Abstract
Background & Aims
Interleukin-10 (IL-10) has been ascribed pro-viral but anti-fibrotic properties in chronic hepatitis C virus (HCV) infection. In this study, we examined the role of HCV-specific T-cell IL-10 response in patients with acute and chronic HCV infection.
Methods
Peripheral HCV-specific T-cell IL-10 and IFNγ responses were measured in cytokine Elispot assay using overlapping HCV-derived peptides in patients with chronic (n=61), resolved (n=15) and acute (n=8) hepatitis C, looking for their onset, quantity, breadth and durability relative to clinical and virological outcomes. The source and effect of HCV-specific IL-10 response were determined in depletion and IL-10 neutralization experiments.
Results
Both HCV-specific IL-10 and IFNγ responses were detected early within 1–2 months of acute clinical hepatitis C. However, only HCV-specific IL-10 response correlated with elevated liver enzymes, increased viremia and suppressed HCV-specific CD4+ T-cell proliferation in acute infection. While these associations were lost in established chronic infection, HCV-specific IL-10 responses were increased in patients without cirrhosis while IL-10 blockade enhanced antiviral effector IFNγ responses.
Conclusions
HCV-specific IL-10 Tr1 responses may play a dual role in HCV infection, dampening effector T-cells to promote viral persistence in acute infection but also protecting against progressive fibrosis in chronic infection.
Keywords: hepatitis C, interleukin-10, interferon-gamma, regulatory T-cells, CD8+ T-cells, IL-10, HCV
INTRODUCTION
T-cells play a critical role in natural hepatitis C virus (HCV) clearance. While HCV is spontaneously cleared with a vigorous and broad virus-specific effector T-cell response, such responses are impaired in persistent infections (1–5). While most studies have focused on the antiviral effector cytokine IFNγ, a role for the immune regulatory cytokine interleukin-10 (IL-10) has been suggested by enhanced HCV titers and reduced hepatic fibrosis during exogenous IL-10 therapy in HCV-infected patients (6) as well as associations between IL-10 promoter polymorphisms and clinical outcomes (7, 8). IL-10 is an important immune regulatory cytokine with pleiotropic effects (9, 10). T-cells that predominantly secrete IL-10 (but not IFNγ, IL-4 or IL-5) with immune regulatory properties have been termed 'Tr1' cells, defining a subset of antigen-specific regulatory T-cells (Tregs) (11) distinct from thymic-derived, naturally occurring CD25+Foxp3+ Tregs (12). Relevant for HCV pathogenesis, circulating CD4+ T-cells with HCV-specific IL-10 production have been described in patients with chronic HCV infection (5, 13, 14). Furthermore, HCV-specific IL-10+ CD8+ Tr1 cells have been detected in the liver of HCV-infected patients with IL-10-dependent effector T-cell suppression (15, 16).
In this study, we report that a broadly specific HCV-specific Tr1 response is induced early during acute hepatitis C associated with reduced HCV-specific CD4+ T-cell proliferation and increased HCV viremia. Both CD4+ and CD8+ T-cells (but not CD25+ Tregs) contributed to HCV-specific Tr1 response that was also associated with reduced fibrosis in established chronic HCV infection. Collectively, these results suggest that HCV-specific Tr1 response may suppresses antiviral effector response, promoting chronic evolution in acute infection while limiting progressive liver damage in established chronic infection (17).
MATERIALS AND METHODS
Patients
Subjects were recruited from the Gastroenterology Clinics at the Philadelphia Veterans Affairs Medical Center and the Clinical Translational Research Center at the University of Pennsylvania following informed consent according to protocols approved by respective institutional review boards. All patients were assessed for baseline demographic, clinical and virological parameters, including serum HCV RNA by Roche COBAS qualitative, quantitative COBAS, or TaqMan reverse-transcriptase polymerase chain reaction (RT-PCR) assay (Roche Diagnostics, Branchburg, NJ) and HCV genotype by InnoLIPA (Innogenetics, Gent, Belgium). They included 15 spontaneously 'recovered' (Group R: HCV Ab+/HCV RNA−) and 11 uninfected control (Group N: HCV Ab−/HCV RNA−) (Table 1) and 61 'chronic' patients with chronic genotype 1 infection (Group C: HCV Ab+/RNA+). Eight patients with acute genotype 1 hepatitis C were also enrolled prior to antiviral therapy (18), including 3 spontaneous resolvers, 3 with chronic evolution, and 2 with early viral control and interferon treatment-associated resolution (Table 2). Exclusion criteria included HIV and/or HBV co-infection, immunosuppressive therapy, and conditions precluding research blood donation. Cirrhosis was diagnosed in 8 based on liver histology (Modified Ishak F4-6) and in 7 with clinical and/or radiological evidence of portal hypertension (encephalopathy, ascites and/or varices). Thirty-one patients were defined as non-cirrhotic based on histology with Modified Ishak score F0-3.
Table 1.
Chronic, Recovered and Normal Control Subjects
| Chornic N=61 | Normal (HCV Ab−/HCV RNA−) N=11 | Recovered (HCV Ab+/HCV RNA−) N=15 | p(Recovered vs. Chronic) | ||||
|---|---|---|---|---|---|---|---|
| Male/Female | 61/0 | 5/6 | 13/2 | p=0.037 | |||
| White/Black/Other | 15/43/3 | 7/3/1 | 8/7/0 | p=0.08 | |||
| Median | Range | Median | Range | Median | Range | ||
| Age (years) | 52 | 35–69 | 44 | 24–46 | 51 | 42–64 | p=0.030 |
| HCA RNA (IU/ml) | 773500 | 8,620–18,900,000 | p<0.0001 | ||||
| ALT (U/ML) | 47 | 13–326 | 22 | 13–43 | 22 | 12–39 | p<0.0001 |
| Albumin (g/dl) | 4.1 | 3.4–5.3 | 4.2 | 3.8–4.7 | 4.4 | 3.9–4.9 | p=0.052 |
| Total Bilirubin (mg/dl) | 0.8 | 0.2–1.9 | 0.4 | 0.2–1.4 | 0.7 | 0.2–1.1 | p=0.11 |
| INR | 1.0 | 0.9–1.12 | 1.0 | 1.0–1.2 | 1.0 | 0.9–1.2 | p=0.47 |
| Platelet (× 1000/ml) | 235 | 96–456 | 243 | 203–346 | 244 | 154–305 | p=0.54 |
Table 2.
Acute Hepatitis C Cohort
| ID | Gender | Ethnicity | Genotype | Age | BMI (kg/m2) | Diabetic | Interferon-treated | 1st HCV RNA (IU/ml) | Peak ALT (U/ml) | Peak Total Bilirubin (mg/dl) |
|---|---|---|---|---|---|---|---|---|---|---|
| AR031 | M | Asian | 1a | 57 | 26.5 | N | N | 23700 | 75 | 1.1 |
| AR041 | F | African-American | 1b | 48 | 29.6 | N | N | 1100 | 42 | 0.7 |
| AR06 | M | Caucasian | 1a | 24 | 29.1 | N | N | 20100000 | 1517 | 7.6 |
| AC101 | M | Caucasian | 1b | 64 | 42.1 | Y (Type II) | N | 133000 | 40 | 0.5 |
| AC12 | F | Caucasian | 1b | 23 | 27.3 | N | N | 2370000 | 115 | 0.8 |
| AC13 | M | Caucasian | 1b | 39 | 29.8 | N | N | 1750000 | 998 | 1.1 |
| A143 | F | African-American | 1a | 54 | 24.7 | Y (Type I) | Y | 7780 | 227 | 1.5 |
| A162 | M | Caucasian | 14 | 68 | 30.5 | N | Y | 11900000 | 1910 | 10.4 |
Patient included in cohort described in Kaplan, DE et al. Gastroenterology 2007; 132:654-666
Patient was HCV RNA-negative prior to initiation of pegylated-interferon alpha/ribavirin therapy at week 8
Patient maintained HCV RNA titer <104IU/ml until week 26, at which point patient was initiated on pegylated-interferon alpha/ribavirin therapy. She tolerated only 3 injections but still cleared infection
Not subtypeable by InnoLIPA assay
Recombinant HCV proteins
Recombinant genotype 1a-derived HCV core, NS3/4, and NS5 and control superoxide dismutase (SOD) proteins known to stimulate CD4+ T-cells were generously provided by Dr. Michael Houghton (Chiron Corporation, Emeryville, CA).(3, 19–21)
Overlapping HCV peptides
361 overlapping 15mer peptides (offset by 6 amino acid residues) spanning the entire HCV core and NS3-NS5 proteins based on HCV-H sequence (genotype 1a) were synthesized (Genemed, San Francisco, CA) and mixed into 12 separate pools containing 20–31 consecutive peptides/pool or 36 pools with 10–11 peptides/pool as previously described.(22, 23) These peptides were immunogenic for CD4+ and CD8+ T-cells.(22, 23)
Peripheral blood mononuclear cells (PBMC)
PBMC were isolated from blood using Ficoll-Histopaque (Sigma, St. Louis MO) density centrifugation.(22, 23) Cramp 2000
HCV-specific CD4+ proliferative T-cell response
CD4+ proliferation assay was performed as previously described (3, 22, 23) with 2×105 cells/well stimulated with recombinant HCV and control SOD proteins (10µg/mL) for 7 days, or phytohemagglutinin (PHA) at 2 µg/ml for day 4 days, with 16 hours of 3H-thymidine uptake (1µCi/well) (Dupont NEN, Boston, MA). The results were expressed as a stimulation index (SI) with mean counts per minute (cpm) in stimulated wells divided by the mean cpm in control wells.(23)
Cytokine Elispot Assay
IL-10 and IFNγ Elispot assay was performed with 2×105 PBMC/well in triplicates as described previously.(23) HCV-specific CD4+ T-cell responses were examined using recombinant HCV and control proteins (10µg/ml). HCV-specific total T-cell IL-10 and IFNγ responses were examined using overlapping HCV-derived 15mers (5µM/peptide) with positive controls including PHA (2µg/mL), tetanus toxoid (0.5µg/mL) and Candida albicans (20µg/mL).(22, 23) IL-10 and IFNγ spot forming units (SFU) were counted using Elispot reader (Hitech Instruments, Media, PA). HCV-specific Tr1 or Th1 frequency was calculated by subtracting the mean SFU in negative control wells from mean SFU in antigen-stimulated wells and expressed as SFU/106 PBMC for each antigen or combined for total HCV-specific Tr1 or Th1 response (22, 23). The cut-off for a positive response for individual peptide pools was defined as >50 SFU/106 PBMC which was the 95% percentile for each peptide pool in 11 healthy controls. The validity of IL-10 SFU counts was confirmed in a subset of assays at multiple sensitivity settings.
Cell subset depletion and isolation
CD4 and CD8 T-cell depletion of PBMC was performed using CD4- and CD8-dynabeads (Dynal/Invitrogen, Carlsbad CA). CD4+CD25hi and CD4+CD25− T-cells were separated by the CD4+CD25+ Regulatory T Cell Isolation Kit (Miltenyi Biotech, Auburn CA) (22). Purity of isolated cell subset was confirmed by flow cytometry.
IL-10 blockade
IFNγ Elispot was performed with the addition of 10-mcg/ml mouse anti-human IL-10 (MAB217, R&D Systems, Minneapolis MN) or isotype control during antigenic stimulation of whole, CD4+-depleted or CD8-depleted PBMC. The effect of anti-IL-10 on nonspecific IFNγ response was first determined by subtracting the IFNγ SFU in media control wells without anti-IL-10 from the IFNγ SFU in media control wells with anti-IL-10. The effect of anti-IL-10 specifically for antigen-specific IFNγ response was assessed by subtracting the IFNγ SFU in media control wells from antigen-stimulated wells, both with added anti-IL-10.
Flow cytometry
Cells were stained with fluorescent antibodies, acquired on FACSCalibur or FACSCanto (Becton Dickinson, Franklin Lakes, NJ), and analyzed by FlowJo (Tree Star Inc., Ashland OR). The cut-off for each marker was based on the isotype antibody. All antibodies were purchased from Becton Dickinson (Becton Dickinson, Franklin Lakes, NJ) except for anti-Foxp3 (eBioscience, San Diego CA).
Multiplex cytokine quantification
Culture supernatant was harvested after 24-hour stimulation of cell subsets 0.04 mcg/ml phorbol myristate acetate (PMA) plus 0.8 mcg/ml ionomycin (Sigma, St Louis MO) or media alone and examined using the BD Cytokine Bead Array Human Th1/Th2 Cytokine Kit (Becton Dickinson, San Jose CA) per manufacturer’s instructions.
Statistical analysis
The median values for clinical and immunologic parameters were compared using the nonparametric Kruskal-Wallis ANOVA, Wilcoxon Rank Sum or Mann-Whitney U test. Frequency data were compared using χ2 or Fisher’s exact test based on sample size. Spearman rank correlation was used for bivariate correlation of variables with log transformation of skewed variables to attain normalization. The correlations were confirmed with Generalized Estimate Equation linear regression to adjust for patient clustering when appropriate. All data were analyzed with JMP 5.1 (SAS Institute Inc, Cary NC). P-values below 0.05 were considered significant.
RESULTS
HCV-specific Tr1 response is associated with increased viremia and liver inflammation but reduced HCV-specific CD4+ proliferative T-cell response in acute hepatitis C. We began by determining the kinetics of HCV-specific IL-10+ Tr1 and IFNγ+ Th1 responses during acute hepatitis C relative to HCV-specific CD4+ T-cell proliferation, serum alanine aminotransferase (sALT) activity and HCV RNA titer as well as virological outcome. As shown in Figure 1A, HCV-specific IL10+ Tr1 response was detected within the first 6 months of clinical onset regardless of virological outcomes: AR06 (week 2, SFU 330) AR04 (week 6, SFU 1,884), AR03 (week 11, SFU 2,508), A16 (week 2, SFU 2214), A14 (week 15, 303), AC13 (week 1, SFU 17,278), AC10 (week 15, SFU 871) and AC12 (week 19, SFU 1,008). Self-limited infections were associated with early reductions of HCV-specific IL-10+ Tr1 response (SFU<400) relative to strong CD4+ T-cell proliferation (sum SI 10–100), a parameter associated with HCV clearance (18, 21, 24). By contrast, at all time points earlier than 24 weeks, chronic evolution was associated with marked HCV-specific IL-10+ Tr1 response (SFU>1000) and suppressed CD4+ T-cell proliferation (sum SI<10). For all observations within the first 24 weeks, weighting to account for multiple observations per subject, there was a significant difference in the median of IL-10 SFU (AC 4548 vs. AR 105, p=0.0013, Figure 1B) and CD4 stimulation index (AC 3.3 versus AR 42.9, p=0.0034), but not IFNγ SFU. For entire cohort, HCV-specific Tr1 response was inversely associated with HCV-specific CD4+ T-cell proliferation (Figure 1C). Accordingly, HCV-specific Tr1 response was directly associated with HCV RNA titers and sALT activity. By contrast, HCV-specific IFNγ response did not associate with HCV-specific CD4+ T-cell proliferation, viral titer, sALT activity or HCV-specific Tr1 response (data not shown). These results suggest that early and prolonged induction of HCV-specific Tr1 response in acute HCV infection may contribute to antiviral CD4+ T-cell suppression and chronic evolution.
Figure 1. Evolution of IL-10 responses in acute hepatitis C relative to clinical, immunological and immunophenotypical parameters.
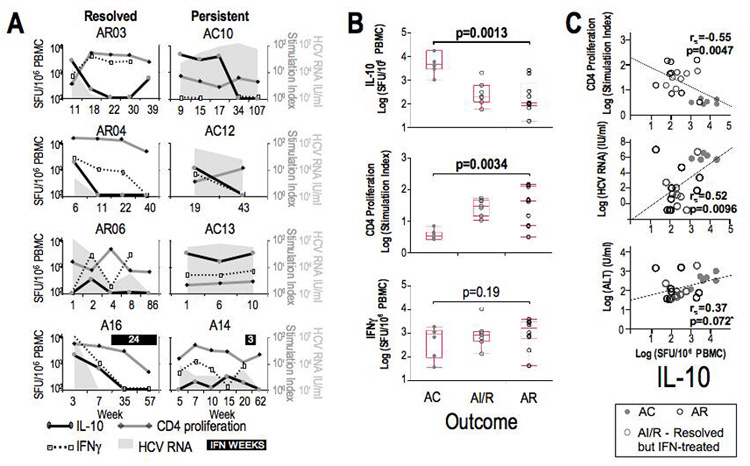
A. Temporal evolution IL-10 SFU/106 PBMC (black line), CD4 T-cell proliferation (expressed as stimulation index) (grey line) and IFNγ SFU/106 PBMC (dotted line) relative to HCV viral titer IU/ml (grey background) in 3 acute resolving (AR03, AR04, and AR06), 3 acute with chronic evolution (AC10, AC12, and AC13), and 2 acute patients who resolved but received interferon (AI/R: A14 - received only 3 PEG-IFN injections, A16 - was RNA-negative before started interferon). B. Weighted comparison of all observations within the first 24 weeks for IL-10 SFU/106 PBMC, CD4 T-cell proliferation and IFNγ SFU/106 PBMC relative to clinical outcome. P-value given for AC versus AR subjects obtained by Wilcoxon Test. C. Weighted Spearman correlations of CD4 proliferation (log stimulation index), HCV RNA titer (log IU/ml), and ALT (log U/ml) with HCV-specific IL-10 SFU/106 PBMC for all patient observations within the first 24 weeks after presentation (n=8 patients, mean 3 observations per patient). Data from AC (black circles), interferon-treated (grey circles) and AR (grey dots) are shown. Spearman rs and p-values are shown for all data. By GEE linear regression modeling, IL-10 SFU and CD4 stimulation index remained highly significant (p=0.0012), as did the association with HCV RNA titer (p=0.0108). *Exclusion of single outlier value from AR06 week 1 increased strength of correlation of IL-10 with ALT (rs=0.53, p=0.0088).
HCV persistence is associated with increased frequency and scope of HCV-specific Tr1 response
We then examined HCV-specific Tr1 response in patients with established chronic HCV infection. HCV-specific IL-10+ T-cells could be detected at ten-fold higher frequencies in the Chronic than the Recovered or Normal subjects (Figure 2A)(median C659 vs. R65 SFU/106 PBMC, p=0.0022). Similarly, HCV-specific Tr1 response in the Chronic subjects was broader than the Recovered or Normal subjects (C 25% vs. R 0% vs. N 0%, p=0.0001). These differences were HCV-specific since IL-10 responses to the tetanus toxoid and C. albicans (data not shown) were similar between the three groups (Figure 2A, right). The opposite was true for HCV-specific IFNγ response, which was greater among the Recovered than the Chronic, or Normal subjects both in frequency (median C 258 vs. R 723 SFU/106 PBMC, p=0.0006) and scope (C 0% vs. R 42% vs. N 0%, p=0.004) (Figure 2B). Interestingly, HCV peptide pools eliciting increased IL-10 responses in Chronic patients were also highly antigenic for IFNγ responses among the Recovered subjects (Figure 2C, black bars), resulting in a significant correlation in the relative immunogenicities of the HCV peptide pools for IL-10 responses among the Chronic patients and IFNγ responses among the Recovered subjects (p=0.035) (Figure 2D). Thus, HCV-specific T-cells were maintained at similar frequencies and antigenic hierarchy in patients with chronic and recovered HCV infection, but polarized towards IL-10 production in chronic HCV infection and to IFNγ in HCV clearance.
Figure 2. Magnitude and breadth of HCV-specific IL-10 Tr1 responses in chronic hepatitis C.
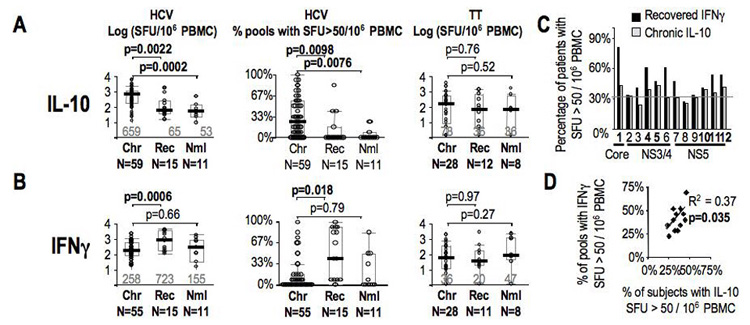
A. Total HCV-specific IL-10 SFU/106 PBMC, percentage of pools with IL-10 SFU/106 PBMC > 50, and Tetanus toxoid-specific IL-10 SFU/106 PBMC by Elispot assay are shown for chronic genotype 1 HCV patients (Chr), recovered controls (Rec) and healthy donors (Nml). Median values are shown in grey with box plots indicating median (black bars), 25th and 75th percentiles. B. Total HCV-specific IFNg SFU/106 PBMC, percentage of pools with IFNγ SFU/106 PBMC > 50, and Tetanus toxoid-specific IFNγ SFU/106 PBMC by Elispot assay are shown for chronic HCV patients (Chr), recovered controls (Rec) and healthy donors (Nml). C. To demonstrate Tr1 regional response hierarchy in chronic patients relative to the effector IFNγ regional hierarchy, for each of the 12 peptide pools (annotated by region within the polyprotein), the percentage of positive IL-10 response in chronic HCV patients (N=61) (grey bars) are compared to the percentage of positive effector IFNγ responses in recovered patients (N=15)(black bars) are plotted. Grey line indicates 33%. D. Pearson correlation of chronic IL-10 and recovered IFNg responses for the 12 peptide pools showing that regional specificities of IL-10 responses in chronic patients resembles specificities for effector response in recovered subjects.
HCV-specific Tr1 response correlates with reduced fibrosis in established chronic HCV infection
While HCV-specific Tr1 response correlated with immunological, virological and clinical parameters during acute hepatitis C, HCV-specific Tr1 response was not associated with HCV-specific CD4+ T-cell proliferation, which was generally suppressed among Chronic patients. Similarly, HCV-specific Tr1 response among the Chronic patients did not correlate with HCV titers, sALT activity (Figure 3B/C) or histological activity (data not shown). However, HCV-specific Tr1 response was significantly lower in 15 patients with histological (n=8) and/or clinical (n=7) cirrhosis, compared to 31 patients without cirrhosis on liver biopsy (median IL-10 SFU: Cirrhotics 418 vs. Non-cirrhotic 899 SFU, p=0.037) (Figure 3D), suggesting an inverse relationship between HCV-specific Tr1 response and cirrhosis.
Figure 3. Correlation of HCV-specific IL-10 responses with immunologic, virologic and clinical parameters in chronic hepatitis C.
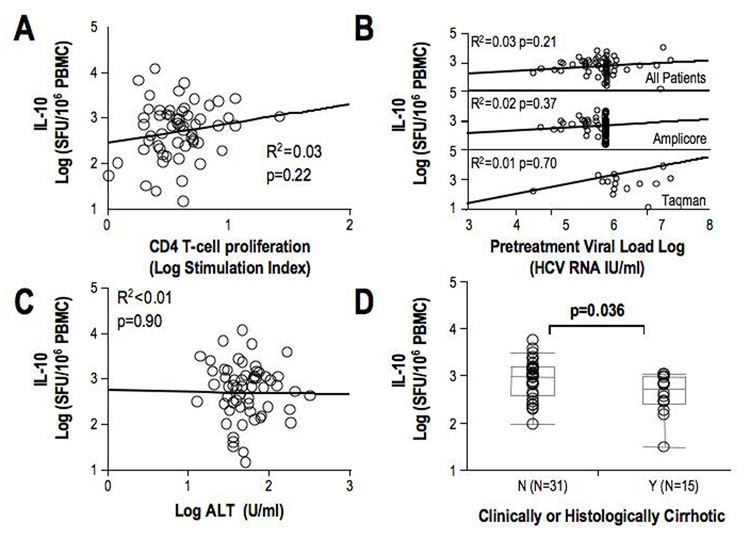
A. Spearman correlation of HCV-specific IL-10 responses and CD4 T-cell proliferation and IFNγ responses in 59 chronic patients. B. Spearman correlation of IL-10 responses with HCV RNA titer in all chronic HCV patients using both Roche COBAS Amplicore (maximum reported value 850,000 IU/ml) and Taqman PCR (top), Amplicore only (middle) and Taqman only (bottom). C. Spearman correlation of HCV-specific IL-10 responses and ALT in 59 chronic HCV patients. D. IL-10 Tr1 responses in patients with or without cirrhosis. Cirrhosis was defined histologically (<F4/6 fibrosis, N=31 versus >F4/6 N=8) or clinically (evidence of portal hypertension or onset of decompensated cirrhosis, N=7). In 15 patients there were inadequate histological or clinical data to determine presence or absence of cirrhosis.
Both CD4+ and CD8+ T-cells contribute to the peripheral HCV-specific IL-10 Tr1 response
The contribution of CD8+ T-cells in HCV-specific IL-10 response was examined in whole and CD8-depleted PBMC samples in IL-10 Elispot assay. As shown in Figure 4A, HCV-specific Tr1 response was variably influenced by CD8+ T-cell depletion, increasing in 4/6 patients in response to HCV Core (pool 1, aa1-195) and 3/6 to C-terminal NS5B (pool 12, aa2827-3015), while decreasing in 4/6 patients in response to HCV core (pool 2). By contrast, HCV-specific IL-10 response was significantly reduced by CD4+ depletion in overall circulating frequency (p=0.006) and in 15/17 patients (Figure 4B). These results suggest that both CD4+ and CD8+ T-cells may contribute to HCV-specific IL-10 response, although to a greater magnitude by the CD4+ T-cells.
Figure 4. Cell subsets and IL-10.
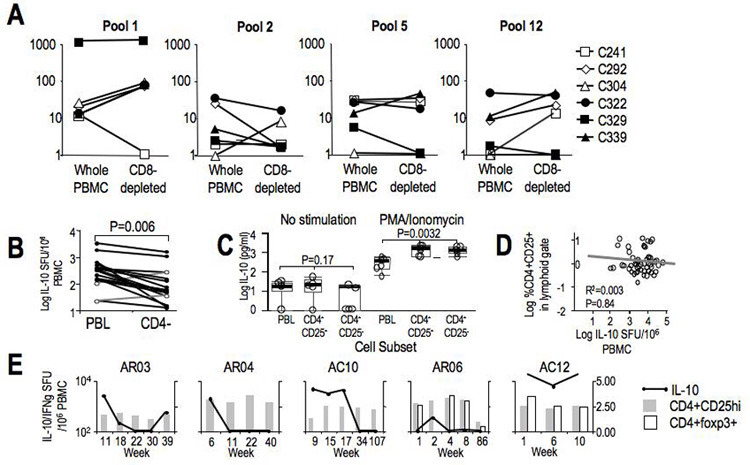
A. HCV-specific (to Pools 1 (Core) and 2 (NS3)), C. albicans-specific, and PHA-induced IL-10 SFU/106 PBMC in unfractionated PBMC and CD8+ T-cell-depleted PBMC in 6 chronic HCV patients. Depleted subset SFU were corrected for changes in T-cell frequency by multiplying by [%CD3PBMC}/[%CD3CD8−]. B. Summed HCV-specific IL-10 SFU/106 PBMC to 12 pools of 15mer overlapping peptides spanning Core, NS3-NS5 with unfractionated and CD4+ T-cell-depleted PBMC in 17 chronic HCV patients. C. IL-10 secretion measured by cytokine bead array (expressed in log IL-10 pg/ml) in 24 hour unstimulated and PMA/ionomycin stimulated cultures of unfractionated PBMC, bead-selected CD4+CD25+ and CD4+CD25− subsets in 5 chronic HCV patients. D. Correlation of frequency of peripheral CD4+CD25−FITC+ T-cells and IL-10 SFU/106 PBMC in 59 chronic HCV patients. E. CD4+CD25hi and CD4+foxp3+ T-cell frequency in acute HCV patients. CD25+ cutoff was defined by 99.9% isotype in the lymphoid gate, while CD25hi was defined by 99.9% of CD8+ T-cells. An example of the gating strategy for CD4+CD25hi population and CD4+foxp3+ gating is shown in Supplementary Figure 1. For 3 AR and 2 AC subjects CD4+CD25hi (grey bars) and CD4+foxp3+ (white bars) are plotted relative to IL-10 SFU/106 PBMC (black line).
We then examined which CD4+ subsets contribute to IL-10 response. As shown in Figure 4C, IL-10 production was significantly greater in both CD4+CD25+ and CD4+CD25− subsets compared to whole PBMC, consistent with the notion that IL-10 is secreted by CD4+ T-cells. However, there was no difference in IL-10 production by CD4+CD25+ and CD4+CD25− T-cells, suggesting that IL-10 production is not specifically mediated by CD25+ Tregs. Furthermore, HCV-specific Tr1 response did not correlate with CD4+CD25+ and/or Foxp3+ Treg frequency cross-sectionally in patients with established chronic infection (Figure 4D) or longitudinally during acute evolving hepatitis C (Figure 4E), suggesting that CD25+Foxp3+ Tregs are not the primary sources of HCV-specific Tr1 response.
IL-10 receptor (IL-10R) blockade results in enhanced HCV-specific IFNγ+ effector T cell response
The functional impact of IL-10 on antigen-specific IFNγ response was examined in PBMC following antigen-specific stimulation with and without IL-10R blockade in IFNγ Elispot. As shown for 3 representative patients in Figure 5A, IL-10 blockade in PBMC with and without CD4 or CD8-depletion led to variable increases in background IFNγ production in some patients. However, IL-10 blockade further increased in IFNγ response to HCV-derived peptides in whole, CD4-depleted and CD8-depleted PBMC (Figure 5B) which was statistically significant despite the small sample size for the responses to HCV Core 127–195 peptides. For example, in CD4-depleted PBMC, IL-10R blockade enhanced IFNγ response in 4/6 patients for C-terminal Core 127–195 and in 3/6 patients for N-terminal NS3 1027–1095. HCV-specific IFNγ response was also augmented in CD8-depleted PBMC in 6/6 patients for HCV Core 127–195 and in 3/6 patients for NS3 1027–1095. The immune augmentation induced by IL-10 blockade in CD4-depleted PBMC suggests that IL-10 produced by non-CD4 T-cells also contributes to HCV-specific effector T-cell suppression ex-vivo. These data collectively suggest that IL-10 can reversibly suppress some antiviral effector T-cell responses in HCV infection, perhaps favoring chronic evolution in acute infection but also limiting liver inflammation during chronic infection.
Figure 5. Effect of IL-10 blockade on IFNγ effector responses.
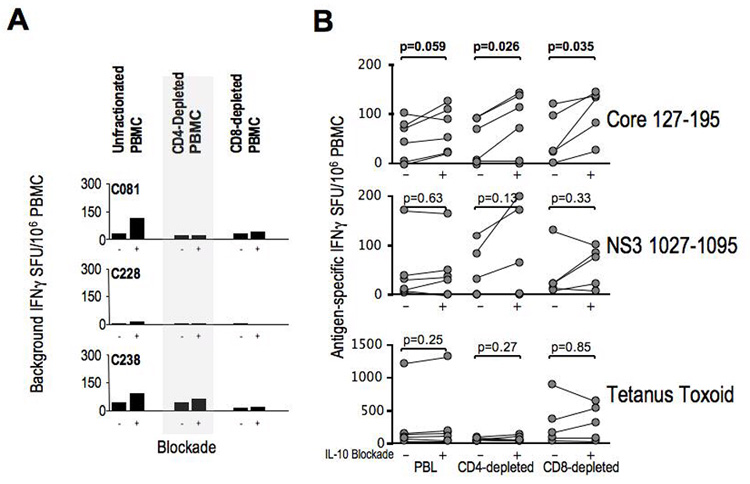
A. Unstimulated (media-control) IFNγ SFU/106 PBMC with IL-10R blocking antibody or isotype control in PBMC, CD4-depleted and CD8-depleted PBMC subsets. 3 representative chronic HCV patients’ data shown. B. IFNγ SFU/106 PBMC after stimulation with HCV Core 127–195 15mer peptides (5uM), NS3 1027–1095 peptides (5uM) and tetanus toxoid (0.1 ug/ml) in PBMC, CD4-depleted/CD8-enriched and CD8-depleted/CD4-enriched PBMC subsets from 6 Chronic HCV subjects. To correct for the effect of IL-10 blockade on background IFNg production, mean SFU values for stimulated wells reflect subtraction of the mean SFU of unstimulated wells. P-values were obtained by matched-pair Wilcoxon sign-rank testing.
DISCUSSION
IL-10+ Tr1 cells represent a subset of Tregs, distinct from CD4+CD25+ regulatory T-cells and with suppressive or regulatory properties (25–27). IL-10 is produced by many cell types including dendritic cells (28, 29), macrophages, monocytes, NK (30, 31) and NKT-cells (32) with pleiotropic effects. For example, IL-10 can downregulate antigen processing by transporter-associated with antigen processing, MHC expression and IL-12 production by antigen-presenting cells (APC) (9, 10), resulting in impaired T-cell proliferation, effector function and memory (33). Virus-specific Tr1 responses have been linked to chronic hepatitis B and C (5, 13, 34–38). Relevant to HCV, HCV-specific IL-10+ CD8 T-cell frequency in the liver correlated inversely to histological activity (16, 39). Additionally, changes in serum or T-cell IL-10 levels have been associated with the efficacy of antiviral therapy in some studies (32, 34, 40–46).
While antigen-specific Tr1 response has been detected in patients with acute and chronic hepatitis C (13, 34, 47, 48), this response begins early within 1–2 months of acute clinical hepatitis in our subjects, contrasting their detection only after 24 weeks of infection in a study of genotype 4 infection(48). HCV-specific Tr1 response was also detected regardless of subsequent virological outcome in our study, similar to Urbani et al (47). However, in the present study the strength of HCV-specific Tr1 response (but not IFNγ response) correlated with clinical, virological and immunological parameters during acute infection. The direct associations between HCV-specific Tr1 response and sALT levels and viremia as well as suppressed antiviral CD4 T-cell proliferation during acute infection suggests that IL-10 response is induced as a compensatory mechanism to dampen the ongoing inflammation (49), leading to antiviral CD4 T-cell suppression and increased viremia with chronic evolution.
One notable finding in our study is that HCV-specific Tr1 response was broadly directed with a similar magnitude and scope as the robust HCV-specific IFNγ response observed with resolved HCV infection. This suggested that HCV-specific T-cells are maintained at similar frequency, scope and antigenic hierarchy in chronic HCV infection as those in recovered HCV infection but are polarized toward IL-10 production rather than the IFNγ production characteristic of spontaneous HCV clearance. Possible explanations for this finding include altered T-cell maturation due to virus-induced dysfunction of antigen-presenting cells (29, 50, 51), viral epitope mutation (52), or altered T-cells costimulation (53–55). In the chronic phase, peripheral HCV-specific Tr1 response did not correlate with CD4+CD25+ regulatory T-cell frequency, distinguishing Tr1 cells from CD4+CD25+ Tregs (26, 27). Although HCV-specific Tr1 response did not correlate with sALT activity in established chronic HCV infection, it correlated inversely with cirrhosis with the caveat that histological or clinical evidence of cirrhosis could only be assessed in 46 of the 61 patients. Based on antifibrotic effect reported by exogenous IL-10 therapy (6), HCV-specific Tr1 response may exert a protective effect that is lost in patients with cirrhosis.
As for the cell subset(s), CD4+CD25− T-cells contributed to peripheral HCV-specific Tr1 response in HCV-infected patients contrasting with previous studies focusing on CD4+CD25+ IL-10+ T-cells (56, 57). Interestingly, IL-10 blockade, similar to results reported by Alatrakchi et al.(58), enhanced HCV-specific IFNγ response to HCV Core peptides in CD4-depleted as well as CD8-depleted PBMC, suggesting that IL-10 production by CD4− and/or CD8− cells (including non-T-cells such as monocytes, B-cells or NK cells) participate in HCV-specific immune regulation. Enhancement of HCV-specific effector T-cell response by IL-10R blockade has interesting therapeutic implications, particularly in light of virus control with restoration of effector T-cell function in murine LCMV infection demonstrated by IL-10 blockade in-vivo (59, 60). However, the inverse association between HCV-specific Tr1 response and cirrhosis in our study suggests further caution in applying IL-10 blockade to HCV infection.
It is important to acknowledge some of the potential limitations in this study. First, peripheral HCV-specific Tr1 cells in our study could represent only a subpopulation of dysfunctional cells without functional relevance to the liver, the site of infection. However, the inverse association between peripheral HCV-specific Tr1 response and cirrhosis suggests that they have a prognostic relevance at the very minimum. Second, because we relied primarily on the Elispot assay to detect IL-10+ cells, we cannot exclude the contribution of non-T-cells in IL-10 production. Nevertheless, HCV-specific nature of the IL-10 responses strongly suggested TCR-mediated signaling using HCV-derived peptides. Third, a third of the subjects did not undergo liver biopsy for histological assessment of cirrhosis. However, we believe that it is justified to assign the 7 clinically cirrhotic patients to the cirrhotic group, as it is neither indicated nor ethical to biopsy such subjects who most likely have had histological cirrhosis for 7–10 years (61). Furthermore, the exclusion of subjects without a liver biopsy still yielded a strong trend towards a difference (p=0.08 with only n=8 in cirrhotic group).
In conclusion, HCV-specific Tr1 response occurred early in acute hepatitis C infection in the setting of active inflammation and viremia with suppressed HCV-specific CD4 T-cell response. During established chronic infection, IL-10+ Tr1 cells persisted at high frequency with a broad specificity. While IL-10 blockade could enhance antiviral effector IFNγ+ T-cell responses ex vivo, HCV-specific Tr1 response was also inversely associated with liver cirrhosis in patients with chronic infection, suggesting an important immune regulatory effect that limits liver disease progression. Thus, our data suggest an association of HCV-specific Tr1 responses with both HCV viral persistence and disease control. These findings have important implications in HCV immune pathogenesis and immunotherapeutic development.
ACKNOWLEDGMENTS
This material is based upon work supported in part by the Office of Research and Development, Department of Veterans Affairs and with the resources and the use of facilities at the Philadelphia VA Medical Center. This study was also supported by NIH grants R01-AI-47519 and R01-AA-12849; the Philadelphia VA Medical Research; NIH/NIDDK Center of Molecular Studies in Digestive and Liver Diseases P30DK50306 and its Molecular Biology and Cell Culture Core Facilities; the NIH Public Health Service Research Grant M01-RR00040. DEK was supported by the NIH T32 DK 07066, NIH Loan Repayment Program, AASLD/Schering Advanced Hepatology Fellowship, NIH K12-RR-017625, and the VA Career Development Award and the VA Stars and Stripes Award. The authors thank Dr Michael Houghton at Chiron Corporation for the generous provision of the recombinant HCV antigens and Colleen Brensinger, MS for statistical support.
Abbreviations
- HCV
hepatitis C virus
- IFN
interferon
- PEG-IFNα
pegylated interferon-alpha
- PBMC
peripheral blood mononuclear cells
- PCR
reverse-transcriptase polymerase chain reaction
- PHA
phytohemagglutinin
- SD
standard deviation
- SFU
spot-forming unit
- SOD
superoxide dismutase
- SVR
sustained virologic response
- Th1
type 1 helper T-cell response
- Th2
type 2 helper T-cell response
- Tr1
type 1 regulatory T-cell response
- Treg
classical regulatory T-cell (CD4+CD25hi or CD4+foxp3+)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Missale G, Bertoni R, Lamonaca V, Valli A, Massari M, Mori C, et al. Different clinical behaviors of acute hepatitis C virus infection are associated with different vigor of the anti-viral cell-mediated immune response. J Clin Invest. 1996;98:706–714. doi: 10.1172/JCI118842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takaki A, Wiese M, Maertens G, Depla E, Seifert U, Liebetrau A, et al. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat Med. 2000;6:578–582. doi: 10.1038/75063. [DOI] [PubMed] [Google Scholar]
- 3.Chang KM, Thimme R, Melpolder JJ, Oldach D, Pemberton J, Moorhead-Loudis J, et al. Differential CD4(+) and CD8(+) T-cell responsiveness in hepatitis C virus infection. Hepatology. 2001;33:267–276. doi: 10.1053/jhep.2001.21162. [DOI] [PubMed] [Google Scholar]
- 4.Wedemeyer H, He XS, Nascimbeni M, Davis AR, Greenberg HB, Hoofnagle JH, et al. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J Immunol. 2002;169:3447–3458. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- 5.Ulsenheimer A, Gerlach JT, Gruener NH, Jung MC, Schirren CA, Schraut W, et al. Detection of functionally altered hepatitis C virus-specific CD4 T cells in acute and chronic hepatitis C. Hepatology. 2003;37:1189–1198. doi: 10.1053/jhep.2003.50194. [DOI] [PubMed] [Google Scholar]
- 6.Nelson DR, Tu Z, Soldevila-Pico C, Abdelmalek M, Zhu H, Xu YL, et al. Long-term interleukin 10 therapy in chronic hepatitis C patients has a proviral and anti-inflammatory effect. Hepatology. 2003;38:859–868. doi: 10.1053/jhep.2003.50427. [DOI] [PubMed] [Google Scholar]
- 7.Abbott WG, Rigopoulou E, Haigh P, Cooksley H, Mullerova I, Novelli M, et al. Single nucleotide polymorphisms in the interferon-gamma and interleukin-10 genes do not influence chronic hepatitis C severity or T-cell reactivity to hepatitis C virus. Liver Int. 2004;24:90–97. doi: 10.1111/j.1478-3231.2004.00904.x. [DOI] [PubMed] [Google Scholar]
- 8.Edwards-Smith CJ, Jonsson JR, Purdie DM, Bansal A, Shorthouse C, Powell EE. Interleukin-10 promoter polymorphism predicts initial response of chronic hepatitis C to interferon alfa. Hepatology. 1999;30:526–530. doi: 10.1002/hep.510300207. [DOI] [PubMed] [Google Scholar]
- 9.Mocellin S, Panelli MC, Wang E, Nagorsen D, Marincola FM. The dual role of IL-10. Trends Immunol. 2003;24:36–43. doi: 10.1016/s1471-4906(02)00009-1. [DOI] [PubMed] [Google Scholar]
- 10.D'Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 12.Sakaguchi S. The origin of FOXP3-expressing CD4+ regulatory T cells: thymus or periphery. J Clin Invest. 2003;112:1310–1312. doi: 10.1172/JCI20274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacDonald AJ, Duffy M, Brady MT, McKiernan S, Hall W, Hegarty J, et al. CD4 T helper type 1 and regulatory T cells induced against the same epitopes on the core protein in hepatitis C virus-infected persons. J Infect Dis. 2002;185:720–727. doi: 10.1086/339340. [DOI] [PubMed] [Google Scholar]
- 14.Graham CS, Wells A, Liu T, Sherman KE, Peters M, Chung RT, et al. Antigen-specific immune responses and liver histology in HIV and hepatitis C coinfection. Aids. 2005;19:767–773. doi: 10.1097/01.aids.0000168970.80551.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abel M, Sene D, Pol S, Bourliere M, Poynard T, Charlotte F, et al. Intrahepatic virus-specific IL-10-producing CD8 T cells prevent liver damage during chronic hepatitis C virus infection. Hepatology. 2006;44:1607–1616. doi: 10.1002/hep.21438. [DOI] [PubMed] [Google Scholar]
- 16.Accapezzato D, Francavilla V, Paroli M, Casciaro M, Chircu LV, Cividini A, et al. Hepatic expansion of a virus-specific regulatory CD8(+) T cell population in chronic hepatitis C virus infection. J Clin Invest. 2004;113:963–972. doi: 10.1172/JCI20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson DR, Lauwers GY, Lau JY, Davis GL. Interleukin 10 treatment reduces fibrosis in patients with chronic hepatitis C: a pilot trial of interferon nonresponders. Gastroenterology. 2000;118:655–660. doi: 10.1016/s0016-5085(00)70134-x. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan DE, Sugimoto K, Newton K, Valiga ME, Ikeda F, Aytaman A, et al. Discordant role of CD4 T-cell response relative to neutralizing antibody and CD8 T-cell responses in acute hepatitis C. Gastroenterology. 2007;132:654–666. doi: 10.1053/j.gastro.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 19.Diepolder HM, Zachoval R, Hoffmann RM, Jung MC, Gerlach T, Pape GR. The role of hepatitis C virus specific CD4+ T lymphocytes in acute and chronic hepatitis C. J Mol Med. 1996;74:583–588. doi: 10.1007/s001090050062. [DOI] [PubMed] [Google Scholar]
- 20.Missale G, Cariani E, Lamonaca V, Ravaggi A, Rossini A, Bertoni R, et al. Effects of interferon treatment on the antiviral T-cell response in hepatitis C virus genotype 1b- and genotype 2c-infected patients. Hepatology. 1997;26:792–797. doi: 10.1053/jhep.1997.v26.pm0009303515. [DOI] [PubMed] [Google Scholar]
- 21.Gerlach JT, Diepolder HM, Jung MC, Gruener NH, Schraut WW, Zachoval R, et al. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gastroenterology. 1999;117:933–941. doi: 10.1016/s0016-5085(99)70353-7. [DOI] [PubMed] [Google Scholar]
- 22.Sugimoto K, Ikeda F, Stadanlick J, Nunes FA, Alter HJ, Chang KM. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex vivo in persistent HCV infection. Hepatology. 2003;38:1437–1448. doi: 10.1016/j.hep.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 23.Sugimoto K, Stadanlick J, Ikeda F, Brensinger C, Furth EE, Alter HJ, et al. Influence of ethnicity in the outcome of hepatitis C virus infection and cellular immune response. Hepatology. 2003;37:590–599. doi: 10.1053/jhep.2003.50103. [DOI] [PubMed] [Google Scholar]
- 24.Folgori A, Spada E, Pezzanera M, Ruggeri L, Mele A, Garbuglia AR, et al. Early impairment of hepatitis C virus specific T cell proliferation during acute infection leads to failure of viral clearance. Gut. 2006;55:1012–1019. doi: 10.1136/gut.2005.080077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sundstedt A, O'Neill EJ, Nicolson KS, Wraith DC. Role for IL-10 in suppression mediated by peptide-induced regulatory T cells in vivo. J Immunol. 2003;170:1240–1248. doi: 10.4049/jimmunol.170.3.1240. [DOI] [PubMed] [Google Scholar]
- 26.Vieira PL, Christensen JR, Minaee S, O'Neill EJ, Barrat FJ, Boonstra A, et al. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5986–5993. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 27.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25− cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 28.Dolganiuc A, Kodys K, Kopasz A, Marshall C, Do T, Romics L, Jr, et al. Hepatitis C virus core and nonstructural protein 3 proteins induce pro- and anti-inflammatory cytokines and inhibit dendritic cell differentiation. J Immunol. 2003;170:5615–5624. doi: 10.4049/jimmunol.170.11.5615. [DOI] [PubMed] [Google Scholar]
- 29.Brady MT, MacDonald AJ, Rowan AG, Mills KH. Hepatitis C virus nonstructural protein 4 suppresses Th1 responses by stimulating IL-10 production from monocytes. Eur J Immunol. 2003;33:3448–3457. doi: 10.1002/eji.200324251. [DOI] [PubMed] [Google Scholar]
- 30.Jinushi M, Takehara T, Tatsumi T, Kanto T, Miyagi T, Suzuki T, et al. Negative regulation of NK cell activities by inhibitory receptor CD94/NKG2A leads to altered NK cell-induced modulation of dendritic cell functions in chronic hepatitis C virus infection. J Immunol. 2004;173:6072–6081. doi: 10.4049/jimmunol.173.10.6072. [DOI] [PubMed] [Google Scholar]
- 31.De Maria A, Fogli M, Mazza S, Basso M, Picciotto A, Costa P, et al. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur J Immunol. 2007;37:445–455. doi: 10.1002/eji.200635989. [DOI] [PubMed] [Google Scholar]
- 32.Amaraa R, Mareckova H, Urbanek P, Fucikova T. Production of interleukins 10 and 12 by activated peripheral blood monocytes/macrophages in patients suffering from chronic hepatitis C virus infection with respect to the response to interferon and ribavirin treatment. Immunol Lett. 2002;83:209–214. doi: 10.1016/s0165-2478(02)00102-5. [DOI] [PubMed] [Google Scholar]
- 33.Shibata Y, Foster LA, Kurimoto M, Okamura H, Nakamura RM, Kawajiri K, et al. Immunoregulatory roles of IL-10 in innate immunity: IL-10 inhibits macrophage production of IFN-gamma-inducing factors but enhances NK cell production of IFN-gamma. J Immunol. 1998;161:4283–4288. [PubMed] [Google Scholar]
- 34.Cramp ME, Rossol S, Chokshi S, Carucci P, Williams R, Naoumov NV. Hepatitis C virus-specific T-cell reactivity during interferon and ribavirin treatment in chronic hepatitis C. Gastroenterology. 2000;118:346–355. doi: 10.1016/s0016-5085(00)70217-4. [DOI] [PubMed] [Google Scholar]
- 35.Yuen MF, Wong DK, Zheng BJ, Chan CC, Yuen JC, Wong BC, et al. Difference in T helper responses during hepatitis flares in hepatitis B e antigen (HBeAg)-positive patients with genotypes B and C: implication for early HBeAg seroconversion. J Viral Hepat. 2007;14:269–275. doi: 10.1111/j.1365-2893.2006.00799.x. [DOI] [PubMed] [Google Scholar]
- 36.Tsai SL, Liaw YF, Chen MH, Huang CY, Kuo GC. Detection of type 2-like T-helper cells in hepatitis C virus infection: implications for hepatitis C virus chronicity. Hepatology. 1997;25:449–458. doi: 10.1002/hep.510250233. [DOI] [PubMed] [Google Scholar]
- 37.Szkaradkiewicz A, Jopek A, Wysocki J, Grzymislawski M, Malecka I, Wozniak A. HBcAg-specific cytokine production by CD4 T lymphocytes of children with acute and chronic hepatitis B. Virus Res. 2003;97:127–133. doi: 10.1016/j.virusres.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Chang JJ, Thompson AJ, Visvanathan K, Kent SJ, Cameron PU, Wightman F, et al. The phenotype of hepatitis B virus-specific T cells differ in the liver and blood in chronic hepatitis B virus infection. Hepatology. 2007;46:1332–1340. doi: 10.1002/hep.21844. [DOI] [PubMed] [Google Scholar]
- 39.Koziel MJ, Dudley D, Wong JT, Dienstag J, Houghton M, Ralston R, et al. Intrahepatic cytotoxic T lymphocytes specific for hepatitis C virus in persons with chronic hepatitis [published erratum appears in J Immunol 1993 Mar 15;150(6):2563] J Immunol. 1992;149:3339–3344. [PubMed] [Google Scholar]
- 40.Kamal SM, Fehr J, Roesler B, Peters T, Rasenack JW. Peginterferon alone or with ribavirin enhances HCV-specific CD4 T-helper 1 responses in patients with chronic hepatiti C. Gastroenterology. 2002;123:1070–1083. doi: 10.1053/gast.2002.36045. [DOI] [PubMed] [Google Scholar]
- 41.Hempel G, Galle PR, Lohr HF. Quantitative analysis of specific Th1/Th2 helper cell responses and IgG subtype antibodies in interferon-alpha-treated patients with chronic hepatitis C. J Med Virol. 2001;64:340–349. doi: 10.1002/jmv.1056. [DOI] [PubMed] [Google Scholar]
- 42.Amati L, Caradonna L, Magrone T, Mastronardi ML, Cuppone R, Cozzolongo R, et al. Modifications of the immune responsiveness in patients with hepatitis C virus infection following treatment with IFN-alpha/ribavirin. Curr Pharm Des. 2002;8:981–993. doi: 10.2174/1381612024607036. [DOI] [PubMed] [Google Scholar]
- 43.Bergamini A, Bolacchi F, Cepparulo M, Demin F, Uccella I, Bongiovanni B, et al. Treatment with ribavirin and interferon-alpha reduces interferon-gamma expression in patients with chronic hepatitis C. Clin Exp Immunol. 2001;123:459–464. doi: 10.1046/j.1365-2249.2001.01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shapiro S, Gershtein V, Elias N, Zuckerman E, Salman N, Lahat N. mRNA cytokine profile in peripheral blood cells from chronic hepatitis C virus (HCV)-infected patients: effects of interferon-alpha (IFN-alpha) treatment. Clin Exp Immunol. 1998;114:55–60. doi: 10.1046/j.1365-2249.1998.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bozkaya H, Bozdayi AM, Aslan N, Turkay C, Sarioglu M, Cetinkaya H, et al. Circulating IL-2 and IL-10 in chronic active hepatitis C with respect to the response to IFN treatment. Infection. 2000;28:309–313. doi: 10.1007/s150100070025. [DOI] [PubMed] [Google Scholar]
- 46.Barnes E, Harcourt G, Brown D, Lucas M, Phillips R, Dusheiko G, et al. The dynamics of T-lymphocyte responses during combination therapy for chronic hepatitis C virus infection. Hepatology. 2002;36:743–754. doi: 10.1053/jhep.2002.35344. [DOI] [PubMed] [Google Scholar]
- 47.Urbani S, Amadei B, Fisicaro P, Tola D, Orlandini A, Sacchelli L, et al. Outcome of acute hepatitis C is related to virus-specific CD4 function and maturation of antiviral memory CD8 responses. Hepatology. 2006;44:126–139. doi: 10.1002/hep.21242. [DOI] [PubMed] [Google Scholar]
- 48.Kamal SM, Rasenack JW, Bianchi L, Al Tawil A, El Sayed Khalifa K, Peter T, et al. Acute hepatitis C without and with schistosomiasis: correlation with hepatitis C-specific CD4(+) T-cell and cytokine response. Gastroenterology. 2001;121:646–656. doi: 10.1053/gast.2001.27024. [DOI] [PubMed] [Google Scholar]
- 49.Luik A, Knapp S, Thursz M, Thomas HC, Schlaak JF. Autoregulatory role of interleukin-10 in hepatitis C patients treated with IFN-alpha. J Interferon Cytokine Res. 2004;24:585–593. doi: 10.1089/jir.2004.24.585. [DOI] [PubMed] [Google Scholar]
- 50.Yakushijin T, Kanto T, Inoue M, Oze T, Miyazaki M, Itose I, et al. Reduced expression and functional impairment of Toll-like receptor 2 on dendritic cells in chronic hepatitis C virus infection. Hepatol Res. 2006;34:156–162. doi: 10.1016/j.hepres.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 51.Szabo G, Dolganiuc A, Mandrekar P, White B. Inhibition of antigen-presenting cell functions by alcohol: implications for hepatitis C virus infection. Alcohol (Fayetteville, NY) 2004;33:241–249. doi: 10.1016/j.alcohol.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 52.Kohyama M, Kakehi M, Totsuka M, Hachimura S, Hisatsune T, Kaminogawa S. Selective induction of CD8+ T cell functions by single substituted analogs of an antigenic peptide: distinct signals for IL-10 production. FEBS Lett. 1998;423:138–142. doi: 10.1016/s0014-5793(98)00082-9. [DOI] [PubMed] [Google Scholar]
- 53.Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, et al. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kohyama M, Sugahara D, Sugiyama S, Yagita H, Okumura K, Hozumi N. Inducible costimulator-dependent IL-10 production by regulatory T cells specific for self-antigen. Proc Natl Acad Sci U S A. 2004;101:4192–4197. doi: 10.1073/pnas.0400214101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Witsch EJ, Peiser M, Hutloff A, Buchner K, Dorner BG, Jonuleit H, et al. ICOS and CD28 reversely regulate IL-10 on re-activation of human effector T cells with mature dendritic cells. Eur J Immunol. 2002;32:2680–2686. doi: 10.1002/1521-4141(200209)32:9<2680::AID-IMMU2680>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 56.Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR, Liu C, et al. An immunomodulatory role for CD4(+)CD25(+) regulatory T lymphocytes in hepatitis C virus infection. Hepatology. 2004;40:1062–1071. doi: 10.1002/hep.20454. [DOI] [PubMed] [Google Scholar]
- 57.Bolacchi F, Sinistro A, Ciaprini C, Demin F, Capozzi M, Carducci FC, et al. Increased hepatitis C virus (HCV)-specific CD4+CD25+ regulatory T lymphocytes and reduced HCV-specific CD4+ T cell response in HCV-infected patients with normal versus abnormal alanine aminotransferase levels. Clin Exp Immunol. 2006;144:188–196. doi: 10.1111/j.1365-2249.2006.03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alatrakchi N, Graham CS, van der Vliet HJ, Sherman KE, Exley MA, Koziel MJ. Hepatitis C virus (HCV)-specific CD8+ cells produce transforming growth factor beta that can suppress HCV-specific T-cell responses. J Virol. 2007;81:5882–5892. doi: 10.1128/JVI.02202-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, et al. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med. 2006;203:2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, et al. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology. 2006;43:1303–1310. doi: 10.1002/hep.21176. [DOI] [PubMed] [Google Scholar]


