Abstract
Diethanolamine (DEA) is a widely used ingredient in many consumer products and in a number of industrial applications. It has been previously reported that dermal administration of DEA to mice diminished hepatic stores of choline and altered brain development in the fetus. The aim of this study was to use mouse neural precursor cells in vitro to assess the mechanism underlying the effects of DEA. Cells exposed to DEA treatment (3mM) proliferated less (by 5-bromo-2-deoxyuridine incorporation) at 48 h (24% of control [CT]), and had increased apoptosis at 72 h (308% of CT). Uptake of choline into cells was reduced by DEA treatment (to 52% of CT), resulting in diminished intracellular concentrations of choline and phosphocholine (55 and 12% of CT, respectively). When choline concentration in the growth medium was increased threefold (to 210μM), the effects of DEA exposure on cell proliferation and apoptosis were prevented, however, intracellular phosphocholine concentrations remained low. In choline kinase assays, we observed that DEA can be phosphorylated to phospho-DEA at the expense of choline. Thus, the effects of DEA are likely mediated by inhibition of choline transport into neural precursor cells and by altered metabolism of choline. Our study suggests that prenatal exposure to DEA may have a detrimental effect on brain development.
Keywords: diethanolamine, choline, neural precursor cells, cell proliferation, apoptosis
Diethanolamine (DEA, CASRN 111−42−2) is an alkanolamine extensively used in many consumer products (cosmetic formulations and pharmaceuticals), as well as in various industrial processes (CIR, 1983, 1986). The most common exposure to DEA in humans is via dermal exposure to personal-care products (i.e., soaps, shampoos, and cosmetics), detergents, and other surfactants that contain DEA or fatty acid conjugates of DEA (TPMC, 2002). Occupational exposure to DEA occurs through the use of lubricating liquids in industrial processes (TPMC, 2002). The National Institute for Occupational Safety and Health estimates that the number of workers potentially exposed to DEA is approximately 800,000 per year (TPMC, 2002). Estimated annual production of DEA in the United States was 106,000 tons in 1995 (TPMC, 2002).
Although most of the studies that focused on the potential toxicity of DEA and its potential as a carcinogen have found no effects in humans (IARC, 2000), there is no available data in humans regarding tissue levels of DEA or quantifying human exposure to DEA (TPMC, 2002). However, studies performed in rodents found an association between long-term exposure to DEA and liver and kidney tumors (Program, 1999). Prolonged DEA administration to rats resulted in DEA accumulation in various tissues at high concentrations (31 μg equivalent DEA/g tissue in rat brains after an 8-week exposure to a daily dose of 7 mg/kg bw), with variable accumulation rates for different tissues (114 vs. 10 tissue per blood ratio in liver and brain, respectively) (Mathews et al., 1997). Because DEA is structurally similar to choline, it was hypothesized that DEA-related outcomes are due to perturbations in choline metabolism; such effects have been demonstrated in liver (Barbee and Hartung, 1979; Lehman-McKeeman et al., 2002; Stott et al., 2000). Similar to choline deficiency, DEA also alters DNA methylation in mouse hepatocytes (Bachman et al., 2006).
Maternal choline deficiency during pregnancy altered the development of fetal mouse hippocampus (Albright et al., 2005; Craciunescu et al., 2003), and this effect was likely mediated by alterations in gene-specific DNA methylation within neuronal precursor cells that will form the hippocampus (Niculescu et al., 2004, 2006). We recently reported that DEA, when dermally administered to pregnant mice, induced similar changes in the fetal hippocampus, i.e., reduced proliferation of neuronal precursor cells and increased apoptosis (Craciunescu et al., 2006).
The aim of the present work was to study a potential mechanism for these alterations. Based on previous studies using in vitro models (Lehman-McKeeman and Gamsky 1999, 2000), we hypothesized that DEA inhibits uptake of choline in mouse neural precursor cells and results in diminished intracellular availability of choline. If this occurs, supplementation with choline should prevent the effects of DEA. This is the first study, in our knowledge, that describes changes induced by DEA on the metabolism of choline in neural precursor cells.
MATERIALS AND METHODS
All reagents were purchased from Sigma Chemicals (St Louis, MO), unless otherwise specified, and solvents were of HPLC grade. DEA (ethylene-d8) was obtained from Cambridge Isotope Laboratories (Andover, MA).
Cell culture
Mouse C57Bl/6J cortical neural precursor cells (at embryonic day 14) were purchased from Cambrex (Walkersville, MD; # M-Cx-300) and were initially plated according to the manufacturer's protocol using Neurobasal medium (Invitrogen, Purchase, NY) with the following ingredients added (Wachs et al., 2003): 1% 2mM L-Glutamine (Invitrogen), 1% 100 U/ml Penicillin-Streptomycin (Invitrogen), 2% B27 Supplement without vitamin A (Invitrogen), 20 ng/ml murine EGF (Invitrogen), 20 ng/ml human bFGF (Invitrogen), and 2 μg/ml heparin. The medium was filtered into a sterile container using a Nalgene 150 ml SFCA membrane 0.2 μm filter (Fisher Scientific, Fair Lawn, NJ). Cells were plated in 10 cm petri dishes (Fisher Scientific) and incubated for 5 days at 37°C and 5% CO2. At day 3, fresh medium and growth factors were added to double the suspension volume. After 5 days, the culture medium containing floating neurospheres was collected in sterile 15 ml tubes and centrifuged at 120g for 5 min at 37°C. The neurosphere pellets were dissociated in Accutase (Innovative Cell Technologies, San Diego, CA), passaged, and reseeded as a suspension. Cells were treated for up to 72 h with Neurobasal medium without choline chloride (D700SA; Atlanta Biologicals, Lawrenceville, GA) containing the above described ingredients but with different concentrations of choline chloride and DEA (n = 4 plates per treatment): 70μM choline chloride (control, CT); 210μM choline chloride (choline supplemented, CS); 70μM choline chloride and 3mM DEA (CT-DEA); 210μM choline chloride and 3mM DEA (choline supplementation with DEA [CS-DEA]). At the end of 48 and 72 h time exposure, the neurospheres were harvested and processed immediately, or stored as pellets at − 20°C for the choline and DEA measurements.
DEA administration to pregnant mice
DEA (80 mg/kg bw/day) was administered dermally as previously described (Craciunescu et al., 2006), from gestation day 7 through 17. At the end of day 17, fetal brains were removed, stored at − 80°C, and subsequently processed for the determination of DEA metabolites. Fetal brains from two different pregnant dams were used.
Cell proliferation
Cell proliferation was assessed using 5-bromo-2-deoxyuridine (BrdU) incorporation. Prior to harvesting, 10μM BrdU was added to the culture media and cells were incubated for 2 h. Cells were harvested and subsequently processed using a BrdU Immunohistochemistry Kit (Chemicon International, Temecula, CA) according to the manufacturer's protocol. BrdU-positive cells (cells in the S phase of the cell cycle) were identified using light microscopy and expressed as a percentage of the total number of cells. At least 300 cells were counted per sample and datapoint.
DNA fragmentation
Terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate (dUTP)-digoxigenin anti-digoxigenin fluorescein conjugate antibody nick end labeling (TUNEL) was used as a marker of apoptosis. Cells with single- and double-stranded DNA breaks were detected using the ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit (Chemicon International), according to the manufacturer's protocol. Using light microscopy, at least 300 cells were counted per sample and datapoint, and TUNEL-positive cells were expressed as a percentage of the total number of cells.
Choline uptake
Radiolabeled choline chloride ([methyl-14C]; 55 mCi/mmol, ICN Biomedicals, Irvine, CA) was used to assess choline uptake into neural precursor cells for all four treatment groups: CT, CS, CT-DEA, and CS-DEA. Briefly, samples were incubated with 14C-choline (10,000 disintegrations per minute [DPM]), where the total concentration of choline was brought to 70 or 210μM, respectively, with unlabeled choline chloride. After incubating at 37°C for 6 h, cells were immediately put on ice. All subsequent procedures were performed on ice. To determine the background caused by extracellular radiolabel, ice-cold media, containing the same amount of radiolabeled choline as the experiment, was added to cells which, in turn, were immediately harvested on ice and subjected to centrifugation. Cell pellets were washed with 15 ml PBS twice, resuspended in distilled water, and sonicated (five pulses using a Sonicator W-225R, Heat Systems-Ultrasonics, Framingdale, NY). Aliquots from the sonicated samples were used to determine DNA concentration (Labarca and Paigen, 1980), and radiolabel was counted after the addition of 3 ml scintillation fluid (ScintiSafe 30%, Fisher Scientific) using a Wallac1409 liquid scintillation counter (PerkinElmer, Boston, MA). DPM values were normalized for DNA amount, and corrected for extracellular background and specific activity of 14C-choline in the initial incubation medium for each treatment. Uptake data were expressed as nmol choline per mg DNA. The external background was less than 3% of the total DPM.
Choline and DEA metabolites
Choline, DEA, and their metabolites were extracted from fetal brains and neural precursor cell samples (after 48 h treatment) using the method of Bligh and Dyer (1959). Aqueous and organic compounds were separated, analyzed, and quantified using liquid chromatography-electrospray ionization-isotope dilution mass spectrometry (LC-ESI-IDMS), as previously described (Koc et al., 2002), after spiking the samples with deuterium-labeled internal standards which were used to correct for recovery. Results were expressed as nmol/mg DNA.
For DEA determination in fetal brains and for the choline kinase assay described below, choline (Cho, mass-to-charge ratio [m/z] 104), phosphocholine (PCho, m/z 184), DEA (m/z 106), and phosphodiethanolamine (phospho-DEA [PDEA], m/z 186) were analyzed by monitoring the mass of the intact cations using the method of Koc et al. (2002). Deuterium-labeled DEA (DEA-d8) was used as the internal standard for quantitation of DEA. DEA had a linear response in the 0−200 nmol range, and 100.3% recovery when spiked in a sample of cells. PCho-d9 was used as the internal standard to calculate the ion peak intensity of PDEA, and to estimate the concentration of PDEA due to the unavailability of an authentic PDEA standard. All compounds were also analyzed in selected reaction monitoring mode to validate the data collected in selected ion monitoring mode. Helium was used as the collision gas; relative collision energy was set at 28% (Cho, DEA) and 30% (PCho, PDEA) of maximum, and dwell time was set to 0.03 s for each transition. The most abundant product ion was used for quantitation as follows: Cho at m/z 60, Cho-d9 at m/z 69, PCho at m/z 86, PCho-d9 at m/z 95, DEA at m/z 88, DEA-d8 at m/z 96, and PDEA at m/z 88.
Choline kinase in vitro assay
Choline kinase (EC 2.7.1.32) was used as previously described (Goldberg and McCaman, 1973), to determine whether DEA could be phosphorylated to PDEA. Briefly, choline (0.5 μmol per reaction) and increasing amounts of DEA (0, 0.5, 2.5, 5, 10, 20 μmol per reaction), in a total volume of 25 μl, was added to 0.5 ml cocktail containing 10mM MgCl2, 6.5mM Glycyl-Glycine, pH 8.5, 4.25mM dithiotreitol, 0.33mM ATP, and 0.05 U choline kinase (yeast origin C7138; Sigma) and incubated at 30°C for 15 min. The reaction was stopped with the addition of 2.5 ml ice-cold H2O, and the samples were stored at − 20°C until analyzed for choline, PCho, DEA, and PDEA. For this experiment, the molar ratio between DEA and choline was 0:1, 1:1, 5:1, 10:1, 20:1, and 40:1, respectively. The highest ratio was similar to the DEA/choline ratio used in the in vivo cell culture study (42:1). All reactions were performed in duplicate.
Statistics
Statistical analysis was performed using JMP 3.2.6 software (SAS Institute Inc, Cary, NC). One-way ANOVA was used to test the null hypothesis between all four treatments (CT, CS, CT-DEA, and CS-DEA), and Tukey-Kramer critical difference test was used to determine the statistical differences between these groups. Differences were considered significant at p value < 0.05.
RESULTS
DEA in Fetal Brains
In fetal brains obtained from pups from two different dams, free DEA concentrations were 0.023 and 0.026mM, and PDEA concentrations were 1.6 and 1.3mM, respectively.
Cell Proliferation and Apoptosis
Exposure of mouse neural precursor cells to DEA (CT-DEA, 3mM) reduced the incorporation of BrdU into cells after 48 and 72 h compared to CT (3.8% BrdU-positive cells ± 0.4 SE and 2.6% BrdU-positive cells ± 0.4 SE vs. 16.0% ± 1.1 SE and 15.4% ± 1.1 SE, respectively, Fig. 1a). CS-DEA almost completely restored BrdU incorporation at 48 h (11.7% ± 0.7 SE), and completely restored it at 72 h (14.0% ± 0.3 SE) compared to CT. No differences were found between CT and CS neural precursor cells. These results indicate that DEA inhibits BrdU incorporation within 48 h exposure. Choline supplementation restores BrdU incorporation in DEA-treated cells, suggesting that DEA inhibits cell proliferation by interfering with availability of intracellular choline.
FIG. 1.
DEA altered BrdU incorporation and apoptosis in mouse neural progenitor cells from embryonic day 14. Neural precursor cells were cultured in Neurobasal medium (see the “Materials and Methods” section) containing 70μM choline (CT), 70μM choline, and 3mM DEA (CT-DEA); 210μM choline (CS); or 210μM choline and 3mM DEA (CS-DEA). Cell proliferation and apoptosis were assessed after 48 and 72 h, using BrdU incorporation and TUNEL, respectively. Results are expressed as percentage of cells that are BrdU or TUNEL positive. Error bars indicate SE. Superscripts denote statistical significance at p < 0.05: *, different than all other groups; a, different than CT; b, different than CS; c, different than CT-DEA. Arrows show significance of change for the same treatment and between time points. (a) BrdU incorporation at 48 and 72 h exposure. (b) DNA fragmentation at 48 and 72 h exposure.
Changes in rates of DNA fragmentation were observed at a later time point than were changes in BrdU incorporation (Fig. 1b). At 48 h of DEA treatment, both CS- and CS-DEA-treated cells had less DNA fragmentation than did CT cells (2.7% TUNEL-positive cells ± 0.3 SE and 3.5% TUNEL-positive cells ± 0.4 SE vs. 6.1% ± 0.3 SE). DEA (CT-DEA) did not induce a significant increase in DNA fragmentation (5.5% ± 1.1 SE) compared to CT at this time. At 72 h, DEA (CT-DEA) induced a threefold increase (to 14.8% ± 2.2 SE) in DNA fragmentation compared to CT (4.8% ± 0.2 SE), while CS-DEA prevented this increase (5.6% ± 0.4 SE). These results suggest that DEA increases apoptosis by interfering with availability of intracellular choline.
Choline Uptake
Uptake of radiolabeled choline into neural precursor cells was similar in CT and CS groups (Fig. 2). Exposure to DEA decreased choline uptake to 51.9% of CT (p < 0.05). CS-DEA prevented this effect.
FIG. 2.

DEA inhibited choline uptake in mouse neural precursor cells from embryonic day 14. Neural precursor cells were cultured in Neurobasal medium (see the “Materials and Methods” section) containing 70μM choline (CT), 70μM choline, and 3mM DEA (CT-DEA); 210μM choline (CS); or 210μM choline and 3mM DEA (CS-DEA). All plates were treated with the same amount of radiolabeled choline. After 6 h of exposure, cells were harvested and intracellular choline uptake was measured. An asterisk denotes statistical significance compared to all other groups at p < 0.05 (Tukey-Kramer). Error bars indicate SE.
Choline Metabolites
Concentrations of choline metabolites in neural precursor cells are indicated in Table 1. No betaine was detected in any of the groups of cells. Choline supplementation increased intracellular concentrations of choline and phosphocholine, but did not change concentrations of other choline metabolites compared to CT. Exposure to DEA (CT-DEA) decreased intracellular concentrations of choline and phosphocholine, but did not change concentrations of other choline metabolites. CS-DEA prevented the decrease in choline concentrations, but only partially prevented the decrease in phosphocholine concentrations; again all other choline metabolites were unchanged.
TABLE 1.
Choline Metabolites in Neural Precursor Cells
| Choline (nmol/mg DNA) | Glycerophosphocholine (nmol/mg DNA) | Phosphocholine (nmol/mg DNA) | Phosphatidylcholine (nmol/mg DNA) | Sphingomyelin (nmol/mg DNA) | |
|---|---|---|---|---|---|
| CT | 43.5 ± 3.5 | 17.5 ± 2.7 | 255.0 ± 5.7 | 1186.8 ± 44.9 | 173.3 ± 17.4 |
| CS | 57.8 ± 1.4a | 16.0 ± 0.9 | 312.3 ± 11.2a | 1155.7 ± 9.0 | 166.0 ± 2.3 |
| CT-DEA | 24.1 ± 0.8a,b | 14.2 ± 0.5 | 31.6 ± 0.5a,b | 1175.0 ± 16.1 | 202.2 ± 6.2 |
| CS-DEA | 43.7 ± 0.4b,c | 14.9 ± 1.2 | 74.6 ± 6.2a,b,c | 1229.5 ± 10.5 | 173.4 ± 2.4 |
Note. Neural precursor cells were cultured in Neurobasal medium (see the “Materials and Methods” section) containing 70μM choline (CT), 70μM choline, and 3mM DEA (CT-DEA); 210μM choline (CS); or 210μM choline and 3mM DEA (CS-DEA). Choline metabolites were measured by LC-MS (see the “Materials and Methods” section), after 48 h treatment. No betaine was detected in any sample. Superscripts denote statistical significance at p < 0.05;
Different than CT
Different than CS
Different than CT-DEA.
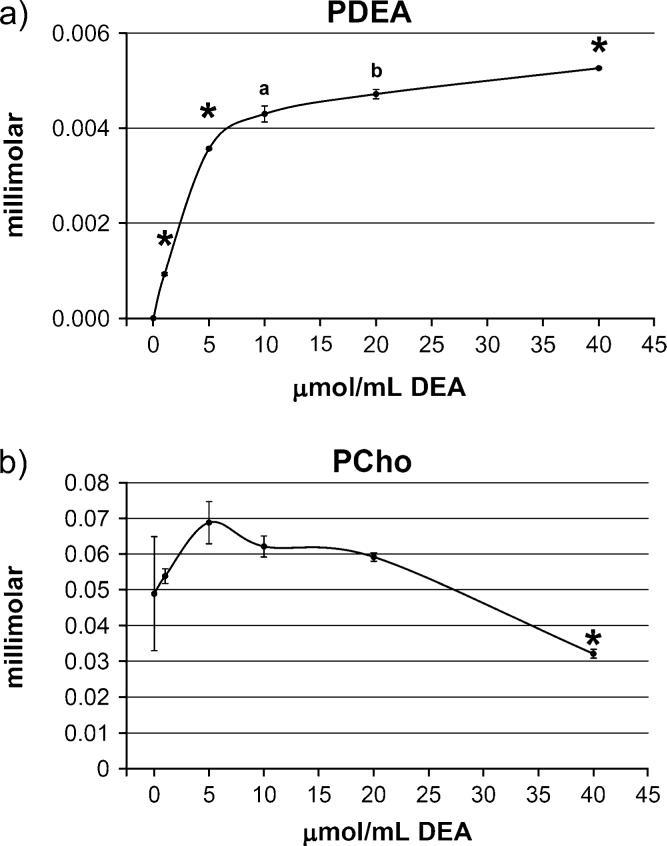
Choline/DEA Competition Study
We observed that DEA was a substrate for choline kinase and that more PDEA was formed as exposure to DEA was increased (Fig. 3a). In the presence of choline, choline kinase phosphorylated DEA to PDEA in a dose-dependent manner. The curve shape suggests that saturation levels of substrate were reached at 40 μmol/ml DEA or above. At the same time, DEA inhibited phosphorylation of choline at the highest concentration (40 μmol/ml) (Fig. 3b).
FIG. 3.
DEA inhibited choline phosphorylation by choline kinase. Increased amounts of DEA (0, 1, 5, 10, 20, or 40 μmol/ml per reaction) were incubated in the presence of choline kinase and 1 μmol/ml choline (see the “Materials and Methods” section). An asterisk denotes statistical significance versus all other groups; a, different from all other groups except the 20 μmol/ml DEA dose; b, different than all other groups except the 10 μmol/ml DEA dose (p < 0.05, Tukey-Kramer). PDEA, phospho-DEA; PCho, phosphocholine. Error bars indicate SE. (a) DEA phosphorylation by choline kinase assay. (b) Choline phosphorylation by choline kinase assay.
DISCUSSION
We report, for the first time that neural precursor cells treated with 3mM DEA in vitro have decreased cell proliferation and increased rates of apoptosis. The DEA concentration used in the cell culture medium (3mM) is similar to the concentration of phosphorylated DEA in fetal mouse brains (1.5mM; this underestimates total DEA compounds in brain) from dams treated with DEA at concentrations that inhibited cell proliferation and increased apoptosis in fetal hippocampus (Craciunescu et al., 2006). While changes in BrdU incorporation (cell proliferation) were first noted at 48 h, increased apoptosis was a later event (72 h). Choline supplementation of the DEA-containing media prevented DEA-induced changes in proliferation and apoptosis. We also found that DEA treatment competes with choline transport into neural precursor cells and that this effect can be overcome by addition of excess choline. These data help to explain why DEA treatment during pregnancy resulted in changes in fetal brain development that are similar to those observed when dams are choline deficient during pregnancy (Craciunescu et al., 2006); DEA competes with choline for transport into cells. Our results are consistent with previous reports of DEA's effects in other cell types. DEA competitively inhibits choline uptake in both CHO-K1 cells and Syrian hamster embryo cells (Lehman-McKeeman and Gamsky, 1999, 2000).
We previously reported that phosphocholine was the metabolite most affected in mouse liver after treatment with DEA in vivo (Lehman-McKeeman et al., 2002). It is interesting that, although choline supplementation could restore intracellular concentrations of choline after DEA treatment, it did not fully restore intracellular concentrations of phosphocholine. This suggests that DEA, in addition to inhibiting choline transport, acts to change the flux of phosphocholine either by inhibiting choline kinase or by accelerating the use of phosphocholine for synthesis of phosphatidylcholine (Fig. 4). The latter mechanism is unlikely because DEA is incorporated into the phospholipid fraction in liver and inhibits phosphatidylcholine synthesis (Lehman-McKeeman and Gamsky, 1999). To determine whether DEA directly inhibits choline phosphorylation, we incubated DEA with purified choline kinase. We found that DEA was phosphorylated at a much lower rate than was choline, suggesting that choline kinase has a very low affinity for DEA, compared to choline. This conclusion is supported by previous studies that indicated that choline kinase has a higher Km for DEA (12mM) than it does for either choline (Km = 0.076mM) or ethanolamine (Km = 0.054mM) for the synthesis of phospholipids in vitro (Barbee and Hartung, 1979). Despite this, using a similar ratio of DEA/choline as in our in vivo study (40:1, see the “Materials and Methods” section), we found that choline phosphorylation was significantly inhibited in the presence of DEA. It is not surprising that DEA treatment did not alter concentrations of phosphatidylcholine (or of glycerophosphocholine which is a breakdown product of phosphatidylcholine, Fig. 4) and sphingomyelin, as the 48-h duration of the experiment was short relative to the rate of turnover of these phospholipids in neural precursor cells.
FIG. 4.

Choline metabolism (selected pathways). Metabolism of choline intersects with the homocysteine metabolism. Choline is phosphorylated to phosphocholine by choline kinase in the first step toward phosphatidylcholine synthesis. In an alternate pathway, choline serves as a methyl donor (through betaine) for the methylation of homocysteine to methionine.
As noted earlier, DEA is a commonly used ingredient in consumer products. However, no human data are available at the present to allow accurate assessment of DEA exposure, and there is no data on DEA metabolite concentrations in human tissues. Therefore, it is impossible to determine whether the various dosages used in animal studies accurately reflect human exposure (TPMC, 2002). Previous studies using rats and mice show that daily DEA administration dermally, intravenously, or orally results in DEA accumulation in various tissues (Mathews et al., 1997; TPMC, 2002). Repeated daily administration of 7 mg DEA/kg bw in rats generated DEA concentrations in rat brain between 0.17mM (2-week exposure) and 0.29mM (8-week exposure) (Mathews et al., 1997). In our study, DEA administration to pregnant mice for 10 days (80 mg/kg bw daily) generated PDEA concentrations of about 1.3 to 1.5mM, indicating that DEA accumulated in fetal tissues and that the DEA concentration we used in the culture medium was appropriate.
Our work suggests that DEA interferes with choline transport and choline phosphorylation in neural precursor cells and this may explain the previously reported effects of DEA on brain development (Craciunescu et al., 2006). Moreover, because choline supplementation can overcome the observed effects of DEA, we suggest that DEA acts by altering intracellular choline availability. We previously established that choline deficiency altered the epigenetic regulation of gene expression in cell culture and in fetal brain by inhibiting DNA methylation, thereby changing expression of cyclin-dependent kinase inhibitors that modulate cell proliferation (Niculescu et al., 2004, 2006). We suggest that the same mechanism may be involved in the DEA-induced outcomes. This hypothesis is also supported by a recent study showing that both choline deficiency and DEA alter the DNA methylation profile in mouse livers (Bachman et al., 2006). We can only speculate about the effects of DEA exposure on brain development. Mouse fetuses born of dams fed a choline deficient diet have permanent alterations in memory and attention (Meck and Williams, 2003). Whether or not these findings can be applied to the human brain development remains to be determined.
ACKNOWLEDGMENTS
This work was funded by grants from the National Institutes of Health (ES012997, AG09525) and was assisted by the University of North Carolina Clinical Nutrition Research Unit (DK56350) and Center for Environmental Health and Susceptibility (ES10126). The authors certify that all research involving human subjects was done under full compliance with all government policies and the Helsinki Declaration. Conflicts of interest: S.H.Z. acknowledges that he has a gift from the American Chemistry Council to do research in this area; this funding organization does not have control over this publication. S.H.Z. also serves on the Dupont Scientific Advisory Board.
REFERENCES
- Albright CD, da Costa KA, Craciunescu CN, Klem E, Mar MH, Zeisel SH. Regulation of choline deficiency apoptosis by epidermal growth factor in CWSV-1 rat hepatocytes. Cell Physiol. Biochem. 2005;15:59–68. doi: 10.1159/000083653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachman AN, Kamendulis LM, Goodman JI. Diethanolamine and phenobarbital produce an altered pattern of methylation in GC-rich regions of DNA in B6C3F1 mouse hepatocytes similar to that resulting from choline deficiency. Toxicol. Sci. 2006;90:317–325. doi: 10.1093/toxsci/kfj091. [DOI] [PubMed] [Google Scholar]
- Barbee SJ, Hartung R. The effect of diethanolamine on hepatic and renal phospholipid metabolism in the rat. Toxicol. Appl. Pharmacol. 1979;47:421–430. doi: 10.1016/0041-008x(79)90511-8. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- CIR Cosmetic ingredients review: Final report on the safety assessment of triethanolamine, diethanolamine, and monoethanolamine. J. Am. Coll. Toxicol. 1983;2:183–235. [Google Scholar]
- CIR Cosmetic ingredients review: Final report on the safety assessment of cocamide DEA, lauramide DEA, linoleamiede DEA, and oleamide DEA. J. Am. Coll. Toxicol. 1986;5:415–454. [Google Scholar]
- Craciunescu CN, Albright CD, Mar MH, Song J, Zeisel SH. Choline availability during embryonic development alters progenitor cell mitosis in developing mouse hippocampus. J. Nutr. 2003;133:3614–3618. doi: 10.1093/jn/133.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craciunescu CN, Wu R, Zeisel SH. Diethanolamine alters neurogenesis and induces apoptosis in fetal mouse hippocampus. FASEB J. 2006;20:1635–1640. doi: 10.1096/fj.06-5978com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer Press (IARC) Diethanolamine (group 3), 5. Summary of data reported and evaluation, 5.1 exposure data, 5.2 human carcinogenicity data. In: Iacono JM, editor. Some Industrial Chemicals. Vol. 77. International Agency for Research on Cancer Press; Lyon, France: 2000. pp. 349–379. [Google Scholar]
- Koc H, Mar MH, Ranasinghe A, Swenberg JA, Zeisel SH. Quantitation of choline and its metabolites in tissues and foods by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry. Anal. Chem. 2002;74:4734–4740. doi: 10.1021/ac025624x. [DOI] [PubMed] [Google Scholar]
- Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal. Biochem. 1980;102:344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lehman-McKeeman LD, Gamsky EA. Diethanolamine inhibits choline uptake and phosphatidylcholine synthesis in Chinese hamster ovary cells. Biochem. Biophys. Res. Commun. 1999;262:600–604. doi: 10.1006/bbrc.1999.1253. [DOI] [PubMed] [Google Scholar]
- Lehman-McKeeman LD, Gamsky EA. Choline supplementation inhibits diethanolamine-induced morphological transformation in Syrian hamster embryo cells: Evidence for a carcinogenic mechanism. Toxicol. Sci. 2000;55:303–310. doi: 10.1093/toxsci/55.2.303. [DOI] [PubMed] [Google Scholar]
- Lehman-McKeeman LD, Gamsky EA, Hicks SM, Vassallo JD, Mar MH, Zeisel SH. Diethanolamine induces hepatic choline deficiency in mice. Toxicol. Sci. 2002;67:38–45. doi: 10.1093/toxsci/67.1.38. [DOI] [PubMed] [Google Scholar]
- Mathews JM, Garner CE, Black SL, Matthews HB. Diethanolamine absorption, metabolism and disposition in rat and mouse following oral, intravenous and dermal administration. Xenobiotica. 1997;27:733–746. doi: 10.1080/004982597240316. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: Implications for memory and attentional processing across the lifespan. Neurosci. Biobehav. Rev. 2003;27:385–399. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J. 2006;20:43–49. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu MD, Yamamuro Y, Zeisel SH. Choline availability modulates human neuroblastoma cell proliferation and alters the methylation of the promoter region of the cyclin-dependent kinase inhibitor 3 gene. J. Neurochem. 2004;89:1252–1259. doi: 10.1111/j.1471-4159.2004.02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTP Toxicology and carcinogenesis studies of diethanolamine (CAS No. 111−42−2) in F344/N rats and B6C3F1 Mice (Dermal Studies). Natl. Toxicol. Program. Tech. Rep. Ser. 1999;478:1–212. Program. [PubMed] [Google Scholar]
- Stott WT, Bartels MJ, Brzak KA, Mar M, Markham DA, Thornton CM, Zeisel SH. Potential mechanisms of tumorigenic action of diethanolamine in mice. Toxicol. Lett. 2000;114:67–75. doi: 10.1016/s0378-4274(99)00197-6. [DOI] [PubMed] [Google Scholar]
- Technology Planning and Management Corporation (TPMC) Report on Carcinogens Background Document for Diethanolamine. In National Toxicology Program N01ES85421. NIEHS; Durham, NC: 2002. p. 229. [Google Scholar]
- Wachs FP, Couillard-Despres S, Engelhardt M, Wilhelm D, Ploetz S, Vroemen M, Kaesbauer J, Uyanik G, Klucken J, Karl C, et al. High efficacy of clonal growth and expansion of adult neural stem cells. Lab. Invest. 2003;83:949–962. doi: 10.1097/01.lab.0000075556.74231.a5. [DOI] [PubMed] [Google Scholar]