Summary
Recent progress in the understanding of the human dietary requirement for choline highlights the importance of genetic variation and epigenetics in human nutrient requirements. Choline is a major dietary source of methyl-groups (one of choline's metabolites, betaine, participates in the methylation of homocysteine to form methionine); also choline is needed for the biosynthesis of cell membranes, bioactive phospholipids and the neurotransmitter acetylcholine. A recommended dietary intake for choline in humans was set in 1998, and a portion of the choline requirement can be met via endogenous de novo synthesis of phosphatidylcholine catalyzed by phosphatidylethanolamine N-methyltransferase (PEMT) in the liver. Though many foods contain choline, many humans do not get enough in their diets. When deprived of dietary choline, most adult men and postmenopausal women developed signs of organ dysfunction (fatty liver, liver or muscle cell damage, and reduces the capacity to handle a methionine load, resulting in elevated homocysteine). However, only a portion of premenopausal women developed such problems. The difference in requirement occurs because estrogen induces expression of the PEMT gene and allows premenopausal women to make more of their needed choline endogenously. In addition, there is significant variation in the dietary requirement for choline that can be explained by common polymorphisms in genes of choline and folate metabolism. Choline is critical during fetal development, when it alters DNA methylation and thereby influences neural precursor cell proliferation and apoptosis. This results in long term alterations in brain structure and function, specifically memory function.
Keywords: Choline, brain development, single nucleotide polymorphism, epigenetics, DNA methylation.
EFFECTS OF A LOW CHOLINE DIET IN HUMANS
Choline is a dietary component essential for normal function of all cells (1). It is the major source of methyl-groups in the diet (one of choline's metabolites, betaine, participates in the methylation of homocysteine to form methionine) (2). In addition, choline is used for the biosynthesis of cell membranes, bioactive phospholipids, and the neurotransmitter acetylcholine (1). The functional consequences of dietary choline deficiency in humans include the development of fatty liver (3, 4) (hepatosteatosis; occurs because a lack of phosphatidylcholine limits the export of excess triglyceride from liver in lipoproteins (5, 6)), liver damage (3, 7 – 10) (elevated serum aminotransferases; secondary to apoptosis (7, 11, 12) and muscle damage (10, 13) (elevated creatine phosphokinase in blood; occurs because muscle membranes are more fragile and because of induction of apoptosis (13)).
In 1998 the US Institute of Medicine's Food and Nutrition Board established an Adequate Intake (AI) and Tolerable Upper Limit (UL) for choline (14).
CHOLINE, FOLATE AND METHIONINE METABOLISM ARE INTERRELATED
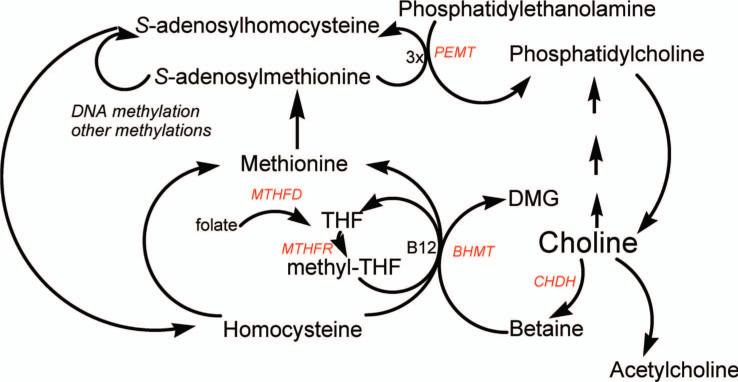
Choline, methionine and folate metabolism interact at the point that homocysteine is converted to methionine (Fig. 1). Thus, any requirement for dietary choline must be considered in relation to these other nutrients. Homocysteine can be methylated to form methionine (15) by two parallel pathways, both of which lower homocysteine concentrations (16). In the first, vitamins B12 and folic acid are involved in a reaction catalyzed by methionine synthase (17). Deficiency of these nutrients (18, 19), or single nucleotide polymorphisms in the genes for the enzymes involved in this pathway (17, 19, 20), can result in elevated plasma homocysteine concentrations. In addition, tetrahydrofolate is needed to scavenge one-carbon groups when betaine is metabolized (21). The alternative pathway for the methylation of homocysteine to form methionine is catalyzed by betaine homocysteine methyltransferase (BHMT) (22), an enzyme whose activity has been reported to increase in rats during methionine excess (23). Betaine, derived from dietary choline by the action of choline dehydrogenase (CHDH), is the methyl group donor in this reaction and supplemental oral betaine can lower plasma homocysteine concentrations (24, 25).
Figure 1.
Choline metabolism and links to methionine and folate metabolism. The pathways described are all present in the liver, with other tissues having one or more of these pathways. PEMT, phosphatidylethanolamine-N-methyltransferase; CHDH, choline dehydrogenase; BHMT, betaine homocysteine methyltransferase; MTHFR, methylene tetrahydrofolate reductase; MTHFD, methylene tetrahydrofolate dehydrogenase.
Perturbing metabolism of one of the methyl-donors results in compensatory changes in the other methyl-donors due to the intermingling of these metabolic pathways (26 – 28). Rats treated with the anti-folate, methotrexate, had diminished pools of choline metabolites in liver (27, 29). Rats ingesting a choline-deficient diet had diminished tissue concentrations of methionine and S-adenosylmethionine (30) and doubled plasma homocysteine concentrations (31). We recently reported that humans who are depleted of choline have diminished capacity to methylate homocysteine and developed elevated homocysteine concentrations in plasma after a methionine loading test (2).
DIETARY INTAKE AND ENDOGENOUS SYNTHESIS OF CHOLINE
Excellent sources of dietary choline include liver, eggs and wheat germ (32, 33). In foods, choline is found free and as choline esters. Though it is likely that these forms are fungible, there is some evidence that they may have different bioavailability (34) because the lipid-soluble forms bypass the liver when absorbed from the diet while the water soluble forms enter the portal circulation and are mostly absorbed by the liver. It is not clear whether normal dietary patterns deliver the recommended amounts of choline for all people. Shaw and colleagues, studying pregnant women in California observed intakes of choline in 25% of the population that were less than needed to prevent birth defects in their fetuses (35, 36).
The only source of choline other than diet is from the de novo biosynthesis of phosphatidylcholine catalyzed by phosphatidylethanolamine-N-methyltransferase (PEMT) in liver. This enzyme uses S-adenosylmethionine as a methyl donor and forms a new choline moiety (37). When fed a diet deficient in choline, Pemt −/− mice developed fatty liver, severe liver damage and died; a choline supplemented diet prevented this (38) and reversed hepatic damage if begun early enough (39). The PEMT pathway is not just a minor pathway that backs up the cytidine diphosphocholine pathway for phosphatidylcholine biosynthesis. Pemt −/− mice have lower choline pools in liver despite being fed sufficient or supplemental amounts of dietary choline (40), suggesting that choline production by PEMT is a significant source of choline relative to dietary intake. When Pemt is deleted in mice, plasma homocysteine concentrations fall 50% and, when it is over expressed, plasma homocysteine concentrations increase 40% (41, 42), demonstrating that PEMT activity is a very major consumer of S-adenosylmethionine (and thereby a producer of homocysteine).
ESTROGEN RESPONSE ELEMENTS AND THE REQUIREMENT FOR CHOLINE
Premenopausal women, relative to males and postmenopausal women, are resistant to developing organ dysfunction when fed a low choline diet (10). The classic actions of estrogen occur through its receptors ERα and ERβ which bind as homodimers or heterodimers to estrogen response elements (EREs) in the promoters of many estrogen-responsive genes (43). The consensus ERE (PuGGTCAnnnTGACCPy) (43) and some imperfect ERE half site motifs (ERE1/2) bind with ERα and ERβ (44 – 46). There are multiple EREs in the promoter region(s) of the PEMT gene (47) and estrogen, at doses bracketing physiological concentrations in humans (0 − 100 nmol/l), causes a marked up-regulation in PEMT mRNA expression and enzyme activity in human hepatocytes (47). Thus, premenopausal women have an enhanced capacity for de novo biosynthesis of choline moiety.
During pregnancy, estradiol concentration rises from approximately 1 nM to 60 nM at term (48, 49), suggesting that capacity for endogenous synthesis of choline should be highest during period when females need to support fetal development. Pregnancy and lactation are times when demand for choline is especially high; transport of choline from mother to fetus (50, 51) depletes maternal plasma choline in humans (52). It is interesting that despite enhanced capacity to synthesize choline, the demand for this nutrient is so high that stores are depleted during pregnancy. Pregnant rats had diminished total liver choline compounds compared to non-mated controls and become as sensitive to choline-deficient diets as were male rats (53). Because milk contains a great deal of choline, lactation further increases maternal demand for choline resulting in further depletion of tissue stores (53, 54). These observations suggest that women depend on high rates of endogenous biosynthesis of choline induced by estrogen, as well as on dietary intake of choline to sustain normal pregnancy. It is biologically plausible that, during evolution, appropriate mechanisms for inducing endogenous choline biosynthesis (via PEMT) were developed to assure that young women are less susceptible to dietary choline deficiency and have adequate stores of choline prior to, and during pregnancy. Pemt −/− mice abort pregnancies around 9 − 10 days gestation unless fed supplemental choline (personal observation; (55)). A better understanding of why there is variation in dietary choline requirements might be important for identifying women at greater risk for choline deficiency during pregnancy. This is a real possibility, as the data of Shaw and colleagues show that women in the USA vary enough in dietary choline intake (from <300 mg/d to >500 mg/d) to influence the risk that they will have a baby with a birth defect (35). Choline nutriture during pregnancy is especially important because it influences brain development in the fetus (56 – 68) and because it is important for maintaining normal plasma homocysteine concentrations during pregnancy (69). High maternal homocysteine concentrations are associated with increased incidence of birth defects (70).
GENE POLYMORPHISMS AND DIETARY CHOLINE REQUIREMENTS
Though some humans develop fatty liver, as well as liver and muscle damage when fed a low choline diet, others do not. Even among premenopausal women who should be resistant to choline deficiency, a significant number develop organ dysfunction when deprived of choline (10). Genetic variation likely underlies these differences in dietary requirements. From what we understand about choline metabolism, several pathways influence how much choline is required from diet and single nucleotide polymorphisms (SNPs) in genes for these pathways might be of importance. Specifically, polymorphisms in the folate pathways could limit the availability of methyltetrahydrofolate and thereby increase use of choline as a methyl donor. Polymorphisms in the PEMT gene might alter endogenous synthesis of choline and polymorphisms in other genes of choline metabolism could influence dietary requirements by changing the utilization of choline moiety.
We developed a clinical methodology for phenotyping individuals with respect to their susceptibility to developing organ dysfunction when fed a low choline diet (2, 10, 13, 71). In a 95-day repeated measure, within subject study with graded repletion, adult men and women (pre- and post-menopausal) ages 18 − 70 were admitted to the General Clinical Research Center and fed a standard diet containing a known amount of choline (550 mg/70 kg/d; baseline). On day 11 subjects were placed on a diet containing <50 mg choline/day for up to 42 days. Blood and urine were collected to measure various experimental parameters of dietary choline status and markers of organ dysfunction (serum creatine phosphokinase (CPK), alanine amino transferase (ALT), aspartate amino transferase (AST)) and liver fat level was assessed by MRI. If at some point during the depletion period, functional markers indicated organ dysfunction associated with choline deficiency, subjects were switched to a diet containing 137 mg/70 kg/d choline (low or normal folate) for 10 days. If the functional markers indicated that deficiency status persisted, subjects were then switched to a diet containing 275 mg/70 kg/d choline (low or normal folate) for 10 days. If markers of organ dysfunction did not indicate repletion at this time, subjects were switched to a diet containing 413 mg/70 kg/d choline (low or normal folate) for 10 days. If still not repleted, subjects were fed a diet containing at least 550 mg/70 kg/d choline (low or normal folate) until repleted.
We examined whether major genetic variants of folate metabolism modify susceptibility of humans to choline deficiency (72). Premenopausal women who were carriers of the very common 5,10-methylenetetrahydrofolate dehydrogenase-1958A (MTHFD1; rs2236225) gene allele were more than 15 times as likely as non-carriers to develop signs of choline deficiency (P < 0.0001) on the low-choline diet. Sixty-three percent of our study population had at least one allele for this SNP. Two reactions, mediated by methylenetetrahydrofolate dehydrogenase and methenyltetrahydrofolate cyclohydrolase can convert 10-formyl tetrahydrofolate to 5,10-methylene tetrahydrofolate. While the formation of 5-methyl tetrahydrofolate is practically irreversible in vivo, the interconversion of 5,10-methylene tetrahydrofolate and 10-formyl tetrahydrofolate is closer to equilibrium (73). This means that 5,10-methylene tetrahydrofolate may be directed either toward homocysteine remethylation or away from it. The MTHFD1 G1958A polymorphism may thus affect the delicately balanced flux between 5,10-methylene tetrahydrofolate and 10-formyl tetrahydrofolate and thereby influence the availability of 5-methyl THF for homocysteine remethylation. This would increase demand for choline as a methyl-group donor. It is of interest that the risk of having a child with a neural tube defect increases in mothers with the G1958A SNP in MTHFD1 (74).
As noted earlier PEMT encodes for a protein responsible for endogenous formation of choline. We identified an SNP in the promoter region of the PEMT gene (−744 G−>C; rs12325817) for which 18 of 23 carriers of the C allele (78%) developed organ dysfunction when fed a low choline diet (odds ratio 25, P = 0.002) (75). Given the sexual differences in the effect of PEMT rs12325817, it is possible that this SNP alters the estrogen responsiveness of the promoter. The frequency of this variant allele was 0.74. A SNP in the PEMT coding region (+5465 G−>A; rs7946) results in a 30% loss of function and is associated with increased risk for nonalcoholic fatty liver disease (76) but was not associated with susceptibility to choline deficiency (75). The first of two SNPs in the coding region of the choline dehydrogenase gene (CHDH; +318 A−>C; rs9001) had a protective effect on susceptibility to choline deficiency, while a second CHDH variant (+432 G−>T; rs12676) was associated with increased susceptibility to choline deficiency (75). A SNP in the betaine:homocysteine methyltransferase gene (BHMT; +742 G−>A; rs3733890) was not associated with susceptibility to choline deficiency (75). Identification of common polymorphisms that affect dietary requirements for choline could enable us to identify individuals for whom we need to assure adequate dietary choline intake.
CHOLINE AND BRAIN DEVELOPMENT IN UTERO
Large amounts of choline are delivered to the fetus across the placenta, where choline transport systems pump it against a concentration gradient (51). Plasma or serum choline concentrations are 6−7-fold higher in the fetus and newborn than they are in the adult (77, 78). High levels of choline circulating in the neonate presumably ensure enhanced availability of choline to tissues. There is a novel form of PEMT in neonatal rat brain that is extremely active (37); this isoform is not present in adult brain. Furthermore, in the brains of newborn rats, S-adenosylmethionine concentrations are 40 − 50 nmol/g of tissue (79) – levels probably sufficient to enable the neonatal form of phosphatidylethanolamine-N-methyltransferase to maintain high rates of activity.
Maternal dietary choline supplementation or choline deficiency during late pregnancy in rodents were associated with significant and irreversible changes in hippocampal function in the adult rodent, including altered long term potentiation (LTP) (64, 80, 81) and altered memory (65, 66, 82 – 85). More choline (about 4 times dietary levels) during days 11 − 17 of gestation in the rodent increased hippocampal progenitor cell proliferation (56, 60), decreased apoptosis in these cells (56, 60), enhanced LTP in the offspring when they were adult animals (64, 80, 81) and enhanced visuospatial and auditory memory by as much as 30% in the adult animals through out their lifetimes (65, 66, 82, 83, 85 – 87). Indeed, adult rodents decrement in memory as they age, and offspring exposed to extra choline in utero do not show this ‘senility’ (83, 86). Mothers fed choline deficient diets during late pregnancy have offspring with diminished progenitor cell proliferation and increased apoptosis in fetal hippocampus (56, 60), insensitivity to LTP when they were adult animals (81), and decremented visuospatial and auditory memory (85). The effects of perinatal choline supplementation on memory were initially found using radial-arm maze tasks and the Sprague-Dawley rat strain, but other laboratories have found similar results using other spatial memory tasks, such as the Morris water maze (88, 89), using passive avoidance paradigms (90), using measures of attention (91), using other strains of rats such as Long-Evans (92 – 94), and using mice (90). The effects of choline supplementation in utero were also detected in studies on effects of fetal alcohol exposure, where supplementation with choline attenuated behavioral alterations but not motor abnormalities (95, 96). Thus, choline supplementation during a critical period in pregnancy causes lifelong changes in brain structure and function.
EPIGENETICS AND THE EFFECTS OF CHOLINE
The effects of choline on neural tube closure and on brain development likely are mediated by changes in the expression of genes. Dietary choline deficiency decreases S-adenosylmethionine concentrations in tissues (30, 97), with resulting hypomethylation of DNA (98, 99). DNA methylation occurs at cytosine bases that are followed by a guanosine (5′-CpG-3′ sites) (100) and influences many cellular events, including gene transcription, genomic imprinting and genomic stability (101 – 103). In mammals, about 60 − 80% of CpG sites in DNA are methylated, while most CpGs within CpG islands are not (104). When this modification occurs in promoter regions, gene expression is altered (105); increased methylation is associated with gene silencing or reduced gene expression (104). In choline deficient cells in culture, and in fetal rodent brains from mothers fed choline deficient diets, methylation of the CDKN3 gene promoter is decreased, resulting in over expression of this gene which inhibits cell proliferation (106, 107). This change in gene promoter methylation likely alters neurogenesis in the hippocampus for life – prenatal choline supplementation in rats resulted in increased neurogenesis that was still detectable at 7 months of age (108). There are other examples where maternal diets high in methyl groups had permanent effects on their offspring. Feeding pregnant Pseudoagouti Avy/a mouse dams a choline methyl-supplemented diet altered epigenetic regulation of agouti expression in their offspring, as indicated by increased agouti/black mottling of their coats (109, 110). In another example, there was increased DNA methylation of the fetal gene axin fused (Axin(Fu)) after methyl donor supplementation of female mice before and during pregnancy which reduced by 50% the incidence of tail kinking in Axin(Fu)/+ offspring. It is clear that the dietary manipulation of methyl donors (either deficiency or supplementation) can have a profound impact upon gene expression and, by consequence, upon the homeostatic mechanisms that ensure the normal function of physiological processes.
Whether these findings in rodents apply as well to humans is not known. Of course human and rat brains mature at different rates, with rat brain comparatively more mature at birth than is the human brain. In humans, the architecture of the hippo-campus continues to develop after birth, and by 4 years of age it closely resembles adult structure (111). This area of brain is one of the few areas in which nerve cells continue to multiply slowly throughout life (112, 113).
CONCLUSION
Understanding dietary choline requirement is an exercise in understanding nutrigenomics. Endogenous biosynthesis is induced by estrogen response elements, DNA methylation is influenced by the availability of choline, and common genetic polymorphisms have major effects on the dietary requirement for this nutrient. These interactions have important health significance because this nutrient is important for brain development and for preventing birth defects. In addition, choline deficiency can be a cause of fatty liver, an extremely common problem in adults (114). Choline deficiency has other health consequences – it is associated with liver and muscle damage and with exaggerated plasma homocysteine rise after a methionine load (2). Elevated plasma homocysteine is an independent risk factor for cardiovascular disease and stroke in humans (115 – 117).
ACKNOWLEDGEMENTS
This work was funded by grants from the National Institutes of Health (DK55865, AG09525). Support for this work was also provided by grants from the NIH to the UNC Clinical Nutrition Research Unit (DK56350).
REFERENCES
- 1.Zeisel SH, Blusztajn JK. Choline and human nutrition. Ann. Rev. Nutr. 1994;14:269–296. doi: 10.1146/annurev.nu.14.070194.001413. [DOI] [PubMed] [Google Scholar]
- 2.da Costa KA, Gaffney CE, Fischer LM, Zeisel SH. Choline deficiency in mice and humans is associated with increased plasma homocysteine concentration after a methionine load. Am. J. Clin. Nutr. 2005;81:440–444. doi: 10.1093/ajcn.81.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeisel SH, daCosta K-A, Franklin PD, Alexander EA, Lamont JT, Sheard NF, Beiser A. Choline, an essential nutrient for humans. FASEB J. 1991;5:2093–2098. [PubMed] [Google Scholar]
- 4.Buchman A, Dubin M, Moukarzel A, Jenden D, Roch M, Rice K, Gornbein J, Ament M. Choline deficiency: a cause of hepatic steatosis during parenteral nutrition that can be reversed with intravenous choline supplementation. Hepatology. 1995;22:1399–1403. [PubMed] [Google Scholar]
- 5.Yao ZM, Vance DE. Head group specificity in the requirement of phosphatidylcholine biosynthesis for very low density lipoprotein secretion from cultured hepatocytes. J. Biol. Chem. 1989;264:11373–11380. [PubMed] [Google Scholar]
- 6.Yao ZM, Vance DE. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J. Biol. Chem. 1988;263:2998–3004. [PubMed] [Google Scholar]
- 7.Albright CD, Lui R, Bethea TC, da Costa K-A, Salganik RI, Zeisel SH. Choline deficiency induces apoptosis in SV40-immortalized CWSV-1 rat hepatocytes in culture. FASEB J. 1996;10:510–516. doi: 10.1096/fasebj.10.4.8647350. [DOI] [PubMed] [Google Scholar]
- 8.Albright CD, Zeisel SH. Choline deficiency causes increased localization of TGFβ1 signaling proteins and apoptosis in rat liver. Pathobiology. 1997;65:264–270. doi: 10.1159/000164137. [DOI] [PubMed] [Google Scholar]
- 9.Albright CD, da Costa KA, Craciunescu CN, Klem E, Mar MH, Zeisel SH. Regulation of choline deficiency apoptosis by epidermal growth factor in CWSV-1 rat hepatocytes. Cell Physiol. Biochem. 2005;15:59–68. doi: 10.1159/000083653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer L, daCosta K, Kwock L, Stewart P, Lu T-S, Stabler S, Allen R, Zeisel S. Gender and menopausal status influence human dietary requirements for the nutrient choline. Am. J. Clin. Nutr. 2007;85(5):1275–1285. doi: 10.1093/ajcn/85.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James SJ, Miller BJ, Basnakian AG, Pogribny IP, Pogribna M, Muskhelishvili L. Apoptosis and proliferation under conditions of deoxynucleotide pool imbalance in liver of folate/methyl deficient rats. Carcinogenesis. 1997;18:287–293. doi: 10.1093/carcin/18.2.287. [DOI] [PubMed] [Google Scholar]
- 12.Shin OH, Mar MH, Albright CD, Citarella MT, daCosta KA, Zeisel SH. Methyl-group donors cannot prevent apoptotic death of rat hepatocytes induced by choline-deficiency. J. Cell. Biochem. 1997;64:196–208. doi: 10.1002/(sici)1097-4644(199702)64:2<196::aid-jcb3>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 13.da Costa KA, Badea M, Fischer LM, Zeisel SH. Elevated serum creatine phosphokinase in choline-deficient humans: mechanistic studies in C2C12 mouse myoblasts. Am. J. Clin. Nutr. 2004;80:163–170. doi: 10.1093/ajcn/80.1.163. [DOI] [PubMed] [Google Scholar]
- 14.Institute of Medicine, and National Academy of Sciences USA . Dietary reference intakes for folate, thiamin, riboflavin, niacin, vitamin B12, panthothenic acid, biotin, and choline. Vol. 1. National Academy Press; Washington, DC: 1998. Choline. pp. 390–422. [PubMed] [Google Scholar]
- 15.Finkelstein JD. Pathways and regulation of homocysteine metabolism in mammals. Semin. Thromb. Hemost. 2000;26:219–225. doi: 10.1055/s-2000-8466. [DOI] [PubMed] [Google Scholar]
- 16.Olthof MR, van Vliet T, Boelsma E, Verhoef P. Low dose betaine supplementation leads to immediate and long term lowering of plasma homocysteine in healthy men and women. J. Nutr. 2003;133:4135–4138. doi: 10.1093/jn/133.12.4135. [DOI] [PubMed] [Google Scholar]
- 17.Weisberg IS, Jacques PF, Selhub J, Bostom AG, Chen Z, Curtis Ellison R, Eckfeldt JH, Rozen R. The 1298A–>C polymorphism in methylenetetrahydrofolate reductase (MTHFR): in vitro expression and association with homocysteine. Atherosclerosis. 2001;156:409–415. doi: 10.1016/s0021-9150(00)00671-7. [DOI] [PubMed] [Google Scholar]
- 18.Shelnutt KP, Kauwell GP, Chapman CM, Gregory JF, 3rd, Maneval DR, Browdy AA, Theriaque DW, Bailey LB. Folate status response to controlled folate intake is affected by the methylenetetrahydrofolate reductase 677C–>T polymorphism in young women. J. Nutr. 2003;133:4107–4111. doi: 10.1093/jn/133.12.4107. [DOI] [PubMed] [Google Scholar]
- 19.Jacques PF, Bostom AG, Wilson PW, Rich S, Rosenberg IH, Selhub J. Determinants of plasma total homocysteine concentration in the Framingham Offspring cohort. Am. J. Clin. Nutr. 2001;73:613–621. doi: 10.1093/ajcn/73.3.613. [DOI] [PubMed] [Google Scholar]
- 20.Watkins D, Ru M, Hwang HY, Kim CD, Murray A, Philip NS, Kim W, Legakis H, Wai T, Hilton JF, Ge B, Dore C, Hosack A, Wilson A, Gravel RA, Shane B, Hudson TJ, Rosenblatt DS. Hyperhomocysteinemia due to methionine synthase deficiency, cblG: structure of the MTR gene, genotype diversity, and recognition of a common mutation, P1173L. Am. J. Hum. Genet. 2002;71:143–153. doi: 10.1086/341354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mudd SH, Ebert MH, Scriver CR. Labile methyl group balances in the human: the role of sarcosine. Metabolism. 1980;29:707–720. doi: 10.1016/0026-0495(80)90192-4. [DOI] [PubMed] [Google Scholar]
- 22.Sunden S, Renduchintala M, Park E, Miklasz S, Garrow T. Betaine-homocysteine methyltransferase expression in porcine and human tissues and chromosomal localization of the human gene. Arch. Biochem. Biophys. 1997;345:171–174. doi: 10.1006/abbi.1997.0246. [DOI] [PubMed] [Google Scholar]
- 23.Holm PI, Bleie O, Ueland PM, Lien EA, Refsum H, Nordrehaug JE, Nygard O. Betaine as a determinant of postmethionine load total plasma homocysteine before and after B-vitamin supplementation. Arterioscler. Thromb. Vasc. Biol. 2004;24:301–307. doi: 10.1161/01.ATV.0000114569.54976.31. [DOI] [PubMed] [Google Scholar]
- 24.Steenge GR, Verhoef P, Katan MB. Betaine supplementation lowers plasma homocysteine in healthy men and women. J. Nutr. 2003;133:1291–1295. doi: 10.1093/jn/133.5.1291. [DOI] [PubMed] [Google Scholar]
- 25.Wendel U, Bremer H. Betaine in the treatment of homocystinuria due to 5,10-methylenetetrahydrofolate reductase deficiency. Eur. J. Pediatr. 1984;142:147–150. doi: 10.1007/BF00445602. [DOI] [PubMed] [Google Scholar]
- 26.Kim Y-I, Miller JW, da Costa K-A, Nadeau M, Smith D, Selhub J, Zeisel SH, Mason JB. Folate deficiency causes secondary depletion of choline and phosphocholine in liver. J. Nutr. 1995;124:2197–2203. doi: 10.1093/jn/124.11.2197. [DOI] [PubMed] [Google Scholar]
- 27.Selhub J, Seyoum E, Pomfret EA, Zeisel SH. Effects of choline deficiency and methotrexate treatment upon liver folate content and distribution. Cancer Res. 1991;51:16–21. [PubMed] [Google Scholar]
- 28.Varela-Moreiras G, Selhub J, da Costa K, Zeisel SH. Effect of chronic choline deficiency in rats on liver folate content and distribution. J. Nutr. Biochem. 1992;3:519–522. [Google Scholar]
- 29.Pomfret EA, da Costa K, Zeisel SH. Effects of choline deficiency and methotrexate treatment upon rat liver. J. Nutr. Biochem. 1990;1:533–541. doi: 10.1016/0955-2863(90)90039-n. [DOI] [PubMed] [Google Scholar]
- 30.Zeisel SH, Zola T, daCosta K, Pomfret EA. Effect of choline deficiency on S-adenosylmethionine and methionine concentrations in rat liver. Biochem. J. 1989;259:725–729. doi: 10.1042/bj2590725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varela-Moreiras G, Ragel C, Perez de Miguelsanz J. Choline deficiency and methotrexate treatment induces marked but reversible changes in hepatic folate concentrations, serum homocysteine and DNA methylation rates in rats. J. Amer. Coll. Nutr. 1995;14:480–485. doi: 10.1080/07315724.1995.10718539. [DOI] [PubMed] [Google Scholar]
- 32.Zeisel SH, Mar MH, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 2003;133:1302–1307. doi: 10.1093/jn/133.5.1302. [DOI] [PubMed] [Google Scholar]
- 33.Zeisel SH, Mar M-H, Howe JC, Holden JM. Erratum: Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. J. Nutr. 2003;133133:1302–1307. 2918–2919. doi: 10.1093/jn/133.5.1302. [DOI] [PubMed] [Google Scholar]
- 34.Cheng W-L, Holmes-McNary MQ, Mar M-H, Lien EL, Zeisel SH. Bioavailability of choline and choline esters from milk in rat pups. J. Nutr. Biochem. 1996;7:457–464. [Google Scholar]
- 35.Shaw GM, Carmichael SL, Yang W, Selvin S, Schaffer DM. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am. J. Epidemiol. 2004;160:102–109. doi: 10.1093/aje/kwh187. [DOI] [PubMed] [Google Scholar]
- 36.Shaw GM, Carmichael SL, Laurent C, Rasmussen SA. Maternal nutrient intakes and risk of orofacial clefts. Epidemiology. 2006;17:285–291. doi: 10.1097/01.ede.0000208348.30012.35. [DOI] [PubMed] [Google Scholar]
- 37.Blusztajn JK, Zeisel SH, Wurtman RJ. Developmental changes in the activity of phosphatidylethanolamine N-methyltransferases in rat brain. Biochem. J. 1985;232:505–511. doi: 10.1042/bj2320505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walkey CJ, Yu L, Agellon LB, Vance DE. Biochemical and evolutionary significance of phospholipid methylation. J. Biol. Chem. 1998;273:27043–27046. doi: 10.1074/jbc.273.42.27043. [DOI] [PubMed] [Google Scholar]
- 39.Waite KA, Cabilio NR, Vance DE. Choline deficiency-induced liver damage is reversible in Pemt(−/−) mice. J. Nutr. 2002;132:68–71. doi: 10.1093/jn/132.1.68. [DOI] [PubMed] [Google Scholar]
- 40.Zhu X, Song J, Mar MH, Edwards LJ, Zeisel SH. Phosphatidylethanolamine N-methyltransferase (PEMT) knockout mice have hepatic steatosis and abnormal hepatic choline metabolite concentrations despite ingesting a recommended dietary intake of choline. Biochem. J. 2003;370:987–993. doi: 10.1042/BJ20021523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacobs RL, Stead LM, Devlin C, Tabas I, Brosnan ME, Brosnan JT, Vance DE. Physiological regulation of phospholipid methylation alters plasma homocysteine in mice. J. Biol. Chem. 2005;280:28299–28305. doi: 10.1074/jbc.M501971200. [DOI] [PubMed] [Google Scholar]
- 42.Shields DJ, Lingrell S, Agellon LB, Brosnan JT, Vance DE. Localization-independent regulation of homocysteine secretion by phosphatidylethanolamine N-methyltransferase. J. Biol. Chem. 2005;280(29):27339–27344. doi: 10.1074/jbc.M504658200. [DOI] [PubMed] [Google Scholar]
- 43.Walter P, Green S, Greene G, Krust A, Bornert JM, Jeltsch JM, Staub A, Jensen E, Scrace G, Waterfield M, et al. Cloning of the human estrogen receptor cDNA. Proc. Natl. Acad. Sci. USA. 1985;82:7889–7893. doi: 10.1073/pnas.82.23.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lopez D, Sanchez MD, Shea-Eaton W, McLean MP. Estrogen activates the high-density lipoprotein receptor gene via binding to estrogen response elements and interaction with sterol regulatory element binding protein-1A. Endocrinology. 2002;143:2155–2168. doi: 10.1210/endo.143.6.8855. [DOI] [PubMed] [Google Scholar]
- 45.Agarwal A, Yeung WS, Lee KF. Cloning and characterization of the human oviduct-specific glycoprotein (HuOGP) gene promoter. Mol. Hum. Reprod. 2002;8:167–175. doi: 10.1093/molehr/8.2.167. [DOI] [PubMed] [Google Scholar]
- 46.Xie T, Ho SL, Ramsden D. Characterization and implications of estrogenic down-regulation of human catechol-O-methyltransferase gene transcription. Mol. Pharmacol. 1999;56:31–38. doi: 10.1124/mol.56.1.31. [DOI] [PubMed] [Google Scholar]
- 47.Resseguie M, Song J, Niculescu M, da Costa K, Randall T, Zeisel S. Phosphatidylethanolamine n-methyltransferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. FASEB J. 2007 2007 April;24 doi: 10.1096/fj.07-8227com. fj.07−8227com. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarda IR, Gorwill RH. Hormonal studies in pregnancy. I. Total unconjugated estrogens in maternal peripheral vein, cord vein, and cord artery serum at delivery. Am. J. Obstet. Gynecol. 1976;124:234–238. [PubMed] [Google Scholar]
- 49.Adeyemo O, Jeyakumar H. Plasma progesterone, estradiol-17 beta and testosterone in maternal and cord blood, and maternal human chorionic gonadotropin at parturition. Afr. J. Med. Med. Sci. 1993;22:55–60. [PubMed] [Google Scholar]
- 50.Sweiry JH, Yudilevich DL. Characterization of choline transport at maternal and fetal interfaces of the perfused guinea-pig placenta. J. Physiol. 1985;366:251–266. doi: 10.1113/jphysiol.1985.sp015795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sweiry JH, Page KR, Dacke CG, Abramovich DR, Yudilevich DL. Evidence of saturable uptake mechanisms at maternal and fetal sides of the perfused human placenta by rapid paired-tracer dilution: studies with calcium and choline. J. Devel. Physiol. 1986;8:435–445. [PubMed] [Google Scholar]
- 52.McMahon KE, Farrell PM. Measurement of free choline concentrations in maternal and neonatal blood by micropyrolysis gas chromatography. Clin. Chim. Acta. 1985;149:1–12. doi: 10.1016/0009-8981(85)90267-0. [DOI] [PubMed] [Google Scholar]
- 53.Zeisel SH, Mar M-H, Zhou Z-W, da Costa K-A. Pregnancy and lactation are associated with diminished concentrations of choline and its metabolites in rat liver. J. Nutr. 1995;125:3049–3054. doi: 10.1093/jn/125.12.3049. [DOI] [PubMed] [Google Scholar]
- 54.Holmes-McNary M, Cheng WL, Mar. MH, Fussell S, Zeisel SH. Choline and choline esters in human and rat milk and infant formulas. Am. J. Clin. Nutr. 1996;64:572–576. doi: 10.1093/ajcn/64.4.572. [DOI] [PubMed] [Google Scholar]
- 55.Zhu X, Mar MH, Song J, Zeisel SH. Deletion of the Pemt gene increases progenitor cell mitosis, DNA and protein methylation and decreases calretinin expression in embryonic day 17 mouse hippocampus. Brain Res. Dev. Brain Res. 2004;149:121–129. doi: 10.1016/j.devbrainres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 56.Albright CD, Friedrich CB, Brown EC, Mar MH, Zeisel SH. Maternal dietary choline availability alters mitosis, apoptosis and the localization of TOAD-64 protein in the developing fetal rat septum. Brain Res. Dev. Brain Res. 1999;115:123–129. doi: 10.1016/s0165-3806(99)00057-7. [DOI] [PubMed] [Google Scholar]
- 57.Albright CD, Mar MH, Craciunescu CN, Song J, Zeisel SH. Maternal dietary choline availability alters the balance of netrin-1 and DCC neuronal migration proteins in fetal mouse brain hippocampus. Brain Res. Dev. Brain Res. 2005;159:149–154. doi: 10.1016/j.devbrainres.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Albright CD, Mar MH, Friedrich CB, Brown EC, Zeisel SH. Maternal choline availability alters the localization of p15Ink4B and p27Kip1 cyclin-dependent kinase inhibitors in the developing fetal rat brain hippocampus. Dev. Neurosci. 2001;23:100–106. doi: 10.1159/000048701. [DOI] [PubMed] [Google Scholar]
- 59.Albright CD, Siwek DF, Craciunescu CN, Mar MH, Kowall NW, Williams CL, Zeisel SH. Choline availability during embryonic development alters the localization of calretinin in developing and aging mouse hippocampus. Nutr. Neurosci. 2003;6:129–134. doi: 10.1080/1028415031000084418. [DOI] [PubMed] [Google Scholar]
- 60.Albright CD, Tsai AY, Friedrich CB, Mar MH, Zeisel SH. Choline availability alters embryonic development of the hippocampus and septum in the rat. Brain Res. Dev. Brain Res. 1999;113:13–20. doi: 10.1016/s0165-3806(98)00183-7. [DOI] [PubMed] [Google Scholar]
- 61.Albright CD, Tsai AY, Mar M-H, Zeisel SH. Choline availability modulates the expression of TGFß1 and cytoskeletal proteins in the hippocampus of developing rat brain. Neurochem. Res. 1998;23:751–758. doi: 10.1023/a:1022411510636. [DOI] [PubMed] [Google Scholar]
- 62.Craciunescu CN, Albright CD, Mar MH, Song J, Zeisel SH. Choline availability during embryonic development alters progenitor cell mitosis in developing mouse hippocampus. J. Nutr. 2003;133:3614–3618. doi: 10.1093/jn/133.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. Faseb J. 2006;20:43–49. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pyapali G, Turner D, Williams C, Meck W, Swartzwelder HS. Prenatal choline supplementation decreases the threshold for induction of long-term potentiation in young adult rats. J. Neurophysiol. 1998;79:1790–1796. doi: 10.1152/jn.1998.79.4.1790. [DOI] [PubMed] [Google Scholar]
- 65.Meck W, Williams C. Perinatal choline supplementation increases the threshold for chunking in spatial memory. Neuroreport. 1997;8:3053–3059. doi: 10.1097/00001756-199709290-00010. [DOI] [PubMed] [Google Scholar]
- 66.Meck WH, Smith RA, Williams CL. Pre- and postnatal choline supplementation produces long-term facilitation of spatial memory. Dev. Psychobiol. 1988;21:339–353. doi: 10.1002/dev.420210405. [DOI] [PubMed] [Google Scholar]
- 67.Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci. Biobehav. Rev. 2003;27:385–399. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- 68.Mellott TJ, Williams CL, Meck WH, Blusztajn JK. Prenatal choline supplementation advances hippocampal development and enhances MAPK and CREB activation. Faseb J. 2004;18:545–547. doi: 10.1096/fj.03-0877fje. [DOI] [PubMed] [Google Scholar]
- 69.Velzing-Aarts FV, Holm PI, Fokkema MR, van der Dijs FP, Ueland PM, Muskiet FA. Plasma choline and betaine and their relation to plasma homocysteine in normal pregnancy. Am. J. Clin. Nutr. 2005;81:1383–1389. doi: 10.1093/ajcn/81.6.1383. [DOI] [PubMed] [Google Scholar]
- 70.Hobbs CA, Cleves MA, Melnyk S, Zhao W, James SJ. Congenital heart defects and abnormal maternal biomarkers of methionine and homocysteine metabolism. Am. J. Clin. Nutr. 2005;81:147–153. doi: 10.1093/ajcn/81.1.147. [DOI] [PubMed] [Google Scholar]
- 71.Busby MG, Fischer L, Da Costa KA, Thompson D, Mar MH, Zeisel SH. Choline- and betaine-defined diets for use in clinical research and for the management of trimethylaminuria. J. Am. Diet Assoc. 2004;104:1836–1845. doi: 10.1016/j.jada.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 72.Kohlmeier M, da Costa KA, Fischer LM, Zeisel SH. Genetic variation of folate-mediated one-carbon transfer pathway predicts susceptibility to choline deficiency in humans. Proc. Natl. Acad. Sci. USA. 2005;102:16025–16030. doi: 10.1073/pnas.0504285102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Horne DW. Neither methionine nor nitrous oxide inactivation of methionine synthase affect the concentration of 5,10-methylenetetrahydrofolate in rat liver. J. Nutr. 2003;133:476–478. doi: 10.1093/jn/133.2.476. [DOI] [PubMed] [Google Scholar]
- 74.Brody LC, Conley M, Cox C, Kirke PN, McKeever MP, Mills JL, Molloy AM, O'Leary VB, Parle-McDermott A, Scott JM, Swanson DA. A polymorphism, R653Q, in the trifunctional enzyme methylenetetrahydrofolate dehydrogenase/methenyltetrahydrofolate cyclohydrolase/formyltetrahydrofolate synthetase is a maternal genetic risk factor for neural tube defects: report of the Birth Defects Research Group. Am. J. Hum. Genet. 2002;71:1207–1215. doi: 10.1086/344213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.da Costa KA, Kozyreva OG, Song J, Galanko JA, Fischer LM, Zeisel SH. Common genetic polymorphisms affect the human requirement for the nutrient choline. Faseb J. 2006;20:1336–1344. doi: 10.1096/fj.06-5734com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Song J, da Costa KA, Fischer L, Kohlmeier M, Kwock L, Wang S, Zeisel SH. Polymorphism of the PEMT gene and susceptibility to nonalcoholic fatty liver disease (NAFLD). Faseb J. 2005;19(10):1266–1271. doi: 10.1096/fj.04-3580com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zeisel SH, Wurtman RJ. Developmental changes in rat blood choline concentration. Biochem. J. 1981;198:565–570. doi: 10.1042/bj1980565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ozarda IY, Uncu G, Ulus IH. Free and phospholipid-bound choline concentrations in serum during pregnancy, after delivery and in newborns. Arch. Physiol. Biochem. 2002;110:393–399. doi: 10.1076/apab.110.5.393.11832. [DOI] [PubMed] [Google Scholar]
- 79.Hoffman DR, Cornatzer WE, Duerre JA. Relationship between tissue levels of S-adenosylmethionine, S-adenosylhomocysteine, and transmethylation reactions. Can. J. Biochem. 1979;57:56–65. doi: 10.1139/o79-007. [DOI] [PubMed] [Google Scholar]
- 80.Montoya DA, White AM, Williams CL, Blusztajn JK, Meck WH, Swartzwelder HS. Prenatal choline exposure alters hippocampal responsiveness to cholinergic stimulation in adulthood. Brain Res. Dev. Brain Res. 2000;123:25–32. doi: 10.1016/s0165-3806(00)00075-4. [DOI] [PubMed] [Google Scholar]
- 81.Jones JP, Meck W, Williams CL, Wilson WA, Swartzwelder HS. Choline availability to the developing rat fetus alters adult hippocampal long-term potentiation. Brain Res. Dev. Brain Res. 1999;118:159–167. doi: 10.1016/s0165-3806(99)00103-0. [DOI] [PubMed] [Google Scholar]
- 82.Meck W, Williams C. Characterization of the facilitative effects of perinatal choline supplementation on timing and temporal memory. Neuroreport. 1997;8:2831–2835. doi: 10.1097/00001756-199709080-00005. [DOI] [PubMed] [Google Scholar]
- 83.Meck W, Williams C. Simultaneous temporal processing is sensitive to prenatal choline availability in mature and aged rats. Neuroreport. 1997;8:3045–3051. doi: 10.1097/00001756-199709290-00009. [DOI] [PubMed] [Google Scholar]
- 84.Meck WH, Smith RA, Williams CL. Organizational changes in cholinergic activity and enhanced visuospatial memory as a function of choline administered prenatally or postnatally or both. Behav. Neurosci. 1989;103:1234–1241. doi: 10.1037//0735-7044.103.6.1234. [DOI] [PubMed] [Google Scholar]
- 85.Meck WH, Williams CL. Choline supplementation during prenatal development reduces proactive interference in spatial memory. Brain Res. Dev. Brain Res. 1999;118:51–59. doi: 10.1016/s0165-3806(99)00105-4. [DOI] [PubMed] [Google Scholar]
- 86.Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: Implications for memory and attentional processing across the lifespan. Neurosci. Biobehav. Rev. 2003;27:385–399. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- 87.Williams CL, Meck WH, Heyer DD, Loy R. Hypertrophy of basal forebrain neurons and enhanced visuospatial memory in perinatally choline-supplemented rats. Brain Res. 1998;794:225–238. doi: 10.1016/s0006-8993(98)00229-7. [DOI] [PubMed] [Google Scholar]
- 88.Schenk F, Brandner C. Indirect effects of peri- and postnatal choline treatment on place-learning abilities in rat. Psychobiology. 1995;23:302–313. [Google Scholar]
- 89.Brandner C. Perinatal choline treatment modifies the effects of a visuo-spatial attractive cue upon spatial memory in naive adult rats. Brain Res. 2002;928:85–95. doi: 10.1016/s0006-8993(01)03363-7. [DOI] [PubMed] [Google Scholar]
- 90.Ricceri L, Berger-Sweeney J. Postnatal choline supplementation in preweanling mice: sexually dimorphic behavioral and neurochemical effects. Behav. Neurosci. 1998;112:1387–1392. [PubMed] [Google Scholar]
- 91.Mohler E, Meck W, Williams C. Sustained attention in adult mice is modulated by prenatal choline availability. Int. J. Comp. Psychology. 2001;14:136–150. [Google Scholar]
- 92.Tees RC. The influences of rearing environment and neonatal choline dietary supplementation on spatial learning and memory in adult rats. Behav. Brain Res. 1999;105:173–188. doi: 10.1016/s0166-4328(99)00074-1. [DOI] [PubMed] [Google Scholar]
- 93.Tees RC. The influences of sex, rearing environment, and neonatal choline dietary supplementation on spatial and nonspatial learning and memory in adult rats. Dev. Psychobiol. 1999;35:328–342. doi: 10.1002/(sici)1098-2302(199912)35:4<328::aid-dev7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 94.Tees RC, Mohammadi E, Adam TJ. Altering the impact of early rearing on the rat's spatial memory with pre- and postnatal choline supplementation. Soc. Neurosci. Abstr. 1999;17:1401. [Google Scholar]
- 95.Thomas JD, Garrison M, O'Neill TM. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol. Teratol. 2004;26:35–45. doi: 10.1016/j.ntt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 96.Thomas JD, O'Neill TM, Dominguez HD. Perinatal choline supplementation does not mitigate motor coordination deficits associated with neonatal alcohol exposure in rats. Neurotoxicol. Teratol. 2004;26:223–229. doi: 10.1016/j.ntt.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 97.Shivapurkar N, Poirier LA. Tissue levels of S-adenosylmethionine and S-adenosylhomocysteine in rats fed methyl-deficient, amino acid-defined diets for one to five weeks. Carcinogenesis. 1983;4:1051–1057. doi: 10.1093/carcin/4.8.1051. [DOI] [PubMed] [Google Scholar]
- 98.Locker J, Reddy TV, Lombardi B. DNA methylation and hepatocarcinogenesis in rats fed a choline-devoid diet. Carcinogenesis. 1986;7:1309–1312. doi: 10.1093/carcin/7.8.1309. [DOI] [PubMed] [Google Scholar]
- 99.Tsujiuchi T, Tsutsumi M, Sasaki Y, Takahama M, Konishi Y. Hypomethylation of CpG sites and c-myc gene overexpression in hepatocellular carcinomas, but not hyperplastic nodules, induced by a choline-deficient L-amino acid-defined diet in rats. Jpn. J. Cancer Res. 1999;90:909–913. doi: 10.1111/j.1349-7006.1999.tb00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Holliday R, Grigg GW. DNA methylation and mutation. Mutation Res. 1993;285:61–67. doi: 10.1016/0027-5107(93)90052-h. [DOI] [PubMed] [Google Scholar]
- 101.Jaenisch R. DNA methylation and imprinting: why bother? Trends Genet. 1997;13:323–329. doi: 10.1016/s0168-9525(97)01180-3. [DOI] [PubMed] [Google Scholar]
- 102.Jones PA, Gonzalgo ML. Altered DNA methylation and genome instability: a new pathway to cancer? Proc. Natl. Acad. Sci. USA. 1997;94:2103–2105. doi: 10.1073/pnas.94.6.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Robertson KD, Wolffe AP. DNA methylation in health and disease. Nat. Rev. Genet. 2000;1:11–19. doi: 10.1038/35049533. [DOI] [PubMed] [Google Scholar]
- 104.Jeltsch A. Beyond Watson and Crick: DNA methylation and molecular enzymology of DNA methyltransferases. Chembiochemistry. 2002;3:382. doi: 10.1002/1439-7633(20020402)3:4<274::AID-CBIC274>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 105.Bird AP. CpG-rich islands and the function of DNA methylation. Nature. 1986;321:209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- 106.Niculescu MD, Yamamuro Y, Zeisel SH. Choline availability modulates human neuroblastoma cell proliferation and alters the methylation of the promoter region of the cyclin-dependent kinase inhibitor 3 gene. J. Neurochem. 2004;89(5):1252–1259. doi: 10.1111/j.1471-4159.2004.02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Niculescu MD, Craciunescu CN, Zeisel SH. Gene expression profiling of choline-deprived neural precursor cells isolated from mouse brain. Brain Res. Mol. Brain Res. 2005;134:309–322. doi: 10.1016/j.molbrainres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 108.Glenn MJ, Gibson EM, Kirby ED, Mellott TJ, Blusztajn JK, Williams CL. Prenatal choline availability modulates hippocampal neurogenesis and neurogenic responses to enriching experiences in adult female rats. Eur. J. Neurosci. 2007;25:2473–2482. doi: 10.1111/j.1460-9568.2007.05505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wolff GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. Faseb J. 1998;12:949–957. [PubMed] [Google Scholar]
- 110.Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J. Nutr. 2002;132:2393S–2400S. doi: 10.1093/jn/132.8.2393S. [DOI] [PubMed] [Google Scholar]
- 111.Dani S, Hori A, Walter G, editors. Principals of neural aging. Elsevier; Amsterdam: 1997. [Google Scholar]
- 112.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999;2:266–270. doi: 10.1038/6368. [see comments] [DOI] [PubMed] [Google Scholar]
- 113.Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J. Comp. Neurol. 1999;406:449–460. [PubMed] [Google Scholar]
- 114.Brunt EM. Nonalcoholic steatohepatitis: definition and pathology. Semin. Liver Dis. 2001;21:3–16. doi: 10.1055/s-2001-12925. [DOI] [PubMed] [Google Scholar]
- 115.Glueck CJ, Shaw P, Lang JE, Tracy T, Sieve-Smith L, Wang Y. Evidence that homocysteine is an independent risk factor for atherosclerosis in hyperlipidemic patients. Am. J. Cardiol. 1995;75:132–136. doi: 10.1016/s0002-9149(00)80061-2. [DOI] [PubMed] [Google Scholar]
- 116.Goddijn-Wessel T, Wouters M, van de Molen E, Spuijbroek M, Steegers-Theunissen R, Blom H, Boers G, Eskes T. Hyperhomocysteinemia: a risk factor for placental abruption or infarction. Eur. J. Obstet. Gynecol. Reprod. Biol. 1996;66:23–29. doi: 10.1016/0301-2115(96)02383-4. [DOI] [PubMed] [Google Scholar]
- 117.McCully K. Homocysteine and vascular disease. Nat. Med. 1996;2:386–389. doi: 10.1038/nm0496-386. [DOI] [PubMed] [Google Scholar]