Abstract
Rationale and objectives
Our previous work uncovered a differential preference of maternal female rats for cues associated with pups versus cues associated with cocaine at three different postpartum time points. Our current study examines the preference for these cues in conjunction with an assessment of the capacity to express the maternal behavior at one of these time points. We examined dams at day 10 postpartum using a procedure that included two additional controls, and a complete assessment of the expression of maternal behavior and locomotor activity.
Methods
A conditioned place-preference procedure was used to determine the preference for cocaine- or pup-associated cues. The two controls were (1) a preconditioning test to verify no initial chamber preference and (2) a separate control group of postpartum day-10 dams exposed to chambers and cues but not to unconditioned stimuli. The expression of maternal behavior was determined by measurement of maternal nest building, retrieval of pups to the nest, grooming, crouching over pups, nursing, and maternal aggression. Locomotor activity was measured with an automated apparatus.
Results
Dams conditioned with cocaine or pups showed a preference for either the cocaine-associated chamber or the pup-associated chamber, confirming the existence of two similar-sized preference groups at this time point. Regardless of preference, dams had equal and robust expression of maternal behavior and similar locomotor capacity. The pre-conditioning test showed no initial chamber preferences and did not alter the conditioned preference response. The use of unconditioned stimuli in the place-preference conditioning procedure was effective and necessary for the preference response.
Conclusion
Our current study has revealed that differences in the motivational state of the maternal dam emerge even while the expression of maternal behavior is constant and substantial. The data suggest that the difference in preference is a very specific appetitive response that is not linked to expression of maternal behavior or locomotor capacity.
Keywords: Maternal behavior, Conditioned place preference, Cocaine, Locomotor activity, Maternal aggression, Postpartum period
Introduction
Previously, we used a conditioned place preference (CPP) procedure to examine the response of maternal, female rats (dams) given a choice between cocaine- and offspring (pup)-associated chambers at three different time points postpartum (Mattson et al. 2001). During the early postpartum period (postpartum day 8), the substantial majority of dams had a preference for the pup-associated chamber, while the substantial majority of dams tested in the late postpartum period (postpartum day 16) had a preference for the cocaine-associated chamber. On postpartum day 10, the preference of the dams had a distribution with two modes of similar size, one with a subset preferring drug-associated cues, the other with a subset preferring pup-associated cues. The preference of the dams changed dramatically during the postpartum period, with day 10 postpartum marking the midpoint of a transition for the population. We now extend our examination of the postpartum day-10 time point.
The CPP procedure assesses the preference for or the motivation to seek a reinforcing stimulus, including a variety of natural reinforcers such as food, water, sex, and offspring, as well as drugs of abuse (Eikelboom and Stewart 1982; Mucha et al. 1982; Khantzian 1985; Carr et al. 1989; Fleming et al. 1989, 1994; Nader and Woolverton 1991, 1992; Parker 1992; Schechter and Calcagnetti 1993, 1998; Erb and Parker 1994; Bardo et al. 1995; Lee et al. 1999; Bardo and Bevins 2000). Animals are given pairings of an unconditioned reinforcing stimulus with a set of unique environmental cues that serve as the conditioned stimulus. The CPP method allows assessment of the preference for a reinforcing stimulus in its absence (Nomikos and Spyraki 1988; Carr et al. 1989; White and Hiroi 1992; Durazzo et al. 1994; Fleming et al. 1994; Shippenberg and Heidbreder 1995; McBride et al. 1999; Campbell et al. 2000).
The reinforcing properties of cocaine in rats are well-documented with both CPP and self-administration procedures, but few studies have been conducted in female rats (Schechter and Calcagnetti 1993, 1998). While non-maternal adult rats vigorously avoid pups, pups are a powerful reinforcing stimulus to the maternal rat (Fahrbach and Pfaff 1982; Numan 1994), as demonstrated with a CPP procedure (Fleming et al. 1994) and an operant conditioning procedure (Lee et al. 1999).
Typically in CPP studies, the preference for only one reinforcing stimulus is assessed, but a few studies have assessed the preference for two known reinforcing stimuli, for example, novelty relative to drugs (Carr et al. 1988; Bardo et al. 1990; Parker 1992). Parker (1992) used the CPP procedure to compare the preference for four choices – a drug-paired chamber, a saline-paired chamber, a novel chamber, and a chamber with prior exposure. Based on these studies in particular, we developed a CPP procedure to assess the compared conditioned response to two reinforcing stimuli, pups and cocaine, by the maternal dam (Mattson et al. 2001).
Many studies have examined the species-specific expression of maternal behavior (consummatory aspect), while few have examined the motivational aspects (appetitive aspect) or the relationship of the two phases to each other. Expression of maternal behavior begins just prior to parturition and remains vigorous throughout the postpartum period (Fahrbach and Pfaff 1982; Numan 1994; Kalinichev et al. 2000a). Expression of maternal behavior in the rat includes pup-directed behaviors such as retrieving the pups to the nest, grouping and nursing them, and non-pup-directed behaviors, like building a nest and maternal aggression (Bridges 1996). Within the postpartum period, both hormonal changes in the dam and pup development influence expression of maternal behavior. During the first four postpartum days, maternal behavior is hormonally dependent. As the postpartum period progresses to weaning, maternal behavior is maintained predominantly by the sensory stimuli provided by the pups. Weaning usually occurs between postpartum days 23 and 25.
We particularly wanted to investigate the preferences and behaviors of dams on postpartum day 10, when substantial subsets of dams with each preference (for the cocaine-associated chamber or the pup-associated chamber) are found. This time point is advantageous because it provides a substantial number of dams with each preference and yet limits the differences in the endocrine and physiological variables that would be much more marked if the two preference subsets were taken from two postpartum time points.
Based on our previous findings (Mattson et al. 2001), we wanted to examine dams at this time point to determine the preference by dams in which we also assessed the expression of maternal behavior and locomotor activity. To increase the precision and rigor of the examination, two forms of controls were added.
A common preconditioning test, in which dams were exposed to the apparatus prior to conditioning with the unconditioned stimuli, was incorporated to determine that they had no initial bias for any particular chamber or cues in the place-preference apparatus (Carr et al. 1989; Schechter and Calcagnetti 1993, 1998; Bardo et al. 1995; Bardo and Bevins 2000).
We also generated a control group of dams exposed to cues and chambers but never exposed to the unconditioned stimuli, cocaine or pups (Bardo and Bevins 2000).
By examining preference and expression of maternal behavior in the same dams, we intended to determine whether the motivation to seek pups and the expression of maternal behavior were separable components of maternal behavior. Measures of locomotor activity were added to ensure that dams of both subsets were of similar general behavioral capacity.
Methods
Subjects
The rats (n=41) were Sprague-Dawley females from our animal colony maintained at the Laboratory Animal Facility (LAF) at Rutgers University (Newark, NJ), originally obtained from Charles River Laboratories (Wilmington, MA). Our animal facility is accredited through the American Association for Accreditation of Laboratory Animal Care (AAALAC). Two separate populations of rats were used for conditioning and testing the effects of unconditioned stimuli: population 1 (n=9) and population 2 (n=27). A control group (n=5) was exposed to the cues used as conditioned stimuli, without exposure to the unconditioned stimuli. Female rats 90–120 days of age were bred to male rats and housed under conditions that provide precise timing of parturition (Mayer and Rosenblatt 1980, 1998). The day after parturition, the females and their pups were moved to testing rooms. As part of our standard procedures, all pups were briefly removed from their dams and placed in a group from which seven pups were taken and returned to the dams for care and nursing. Therefore, each dam had a similar composition of their litter, consisting of some of their genetic pups and some from other dams. Those pups then were considered her litter and remained with her in her home cage and used during her conditioning. All further details are as described by Mattson et al. (2001).
CPP procedure
Dams were conditioned on postpartum days 6–9 and tested on postpartum day 10. The dams were conditioned with the pups from the litter, for which they were caring in their home cages and which were age-matched to the postpartum day of the dam.
Apparatus
The CPP procedure used a Plexiglas apparatus, which consisted of three equal-sized chambers (21.6 cm wide × 27.9 cm long × 17.6 cm high), covered with a clear Plexiglas lid with air holes. The conditioned stimuli were unique wall and floor covering combinations that were paired with the unconditioned stimuli in a restricted randomized counterbalanced sequence (Mattson et al. 2001).
Pre-conditioning test
On postpartum day 5, the day prior to the start of conditioning, dams were allowed to explore the entire apparatus for 30 min. The rat was initially placed in the center chamber. The contextual cues that would become the conditioned stimuli during the subsequent conditioning phase were in place without unconditioned stimuli. The time that the dam spent in each chamber was recorded.
Conditioning phase
A barrier was placed in the center chamber, confining the dam to the one chamber for each conditioning session. Females were exposed to unconditioned stimuli in the presence of conditioned stimuli cues for 2 h on 4 days. The stimuli were a 10-mg/kg s.c. injection of cocaine or three pups, presented in an alternating sequence one stimulus per day. The order of the presentation of the unconditioned stimuli was counterbalanced; therefore, half of the dams received cocaine on the first conditioning day, the other half received pups; the corollary is that the unconditioned stimulus received on the final conditioning day alternated as well. On the days that the females received the pup stimulus, they were pup deprived for 1 h prior to conditioning.
CPP testing
The dams were tested for their conditioned cue preference on postpartum day 10, which was the day after the final day of unconditioned stimulus-cue conditioning. After 1 h of pup deprivation, the dams were placed into the center chamber of the apparatus and allowed to move freely throughout the apparatus for 1 h. The contextual cues (conditioned stimuli) of each chamber were arranged as in the conditioning phase, but no unconditioned stimuli were present. No conditioning had occurred in the middle chamber, but the rats had been exposed to it in preconditioning testing. Therefore, the middle chamber was neutral, not habituated, but not entirely novel. The time the dams spent in each chamber and their behavior were recorded for 1 h (Mattson et al. 2001). Dams in population 2 had a 2-h testing session since their brains were taken for further experiments (Mattson and Morrell 2001); for the second hour, behavior and location were noted every 10 min.
Controls exposed to chambers and cues but not unconditioned stimuli
A control group (n=5) were exposed with only the chamber cues (conditioned stimuli for experimental dams) (Rescorla 1967; Bardo and Bevins 2000), and were never exposed to unconditioned stimuli. The control group was never administered cocaine, and their only contact with pups was in the home cage. Initial testing with free access to all chambers of the apparatus occurred on postpartum day 5; on days 6–9 dams were confined to one chamber with cues in place per day; preference testing for the cued chambers occurred on postpartum day 10; all further details as for experimental animals.
Preference criterion
A dam was determined to have a preference for a particular chamber if she spent at least 50% of the testing time in that chamber, with the caveat that this time had to be at least 25% greater than the time spent in either of the two remaining chambers. Further explanation of our preference criterion is described by Mattson et al. (2001).
Additional behavioral characterization
The females in population 2 (n=27) had additional behavioral testing. A subset (n=9) was also tested on locomotor activity after cocaine administration. All of these tests were sequenced to ensure no interference with the CPP procedures.
Home-cage maternal behavior
The maternal behaviors of the dams were observed daily, including building of a nest site, pup-retrieval, grouping the pups in the nest, crouching, nursing, and licking the pups (Vernotica et al. 1996a). Maternal aggression is another normal component of maternal behavior by which the dam defends her nest and pups against intruders, and it is especially prominent in the peripartum period, from about 2 days before parturition through days 4–5 postpartum. To test maternal aggression, an unfamiliar, juvenile male or “intruder” (approximately 40 days old; used only once) of smaller size than the dam was placed into her home cage, in the presence of her litter and the nest site. Maternal aggression was measured on postpartum day 2, according to our established protocol with components summed into a single numerical aggression score from a minimum of 1 to a maximum of 16 (Mayer et al. 1990; Vernotica et al. 1996a).
Locomotor activity
Locomotor activity was measured for 1 h in an automated locomotor activity system (Digiscan Animal Activity Monitor, Model RXYZCM, Omnitech Electronics Trambue, OH) with chambers (40×40×40 cm) equipped with photocell beams. We report only horizontal distance traveled and center time, since other locomotor measures co-varied with them (Vernotica and Morrell 1998). Dams were injected s.c. with physiological saline (equivalent to saline volume used for cocaine injections) and tested for locomotor activity on postpartum day 4, prior to place preference procedures. On postpartum day 11, the day after place-preference testing, nine of the dams were again injected with cocaine (10 mg/kg s.c.), and locomotor activity under the influence of cocaine was recorded.
Drug administration
Cocaine hydrochloride in highly (>90%) purified powered form was provided by the National Institute of Drug Abuse, Research Triangle Park, NC, USA. The animals received s.c. injections in alternating dorsal, caudal flank regions of 10 mg/kg cocaine dissolved in physiological saline to obtain a concentration of 4.5 mg/ml. Dams were hand held during injections, so the injection would not be associated with the apparatus; the site was massaged to avoid skin ischemia (Scott et al. 1997). Saline of equivalent volume was administered for control injections.
Measures of general physiology and health
All animals were healthy throughout the experiment as assessed by physical examination by the investigators and staff of the LAF. The weight of dams and gain by the pups was normal. Based on published accounts of skin lesions caused by s.c. cocaine injections in laboratory rats (Bruckner et al. 1982; Spear et al. 1989; Scott et al. 1997), we made daily observations of the injection areas; no skin lesions were found.
Statistical analysis
Chi-square test was used to analyze percentages of populations. Aggression scores, pup retrieval latencies, locomotor activity and place-preference times were normally distributed and analyzed by t-test or analysis of variance followed by Tukey's post-hoc test. Paired t-test was used to analyze locomotor activity data saline and cocaine treatment effects. Repeated-measures analysis of variance followed by Wilks' Lambda test was used to analyze pre- and post-conditioning times. Multi-variate analysis of variance followed by Wilks' Lambda test was used to analyze the combined effect of all independent and dependent variables in the place preference data. For all tests, the significance level was set at P<0.05.
Results
CPP responses to cocaine and pup exposure
Pre- and post-conditioning comparisons. Before conditioning by exposure to unconditioned stimuli, the dams spent approximately 33% of their time in each chamber. After such conditioning, they spent 41% of their time in each of the stimulus-associated chambers and 18% of their time in the center/neutral chamber, which was not paired with an unconditioned stimulus. These changes were statistically significant (F2,52=7.11, P<0.05; Wilks' Lambda=0.81). Further, there was no correlation between chamber preference before and after conditioning with the unconditioned stimuli. A multi-variate ANOVA verified that the orientation of the chambers and cue-combinations did not affect chamber preference.
Preference
In each of the two experimental populations, approximately 50% (n=17) of the dams met the criterion for a preference for the cocaine-associated chamber and 37% (n=15) met the criterion for a preference for the pup-associated chamber, with 13% having no preference (Fig. 1A). On average, dams from each of these experimental populations spent 48% of their time in the cocaine-associated chamber, 38% of their time in the pup-associated chamber (Fig. 1B) and about 15% of their time in the neutral chamber. When each preference subset was considered specifically, the dams that met the criterion for a cocaine-associated or pup-associated preference spent an average of 80% of their time in that chamber. The two experimental populations did not differ from each other statistically on any measure.
Fig. 1A, B.
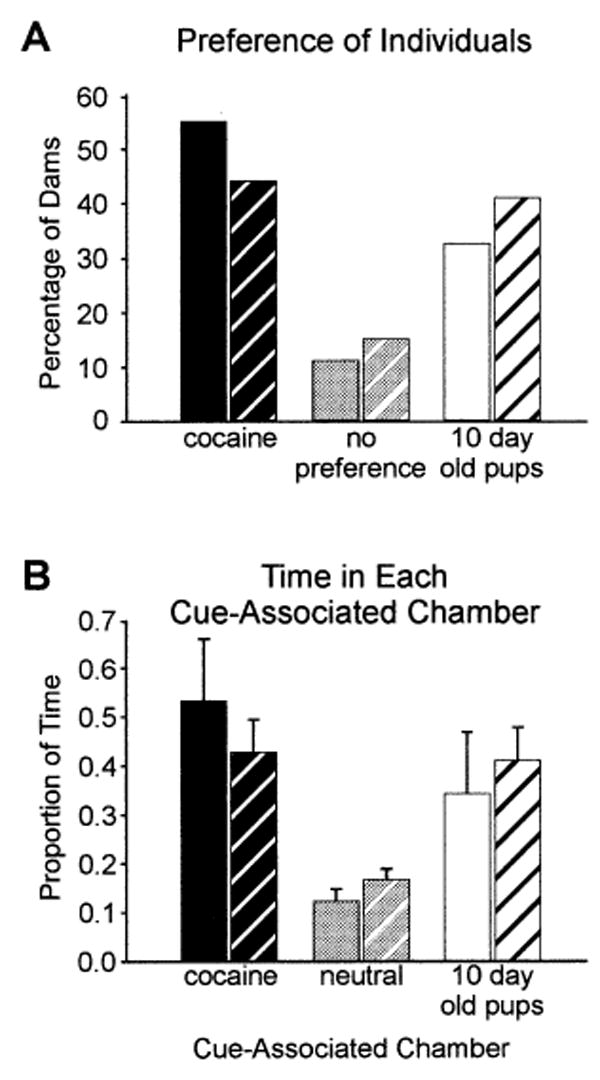
These graphs represent the response of dams to place-preference conditioning with cocaine and pups. A Percentage of dams that reached preference criterion for either cocaine- or pup-associated chambers or dams that had no preference. B Time spent by these dams in each of the three chambers, either cocaine- or pup-associated or neutral. Population 1 (n=9) and population 2 (n=27). A similar and statistically significant number of dams in both populations had a preference for either cocaine- or pup-related cues; the two populations had the same preference patterns. Solid bars population 1, hatched bars population 2
Dams spent their test time investigating, sniffing, and grooming; time in the preferred chamber was not associated with sleeping or failure to investigate the other chambers. During the second hour of testing, the dams remained in their preferred cue-associated chamber; only two exited but immediately returned. In the second hour, investigative behaviors decreased, and the dams primarily rested and groomed. No dam switched preference during the second hour.
Place-preference responses without cocaine or pup exposure
In their initial responses to the chambers with cues, the control dams (n=5) spent approximately 33% of their time in each chamber. After exposure to the chambers in the procedure (without association to unconditioned stimuli), their behavior was unchanged and no dam met the criteria for a chamber preference (Fig. 2).
Fig. 2.
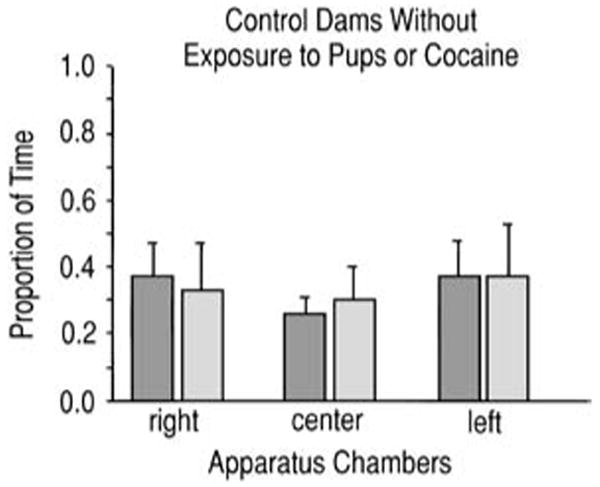
This graph shows the time spent in each apparatus chamber during initial apparatus exposure and then during the testing period after exposure only to the cues that would have served as conditioned cues in the complete procedure by a control group of dams (n=5) that were never exposed to unconditioned stimulus. It illustrates that exposure to the apparatus without pairing the chambers with the unconditioned stimuli did not induce a conditioning effect. Dark bars pre-conditioning, light bars post-conditioning
Expression of maternal behavior
All experimental dams had robust expression of maternal behavior, regardless of whether their conditioned preference was for a chamber associated with the unconditioned stimuli pups or cocaine, or if they had no preference. The dams rapidly retrieved pups displaced from their established nest site, engaged in ano-genital licking of pups, crouching, and nursed their pups. All quantitative measures of the expression of maternal behavior, including retrieval latencies, nest scores, nursing times, and grooming behaviors, were at maximal levels. The weight gain by the pups in all litters was equal and normal.
Maternal aggression was also high and equal in both preference subsets as well as dams with no preference. There were no differences in the time to initial attack between the cocaine- and pup-cue associated preference subsets. All dams attacked the intruder rat in less than 2 min after it was placed in their home cage (0.98±0.15), and had high composite aggression scores (14.16±0.49).
Locomotor activity
Dams that were given a saline injection traveled on average a total distance of 763±61 cm in 1 h. The two preference subsets were not different from each other nor those that had no preference (n=27; Fig. 3A). A small subset of the dams were subsequently tested for locomotor activity after cocaine, and within 1 h after injection traveled 228% farther than the distance traveled after saline administration (1607±266 cm versus 492±59 cm). This was statistically significantly greater (t8=4.09, P<0.05; n=9; Fig. 3B). There were no differences in the level of cocaine-induced locomotion across the two preference subsets.
Fig. 3A–D.
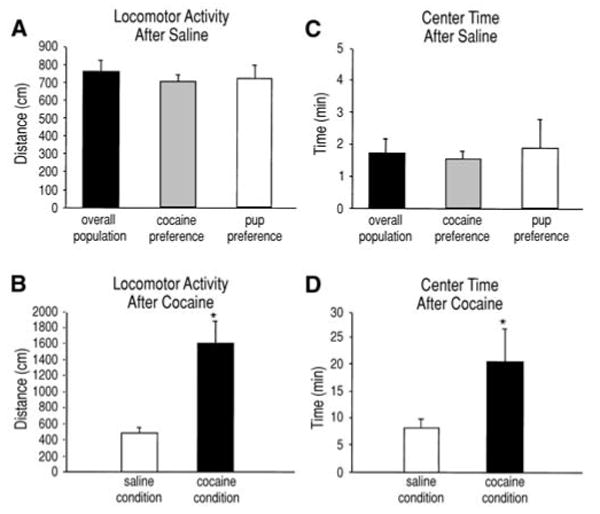
These graphs show two aspects of locomotor activity for both the total population (n=27) and the dams separated by preference for a stimulus-associated chamber. Mean (±SEM) horizontal distance traveled after (A) saline administration and (B) cocaine administration (n=9). Mean (±SEM) time spent in the center after (C) saline administration (n=27) and (D) cocaine administration (n=9). There were no differences between preference subsets, but there was a significant increase in locomotor activity after cocaine administration. Asterisk indicates a significant difference from the saline condition, P<0.05
Center time
After saline administration, dams spent only 3% of their test time in the center area of the locomotor apparatus (1.7±0.4 min/60 min; n=27; Fig. 3C). The small subset of dams that were tested for locomotor activity and center time under the influence of cocaine spent nearly three times longer in the center area after cocaine (21±6 min) than their center time after saline (8±2 min; n=9). This increase was statistically significant (Fig. 3D; t8=2.2, P<0.05). There were no differences in either center time test between the preference subsets.
Discussion
These experiments show that there is a bimodal distribution of the preference of the dams with substantial subsets preferring either pup- or drug-associated cues. Regardless of their conditioning exposure or their preference responses, dams did not differ in their expression of all active components of maternal behavior. Dams that displayed a very strong preference for the cocaine-associated chamber also expressed maternal behavior at robust levels, which was indistinguishable from dams with a strong preference for the pup-associated chamber. Taken together, the place preference and data on the expression of maternal behavior suggest that the motivation to seek the pup stimulus (appetitive component) can be regulated by processes, in part, independent from processes underlying the expression of maternal behavior (consummatory component). Furthermore, these dams must be detecting a sufficient difference in the reinforcing properties of the two stimuli, cocaine (pharmacological) versus pups (natural), such that a different behavioral outcome could occur regardless of the identical capacity to express maternal behavior.
Previously, we and others found that dams express high levels of maternal aggression on postpartum day 2 (Vernotica et al. 1996a) and that the expression of maternal aggression depends on many experimental variables including whether the rats are under the influence of cocaine (Johns at al. 1994, 1997, 1998; Vernotica et al. 1996a). While we did not perform any aggression testing while the dams were under the influence of cocaine in the current study, due to the constraints of our CPP procedure, we would predict that no differences would be detected across the preference subsets.
The current study also confirms that our initial findings at postpartum day 10 are stable even with the use of preconditioning exposure to the apparatus. These data also demonstrate that only exposure to cocaine and pup stimuli in the apparatus chambers is effective in altering the preference responses of the dams. Exposure to only the cues used as conditioned stimuli without exposure to unconditioned stimuli does not establish a place preference. This is a simple but fundamental demonstration that our apparatus and place-preference conditioning procedure do not in and of themselves stimulate a preference response from the dams indicating they are unbiased.
Systematic examination of locomotor activity including center time revealed no differences across the preference subsets. Locomotor activity increased after cocaine, in accord with our previous findings in the maternal female rat showing that 10 mg/kg cocaine increases locomotion modestly, much less than 20 mg/kg in male or female rats (Vernotica et al. 1996a, 1999; Vernotica and Morrell 1998; Blanchard and Blanchard 1999; Blanchard et al. 1999).
We found a significant increase in the total amount of time spent in the center field after cocaine, which would suggest that cocaine decreases the natural tendency of rats to avoid the center (Welker 1957; Belzung and Le Pape 1994). This suggestion is in contrast to the findings of Blanchard et al. (1999) and Herbert et al. (1999) that acute cocaine (at higher doses) can induce anxiety-like effects, while simultaneously increasing locomotion, possibly indicative of a flight response. These investigators have studied the phenomena in depth, and our finding is a modest analysis, simply demonstrating that the two preference subsets did not differ in center time whether or not they were under the influence of cocaine. Further, dams in the current study were tested after their third injection of cocaine and we cannot exclude the influence of a subtle drug sensitization as well.
In summary, a comparison of the preferences of the dams and their performance on other behavioral measures indicate that the differences in appetitive responses are very specific and cannot be linked to other features of the behavior of the dams. These conclusions accord with those of Gong et al. (1996) and Kosten and Miserendino (1998), who found no correlations among responses to novelty, locomotor activity, and cocaine place conditioning. Erb and Parker (1994) using a different novelty procedure and a different psychostimulant found no relationship between the strength of amphetamine place conditioning and responding in the novel environment. However, the data with amphetamine are more complex, since there is some evidence that place preference for amphetamine-associated cues could be predicted by reactivity to novelty (Klebaur and Bardo 1999; Klebaur et al. 2001).
We hypothesize that the relative strength of the cocaine and pup stimuli used in this study are reasonably well matched for the following reasons. We chose the minimum amount of each stimulus effective in our previous experimental procedures. Three pups are a sufficient stimulus to induce maternal behavior in previously non-maternal animals (Kalinichev et al. 2000a, 2000b); and, 10 m/kg cocaine injected s.c. is sufficient to impair the expression of maternal behavior and to increase locomotor activity in the postpartum dam (Vernotica et al. 1996b; Vernotica and Morrell 1998). The fact that dams at different points in the postpartum period largely switch their preference from pup-associated chambers to cocaine-associated chambers also supports our hypothesis.
There is also literature suggesting that a standard dose–response curve for drugs with abuse potential is not found using CPP procedures (Carr et al. 1989; Schechter and Calcagnetti 1993, 1998; Bardo et al. 1995). Once animals reach a dose that induces a CPP, the response is not strengthened by increasing the drug dose. This is different from the sensitive dose–response relationship that is found with most drug self-administration procedures and suggests that once a stimulus response is achieved with CPP procedures, stimulus strength is not sensitively registered.
In the most commonly accepted interpretation of CPP data, animals are thought to prefer the chamber with the greatest reinforcing properties (Carr et al. 1989; Wise 1989; Bardo and Bevins 2000). There are two alternative explanations for the expression of a CPP that have been proposed (Scoles and Siegel 1986; Parker 1992; Schechter and Calcagnetti 1993; Bardo et al. 1995). First, the response could be indicative of the chamber most novel to the animal at the time of testing. Second, the response could be avoidance of the non-preferred chamber. These alternative explanations could be viable if the CPP uses only a two-chambered apparatus or one with a small center chamber that is essentially a passageway. Usually the time the animal spends in the center chamber is not considered in the analysis.
Our apparatus had three chambers of equal size, one being neutral, or virtually novel, which provides an alternate choice to the two chambers paired with unconditioned stimuli (Carr et al. 1989; Parker 1992), and our analysis includes center time. We can, therefore, reasonably interpret increased time in a given chamber as a measure of the deliberate choice of the dam for that conditioned stimulus, not a preference for novelty or avoidance of the other conditioned stimulus.
Our prior work shows that the preference of the majority of the dams made a marked transition during the postpartum period (Mattson et al. 2001), but we have yet to approach the question of which variables are necessary or sufficient for this transition. This change in preference may be due to variables intrinsic to the dam or to changes in the stimulus properties of the pup due to their development. The variables intrinsic to the dam include the normal hormonal and physiological alterations of the postpartum period (Fahrbach and Pfaff 1982; Numan 1994). This hypothesis is supported by data showing that female rats substantially decrease their self-administration of cocaine during late pregnancy and the first 7 days of the postpartum period, relative to the period before pregnancy or the initial two-thirds of pregnancy (Hecht et al. 1999).
Pups develop rapidly in the postpartum period and their characteristics change as they develop. These changing characteristics are also likely to be important variables in the formation and transition of the dam's preferences. Maternal rats seemingly prefer younger rather than older pups, but there is no quantitative analysis of the effect of pup age on the reinforcing properties of the pup stimulus. Nor is there a clear examination of the purely appetitive aspects of the stimulus process, because prior work on the preference for proximity to pups (Fleming et al. 1994), and bar pressing for pups (Lee et al.1999) provided the stimulus and allowed the consummatory aspects of the behavior to proceed while appetitive measures were being taken.
In on-going experiments, we are investigating the independent variables that mediate the transition in preference across the postpartum period (Wansaw et al. 2001, 2002). Additional experiments will investigate the neural substrates that are engaged when the dam expresses each of the two preferences (Mattson and Morrell 2001; Smith et al. 2002).
Acknowledgments
This research was supported by PHS 5 S06 GM 08223–15.
Contributor Information
Brandi J. Mattson, Center for Molecular and Behavioral Neuroscience, Rutgers University, Newark, NJ 07102, USA, Behavioral Neuroscience Branch, National Institute on Drug Abuse, Building C, 5500 Nathan Shock Drive, Baltimore, MD 21224, USA, e-mail: bmattson@intra.nida.nih.gov, Tel.: +1-410–550–1815 ×24, Fax: +1-410–550–1612
Sharon E. Williams, Center for Molecular and Behavioral Neuroscience, Rutgers University, Newark, NJ 07102, USA
Jay S. Rosenblatt, Department of Psychology, Rutgers University, Newark, NJ 07102, USA
Joan I. Morrell, Center for Molecular and Behavioral Neuroscience, Rutgers University, Newark, NJ 07102, USA
References
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Neisewander JL, Pierce RC. Novelty-induced place preference behavior in rats: effects of opiate and dopaminergic drugs. Pharmacol Biochem Behav. 1990;32:683–687. doi: 10.1016/0091-3057(89)90018-x. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Rowlett JK, Harris MJ. Conditioned place preference using opiate and stimulant drugs: a meta- analysis. Neurosci Biobehav Rev. 1995;19:39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- Belzung C, Le Pape G. Comparison of different behavioral test situations used in psychopharmacology for measurement of anxiety. Physiol Behav. 1994;56:623–628. doi: 10.1016/0031-9384(94)90311-5. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. Cocaine potentiates defensive behaviors related to fear and anxiety. Neurosci Biobehav Rev. 1999;23:981–991. doi: 10.1016/s0149-7634(99)00031-7. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Kaawaloa JN, Hebert MA, Blanchard DC. Cocaine produces panic-like flight responses in mice in the mouse defense test battery. Pharmacol Biochem Behav. 1999;64:523–528. doi: 10.1016/s0091-3057(99)00126-4. [DOI] [PubMed] [Google Scholar]
- Bridges RS. Biochemical basis of parental behavior in the rat. In: Rosenblatt JS, Snowden CT, editors. Advances in the study of behavior. Vol. 25. Academic Press; New York: 1996. pp. 215–242. [Google Scholar]
- Bruckner JV, Jiang WD, Ho BT, Levy BM. Histopathological evaluation of cocaine-induced skin lesions in the rat. J Cutan Pathol. 1982;9:83–95. doi: 10.1111/j.1600-0560.1982.tb01045.x. [DOI] [PubMed] [Google Scholar]
- Campbell JO, Wood RD, Spear LP. Cocaine and morphine-induced place conditioning in adolescent and adult rats. Physiol Behav. 2000;68:487–493. doi: 10.1016/s0031-9384(99)00225-5. [DOI] [PubMed] [Google Scholar]
- Carr GD, Phillips AG, Fibiger HC. Independence of amphetamine reward from locomotor stimulation demonstrated by conditioned place preference. Psychopharmacology. 1988;94:221–226. doi: 10.1007/BF00176849. [DOI] [PubMed] [Google Scholar]
- Carr GD, Fibiger HC, Phillips AG. Conditioned place preference as a measure of drug reward. In: Liebman JM, Cooper SJ, editors. The neuropharmacological basis of reward. Oxford University Press; New York: 1989. pp. 264–319. [Google Scholar]
- Durazzo TC, Gauvin DV, Goulden KL, Briscoe RJ, Holloway FA. Cocaine-induced conditioned place approach in rats: the role of dose and route of administration. Pharmacol Biochem Behav. 1994;49:1001–1005. doi: 10.1016/0091-3057(94)90255-0. [DOI] [PubMed] [Google Scholar]
- Eikelboom R, Stewart J. Conditioning of drug-induced physiological responses. Psychol Rev. 1982;89:507–528. [PubMed] [Google Scholar]
- Erb SM, Parker LA. Individual differences in novelty-induced activity do not predict strength of amphetamine-induced place conditioning. Pharmacol Biochem Behav. 1994;48:581–586. doi: 10.1016/0091-3057(94)90317-4. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE, Pfaff DW. Hormonal and neural mechanisms underlying maternal behavior. In: Pfaff DW, editor. The physiological mechanisms of motivation. Springer; Berlin Heidelberg New York: 1982. pp. 253–285. [Google Scholar]
- Fleming AS, Cheung U, Myhal N, Kessler Z. Effects of maternal hormones on ‘timidity’ and attraction to pup-related odors in female rats. Physiol Behav. 1989;46:449–453. doi: 10.1016/0031-9384(89)90019-x. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Korsmit M, Deller M. Rat pups are potent reinforcers to the maternal animal: effects of experience parity, hormones, and dopamine function. Psychobiology. 1994;22:44–53. [Google Scholar]
- Gong W, Neill DB, Justice JB., Jr Locomotor response to novelty does not predict cocaine place preference conditioning in rats. Pharmacol Biochem Behav. 1996;53:191–196. [PubMed] [Google Scholar]
- Hebert MA, Blanchard DC, Blanchard RJ. Intravenous cocaine precipitates panic-like flight responses and lasting hyperdefensiveness in laboratory rats. Pharmacol Biochem Behav. 1999;63:349–360. doi: 10.1016/s0091-3057(98)00255-x. [DOI] [PubMed] [Google Scholar]
- Hecht GS, Spear NE, Spear LP. Changes in progressive ratio responding for intravenous cocaine throughout the reproductive process in female rats. Dev Psychobiology. 1999;20:136–145. [PubMed] [Google Scholar]
- Johns JM, Noonan LR, Zimmerman LI, Li L, Pedersen CA. Effects of chronic and acute cocaine treatment on the onset of maternal behavior and aggression in Sprague-Dawley rats. Behav Neurosci. 1994;108:107–112. doi: 10.1037//0735-7044.108.1.107. [DOI] [PubMed] [Google Scholar]
- Johns JM, Lubin DA, Walker CH, Meter KE, Mason GA. Chronic gestational cocaine treatment decreases oxytocin levels in the medial preoptic area, ventral tegmental area and hippocampus in Sprague-Dawley rats. Neuropeptides. 1997;31:439–443. doi: 10.1016/s0143-4179(97)90037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JM, Nelson CJ, Meter KE, Lubin DA, Couch CD, Ayers A, Walker CH. Dose-dependent effects of multiple acute cocaine injections on maternal behavior and aggression in Sprague-Dawley rats. Dev Neurosci. 1998;20:525–532. doi: 10.1159/000017353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinichev M, Rosenblatt JS, Morrell JI. The medial preoptic area, necessary for adult maternal behavior in rats, is only partially established as a component of the neural circuit that supports maternal behavior in juvenile rats. Behav Neurosci. 2000a;114:196–210. doi: 10.1037//0735-7044.114.1.196. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Rosenblatt JS, Nakabeppu Y, Morrell JI. Induction of c-fos-like and fosB-like immunoreactivity reveals forebrain neuronal populations involved differentially in pup-mediated maternal behavior in juvenile and adult rats. J Comp Neurol. 2000b;416:45–78. doi: 10.1002/(sici)1096-9861(20000103)416:1<45::aid-cne5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am J Psychiatry. 1985;142:1259–1264. doi: 10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- Klebaur JE, Bardo MT. Individual differences in novelty seeking on the playground maze predict amphetamine conditioned place preference. Pharmacol Biochem Behav. 1999;63:131–136. doi: 10.1016/s0091-3057(98)00258-5. [DOI] [PubMed] [Google Scholar]
- Klebaur JE, Bevins RA, Segar TM, Bardo MT. Individual differences in behavioral responses to novelty and amphetamine self-administration in male and female rats. Behav Pharmacol. 2001;12:267–275. doi: 10.1097/00008877-200107000-00005. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ. Dissociation of novelty- and cocaine-conditioned locomotor activity from cocaine place conditioning. Pharmacol Biochem Behav. 1998;60:785–791. doi: 10.1016/s0091-3057(97)00388-2. [DOI] [PubMed] [Google Scholar]
- Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: effects of lesions of the MPOA and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav Brain Res. 1999;100:15–31. doi: 10.1016/s0166-4328(98)00109-0. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Morrell JI. Neuronal fos expression in dams with preferences for cocaine- or pup-conditioned contexts (abstract) Soc Neurosci. 2001;31:647.15. [Google Scholar]
- Mattson BJ, Williams S, Rosenblatt JS, Morrell JI. Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav Neurosci. 2001;115:683–694. doi: 10.1037//0735-7044.115.3.683. [DOI] [PubMed] [Google Scholar]
- Mayer AD, Rosenblatt JS. Hormonal interaction with stimulus and situational factors in the initiation of maternal behavior in nonpregnant rats. J Comp Physiol Psychol. 1980;94:1040–1059. doi: 10.1037/h0077744. [DOI] [PubMed] [Google Scholar]
- Mayer AD, Rosenblatt JS. A method for regulating the duration of pregnancy and the time of parturition in Sprague-Dawley rats (Charles River CD strain) Dev Psychobiol. 1998;32:131–136. [PubMed] [Google Scholar]
- Mayer AD, Monroy MA, Rosenblatt JS. Prolonged estrogen-progesterone treatment of nonpregnant ovariectomized rats: factors stimulating home-cage and maternal aggression and short- latency maternal behavior. Horm Behav. 1990;24:342–364. doi: 10.1016/0018-506x(90)90014-o. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Ikemoto S. Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res. 1999;101:129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- Mucha RF, van der KD, O'Shaughnessy M, Bucenieks P. Drug reinforcement studied by the use of place conditioning in rat. Brain Res. 1982;243:91–105. doi: 10.1016/0006-8993(82)91123-4. [DOI] [PubMed] [Google Scholar]
- Nader MA, Woolverton WL. Effects of increasing the magnitude of an alternative reinforcer on drug choice in a discrete-trials choice procedure. Psychopharmacology. 1991;105:169–174. doi: 10.1007/BF02244304. [DOI] [PubMed] [Google Scholar]
- Nader MA, Woolverton WL. Effects of increasing response requirement on choice between cocaine and food in rhesus monkeys. Psychopharmacology. 1992;108:295–300. doi: 10.1007/BF02245115. [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Spyraki C. Cocaine-induced place conditioning: importance of route of administration and other procedural variables. Psychopharmacology. 1988;94:119–125. doi: 10.1007/BF00735892. [DOI] [PubMed] [Google Scholar]
- Numan M. Maternal behavior. In: Knobil E, Neill J, editors. The physiology of reproductive behavior. 2nd. Raven Press; New York: 1994. pp. 221–302. [Google Scholar]
- Parker LA. Place conditioning in a three or four choice apparatus: role of stimulus novelty in drug-induced place conditioning. Behav Neurosci. 1992;106:294–306. doi: 10.1037//0735-7044.106.2.294. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Pavlovian conditioning and its proper control procedures. Psych Rev. 1967;74:71–80. doi: 10.1037/h0024109. [DOI] [PubMed] [Google Scholar]
- Schechter MD, Calcagnetti DJ. Trends in place preference conditioning with a cross-indexed bibliography, 1957–1991. Neurosci Biobehav Rev. 1993;17:21–41. doi: 10.1016/s0149-7634(05)80228-3. [DOI] [PubMed] [Google Scholar]
- Schechter MD, Calcagnetti DJ. Continued trends in the conditioned place preference literature from 1992 to 1996, inclusive, with a cross-indexed bibliography. Neurosci Biobehav Rev. 1998;22:827–846. doi: 10.1016/s0149-7634(98)00012-8. [DOI] [PubMed] [Google Scholar]
- Scoles MT, Siegel S. A potential role of saline trials in morphine-induced place preference conditioning. Pharmacol Biochem Behav. 1986;35:583–587. doi: 10.1016/0091-3057(86)90106-1. [DOI] [PubMed] [Google Scholar]
- Scott DW, Morrell JI, Vernotica EM. Focal necrotizing panniculitis and vascular necrosis in rats given subcutaneous injections of cocaine hydrochloride. J Cutan Pathol. 1997;24:25–29. doi: 10.1111/j.1600-0560.1997.tb00781.x. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Heidbreder C. Sensitization to the conditioned rewarding effects of cocaine: pharmacological and temporal characteristics. J Pharmacol Exp Ther. 1995;273:808–815. [PubMed] [Google Scholar]
- Smith KS, Mattson BJ, Morrell JI. A quantitative analysis of neural substrates engaged in the motivation to perform maternal behavior (abstract) Soc Neurosci. 2002;32:878.13. [Google Scholar]
- Spear LP, Frambes NA, Kirstein CL. Fetal and maternal brain and plasma levels of cocaine and benzoylecgonine following chronic subcutaneous administration of cocaine during gestation in rats. Psychopharmacology. 1989;97:427–431. doi: 10.1007/BF00439542. [DOI] [PubMed] [Google Scholar]
- Vernotica EM, Morrell JI. Plasma cocaine levels and locomotor activity after systemic injection in virgin and in lactating maternal female rats. Physiol Behav. 1998;64:399–407. doi: 10.1016/s0031-9384(98)00092-4. [DOI] [PubMed] [Google Scholar]
- Vernotica EM, Lisciotto CA, Rosenblatt JS, Morrell JI. Cocaine transiently impairs maternal behavior in the rat. Behav Neurosci. 1996a;110:315–323. doi: 10.1037//0735-7044.110.2.315. [DOI] [PubMed] [Google Scholar]
- Vernotica EM, Rosenblatt JS, Morrell JI. Acute cocaine disrupts all components of established maternal behavior in the rat (abstract) Soc Neurosci. 1996b;1887 [Google Scholar]
- Vernotica EM, Rosenblatt JS, Morrell JI. Microinfusion of cocaine into the medial preoptic area or nucleus accumbens transiently impairs maternal behavior in the rat. Behav Neurosci. 1999;113:377–390. doi: 10.1037//0735-7044.113.2.377. [DOI] [PubMed] [Google Scholar]
- Wansaw M, Williams SE, Rosenblatt JS, Morrell JI. Stress and habituation: variables affecting conditioned place preference in maternal rodents (abstract) Soc Neurosci. 2001;31:77.8. [Google Scholar]
- Wansaw M, Williams SE, Rosenblatt JS, Morrell JI. Cocaine- and pup-induced conditioned place preference in the lactating, maternal rat (abstract) Soc Neurosci. 2002;32:181.15. [Google Scholar]
- Welker WI. “Free” versus “forced” exploration of a novel situation by rats. Psychol Rep. 1957;3:95–108. [Google Scholar]
- Wise RA. The brain and reward. In: Leibman JM, Cooper SJ, editors. The neuropharmacological basis of reward. Clarendon Press; Oxford: 1989. pp. 377–424. [Google Scholar]
- White NM, Hiroi N. Pipradol conditioned place preference is blocked by SCH23390. Pharmacol Biochem Behav. 1992;43:377–380. doi: 10.1016/0091-3057(92)90165-c. [DOI] [PubMed] [Google Scholar]


