Abstract
Sphingosine1-phosphate (S1P) is a lipid mediator involved in diverse biological processes, from vascular and neural development to the regulation of lymphocyte trafficking. Many of its functions are regulated by five widely expressed S1P G-protein coupled receptors (S1P1-5). S1P is produced mostly intracellularly, thus, much of its potential as an autocrine and paracrine mediator depends on how, when, and where it is generated or secreted out of the cells. However, S1P can also have intracellular activity independent of its receptors, adding to the complexity of S1P function. The mast cell, a major effector cell during an allergic response, has proven instrumental towards understanding the complex regulation and function of S1P. Antigen (Ag) engagement of the IgE receptor in mast cells stimulates sphingosine kinases, which generate S1P and are involved in the activation of calcium fluxes critical for mast cell responses. In addition, mast cells secrete considerable amounts of S1P upon activation, thus affecting the surrounding tissues and recruiting inflammatory cells. Export of S1P is also involved in the autocrine transactivation of S1P receptors present in mast cells. The in vivo response of mast cells, however, is not strictly dependent on their ability to generate S1P, but they are also affected by changes in S1P in the environment previous to Ag challenge. This review will discuss the recent advances towards understanding the intricacies of S1P generation, secretion and regulation in mast cells. In addition, how S1P receptors are activated and their involvement in mast cell functions will also be covered, including new insights on the role of S1P in the mast cell-mediated allergic response of systemic anaphylaxis.
Keywords: Sphingosine kinase, sphingosine-1-phosphate, mast cells, anaphylaxis
1. The many strategies for Sphingosine 1-phosphate’s control of biological processes
Sphingosine (SPH), first described in the eighteen hundreds, was named after the Sphinx, a Greek mythological character with a human face and a winged lion body, owing to the mysterious properties of this compound. Its phosphorylated derivative, Sphingosine-1-phosphate (S1P), was later described as a metabolite of SPH and complex sphingolipids. In due course, it has been proven that the properties and functions of S1P were not less enigmatic than those of SPH and that the multiple facets of its mode of action fit well with the multiple-natured creature of its Sphinx-rooted name.
Over the years we have become cognizant of the critical involvement of S1P in multiple physiological and pathological processes in cells and organisms and its versatile mechanisms of action. S1P promotes proliferation, cell survival, and chemotaxis in diverse cell types, differentiation of endothelial cells, neural and vascular development, vascular permeability, angiogenesis, tumorigenesis and lymphocyte trafficking [1-8]. S1P binds specifically to five membrane-bound G-protein coupled receptors (GPCR), named S1P1-5, that exhibit similar affinities overall but engage distinct signaling pathways [4, 9]. The expression patterns of these receptors vary from cell to cell and are subject to regulation by various factors in the cellular environment. The resulting qualitative and quantitative combinations of the receptors partly explain the diverse actions of this lysophospholipid. In addition, S1P, similar to other sphingolipid metabolites, has been shown to affect cell function independently of cell surface receptors [2]. This could be achieved by binding and modifying yet unknown intracellular targets, or by modifying the relative levels of other bioactive lipid products, particularly SPH and ceramide whose effects generally oppose those of S1P, or other lipids with essential cellular functions (Figure 1) [2, 3, 10-12]. To this date, the exact mechanisms for its intracellular effects remain unresolved.
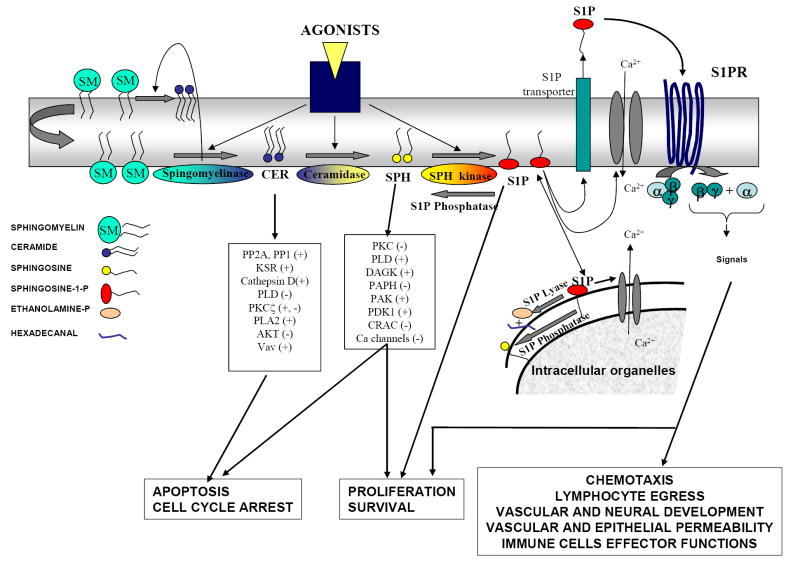
FIGURE 1. Intracellular regulation of ceramide, sphingosine and sphingosine-1-phosphate.
A variety of cell receptor agonists and environmental stimuli sequentially and/or selectively activate sphingomyelinases (that cleave the phosphocholine group of sphingomyelin to yield ceramide), ceramidases (that cleave the fatty acid chain of ceramide to form SPH), and sphingosine kinases (that phosphorylate SPH in its primary hydroxyl group to form S1P). Consequently, the levels of ceramide, SPH, S1P, or a combination of these lipids are elevated in cells [10]. All of them are bioactive lipids that can activate or inhibit various signaling pathways by affecting key signaling proteins. The actions of ceramide and SPH in many cell systems oppose those of S1P. S1P can act as a second messenger inside cells. Although the exact targets are unknown, it has been reported to affect a variety of calcium channels. S1P can be exported outside cells by transporters and bind a family of GPCR coupled receptors (S1P1-5) present at the plasma membrane. Some isoforms of sphingomyelinase, ceramidase, and sphingosine kinase can be secreted under certain conditions, and the activation of these secreted enzymes can generate sphingolipid metabolites in the extracellular environment. Enzymes involved in the degradation of S1P (S1P phosphatases and S1P lyase) are also critical for the fine-tuning of S1P levels inside and outside cells. For clarity, the generation of sphingolipid metabolites is depicted at the inner leaflet of the plasma membrane, probably the major active signaling pool, but other intracellular membrane locations are possible (see reviews for more details [2, 10, 107, 108]).
S1P is formed in cells by the phosphorylation of SPH mediated by two phylogenically conserved sphingosine kinases (SphK1 and SphK2) [13] and may also be produced outside cells by a secreted form of SphK1 [14]. The activity and cellular location of SphK1 and SphK2 are regulated by stimuli resulting in rapid increases in the levels of S1P. However, these increases are generally short-lived and the resting levels of S1P are maintained low, due to its irreversible degradation by a S1P lyase, its dephosphorylation to SPH by S1P phosphatases, and the relocation of sphingosines kinases from membranes to the cytosol, away from their substrate [15]. Other enzymes in the metabolic pathway of sphingolipids may also be involved in the regulation of S1P levels or function (Figure 1) [10]. S1P formed intracellularly can be exported to the extracellular media engaging S1P receptors in an autocrine or paracrine fashion (Figure 1), adding to the repertoire of possible physiological actions of this lipid mediator in vivo [16]. Interestingly, there is a reservoir of S1P in plasma (0.4 to 2 μM, which is well above the binding affinity for its receptors), mostly bound to albumin or lipoproteins [17]. Recent studies pointed to hematopoietic cells, particularly erythrocytes [18, 19], as the cell type responsible for maintaining high plasma S1P, but not lymph S1P. Other blood cells and endothelial cells may also participate in the regulation of circulating S1P [17]. Evidence suggests that the concentrations in the interstitium of lymph nodes and spleen, and probably other tissues, is significantly lower due to the presence of S1P lyase activity [20] and phosphatase activities [21], allowing cells in those microenvironments to become susceptible to local changes in S1P concentration.
The recent discovery that S1P is involved in lymphocyte egress from the thymus and secondary lymph nodes into the circulation has also highlighted the importance of maintaining the physiological gradients of S1P and regulating the expression S1P receptors for normal immune function. Lymphocytes migrate out of the lymphoid organs, where S1P levels are low, toward higher concentrations of S1P in blood and lymph, a process mediated by the S1P1 receptor. Lymphocytes exiting the thymus and lymph nodes show an upregulated expression of S1P1 and respond chemotactically towards S1P [22], but once exposed to the high amounts of S1P in the circulation the receptor expression is downregulated and not restored again until they reach the lymph nodes [4, 6]. Remarkably, downregulation of S1P1 after T cell receptor activation [23] appear to contribute to the retention and expansion of antigen-bearing cells in the lymph nodes [22]. Similarly, the homing of natural killer cells to blood, and peripheral tissues is partly regulated by S1P1 [24] and by S1P5 [25]. In addition to the role of S1P gradients in the homing of lymphocytes, local S1P production or regulation of its receptors may alter the phenotypic outcome of immune cells (see sections 2.3.1 and 2.4) and modify the type of immune response [26-29]. Thus, maintenance of blood or lymph to tissue gradients, as well as changes in S1P within the tissues may regulate the emerging and differentiation of immune cells. The intricate regulation of S1P levels in cells and in the organism as a whole, its receptors and its mode of action (receptor-mediated or receptor independent), allows for plasticity in the overall responsiveness of cells to this single molecule (Figure 2).
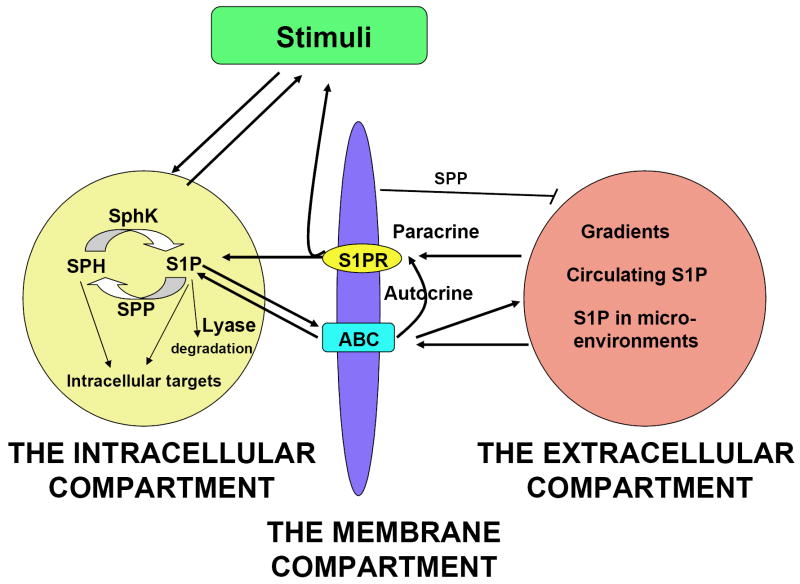
FIGURE 2. Schematic representation of the different compartments involved in the regulation and function of S1P in vivo.
At the intracellular compartment, S1P is formed after stimulation by different agonists, and it signals as an intracellular mediator. Other enzymes involved in the metabolism of sphingolipids (see Figure 1) can alter its levels and the levels of other metabolites to shape the cellular response. At the membrane compartment, S1P can exit the cell via plasma membrane transporters and engage autocrine (via binding to S1P receptors in the same cell type) or paracrine loops (via binding to S1P receptors in other cell types). At the extracellular compartment, local tissue S1P levels, circulating S1P or blood-tissue gradients may affect the expression of S1P receptors in different cells and their function in proximal or distant cells. The S1P present in the extracellular environment originates from the export of intracellularly generated S1P. Alternatively, secreted sphingolipid enzymes (see Figure 1) or, potentially, autotaxin can generate S1P extracellularly. Various stimuli or changes in physiological conditions can indirectly change S1P levels in the extracellular environment (i.e regulation of S1P lyase or S1P phosphatases (SPP), activation of platelets or mast cells, etc), affecting other cells responses. Reciprocally, activation of S1PR may alter the composition of external stimuli in the physiological environment.
2. Mast cells and the multifaceted function of S1P
A growing body of evidence has implicated S1P metabolism and its receptors in mast cell function. A great deal of the complexity described for S1P regulation and function is represented in the mast cell. Both SphK1 and SphK2 are activated in these cells after the engagement of the high affinity receptor for IgE and S1P is produced in cells within minutes and secreted into the extracellular space as a late response. The activities of SphKs are key to the activation and expression of the receptors for S1P present on mast cells but also essential to S1P receptor-independent mast cell function. When activated, mast cells produce gradients of S1P within resident tissues that may contribute to the recruitment and activation of other immune cells. Furthermore, recent evidence suggests that deregulated S1P levels in circulation can affect the responsiveness of mast cells and the allergic response. This review will summarize, following the structure illustrated in Figure 2, our current understanding of these processes in mast cells and discuss their overall significance.
2.1- What are mast cells?
Mast cells are tissue-resident cells that are critical for innate and acquired immunity [30]. Mast cells bear various receptor types and thus, they respond to a variety of stimuli. However, they are characterized by the presence of the high affinity receptor for IgE, FcεRI, which is a critical player in allergic disease [31]. IgE bound to this receptor is crosslinked by exposure to a multivalent antigen, clustering receptors and initiating an intricate cascade of signals that culminate in the compound exocytosis of preformed granules (a process known as degranulation) and the production and secretion of cytokines and lipid mediators [32]. The result is the appearance into the extracellular space of an impressive array of vasoactive mediators, proteases, chemokines, and cytokines that enhance vascular permeability, recruitment and function of leukocytes, and cause local inflammation. Besides engagement of the IgE receptor, other stimuli can trigger the selective release of certain cytokines or other mediators, and thus, the function of mast cells is not restricted to allergic reactions triggered by an antigen [32, 33]. However, the dramatic increase in the prevalence of allergic disease over the last 20 years has prompted more intense research on the signaling mechanisms essential for mast cell responses, with the intent of finding alternative targets for pharmacological intervention.
2.2- The intracellular compartment: generation of S1P by mast cells
2.2.1- Sphingosine kinases, key players in mast cell function
Activation of the IgE receptor in mast cells results in the intracellular formation of S1P and its export into the extracellular space [34-38]. Using mast cells derived from embryonic liver progenitors of mice deficient in SphK1, SphK2 or both, SphK2 was shown to be the major producer of S1P [39]. The functional consequence of SphK2 deficiency was broad, including a reduction in the extent of degranulation and in the production of various cytokines and eicosanoid products, all of which are key in the induction of the allergic response and inflammation. The underlying cause for the overall shutdown in mast cell responsiveness was an impairment of calcium influx and PKC activation [39] (Figure 3). Calcium influx is known to be an essential process for IgE receptor-induced mast cell degranulation and cytokine production. Thus, the placement of SphK2 activity as a control mechanism for calcium flux and mast cell responses may be of critical importance to the understanding of calcium regulation in these cells and to the development of drugs that alleviate the allergic response.
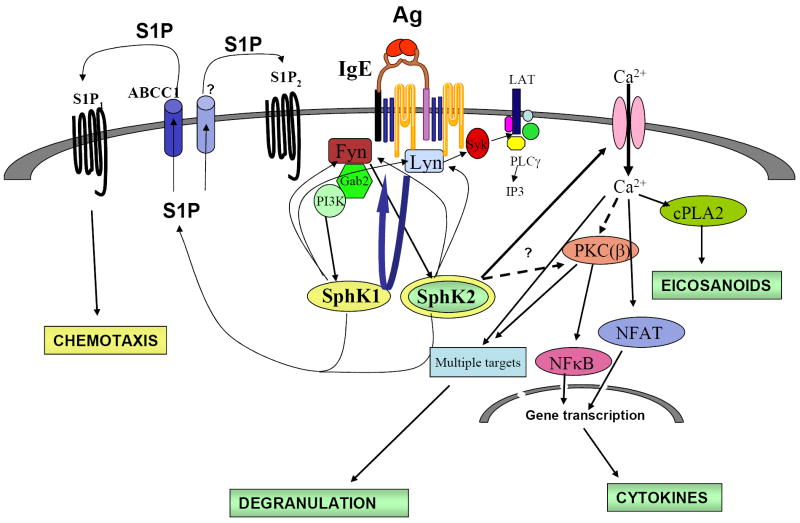
FIGURE 3. Activation and function of sphingosine kinases in murine bone-marrow derived mast cells.
After FcεRI engagement, the membrane-localized Src Kinases Lyn and Fyn form complexes with SphKs. This localizes SphKs to the membrane and to lipid rafts, where their substrate, SPH, is enriched. Fyn is necessary also for the activation of SphK1 and SphK2. Activated Fyn provides both Gab2/PI3K-dependent and -independent signals that are key to full activation of SphK1 and SphK2, respectively. The dependence on PI3K can be related to a direct effect of PIP3 on SphKs, its importance in PLD activation and phosphatidic acid generation, or other downstream signaling partners. SphK activities, in turn, may enhance Fyn and Lyn activities. SphK2 is essential for FcεRI-induced calcium influx from the extracellular media, PKC activation and consequent NFκB activation and thus, it affects degranulation, arachidonic acid, leukotriens and cytokine production. Furthermore, S1P production is required for the transactivation of the receptors S1P1 and S1P2. Presence of S1P2 is important for proper degranulation, and S1P1 for the to the movement of mast cells towards an Ag gradient. In murine mast cells, both SphK1 and SphK2 are necessary for chemotaxis towards Ag. An ABCC1-type of transporter is involved in the secretion of S1P into the media and in the transactivation of S1P1 but not S1P2. Other transporters may also participate in the secretion of S1P. Mast cell secreted S1P can promote inflammation by activating and recruiting other immune cells involved in allergic and inflammatory responses.
The finding of this significant role of SphK2 in mast cell effector function is surprising since most the studies in mammalian cells have implicated a function for SphK1 [16], while the function of SphK2 has remained elusive. It has been suggested that SphK1 may have evolved later in phylogeny as complexity in functions increased, while SphK2 may have retained more basic functions such as the regulation of sphingolipid levels in cells [40, 41]. This was based on a higher degree of homology of SphK2 to sphingosine kinase genes in lower organisms and the wider range of substrates SphK2 can phosphorylate as compared to SphK1, including sphingomimetics like the immunosuppressor FTY720 and sphingoid bases other than SPH that are found in less evolved organisms [3, 42-44]. In organisms such as Drosophila, the SphK2 homolog is critical for proper flight performance and fecundity [40]. Recent reports indicate that the mast cell, unlike other immune cells, is an ancient cell type found in urochordata that probably played a role in early innate immunity [45], so it is tempting to speculate that an earlier form of sphingosine kinase, SphK2, gained functional prevalence during evolution in this “mast cell-like” cell. The predominance of SphK2 found in the genetic deletion model contrasted with the findings in other mast cell types using messenger knockdown approaches. In the transformed rat mucosal mast cell-like RBL2H3 line, activation of SphK1, but not SphK2, was involved in degranulation upon Ag challenge and chemotaxis towards Ag [35]. In human mast cells, the observations have been variable. In human bone marrow-derived mast cells, antisense oligonucleotide techniques showed that SphK1 was involved in degranulation, while SphK2 message was not found [36]. In contrast, in CD34+-derived human mast cells derived from blood, SphK2 and SphK1 activities and their corresponding messages were present and both were activated by stimulation of the IgE receptor [37]. A more recent study, using silencing of SphK1 and SphK2 by RNAi in cord blood derived human mast cells and in the transformed human mast cell line LAD2, showed that SphK1 was important for degranulation, migration toward antigen and secretion of the chemokine CCL2/MCP1, while SphK2 drastically affected cytokine production [46]. It is important to note that in mammalians, mast cells are not a homogeneous cell population [32]. Their phenotype varies depending on the environment they populate in vivo, and on the experimental conditions under which the primary cultures are differentiated in vitro, and thus the reasons for the discrepancies related above might reflect differences in the phenotype of these cells, in the experimental conditions used in the studies or in the species of origin. This issue is of relative importance when considering one of these isoforms as a target for drug discovery to treat diseases such as asthma, allergic dermatitis or other allergic diseases in which different mast cell populations participate, and thus more evidence is needed to determine whether SphK1 and SphK2 differ in their functional roles depending on the mast cell population or the species. Regardless, the studies clearly demonstrate a critical role of SphKs in mast cell function and potential overlapping roles for these isoforms since both can mediate degranulation and cytokine production in phenotypically different mast cells. However, their role in a particular cell appears to be specific and non redundant. In agreement, SphK2 has been reported to enable EGF-mediated chemotaxis in the breast cancer cell line MDA-MB-543 but not in HEK293, where SphK1 is important [47]. Additionally, opposite cellular functions for SphK1 and SphK2 such as cell survival have also been described in other cell types [48]. The division of function of the two SphK isoforms may be a reflection of their cellular distribution in cells, their relative abundance, the signaling mechanisms leading to their activation or the proximity to the targets they modify. These complexities add to the flexibility of spatio-temporal regulation of S1P generation and enhance the range of S1P actions. A deeper understanding of the localization of SphK1 or SphK2 during activation with respect to other signaling components as well as S1P receptors will also aid in the understanding of their relative contribution and function under particular stimuli.
2.2.2- SphK2-induced calcium regulation
The critical importance of calcium mobilization in the regulation of mast cell responses by FcεRI has been long recognized [31]. Calcium release from intracellular stores by IgE/Ag involves PLCγ activation and inositol (3,4,5)-trisphosphate (IP3) formation [49], although others have also implicated SphK1 and S1P in mast cell calcium mobilization [34, 36]. However, release from intracellular stores cannot account for the overall calcium responses in mast cells, and instead, the extracellular calcium pool appears to be the major contributor for calcium and mast cell responses [31]. A type of store-operated channel (SOC), the calcium-release activated channel (CRAC), was identified in mast cells [50] and considered a major component responsible for calcium flux across the plasma membrane. The real nature and the regulation of this channel have, however, remained elusive. Just recently, a pore-forming component (Orai1) [51-53] and a regulatory component or “store calcium sensor” (STIM1) [54, 55] of the CRAC channel were identified and demonstrated to be key for IgE/Ag-mediated calcium influx, mast cell degranulation and cytokine production in vitro and in vivo [56, 57]. The mechanism by which STIM1 regulates Orai1 activity is still unclear. Interestingly, the findings in SphK2-deficient mast cells indicated that the activation of SphK2 is crucial for calcium uptake upon Ag stimulation (Figure 3), but not for calcium mobilization from ER stores [39] as the previously reported function of SphK1 in RBL2H3 and cord-blood derived human mast cells [34, 36, 58]. Thus, full exploration of the type of channel targeted by SphK2 may provide some clues into the mechanism of activation of CRAC channels or other types of calcium channels also involved in calcium entry in mast cells and a variety of immune or non-immune cells [59-62].
The regulation of calcium influx by SphK2 did not involve S1P1 or S1P2, the S1P receptors expressed in mast cells [35]. Unlike other cell types [63], wild type or SphK2-deficient mast cells did not mobilize calcium in response to S1P added exogenously alone or in combination with IgE/Ag [39]. Since SphK2 controls the intracellular levels of S1P and SPH in mast cells in the resting and activated states, it is possible that an elevation in intracellular S1P leads to the opening of a calcium gate, or that a reduction of SPH releases a standing inhibition of the calcium channel [39, 64]. Both mechanisms have been documented in various cell types. Intracellular S1P promotes calcium influx in yeast [65], activates calcium-induced calcium entry in human neutrophils [66], activates TRPC5 calcium channels in vascular smooth muscle cells [67], and calcium-activated K+ (BKCa) channels in endothelial cells [68]. In contrast, SPH has been described to inhibit voltage-operated calcium channels [69, 70], the sodium/calcium exchanger [71] and CRAC channels in RBL2H3 cells [64]. Mathes et al proposed that steady state levels of SPH would maintain CRAC currents in a blocked, resting state. After FcεRI stimulation, the levels SPH drop as S1P increases favoring the proposed responsive state of mast cells, and allowing for the full calcium responses induced by FcεRI. In support for this model, Prieschl and colleagues observed that addition of exogenous SPH inhibited mast cell degranulation, leukotriene and TNFα production, effects that could be effectively reversed by S1P, proposing that the balance between SPH and S1P is decisive for the excitability of mast cells [38]. It is interesting to note that an essential characteristic of some calcium channels is their modulation by lipids [72]. The various observations that S1P or SPH are able to modify the function of diverse types of ion channels, raises the possibility of their direct interactions with the channels or modification of the lipid environment which may influence conformation and function of the channel. Resolving whether the effects on calcium by SphK2 activation reflect the elimination of the negative regulator SPH or production of an activator, S1P, or both, can be difficult without determining which is the specific channel. Equally challenging is to understand mechanistically how sphingolipid metabolites may affect this channel. However, answering these questions may be of considerable general interest and of importance in understanding diverse physiological and pathological conditions.
2.2.3- Mechanisms of activation of SphKs
Sphingosine kinases are activated in most cells by growth factor receptors, GPCR coupled receptors, cytokines and immunoglobulin receptors. Several studies have partially revealed the mechanisms of such activation, demonstrating the involvement of messenger molecules, post-translational modifications, different interactions with signaling proteins, and changes in localization (reviewed in [15, 16]). It generally appears that full activation is accomplished by an enhancement of the catalytic activity and a translocation of SphKs to membrane locations where the substrate is present. These two steps are interconnected and not necessarily mediated by a single mechanism. For example, a well characterized phosphorylation of SphK1 in Ser225 by ERK1/2 is essential for SphK1 activation and translocation to the membrane by TNFα and phorbol esthers in HEK293 cells [73]; however, phosphorylation alone may not be sufficient for the stabletranslocation of SphK1 since a mutated form of SphK1 that is unable to bind calmodulin but is phosphorylated normally in response to PMA or TNFα, did not translocate to the membrane, suggesting the additional involvement of a calcium/calmodulin dependent mechanism [74]. Along with ERK1/2 mediated phosphorylation and calcium/calmodulin binding, association with other protein partners, cytoskeleton [75], and interactions with negatively charged lipids in the membrane [76-78] may be important for the activation of SphK1 (reviewed in [15]) depending on the cell type, stimulus or locale they are distributed into.
Much less is known about the mechanisms of activation of SphK2, due in part to the more recent discovery of its agonist-mediated activation and participation in biological processes in mammalian cells. Unlike SphK1, which has been commonly described as a cytosolic enzyme in a resting state, the localization of SphK2 varies depending on the cell type and the experimental conditions, probably owing to the presence of a nuclear localization signal [79] and putative membrane domains [43]. Phosphorylation of SphK2 in Ser351 (equivalent to Ser225 of SphK1) and Thr578 by ERK1 in the cancer cell line MDA-MB-593, mediates EGF-induced SphK2 activation but not its translocation, as in these cells SphK2 is already present at the plasma membrane [80], while phosphorylation of SphK2 by protein kinase D in Ser419/Ser421 in HeLa cells treated with PMA induced the exit of SphK2 from the nucleus [81]. In mast cells, however, SphK2 is mostly cytosolic and it has little representation in the cell membrane under resting conditions [37]. Even after engagement of the IgE receptor, which increases the proportion of membrane-associated SphK2, most of it remains cytosolic, indicating that activation of discrete SphK pools is sufficient for proper signaling. Exploration of the signaling events necessary for the activation of SphK1 and 2 by FcεRI, indicated a complex interplay of protein kinase- and lipid-derived signals and suggested that alternative mechanisms to those described above may be in place in mast cells [37].
The FcεRI is a tetrameric receptor (α, β and two γ chains) containing in its β and γ chains immunoreceptor tyrosine-based activation motifs (ITAMs) necessary for signal propagation [31]. Multivalent antigens bring IgE receptors in proximity allowing the ITAMs to be transphosphorylated by the Src kinase Lyn, which is associated to the β subunit of the receptors [82]. Phosphorylated ITAMS can bind a variety of positive (such as the essential tyrosine kinase Syk, which is itself phosphorylated by Lyn) as well as negative regulators of signaling, that altogether shape the signaling responses. Another Src kinase, Fyn, is essential in the initiation of signaling cascades and mast cells responses although the details on how Fyn is activated upon receptor engagement are still tenuous. The signaling of FcεRI is intricate and redundant and involves the initiating tyrosine kinases Lyn and Fyn, and a number of adaptor proteins (including LAT and Gab2) that once phosphorylated by tyrosine kinases recruit other kinases and lipid enzymes that control mast cell responsiveness (reviewed in [82]). The initiation and propagation of signals is highly compartmentalized due to the formation of multi-molecular signaling complexes (termed signalsomes) assembled by the adaptor proteins and localized to specific regions within the plasma membrane [83]. Some of these regions are liquid-ordered phase domains in the plasma membrane, which are enriched in cholesterol, sphingolipids and other saturated phospho-lipids (lipid rafts). Thus, the rapid activation of SphK1 and SphK2 must occur in the midst of an extremely organized, compartmentalized and redundant network of signals and messenger molecules, many of which are in turn affected by SphK activity.
Fyn and Lyn kinases were found to directly interact with SphK1 and SphK2. Furthermore the presence of Fyn activity is essential for activation of SphKs by FcεRI whereas Lyn is partly dispensable [37, 84]. Neither SphK1 nor SphK2 are phosphorylated by these tyrosine kinases, and the interaction of SphK2 with Fyn or Lyn did not affect its catalytic activity in vitro. However, Fyn or Lyn-deficient cells could not effectively induce early translocation of SphKs to the membrane, suggesting that the interaction with these Src kinases facilitate both the location of SphKs to the membrane and their activation. The involvement of Fyn and Lyn, initiators of FcεRI signaling, places SphKs activation proximal to the receptor, lipid rafts where sphingosine is located, and to the signalosomes where signals are generated. Fyn phosphorylates the adapter Grb2-associated binder 2 (Gab2), which then binds the p85 regulatory subunit of phosphatidylinositol 3-OH kinase (PI3K) [85]. Both Gab2 and PI3K activity were essential for SphK1 activation, and partially responsible for SphK2 activation, indicating additional Fyn-dependent, PI3K- independent signals required for the activation of SphK2 (Figure 3). In contrast to other cells [15, 16], calcium elevations or PKC activation were not amongst the signals participating in the activation or translocation of SphKs in mast cells. Instead, SphK2 activation is upstream of these events as discussed earlier [39]. A possible signal for the activation of SphK2 could be an ERK1/2- mediated Ser/Thr phosphorylation of SphK2 as observed in other cells [80]. However, Fyn deficient mast cells showed normal ERK1/2 phosphorylation but no activation of SphK2 in response to Ag. Thus, if ERK1/2 were a player, one would have to envision a scenario where the presence of Fyn is required for a localized, ERK1/2-mediated activation of SphK2, similar to the described requirement of TRAF2 for ERK1/2-mediated phosphorylation and activation of SphK1 in TNFα-activated HEK293T cells [73].
Although the specific mechanistic detail of activation of SphK1 and 2 in these cells is not entirely clear, one can conclude that tyrosine kinases, particularly Fyn, the adaptor protein Gab2 and the lipid kinase PI3K, as well as other undefined signals, provide the environment necessary for the efficient and proper redistribution and activation of SphKs. It is interesting to note that all the components of these particular signaling complexes have been defined as crucial for proper functioning of allergically stimulated mast cells. Loss of Fyn or Gab2 in mast cells impairs cytokine production and degranulation [85, 86]; the intracellular level of phosphatidylinositol (3,4,5)-trisphosphate (PIP3), produced by PI3K downstream of Fyn/Gab2, plays an important role in determining the extent of a mast cell response to a stimulus [82]; and loss of SphK2 impairs cytokine production and degranulation [39]. The observations support the idea that these events are in a common signaling axis and suggest the importance of maintaining the integrity and function of these complexes, since not only the Src kinases can affect SphKs activity, the reciprocal is also true (i.e, SphK activities enhance the tyrosine kinase activity of both Lyn and Fyn [37, 84]) (Figure 3). From a broader perspective, our and other investigator’s findings indicate that SphK1 and 2 have distinct regulatory requirements, but neither is activated by an entirely unique mechanism. The involvement of varied mechanisms may direct the signaling potential of SphKs towards different outcomes in cells in response to diverse stimuli. This is also exemplified in mast cells, where the relative contribution of Fyn and Lyn tyrosine kinases in the activation of SphKs by stem cell factor or interleukin-3 differs from that by IgE/Ag [37]. In-depth knowledge of the mechanisms involved can be of importance from a pharmacological perspective, particularly to intercept unwanted cell responses where SphKs are critical players, such as allergy.
2.3- The membrane compartment: inside-out and outside-in
2.3.1- Pumping out S1P
Activation of SphKs induced by FcεRI in mast cells leads to elevations in the cellular content of S1P and to a substantial release of this mediator into the extracellular space [35, 37, 38]. The export of S1P under certain stimulatory conditions has long been recognized in other cells, mostly because of its involvement in autocrine loops that cause the activation and internalization or desensitization of S1P receptors [2]. However, in those instances S1P in the media was frequently undetectable due to the minute overall amounts required for those actions [2, 87], suggesting a localized process and a tight spatial coordination between S1P production, export and S1P receptor activation. Mast cells and platelets are amongst the few cell types known to release abundant amounts of S1P under agonist stimulation [38, 88]. Secretion of S1P was also observed in unchallenged mast cells treated with its precursor [89], SPH, or in cells overexpressing SphK1 [90], but in either case antigen challenge substantially enhanced the export of S1P, indicating a transport driven by S1P concentrations or an active regulation by the IgE-receptor signaling. The release of S1P is maximal within 30 min to hours of antigen stimulation [37, 38], while an autocrine SphK-dependent transactivation of the S1P receptors in the mast cell (S1P1 and S1P2) is apparent within minutes [35], suggesting that an early, albeit not readily detectable, export of S1P is sufficient for the onset of the autocrine loops. On the other hand, one can deduce that the delayed appearance of abundant S1P in the supernatant from mast cells may have a wider, paracrine impact on surrounding cells [91]. The consequent production of local S1P gradients by stimulated mast cells in the skin, gut and other tissues where mast cells reside may not only recruit a number of immune cells [22, 23, 25, 26, 35, 92, 93], but also influence the type of the immune response. For example, exposure of mature dendritic cells to S1P impairs their ability to initiate T helper 1 (Th-1) but promotes T helper 2 (Th-2) responses of naïve CD4 T cells [26], while exposure of T cell receptor (TCR)- and cytokine-activated CD4 T cells to S1P, increases their differentiation into T helper 17 (Th-17) cells and suppresses Th1 and Th2 cytokine production profiles [28, 29]. Besides immune cell recruitment and function, local production of S1P can influence the response of non-immune cells in the surrounding tissue and contribute to the pathology of diseases such as asthma (reviewed in [91, 94]). The finding that S1P was elevated in the airways of asthmatic individuals after challenge [95] and in the joints of arthritic individuals [96] supports its potential role in allergic inflammation, although the exact significance of such elevation remains to be demonstrated.
An intriguing question is how an amphiphilic molecule such as S1P, can be secreted by cells and how is the process regulated. Activated platelets and mast cells secrete their granular contents by exocytosis, but this process is very unlikely to be the mechanism for S1P secretion because of the difference in kinetics for granule secretion compared to S1P appearance in the media. In addition, in mast cells, unlike platelets, S1P is synthesized after stimulation and not pre-accumulated, making unlikely its storage in exocytotic granules. It was recently shown that degranulation of mast cells can be inhibited without affecting Ag-induced S1P secretion, demonstrating the dissociation between these processes [89]. The involvement of ATP binding cassette (ABC) family of proteins has been reported in the transport of S1P [89, 97, 98] and other structural derivatives of S1P [99] across the membrane. In platelets, however, both ATP-independent and ATP-dependent transport mechanisms have been described [98, 100]. The ATP-dependent mechanism of S1P export was identified as an ABCA-like transporter, sensitive to glyburide but not to MK571 or cyclosporine A [98]. In contrast, in murine and human mast cells, the export of S1P is markedly reduced by MK571, an inhibitor of the ABCC1, and by downregulation of this transporter with siRNA, but not by inhibitors of the ABCB1 type transporters [89]. Thus, different transporters may mediate S1P export and the predominance of a particular type may depend on their expression pattern in a cell, its location in relationship with the site of S1P generation, or the type of trigger. The task of sorting out these queries is a significant one, particularly in the light of the potential contributory role of S1P released by different populations of mast cells in allergic airway hypersensitivity and asthma [94].
2.3.2- S1P receptors in mast cells: enhancers of IgE/Ag-mediated responses
Secretion of S1P by mast cells can enhance their function by binding to S1P1 and S1P2 receptors on mast cells [35]. The activation of S1P1 and S1P2 in mast cells in response to IgE/Ag stimulation was recognized by the re-distribution of β-arrestin from cytosol to membrane where it interacts with the activated S1P receptor, and the ensuing endocytosis of the receptor complexes, two general GPCR-triggered events. IgE/Ag-induced endocytosis of S1P1 or S1P2 seemed to be ligand dependent since a competitive inhibitor of SphK, dimethylsphingosine, blocked it [35]. Internalization of S1P receptors independently of FcεRI engagement was also observed in RBL2H3 cells under conditions of increased presence of SphK1 at the plasma membrane (i.e, enforced expression of SphK1 in the presence of serum in the culture media) [90], stressing the importance of the generation of S1P at this location for proper receptor activation. Using antisense oligonucleotide-mediated knockdown of S1P1 or S1P2 as well as S1P2-deficient bone marrow derived mast cells, it became clear that each of these receptors had a specific, non-overlapping role in FcεRI stimulated mast cells [35]. S1P1 is involved in the migration of mast cells toward low concentrations of antigen, while S1P2 participates in FcεRI-induced degranulation. The involvement of the SphK/S1P receptor activation in mast cell responses is further highlighted by the faulty degranulation and migration of Fyn-deficient mast cells, which also show impaired SphK activation and S1P production. Partial restoration of the defective degranulation in these cells was achieved by addition of exogenous S1P [37]. Interestingly, while knockdown of S1P1 or ectopic expression of S1P1 did not affect degranulation, overexpression of S1P2 negatively regulated chemotactic motility of RBL cells towards Ag [35], suggesting a countermigratory role of S1P2 in mast cells as has been documented in other cell types. Since S1P2 but not S1P1 mRNA expression is enhanced as a late consequence of FcεRI cross-linking, a plausible hypothesis is that S1P1 participates in the recruitment of mast cells to the site of action driven by an Ag gradient, while S1P2 is involved in the resolution of migration and contributes to degranulation once they have reached the site.
It is interesting to note that inhibition of ABCC1-mediated S1P export blocked migration of mast cells to Ag but not degranulation, underlining the dependence of S1P1 transactivation on newly generated S1P and export [89]. As for S1P2, it is possible that a yet unidentified transporter brings S1P to its proximity, which would implicate the presence of specialized transporters connecting distinct pools of S1P (generated by SphK1 or SphK2, for example) to distinct S1P receptors upon FcεRI engagement (Figure 3). However, one cannot exclude the possibility that a constitutively active S1P2 is needed as a platform for the activation of FcεRI, similar to a model described by Pyne and colleagues for the growth factor receptor PDGF and S1P1 [101]. In agreement with this possibility, S1P added exogenously, even at micromolar concentrations, alone or in combination with IgE/Ag is a poor inducer of degranulation in mast cells while, in contrast, S1P2 deficiency results in a 40-50% decrease in degranulation. This model would also be consistent with the findings that a reduction in the endogenous levels of S1P (as found in Fyn or in SphK2 deficient cells) would result in loss of the constitutively active state of S1P2 and disruption of FcεRI signaling. Although the mast cell is a tissue resident cell and it is found in the circulation mostly as an immature progenitor, they can be exposed to the higher concentrations of circulating S1P in the proximity of blood vessels, where mast cells are normally present. Teleologically speaking it would not be physiologically cost-effective that mast cells degranulate readily to a lipid present normally in high concentration in circulating fluids. However, having a “constitutively active” S1P2 receptor, particularly in those locations more exposed to circulating S1P, could lower the threshold of activation of the FcεRI upon encounter with an Ag. Another question that remains unresolved is whether the impaired degranulation that results from loss in SphK2 can be attributed solely to a lack of S1P generation and S1P2 activation (or constitutive activation), or also to SphK2-dependent, S1P2-independent mechanisms. The partial restoration of degranulation in SphK2-deficient mast cells by treatment with S1P suggest that SphK2 participates in some aspects of FcεRI signaling independently of S1P2. Further studies using SphK2/S1P2 double knockout mast cells would be instrumental in clarifying the participation of S1P2 dependent and/or independent mechanisms in the release of mediators by mast cells.
2.4- The extracellular compartment: S1P from non-mast cells sources affecting mast cell-induced anaphylaxis
An intriguing recent finding is the role of SphKs in systemic anaphylaxis. Mast cells are known as the effector cells responsible for the cardiopulmonary changes and death during IgE-dependent anaphylaxis [32]. This IgE-dependent immediate-type allergic reaction can be studied in mouse models of passive systemic anaphylaxis (PSA) or passive cutaneous anaphylaxis (PCA) where mice are passively sensitized with a systemic or intradermal injection of IgE antibodies, respectively, and challenged with a specific Ag. The immediate release of mediators from mast cells, particularly histamine [102], mediates most of the changes observed during the anaphylactic reaction. Thus, it was expected that SphK2 deficient mice, whose in vitro derived mast cells failed to produce S1P, degranulate and produce cytokines, would show reduced histamine production upon systemic challenge. Against expectations, SphK2-deficient mice responded normally (i.e normal histamine release into circulation) to a systemic challenge, while SphK1-null mice were resistant to anaphylaxis (i.e reduced histamine release), even though mast cells from these mice functioned normally in vitro [39]. These data suggest that the overall systemic responses are complicated by the contributions of multiple mast cell populations and possibly other systemic factors. In fact, our recent studies in wild type mice revealed a close correlation between the levels of S1P in circulation and the histamine levels following anaphylactic systemic challenge, suggesting that plasma levels of S1P could be one of those factors. Mice deficient in SphK1 had greatly reduced levels of circulating S1P [39, 103] and lower histamine responses, while mice deficient in SphK2 had elevated levels of circulating S1P [39, 104] and higher histamine levels than expected, providing further evidence for this correlation. Moreover, reduction of circulating S1P levels in the Sphk2-null genetic background by deletion of one allele of SphK1 (Sphk2-/-Sphk1+/-) resulted in low histamine release upon systemic challenge, even though the mast cells derived from these mice had approximately equal SphK activity and S1P levels as those from Sphk2-/- [39]. These findings pointed to yet another complexity in the function of S1P: the regulation of the in vivo S1P homeostasis extrinsic to the mast cell. It is important to note that although mast cells secrete S1P under activation as a late response and can alter S1P levels in their environment or in blood (for example, they could be responsible for the elevated S1P levels in the bronchial lavage of asthmatics hours after challenge), they do not contribute to the normal S1P homeostasis in plasma, since mast cell-deficient mice had similar levels of plasma S1P than mast-deficient mice with engrafted mast cells [39]. Thus, circulating levels of S1P, regulated by cells other than mast cells via SphK1, were also important in determining mast cell responsiveness (Figure 4). This component is distinguishable from the intrinsic regulation of SphK2 by FcεRI and the role in mast cell function described above, but both appear to contribute, via SphK1 and SphK2, respectively, to the mast cell responses in vivo, since high enough levels of circulating S1P could overcome the intrinsic defects of SphK2-null mast cells. The mechanisms mediating this function of extrinsic S1P are currently unclear. Some possible mechanisms are discussed in figure 4.
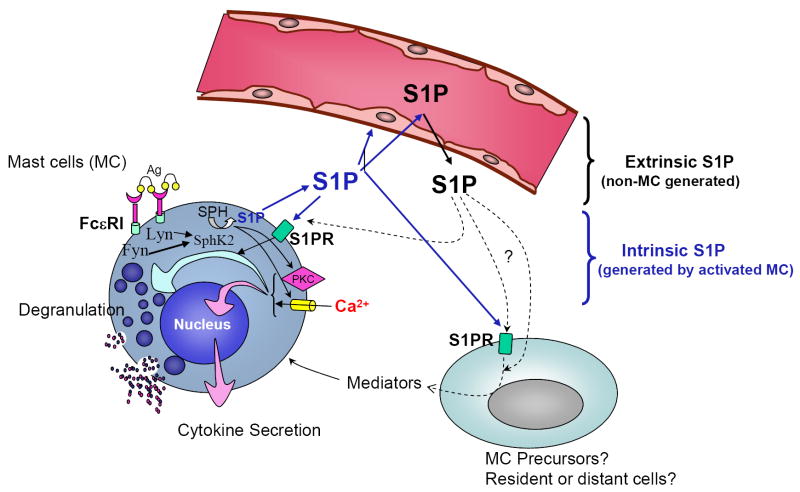
FIGURE 4. In vivo S1P networks affect mast cell function and anaphylaxis.
This figure depicts the mast cell in its physiological environment, and the partnership between extrinsic S1P generated by cells other than mast cells (black arrows; “extracellular compartment” in Figure 2), and intrinsic S1P or S1P generated by stimulated mast cells (blue arrows; corresponding to the “intracellular compartment” in Figure 2), in the regulation of mast cell responsiveness. Loss of SphK2 dramatically reduces the intracellular levels of S1P in mast cells, while loss of SphK1 has no effect. In contrast, deficiency in SphK1 results in reduced levels of S1P in circulation while loss of SphK2 increases the levels. Mast cells may be affected by those changes in different ways. It is possible that changes in S1P in the circulation have a domino effect on the interstitial levels of S1P in tissues, particularly on cells in the proximity of blood vessels where mast cells are present. Changes in those levels may directly affect the priming of S1P2 in the mast cell, impacting on degranulation once the cells are activated. This possibility implies interference of extrinsic S1P with the autocrine loop of S1P2 activation by intrinsic S1P. Other possibilities include an effect of extrinsic S1P on mast cell precursors in the blood stream or mast cells in tissues that will change their phenotypic outcome towards a more or less responsive phenotype. Constant exposure to higher or lower levels of S1P could also alter the phenotype of immune or non-immune cells inducing the generation of mediators that secondarily might influence the differentiation of mast cells. As a consequence of these direct or indirect effects of circulating S1P, mast cells numbers or their phenotypic outcome could be modified.
Although these findings strongly support an effect of the extrinsic S1P in vivo as a key regulator of mast cell responsiveness and anaphylaxis, additional studies are needed to establish a direct cause-effect in models other than SphK-null mice. In this direction, our preliminary data indicates that mice with a genetic background (SV129) known to induce stronger Th2-type responses and anaphylaxis, had also higher levels of circulating S1P than a C57/B6 background known for weak Th2-type responses [105], supporting the observed correlation between circulating S1P and susceptibility to anaphylaxis. SphK1 [39, 103] and erythrocytes [18, 19] are important for the maintenance of high S1P concentrations in blood, although little is known about the exact mechanism of regulation or the involvement of other sphingolipid enzymes, such as S1P lyase and S1P phosphatases, and other cell types, such as endothelial cells, in S1P homeostasis [3, 20, 21]. Genetic alterations in any of the steps implicated in this process or dietary factors impinging on its regulation, may result in alterations in the susceptibility to an anaphylactic response. Further exploration of all these issues may begin to unravel the mystery of why very few but not most individuals with similar allergies are susceptible to an anaphylactic response.
1- Multiple levels of complexity: the challenge
The understanding of the complexity of S1P regulation and function, and of sphingolipid metabolites overall, has come a long way in the last few years. Different aspects of it have been dissected and studied in various model systems, but few incorporate all of those aspects as the mast cell model. Studies in murine mast cells have revealed that SphK2 activation and translocation to membranes is quite important for sustaining normal responsiveness of mast cells during an allergic stimulus. This is partly due to the generation of S1P and the rapid activation of S1P receptors in the mast cells, but also to receptor-independent effects on a calcium channel at the membrane whose nature is yet unknown. Furthermore, the regulation of S1P generation and export from mast cells and its actions as a paracrine mediator may also be of critical importance for allergic inflammation. Development of animal models to demonstrate this potential role for S1P and to study the receptors involved should be useful to open new avenues for treatment of allergic disease. Also, if additional roles for S1P in inflammatory conditions are demonstrated, the recent finding of ABCC1 as a transporter of S1P in mast cells (or other transporters of S1P that may be functioning in different populations of mast cells in vivo) can be of relevance from a pharmacological perspective.
Another interesting aspect yet to be further explored, is the effect of S1P in the in vivo environment. Preserving in vivo S1P gradient from blood to tissues is critical for lymphocyte egress, but additionally, maintaining S1P homeostasis may be important to sustain a balanced cell function (Figure 2), as it seems to be key to systemic anaphylaxis. Interestingly, although unclear whether a cause or consequence of the disease, the levels of circulating S1P were abnormally high in patients with coronary artery disease and were found to be a better predictive factor than any other traditional risk factors [106], underlying the importance of this homeostasis. The changes in the levels of S1P in the circulation by loss of either of the SphK isoforms individually [39], unlike loss of S1P lyase activity [20], does not result in ablation of the blood to tissue gradient, and thus lymphocyte egress is intact. However, the actual levels of circulating S1P have an impact on the responsiveness of mast cells to an allergic stimulus, and probably those of T cells and dendritic cells as supported by several in vitro findings [26-28]. Further efforts trying to understand how extrinsic S1P may affect susceptibility to anaphylaxis (Figure 4), how its levels are regulated in this compartment, why they are sustained and how dietary components modify S1P regulation and influence immunity, may begin to provide some clues in the knowledge of the allergic or other diseases and promote the possibility of intervention.
Acknowledgments
The author acknowledges the support from the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health, and the comments of Dr. Juan Rivera.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allende ML, Proia RL. Sphingosine-1-phosphate receptors and the development of the vascular system. Biochim Biophys Acta. 2002;1582:222–227. doi: 10.1016/s1388-1981(02)00175-0. [DOI] [PubMed] [Google Scholar]
- 2.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 3.Saba JD, Hla T. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ Res. 2004;94:724–734. doi: 10.1161/01.RES.0000122383.60368.24. [DOI] [PubMed] [Google Scholar]
- 4.Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5:560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 5.Yatomi Y. Sphingosine 1-phosphate in vascular biology: possible therapeutic strategies to control vascular diseases. Curr Pharm Des. 2006;12:575–587. doi: 10.2174/138161206775474404. [DOI] [PubMed] [Google Scholar]
- 6.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 7.Rosen H, Sanna MG, Cahalan SM, Gonzalez-Cabrera PJ. Tipping the gatekeeper: S1P regulation of endothelial barrier function. Trends Immunol. 2007;28:102–107. doi: 10.1016/j.it.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, et al. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- 10.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 11.Kohno M, Momoi M, Oo ML, Paik JH, Lee YM, et al. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol Cell Biol. 2006;26:7211–7223. doi: 10.1128/MCB.02341-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizugishi K, Li C, Olivera A, Bielawski J, Bielawska A, et al. Maternal disturbance in activated sphingolipid metabolism causes pregnancy loss in mice. J Clin Invest. 2007;117:2993–3006. doi: 10.1172/JCI30674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olivera A, Spiegel S. Sphingosine kinase: a mediator of vital cellular functions. Prostaglandins. 2001;64:123–134. doi: 10.1016/s0090-6980(01)00108-3. [DOI] [PubMed] [Google Scholar]
- 14.Ancellin N, Colmont C, Su J, Li Q, Mittereder N, et al. Extracellular export of sphingosine kinase-1 enzyme. Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J Biol Chem. 2002;277:6667–6675. doi: 10.1074/jbc.M102841200. [DOI] [PubMed] [Google Scholar]
- 15.Taha TA, Hannun YA, Obeid LM. Sphingosine kinase: biochemical and cellular regulation and role in disease. J Biochem Mol Biol. 2006;39:113–131. doi: 10.5483/bmbrep.2006.39.2.113. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez SE, Milstien S, Spiegel S. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol Metab. 2007;18:300–307. doi: 10.1016/j.tem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Yatomi Y. Plasma sphingosine 1-phosphate metabolism and analysis. Biochim Biophys Acta. 2007 doi: 10.1016/j.bbagen.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 19.Hanel P, Andreani P, Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. Faseb J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 20.Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 21.Peest U, Sensken SC, Andreani P, Hanel P, Van Veldhoven PP, et al. S1P-lyase independent clearance of extracellular sphingosine 1-phosphate after dephosphorylation and cellular uptake. J Cell Biochem. 2008 doi: 10.1002/jcb.21665. [DOI] [PubMed] [Google Scholar]
- 22.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 23.Graeler M, Goetzl EJ. Activation-regulated expression and chemotactic function of sphingosine 1-phosphate receptors in mouse splenic T cells. Faseb J. 2002;16:1874–1878. doi: 10.1096/fj.02-0548com. [DOI] [PubMed] [Google Scholar]
- 24.Allende ML, Zhou D, Kalkofen DN, Benhamed S, Tuymetova G, et al. S1P1 receptor expression regulates emergence of NKT cells in peripheral tissues. Faseb J. 2008;22:307–315. doi: 10.1096/fj.07-9087com. [DOI] [PubMed] [Google Scholar]
- 25.Walzer T, Chiossone L, Chaix J, Calver A, Carozzo C, et al. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat Immunol. 2007;8:1337–1344. doi: 10.1038/ni1523. [DOI] [PubMed] [Google Scholar]
- 26.Idzko M, Panther E, Corinti S, Morelli A, Ferrari D, et al. Sphingosine 1-phosphate induces chemotaxis of immature and modulates cytokine-release in mature human dendritic cells for emergence of Th2 immune responses. Faseb J. 2002;16:625–627. doi: 10.1096/fj.01-0625fje. [DOI] [PubMed] [Google Scholar]
- 27.Martino A, Volpe E, Auricchio G, Izzi V, Poccia F, et al. Sphingosine 1-phosphate interferes on the differentiation of human monocytes into competent dendritic cells. Scand J Immunol. 2007;65:84–91. doi: 10.1111/j.1365-3083.2006.01860.x. [DOI] [PubMed] [Google Scholar]
- 28.Liao JJ, Huang MC, Goetzl EJ. Cutting edge: Alternative signaling of Th17 cell development by sphingosine 1-phosphate. J Immunol. 2007;178:5425–5428. doi: 10.4049/jimmunol.178.9.5425. [DOI] [PubMed] [Google Scholar]
- 29.Huang MC, Watson SR, Liao JJ, Goetzl EJ. Th17 augmentation in OTII TCR plus T cell-selective type 1 sphingosine 1-phosphate receptor double transgenic mice. J Immunol. 2007;178:6806–6813. doi: 10.4049/jimmunol.178.11.6806. [DOI] [PubMed] [Google Scholar]
- 30.Dawicki W, Marshall JS. New and emerging roles for mast cells in host defence. Curr Opin Immunol. 2007;19:31–38. doi: 10.1016/j.coi.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Turner H, Kinet JP. Signalling through the high-affinity IgE receptor FceRI. Nature. 1999;402:B24–30. doi: 10.1038/35037021. [DOI] [PubMed] [Google Scholar]
- 32.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, et al. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 33.Kuehn HS, Gilfillan AM. G protein-coupled receptors and the modification of FcεRI-mediated mast cell activation. Immunol Lett. 2007;113:59–69. doi: 10.1016/j.imlet.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi OH, Kim J-H, Kinet J-P. Calcium mobilization via sphingosine kinase in signalling by the FcεRI antigen receptor. Nature. 1996;380:634–636. doi: 10.1038/380634a0. [DOI] [PubMed] [Google Scholar]
- 35.Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, et al. Transactivation of Sphingosine-1-Phosphate Receptors by FcεRI Triggering Is Required for Normal Mast Cell Degranulation and Chemotaxis. J Exp Med. 2004;199:959–970. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melendez AJ, Khaw AK. Dichotomy of Ca2+ signals triggered by different phospholipid pathways in antigen stimulation of human mast cells. J Biol Chem. 2002;277:17255–17262. doi: 10.1074/jbc.M110944200. [DOI] [PubMed] [Google Scholar]
- 37.Olivera A, Urtz N, Mizugishi K, Yamashita Y, Gilfillan AM, et al. IgE-dependent activation of sphingosine kinases 1 and 2 and secretion of sphingosine 1-phosphate requires Fyn kinase and contributes to mast cell responses. J Biol Chem. 2006;281:2515–2525. doi: 10.1074/jbc.M508931200. [DOI] [PubMed] [Google Scholar]
- 38.Prieschl EE, Csonga R, Novotny V, Kikuchi GE, Baumruker T. The balance between sphingosine and sphingosine-1-phosphate is decisive for mast cell activation after FcεRI triggering. J Exp Med. 1999;190:1–8. doi: 10.1084/jem.190.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olivera A, Mizugishi K, Tikhonova A, Ciaccia L, Odom S, et al. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity. 2007;26:287–297. doi: 10.1016/j.immuni.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Herr DR, Fyrst H, Creason MB, Phan VH, Saba JD, et al. Characterization of the Drosophila sphingosine kinases and requirement for Sk2 in normal reproductive function. J Biol Chem. 2004;279:12685–12694. doi: 10.1074/jbc.M310647200. [DOI] [PubMed] [Google Scholar]
- 41.Le Stunff H, Giussani P, Maceyka M, Lepine S, Milstien S, et al. Recycling of sphingosine is regulated by the concerted actions of sphingosine-1-phosphate phosphohydrolase 1 and sphingosine kinase 2. J Biol Chem. 2007;282:34372–34380. doi: 10.1074/jbc.M703329200. [DOI] [PubMed] [Google Scholar]
- 42.Billich A, Bornancin F, Devay P, Mechtcheriakova D, Urtz N, et al. Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem. 2003;278:47408–47415. doi: 10.1074/jbc.M307687200. [DOI] [PubMed] [Google Scholar]
- 43.Liu H, Chakravarty D, Maceyka M, Milstien S, Spiegel S. Sphingosine kinases: a novel family of lipid kinases. Prog Nucleic Acid Res Mol Biol. 2002;71:493–511. doi: 10.1016/s0079-6603(02)71049-0. [DOI] [PubMed] [Google Scholar]
- 44.Paugh SW, Payne SG, Barbour SE, Milstien S, Spiegel S. The immunosuppressant FTY720 is phosphorylated by sphingosine kinase type 2. FEBS Lett. 2003;554:189–193. doi: 10.1016/s0014-5793(03)01168-2. [DOI] [PubMed] [Google Scholar]
- 45.Stevens RL, Adachi R. Protease-proteoglycan complexes of mouse and human mast cells and importance of their beta-tryptase-heparin complexes in inflammation and innate immunity. Immunol Rev. 2007;217:155–167. doi: 10.1111/j.1600-065X.2007.00525.x. [DOI] [PubMed] [Google Scholar]
- 46.Oskeritzian CA, Alvarez SE, Hait NC, Price MM, Milstien S, et al. Distinct roles of sphingosine kinases 1 and 2 in human mast cell functions. Blood. 2008 doi: 10.1182/blood-2007-09-115451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hait NC, Sarkar S, Le Stunff H, Mikami A, Maceyka M, et al. Role of sphingosine kinase 2 in cell migration towards epidermal growth factor. J Biol Chem. 2005;280:29462–29469. doi: 10.1074/jbc.M502922200. [DOI] [PubMed] [Google Scholar]
- 48.Maceyka M, Sankala H, Hait NC, Le Stunff H, Liu H, et al. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J Biol Chem. 2005;280:37118–37129. doi: 10.1074/jbc.M502207200. [DOI] [PubMed] [Google Scholar]
- 49.Michell RH, Conroy LA, Finney M, French PJ, Bunce CM, et al. Inositol lipids and phosphates in the proliferation and differentiation of lymphocytes and myeloid cells. Ciba Found Symp. 1992;164:2–11. doi: 10.1002/9780470514207.ch2. discussion 12-6. [DOI] [PubMed] [Google Scholar]
- 50.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 51.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 52.Vig M, Beck A, Billingsley JM, Lis A, Parvez S, et al. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, et al. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 54.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, et al. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281:20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- 56.Vig M, Dehaven WI, Bird GS, Billingsley JM, Wang H, et al. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 2008;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baba Y, Nishida K, Fujii Y, Hirano T, Hikida M, et al. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nat Immunol. 2008;9:81–88. doi: 10.1038/ni1546. [DOI] [PubMed] [Google Scholar]
- 58.Lee HS, Park CS, Lee YM, Suk HY, Clemons TC, et al. Antigen-induced Ca2+ mobilization in RBL-2H3 cells: role of I(1,4,5)P3 and S1P and necessity of I(1,4,5)P3 production. Cell Calcium. 2005;38:581–592. doi: 10.1016/j.ceca.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 59.Ma HT, Peng Z, Hiragun T, Iwaki S, Gilfillan AM, et al. Canonical Transient Receptor Potential 5 Channel in Conjunction with Orai1 and STIM1 Allows Sr2+ Entry, Optimal Influx of Ca2+, and Degranulation in a Rat Mast Cell Line. J Immunol. 2008;180:2233–2239. doi: 10.4049/jimmunol.180.4.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vennekens R, Olausson J, Meissner M, Bloch W, Mathar I, et al. Increased IgE-dependent mast cell activation and anaphylactic responses in mice lacking the calcium-activated nonselective cation channel TRPM4. Nat Immunol. 2007;8:312–320. doi: 10.1038/ni1441. [DOI] [PubMed] [Google Scholar]
- 61.Vig M, Kinet JP. The long and arduous road to CRAC. Cell Calcium. 2007;42:157–62. doi: 10.1016/j.ceca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smyth JT, Dehaven WI, Jones BF, Mercer JC, Trebak M, et al. Emerging perspectives in store-operated Ca2+ entry: roles of Orai, Stim and TRP. Biochim Biophys Acta. 2006;1763:1147–1160. doi: 10.1016/j.bbamcr.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 63.Meyer D, Heringdorf Zu. Lysophospholipid receptor-dependent and -independent calcium signaling. J Cell Biochem. 2004;92:937–948. doi: 10.1002/jcb.20107. [DOI] [PubMed] [Google Scholar]
- 64.Mathes C, Fleig A, Penner R. Calcium release-activated calcium current (ICRAC) is a direct target for sphingosine. J Biol Chem. 1998;273:25020–25030. doi: 10.1074/jbc.273.39.25020. [DOI] [PubMed] [Google Scholar]
- 65.Birchwood CJ, Saba JD, Dickson RC, Cunningham KW. Calcium influx and signaling in yeast stimulated by intracellular sphingosine 1-phosphate accumulation. J Biol Chem. 2001;276:11712–11718. doi: 10.1074/jbc.M010221200. [DOI] [PubMed] [Google Scholar]
- 66.Itagaki K, Hauser CJ. Sphingosine 1-phosphate, a diffusible calcium influx factor mediating store-operated calcium entry. J Biol Chem. 2003;278:27540–27547. doi: 10.1074/jbc.M301763200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu SZ, Muraki K, Zeng F, Li J, Sukumar P, et al. A sphingosine-1-phosphate-activated calcium channel controlling vascular smooth muscle cell motility. Circ Res. 2006;98:1381–1389. doi: 10.1161/01.RES.0000225284.36490.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim MY, Liang GH, Kim JA, Kim YJ, Oh S, et al. Sphingosine-1-phosphate activates BKCa channels independently of G protein-coupled receptor in human endothelial cells. Am J Physiol Cell Physiol. 2006;290:C1000–1008. doi: 10.1152/ajpcell.00353.2005. [DOI] [PubMed] [Google Scholar]
- 69.Titievsky A, Titievskaya I, Pasternack M, Kaila K, Tornquist K. Sphingosine inhibits voltage-operated calcium channels in GH4C1 cells. J Biol Chem. 1998;273:242–247. doi: 10.1074/jbc.273.1.242. [DOI] [PubMed] [Google Scholar]
- 70.Blom T, Bergelin N, Slotte JP, Tornquist K. Sphingosine kinase regulates voltage operated calcium channels in GH4C1 rat pituitary cells. Cell Signal. 2006;18:1366–1375. doi: 10.1016/j.cellsig.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 71.Condrescu M, Opuni K, Hantash BM, Reeves JP. Cellular regulation of sodium-calcium exchange. Ann N Y Acad Sci. 2002;976:214–223. doi: 10.1111/j.1749-6632.2002.tb04744.x. [DOI] [PubMed] [Google Scholar]
- 72.Hardie RC. TRP channels and lipids: from Drosophila to mammalian physiology. J Physiol. 2007;578:9–24. doi: 10.1113/jphysiol.2006.118372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pitson SM, Moretti PA, Zebol JR, Lynn HE, Xia P, et al. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003;22:5491–5500. doi: 10.1093/emboj/cdg540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sutherland CM, Moretti PA, Hewitt NM, Bagley CJ, Vadas MA, et al. The calmodulin-binding site of sphingosine kinase and its role in agonist-dependent translocation of sphingosine kinase 1 to the plasma membrane. J Biol Chem. 2006;281:11693–11701. doi: 10.1074/jbc.M601042200. [DOI] [PubMed] [Google Scholar]
- 75.Kusner DJ, Thompson CR, Melrose NA, Pitson SM, Obeid LM, et al. The localization and activity of sphingosine kinase 1 are coordinately regulated with actin cytoskeletal dynamics in macrophages. J Biol Chem. 2007;282:23147–23162. doi: 10.1074/jbc.M700193200. [DOI] [PubMed] [Google Scholar]
- 76.Olivera A, Rosenthal J, Spiegel S. Effect of acidic phospholipids on sphingosine kinase. J Cell Biochem. 1996;60:529–537. doi: 10.1002/(sici)1097-4644(19960315)60:4<529::aid-jcb9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 77.Delon C, Manifava M, Wood E, Thompson D, Krugmann S, et al. Sphingosine kinase 1 is an intracellular effector of phosphatidic acid. J Biol Chem. 2004;279:44763–44774. doi: 10.1074/jbc.M405771200. [DOI] [PubMed] [Google Scholar]
- 78.Stahelin RV, Hwang JH, Kim JH, Park ZY, Johnson KR, et al. The mechanism of membrane targeting of human sphingosine kinase 1. J Biol Chem. 2005;280:43030–43038. doi: 10.1074/jbc.M507574200. [DOI] [PubMed] [Google Scholar]
- 79.Igarashi N, Okada T, Hayashi S, Fujita T, Jahangeer S, et al. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J Biol Chem. 2003;278:46832–46839. doi: 10.1074/jbc.M306577200. [DOI] [PubMed] [Google Scholar]
- 80.Hait NC, Bellamy A, Milstien S, Kordula T, Spiegel S. Sphingosine kinase type 2 activation by ERK-mediated phosphorylation. J Biol Chem. 2007;282:12058–12065. doi: 10.1074/jbc.M609559200. [DOI] [PubMed] [Google Scholar]
- 81.Ding G, Sonoda H, Yu H, Kajimoto T, Goparaju SK, et al. Protein kinase D-mediated phosphorylation and nuclear export of sphingosine kinase 2. J Biol Chem. 2007;282:27493–27502. doi: 10.1074/jbc.M701641200. [DOI] [PubMed] [Google Scholar]
- 82.Rivera J, Olivera A. Src family kinases and lipid mediators in control of allergic inflammation. Immunol Rev. 2007;217:255–268. doi: 10.1111/j.1600-065X.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- 83.Rivera J. Molecular adapters in FcεRI signaling and the allergic response. Curr Opin Immunol. 2002;14:688–693. doi: 10.1016/s0952-7915(02)00396-5. [DOI] [PubMed] [Google Scholar]
- 84.Urtz N, Olivera A, Bofill-Cardona E, Csonga R, Billich A, et al. Early activation of sphingosine kinase in mast cells and recruitment to FcεRI are mediated by its interaction with Lyn kinase. Mol Cell Biol. 2004;24:8765–8777. doi: 10.1128/MCB.24.19.8765-8777.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Parravicini V, Gadina M, Kovarova M, Odom S, Gonzalez-Espinosa C, et al. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat Immunol. 2002;3:741–748. doi: 10.1038/ni817. [DOI] [PubMed] [Google Scholar]
- 86.Gu H, Saito K, Klaman LD, Shen J, Fleming T, et al. Essential role for Gab2 in the allergic response. Nature. 2001;412:186–190. doi: 10.1038/35084076. [DOI] [PubMed] [Google Scholar]
- 87.Hobson JP, Rosenfeldt HM, Barak LS, Olivera A, Poulton S, et al. Role of the sphingosine-1-phosphate receptor EDG-1 in PDGF-induced cell motility. Science. 2001;291:1800–1803. doi: 10.1126/science.1057559. [DOI] [PubMed] [Google Scholar]
- 88.Yang L, Yatomi Y, Miura Y, Satoh K, Ozaki Y. Metabolism and functional effects of sphingolipids in blood cells. Br J Haematol. 1999;107:282–293. doi: 10.1046/j.1365-2141.1999.01697.x. [DOI] [PubMed] [Google Scholar]
- 89.Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, et al. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci U S A. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jolly PS, Bektas M, Watterson KR, Sankala H, Payne SG, et al. Expression of SphK1 impairs degranulation and motility of RBL-2H3 mast cells by desensitizing S1P receptors. Blood. 2005;105:4736–42. doi: 10.1182/blood-2004-12-4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Olivera A, Rivera J. Sphingolipids and the Balancing of Immune Cell Function: Lessons from the Mast Cell. J Immunol. 2005;174:1153–1158. doi: 10.4049/jimmunol.174.3.1153. [DOI] [PubMed] [Google Scholar]
- 92.Roviezzo F, Del Galdo F, Abbate G, Bucci M, D’Agostino B, et al. Human eosinophil chemotaxis and selective in vivo recruitment by sphingosine 1-phosphate. Proc Natl Acad Sci U S A. 2004;101:11170–11175. doi: 10.1073/pnas.0401439101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Czeloth N, Schippers A, Wagner N, Muller W, Kuster B, et al. Sphingosine-1 phosphate signaling regulates positioning of dendritic cells within the spleen. J Immunol. 2007;179:5855–5863. doi: 10.4049/jimmunol.179.9.5855. [DOI] [PubMed] [Google Scholar]
- 94.Oskeritzian CA, Milstien S, Spiegel S. Sphingosine-1-phosphate in allergic responses, asthma and anaphylaxis. Pharmacol Ther. 2007;115:390–399. doi: 10.1016/j.pharmthera.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ammit AJ, Hastie AT, Edsall LC, Hoffman RK, Amrani Y, et al. Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma. Faseb J. 2001;15:1212–1214. doi: 10.1096/fj.00-0742fje. [DOI] [PubMed] [Google Scholar]
- 96.Kitano M, Hla T, Sekiguchi M, Kawahito Y, Yoshimura R, et al. Sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 signaling in rheumatoid synovium: regulation of synovial proliferation and inflammatory gene expression. Arthritis Rheum. 2006;54:742–753. doi: 10.1002/art.21668. [DOI] [PubMed] [Google Scholar]
- 97.Boujaoude LC, Bradshaw-Wilder C, Mao C, Cohn J, Ogretmen B, et al. Cystic fibrosis transmembrane regulator regulates uptake of sphingoid base phosphates and lysophosphatidic acid: modulation of cellular activity of sphingosine 1-phosphate. J Biol Chem. 2001;276:35258–35264. doi: 10.1074/jbc.M105442200. [DOI] [PubMed] [Google Scholar]
- 98.Kobayashi N, Nishi T, Hirata T, Kihara A, Sano T, et al. Sphingosine 1-phosphate is released from the cytosol of rat platelets in a carrier-mediated manner. J Lipid Res. 2006;47:614–621. doi: 10.1194/jlr.M500468-JLR200. [DOI] [PubMed] [Google Scholar]
- 99.Honig SM, Fu S, Mao X, Yopp A, Gunn MD, et al. FTY720 stimulates multidrug transporter- and cysteinyl leukotriene-dependent T cell chemotaxis to lymph nodes. J Clin Invest. 2003;111:627–637. doi: 10.1172/JCI16200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Anada Y, Igarashi Y, Kihara A. The immunomodulator FTY720 is phosphorylated and released from platelets. Eur J Pharmacol. 2007;568:106–111. doi: 10.1016/j.ejphar.2007.04.053. [DOI] [PubMed] [Google Scholar]
- 101.Waters CM, Long J, Gorshkova I, Fujiwara Y, Connell M, et al. Cell migration activated by platelet-derived growth factor receptor is blocked by an inverse agonist of the sphingosine 1-phosphate receptor-1. Faseb J. 2006;20:509–511. doi: 10.1096/fj.05-4810fje. [DOI] [PubMed] [Google Scholar]
- 102.Makabe-Kobayashi Y, Hori Y, Adachi T, Ishigaki-Suzuki S, Kikuchi Y, et al. The control effect of histamine on body temperature and respiratory function in IgE-dependent systemic anaphylaxis. J Allergy Clin Immunol. 2002;110:298–303. doi: 10.1067/mai.2002.125977. [DOI] [PubMed] [Google Scholar]
- 103.Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, et al. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem. 2004;279:52487–52492. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- 104.Zemann B, Kinzel B, Muller M, Reuschel R, Mechtcheriakova D, et al. Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood. 2006;107:1454–1458. doi: 10.1182/blood-2005-07-2628. [DOI] [PubMed] [Google Scholar]
- 105.Rivera J, Tessarollo L. Genetic Background and the dilema of translating mouse studies to humans. Immunity. 2008 doi: 10.1016/j.immuni.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 106.Deutschman DH, Carstens JS, Klepper RL, Smith WS, Page MT, et al. Predicting obstructive coronary artery disease with serum sphingosine-1-phosphate. Am Heart J. 2003;146:62–68. doi: 10.1016/S0002-8703(03)00118-2. [DOI] [PubMed] [Google Scholar]
- 107.Levade T, Jaffrezou JP. Signalling sphingomyelinases: which, where, how and why? Biochim Biophys Acta. 1999;1438:1–17. doi: 10.1016/s1388-1981(99)00038-4. [DOI] [PubMed] [Google Scholar]
- 108.Gulbins E. Regulation of death receptor signaling and apoptosis by ceramide. Pharmacol Res. 2003;47:393–399. doi: 10.1016/s1043-6618(03)00052-5. [DOI] [PubMed] [Google Scholar]