Abstract
Delay in follow-up after an abnormal mammogram is associated with advanced disease stage, poorer survival, and increased anxiety. Despite the implementation of many patient navigator programs across the country, there are few published, peer-reviewed studies documenting its effectiveness. We tested the effectiveness of a patient navigator in improving timeliness to diagnosis, decreasing anxiety, and increasing satisfaction in urban minority women after an abnormal mammogram. Women with suspicious mammograms were randomly assigned to usual care (N = 50) or usual care plus intervention with a patient navigator (N = 55). There were no demographic differences between the two groups. Women in the intervention group had shorter times to diagnostic resolution (mean 25.0 vs. 42.7 days; p = .001), with 22% of women in the control group without a final diagnosis at 60 days vs. 6% in the intervention group. The intervention group also had lower mean anxiety scores (decrease of 8.0 in intervention vs. increase of 5.8 in control; p < .001), and higher mean satisfaction scores (4.3 vs. 2.9; p < .001). Patient navigation is an effective strategy to improve timely diagnostic resolution, significantly decrease anxiety, and increase patient satisfaction among urban minority women with abnormal mammograms.
Keywords: Mammography, Minority groups, Urban health, Anxiety, Patient satisfaction
INTRODUCTION
Breast cancer is the most common cancer in women and the second leading cause of cancer deaths in women in the United States. In 2007, there will be over 180,500 new cases and almost 50,000 deaths from breast cancer in the United States.1 The 5-year survival rate is 98% for patients diagnosed with local stage, but drops to 84% for regional spread, and 26% for distant disease. Despite the success of the Center for Disease Control’s Breast and Cervical Cancer Early Detection Program in increasing mammography screening in poor and minority women,2 underserved women are still diagnosed with breast cancer at later stages, and African-American women continue to have the highest mortality rates from breast cancer.1,3 The potential of mammography screening in improving breast cancer outcomes in poor and minority women cannot be realized without regular repeat screening, and timely and adequate follow-up and treatment after an abnormal mammogram. Delay in breast cancer diagnosis and treatment after an abnormal screening mammogram is associated with larger tumor size, advanced disease stage, and poorer survival.4 For patients without breast cancer, delay in timely follow-up after an abnormal screening result is associated with considerable anxiety and emotional distress.5
Minority women face many potential barriers that hinder timely follow-up of suspicious mammograms.6 Barriers to obtaining timely diagnostic and treatment services include lack of continuity of medical care and social support, mistrust, communication problems, cultural factors, health beliefs, and other economic, personal and family health priorities. One intervention that has been employed to decrease barriers and improve high-quality cancer care to underserved patients is the use of a patient navigator. Since the establishment of the first patient navigator program at Harlem Hospital in 1990,7 over 200 cancer care programs in the U.S. have implemented some form of patient navigation. However, there are few published, peer-reviewed studies documenting its effectiveness.6 Observational studies suggest that patient navigation is associated with increased screening and follow-up rates, improved timeliness in follow-up and diagnosis of breast abnormalities, lower breast cancer stage at diagnosis, and high patient satisfaction.7–17 There is only one published randomized trial testing the effectiveness of patient navigation after an abnormal mammogram.18 This study, conducted in mostly foreign born, non-English-speaking Latino women, found that patient navigation and structured counseling significantly increased follow-up adherence rates and timely diagnostic resolution after an abnormal mammogram. There are no controlled studies evaluating the effectiveness of patient navigation after an abnormal mammogram in other populations or in decreasing patient anxiety or increasing satisfaction.
The purpose of this study was to use a randomized controlled trial design to examine the effectiveness of a patient navigator in improving quality of care after a suspicious mammogram in urban minority women. We hypothesized that the benefit of a familiar face and culturally sensitive advocate would increase trust and provide emotional support as well as assist women to navigate the healthcare system, thus leading to improved timeliness in diagnosis, decreased anxiety, and increased satisfaction.
METHODS
Study Site
This randomized controlled trial was conducted at an urban university hospital in Newark, New Jersey. This public hospital serves a predominantly low-income minority population with over 50% African-American and 30% Hispanic patients. Forty percent of patients are uninsured, and 30% have Medicaid insurance. This study was approved by the Institutional Review Board of the UMDNJ-New Jersey Medical School.
Subjects
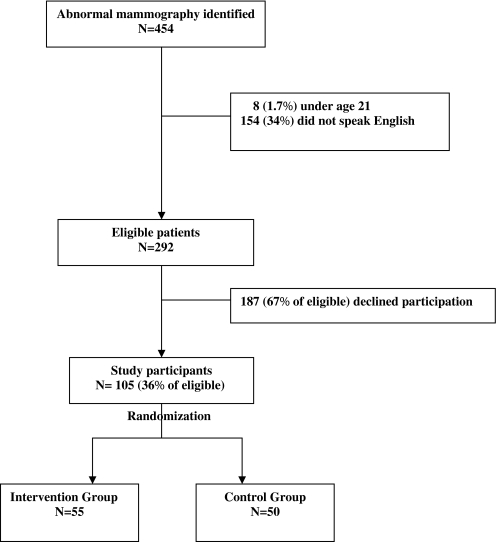
Women with suspicious mammogram results (American College of Radiology Breast Imaging and Reporting Data Systems [BI-RADS]) of 4 (suspicious abnormality) or 5 (highly suggestive of malignancy) from May 2005 to April 2007 (N = 454) were sequentially identified from weekly radiology logs and recruited into the study (Figure 1). The women were contacted by phone and then met in person by the patient navigator and asked to participate in the study within 1 week of their abnormal mammogram. To blind women from the intervention, they were advised that the hospital was conducting a study to “better understand women’s experience with the hospital system after an abnormal mammogram.” Women under age 21(N = 8) and those who did not speak English (N = 154) were ineligible because our patient navigator was not bilingual. Of 292 study eligible women, 105 (36%) agreed to participate and provided informed consent. Patients were randomly assigned to control (usual care, N = 50) or intervention (usual care plus patient navigation, N = 55) groups using a computer-generated table of random numbers. Women randomized to the control group received usual care, consisting of the following: 1. The radiologist informed the patient of the result; 2. The radiologist notified the referring physician of the suspicious mammogram; and if needed, 3. The radiologist notified the breast surgeon and scheduled the patient for an appointment with the breast clinic. Women in the intervention group received, in addition to usual care, services of an African-American patient navigator.
FIGURE 1.
Study sample of randomized controlled trial of patient navigation among 105 women with an abnormal mammogram (2005–2007).
Navigation
The patient navigator focused on specific needs of the women and guided those patients through the healthcare system. For example, the patient navigator provided patients with emotional and social support; helped patients make appointments and arrive at scheduled appointments on time and prepared; facilitated applications for financial assistance; connected patients with resources and support systems; and facilitated interaction and communication with healthcare staff and providers.
Qualifications and Training of Patient Navigator
Criteria for hiring the navigator included a bachelor’s degree in a social science or related field, at least 2 years of clinical, social work, or outreach experience, and knowledge of the community and existing health resources. The patient navigator that was hired had a bachelor’s degree in social relations and had previous experience as a youth advocate, habilitation counselor, and breast cancer support group volunteer. We chose an educated and experienced person who would be knowledgeable about the hospital system and could help translate medical terminology and facilitate communication to the patients. Training before initiation of the project included education on breast health, public speaking skills workshop, and orientations and observations with the mammography van unit, radiologists, breast surgeons, oncologists, social workers, and financial assistance personnel. Contacts were made with other community organizations providing breast cancer outreach and support. Ongoing mentoring with the oncology social worker is provided, and ongoing training is provided through conferences for health and social service workers and breast cancer symposiums.
Instruments and Measures
The main outcome measures were the diagnostic interval, change in patient anxiety, and patient satisfaction. The diagnostic interval was defined as the time (in days) from date of suspicious mammogram to date of final diagnosis (benign or malignant pathology report). Anxiety was chosen as an outcome measure because studies have shown that for patients without breast cancer, delay in timely follow-up after an abnormal screening result is associated with considerable anxiety and emotional distress.5 Change in anxiety was measured by the Zung Anxiety Self-Assessment Scale.19 This was a self-administered survey given to all patients at enrollment and 1 month after final resolution (benign diagnosis or for cancer patients, after initiation of cancer treatment). One month after final resolution was selected as the time to administer the anxiety and satisfaction scales because administering them right at the time of resolution may reflect higher anxiety during the time while awaiting results. The survey scored 20 items on a 4-point Likert-type scale (1 = none or a little of the time to 4 = most or all of the time). The sum of responses from the anxiety survey was converted to an anxiety index based on the Zung Anxiety Assessment Tool.19 The index ranges from below 45 (within normal range) to over 75 (most extreme anxiety). The Cronbach alpha for the Zung scale was 0.88 at baseline and 0.93 at follow-up.
Patient satisfaction with care was measured by an adaptation of the “Satisfaction with Hospital Care Questionnaire”20 1 month after final resolution of the suspicious mammogram, or in cancer patients, after initiation of cancer treatment. This questionnaire scored 15 items on a 5-point Lifetree-type scale (1=dissatisfied to 5= very satisfied) and included questions regarding: encounters with personnel, waiting times between and during appointments, accessibility of hospital personnel by telephone, approachability of staff, amount and clarity of information given, opportunities given to talk about problems, guidance and support, ease of finding way around the hospital, the way information was passed to their doctor, the way doctors treated them, and their experience with the biopsy. Questions from the original survey that pertained to inpatient hospital care were not included. An overall mean satisfaction score was calculated for all subjects based on the sum of their responses to the questionnaire divided by the number of items. The Cronbach alpha for the patient satisfaction scale was 0.95.
Other predictor variables such as demographic data and clinical information that may potentially affect the main outcomes of interest were collected at baseline through a patient survey. These included age, race/ethnicity, marital status, education, employment, insurance, smoking status, reason for mammogram, previous abnormal mammogram, history of breast cancer, family history of cancer, and usual source of care. Information on outcomes (dates of mammogram, dates of biopsy, biopsy result, and, if cancer diagnosis, date of initial treatment) was collected through medical chart abstraction by the principal investigator.
Statistical Analysis
To determine whether randomization resulted in equivalent control and intervention groups, univariate relations between predictor variables and the two groups were obtained using the t test for continuous variables and the chi-square or Fisher’s exact test for categorical variables. We also compared the mean anxiety index at baseline between the intervention and control groups, using the t test for difference in means.
Survival analysis was used to compare the diagnostic interval for the intervention and control groups to account for patients that were lost to follow-up, dropped out, or decided to get follow-up care elsewhere. Last date of contact was used as censor dates for these patients. We used Kaplan–Meier estimates to generate survival curves and compared differences in the diagnostic interval with the log-rank test. The mean change in the anxiety index, and mean satisfaction score were compared for the control and intervention groups, using the t test. Analyses used two-sided p values with significance set at 0.05. All analyses were performed using SPSS (version 14.0.2, Chicago, IL).
RESULTS
The study enrolled 105 women, 50 in the control group, and 55 in the intervention group. One woman went elsewhere for follow-up (intervention group) and one was lost to follow-up (control group). One other woman in the intervention group dropped out of the study after her diagnosis and did not complete the follow-up anxiety or satisfaction surveys. Table 1 shows the baseline characteristics of the study population. The majority of women were black, unmarried, unemployed, with a high school education or less. Sixty percent of women had no insurance. Women in the intervention and control groups were similar in age, race/ethnicity, marital status, education, employment, insurance, smoking status, reason for mammogram, previous abnormal mammogram, history of breast cancer, family history of cancer, and usual source of care. There were 15 women in the intervention group diagnosed with cancer, whereas 11 women in the control group were diagnosed with cancer (p = .651).
TABLE 1.
Baseline characteristics of the control and intervention groups in study (N = 105)
| Characteristic | Total (%) | Control (%) | Intervention (%) | P value |
|---|---|---|---|---|
| Mean age, years (SD) | 50.1 (11.6) | 50.3 (11.1) | 49.9 (12.2) | 0.866 |
| Race/ethnicity | ||||
| Black | 59.0 | 58.0 | 60.0 | |
| Hispanic | 27.6 | 30.0 | 25.5 | 0.843 |
| Other | 13.3 | 12.0 | 14.5 | |
| Marital status | ||||
| Single | 46.7 | 48.0 | 45.5 | 0.960 |
| Married | 32.4 | 32.0 | 32.7 | |
| Separated, divorced, or widowed | 21.0 | 20.0 | 21.8 | |
| Educational level | ||||
| High school and less | 76.2 | 82.0 | 70.9 | 0.252* |
| College or more | 23.8 | 18.0 | 29.1 | |
| Employment status | ||||
| Unemployed | 65.7 | 68.0 | 63.6 | 0.684* |
| Employed | 34.3 | 32.0 | 36.4 | |
| Insurance | ||||
| None | 60.0 | 56.0 | 63.6 | 0.706 |
| Government | 24.8 | 28.0 | 21.8 | |
| Private | 15.2 | 16.0 | 14.5 | |
| Smoking | ||||
| Never | 48.6 | 48.0 | 49.1 | 0.270 |
| Current | 36.2 | 42.0 | 30.9 | |
| Former | 15.2 | 10.0 | 20.0 | |
| Reason for mammogram | ||||
| Screening | 41.0 | 38.0 | 43.6 | 0.821 |
| Follow-up abnormal | 13.3 | 12.0 | 14.5 | |
| Lump on self breast exam | 32.4 | 34.0 | 30.9 | |
| Lump on clinical breast exam | 13.3 | 16.0 | 10.9 | |
| Previous abnormal mammogram | ||||
| Yes | 37.1 | 34.0 | 40.0 | 0.551* |
| No | 62.9 | 66.0 | 60.0 | |
| Personal history of breast cancer | ||||
| Yes | 4.8 | 4.0 | 5.5 | 1.00* |
| No | 95.2 | 96.0 | 94.5 | |
| Family history of breast cancer | ||||
| Yes | 19.2 | 18.0 | 20.4 | 0.808* |
| No | 80.8 | 82.0 | 79.6 | |
| Usual source of care | ||||
| Primary care | 48.6 | 48.0 | 49.1 | 1.00* |
| No primary care | 51.4 | 52.0 | 50.9 |
*Fisher’s exact test was used.
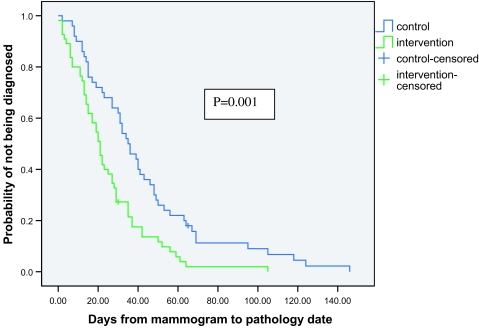
Figure 2 shows the Kaplan–Meier plot for the diagnostic interval among the intervention and control groups. Women in the intervention group had shorter diagnostic intervals than women in the control group (p = .001). The proportion of women without a final diagnosis at 60 days was 22% in the control group vs. 6% in the intervention group. Table 2 compares the results of our main outcomes for the two groups. The mean diagnostic interval in the control group was 42.7 days vs. 25.0 days for the intervention group (p = .001). At baseline, there was no difference in mean anxiety index between the intervention and control group (38.7 vs. 36.6, respectively; p = .346). However, after diagnosis, the mean anxiety index was lower in the intervention group (30.2) than in the control group (42.8; p < .001). Likewise, the change in anxiety index from baseline to follow-up was statistically different among the groups (decrease of 8.0 in intervention vs. increase of 5.8 in control; p < .001). In addition, the mean satisfaction score was higher in the intervention group (4.3) than in the control group (2.9; p < .001).
FIGURE 2.
Kaplan–Meier estimates of diagnostic interval among the control and intervention groups.
TABLE 2.
Diagnostic interval, anxiety, and satisfaction for the control and intervention groups (Total Sample)
| Main outcomes | Total | Control | Intervention | p value |
|---|---|---|---|---|
| Diagnostic interval | ||||
| Mean days (95% C.I.) | 33.5 (28.0, 38.9) | 42.7 (33.5, 51.8) | 25.0 (19.6, 30.3) | 0.001* |
| Mean anxiety index (S.D.) | ||||
| Baseline | 37.7 (11.4) | 36.6 ( 9.3) | 38.7 (13.0) | 0.346 |
| Follow-up | 36.1 (12.4) | 42.8 (13.3) | 30.2 (7.6) | <0.001 |
| Change in anxiety index | −1.5 (14.1) | 5.8 (14.0) | −8.0 (10.6) | <0.001 |
| Mean satisfaction score (S.D.) | 3.6 ( 0.9) | 2.9 ( 0.7) | 4.3 ( 0.5) | <0.001 |
*Log-rank test
Subgroup analyses were conducted to compare those with benign diagnosis (N = 77) and those with cancer diagnosis (N = 26). The mean anxiety index at baseline, the mean anxiety index at follow-up, and the change from baseline to follow-up were similar in the group of women without cancer and those with cancer (baseline: 34.4 vs. 34.7, p = .284; follow-up: 32.4 vs. 33.4, p = .068; change: −5.1 vs. −6.7; p = .483). Tables 3 and 4 show the results of all outcomes stratified by benign or cancer diagnosis. Results were similar to the overall analysis except, in the smaller subgroup of patients with cancer, the diagnostic intervals were not significantly different between the intervention and control group (mean = 14.3 vs. 33.9 days, respectively; p = .130). However, the magnitude of the difference was substantial. The difference may not have been detectable as statistically significant because of the small sample size in the subgroup analysis.
TABLE 3.
Diagnostic interval, anxiety, and satisfaction for patients without cancer
| Main outcomes | Total | Control | Intervention | p value |
|---|---|---|---|---|
| Diagnostic interval | ||||
| Mean days (95% C.I.) | 36.0 (30.2, 41.8) | 43.7 (34.3, 53.1) | 28.4 (22.4, 34.5) | 0.005* |
| Mean anxiety index (S.D.) | ||||
| Baseline | 36.9 (11.0) | 36.7 ( 10.1) | 37.0 (11.9) | 0.925 |
| Follow-up | 34.9 (10.8) | 40.7 (12.1) | 29.3 (4.9) | <0.001 |
| Change in anxiety index | −2.1 (13.3) | 3.8 (13.3) | −7.7 (10.7) | <0.001 |
| Mean satisfaction score (S.D.) | 3.7 ( 0.9) | 3.0 ( 0.7) | 4.3 ( 0.5) | <0.001 |
*Log-rank test
TABLE 4.
Diagnostic interval, anxiety, and satisfaction for patients with cancer
| Main outcomes | Total | Control | Intervention | P value |
|---|---|---|---|---|
| Diagnostic interval | ||||
| Mean days (95% C.I.) | 22.6 (11.1, 34.0) | 33.9 (10.7, 57.2) | 14.3 (5.4, 23.1) | 0.130* |
| Mean anxiety index (S.D.) | ||||
| Baseline | 39.6 (12.2) | 36.5 ( 6.9) | 41.9 (14.7) | 0.264 |
| Follow-up | 40.1 (16.0) | 50.3 (15.6) | 32.9 (12.2) | 0.006 |
| Change in anxiety index | 0.3 (16.4) | 13.2 (14.6) | −9.0 (10.4) | <0.001 |
| Mean satisfaction score (S.D.) | 3.5 ( 1.0) | 2.5 ( 0.5) | 4.2 ( 0.5) | <0.001 |
*Log-rank test.
We also conducted analyses to examine whether demographic characteristics and clinical factors (from Table 1) were associated with diagnostic interval, change in patient anxiety, and patient satisfaction. Results showed that the effectiveness of patient navigation did not differ in terms of demographic characteristics and clinical factors, although the sample size in the different subgroups may have been too small to detect differences.
DISCUSSION
This is the first publication, to our knowledge, of a randomized controlled trial testing the benefits of a patient navigator after abnormal mammograms in urban mostly African-American women. Whereas it supports findings from other uncontrolled studies showing that a patient navigator significantly improves follow-up and timeliness to diagnosis, this is the first randomized controlled study to show that patient navigation significantly decreases anxiety levels and increases patient satisfaction.
Despite the fact that this hospital already had a system in place to facilitate timely follow-up after an abnormal mammogram, the use of a patient navigator still significantly decreased time to diagnostic resolution. Only 6% of women in the intervention group did not have a final diagnosis at 60 days (program standard set by the CDC),21 compared to 22% of women in the control group. Although a 2- to 3-week difference in time to diagnosis may have little biological consequence in cancer outcomes, patient navigation has the added benefit of significantly decreasing anxiety and improving patient satisfaction. Despite more women in the intervention group being diagnosed with cancer (who would be expected to have higher anxiety levels), the anxiety levels of women in the intervention group after diagnosis were significantly lower than the women in the control group. They were also more satisfied with the care they received than women in the control group. Patient navigation provides women with abnormal mammograms with more timely reassurance for women with benign diagnoses and potentially earlier and higher completion of treatment for those with breast cancer.
This study has all the strengths of a randomized controlled trial, but there are several limitations. First, it was conducted in a small sample at one urban university hospital serving poor minority patients. However, the results may be broadly applied to poor English-speaking minority patients receiving care at other urban public hospitals. These patients are at higher risk for suboptimal follow-up and worse breast cancer outcomes. Second, this study excluded a high proportion of the study population who did not speak English, as the patient navigator was not bilingual. Our subgroup analysis based on race/ethnicity showed no differences in outcomes probably because of low sample size. The one other published randomized controlled trial on patient navigation showed that it was effective in increasing follow-up adherence rates and timely diagnostic resolution after an abnormal mammogram in Spanish-speaking women.18 Finally, we had a low enrollment rate of 36% of eligible patients participating, with distrust cited as the most common reason given for refusal. This difficulty in recruiting African-Americans to research studies has been widely documented, and points to the urgent need for innovative strategies to enhance participation of African Americans in clinical trials. Unfortunately, because of patient consent issues, we could not collect information on the patients who refused participation to compare them with the participants. When comparing participants to the general hospital population, participants were more likely to be uninsured (probably because of our free mammography screening program for uninsured women), but the racial distribution was similar to the hospital population.
Patient navigation is an effective strategy to improve timely diagnostic resolution, significantly decrease anxiety, and increase patient satisfaction among urban minority women with abnormal mammograms. Further randomized controlled trials on the effectiveness of patient navigation are needed in larger samples and other settings, and studies are needed to determine the cost-effectiveness of patient navigation.
Acknowledgment
This patient navigation program was funded by the Susan G. Komen Foundation North Jersey Affiliate.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer Statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. [DOI] [PubMed]
- 2.Center for Disease Control and Prevention. The National Breast and Cervical Cancer Early Detection Program: Saving Lives Through Screening—2006 Program Fact Sheet. Available at: http://www.cdc.gov/cancer/nbccedp/about.htm. Accessed December 2006.
- 3.Hunter CP. Epidemiology, stage at diagnosis, and tumor biology of breast carcinoma in multiracial and multiethnic populations. Cancer. 2000;88(5 Suppl):1193–1202. [DOI] [PubMed]
- 4.Kothari A, Fentiman IS. Diagnostic delays in breast cancer and impact on survival. Int J Clin Pract. 2003;57(3):200–203. [PubMed]
- 5.Heckman BD, Fisher EB, Monsees B, Merbaum M, Ristvedt S, Bishop C. Coping and anxiety in women recalled for additional diagnostic procedures following an abnormal screening mammogram. Health Psychol. 2004;23(1):42–48. [DOI] [PubMed]
- 6.Dohan D, Schrag D. Using navigators to improve care of underserved patients: current practices and approaches. Cancer. 2005;104(4):848–855. [DOI] [PubMed]
- 7.Freeman HP, Muth BJ, Kerner JF. Expanding access to cancer screening and clinical follow-up among the medically underserved. Cancer Pract. 1995;3(1):19. [PubMed]
- 8.Weinrich SP, Boyd MD, Weinrich M, Greene F, Reynolds WA, Jr., Metlin C. Increasing prostate cancer screening in African American men with peer-educator and client-navigator interventions. J Cancer Educ. 1998;13(4):213–219. [DOI] [PubMed]
- 9.Tingen MS, Weinrich SP, Heydt DD, Boyd MD, Weinrich MC. Perceived benefits: a predictor of participation in prostate cancer screening. Cancer Nurs. Oct 1998;21(5):349–357. [DOI] [PubMed]
- 10.Frelix GD, Rosenblatt R, Solomon M, Vikram B. Breast cancer screening in underserved women in the Bronx. J Natl Med Assoc. 1999;91(4):195–200. [PMC free article] [PubMed]
- 11.Ell K, Padgett D, Vourlekis B, et al. Abnormal mammogram follow-up: a pilot study women with low income. Cancer Pract. 2002;10(3):130–138. [DOI] [PubMed]
- 12.Ell K, Vourlekis B, Muderspach L, et al. Abnormal cervical screen follow-up among low-income Latinas: Project SAFe. Journal of Womens Health and Gender-Based Med. 2002;11(7):639–651. [DOI] [PubMed]
- 13.Psooy BJ, Schreuer D, Borgaonkar J, Caines JS. Patient navigation: improving timeliness in the diagnosis of breast abnormalities. Can Assoc Radiol J. 2004;55(3):145–150. [PubMed]
- 14.Dignan MB, Burhansstipanov L, Hariton J, et al. A comparison of two Native American Navigator formats: face-to-face and telephone. Cancer Control. Nov 2005;12(Suppl 2):28–33. [DOI] [PMC free article] [PubMed]
- 15.Jandorf L, Gutierrez Y, Lopez J, Christie J, Itzkowitz SH. Use of a patient navigator to increase colorectal cancer screening in an urban neighborhood health clinic. J Urban Health. 2005;82(2):216–224. [DOI] [PMC free article] [PubMed]
- 16.Nash D, Azeez S, Vlahov D, Schori M. Evaluation of an intervention to increase screening colonoscopy in an urban public hospital setting. J Urban Health. 2006;83(2):231–243. [DOI] [PMC free article] [PubMed]
- 17.Battaglia TA, Roloff K, Posner MA, Freund KM. Improving follow-up to abnormal breast cancer screening in an urban population. Cancer. 2007;109(S2):359–367. [DOI] [PubMed]
- 18.Ell K, Vourlekis B, Lee P-J, Xie B. Patient navigation and case management following an abnormal mammogram: a randomized clinical trial. Prev Med. 2007;44(1):26. [DOI] [PubMed]
- 19.Zung WK. A rating instrument for anxiety disorders. Psychosomatics. 1971;12(6):371–384. [DOI] [PubMed]
- 20.Hendriks AAJ, Oort FJ, Vrielink MR, Smets EMA. Reliability and validity of the Satisfaction with Hospital Care Questionnaire. Int J Qual Health Care. 2002;14(6):471–482. [DOI] [PubMed]
- 21.Centers for Disease Control and Prevention. Annual performance plan and report 2004. Atlanta, GA: Centers for Disease Control and Prevention; 1994.