Abstract
The complexity of gonadal steroid hormone actions is reflected in their broad and diverse effects on a host of integrated systems including reproductive physiology, sexual behavior, stress responses, immune function, cognition, and neural protection. Understanding the specific contributions of androgens and estrogens in neurons that mediate these important biological processes is central to the study of neuroendocrinology. Of particular interest in recent years has been the biological role of androgen metabolites. The goal of this review is to highlight recent data delineating the specific brain targets for the dihydrotestosterone metabolite, 5α-androstane, 3β, 17β-diol (3β-Diol). Studies using both in vitro and in vivo approaches provide compelling evidence that 3β-Diol is an important modulator of the stress response mediated by the hypothalmo-pituitary-adrenal axis. Further, the actions of 3β-Diol are mediated by estrogen receptors, and not androgen receptors, often through a canonical estrogen response element in the promoter of a given target gene. These novel findings compel us to re-evaluate the interpretation of past studies and the design of future experiments aimed at elucidating the specific effects of androgen receptor signaling pathways.
Keywords: Androgen, 5alpha androstane 3beta, 17beta-diol, dihydrotestosterone, estrogen receptor beta, corticosterone, ACTH, vasopressin, estrogen response element
INTRODUCTION
A recurring theme in the behavioral neuroendocrinology literature is the close relationship and overlapping function between what is classically considered a predominantly “male hormone”, testosterone, and the “female hormone” estradiol. This relationship is based on numerous observations made over the years that testosterone can be converted to estradiol in a number of tissues, including brain (Naftolin et al., 1975), by the P450 enzyme, aromatase. Further, testosterone can also be converted to a more potent androgen, dihydrotestosterone, by the 5-alpha reductase enzyme (Selmanoff et al., 1977). Studies have shown that the metabolism of testosterone to estradiol is often critical for its behavioral actions in males, thus dispensing with the notion that estrogen is a “female hormone”. For example, when administered separately to castrated adult male rats, neither testosterone's aromatized metabolite estradiol, nor its reduced metabolite dihydrotestosterone, are able to restore the entire array of male sexual behaviors (Davidson, 1969, McDonald et al., 1970) to the level of testosterone alone. However, the ability of estrogens and androgens to work in tandem to regulate male behavior was confirmed by investigators who demonstrated that co-treatment of castrated male animals with estradiol and dihydrotestosterone restored all parameters of male sexual behavior comparable to that of animals treated with testosterone alone (Baum and Vreeburg, 1973).
Several brain regions important for controlling male sexual behavior contain aromatase activity (Naftolin, 1975, Selmanoff, 1977, Roselli et al., 1998) providing support for the concept that testosterone can be converted locally to estradiol within selective brain sites. Recent studies using the aromatase null mouse model (ArKO) underscore the importance of brain aromatase and estrogen in male rodent sexual behavior. Gonadally intact male ArKO mice fail to display copulatory behaviors in the presence of an estrous female (Matsumoto et al., 2003), whereas subcutaneous treatment with estradiol restored the display of sexual behaviors to that of wild type mice (Bakker et al., 2004). Such studies implicate estrogen receptors, activated by the estradiol formed from local testosterone aromatization, as important in male behaviors. This does not minimize the importance of androgen in male behaviors, and in most cases, the physiological contribution of androgen receptors (ARs) is examined by administering dihydrotestosterone. This 5α-reduced form of testosterone has been classically considered a prototypical AR agonist with no ability to be aromatized to estrogen-like metabolites. In this review, we will consider recent data that indicates that dihydrotestosterone, in a fashion similar to testosterone, may be converted to products with estrogen-like activity, but by enzymes other than aromatase (Figure 1). These products may subsequently play an important role in regulating non-reproductive functions such as stress reactivity of the male rodent. Such a paradigm shift in our thinking could have important ramifications regarding how we interpret previous studies and design future studies concerning the actions of gonadal steroid hormones and brain function.
Figure 1.
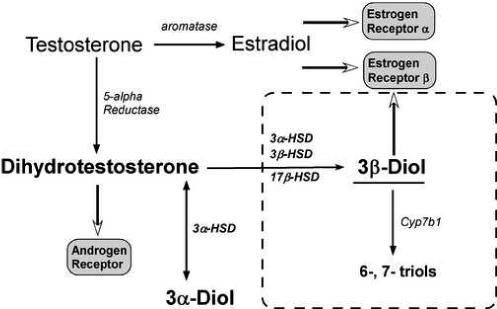
Diagram depicting central dogma concerning the actions of testosterone through metabolism by aromatase to estradiol or 5-alpha reductase to dihydrotestosterone. A novel mechanism of action for dihydrotestosterone is outlined by dotted lines. According to this hypothesis, dihydrotestosterone is converted to the estrogen receptor beta binding molecule, 3β-Diol (5α- androstan-3β, 17β-diol) by the combined actions of the enzymes 3 alpha hydroxysteroid dehydrogenase (3α-HSD), 3beta hydroxysteroid dehydrogenase (3β-HSD) or 17beta hydroxysteroid dehydrogenase (17β-HSD). The actions of 3β-Diol can be reduced by their further metabolism to the inactive compounds, 6-, or 7- triol by the enzyme cyp7b1. The molecule, 3α-Diol undergoes a bidirectional conversion from DHT by 3α-HSD and as such can act as a sink for further production of DHT.
THE HYPOTHALAMO-PITUITARY ADRENAL AXIS
In rodents, adrenal corticosterone secretion is controlled by the activity of a neuroendocrine axis that involves the hypothalamus, the anterior pituitary and the adrenal gland. This hypothalamo-pituitary-adrenal (HPA) axis represents a cascade of neural and humoral signals driven by both the circadian pacemaker as well as the environment. Changing environmental conditions or perceived threats to homeostasis activate the HPA by funneling information through neurons located in the paraventricular n. of the hypothalamus (PVN), a critical brain region that integrates positive and negative inputs. Central to HPA axis regulation are selective neurons in the parvicellular part of the PVN that contain corticotropin-releasing hormone (CRH). The release of CRH to the hypophyseal portal system enhances synthesis and release of adrenocorticotrophic hormone (ACTH) from the anterior pituitary. In turn, ACTH acts on the adrenal cortex to stimulate secretion and cause a rise in plasma corticosterone. Circulating corticosterone subsequently acts at the level of the pituitary, hypothalamus and higher brain areas to limit further hormone secretion (for review, see DeKloet, 1991; Holsboer and Barden, 1996). Other neuropeptides also play neuromodulatory roles in regulating HPA axis function. Of particular importance, vasopressin, another neuropeptide found in PVN neurons, is also an ACTH secretogogue alone (Gillies et al, 1982) and it amplifies the actions of CRH at the level of the anterior pituitary corticotroph (Rivier and Vale, 1983, Schloser et al, 1994).
SEX DIFFERENCES ARE FOUND IN THE HPA AXIS RESPONSE TO STRESS
Sex differences in the ACTH and corticosterone response to a stressor have been consistently reported in the literature (Gaskin and Kitay, 1970; Handa et al., 1994b). However, the mechanisms underlying these differences have only recently begun to be explored in detail. In general, the neuroendocrine response of female rodents to acute stress is greater than that of males. This hyperreactivity of the female stress response is characterized by a greater and prolonged secretion of ACTH and corticosterone suggesting enhanced stimulus as well as a reduced negative feedback (Burgess and Handa, 1992). Consistent with these findings, females also have higher levels of corticosteroid binding globulin (CBG), a liver-derived plasma protein that binds and sequesters corticosterone from its receptor (McCormick et al. 2002). Moreover, evidence that sex steroid hormones can interact with the regulatory elements of the HPA axis comes from studies showing that gonadectomy of both males and females reduces the sex difference and hormone replacement to gonadectomized animals can reinstate the sex difference (Handa et al, 1994a,b). Whereas estradiol treatment appears to enhance, and testosterone treatment inhibits HPA reactivity, the mechanisms by which testosterone and estradiol act to influence HPA function have not been completely resolved. Evidence of estradiol and testosterone acting at the adrenal gland (Kitay 1965), anterior pituitary (Coyne and Kitay, 1969, 1971, Viau and Meaney, 2004) and hypothalamus (Viau and Meaney, 1996; Viau et al., 2003, Handa et al., 1994a) has been reported. Although contributions of each level of the axis likely mediate the sex differences in HPA function, in this review we will focus our attention on the hypothalamic effects of the androgenic component of this regulation.
ANDROGEN REGULATION OF THE NEUROENDOCRINE RESPONSE TO STRESS
Testosterone appears to have an action opposite that of estradiol in regulating the HPA axis (Gaskin and Kitay, 1970; Coyne and Kitay, 1971). Gonadectomy of male rats increases corticosterone and ACTH responses to stress, and correspondingly, c-fos mRNA expression in the PVN is elevated (Handa et al, 1994a, Viau et al., 2003, Lund et al., 2004a). Hormone replacement of gonadectomized rats with either testosterone or the non-aromatizable androgen, dihydrotestosterone returns stress-responsive plasma corticosterone and ACTH levels back to that of the intact male (Lund et al. 2004a). Treatment of gonadectomized animals with dihydrotestosterone also inhibits the stress-induction of cfos mRNA in the PVN (Kerr et al. 1996, Viau et al. 2003; Lund et al., 2004a, Lund et al. 2006.) demonstrating that testosterone's effects are likely not due to the aromatization of testosterone to estradiol. Further evidence that androgen can regulate the HPA axis comes from studies examining the hormonal stress response of male rats before and after puberty. Prior to puberty, when testosterone levels are low, the corticosterone response to acute and chronic stress is high relative to the response seen after puberty (Viau et al. 2005, Romeo et al. 2006) which corresponds to elevations in testosterone that occur during the pubertal transition of males. However, the involvement of testosterone in this mechanism is not absolute since Romeo et al, (2004) have demonstrated that testosterone alone cannot shift the pattern of HPA regulation in pre-pubertal males to that of post-pubertal males.
Although androgens can inhibit HPA axis function (Handa et al., 1994a) and reduce CRH immunoreactivity in the PVN (Bingaman et al., 1994b), androgen receptors are not localized in CRH or AVP neurons within the PVN (Bingaman et al. 1994a). Androgen receptor immunoreactivity (AR-ir) has been found in some PVN neurons, but these AR-ir neurons are in the dorsal cap and the ventral medial parvocellular parts of the PVN, which are non-neuroendocrine neurons that project to spinal cord and brainstem autonomic nuclei (Bingham et al. 2006). Consequently, it has been hypothesized that androgens regulate PVN neuropeptide expression and secretion transsynaptically. Data supporting this hypothesis comes from studies showing that the implantation of testosterone into the medial preoptic area (MPOA) and bed nucleus of the stria terminalis (BnST), brain regions that provide afferent input to the PVN, can reduce the corticosterone response to acute stress (Viau and Meaney, 1996). Further, retrograde tracing studies have shown that AR-ir can be found in neurons of the BnST, but not the septum, that project to the PVN (Suzuki et al, 2001). However, the BnST does not appear to be the only brain site mediating androgen's inhibitory effect on HPA reactivity since stereotaxic application of dihydrotestosterone to a region just above the PVN (to prevent mechanical disruption of the PVN) is as effective as peripherally administered dihydrotestosterone in inhibiting HPA function (Lund et al, 2006). Such data acan be interpreted as indicating that dihydrotestosterone can have a direct action on PVN neurons. However, given that AR are not found in neuroendocrine neurons of the rodent PVN, several other possibilities as to how this might occur must be explored.
In regard to the local action of dihydrotestosterone on PVN neurons to reduce the HPA reactivity to stress; one could argue, based on AR distribution, that the inhibitory effect observed occurs through a multisynaptic pathway that involves activation/inhibition of pathways controlling the autonomic nervous system and the resulting feedback loops that inhibit activity of neurosecretory PVN neurons. An alternative hypothesis is that dihydrotestosterone does not act through the androgen receptors found in PVN neurons, but rather, is activating another type of receptor found in or near the PVN. Although dihydrotestosterone has been historically viewed as a pure AR agonist, recent studies suggest that, like testosterone, dihydrotestosterone can be metabolized to compounds that can bind estrogen receptors, particularly estrogen receptor beta (ERbeta). This possibility is considered in more detail in the ensuing sections of this review.
NEURAL ANDROGEN RECEPTOR and ESTROGEN RECEPTOR DISTRIBUTION RELATIVE TO HPA AXIS FUNCTION
Androgen receptors and estrogen receptors (ER) belong to a superfamily of ligand activated transcription factors that are characterized by their ability to directly alter gene transcription by binding to cognate DNA response elements (Beato, 1989). The classical DNA target for ER is the estrogen response element (ERE) and for AR, is the androgen response element (ARE), however, many hormone responsive genes lack these elements (Massie et al, 2007). Alternate sites in some gene promoters, such as activator protein-1 (AP-1) and SP-1 could serve as targets through which some of these hormone receptors can modify transcription through protein:protein interactions (Webb et al., 1995; Price et al, 2001; Kim et al., 2005).
Using in vivo autoradiographic techniques (Sar and Stumpf, 1977. Stumpf and Sar, 1980; Stumpf, 1971), it has been shown that 3H-estradiol and 3H-dihydrotestosterone are selectively taken up by a number of brain areas including neurons in the PVN. In the PVN there was some 3H-estradioluptake into magnocellular regions, although the amounts were small compared to periventricular and preoptic areas. 3H-estradiol-uptake was also demonstrated in arginine vasopressin (AVP) containing magnocellular neurons (Rhodes et al., 1982). Although for years sensitivity (and in retrospect, specificity) was the limiting factor with such approaches, the recent development of high specific activity ligands demonstrated robust estradiol-uptake by PVN neurons (Shughrue et al., 2000).
Following early reports describing a novel ER, termed ERbeta (Kuiper et al., 1996), ERbeta mRNA was shown to be expressed at high levels by neurons within the PVN (Shugrue et al., 1997) and this localization corresponded with ERbeta-immunoreactivity (-ir; Shughrue and Merchenthaler, 2001; LaFlamme et al, 1998; Suzuki and Handa, 2004). A large percent of ERbeta-ir cells in PVN are AVP positive, but ERbeta is also found in many oxytocin, prolactin (Somponun and Sladek, 2004a, Hrabovsky et al, 1998; Alves et al., 1998; Suzuki and Handa, 2005) and some CRH containing neurons of the PVN (LaFlamme et al., 1998; Miller et al., 2004, Suzuki and Handa, 2005). This suggests that by binding to ERbeta, estradiol could directly alter the function of PVN neuropeptide neurons. In contrast, ERalpha is found at low levels and only in the periventricular PVN and never in CRH, AVP or OXY neurons (Simerly et al, 1991; Estacio et al, 1996; Suzuki and Handa, 2005) thereby ruling out a direct estradiol action mediated through ERalpha, on PVN responses to stress.
Similarly, a number of approaches have been taken to localize AR in neural tissues, including in vivo autoradiography (Sar and Stumpf, 1980), in vitro binding in microdissected brain tissues (Handa et al. 1987), immunohistochemistry (Bingaman et al. 1994a, b; Wood and Newman, 1993 and Zhou et al., 1994), and in situ hybridization (Kerr et al. 1995, Simerly et al., 1991). These studies have all shown that the AR is expressed in brain areas now known to mediate reproductive functions. Moreover, AR has been reported in non-reproductive brain areas such as the hippocampal CA1 region and the parvicellular region of the PVN. AR has also been found in areas that project to the PVN, most notably the BnST and MPOA, two areas providing inhibitory signals to PVN function (Viau and Meaney, 1996). Interestingly, the distribution of AR overlaps considerably with glutamic acid decarboxylase (GAD) in the MPOA and BnST. Since GAD is an enzyme necessary for production of the inhibitory neurotransmitter, gamma-amino butyric acid (GABA), these data suggest this as a potential mechanism for androgenic inhibition of PVN reactivity to stress.
Androgens may also regulate HPA reactivity by direct action at the level of the PVN. Zhou et al (1994) have shown that AR-ir is present in neurons in the parvicellular part of the PVN and that a small population of AR-ir neurons contain AVP, although another study failed to see AR-ir in AVP and CRH neurons (Bingaman et al., 1994a). Alternatively, it is also possible that androgens can work though non-genomic mechanisms to inhibit HPA reactivity. However, few studies to date have explored such rapid membrane associated effects of androgens in any neural function (for review, see Foradori et al., 2008). Nonetheless, the possibility that androgens can work at multiple levels to inhibit the neuroendocrine responses to stress must be considered.
ANDROGEN METABOLISM
The metabolism of steroid hormones in both central and peripheral tissues has been studied for many years. Figure 2 shows the pathway of androgen synthesis and some of the main enzymes involved in the synthesis of testosterone and estradiol from the steroid precursor, cholesterol. In both males and females, testosterone serves, not only as a ligand for AR, but as a precursor for other steroids. It is well established that testosterone can be intracellularly converted in brain tissue to estradiol by the aromatase enzyme (Roselli et al., 1985), or to dihydrotestosterone by the 5-alpha-reductase enzyme (5αR; Lephart et al., 2001). Dihydrotestosterone has historically been used as a potent and selective agonist for androgen receptors since it is not a substrate for aromatization to estradiol. Further, whereas estradiol binds both ERalpha and ERbeta with an affinity in the sub-nanomolar range (Kuiper et al, 1998), it does not bind well to AR (Handa et al 1986). Correspondingly, dihydrotestosterone binds with high affinity to AR but does not bind ER (Handa et al. 1986, Kuiper et al. 1997).
Figure 2.
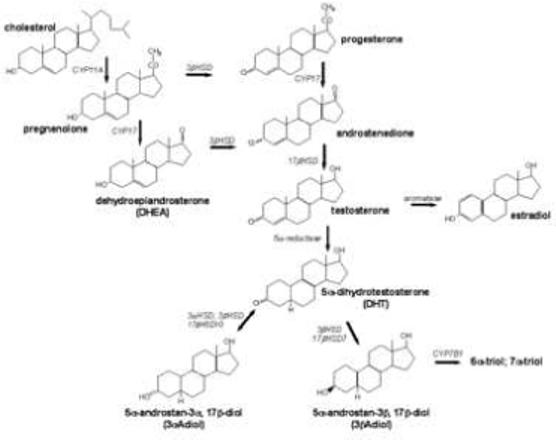
Diagram depicting synthetic pathway for androgens from cholesterol precursor. Bold lettering denotes the common or chemical name of the hormone, italics indicates enzyme required for the conversion of precursor to product. Arrows indicate the direction in which the reaction proceeds.
The selectivity of the steroid metabolizing enzymes is not absolute. For example, dihydrotestostoerne can be further metabolized to 5α -androstane-3α, 17β-diol (3α- Diol) or 5α- androstane-3β, 17β-diol (3β- Diol) by the actions of a number of p450 enzymes including 3α hydroxysteroid dehydrogenase (3α-HSD) , 3β hydroxysteroid dehydrogenase (3β-HSD), and 17β-hydroxysteroid dehydrogenase (Jin and Penning, 2001; Weihua et al., 2002; Gangloff et al. 2003, Torn et al. 2003, Steckelbroeck et al., 2004). Of these enzymes, 5 alpha reductase and 3β-HSD are also involved in the pathways for synthesis and metabolism of other steroids (see figure 2).
Whereas the androgen metabolites, 3α-Diol and 3β-Diol, possess only weak androgen receptor binding activity, they may also initiate responses through other receptor types. For example, 3α-Diol is a neuroactive steroid, and like other 3α tetrahydrosteroids, is a potent allosteric modulator of GABAa receptors. As a result, 3α-Diol has been implicated in the regulation of a number of different behaviors (Rupprecht and Holsboer, 1999, Rosellini et al., 2001, Fernandez-Guasti and Martinez-Mota 2005, Reddy, 2004). In contrast, 3β-Diol cannot bind the benzodiazepine receptor (unpublished) but, if its actions are similar to other 3β-tetrahydrosteroids, it may be an antagonist of 3α-tetrahydrosteroids at the GABAa receptor (Steckelbroeck et al. 2004). Importantly, 3β-Diol has been reported to preferentially bind ERbeta, whereas 3α-Diol has little affinity for ERbeta or ERalpha (Kuiper et al., 1998). Moreover, the conversion of DHT to 3α-Diol is reversible and therefore, 3α-Diol can serve as a sink for subsequent DHT synthesis (Bauman et al. 2006). This is not the case for 3β-Diol where conversion is unidirectional. Ultimately, 3β-Diol is converted to inactive 6α- or 7α-triols by the actions of the enzyme CYP7B1, thereby providing another potential site of regulation for this system (Sundin et al. 1989, Weihua et al. 2002).
The brain contains the necessary steroid metabolizing enzymes that can convert DHT to 3β-Diol (Guennon et al. 1995) and we have hypothesized that in some brain areas the actions of testosterone could be mediated by its 5-α reduction to dihydrotestosterone and its subsequent conversion of dihydrotestosterone to 3β-Diol. The net result is a product that can bind to and activate ERbeta and not AR. This endocrine pathway has been shown to exist in numerous tissues, but its functional significance was first suggested for prostate (Weihua et al., 2002), where it has been proposed that 3β-Diol is the predominant endogenous estrogen. Moreover, our data indicate that the mRNAs for 5α reductase, 3α HSD, 17α HSD and CYP7B1 are present in the PVN of male rats. (Lund et al. 2006). Curiously, 3β-HSD mRNA has not been found in the microdissected PVN (Lund et al., 2006), however, enzyme activity assays indicate that cells within the microdissected PVN are capable of making 3β-Diol from a 3H-DHT precursor in vitro (unpublished data), perhaps implicating other members of the aldo-keto reductase superfamily, such as 3α-HSD or 17β-HSD, as responsible for this conversion (Stoekelbroeck et al., 2004).
3-β-DIOL REGULATION OF THE HPA AXIS
To address the hypothesis that the inhibitory effects of DHT on HPA axis reactivity to stress might be mediated by 3β-Diol, Lund et al. (2004) examined the ability of peripherally administered 3β-Diol to alter stress-responsive CORT and ACTH secretion in castrated adult male mice. Peripheral 3β-Diol treatment was as effective as peripheral DHT administration in reducing CORT and ACTH increases in response to restraint stress (Figure 3). These effects of 3β-Diol could be blocked by co-administration of the ER antagonist, tamoxifen, but not by the AR antagonist, flutamide. Furthermore, the ERbeta agonist, diarylpropionitrile (DPN), was also capable of inhibiting HPA reactivity in a fashion similar to dihydrotestosterone and 3β-Diol. Together, these results provide correlative evidence that 3β-Diol mediates the effects of dihydrotestosterone on corticosterone and ACTH secretion by binding ERbeta.
Figure 3.
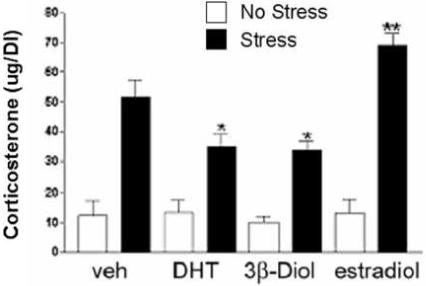
Plasma corticosterone levels in gonadectomized male mice following restraint stress. Animals were treated subcutaneously with oil (veh), dihydrotestosterone propionate (DHT, 1 mg/kg BW) 3β-Diol (1 mg/kg BW) or estradiol benzoate (25 ug/kg BW) daily for 4 days. On the fourth day, animals were restrained for 30 min. All stress groups were significantly increased versus non stressed controls. * indicates those groups with post stress corticosterone levels significantly reduced versus vehicle controls (p<0.05). ** indicates group with post stress corticosterone levels significantly elevated versus vehicle controls (p<0.05). Each bar represents the mean +/− SEM of 8 animals. Figure adapted from Lund et al., 2004.
Further evidence as to the site of 3β-Diol's HPA inhibiting activity was reported recently by Lund et al (2006). By using small pellets of beeswax as a carrier for hormone, they discovered that the stereotaxic application of 3β-Diol to the PVN of castrated male rats mimics the actions of both central and peripherally administered dihydrotestosterone. Furthermore, local application of an ERbeta selective agonist, DPN, could also mimic the actions of dihydrotestosterone. These inhibitory actions of 3β-Diol and DPN can be blocked by the co-administration of the ER antagonist, tamoxifen, whereas the AR antagonist, flutamide has little effect. In contrast, both tamoxifen and flutamide could only partially block the inhibitory actions of local dihydrotestosterone application. Such data suggest the possibility that local synthesis of 3β-Diol by cells in or around the PVN could profoundly impact the function of the HPA reactivity to stressors. Furthermore, these data indicate that compounds that bind ERbeta can act in an inhibitory fashion. This latter point appears to be counter-intuitive to results demonstrating that estradiol treatment of female rats increases their corticosterone response to stress. However it should be considered that estradiol appears to act through ERalpha to augment HPA reactivity. Using the ERalpha selective agonist propylpyrazole triol (PPT) it has been shown that binding to ERalpha has the opposite action of ERbeta agonists and causes increases in HPA reactivity to restraint stress (Lund et al. 2006). The possibility arises that estrogen acts to increase HPA reactivity by binding ERalpha in females and that 3β-Diol works to inhibit HPA reactivity by binding ERbeta in males.
How then, can the HPA axis distinguish the enhancing from inhibiting actions of compounds, such as estradiol, that bind equivalently to both ERalpha and ERbeta? Since it appears that aromatase is present in or near the PVN (Lund et al. 2006, Sanghera et al., 1991) this presents a potential interpretive problem for the HPA of both males (as a result of testosterone aromatization to estradiol) and females. One possibility lies in the ratio of ERalpha to ERbeta that exists within neurons in and around of the PVN. One could argue that a greater ratio of ERalpha to ERbeta could result in a shift towards greater estradiol-induced stimulation and the opposite would be true under conditions where ERbeta was greater than ERalpha. Indeed, it has been demonstrated that levels of receptor might change in response to circulating concentrations of hormones. Although one study showed that stress and adrenalectomy can increase ERbeta mRNA levels in the PVN (Somponpun et al. 2004b) and the effect of adrenalectomy can be partially blocked by corticosterone, another related study has found that adrenalectomy decreases ERbeta mRNA levels and corticosterone prevents this response (Isgor et al., 2003). Furthermore, glucocorticoid receptor stimulation with dexamethasone (DEX) causes increases in ERbeta mRNA and immunoreactive cell numbers without changing ERalpha (Suzuki and Handa, 2004), thus shifting the balance toward inhibition. In contrast, estradiol appears to reduce ERbeta immunoreactivity in neurons around the PVN (Suzuki and Handa, 2004), thereby shifting the balance toward activation
An alternative possibility lies in the observation that transactivation of a gene promoter sequence by ERbeta after binding estradiol, does not completely mimic the activation of the same promoter by 3β-Diol (Pak et al. 2007, see discussion below). Hence, there is the potential for ligand identity in controlling ERbeta's inhibitory actions, and this may be a unique feature for 3β-Diol gene activation which is different from that seen following estradiol binding.
ERbeta protein may be expressed as a number of different splice variants in brain tissues (Petersen et al. 1998; Price et al., 2000), raising the distinct possibility that differential responses to estrogen may be mediated by differing splice variants of ERbeta (for review see Weiser et al., 2007). The originally described estrogen receptor beta is now more appropriately termed ERbeta1 and at least 5 different splice variants of ERbeta have been described. These include a beta2 variant which contains an 18 amino acid insert in the ligand binding domain, a delta3 variant where transcription of exon three is skipped resulting in a protein that does not bind DNA and a delta 4 variant where the fourth exon is skipped resulting in a protein that is largely cytoplasmic in localization (Petersen et al., 1998, Price et al, 2000, 2001). Different combinations of these splice variations can exist within a given receptor protein and differing combinations of receptor variants can exist within a cell thereby altering the way in which an estrogenic signal might be transduced (Weiser et al., 2007). Unfortunately, at present, little is known regarding their interactions with androgen metabolites such as 3β-Diol.
3-β-DIOL REGULATION OF GENE PROMOTERS
To test the hypothesis that 3β-Diol can have direct biological effects in neuronal function, we have used reporter gene assays in neuronal cell lines (Pak et al., 2007; Pak et al., 2005). Initially, we examined whether the signaling pathway of the ligand:receptor complex 3β-Diol:ERbeta was mediated by an ERE or the upstream promoter enhancer element, AP-1. For these studies, the hippocampal-derived neuronal cell line, HT-22, was co-transfected with an expression vector containing ERbeta and a firefly luciferase reporter construct containing an ERE or an AP-1 enhancer site coupled to a minimal promoter. The results showed that 3β-Diol significantly increased ERE-mediated promoter activity to levels greater than that of estradiol (Pak et al., 2005), a surprising result given the significantly lower binding affinity of 3β-Diol for ERbeta compared with estradiol (Ki = 0.1 nM for estradiol and 1.7 nM for 3β-Diol; Pak et al., 2005). Moreover, there was no effect when the reporter was coupled to a promoter containing an AP-1 site, suggesting that 3β-Diol activates a classical, genomic pathway when mediated by ERbeta. Importantly, 3β-Diol had no effect at an AP-1 or ERE site when mediated by ERalpha, or by the ERbeta splice variant, ERbeta2. These responses differ from those elicited by estradiol, whose responses vary depending on the nature of the promoter element placed upstream of the reporter gene. These data also demonstrate a high degree of receptor specificity for 3β-Diol which might be indicative of a highly conserved mechanism for steroid hormone signaling. Further, these results provide support for the concept that dihydrotestosterone could have direct effects on neuronal cell populations that do not express AR provided that the appropriate steroid metabolizing enzymes and ERbeta are present in the target cell.
One potential gene target for ERbeta regulation is arginine vasopressin (Shapiro et al. 2000), an important hormone required for osmoregulation, social, paternal, and aggressive behaviors (Blanchard et al., 2005; Scordalakes and Rissman, 2004; Wang and Aragona, 2004), as well as for the modulation of stress responses through its synergistic action with CRH (Rivier and Vale, 1983, Schloser et al, 1994). Vasopressin containing cells in the PVN and SON contain ERbeta (Suzuki and Handa, 2005; LaFlamme et al, 1998) suggesting a direct regulation of AVP gene expression by ERbeta. To test whether 3β-Diol can have direct effects on the AVP promoter, reporter gene assays were employed. Using the human neuroblastoma-derived cell line SK-N-SH, expression vectors containing full length ERbeta1, or the splice variants ERbeta2 and ERbeta1, delta3, were co-transfected with a firefly luciferase reporter construct containing the full length AVP promoter. The results showed that 3β-Diol increased AVP promoter activity when mediated by ERbeta1 and ERbeta2, but not by ERbeta1,delta3 (Figure 4, Pak et al., 2007). Since it had been previously shown that ERbeta1,delta3 is lacking exon 3 which encodes for the second finger of the DNA binding domain (Petersen et al., 1998), this alteration precludes ERbeta1,delta3 from binding DNA. Such results suggest that receptor:DNA binding is required for 3β-Diol to achieve its effects. Using site-directed mutagenesis we showed that ERbeta1 and ERbeta2 were not binding to a known ERE and were acting at different locations on the AVP promoter (Pak et al., 2007).
Figure 4.
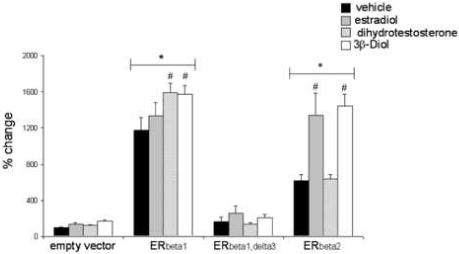
Effects of ERbeta splice variants on AVP promoter activity. Cotransfection of SK-N-SH cells with the full-length AVP promoter (5.5 kb) – luciferase reporter construct and an expression vector containing 1.0 ug/well of ERbeta1, ERbeta1,delta3, or ERbeta2. After transfection, cells were treated with 0.009% ETOH (vehicle), 100 nM estradiol, 100 nM dihydrotestosterone, or 100 nM 3β-Diol for 15 hrs. Data are represented as percent change +/− SEM in relative light units from vehicle treated empty vector controls (p<0.05). * indicates significant difference from empty vector controls. # indicates significant difference among groups (p<0.05). Figure adapted from Pak et al., 2007.
Ligand-bound nuclear receptors interact with a variety of co-regulatory proteins that facilitate gene transcription. Of these, glucocorticoid receptor interacting protein 1 (GRIP1; also called SRC-2, TIF2, and NCoA2) has been shown to be an important regulator of ER signaling (Hong et al., 1996; Norris et al., 1998). Moreover, ERbeta differentially recruits coactivators in response to different ligands (Kraichely et al., 2000). To begin to elucidate some of the downstream molecular participants involved in ERbeta-mediated regulation of the AVP promoter, we used a dominant-negative GRIP1 expression vector (Chang et al., 1999) and functionally depleted endogenous GRIP1 expression in the SK-N-SH cell line. Using this strategy, 3ß-Diol-induced activation of the AVP promoter was completely abolished (Pak et al., 2007). Together, these studies confirm that 3β-Diol utilizes similar signaling pathways as estradiol and emphasizes the redundancy of the system by which gonadal steroid hormones are able to achieve their effects. Nonetheless, the effects of 3β-Diol on gene expression can be vastly different from that observed by estradiol alone.
3β-DIOL REGULATION OF BEHAVIORS
Although 3α-Diol has well known effects on behavior, presumably through its ability to modify GABA signaling (Frye, 2007; Rosellini et al. 2001), behavioral data regarding the physiological effects of 3ß-Diol are limited. In rats, intromissive and ejaculatory behaviors can be fully and partially recovered following exogenous administration of testosterone and its metabolite dihydrotestosterone, respectively (Parrott, 1974, 1975). Although initial reports indicated that mounting and ejaculatory behaviors were not recovered to any extent in castrated rats following 3ß-Diol treatment (Parrott, 1974), it was later shown that 3ß-Diol administered in conjunction with dihydrotestosterone (Baum and Vreeburg, 1976) could restore these behaviors, but to a lesser extent than did testosterone. This may suggest that insufficient dosage or sub-optimal timing of 3β-Diol administration occurred in the initial study. When male copulatory behavior was assessed following castration, it was found that castrates treated with testosterone propionate maintained their ejaculatory ability and that the refractory period between ejaculations remained relatively constant (Parrott, 1975). However, only a small portion of castrated males, treated with 3ß-Diol alone, showed ejaculatory behavior, and of these, the refractory period between ejaculations was greatly increased (Parrott, 1975). It was subsequently found that dihydrotestosterone treatment can, in fact, restore mounting and ejaculatory behaviors in some animals (Paup et al., 1975; Baum and Vreeburg, 1976). These findings were attributed, in part, to the conversion of dihydrotestostorone to 3ß-Diol.
Based on our recent data showing that 3β-Diol treatment can inhibit the hormonal response to stress in rodent models (Lund et al., 2004a, 2006), we investigated the possibility that administration of 3ß-Diol can also elicit a decrease in anxiety-related behaviors in a manner similar to ERbeta agonists. Previous studies have shown that ERbeta agonists such as DPN can prevent anxiety related behaviors in female and male rats (Lund et al., 2005). Because 3β-Diol can selectively bind ERbeta, these results predict that 3ß-Diol may similarly act to decrease anxiety-related behaviors in rats. To test this hypothesis, gonadectomized female rats were tested in the elevated plus maze (EPM) following treatment with DHT, DPN, 3ß-Diol or VEH. The EPM is a standardized test for anxiolytic actions of pharmacological compounds. Substances with anxiety-reducing activity will increase a number of parameters of the EPM including time spent on the open arms of the maze, rearing and other exploratory activity.
Initial findings indicate that 3ß-Diol does indeed decrease anxiety-related behaviors in female rats in a manner similar to DPN (Figure 5). Briefly, adult gonadectomized female rats were treated once daily for 5 days with DHT, DPN, 3ß-Diol or vehicle and tested for anxiety-like behavior using the EPM paradigm. We found that female rats treated with 3ß-Diol spent a significantly greater percentage of time in the open arms and displayed more head dips while on the open arm as compared to all other groups, with the exception of DPN treated females. In addition, both 3ß-Diol- and DPN- treated females exhibited significantly more open arm entries as compared to their dihydrotestosterone and vehicle treated counterparts. These data argue that, similar to the actions of DPN, 3β-Diol can act as an agonist for ERbeta to reduce anxiety related behaviors. Future studies targeted at the downstream mechanisms of ERbeta and incorporating other behavioral endpoints are still required to completely interpret such findings.
Figure 5.
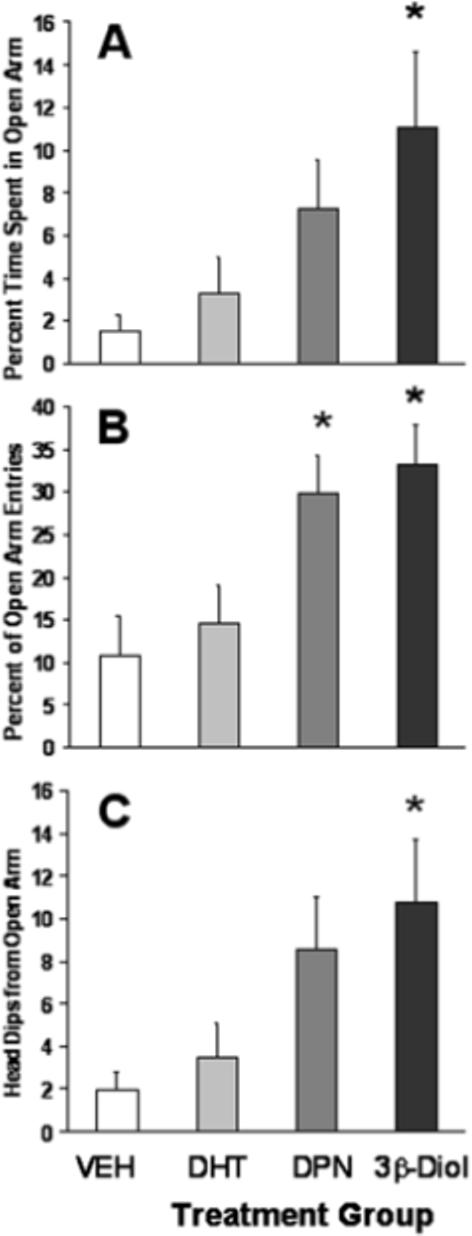
Mean percentage of time (± SEM) spent on the open arms of the elevated plus maze along with the percentage of entries onto the open arm (B) during a 5 minute testing period. Panel C represents the mean number (± SEM) of head dips displayed by animals within each treatment group. One week following ovariectomy, all subjects (n=10/group) received 5 daily s.c. injections of either 5a- androstan-3ß, 17ß-diol (3ß-Diol; 1mg/kg), diarylpropionitrile (DPN; 2mg/kg), dihydrotestosterone (DHT; 1mg/kg) or vehicle (VEH). Behavioral testing commenced 4 hours after the fifth injection. * indicates significant difference from both VEH and DHT groups (p<0.05)
OTHER ROLES FOR 3BETA -DIOL IN BRAIN FUNCTION
Because of its ability to bind and activate ERbeta, 3β-Diol could also be involved in a number of other neural functions. Mouse models where ERbeta has been knocked out have implicated this receptor in controlling behaviors such as anxiety (Osterlund et al., 2005, Krezel et al., 2001) and cognitive function (Fugger et al., 2000) as well as sexual differentiation of the brain (Kudwa et al. 2006). Another interesting possibility is that 3β-Diol could mediate some of the observed effects of androgens on the hypothalamo-pitutiary-gonadal (HPG) axis. Puberty is delayed and fertility is suboptimal in male ERbeta-null mice (Temple et al., 2003), providing some evidence that ERbeta is a potential regulatory component of the reproductive axis. In the brain, gonadotropin-releasing hormone (GnRH) is the primary upstream regulator of reproductive function and it is well accepted that testosterone and estradiol exert tight control over the negative feedback pathways that regulate GnRH synthesis and release. However, since initial efforts aimed at detecting AR and ER in GnRH neurons failed (Huang and Harlan, 1993, Shivers et al., 1983),, many investigators concluded that the effects of steroid hormones on GnRH were mediated indirectly (Herbison et al., 1995; Herbison and Theodosis, 1992; Lehman and Karsch, 1993). However, the identification of ERbeta resurrected this long-standing hypothesis, as several laboratories subsequently showed the co-expression of GnRH and ERβ (Hrabovszky et al., 2000; Hrabovszky et al., 2001; Kallo et al., 2001; Skynner et al., 1999). Nonetheless, evidence for a functional role for ERbeta in regulating GnRH neuronal function in vivo is still forthcoming. Functionally, ERbeta increased GnRH promoter activity in the GnRH-producing cell line, GT1−7 (Pak et al., 2006). Although the effects of ERbeta on the GnRH promoter were largely ligand-independent, this does not preclude the possibility that 3β-Diol could further augment the ligand-independent increase, as this effect has been observed to occur with the AVP promoter (Pak et al., 2007). Moreover, studies using the GT1 cell line have demonstrated their ability to efficiently convert testosterone to dihydrotestosterone and the metabolite 3α-Diol (Poletti et al., 1994) which suggests that GnRH neurons might also express the functional enzymes necessary to convert dihydrotestosterone in situ.
HPA ALTERATIONS IN CLINICAL DISORDERS
Major Depressive Disorder, (MDD) is a major public health concern with substantial economic and social burden (Murray, 1997; Ustun, 2004). During their lifetime, women are up to 2.5 times more likely than men to be diagnosed with MDD (Kessler, 2003; Fava & Kendler 2006). Further, women have a significantly higher heritability of MDD than men and some genes and/or environmental influences associated with MDD may be sex-specific (Kendler et al. 2006; Kendler et al 2001). Clinical studies of depressed patients show gender differences that arise at adolescence as reflected by an increased incidence of MDD in girls and decreased incidence in boys. Studies of prepubertal children show no sex differences in frequency whereas by age 15 there is a female predominance (Angold and Worthman, 1993). Changes in the incidence of depression coincide with the hormonal and physical changes occurring at puberty. However, physical changes of puberty do not necessarily correlate with the onset of depressive state, whereas hormonal changes may (Angold and Worthman, 1993; Brooks-Gunn and Warren, 1989). Thus, these data suggest that hormone sensitivity may be an etiological factor that confers susceptibility to depression and anxiety.
Clinical and preclinical studies provide evidence showing a causal link between the dysregulation of the HPA axis and pathology. Depressed patients have increased ACTH and cortisol secretory responses (Rubin et al, 1987) and elevated cortisol and CRH in CSF (Nemeroff et al. 1984). In addition, 20−50% of depressed patients are dexamethasone (DEX) non-suppressors (e.g. DEX suppresses ACTH and cortisol to a much less extent than healthy controls). Moreover, a combination of DEX suppression and CRH stimulation can distinguish 90% or greater of depressed patients from non-depressed controls (Heuser et al., 1994) as depressed patients require a higher DEX dosage to suppress ACTH and cortisol following CRH infusion. Furthermore, the administration of AVP and DEX to normal patients prior to CRH administration results in a hormone response similar to those following CRH stimulation of DEX-only pretreated depressives (von Bardeleben and Holsboer et al., 1985). Thus, it has been proposed that AVP is elevated in portal blood of depressed patients and that AVP causes HPA hyperactivity during depressive states (von Bardeleban and Holsboer, 1989). Clinical studies have also addressed the hypothesis that AVP is associated with depressive state. Raadsheer et al. (1994) showed that the PVN of depressed patients contains 4 times the number of CRH expressing cells and 3 times the number of CRH neurons that coexpressed AVP as compared to normal controls. Similarly, the number of AVP-ir neurons is increased in the PVN of depressed patients (Purba et al., 1996). Thus, a working model is that reduced glucocorticoid feedback in depressed patients results in an escape of the vasopressinergic system from inhibition and thereby augments HPA activity, particularly following a stressor. A role for ERbeta and 3β-Diol in the regulation of HPA reactivity, may provide another level of complexity to this system, but also provide a potential biological handle for studies examining treatment of such disease states. Unfortunately, at present, no studies have examined a role for 3β-Diol in human affective disorders.
SUMMARY
Increasing data indicate that the potent androgen, dihydrotestosterone, can be metabolized to 3β-Diol, a steroid that can selectively bind ERbeta. Because of its ability to bind ERbeta, 3β-Diol can act to inhibit hormonal responses to stress and stress related behaviors. It remains to be determined whether androgen metabolites that selectively bind ERbeta, but with limited androgenic properties, may be useful pharmacological tools in the treatment of behavioral disorders that involve hyperreactivity of the HPA axis. Nonetheless, increasing data have now demonstrated that dihydrotestosterone's activity may not occur solely through its activation of androgen receptors. Further consideration of potential estrogenic actions of DHT metabolites are likely important for interpretation of a growing literature regarding the non-reproductive actions of sex steroid hormones.
Acknowledgements
These studies were supported by the USPHS grant NS 033917 (RJH).
Abbreviations
- ACTH
adrenocorticotropic hormone
- AP-1
activator protein-1
- ArKO
Aromatase kno ckout mouse
- AR
Androgen Receptor
- AVP
arginine vasopressin
- BnST
Bed nucleus of the stria terminalis
- CBG
corticosteroid binding globulin
- CRH
corticotropin releasing hormone
- CSF
cerebral spinal fluid
- DEX
dexamethasone
- DHT
dihydrotestosterone
- DNA
deoxyribonucleic acid
- DPN
diarylpropionitrile
- EPM
elevated plus maze
- ERbeta
estrogen receptor beta
- ERalpha
estrogen receptor alpha
- ERE
estrogen response element
- GABA
gamma amino butyric acid
- GAD
glutamic acid decarboxylase
- GnRH
gonadotropin releasing hormone
- GRIP-1
glucocorticoid receptor interacting protein 1
- HPA
hypothalamo-pituitary-adrenal
- -ir
immunoreactivity
- MDD
major depressive disorder
- MPOA
medial preoptic area
- OXY
oxytocin
- PPT
propylpyrazoletriol
- PVN
paraventricular nucleus
- 3α-Diol
5alpha androstane 3alpha, 17beta diol
- 3α-HSD
3alpha hydroxysteroid dehydrogenase
- 3β-Diol
5alpha androstane-3beta, 17beta diol
- 3β-HSD
3 beta hydroxysteroid dehydrogenase
- 17β-HSD
17 beta hydroxysteroid dehydrogenase
- 5αR
5 alpha reductase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alves SE, Lopez V, McEwen BS, Weiland NG. Differential colocalization of estrogen receptor beta (ERbeta) with oxytocin and vasopressin in the paraventricular and supraoptic nuclei of the female rat brain: an immunocytochemical study. Proc. Natl. Acad. Sci. USA. 1998;95:3281–3286. doi: 10.1073/pnas.95.6.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A, Worthman CW. Puberty onset of gender differences in rates of depression: a developmental, epidemiologic and neuroendocrine perspective. J Affect Disord. 1993;29(2−3):145–58. doi: 10.1016/0165-0327(93)90029-j. [DOI] [PubMed] [Google Scholar]
- Bakker J, Honda S, Harada N, Balthazart J. Restoration of male sexual behavior by adult exogenous estrogens in male aromatase knockout mice. Horm Behav. 2004;46:1–10. doi: 10.1016/j.yhbeh.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Vreeburg JT. Copulation in castrated male rats following combined treatment with estradiol and dihydrotestosterone. Science. 1973;182:283–5. doi: 10.1126/science.182.4109.283. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Vreeburg JT. Differential effects of the anti-estrogen MER-25 and of three 5α-reduced androgens on mounting and lordosis behavior in the rat. Horm. Behav. 1976;7:87–104. doi: 10.1016/0018-506x(76)90007-6. [DOI] [PubMed] [Google Scholar]
- Bauman DR, Steckelbroeck S, Williams MV, Peehl DM, Penning TM. Identification of the major oxidative 3alpha-hydroxysteroid dehydrogenase in human prostate that converts 5alpha-androstane-3alpha, 17beta-diol to 5alpha-dihydrotestosterone: a potential therapeutic target for androgen-dependent disease. Mol Endocrinol. 2006;20(2):444–58. doi: 10.1210/me.2005-0287. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 1989 Feb 10;56(3):335–44. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Bingaman EW, Magnusson D, Gray TS, Handa RJ. Androgen inhibits the increases in hypothalamic corticotropin releasing hormone (CRH) and CRH immunoreactivity following gonadectomy. Neuroendocrinology. 1994a;59:228–234. doi: 10.1159/000126663. [DOI] [PubMed] [Google Scholar]
- Bingaman EW, Baeckman LM, Yracheta JM, Handa RJ, Gray TS. Localization of androgen receptor immunoreactivity within peptidergic neurons of rat brain. Brain Res. Bull. 1994b;35:379–382. doi: 10.1016/0361-9230(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Bingham B, Williamson M, Viau V. Androgen and estrogen receptor-beta distribution within spinal-projecting and neurosecretory neurons in the paraventricular nucleus of the male rat. J Comp Neurol. 2006;499(6):911–23. doi: 10.1002/cne.21151. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Griebel G, Farrokhi C, Markham C, Yang M, Blanchard DC. AVP V1b selective antagonist SSR149415 blocks aggressive behaviors in hamsters. Pharmacol Biochem Behav. 2005;80:189–194. doi: 10.1016/j.pbb.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J, Warren MP. Biological and social contributions to negative affect in young adolescent girls. Child Dev. 1989;60(1):40–55. doi: 10.1111/j.1467-8624.1989.tb02693.x. [DOI] [PubMed] [Google Scholar]
- Burgess LH, Handa RJ. Estrogen alters adrenocorticotropic hormone and corticosterone secretion and glucocorticoid receptor mediated function. Endocrinology. 1992;131:1261–1269. doi: 10.1210/endo.131.3.1324155. [DOI] [PubMed] [Google Scholar]
- Chang C, Norris JD, Gron H, Paige LA, Hamilton PT, Kenan DJ, Fowlkes D, McDonnell DP. Dissection of the LXXLL nuclear receptor-coactivator interaction motif using combinatorial peptide libraries: discovery of peptide antagonists of estrogen receptors alpha and beta. Mol Cell Biol. 1999;19:8226–8239. doi: 10.1128/mcb.19.12.8226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne MD, Kitay JI. Effect of ovariectomy on pituitary secretion of ACTH. Endocrinology. 1969;85:1097–1102. doi: 10.1210/endo-85-6-1097. [DOI] [PubMed] [Google Scholar]
- Coyne MD, Kitay JI. Effect of orchiectomy on pituitary secretion of ACTH. Endocrinology. 1971;89:1024–1028. doi: 10.1210/endo-89-4-1024. [DOI] [PubMed] [Google Scholar]
- DeKloet ER. Brain Corticosteroid receptor balance and homeostatic control. Front. Neuroendocrinol. 1991;12:95–164. [Google Scholar]
- Davidson JM. Effects of estrogen on the sexual behavior of male rats. Endocrinology. 1969;84:1365–72. doi: 10.1210/endo-84-6-1365. [DOI] [PubMed] [Google Scholar]
- Estacio MAC, Yamada S, Tsukamura H, Hirunagi K, Maeda K-I. Effect of fasting and immobilization stress on estrogen receptor immunoreactivity in the brain in ovariectomized female rats. Brain Res. 1996;717:55–61. doi: 10.1016/0006-8993(96)00022-4. [DOI] [PubMed] [Google Scholar]
- Fava M, Kendler KS. Major depressive disorder. Neuron. 2000;28:335–341. doi: 10.1016/s0896-6273(00)00112-4. [DOI] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Martinez-Mota L. Anxiolytic-like actions of testosterone in the burying behavior test: role of androgen and GABA-benzodiazepine receptors. Psychoneuroendocrinology. 2005;30(8):762–70. doi: 10.1016/j.psyneuen.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Weiser MJ, Handa RJ. Non-genomic actions of androgens. Frontiers in Neuroendocrinology. 2008 doi: 10.1016/j.yfrne.2007.10.005. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA. Some rewarding effects of androgens may be mediated by actions of its 5alpha-reduced metabolite 3alpha-androstanediol. Pharmacol Biochem Behav. 2007;86(2):354–67. doi: 10.1016/j.pbb.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugger HN, Foster TC, Gustafsson J, Rissman EF. Novel effects of estradiol and estrogen receptor alpha and beta on cognitive function. Brain Res. 2000;883(2):258–64. doi: 10.1016/s0006-8993(00)02993-0. [DOI] [PubMed] [Google Scholar]
- Gangloff A, Shi R, Nahoum V, Lin SX. Pseudo-symmetry of C19 steroids, alternative binding orientations, and multispecificity in human estrogenic 17beta-hydroxysteroid dehydrogenase. FASEB J. 2003;17(2):274–6. doi: 10.1096/fj.02-0397fje. [DOI] [PubMed] [Google Scholar]
- Gaskin JH, Kitay JI. Adrenocortical function in the hamster. Sex differences and effects of gonadal hormones. Endocrinology. 1970;87:779–786. doi: 10.1210/endo-87-4-779. [DOI] [PubMed] [Google Scholar]
- Gillies GE, Linton EA, Lowry PJ. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature. 1982;299:355–357. doi: 10.1038/299355a0. [DOI] [PubMed] [Google Scholar]
- Guennoun R, Fiddes RJ, Gouezou M, Lombes M, Baulieu EE. A key enzyme in the biosynthesis of neurosteroids, 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4-isomerase (3 beta-HSD), is expressed in rat brain. Brain Res Mol Brain Res. 1995;30:287–300. doi: 10.1016/0169-328x(95)00016-l. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Stadelman HL, Resko JA. Effect of estrogen on androgen receptor dynamics in the female rat pituitary. Endocrinology. 1986;121:83–89. doi: 10.1210/endo-121-1-84. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Roselli CE, Horton L, Resko JA. The quantitative distribution of cytosolic androgen receptor in microdissected areas of the male rat brain: effects of estrogen treatment. Endocrinology. 1987b;121:233–240. doi: 10.1210/endo-121-1-233. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Nunley KA, Lorens SA, Louie JP, McGivern RF, Bollnow MR. Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and footshock stressors. Physiol. Behav. 1994a;55:117–124. doi: 10.1016/0031-9384(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm. Behav. 1994b;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Horvath TL, Naftolin F, Leranth C. Distribution of estrogen receptor-immunoreactive cells in monkey hypothalamus: relationship to neurones containing luteinizing hormone-releasing hormone and tyrosine hydroxylase. Neuroendocrinology. 1995;61:1–10. doi: 10.1159/000126810. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Theodosis DT. Localization of oestrogen receptors in preoptic neurons containing neurotensin but not tyrosine hydroxylase, cholecystokinin or luteinizing hormone-releasing hormone in the male and female rat. Neuroscience. 1992;50:283–298. doi: 10.1016/0306-4522(92)90423-y. [DOI] [PubMed] [Google Scholar]
- Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. J Psychiatr Res. 1994;28(4):341–56. doi: 10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Kallo I, Hajszan T, Shughrue PJ, Merchenthaler I, Liposits Z. Expression of estrogen receptor-beta messenger ribonucleic acid in oxytocin and vasopressin neurons of the rat supraoptic and paraventricular nuclei. Endocrinology. 1998;139:2600–2604. doi: 10.1210/endo.139.5.6024. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszan T, Carpenter CD, Liposits Z, Petersen SL. Detection of estrogen receptor-beta messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2000;141:3506–3509. doi: 10.1210/endo.141.9.7788. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Steinhauser A, Barabas K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z. Estrogen receptor-beta immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2001;142:3261–3264. doi: 10.1210/endo.142.7.8176. [DOI] [PubMed] [Google Scholar]
- Hoelsboer F, Barden N. Antidepressants and hypothalamic-pituitary-adrenocortical regulation. Endocr. Rev. 1996;17:187–205. doi: 10.1210/edrv-17-2-187. [DOI] [PubMed] [Google Scholar]
- Hong H, Kohli K, Trivedi A, Johnson DL, Stallcup MR. GRIP1, a novel mouse protein that serves as a transcriptional coactivator in yeast for the hormone binding domains of steroid receptors. Proc Natl Acad Sci U S A. 1996;93:4948–4952. doi: 10.1073/pnas.93.10.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Harlan RE. Absence of androgen receptors in LHRH immunoreactive neurons. Brain Res. 1993;624(1−2):309–11. doi: 10.1016/0006-8993(93)90094-4. [DOI] [PubMed] [Google Scholar]
- Isgor C, Cecchi M, Kabbaj M, Akil H, Watson SJ. Estrogen receptor beta in the paraventricular nucleus of hypothalamus regulates the neuroendocrine response to stress and is regulated by corticosterone. Neuroscience. 2003;121(4):837–45. doi: 10.1016/s0306-4522(03)00561-x. [DOI] [PubMed] [Google Scholar]
- Jin Y, Penning TM. Steroid 5alpha-reductases and 3alpha-hydroxysteroid dehydrogenases: key enzymes in androgen metabolism. Best. Pract. Res. Clin. Endocrinol. Metab. 2001;15(1):79–94. doi: 10.1053/beem.2001.0120. [DOI] [PubMed] [Google Scholar]
- Kallo I, Butler JA, Barkovics-Kallo M, Goubillon ML, Coen CW. Oestrogen receptor beta-immunoreactivity in gonadotropin releasing hormone-expressing neurones: regulation by oestrogen. J Neuroendocrinol. 2001;13:741–748. doi: 10.1046/j.1365-2826.2001.00708.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Prescott CA. Toward a comprehensive developmental model for major depression in men. Am J Psychiatry. 2006;163(1):115–24. doi: 10.1176/appi.ajp.163.1.115. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Neale MC, Prescott CA. Genetic risk factors for major depression in men and women: similar or different heritabilities and same or partly distinct genes? Psychol Med. 2001;31(4):605–16. doi: 10.1017/s0033291701003907. [DOI] [PubMed] [Google Scholar]
- Kerr JE, Allore RJ, Beck SG, Handa RJ. Distribution and hormonal regulation of androgen receptor (AR) and AR messenger ribonucleic acid in the rat hippocampus. Endocrinology. 1995;136(8):3213–21. doi: 10.1210/endo.136.8.7628354. [DOI] [PubMed] [Google Scholar]
- Kerr JE, Beck SE, Handa RJ. Androgens selectively modulate c-fos mRNA induction in the rat hippocampus following novelty. Neuroscience. 1996;74:757–766. doi: 10.1016/0306-4522(96)00219-9. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. National Comorbidity Survey Replication. 2003 The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 289(23):3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kim K, Barhoumi R, Burghardt R, Safe S. Analysis of estrogen receptor alpha-Sp1 interactions in breast cancer cells by fluorescence resonance energy transfer. Mol Endocrinol. 2005;19(4):843–54. doi: 10.1210/me.2004-0326. [DOI] [PubMed] [Google Scholar]
- Kitay JI. Depression of adrenal corticosterone production in oophorectomized rats. Endocrinology. 1965;77(6):1048–52. doi: 10.1210/endo-77-6-1048. [DOI] [PubMed] [Google Scholar]
- Kraichely DM, Sun J, Katzenellenbogen JA, Katzenellenbogen BS. Conformational changes and coactivator recruitment by novel ligands for estrogen receptor-alpha and estrogen receptor-beta: correlations with biological character and distinct differences among SRC coactivator family members. Endocrinology. 2000;141:3534–3545. doi: 10.1210/endo.141.10.7698. [DOI] [PubMed] [Google Scholar]
- Krezel W, Dupont S, Krust A, Chambon P, Chapman PF. Increased anxiety and synaptic plasticity in estrogen receptor beta -deficient mice. Proc Natl Acad Sci U S A. 2005;98(21):12278–82. doi: 10.1073/pnas.221451898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudwa AE, Michopoulos V, Gatewood JD, Rissman EF. Roles of estrogen receptors alpha and beta in differentiation of mouse sexual behavior. Neuroscience. 2006;138(3):921–8. doi: 10.1016/j.neuroscience.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Kuiper GGJM, Enmark e., Pelt-Huikko M, Nilsson S, Gustafsson J-A. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interactions of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138(3):863–70. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S. Expression and neuropeptidergic characterization of estrogen receptors (ERalpha and ERbeta) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol. 1998;36(3):357–78. doi: 10.1002/(sici)1097-4695(19980905)36:3<357::aid-neu5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Karsch FJ. Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and beta-endorphin-immunoreactive neurons contain estrogen receptors? A double-label immunocytochemical study in the Suffolk ewe. Endocrinology. 1993;133:887–895. doi: 10.1210/endo.133.2.8102098. [DOI] [PubMed] [Google Scholar]
- Lephart ED, Lund TD, Horvath TL. Brain androgen and progesterone metabolizing enzymes: biosynthesis, distribution and function. Brain Res. Brain Res Rev. 2001;37:25–37. doi: 10.1016/s0165-0173(01)00111-4. [DOI] [PubMed] [Google Scholar]
- Lund TD, Munson DJ, Haldy ME, Handa RJ. Dihydrotestosterone may inhibit hypothalamo-pituitary-adrenal activity by acting through estrogen receptor in the male mouse. Neurosci Lett. 2004a;365(1):43–7. doi: 10.1016/j.neulet.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Lund TD, Munson DJ, Haldy ME, Handa RJ. Androgen inhibits, while oestrogen enhances, restraint-induced activation of neuropeptide neurones in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2004b;16(3):272–8. doi: 10.1111/j.0953-8194.2004.01167.x. [DOI] [PubMed] [Google Scholar]
- Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146(2):797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci. 2006;26(5):1448–56. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie CE, Adryan B, Barbosa-Morais NL, Lynch AG, Tran MG, Neal DE, Mills IG. New androgen receptor genomic targets show an interaction with the ETS1 transcription factor. EMBO Rep. 2007;8(9):871–8. doi: 10.1038/sj.embor.7401046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Honda S, Harada N. Alteration in sex-specific behaviors in male mice lacking the aromatase gene. Neuroendocrinology. 2003;77:416–24. doi: 10.1159/000071313. [DOI] [PubMed] [Google Scholar]
- McDonald P, Beyer C, Newton F, Brien B, Baker R, Tan HS, Sampson C, Kitching P, Greenhill R, Pritchard D. Failure of 5alpha-dihydrotestosterone to initiate sexual behaviour in the castrated male rat. Nature. 1970;227:964–5. doi: 10.1038/227964a0. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Linkroum W, Sallinen BJ, Miller NW. Peripheral and central sex steroids have differential effects on the HPA axis of male and female rats. Stress. 2002;5(4):235–47. doi: 10.1080/1025389021000061165. [DOI] [PubMed] [Google Scholar]
- Miller WJ, Suzuki S, Miller LK, Handa R, Uht RM. Estrogen receptor (ER)beta isoforms rather than ERalpha regulate corticotropin-releasing hormone promoter activity through an alternate pathway. J Neurosci. 2004;24(47):10628–35. doi: 10.1523/JNEUROSCI.5540-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349:1269–76. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Davies IJ, Reddy VV, Flores F, Petro Z, Kuhn M, White RJ, Takaoka Y, Wolin L. The formation of estrogens by central neuroendocrine tissues. Recent Prog Horm Res. 1975;31:295–319. doi: 10.1016/b978-0-12-571131-9.50012-8. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226(4680):1342–4. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- Norris JD, Fan D, Stallcup MR, McDonnell DP. Enhancement of estrogen receptor transcriptional activity by the coactivator GRIP-1 highlights the role of activation function 2 in determining estrogen receptor pharmacology. J Biol Chem. 1998;273:6679–6688. doi: 10.1074/jbc.273.12.6679. [DOI] [PubMed] [Google Scholar]
- Osterlund MK, Witt MR, Gustafsson JA. Estrogen action in mood and neurodegenerative disorders: estrogenic compounds with selective properties-the next generation of therapeutics. Endocrine. 2005;28(3):235–42. doi: 10.1385/ENDO:28:3:235. [DOI] [PubMed] [Google Scholar]
- Pak TR, Chung WC, Lund TD, Hinds LR, Clay CM, Handa RJ. The androgen metabolite, 5alpha-androstane-3beta, 17beta-diol, is a potent modulator of estrogen receptor-beta1-mediated gene transcription in neuronal cells. Endocrinology. 2005;146:147–155. doi: 10.1210/en.2004-0871. [DOI] [PubMed] [Google Scholar]
- Pak TR, Chung WC, Roberts JL, Handa RJ. Ligand-independent effects of estrogen receptor beta on mouse gonadotropin-releasing hormone promoter activity. Endocrinology. 2006;147(4):1924–31. doi: 10.1210/en.2005-1297. [DOI] [PubMed] [Google Scholar]
- Pak TR, Chung WC, Hinds LR, Handa RJ. Estrogen receptor-beta mediates dihydrotestosterone-induced stimulation of the arginine vasopressin promoter in neuronal cells. Endocrinology. 2007;148(7):3371–82. doi: 10.1210/en.2007-0086. [DOI] [PubMed] [Google Scholar]
- Parrott RF. Proceedings: Studies on the maintenance of sexual behaviour in castrated rats treated with various androgens. J Endocrinol. 1974;61(1):XIX–XX. [PubMed] [Google Scholar]
- Parrott RF. Aromatizable and 5alpha-reduced androgens: differentiation between central and peripheral effects on male rat sexual behavior. Horm Behav. 1975;6(2):99–108. doi: 10.1016/0018-506x(75)90026-4. [DOI] [PubMed] [Google Scholar]
- Paup DC, Mennin SP, Gorski RA. Androgen- and estrogen-induced copulatory behavior and inhibition of luteinizing hormone (LH) secretion in the male rat. Horm Behav. 1975;6(1):35–46. doi: 10.1016/0018-506x(75)90021-5. [DOI] [PubMed] [Google Scholar]
- Petersen DN, Tkalcevic GT, Koza-Taylor PH, Turi TG, Brown TA. Identification of estrogen receptor beta2, a functional variant of estrogen receptor beta expressed in normal rat tissues. Endocrinology. 1998;139:1082–1092. doi: 10.1210/endo.139.3.5840. [DOI] [PubMed] [Google Scholar]
- Poletti A, Melcangi RC, Negri-Cesi P, Maggi R, Martini L. Steroid binding and metabolism in the luteinizing hormone-releasing hormone-producing neuronal cell line GT1−1. Endocrinology. 1994;135:2623–2628. doi: 10.1210/endo.135.6.7988451. [DOI] [PubMed] [Google Scholar]
- Price RH, Jr, Lorenzon N, Handa RJ. Differential expression of estrogen receptor beta splice variants in rat brain: identification and characterization of a novel variant missing exon 4. Brain Res Mol Brain Res. 2000;80(2):260–8. doi: 10.1016/s0169-328x(00)00135-2. [DOI] [PubMed] [Google Scholar]
- Price RH, Jr., Butler CA, Webb PC, Uht RA, Kushner PJ, Handa RJ. A splice variant of Estrogen Receptor Beta missing exon 3 displays altered nuclear localization and capacity for transcriptional activation. Endocrinology. 2001;142(5):2039–2049. doi: 10.1210/endo.142.5.8130. [DOI] [PubMed] [Google Scholar]
- Purba JS, Hoogendijk WJ, Hofman MA, Swaab DF. Increased number of vasopressin and oxytocin expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Arch Gen Psychiatry. 1996;53:137–143. doi: 10.1001/archpsyc.1996.01830020055007. [DOI] [PubMed] [Google Scholar]
- Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60(4):436–44. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Anticonvulsant activity of the testosterone-derived neurosteroid 3alpha-androstanediol. Neuroreport. 2004;15(3):515–8. doi: 10.1097/00001756-200403010-00026. [DOI] [PubMed] [Google Scholar]
- Rhodes CH, Morrell JI, Pfaff DW. Estrogen concentrating neurophysin-containing hypothalamic neurons in the vasopressin-deficient (Brattleboro) rat: a study combining steroid autoradiography and immunocytochemistry. J. Neurosci. 1982;2:1718–1724. doi: 10.1523/JNEUROSCI.02-12-01718.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C, Vale W. Interaction of corticotrophin-releasing factor and arginine vasopressin on adrenocorticotropin secretion in vivo. Endocrinology. 1983;113:939–942. doi: 10.1210/endo-113-3-939. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, Chhua N, McPherson CR, McEwen BS. Testosterone cannot activate an adult-like stress response in prepubertal male rats. Neuroendocrinology. 2004;79(3):125–32. doi: 10.1159/000077270. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006;147(4):1664–74. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Horton LE, Resko JA. Distribution and regulation of aromatase activity in the rat hypothalamus and limbic system. Endocrinology. 1985;117(6):2471–7. doi: 10.1210/endo-117-6-2471. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Abdelgadir SE, Ronnekleiv OK, Klosterman SA. Anatomic distribution and regulation of aromatase gene expression in the rat brain. Biol Reprod. 1998 1998 Jan;58(1):79–87. doi: 10.1095/biolreprod58.1.79. [DOI] [PubMed] [Google Scholar]
- Rosellini RA, Svare BB, Rhodes ME, Frye CA. The testosterone metabolite and neurosteroid 3alpha-androstanediol may mediate the effects of testesterone on conditioned place preference. Brain Res Brain Res Rev. 2001;37(1−3):162–71. doi: 10.1016/s0165-0173(01)00116-3. [DOI] [PubMed] [Google Scholar]
- Rubin RT, Poland RE, Lesser IM, Winston RA, Blodgett AL. Neuroendocrine aspects of primary endogenous depression. I. Cortisol secretory dynamics in patients and matched controls. Arch Gen Psychiatry. 1987;44(4):328–36. doi: 10.1001/archpsyc.1987.01800160032006. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Holsboer R. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 1999;22:410–416. doi: 10.1016/s0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- Sanghera MK, Simpson ER, McPhaul MJ, Kozlowski G, Conley AJ, Lephart ED. Immunocytochemical distribution of aromatase cytochrome P450 in the rat brain using peptide-generated polyclonal antibodies. Endocrinology. 1991;129(6):2834–44. doi: 10.1210/endo-129-6-2834. [DOI] [PubMed] [Google Scholar]
- Sar M, Stumpf WE. Simultaneous localization of 3H estradiol and neurophysin I or arginine vasopressin in hypothalamic neurons demonstrated by a combined technique of dry-mount autoradiography and immunohistochemistry. Neurosci. Lett. 1980;17:179–184. doi: 10.1016/0304-3940(80)90081-6. [DOI] [PubMed] [Google Scholar]
- Schlosser SF, Almeida OF, Patchev VK, Yassouridis A, Elands J. Oxytocin stimulated release of adrenocorticotropin from the rat pituitary is mediated by arginine vasopresin receptors of the V1b type. Endocrinology. 1994;135:2058–2063. doi: 10.1210/endo.135.5.7956927. [DOI] [PubMed] [Google Scholar]
- Scordalakes EM, Rissman EF. Aggression and arginine vasopressin immunoreactivity regulation by androgen receptor and estrogen receptor alpha. Genes Brain Behav. 2004;3:20–26. doi: 10.1111/j.1601-183x.2004.00036.x. [DOI] [PubMed] [Google Scholar]
- Selmanoff MK, Brodkin LD, Weiner RI, Siiteri PK. Aromatization and 5alpha-reduction of androgens in discrete hypothalamic and limbic regions of the male and female rat. Endocrinology. 1977;101(3):841–8. doi: 10.1210/endo-101-3-841. [DOI] [PubMed] [Google Scholar]
- Shapiro RA, Xu C, Dorsa DM. Differential transcriptional regulation of rat vasopressin gene expression by estrogen receptor alpha and beta. Endocrinology. 2000;141(11):4056–64. doi: 10.1210/endo.141.11.7796. [DOI] [PubMed] [Google Scholar]
- Shivers BD, Harlan RE, Morrell JI, Pfaff DW. Absence of oestradiol concentration in cell nuclei of LHRH-immunoreactive neurones. Nature. 1983;304(5924):345–7. doi: 10.1038/304345a0. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. The comparative distribution of estrogen receptor -α and β mRNA in the rat central nervous system. J. Comp. Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Evidence for novel estrogen binding sites in the rat hippocampus. Neuroscience. 2000;99(4):605–12. doi: 10.1016/s0306-4522(00)00242-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. J Comp Neurol. 2001;436(1):64–81. [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA containing cells in the rat brain. J. Comp. Neurol. 1991;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Skynner MJ, Sim JA, Herbison AE. Detection of estrogen receptor alpha and beta messenger ribonucleic acids in adult gonadotropin-releasing hormone neurons. Endocrinology. 1999;140:5195–5201. doi: 10.1210/endo.140.11.7146. [DOI] [PubMed] [Google Scholar]
- Somponpun SJ, Sladek CD. Depletion of oestrogen receptor-beta expression in magnocellular arginine vasopressin neurones by hypovolaemia and dehydration. J Neuroendocrinol. 2004;16(6):544–9. doi: 10.1111/j.1365-2826.2004.01200.x. [DOI] [PubMed] [Google Scholar]
- Somponpun SJ, Holmes MC, Seckl JR, Russell JA. Modulation of oestrogen receptor-beta mRNA expression in rat paraventricular and supraoptic nucleus neurones following adrenal steroid manipulation and hyperosmotic stimulation. J Neuroendocrinol. 2004;16(5):472–82. doi: 10.1111/j.1365-2826.2004.01190.x. [DOI] [PubMed] [Google Scholar]
- Steckelbroeck S, Jin Y, Gopishetty S, Oyesanmi B, Penning TM. Human cytosolic 3alpha-hydroxysteroid dehydrogenases of the aldo-keto reductase superfamily display significant 3beta-hydroxysteroid dehydrogenase activity: implications for steroid hormone metabolism and action. J Biol Chem. 2004;279(11):10784–95. doi: 10.1074/jbc.M313308200. [DOI] [PubMed] [Google Scholar]
- Stumpf WE. Estrogen neurons and estrogen neuron systems in the periventricular brain. Am. J. Anat. 1971;129:207–218. doi: 10.1002/aja.1001290209. [DOI] [PubMed] [Google Scholar]
- Stumpf WE, Sar M. Steroid hormone target sites in the brain: the differential distribution of estrogin, progestin, androgen and glucocorticosteroid. J Steroid Biochem. 1977;7(11−12):1163–70. doi: 10.1016/0022-4731(76)90050-9. [DOI] [PubMed] [Google Scholar]
- Sundin M, Warner M, Haaparanta T, Gustafsson J-Å. Isolation and catalytic activity of cytochrome P-450 from ventral prostate of control rats. J. Biol. Chem. 1987;262:12293–12297. [PubMed] [Google Scholar]
- Suzuki S, Lund TD, Price RH, Jr., Handa RJ. Recent Research Developments in Endocrinology. Vol. 2. Transworld Research Network; 2001. Sex differences in the hypothalamo-pituitary-adrenal axis: Novel roles for androgen and estrogen receptors. pp. 69–86. [Google Scholar]
- Suzuki S, Handa RJ. Regulation of estrogen receptor-beta expression in the female rat hypothalamus: differential effects of dexamethasone and estradiol. Endocrinology. 2004;145(8):3658–7. doi: 10.1210/en.2003-1688. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Handa RJ. Estrogen receptor-beta, but not estrogen receptor-alpha, is expressed in prolactin neurons of the female rat paraventricular and supraoptic nuclei: comparison with other neuropeptides. J Comp Neurol. 2005 2005 Mar 28;484(1):28–42. doi: 10.1002/cne.20457. [DOI] [PubMed] [Google Scholar]
- Temple JL, Scordalakes EM, Bodo C, Gustafsson JA, Rissman EF. Lack of functional estrogen receptor beta gene disrupts pubertal male sexual behavior. Horm Behav. 2003;44:427–434. doi: 10.1016/j.yhbeh.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Torn S, Nokelainen P, Kurkela R, Pulkka A, Menjivar M, Ghosh S, Coca-Prados M, Peltoketo H, Isomaa V, Vihko P. Production, purification, and functional analysis of recombinant human and mouse 17beta-hydroxysteroid dehydrogenase type 7. Biochem Biophys Res Commun. 2003;305(1):37–45. doi: 10.1016/s0006-291x(03)00694-6. [DOI] [PubMed] [Google Scholar]
- Ustun TB, Ayuso-Mateos JL, Chatterji S, Mathers C, Murray CJ. Global burden of depressive disorders in the year 2000. Br J Psychiatry. 2004;184:386–92. doi: 10.1192/bjp.184.5.386. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal response to stress is mediated by the medial preoptic area. J. Neurosci. 1996;16:1866–1876. doi: 10.1523/JNEUROSCI.16-05-01866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V, Lee P, Sampson J, Wu J. A testicular influence on restraint-induced activation of medial parvocellular neurons in the paraventricular nucleus in the male rat. Endocrinology. 2003;144(7):3067–75. doi: 10.1210/en.2003-0064. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. Testosterone -dependent variations in plasma and intrapituitary corticosteroid binding globulin and stress hypothalamic-pituitary-adrenal activity in the male rat. J Endocrinol. 2004;181(2):223–31. doi: 10.1677/joe.0.1810223. [DOI] [PubMed] [Google Scholar]
- Viau V, Bingham B, Davis J, Lee P, Wong M. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology. 2005;146(1):137–46. doi: 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]
- von Bardeleben U, Holsboer F. Human corticotropin releasing hormone: clinical studies in patients with affective disorders, alcoholism, panic disorder and in normal controls. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12(Suppl):S165–87. doi: 10.1016/0278-5846(88)90079-6. [DOI] [PubMed] [Google Scholar]
- Wang Z, Aragona BJ. Neurochemical regulation of pair bonding in male prairie voles. Physiol Behav. 2004;83:319–328. doi: 10.1016/j.physbeh.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Webb P, Lopez GN, Uht RM, Kushner PJ. Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin for the cell-specific estrogen-like effects of antiestrogens. Mol. Endocrinol. 1995;9:443–456. doi: 10.1210/mend.9.4.7659088. [DOI] [PubMed] [Google Scholar]
- Weihua Z, Lathe R, Warner M, Gustafsson JA. An endocrine pathway in the prostate, ERbeta, AR, 5alpha- androstane-3beta,17beta-diol, and CYP7B1, regulates prostate growth. Proc. Natl. Acad. Sci. U.S.A. 2002;99:13568–13594. doi: 10.1073/pnas.162477299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser MJ, Foradori CD, Handa RJ. Estrogen receptor beta in the brain: From form to function. Brain Res Rev. 2007 2007 Jun 26; doi: 10.1016/j.brainresrev.2007.05.013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RI, Newman SW. Intracellular partitioning of androgen receptor immunoreactivity in the brain of the male Syrian hamster: effects of castration and steroid replacement. J Neurobiol. 1993;24(7):925–38. doi: 10.1002/neu.480240706. [DOI] [PubMed] [Google Scholar]
- Zhou L, Blaustein JD, De Vries GJ. Distribution of androgen receptor immunoreactivity in vasopressin- and oxytocin-immunoreactive neurons in the male rat brain. Endocrinology. 1994;134(6):2622–7. doi: 10.1210/endo.134.6.8194487. [DOI] [PubMed] [Google Scholar]


