Abstract
Background
Insufficient tissue perfusion underlies many acute and chronic diseases. Tissue perfusion in turn requires adequate blood flow, determined in large part by the relative state of relaxation or constriction of arterial vessels. Nitric oxide (NO) produced by vascular cells modulates blood flow and tissue perfusion by relaxing and dilating arteries. Recently we reported that the secreted protein thrombospondin-1 (TSP1), through its cell surface receptor CD47, limits the ability of NO to relax and dilate blood vessels and thus decreases tissue perfusion. In the present study we tested the hypothesis that blocking TSP1-CD47 signaling increases ischemic tissue survival in random cutaneous porcine flaps.
Methods
Random cutaneous flaps 2 × 10 cm2 were raised in white hairless Yucatan miniature pigs and were treated with a monoclonal antibody to TSP1, an antisense morpholino oligonucleotide to CD47 or control agents and tissue survival assessed. Primary vascular cells (VSMC) cultured from Yucatan pigs were also treated with the same agents ± and an NO donor (DEA/NO) and cGMP quantified.
Results
Antibody blockade of TSP1 or morpholino suppression of CD47 dramatically enhanced survival of random tissue flaps. These responses correlated with increased blood vessel patency and tissue blood flow on vessel injection studies. NO-stimulated cGMP flux in Yucatan VSMC was abrogated after antibody or morpholino treatment.
Conclusion
Antibody ligation of TSP1 or antisense morpholino knock down of CD47 greatly increased tissue survival to ischemia. Given the homology between porcine and human soft tissues these results suggest significant therapeutic potential for people.
Introduction
Impaired tissue healing is a well recognized phenomenon in the elderly.1, 2 Among the many factors that contribute to this consequence of aging, alterations in blood flow is central.3 Decreased tissue blood flow secondary to vascular disease not only impairs tissue responses to trauma, surgical or otherwise, but also leads to eventual ischemic tissue death.4 A majority of the population over 65 years of age will have varying degrees of vascular pathology and progressive diseases arising from the same.5
Nitric oxide (NO) is a central regulators of vascular health and blood flow.6 This bioactive gas increases blood flow in mature vasculature through its ability to relax vascular smooth muscle cells7 and increases new blood vessel formation (angiogenesis) by stimulating vascular cell proliferation and migration.8, 9 In the elderly, NO production in blood vessels is dramatically decreased,10 a problem that is further accelerated in the presence of vascular pathology.11 Recently, we discovered that the secreted matricellular protein, thrombospondin-1 (TSP1) is the central modulator of NO stimulation of vascular cells.12, 13 In the absence of endogenous TSP1, NO-driven increases in tissue blood flow are dramatically increased.14 Likewise, the absence of TSP1 or its necessary receptor CD47 confers significant survival advantages to complex tissue units following ischemic insult and correlates directly with markedly improved blood flow. Blocking TSP1 directly with antibody engagement or suppressing CD4715 with a morpholino oligonucleotide leads to heightened blood flow under ischemic stress16, 17 and atherosclerotic vasculopathy in murine models.18
The use of an appropriate animal model is required to critically evaluate the therapeutic potential of this strategy in humans. The pig (Sus scrofa) represents an ideal large animal model for analysis of therapeutic agents that modify blood flow 19 and tissue perfusion because the structure and morphology of pig vasculature and skin is nearly identical to human.20, 21 Herein we test the hypothesis that blocking TSP1-CD47 signaling increases ischemic tissue survival in random cutaneous porcine flaps. We demonstrate that using monoclonal antibodies to target TSP1 or an antisense morpholino oligonucleotide to suppress CD47 expression greatly enhances ischemic soft tissue survival in a porcine tissue injury model. These results suggest that the identified therapeutics may be clinically efficacious in improving tissue blood flow and healing in people.
Materials and Methods
Cells and reagents
Vascular smooth muscle cell (VSMC) growth medium was obtained from Cambrex (SM-GM, Lonza, Basel, Switzerland). A CD47 morpholino oligonucleotide complementary to a 5′-UTR sequence conserved between murine and human CD47 mRNAs (CGTCACAGGCAGGACCCACTGCCCA) and 5 base mismatched control morpholino were purchased from GeneTools (Philmonth, Oregon). A monoclonal antibody to TSP1 (clone A6.1) was prepared as described 22. TSP1 antibody HB8432 was purified from conditioned medium of the corresponding hybridoma, obtained from the American Type Culture Collection (Manassas, VA), by affinity chromatography on immobilized protein A. Fluorescein was purchased from Pierce (Rockford, IL). Monomeric type I collagen was obtained from Inamed (Fremont, CA).
Animals
White hairless Yucatan miniature swine ((Sus scrofa (Yuc:scr), Sinclair Research Center, Columbia, MO)) between 15 and 30 kg and 6 months of age were housed in an AAALAC accredited facility in accordance with the standards in the Guide for the Care and Use of Laboratory Animals (NRC 1996). Animals were fed once daily with ~750 gm of Zeigler NIH SWINE (Zeigler Bros. Inc., Gardens, PA) and received water ad libitum. The animals were maintained on a 12:12 light dark cycle at 24 ± 2 C and 30–70% relative humidity. Animals were anesthetized by an intramuscular injection of ketamine 10–12 mg/kg and medetomidine 20–30 μg/kg followed by IV catheter placement and administration of propofol to effect. Following intubation, they were placed on isoflurane 1–3%. Buprenorphine 0.01mg/kg was given intramuscularly and cefazolin 25 mg/kg was given intravenously before surgery. Animals received warm LRS at ~10 ml/kg/hr and were maintained on a hot-water blanket during the procedure. Following the indicated post-operative intervals animals were again anesthetized with 10–12 mg/kg ketamine and 20–30 μg/kg medetomidine and an intravenous catheter was placed, data acquisition was performed, and the animals were euthanized by administration of 80–100 mg/kg Euthasol intravenous.
White hairless Yucatan miniature pig VSMC cultures
Under sterile technique, segments of the femoral artery were harvested, and the tissue was cut into small pieces and incubated in smooth muscle cell growth medium with 1% collagenase type II (Worthington Biochemical Corp., Lakewood, NJ). The resultant cell suspension was then plated in sterile culture flasks in growth medium and cultured until confluence obtained.
Ischemic soft tissue flap model
Animals underwent shaving of the dorsal surface hair and cleansing of the skin with soap and alcohol. Random dorsal cutaneous flaps 2 × 10 × 0.5 cm were elevated and immediately sutured in place with interrupted 4-0 nylon suture (Ethicon, Johnson & Johnson, NJ). Treated flaps were injected with the indicated amounts of drug in sterile normal saline. On the 3 and 7 post-operative days tissue survival was determined and flaps excised for histology.
Determination of flap survival
Clinical assessment of flap perfusion was performed by two different surgically trained investigators. Notation of flap color, capillary refill and bleeding to needle stick were recorded. Flap dimensions were then traced onto a clear plastic sheet and with demarcation of viable and non-viable areas made. Weights of segments of sheeting corresponding to viable versus non-viable portions of flaps were then determined and % survival expressed as a percentage versus total as previously described.14 In instances were flap survival via clinical assessment was determined to be less than 90% animals received intravenous fluorescein prior to euthanasia and flap perfusion assessed under ultraviolet illumination.
Indian ink studies
The internal jugular vein and common carotid arteries were cannulated and the animals perfused with a 5 L volume of heparinized (50 U/mL) normal saline heated to 50 °C. A mixture of India ink and gelatin (5%) heated to 50 °C (1 L) was administered by gently pressing and then releasing the plunger of the syringe to simulate the arterial pulse. The flaps were excised and placed in 100% glycerin and stored at 4°C for 10 days. During this time flap tissue became thin, desiccated and transparent and allowed visualization of the vascular architecture under direct transillumination. Using 5X magnification patent vessels (defined as those demonstrating filling with India ink) were quantified and expressed as per cm2 flap area.
Adhesion assay
White hairless Yucatan miniature pig VSMC were plated at 10,000 cells/well onto 96-well plates (Maxisorp, Nunc) precoated with type I collagen (3 μg/ml). Cells were incubated for 1 h in basal medium (lacking additives and serum) with 0.1% BSA and the indicated concentrations of TSP1 and DEA/NO. Plates were then washed with PBS, fixed with glutaraldehyde, stained with crystal violet, and washed. Absorbed stain was solubilized with acetic acid from fixed cells and the resulting color signal determined on a MR580 Microelisa Auto Reader, (Dynatech, Alexandria, VA) at 450 nm wavelength. In other experiments cell were treated with a CD47 or mismatch control morpholino as described for 48 h prior to harvesting.
Morpholino suppression of CD47
White hairless Yucatan miniature pig VSMC were plated onto culture plates (Nunc, Roskilde, Denmark) at a the indicated cell density in smooth muscle cell growth medium + 2% FCS and cultured until approximately 90% confluent. Cultured cells were treated according to the recommendation of the manufacturer with morpholinos (10 μM) and delivery agent (Endoporter® 6 μl/ml) and used within 48 hours of treatment.
Intracellular cyclic nucleotide measurement
White hairless Yucatan miniature pig VSMC (5 × 105 cells/well) grown to 90% surface saturation in 12-well culture plates (Nunc) in SM-GM containing 2% FCS and then weaned off serum over 48 h before treatment with NO donors and other agents in serum/additive free medium + 0.1% BSA. Intracellular cGMP levels were determined using an enzyme immunoassay (Amersham, GE Health Care, UK).
Analysis of white hairless Yucatan CD47 mRNA sequence
Approximately 2 × 106 white hairless Yucatan miniature pig lung endothelial cells were suspended in 1 mL TriZOL reagent (Invitrogen, Carlsbad, CA) and processed according to the manufacturer’s specifications. 5 μg total RNA was used to create cDNA using SuperScript III (Invitrogen, Carlsbad, CA). A primer approximately 60 bp upstream of the predicted CD47 start codon and a primer approximately 60 bp downstream of the predicted CD47 stop codon were used to amplify CD47 cDNA with PCR Supermix (Invitrogen, Carlsbad, CA). Two independently-derived PCR products were purified using the Wizard purification kit (Promega, Madison, WI) and subjected to sequencing at the NCI Minicore with the primers used for PCR and two internal primers (to obtain higher-quality sequence of the ends of the PCR products). The following primers were employed: (porcine CD47) forward CTGCTCCAGACACCTGAGG and reverse CGTCTTAGTACTCTCCAATC.
Histology
Flaps were excised, fixed in 10% buffered formaldehyde, paraffin embedded and sectioned at a thickness of 5 μm. Sections were then stained with hematoxylin and eosin (H & E) according to standard procedures. Review of each slide was performed by an independent pathologist blinded to the origin of each tissue slide.
Immunohistochemistry
Paraffin embedded porcine flaps were sectioned at a thickness of 5 μm and applied to charged glass slides and processed for immunohistology. Briefly tissue sections were deparaffinized with xylene and rehydrated in alcohol. Slides were then heat inactivated in 10 mmol/L sodium citrate (pH 6.0) in a microwave for 5 minutes. Cooled slides were rinsed with PBS and then incubated with 3% H2O2 for 30 minutes at room temperature. Sections were then blocked with 5% normal goat serum in PBS for 30 minutes at room temperature followed by 1 hour incubation in a humidified chamber at 37ºC with TSP1 antibody A6.1 (provided generously by William A. Frazier, Washington University, St. Louis, MO) at a 1:25 dilution. Slides were washed and then incubated with goat anti-rabbit IgG-biotin conjugate (BD PharMingen, San Diego, CA) diluted at 1:100 for 45 minutes, washed with PBS and incubated in pre-diluted Streptavidin-HRP conjugate (BD PharMingen) for 45 minutes at room temperature. Color was developed by DAB substrate kit (BD PharMingen). Slides were counterstained with Mayer’s hematoxylin for 2 minutes, dehydrated, and mounted.
Statistics
Results in a porcine model are presented as the mean ± SD of a total of 23 animals. Each animal underwent 2–4 flaps dependent on the degree on non-pigment dorsal skin for a total of 76 random dorsal cutaneous flaps distributed into the following treatment groups: Untreated (vehicle only) n = 22; CD47 morpholino n = 22; mismatch morpholino (negative control) n = 10; TSP1 monoclonal antibody A6.1 n = 16; TSP1 monoclonal antibody HB8432 n = 6. Experiments with harvested primary femoral artery vascular smooth muscle cells from the Yucatan white hairless pig were repeated a least four times and results are presented as the mean ± SD. India ink analysis of flap perfusion was performed on 6 flaps from each treatment group described and results presented as the mean ± SD. Significance was calculated with Student’s t test or where appropriate one way and two way ANOVA with Tukey post hoc test using a standard soft ware package (Origin) with p < 0.05.
Results
Yucatan pig CD47 mRNA
The antisense morpholino was designed to complement human and murine CD47, but the published sequence for porcine CD47 has four mismatched bases within this sequence (NM_213982, 23)(Fig. 1A). We first confirmed the sequence of Yucatan miniature pig CD47. Tissue from the Yucatan white hairless mini pig was digested, and a CD7 cDNA was prepared and sequenced (accession number giEU179507; NCBI). Based upon DNA sequencing of two independently derived CD47 cDNAs, white hairless Yucatan miniature pig CD47 mRNA and predicted protein sequences are identical to those annotated for Sus scrofa (NM_213982). Based on their distribution in the sequence, 4 mismatches were not predicted to impact targeting (Gene Tools, unpublished communication). The strength of interaction between the target RNA sequence and the CD47 morpholino was estimated by analysis of melting temperature. At concentrations of 1 μM and 10 μM morpholino, the calculated TM are 99.8 ºC and 104.0 ºC respectively for porcine and human CD47. Thus, the morpholino should form stable complexes with the porcine CD47 mRNA under physiological conditions.
Figure 1. Morpholino suppression of CD47 modulates TSP1 inhibition of NO signaling in porcine VSMC.
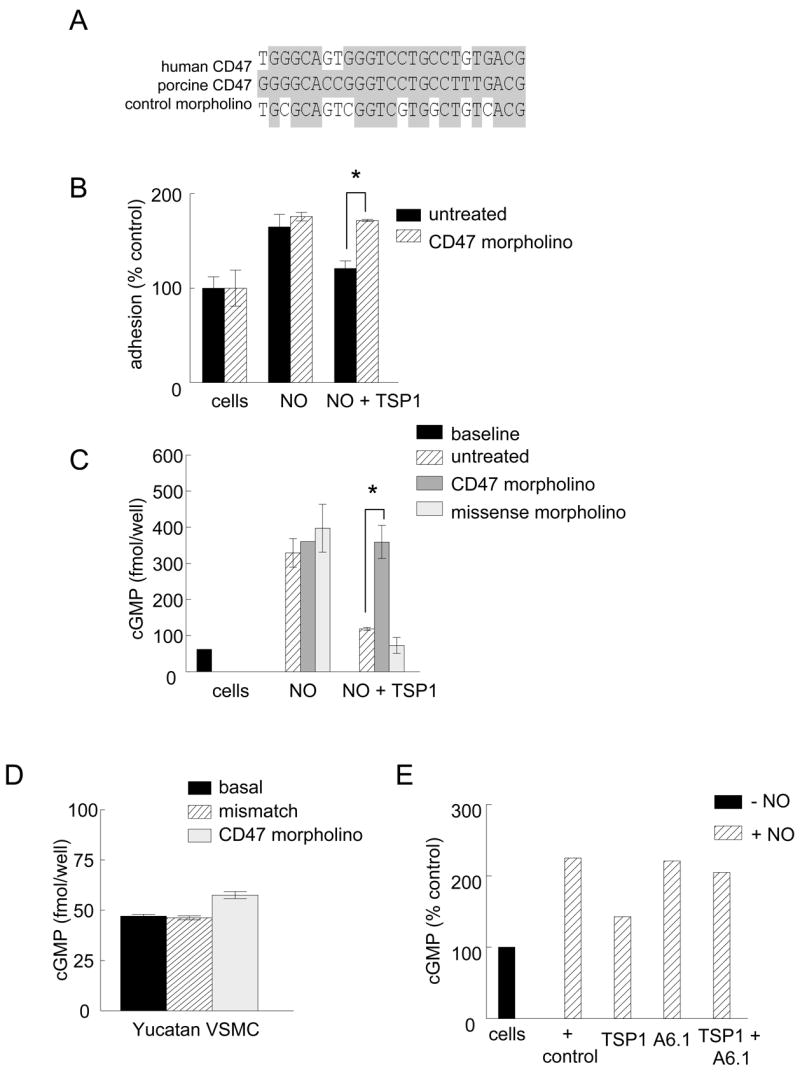
Comparison of the 5′-UTR sequences of human and porcine CD47 mRNA showing complementarity to the antisense and control morpholinos (A). VSMC from the femoral artery of white hairless Yucatan miniature pigs were plated at a density of 1 × 105 cells/well in 96-well plates pre-coated with type I collagen (3 μg/ml) and treated with TSP1 (0.022 – 2.2 nM) ± DEA/NO (10 μM) and adhesion measured as described (B). VSMC from the femoral artery of white hairless Yucatan miniature pigs were plated at a density of 5 × 105 cells/well in 12-well culture plates (Nunc) in growth medium. Cells were pre-treated with a gene silencing CD47 morpholino or 4 base mismatch control morpholino (10 μM) for 48 hours and then treated with TSP1 (2.2 nM) ± DEA/NO (10 μM) for 5 min and cGMP measured via immunoassay, (C). Porcine VSMC were treated for 48 with a CD47 morpholino or mismatch control (10 μM), and then incubated in minimal growth medium (without serum) for 5 min and cGMP measured via immunoassay (D). Results represent the mean ± SD. * P < 0.05. Porcine VSMC were pre-incubated with TSP1 (2.2 nM) and TSP1 antibody A6.1 (10 μg/ml) in basal medium (without serum) and an exogenous NO donor added (DEA/NO 10 μM) and cGMP determined (E). Results presented are representative of three separate experiments.
Morpholino suppression of CD47 prevents TSP1 inhibition of NO-stimulated VSMC adhesion
To confirm that the morpholino effectively targets CD47 in porcine vascular cells, we first replicated our published results in human VSMC, where down regulation of CD47 using the same antisense morpholino blocked the ability of TSP1 to inhibit NO-stimulated effects.16 Porcine VSMC demonstrated NO-stimulated enhancement of adhesion to type I collagen, which was inhibited by exogenous TSP1 at very low doses (Fig. 1B).
NO-driven cGMP accumulation in CD47 morpholino-treated Yucatan pig VSMC is not blocked by TSP1
To further validate the CD47 morpholino, VSMC from the femoral arteries of Yucatan white hairless pigs were pre-treated with a CD47 oligonucleotide morpholino or a mismatched control, and cGMP responses to exogenous NO were measured. As expected untreated, mismatch- and target morpholino-treated cells showed similar increases in cGMP after incubation with an NO donor (Fig. 1C). Pre-incubation with TSP1 (2.2 nM) blocked the increase in cGMP in control and mismatch treated cells. However, NO-stimulated cGMP levels in VSMC treated with a CD47 morpholino were resistant to suppression by TSP1. Importantly, basal cGMP levels were also elevated in resting cells following treatment with the CD47 morpholino compared to untreated and mismatch treated cells (Fig. 1D).
TSP1 antibody treatment of porcine VSMC blocks TSP1 inhibition of NO-stimulated cGMP accumulation
Antibody A6.1 was raised against human TSP1 but recognizes TSP1 across several species of mammals.24 This TSP1 antibody improved survival of ischemic tissue in mice, but its ability to block cGMP signaling via CD47 has not been confirmed in vitro.16, 17 To validate A6.1, white hairless Yucatan miniature pig VSMC were incubated in the presence of A6.1 (10 μg/ml) and TSP1 (2.2 nM), and then NO added. The ability of TSP1 to block an NO-driven accumulation of cGMP was substantially reduced in the cells that were pretreated with A6.1 (Fig. 1E).
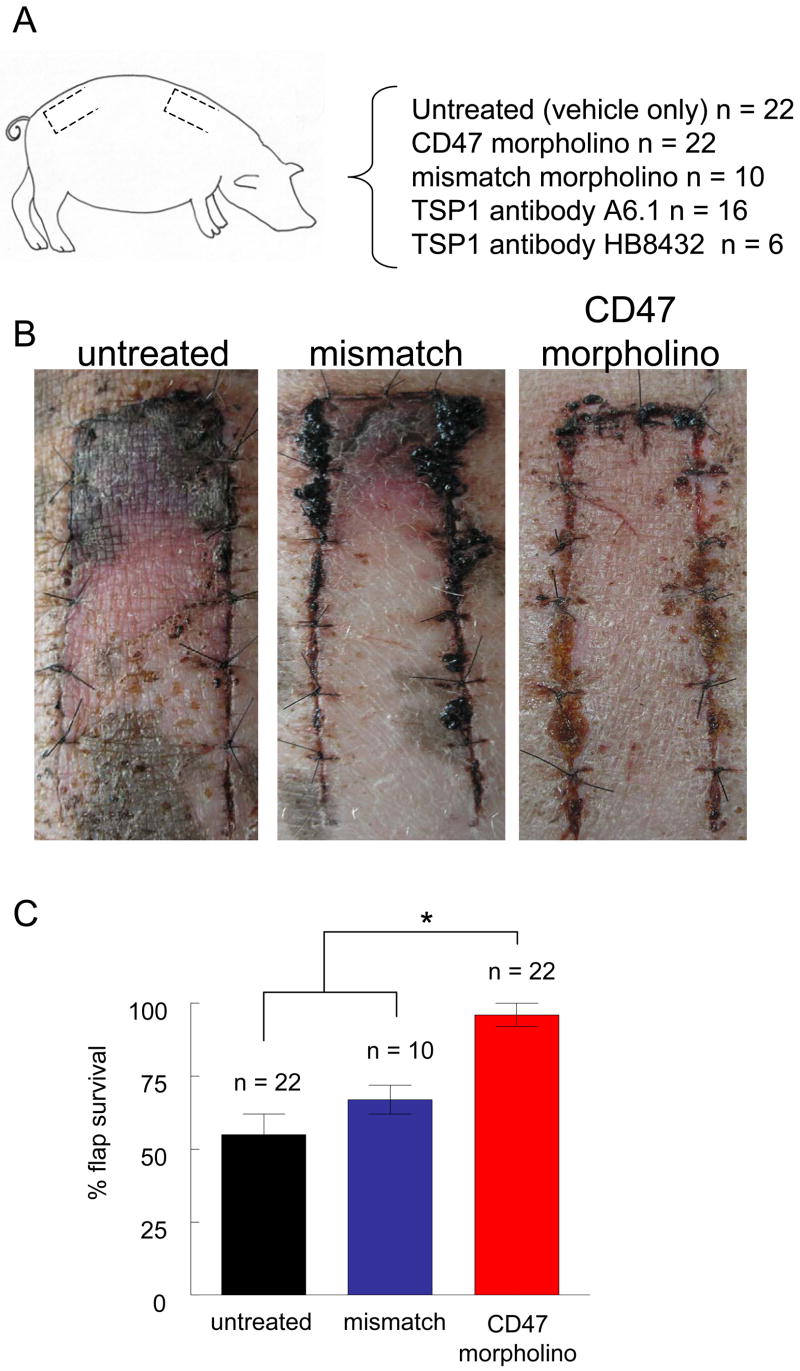
Suppression of CD47 increases random cutaneous flap survival in a porcine model
20 cm2 random cutaneous flaps were created in 6 month old white hairless Yucatan miniature pigs and flaps were treated as indicated (Fig. 2A). Flaps treated with a CD47 morpholino via infiltration injection showed significantly more tissue survival compared to untreated flaps or flaps treated with a control morpholino (Fig. 2B, C). Untreated and flaps treated with a mismatch control morpholino showed 47 ± 5% and 34 ± 6% necrosis respectively. In contrast morpholino treated flaps had minimal necrosis (12 ± 3 %).
Figure 2. CD47 suppression increases random cutaneous flap survival.
White hairless Yucatan miniature pigs underwent 2 × 10 cm dorsal random cutaneous flaps with treatments to flaps as indicated (A). At the time of surgery flaps were injected with vehicle, mismatched or CD47 antisense morpholino and flap survival was determined on post-operative day 7 (B). The degree of flap necrosis was determined at 72 hours postoperatively as described (C). Results represent the mean ± SD.* P < 0.05.
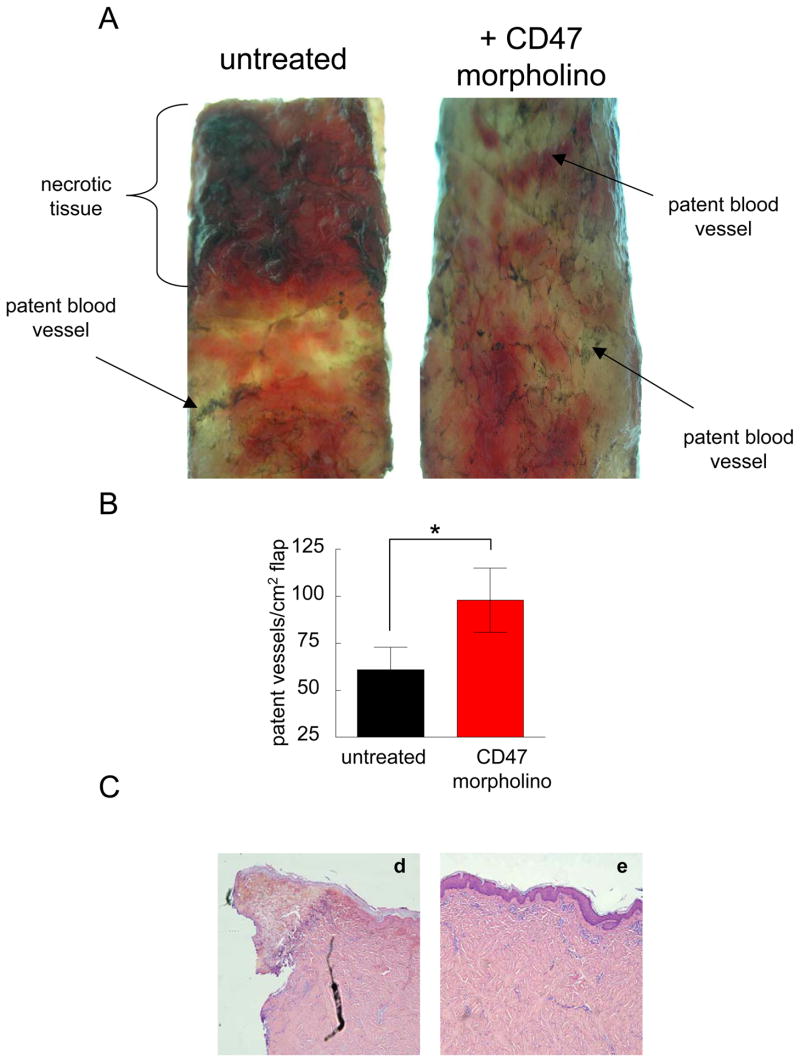
Morpholino suppression of CD47 is associated with increased perfusion of random ischemic flaps
Perfusion injection studies of freshly euthanized white hairless Yucatan pigs demonstrated alterations in flap staining by India ink consistent with clinical findings of tissue survival (Fig. 3A, B). Untreated flaps and flaps treated with a mismatched control morpholino showed lack of India ink staining of vessels that paralleled areas of tissue necrosis (Fig. 3C).
Figure 3. CD47 suppression is associated with increased vascular patency in ischemic cutaneous units.
Six month old white hairless Yucatan miniature pigs underwent random dorsal cutaneous flaps. On post-operative day 3 animals were euthanized and India ink injection of the central and peripheral vasculature performed as described. Flap vasculature in treated and untreated flaps was quantified (A, B). Results represent the mean ± SD. * P < 0.05. Representative H & E staining of untreated (d) and CD47 morpholino treated (e) random cutaneous porcine flaps (C).
Antibody ligation of TSP1 alters ischemic tissue survival
Random dorsal cutaneous flaps were treated with a monoclonal antibody to TSP1 (A6.1), which binds to the type 3 repeats of TSP1 and was previously shown to enhance ischemic tissue survival in a murine model.16 Treated flaps demonstrated significantly increased tissue preservation compared to untreated flaps (Fig. 4A, B). In contrast, a monoclonal antibody that binds to the adjacent EGF repeats of TSP1 (HB8432)24 did not increase tissue survival. Determination of tissue survival correlated with areas of fluorescein staining in flaps obtained in living animals.
Figure 4. Antibody ligation of TSP1 increases ischemic tissue survival in a porcine model.
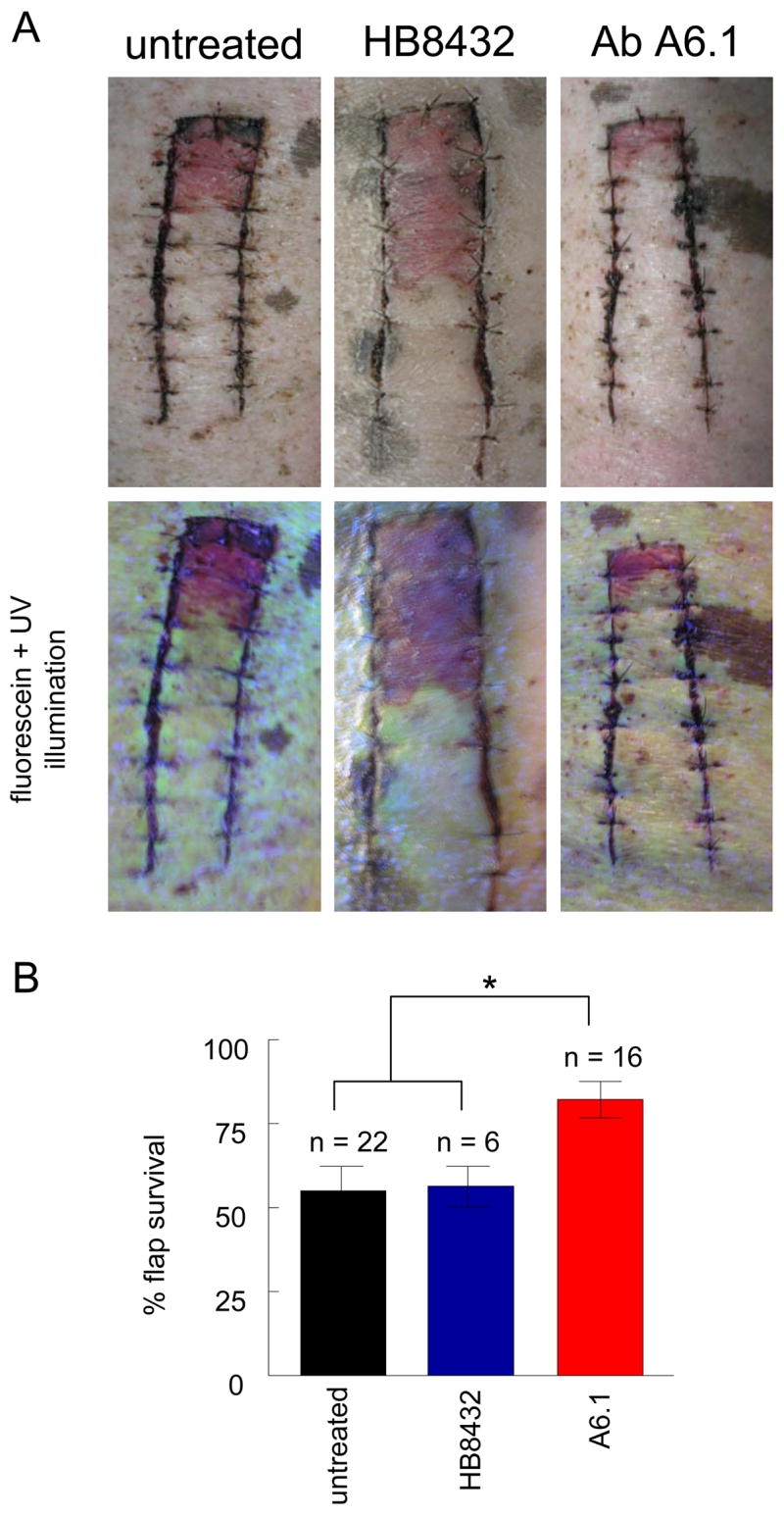
White hairless Yucatan miniature pigs underwent 2 × 10 cm dorsal random cutaneous flaps. Flaps were treated with vehicle (normal saline) or a monoclonal TSP1 antibody (clone HB8432, n = 2 or clone A6.1, n = 16). Therapeutic agents were delivered via injection at the time of surgery directly to the flap (A). At 72 hours post-operatively flap viability was determined (B). Results represent the mean ± SD. * P < 0.05. In instances were flap survival was determined to be less than 90% animals received intravenous fluorescein and flaps photographed under ultraviolet illumination. Representative images are presented.
TSP1 expression is decreased in CD47 morpholino treated flaps compared to untreated controls
TSP1 expression in uninjured soft tissues is minimal. In contrast, TSP1 expression increases dramatically following wounding and then rapidly returns to pre-injury levels as the wound heals.25 TSP1 expression is increased at the site of colonic anastomotic healing. 26 In fetal tissue healing models TSP1 expression has been found to increase acutely and then rapidly return to baseline. 27 In contrast, post-operative human lenses demonstrated decreased TSP1 expression compared to un-operated lenses,28 suggesting some anatomic specificity to the expression of TSP1. Hypoxia is known to increase both transcription of TSP1 and protein expression,29 and critical limb ischemia is associated with over-expression of TSP1.30 Decreased tissue hypoxia and ischemia as a result of improved tissue perfusion secondary to TSP1-CD47 blockade would be expected to be associated with less TSP1 expression. Immunohistochemical analysis of porcine cutaneous flaps demonstrated TSP1 expression (brown color) in both CD47 morpholino treated and untreated flaps (Fig. 5). Staining was found in perivascular cells and blood vessels and in infiltrating immune cells in adipose tissue. Qualitatively increased TSP1 staining was found in untreated compared to treated flaps. TSP1 staining was present in both proximal and distal regions of treated flaps but absent in obviously necrotic distal areas of untreated flaps (data not shown).
Figure 5. TSP1 expression in random porcine cutaneous flaps.
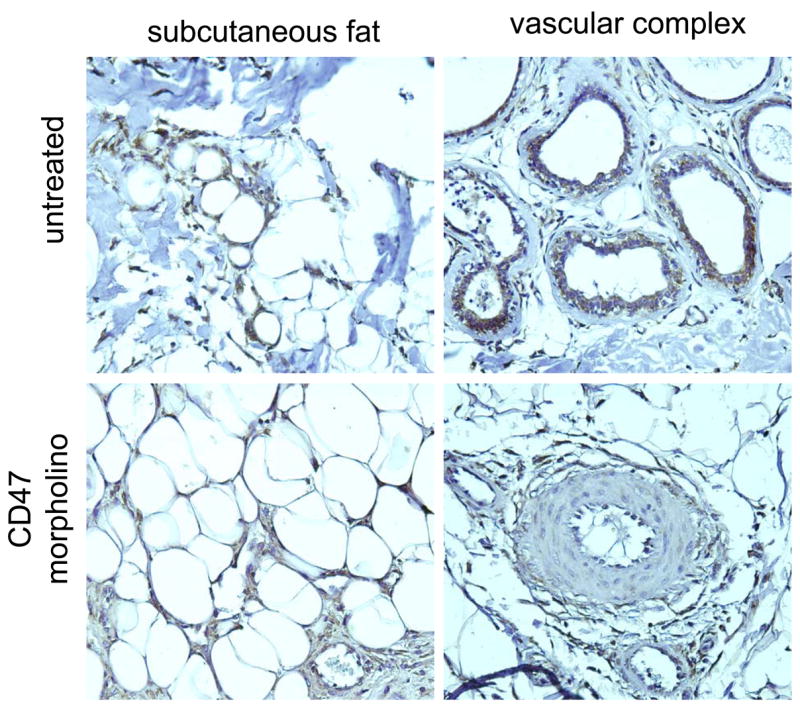
2 × 10 cm random cutaneous flaps were fixed in 10% paraformaldehyde, embedded in paraffin, cut lengthwise into 5 μm sections and mounted onto charged glass slides. Immunohistochemical staining for TSP1 was performed (brown stain). Images were obtained using a 20x objective.
Discussion
Tissue necrosis is a common complication secondary to traumatic injury or in the post-operative interval following elective surgery,31, 32 problems further enhanced by aging.33, 34 Significant morbidity and mortality arise as a result. A number of agents designed to increase tissue blood flow and enhance tissue survival have been utilized in several experimental models, though results in the clinical environment have been disappointing.35 A majority of currently employed agents are administered systemically and have non-specific and troublesome side effects. Thus, new agents are needed that can enhance tissue survival and wound healing and at the same time avoid systemic complications.
Nitric oxide is one of the central regulators of blood flow in the body based on its ability to relax VSMC and dilate blood vessels 6, 36. NO-releasing agents have been used in experimental models to enhanced tissue blood flow and survival under ischemic insult 37, 38. In people NO elevating agents such as nitroglycerine and isosorbide dinitrate have been employed for many years in treating ischemic heart and vascular disease. Recently we discovered that TSP1, through CD47, potently blocks the ability of NO to relax contracting VSMC.14 In murine models of ischemia tissue survival, blood flow was dramatically enhanced by targeting TSP1 and CD47. Additionally tissue blood flow in both young and aged animals is substantially greater following an exogenous NO challenge in mice lacking TSP1.14, 18 However, it is widely appreciated that therapeutic results in murine disease models often do not translate into clinical gains for people.
We now present evidence that targeting TSP1-CD47 enhances NO-signaling in porcine vascular cells and increases ischemic tissue survival in higher mammals. In several in vitro assays, porcine VSMC demonstrated TSP1 inhibition of NO-driven cell signaling. NO-stimulated adhesion to collagen was blocked by TSP1. NO driven intracellular cGMP flux was also sensitive to blockade by TSP1. Treating porcine VSMC with a CD47 morpholino that decreases expression of the cell receptor renders the cells functionally insensitive to TSP1 blockade of NO-stimulated signals including alterations in adhesion and intracellular cGMP flux. Even more significant was the finding that TSP1-CD47 signaling modulated basal levels of intracellular cGMP in porcine VSMC. We previously reported that endogenous TSP1 altered resting levels of cGMP in murine endothelial and aortic-derived VSMC 12, 13 by inhibiting NO-stimulation of soluble guanylate cyclase (sGC)12 and that CD47 is necessarily for this response.15 These findings are consistent with reports from others 39 and our own data 13 that treatment of VSMC with the nitric oxide synthase (NOS) inhibitor N (G)-nitro-L- arginine methyl ester (L-NAME) decreases cGMP and with the demonstration of endothelial NOS 40, 41 and inducible NOS 42, 43 expression in VSMC. We also recently reported that TSP1 limits tissue cGMP at rest and after ischemic insult.14, 18 Herein we extend these findings and show that interrupting TSP1-CD47 signaling by suppressing CD47 levels leads to elevation of basal cGMP in porcine VSMC. Additionally, a TSP1 monoclonal antibody VSMC. Together these findings suggest that in a porcine model of tissue ischemia a CD47 morpholino and a TSP1 antibody enhance tissue survival by increasing cGMP, maximizing vascular relaxation and increasing tissue perfusion.
The results of the present study support the necessary role CD47 plays in TSP1 regulation of NO stimulation of sGC, and support our finding that cells lacking CD47 are immune to inhibition of NO signaling by agents acting through CD36.15 However, CD36 also plays a role in this signaling pathway15, 44 since recombinant TSP1 domains that signal through CD36 can also block NO-driven sGC activation. Furthermore, blockade of CD36 by TSP1 and TSP1-based peptides decreases fatty acid uptake by vascular cells and limits endogenous NO production.44 Finally, TSP1 inhibits NO signaling by blocking both sGC12 and cGMP-dependent protein kinase activation,45 demonstrating that TSP1 blockade of NO/cGMP signaling occurs at multiple levels.
To develop an experimental system with greater similarity to people we employed a porcine model of tissue ischemia. Pigs have been utilized in numerous studies of tissue healing and blood flow since they mimic human cutaneous and vascular anatomy 46–49. The distribution of cutaneous vessels in the pig has recently been described and follows the same general rule as in humans with vessels traveling from deep to superficial and branching step-by-step into reduced-caliber divisions though the total number of segmental arcades in the porcine cutaneous envelope is greater than found in people.20 Clinically this enhanced redundancy in cutaneous perfusion required alteration of the traditional random flap dimensions from a ratio of 1:2 between width and length to 1:5 to insure a predictable degree of tissue necrosis. We have recently reported therapeutic efficacy in enhancing ischemic tissue survival with a CD47 morpholino also delivered directly to murine tissue units through syringe injection.16, 18 Similar increases in tissue survival were obtained in flaps treated with a specific monoclonal antibody to TSP1 (clone A6.1). The monoclonal antibody may prevent TSP1 binding to its receptors at the cell surface and should persist in the soft tissues in the extravascular compartment for some time. In a murine model of tissue ischemia the A6.1 antibody was also tissue protective and enhanced survival and blood flow.16 This finding is consistent with the ability of A6.1 to bind to both human and murine TSP1.24 In contrast another TSP1 monoclonal antibody, clone HB8432, did not confer a tissue survival advantage under ischemic challenge. The epitopes recognized by antibodies A6.1 and HB8433 have been localized to adjacent domains of TSP1,24 which may account for their different activities in vivo.
The results herein presented using a porcine model provide additional confirmation that blockade of TSP1-CD47 signaling regionally, via morpholino suppression of CD47, or direct ligation of TSP1 with a monoclonal antibody greatly enhances ischemic tissue survival. These therapies can be expected to synergize with NO-replacement approaches to further enhance ischemic tissue survival. In a murine model of soft tissue ischemia exogenous NO delivered by isosorbide dinitrate enhanced flap survival alone, a response that was significantly increased in the absence of TSP1 or CD47.13 Combined with cellular data obtained in vascular cells from several different species including murine,12, 13 bovine, porcine and human14 these findings provide strong evidence for the universality of NO regulation by TSP1 in mammals and suggest therapeutic potential for people.
Acknowledgments
We wish to thank Dr. Jon Moulton (Gene Tools) for help in designing a CD47 and control morpholino and Drs. Larry Keefer and Joseph Saavedra (NIH, Frederick, MD) for providing NO donors.
SOURCES OF FUNDING
This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research (D.D.R.) and NIH grants HL54390 and GM57573 (W.A.F.).
References
- 1.Ashcroft GS, Horan MA, Ferguson MW. The effects of ageing on cutaneous wound healing in mammals. J Anat. 1995;187(Pt 1):1–26. [PMC free article] [PubMed] [Google Scholar]
- 2.Ashcroft GS, Mills SJ, Ashworth JJ. Ageing and wound healing. Biogerontology. 2002;3(6):337–45. doi: 10.1023/a:1021399228395. [DOI] [PubMed] [Google Scholar]
- 3.Marin J. Age-related changes in vascular responses: a review. Mech Ageing Dev. 1995;79(2–3):71–114. doi: 10.1016/0047-6374(94)01551-v. [DOI] [PubMed] [Google Scholar]
- 4.Reed MJ, Edelberg JM. Impaired angiogenesis in the aged. Sci Aging Knowledge Environ. 2004;2004(7):pe7. doi: 10.1126/sageke.2004.7.pe7. [DOI] [PubMed] [Google Scholar]
- 5.Sigvant B, Wiberg-Hedman K, Bergqvist D, Rolandsson O, Andersson B, Persson E, Wahlberg E. A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. J Vasc Surg. 2007;45(6):1185–91. doi: 10.1016/j.jvs.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Ignarro LJ. Nitric oxide as a unique signaling molecule in the vascular system: a historical overview. J Physiol Pharmacol. 2002;53(4 Pt 1):503–14. [PubMed] [Google Scholar]
- 7.Ignarro LJ. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ Res. 1989;65(1):1–21. doi: 10.1161/01.res.65.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Murad F. Cyclic GMP: synthesis, metabolism, and function. Introduction and some historical comments. Adv Pharmacol. 1994;26:1–5. [PubMed] [Google Scholar]
- 9.Cooke JP. NO and angiogenesis. Atheroscler Suppl. 2003;4(4):53–60. doi: 10.1016/s1567-5688(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 10.Napoli C, de Nigris F, Williams-Ignarro S, Pignalosa O, Sica V, Ignarro LJ. Nitric oxide and atherosclerosis: an update. Nitric Oxide. 2006;15(4):265–79. doi: 10.1016/j.niox.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Desjardins F, Balligand JL. Nitric oxide-dependent endothelial function and cardiovascular disease. Acta Clin Belg. 2006;61(6):326–34. doi: 10.1179/acb.2006.052. [DOI] [PubMed] [Google Scholar]
- 12.Isenberg JS, Ridnour LA, Perruccio EM, Espey MG, Wink DA, Roberts DD. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc Natl Acad Sci U S A. 2005;102(37):13141–6. doi: 10.1073/pnas.0502977102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isenberg JS, Wink DA, Roberts DD. Thrombospondin-1 antagonizes nitric oxide-stimulated vascular smooth muscle cell responses. Cardiovasc Res. 2006;71(4):785–93. doi: 10.1016/j.cardiores.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 14.Isenberg JS, Hyodo F, Matsumoto K, Romeo MJ, Abu-Asab M, Tsokos M, Kuppusamy P, Wink DA, Krishna MC, Roberts DD. Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation. Blood. 2007;109(5):1945–52. doi: 10.1182/blood-2006-08-041368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isenberg JS, Ridnour LA, Dimitry J, Frazier WA, Wink DA, Roberts DD. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem. 2006;281(36):26069–80. doi: 10.1074/jbc.M605040200. [DOI] [PubMed] [Google Scholar]
- 16.Isenberg JS, Romeo MJ, Abu-Asab M, Tsokos M, Oldenborg A, Pappan L, Wink DA, Frazier WA, Roberts DD. Increasing survival of ischemic tissue by targeting CD47. Circ Res. 2007;100(5):712–20. doi: 10.1161/01.RES.0000259579.35787.4e. [DOI] [PubMed] [Google Scholar]
- 17.Isenberg JS, Pappan LK, Romeo MJ, Abu-Asab M, Tsokos M, Wink DA, Frazier WA, Roberts DD. Blockade of thrombospondin-1-CD47 interactions prevents necrosis of full thickness skin grafts. Ann of Surg. 2007 doi: 10.1097/SLA.0b013e31815685dc. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isenberg JS, Hyodo F, Pappan LK, Abu-Asab M, Tsokos M, Krishna MC, Frazier FA, Roberts DD. Blocking thrombospondin-1/CD47 signaling alleviates deleterious effects of aging on tissue responses to ischemia. Arterioscler Thromb Vasc Biol. 2007 doi: 10.1161/ATVBAHA.107.155390. In press. [DOI] [PubMed] [Google Scholar]
- 19.de Smet BJ, van der Zande J, van der Helm YJ, Kuntz RE, Borst C, Post MJ. The atherosclerotic Yucatan animal model to study the arterial response after balloon angioplasty: the natural history of remodeling. Cardiovasc Res. 1998;39(1):224–32. doi: 10.1016/s0008-6363(98)00085-6. [DOI] [PubMed] [Google Scholar]
- 20.Zhang HM, Yan YP, Sun GC, Hum HX, Liu ZF, Feng YJ. Cutaneous blood vessels in scent pigs. Plast Reconstr Surg. 2000;106(7):1555–65. doi: 10.1097/00006534-200012000-00017. [DOI] [PubMed] [Google Scholar]
- 21.Guyuron B, Michelow B, Schmelzer R, Thomas T, Ellison MA. Delayed healing of rhytidectomy flap resurfaced with CO2 laser. Plast Reconstr Surg. 1998;101(3):816–9. doi: 10.1097/00006534-199803000-00037. [DOI] [PubMed] [Google Scholar]
- 22.Dixit VM, Galvin NJ, O’Rourke KM, Frazier WA. Monoclonal antibodies that recognize calcium-dependent structures of human thrombospondin. Characterization and mapping of their epitopes. J Biol Chem. 1986;261(4):1962–8. [PubMed] [Google Scholar]
- 23.Shahein YE, de Andres DF, Perez de la Lastra JM. Molecular cloning and functional characterization of the pig homologue of integrin-associated protein (IAP/CD47) Immunology. 2002;106(4):564–76. doi: 10.1046/j.1365-2567.2002.01465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Annis DS, Murphy-Ullrich JE, Mosher DF. Function-blocking antithrombospondin-1 monoclonal antibodies. J Thromb Haemost. 2006;4(2):459–68. doi: 10.1111/j.1538-7836.2006.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agah A, Kyriakides TR, Lawler J, Bornstein P. The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am J Pathol. 2002;161(3):831–9. doi: 10.1016/S0002-9440(10)64243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roth JJ, Buckmire MA, Rolandelli RH, Granick MS, Tuszynski GP. Localization of thrombospondin-1 and its cysteine-serine-valine-threonine-cysteine-glycine receptor in colonic anastomotic healing tissue. Histol Histopathol. 1998;13(4):967–71. doi: 10.14670/HH-13.967. [DOI] [PubMed] [Google Scholar]
- 27.Roth JJ, Sung JJ, Granick MS, Solomon MP, Longaker MT, Rothman VL, Nicosia RF, Tuszynski GP. Thrombospondin 1 and its specific cysteine-serine-valine-threonine-cysteine-clycine receptor in fetal wounds. Ann Plast Surg. 1999;42(5):553–63. doi: 10.1097/00000637-199905000-00016. [DOI] [PubMed] [Google Scholar]
- 28.Saika S, Miyamoto T, Ishida I, Barbour WK, Ohnishi Y, Ooshima A. Accumulation of thrombospondin-1 in post-operative capsular fibrosis and its down-regulation in lens cells during lens fiber formation. Exp Eye Res. 2004;79(2):147–56. doi: 10.1016/j.exer.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Phelan MW, Forman LW, Perrine SP, Faller DV. Hypoxia increases thrombospondin-1 transcript and protein in cultured endothelial cells. J Lab Clin Med. 1998;132(6):519–29. doi: 10.1016/s0022-2143(98)90131-7. [DOI] [PubMed] [Google Scholar]
- 30.Favier J, Germain S, Emmerich J, Corvol P, Gasc JM. Critical overexpression of thrombospondin 1 in chronic leg ischaemia. J Pathol. 2005;207(3):358–66. doi: 10.1002/path.1833. [DOI] [PubMed] [Google Scholar]
- 31.Levin LS. The reconstructive ladder. An orthoplastic approach. Orthop Clin North Am. 1993;24(3):393–409. [PubMed] [Google Scholar]
- 32.Raghunathan A, Rapp JH, Littooy F, Santilli S, Krupski WC, Ward HB, Thottapurathu L, Moritz T, McFalls EO. Postoperative outcomes for patients undergoing elective revascularization for critical limb ischemia and intermittent claudication: a subanalysis of the Coronary Artery Revascularization Prophylaxis (CARP) trial. J Vasc Surg. 2006;43(6):1175–82. doi: 10.1016/j.jvs.2005.12.069. [DOI] [PubMed] [Google Scholar]
- 33.Ryan T. The ageing of the blood supply and the lymphatic drainage of the skin. Micron. 2004;35(3):161–71. doi: 10.1016/j.micron.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 34.Harris NR, Rumbaut RE. Age-related responses of the microcirculation to ischemia-reperfusion and inflammation. Pathophysiology. 2001;8(1):1–10. doi: 10.1016/s0928-4680(01)00064-5. [DOI] [PubMed] [Google Scholar]
- 35.Hankey GJ, Norman PE, Eikelboom JW. Medical treatment of peripheral arterial disease. Jama. 2006;295(5):547–53. doi: 10.1001/jama.295.5.547. [DOI] [PubMed] [Google Scholar]
- 36.Bolz SS, Vogel L, Sollinger D, Derwand R, de Wit C, Loirand G, Pohl U. Nitric oxide-induced decrease in calcium sensitivity of resistance arteries is attributable to activation of the myosin light chain phosphatase and antagonized by the RhoA/Rho kinase pathway. Circulation. 2003;107(24):3081–7. doi: 10.1161/01.CIR.0000074202.19612.8C. [DOI] [PubMed] [Google Scholar]
- 37.Isenberg JS, Ridnour LA, Espey MG, Wink DA, Roberts DD. Nitric oxide in wound-healing. Microsurgery. 2005;25(5):442–51. doi: 10.1002/micr.20168. [DOI] [PubMed] [Google Scholar]
- 38.Knox LK, Stewart AG, Hayward PG, Morrison WA. Nitric oxide synthase inhibitors improve skin flap survival in the rat. Microsurgery. 1994;15(10):708–11. doi: 10.1002/micr.1920151008. [DOI] [PubMed] [Google Scholar]
- 39.Mullershausen F, Russwurm M, Koesling D, Friebe A. The enhanced NO-induced cGMP response induced by long-term L-NAME treatment is not due to enhanced expression of NO-sensitive guanylyl cyclase. Vascul Pharmacol. 2003;40(3):161–5. doi: 10.1016/s1537-1891(03)00049-1. [DOI] [PubMed] [Google Scholar]
- 40.Pandolfi A, Grilli A, Cilli C, Patruno A, Giaccari A, Di Silvestre S, De Lutiis MA, Pellegrini G, Capani F, Consoli A, Felaco M. Phenotype modulation in cultures of vascular smooth muscle cells from diabetic rats: association with increased nitric oxide synthase expression and superoxide anion generation. J Cell Physiol. 2003;196(2):378–85. doi: 10.1002/jcp.10325. [DOI] [PubMed] [Google Scholar]
- 41.Buchwalow IB, Podzuweit T, Samoilova VE, Wellner M, Haller H, Grote S, Aleth S, Boecker W, Schmitz W, Neumann J. An in situ evidence for autocrine function of NO in the vasculature. Nitric Oxide. 2004;10(4):203–12. doi: 10.1016/j.niox.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 42.Hecker M, Cattaruzza M, Wagner AH. Regulation of inducible nitric oxide synthase gene expression in vascular smooth muscle cells. Gen Pharmacol. 1999;32(1):9–16. doi: 10.1016/s0306-3623(98)00082-2. [DOI] [PubMed] [Google Scholar]
- 43.MacNaul KL, Hutchinson NI. Differential expression of iNOS and cNOS mRNA in human vascular smooth muscle cells and endothelial cells under normal and inflammatory conditions. Biochem Biophys Res Commun. 1993;196(3):1330–4. doi: 10.1006/bbrc.1993.2398. [DOI] [PubMed] [Google Scholar]
- 44.Isenberg JS, Jia Y, Fukuyama J, Switzer CH, Wink DA, Roberts DD. Thrombospondin-1 inhibits nitric oxide signaling via CD36 by inhibiting myristic acid uptake. J Biol Chem. 2007;282(21):15404–15. doi: 10.1074/jbc.M701638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Isenberg JS, Romeo MJ, Yu C, Yu CK, Nghiem K, Monsale J, Rick ME, Wink DA, Frazier WA, Roberts DD. Thrombospondin-1 stimulates platelet aggregation by blocking the anti-thrombotic activity of nitric oxide/cGMP signaling. Blood. 2007 doi: 10.1182/blood-2007-06-098392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bostick AC, Stoffregen DE, Johnson TE. Response of lightly and highly pigmented porcine skin (Sus scrofa domestica) to single 3.8-microm laser radiation pulses. J Am Assoc Lab Anim Sci. 2006;45(3):33–7. [PubMed] [Google Scholar]
- 47.Van Dorp AG, Verhoeven MC, Koerten HK, Van Der Nat-Van Der Meij TH, Van Blitterswijk CA, Ponec M. Dermal regeneration in full-thickness wounds in Yucatan miniature pigs using a biodegradable copolymer. Wound Repair Regen. 1998;6(6):556–68. doi: 10.1046/j.1524-475x.1998.60608.x. [DOI] [PubMed] [Google Scholar]
- 48.Russell JA, Conforti ML, Connor NP, Hartig GK. Cutaneous tissue flap viability following partial venous obstruction. Plast Reconstr Surg. 2006;117(7):2259–66. doi: 10.1097/01.prs.0000225472.57337.2e. discussion 2267–8. [DOI] [PubMed] [Google Scholar]
- 49.Harder Y, Contaldo C, Klenk J, Banic A, Jakob SM, Erni D. Improved skin flap survival after local heat preconditioning in pigs. J Surg Res. 2004;119(1):100–5. doi: 10.1016/j.jss.2003.11.002. [DOI] [PubMed] [Google Scholar]