose receptor-mediated uptake of lipoarabinomannan from virulent and attenuated strains of Mycobacterium tuberculosis by human macrophages. J Immunol. 1996;157:4568–4575. [PubMed] [Google Scholar]
- 122.Tailleux L, Schwartz O, Herrmann JL, Pivert E, Jackson M, Amara A, Legres L, Dreher D, Nicod LP, Gluckman JC, Lagrange PH, Gicquel B, Neyrolles O. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J Exp Med. 2003;197:121–127. doi: 10.1084/jem.20021468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maeda N, Nigou J, Herrmann JL, Jackson M, Amara A, Lagrange PH, Puzo G, Gicquel B, Neyrolles O. The cell surface receptor DC-SIGN discriminates between Mycobacterium species through selective recognition of the mannose caps on lipoarabinomannan. J Biol Chem. 2003;278:5513–5516. doi: 10.1074/jbc.C200586200. [DOI] [PubMed] [Google Scholar]
- 124.Dao DN, Kremer L, Guerardel Y, Molano A, Jacobs WR, Jr, Porcelli SA, Briken V. Mycobacterium tuberculosis lipomannan induces apoptosis and interleukin-12 production in macrophages. Infect Immun. 2004;72:2067–2074. doi: 10.1128/IAI.72.4.2067-2074.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lopez M, Sly LM, Luu Y, Young D, Cooper H, Reiner NE. The 19-kDa Mycobacterium tuberculosis protein induces macrophage apoptosis through Toll-like receptor-2. J Immunol. 2003;170:2409–2416. doi: 10.4049/jimmunol.170.5.2409. [DOI] [PubMed] [Google Scholar]
- 126.Abel B, Thieblemont N, Quesniaux VJ, Brown N, Mpagi J, Miyake K, Bihl F, Ryffel B. Toll-like receptor 4 expression is required to control chronic Mycobacterium tuberculosis infection in mice. J Immunol. 2002;169:3155–3162. doi: 10.4049/jimmunol.169.6.3155. [DOI] [PubMed] [Google Scholar]
- 127.Drennan MB, Nicolle D, Quesniaux VJ, Jacobs M, Allie N, Mpagi J, Fremond C, Wagner H, Kirschning C, Ryffel B. Toll-like receptor 2-deficient mice succumb to Mycobacterium tuberculosis infection. Am J Pathol. 2004;164:49–57. doi: 10.1016/S0002-9440(10)63095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jounblat R, Kadioglu A, Iannelli F, Pozzi G, Eggleton P, Andrew PW. Binding and agglutination of Streptococcus pneumoniae by human surfactant protein D (SP-D) vary between strains, but SP-D fails to enhance killing by neutrophils. Infect Immun. 2004;72:709–716. doi: 10.1128/IAI.72.2.709-716.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jounblat R, Clark H, Eggleton P, Hawgood S, Andrew PW, Kadioglu A. The role of surfactant protein D in the colonisation of the respiratory tract and onset of bacteraemia during pneumococcal pneumonia. Respir Res. 2005;6:126. doi: 10.1186/1465-9921-6-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Echchannaoui H, Frei K, Schnell C, Leib SL, Zimmerli W, Landmann R. Toll-like receptor 2-deficient mice are highly susceptible to Streptococcus pneumoniae meningitis because of reduced bacterial clearing and enhanced inflammation. J Infect Dis. 2002;186:798–806. doi: 10.1086/342845. [DOI] [PubMed] [Google Scholar]
- 131.Malley R, Henneke P, Morse SC, Cieslewicz MJ, Lipsitch M, Thompson CM, Kurt-Jones E, Paton JC, Wessels MR, Golenbock DT. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc Natl Acad Sci U S A. 2003;100:1966–1971. doi: 10.1073/pnas.0435928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]
- 133.Giannoni E, Sawa T, Allen L, Wiener-Kronish J, Hawgood S. Surfactant proteins A and D enhance pulmonary clearance of Pseudomonas aeruginosa. Am J Respir Cell Mol Biol. 2006;34:704–710. doi: 10.1165/rcmb.2005-0461OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 135.Hajjar AM, Ernst RK, Tsai JH, Wilson CB, Miller SI. Human Toll-like receptor 4 recognizes host-specific LPS modifications. Nat Immunol. 2002;3:354–359. doi: 10.1038/ni777. [DOI] [PubMed] [Google Scholar]
- 136.Adamo R, Sokol S, Soong G, Gomez MI, Prince A. Pseudomonas aeruginosa flagella activate airway epithelial cells through asialoGM1 and toll-like receptor 2 as well as toll-like receptor 5. Am J Respir Cell Mol Biol. 2004;30:627–634. doi: 10.1165/rcmb.2003-0260OC. [DOI] [PubMed] [Google Scholar]
- 137.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 138.Travassos LH, Carneiro LA, Girardin SE, Boneca IG, Lemos R, Bozza MT, Domingues RC, Coyle AJ, Bertin J, Philpott DJ, Plotkowski MC. Nod1 participates in the innate immune response to Pseudomonas aeruginosa. J Biol Chem. 2005;280:36714–36718. doi: 10.1074/jbc.M501649200. [DOI] [PubMed] [Google Scholar]
- 139.Anzueto A, Baughman RP, Guntupalli KK, Weg JG, Wiedemann HP, Raventos AA, Lemaire F, Long W, Zaccardelli DS, Pattishall EN. Aerosolized surfactant in adults with sepsis-induced acute respiratory distress syndrome. Exosurf Acute Respiratory Distress Syndrome Sepsis Study Group. N Engl J Med. 1996;334:1417–1421. doi: 10.1056/NEJM199605303342201. [DOI] [PubMed] [Google Scholar]
- 140.LeVine AM, Lotze A, Stanley S, Stroud C, O'Donnell R, Whitsett J, Pollack MM. Surfactant content in children with inflammatory lung disease. Crit Care Med. 1996;24:1062–1067. doi: 10.1097/00003246-199606000-00029. [DOI] [PubMed] [Google Scholar]
- 141.Schmidt R, Markart P, Ruppert C, Temmesfeld B, Nass R, Lohmeyer J, Seeger W, Gunther A. Pulmonary surfactant in patients with Pneumocystis pneumonia and acquired immunodeficiency syndrome. Crit Care Med. 2006;34:2370–2376. doi: 10.1097/01.CCM.0000234036.19145.52. [DOI] [PubMed] [Google Scholar]
- 142.Strong P, Reid KB, Clark H. Intranasal delivery of a truncated recombinant human SP-D is effective at down-regulating allergic hypersensitivity in mice sensitized to allergens of Aspergillus fumigatus. Clin Exp Immunol. 2002;130:19–24. doi: 10.1046/j.1365-2249.2002.01968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mansour MK, Schlesinger LS, Levitz SM. Optimal T cell responses to Cryptococcus neoformans mannoprotein are dependent on recognition of conjugated carbohydrates by mannose receptors. J Immunol. 2002;168:2872–2879. doi: 10.4049/jimmunol.168.6.2872. [DOI] [PubMed] [Google Scholar]
- 145.Gac S, Coudane J, Boustta M, Domurado M, Vert M. Synthesis, characterisation and in vivo behaviour of a norfloxacin-poly(L-lysine citramide imide) conjugate bearing mannosyl residues. J Drug Target. 2000;7:393–406. [PubMed] [Google Scholar]