Abstract
Epithelialization of normal acute wounds occurs by an orderly series of events whereby keratinocytes migrate, proliferate, and differentiate to restore barrier function. The keratinocytes in the epidermis of chronic ulcers fail to execute this series of events. To better understand the epithelial dynamics of chronic ulcers, we used immunohistochemistry to evaluate proliferation, differentiation, adhesion, and migration in keratinocytes along the margin of chronic ulcers from patients with diabetes mellitus. We compared these features with keratinocytes from the migrating epithelial tongues of acute incisional and excisional wounds from normal volunteers. Keratinocytes at the chronic ulcer edge are highly proliferative (Ki67 proliferation marker), have an activated phenotype (K16), do not stain for keratins involved in epidermal differentiation (K10 and K2), and show a reduced expression of LM-3A32 (uncleaved, precursor of the α3 chain of laminin 5), a key molecule present on migrating epithelium. In contrast, keratinocytes in normal acute wound migrating epithelium do not express the proliferation marker Ki67 but do express K10, K2, and LM-3A32. A better understanding of molecular mechanisms involved in keratinocyte migration may lead to molecular targets for therapies for impaired wound healing. (J Histochem Cytochem 56:687–696, 2008)
Keywords: epithelialization, immunohistochemistry, laminin 332
Chronic cutaneous ulcers in patients with diabetes mellitus are the most common cause of lower limb amputation (Pecoraro et al. 1990). Although an orderly and carefully orchestrated series of events leads to successful repair of acute wounds (Martin 1997; Singer and Clark 1999; Coulombe 2003), repair of chronic ulcers is compromised. Inability to heal chronic ulcers has been attributed to impairment of a variety of biological mechanisms: ischemia, neuropathy, infection, increased proteases, lack of protease inhibitors, prolonged inflammatory response, cytokine and growth factors deficits, senescent fibroblasts, and advanced age to name a few (Falanga 2004,2005; Mustoe 2004; Tomic-Canic et al. 2004; Medina et al. 2005). Failure to epithelialize may be the consequence of any combination of the aforementioned affected biological processes.
Basal keratinocytes at the dermal–epidermal junction (DEJ) in uninjured skin undergo differentiation to ultimately create the strong skin barrier to the external environment. The stratified layers of complex epidermis can be distinguished by expression of differentiation-specific pairs of keratin intermediate filaments (Fuchs and Weber 1994; Fuchs 1995). Interfollicular basal keratinocytes express keratins 5, 14, and 15 and are attached to the dermis at the DEJ. Expression of keratins 1, 10, and K2 in suprabasal keratinocytes is an indication of keratinocyte differentiation.
In response to acute wound injury, suprabasal keratinocytes adjacent to the site of injury and ∼60–80 cells from the wound margin begin to express keratins 6 and 16 at ∼8–24 hr after wounding (Usui et al. 2005). Keratins 6 and 16, not normally expressed in interfollicular keratinocytes, are associated with a change in keratin filament arrangement, conferring keratinocytes with a more activated phenotype (Paladini et al. 1996). Approximately 48 hr after wounding, keratinocytes in the migrating tongue of acute excisional wounds begin to downregulate expression of keratins 1, 10, and 2 (Garlick and Taichman 1994; Usui et al. 2005) while expressing K6 and 16. Normal differentiation resumes after epidermal wound closure is achieved.
As keratinocytes begin to migrate to close a wound (between 6 and 24 hr after wounding), in addition to the changes in keratin expression, leading edge keratinocytes begin to deposit LM-332 (laminin 5, epiligrin, kalinin, nicein), a key basement membrane heterotrimeric protein with α3β3γ2 chains (Carter et al. 1991; Rousselle et al. 1991; Marinkovich et al. 1992; Hamill and McLean 2005). Changes also take place in expression of transmembrane receptors (integrins) to which LM-332 ligates. Integrins function as adhesive proteins and play a role in cell signaling (Larjava et al. 1993; Watt 2002). In wounded keratinocytes, integrin α3β1 ligates with the unprocessed, precursor form of the α3 (LM-3A32) chain of the newly synthesized LM-332 to mediate keratinocyte migration (Carter et al. 1991). LM-3A32 undergoes extracellular proteolytic processing to LM-332 (Amano et al. 2000; Sigle et al. 2004) and ligates with integrin α6β4 to mediate stable anchorage of keratinocytes to the basement membrane (Mercurio et al. 2001).
In normal acute wounds, a proliferative burst of keratinocytes behind the migratory keratinocyte wound margin occurs between 24 and 72 hr after injury, with resumption to a constitutive proliferative population of ∼10% of the basal keratinocytes after wound closure (Usui et al. 2005).
We used indirect immunohistochemistry to compare the presence of adhesive-, migratory-, proliferative-, and differentiation-specific proteins in non-healing ulcers from patients with diabetes to normal acute wounds. In addition, we used in situ hybridization to evaluate the expression of the α3 chain of LM-3A32 in both the ulcers and acute wounds. Understanding key signaling and structural proteins necessary for keratinocyte migration may lead to the development of therapies to promote healing of chronic ulcers.
Materials and Methods
Tissue Samples
Specimens were obtained with approval from the University of Washington Institutional Review Board and with consent from the Research and Development Committee, Department of Veterans Affairs Puget Sound Health Care System (Seattle, WA).
Human Incisional Wounds
Simplate II bleeding-time devices (General Diagnostics; Organon Teknika, Durham, NC) were used to create uniform incisional wounds on both legs of 12 normal male and 3 normal female volunteers [mean, 66 ± 6 (SD) years of age], which is the same volunteer population described in Olerud et al. (1999). This human wound model has previously been described in detail (Olerud et al. 1995). Briefly, pairs of wounds, 5 mm long × 1 mm in depth, were created, covered with an adhesive bandage, left to heal by secondary intention (unsutured), and harvested using a 4-mm biopsy punch, from 1 to 28 days after wounding. Biopsies were frozen in OCT (FineTek; Sakura, Torrance, CA) and stored at −70C for immunohistochemistry and in situ hybridization. Site-matched unwounded skin was biopsied and served as a control. A total of 9–14 wound samples were collected for each time point.
Human Excisional Wounds
A 3-mm punch biopsy tool was used to create wounds on the leg or arm of three normal volunteers (mean, 55 ± 5 years of age), covered with an adhesive bandage, and left to heal by secondary intention. Wounds were harvested at 1, 2, and 7 days after wounding using a 6-mm punch biopsy tool. Wound specimens were processed similarly to the incisional wounds.
Ulcers From Patients With Diabetes
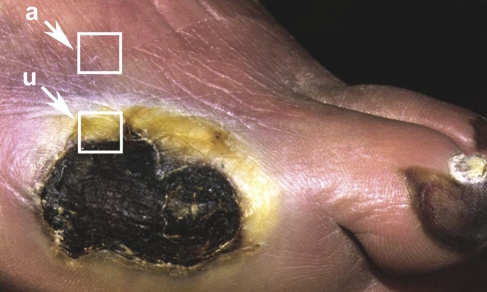
Thirteen patients with diabetes scheduled to undergo lower extremity amputation for chronic ulcers agreed to allow tissue retrieval after amputation. All patients had adult onset diabetes and were treated with insulin. Their mean age was 65 ± 10 years. The mean hemoglobin (Hb)A1c percent was 9.6 ± 5. All patients had an absence of protective sensation as shown by an inability to feel a 5.07 Semmes-Weinstein filament. Full-thickness samples of skin were taken from the ulcer margin [ulcer (u)] (Figure 1), from 1 cm away from, but adjacent to, the ulcer [adjacent (a)], and from skin on the proximal lateral leg, 10–15 cm distal to the patella (proximal). Ulcer, adjacent, and proximal tissue were processed similarly to the incisional wounds.
Figure 1.
Chronic ulcer on the foot of a patient with diabetes. Tissue samples were taken from the ulcer (u) and ∼1 cm adjacent to the ulcer edge (a).
Immunohistochemistry
Six-μm frozen sections of incisional and excisional wounds and tissue from patients with diabetes were immunolabeled using indirect horseradish peroxidase techniques as previously described (Usui et al. 2005). Briefly, tissue sections were postfixed in cold acetone or postfixed with 2% paraformaldehyde/Sorensons buffer, rinsed in TBS, and blocked with 0.3% H2O2 for 30 min. All sections were blocked with 1.3% goat serum/TBS for 30 min. Primary antibodies used were mouse monoclonal keratin 14 (K14; dilution 1:250, gift from Irwin McLean/Irene Leigh, Dundee, UK), mouse monoclonal keratin 10 (K10; dilution 1:100; Dako, Carpentaria, CA), rabbit polyclonal keratin 2 (K2; dilution 1:2000, gift from Irwin McLean/Irene Leigh), mouse monoclonal keratin 16 (K16; dilution 1:500; NeoMarker, Fremont, CA), proliferation marker: mouse monoclonal Ki67 (dilution 1:100; Novocastra, Burlingame, CA), mouse monoclonal integrins α3 subunit and β4 subunit (dilutions 1:4 and 1:25, respectively; William Carter, Seattle, WA), mouse monoclonal LM-3A32 (precursor laminin 5), and LM-332 (dilutions 1:4 and 1:10, respectively; William Carter). Diluent for all antibodies was BSA/TBS at 1 mg/ml. Sections were incubated with primary antibody for 1 hr, rinsed in TBS, and incubated for 30 min with species-specific secondary antibodies: biotinylated goat anti-rabbit at 1:300 (Vector; Burlingame, CA) or biotinylated goat anti-mouse at 1:200 (Vector). Sections were incubated with streptavidin (SABC kit; Vector) at 1:50 for 30 min, followed by 0.12% DAB as chromogen and Glycergel as mounting media (Dako). All incubations were conducted at room temperature in a humidified chamber. Control sections were incubated without primary antibody. Some tissue sections were counterstained with hematoxylin stain (Gill 2; Ricca Chemical Co., Arlington, TX).
In Situ Hybridization
The 600-bp laminin α3 chain cDNA fragment was cloned (Ryan et al. 1994) into Bluescript expression vector pBS II SK+. Antisense laminin cRNA probes were prepared by linearizing the plasmid 7-5-4 with BamHI and transcribing with RNA polymerase T7 in the presence of digoxigenen-11 uridine triphosphate (UTP). Sense laminin cRNA probes were prepared by linearizing the plasmid 7-5-4 with HindIII and transcribing with RNA polymerase T3 in the presence of digoxigenen-11 UTP. Eight-μm frozen sections from unfixed biopsies were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) and processed using standard non-radiolabeled in situ procedures. Reactivity was visualized using alkaline phosphatase.
Photomicrography
All sections were viewed on a Nikon Microphot-SA microscope using either standard brightfield (BF) or differential interference contrast. Image acquisition was controlled through IP Lab Spectrum software (Scanalytics; Fairfax, VA) running on a PowerMac G4 dual processor computer. For BF, the separate 12-bit grayscale images captured through red, green, and blue filters were merged and saved as 24-bit color PICT files. Photoshop (Adobe Systems; San Jose, CA) was used for image color adjustment.
Results
Clinical Assessment
There was vast heterogeneity among ulcers from patients with diabetes. Some ulcers showed no sign of infection and were desiccated, whereas others were gangrenous and fluid filled. Thickness of ulcer edge epidermis ranged from normal (6–10 cell layers) to very hyperplastic (20–40 cell layers). Documentation of dates and frequency of treatments, such as debridement, was not recorded for the chronic wound tissue harvested for this study and may account for some of the variability of results, although once the decision for amputation is made, very little intervention typically occurs in the days immediately preceding amputation.
Immunohistochemistry
All of the samples from the 13 patients were not tested with all of the antibodies. A summary of numbers of samples tested for each antibody and qualitative evaluation of immunohistochemical data are listed in Table 1.
Table 1.
Qualitative analysis of histochemical results from normal subjects and ulcers from patients with diabetes
| One-day normal
|
Two-day normal
|
Three-day normal
|
Seven-day normal
|
|||||
|---|---|---|---|---|---|---|---|---|
| Antibody | Non-wounded | Incisional | Excisional | Excisional | Incisional | Incisional | Excisional | Ulcer |
| Ki67 | 14/14 = <10% (+) | 7/7 = <10% | 1/1 = <10% | 1/1 = ∼55% (+) behind migrating tongue | 7/7 = ∼80% adjacent to wound bed | 8/8 = ∼80% adjacent to wound bed | 2/2 = ∼80% adjacent to wound bed | 8/8 = ∼80–90% ulcer edge |
| K14 | 2/2 = +3a | 3/3 = +3ab | 1/1 = +3ab | 1/1/ = +3ab | 4/4 = +3ab | 4/4 = +3ab | 2/2 = +3ab | 4/4 = +3ab |
| K10 | 3/3 = +3c | 5/5 = +3 | 1/1 = +3 | 1/1 = +3 | 6/6 = +3 | 5/5 = +3 | 2/2 = 0d | 4/4 = 0d |
| K2 | 2/2 = +3c | 3/3 = +3 | 1/1 = +2 | 1/1 = +2 | 2/2 = 0d | 2/2 = +3 | 2/2 = 0d | 2/2 = 0d |
| K16 | 5/5 = 0c | 5/5 = +3 | 1/1 = +3 | 1/1 = +3 | 4/4 = +3ab | 4/4 = +3ab | 2/2 = +3ab | 4/4 = +3ab |
| α3 integrin | 2/2 = +3a | 4/4 = +3ab | 1/1 = +3ab | 1/1 = +3ab | 1/1 = +3ab | 1/1 = +3a | 2/2 = +3ab | 6/6 = +3ab |
| β4 integrin | 3/3 = +3a | 5/5 = +3ab | 1/1 = +3ab | 1/1 = +3ab | 2/2 = +3a | 1/1 = +3a | 2/2 = +3 | 7/7 = +3a, 6/7 = +3ab |
| LM 3A32 | 3/3 = 0c | 3/3 = +3 | 1/1 = +3 | 1/1 = +3 | 3/3 = +3 | 3/3 = +3 | 2/2 = +3 | 1 = +3, 2 = +2, 1 = +1, 2 = 0 |
| LM 332 | 4/4 = +3 | 4/4 = +3 | 1/1 = +3 | 1/1 = +3 | 2/2 = +3 | 3/3 = +3 | 2/2 = +3 | 6/6 = +3 |
Basal keratinocytes.
Suprabasal keratinocytes.
Normal.
Central region of wound epithelium.
Columns indicate number of specimens qualitatively evaluated. 0, no staining; +1, faint staining; +2, moderate staining; +3, strong staining. Bold italic text indicates results differing from normal acute incisional wound.
Cell Proliferation (Ki67)
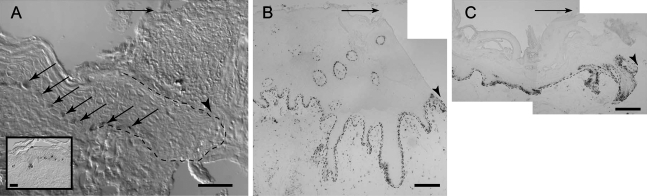
Approximately 10% of interfollicular basal keratinocytes in unwounded skin showed Ki67 immunolabeling (Figure 2A, inset). Basal keratinocytes behind the migrating began proliferating 1 day after wounding; however, the basal keratinocytes in the migrating tongue do not show an increase in proliferation (Figure 2A). In contrast, all ulcer tissue showed increased numbers of Ki67 immunostained basal keratinocytes, not only at the margin of the ulcers (Figures 2B and 2C) but in the tissue adjacent to the ulcer margin (data not shown). Tissue proximal from the ulcer edge showed an immunostaining pattern that correlated with unwounded normal skin (data not shown).
Figure 2.
Keratinocyte proliferation. Tissue sections of normal, unwounded skin (A, inset), normal 2-day excisional wound (A), and ulcer tissue from two patients with diabetes (B,C). Approximately 10% of the basal keratinocytes population immunostain with Ki67. Ki67 immunostaining is absent in the actively migrating tongue (outlined by the dotted line) of a normal, 2-day excisional acute wound; however, it is present in basal keratinocytes in the epidermal region behind the migrating tongue. Tissue sections of ulcers from two patients with diabetes shows strong numbers of Ki67-immunolabeled keratinocytes at the margin of the ulcer edge and a great distance from the ulcer edge (B,C). Top arrow indicates direction of epidermal migration, small arrows in A indicate Ki67-immunostained keratinocytes, and arrowheads in A–C indicate migrating tongue. Bars: A = 50 μm; B,C = 100 μm.
Keratins 14, 10, 2, and 16
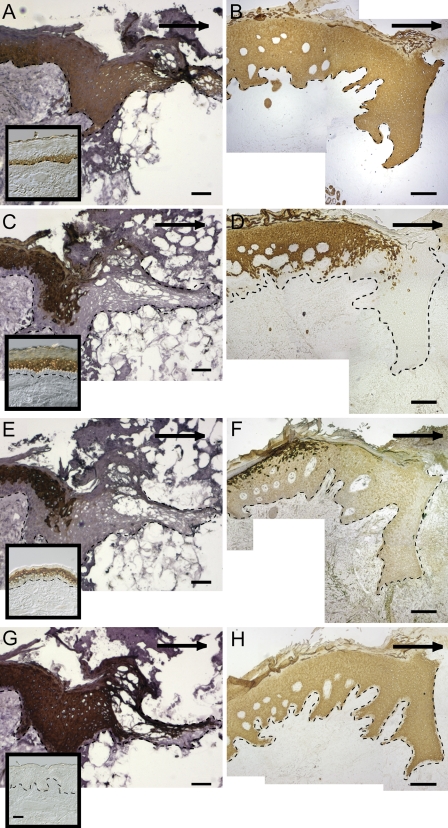
In the unwounded epidermis, K14 was localized in only basal keratinocytes (Figure 3A, inset), whereas both the 7-day acute excisional wound (Figure 3A) and the ulcer sample (Figure 3B) showed K14 immunostaining throughout the entire epidermis. In the unwounded epidermis, K10 was localized in only suprabasal keratinocytes (Figure 3C, inset). K10 was not found in the migrating tongue of the 7-day acute excisional wound (Figure 3C) or in the margin of the ulcer section (Figure 3D). In unwounded skin, K2 was localized in the upper suprabasal layer of the epidermis (Figure 3E, inset). K2 was absent in the migrating tongue of the 7-day acute excisional wound (Figure 3E) and absent in the wound margin of tissue from the ulcer section (Figure 3F). K16, not present in interfollicular normal skin (Figure 3G, inset), was found in all suprabasal keratinocytes and in a few basal keratinocytes in the migrating tongue of the 7-day acute excisional wound (Figure 3G) and in all suprabasal keratinocytes and in some basal keratinocytes the ulcer tissue (Figure 3H).
Figure 3.
Keratin expression in tissue sections of a normal acute 7-day excisional wound (A,C,E,G) and ulcer (B,D,F,H). K14 is found in only basal keratinocytes in unwounded skin (A, inset) and throughout the entire epidermis of the normal acute 7-day excisional migrating tongue and tissue proximal to the tongue (A), as well as the ulcer edge and tissue proximal to the ulcer (B). K10 is found in only suprabasal keratinocytes in unwounded skin (C, inset) and is not found in the migrating tongue of the normal acute 7-day excisional wound (C) or the ulcer margin (D). K2 is found in the upper suprabasal layer of the epidermis of unwounded skin (E, inset) and is not found in the migrating tongue of the normal acute 7-day excisional wound (E) or the ulcer margin (F). K16, not present in interfollicular normal skin (G, inset), is found throughout the suprabasal layers of the migrating tongue of the normal acute 7-day excisional wound (G) and the ulcer margin (H). Dotted line indicates dermal–epidermal junction (DEJ) and arrows indicate direction of migration. Bar = 100 μm.
LM-332
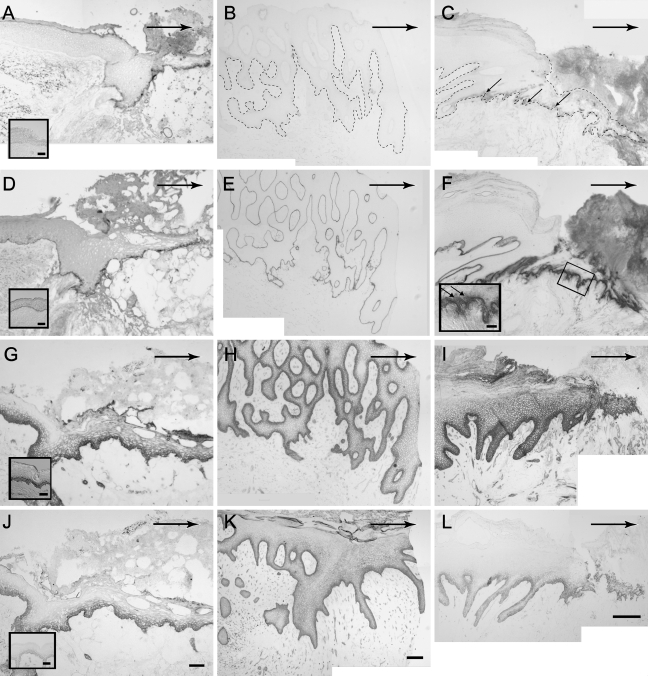
Immunostaining with LM-3A32 (full-length α3 chain of LM 332) antibody showed no staining in the unwounded epidermis (Figure 4A, inset) but strongly stained the entire migrating tongue of a 7-day excisional wound (Figure 4A). Immunostaining with LM-3A32 antibody in ulcer tissue ranged from absent (Figure 4B) to moderate (Figure 4C). None of the staining intensity for ulcer tissue reached levels found in normal acute wounds. Immunostaining with the LM-332 antibody showed a linear pattern along the DEJ in the acute 7-day excisional wound (Figure 4D) and in all ulcer tissue (Figures 4E and 4F).
Figure 4.
LM 332 and integrin expression in tissue sections of a 7-day normal acute excisional wound (A,D,G,J) and tissue sections of ulcers from patient with diabetes 1 (B,E,H,K) and patient with diabetes 2 (C,F,I,L). Immunostaining of LM 3A32 is present in the excisional wound tongue (A), absent in the margin of ulcer tissue (B), and weakly present in ulcer tissue (C). Inset in A shows absence of LM 3A32 staining in normal epidermis. Small arrows indicate LM 3A32 immunostaining. Dotted line indicates DEJ. LM 332 is present along the DEJ of the migrating tongue of the normal 7-day excisional wound (D) and in both examples of ulcer margins (E,F). Inset in D shows LM 332 staining in normal epidermis. LM 332 is found intracellularly in keratinocytes along the margin of ulcer 2 (F, inset). Immunostaining of integrin α3 is restricted to the basal layer in normal interfollicular epidermis (G, inset). Immunostaining of integrin α3 can be seen in basal and suprabasal keratinocytes along the DEJ of the 7-day excisional wound margins (G). Both basal and suprabasal keratinocytes stain for α3 integrin in ulcer samples 1 and 2 (H,I). Immunostaining of integrin β4 is restricted to basal keratinocytes in the interfollicular epidermis (J, inset) and can be seen in basal keratinocytes along the DEJ and a few suprabasal keratinocytes of the 7-day excisional wound margin (J). Immunostaining for integrin β4 in ulcer 1 is stronger than in ulcer 2 (K,L). Large arrows indicate direction of migration. Bars: panels = 100 μm; insets = 50 μm.
Integrin α3 Subunit
The integrin α3 subunit was restricted to basal keratinocytes in unwounded skin (Figure 4G, inset) and was both basal and suprabasal in the migrating tongue of an acute 7-day excisional wound (Figure 4G). All ulcer tissue showed strong membranous integrin α3 immunostaining in basal and suprabasal keratinocytes (Figures 4H and 4I).
Integrin β4 Subunit
In normal, unwounded skin, the integrin β4 subunit was restricted to basal keratinocytes (Figure 4J, inset). In the 7-day acute excisional wound, integrin β4 immunostaining was found in both basal and suprabasal keratinocytes (Figure 4J). All ulcer tissue showed strong membranous integrin β4 immunostaining in basal keratinocytes and variable suprabasal keratinocyte immunostaining (Figures 4K and 4L).
In Situ Hybridization
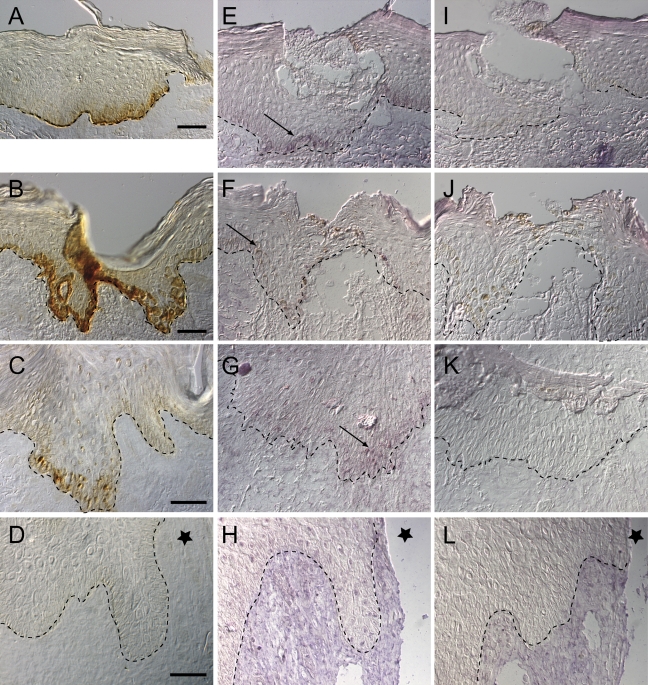
Immunostaining with the antibody to LM-3A32 was strong in keratinocytes found in the migrating tongue of 1-, 3-, and 7-day acute wounds as shown in Figures 5A–5C, respectively, whereas it was absent in the ulcer margin Figure 5D (also in Figure 4B, low magnification). Using the antisense LM-3A32 cRNA probe, LM-3A32 mRNA was detected in keratinocytes along the migrating tongue of acute 1- and 3-day incisional wounds (Figures 5E and 5F) and in a few basal keratinocytes along the DEJ of the 7-day closed wound (Figure 5G). LM-3A32 mRNA, however, was absent in the migrating edge of the ulcer tissue section from a patient with diabetes (Figure 5H), as well as absent in the adjacent and proximal tissue (data not shown). Tissue hybridized with the sense probe served as controls (Figures 5I–5L).
Figure 5.
LM 3A32 immunohistochemistry and in situ hybridization in normal acute incisional wounds of 1 day (A,E,I), 3 days (B,F,J), 7 days (C,G,K), and ulcer (D,H,L). Immunostaining with LM 3A32 antibody shows staining of the 1-, 3-, and 7-day acute incisional wounds (A–C) but not in the ulcer (D). LM 3A32 mRNA using a digoxigenin-labeled LM 3A32 RNA antisense probe is present on the migrating epithelia of 1-, 3-, and 7-day acute incisional wounds (E–G) and not present in the ulcer margin tissue from a patient with diabetes (H). Arrows indicate presence of LM 3A325 mRNA. In situ hybridization using sense probes (I–L) served as controls. Dotted line indicates DEJ and star indicates ulcer margin.
Discussion
In agreement with studies of venous ulcers conducted by Andriessen et al. (1995), our results showed that the epidermis of ulcer margins from patients with diabetes is highly proliferative, no matter the thickness of the ulcer edge. The epidermis adjacent to the ulcer (1 cm), with no gross indications of being affected by the ulcer, is also highly proliferative. Signals initiating the keratinocyte activation cycle (Ki67) (Freedberg et al. 2001) seem to result in sustained proliferation in the epidermis even a great distance from the ulcer margin. Studies by Natarajan et al. (2003,2006) showed that p16 (a cell cycle protein that suppresses G1-Cdk activity in G1) is activated in migrating keratinocytes, coexpressed with a precursor γ2 chain of LM-332, and is associated with growth arrest. In the absence of p16, transcription for genes encoding proteins necessary to initiate chromosome replication takes place. Keratinocytes that express p16 are migratory and not mitotic. Their studies excluded evaluating tissue from patients with diabetes; thus, it has yet to be determined whether the epidermal margins of patients with diabetes lack p16 expression. Although proliferation of keratinocytes is necessary to fill the defect, the lack of appropriate signals to downregulate proliferation and allow migration may delay wound closure.
The immunostaining patterns for keratins K14, K10, and K2 are strikingly different between chronic ulcer margin and normal acute incisional wound epithelium. In acute incisional wounds, K14 staining in a 1-day wound is limited to the basal cell layer and a few suprabasal cells immediately in contact with the wound matrix. By 2 days after wounding, the entire new acute wound epithelium stains for K14, and this staining pattern persists for ∼14 days after wounding. By 28 days after wounding, K14 immunostaining is present only in the basal keratinocytes found along the DEJ (Usui et al. 2005). All keratinocytes layers of the chronic wound tongue stained with K14 similarly to 2- and 7-day excisional wound keratinocytes. K10 and K2 immunostaining is present in the migrating wound tongue of acute normal wounds for the first 48 hr, but if the migrating tongue has a longer path than can be closed in 48–72 hr (as seen in excisional normal wounds), K10 and K2 immunostaining is no longer present in the central portion of the new wound epithelium (Usui et al. 2005). This pattern is similar to the immunostaining seen in chronic ulcer margins, where K10 and K2 are notably absent. K16 staining is seen within hours of wounding in acute incisional wounds and is seen during the activation period of epidermal repair for at least 28 days after wound closure (Usui et al. 2005). In chronic ulcer margins and in apparently normal tissue adjacent (1 cm proximal) to the ulcer, K16 is dramatically stained throughout the epidermis. Despite the presence of K16, an activated, migratory keratinocyte phenotype, keratinocytes at the ulcer margin are not activated to migrate. Although our immunohistochemical studies indicated that all of the samples tested with a K16 antibody showed intense staining equivalent to the migrating tongue of acute wounds, our results are in contrast to that found by Stojadinovic et al. (2005), in which they found a significant reduction of K6 (partner of K16) in the chronic wound margin. This discrepancy could be caused by differences in timing of patient therapy (i.e., debridement), type of ulcer (venous stasis, decubitus, etc.), tissue preparation, or staining technique. Studies by Paladini and Coulombe (1999) indicated that there is not a straightforward correlation between regulation of K16 mRNA and K16 protein expression. Perhaps the strong expression of K16 in the ulcer tissue results ultimately in hyperproliferation (Takahashi et al. 1994). The hyperproliferation we see in the diabetic ulcer tissue may impact keratinocyte migration.
Studies have shown that when LM-3A32 (Ryan et al. 1999) binds to α3β1 integrin, keratinocytes are activated to migrate. Recent studies have shown that Rac activator Tiam1 (T-lymphomas invasion and metastasis 1) is required for α3β1-mediated LM-332 deposition and cell migration (Hamelers et al. 2005). Our studies showed that LM-3A32 is strongly present in keratinocytes along the margin of normal acute wounds; however, in ulcers, LM-3A32 is often absent, or at best, moderately present. One possible explanation for variability of staining of LM-3A32 may be related to ulcer debridement as a clinical intervention before amputation. Debridement may create an acute wound along the ulcer margin and “re”-stimulate keratinocytes to produce new LM-332. Because clinical information regarding debridement of ulcers was not documented for our ulcer tissue library, we cannot infer that there is a correlation between staining variability of LM-3A32 and debridement. Based on our immunohistochemical results, integrin α3β1 that ligates with LM-3A32 is present in ulcer keratinocytes; therefore, we hypothesize that failure of keratinocytes to migrate may be the result of failure of keratinocytes to actively sustain LM-3A32 production or that LM-3A32 is quickly cleaved by proteases, because LM-332 is present along the entire basement membrane of ulcers. It seems that keratinocytes in the ulcer are capable of producing, depositing, and processing precursor forms of LM-332 to establish a basement membrane. Because there is strong immunostaining for integrin α6β4 in ulcers, deposited and processed LM-332 can bind to integrin α6β4 for strong anchorage. Our in situ data showed the presence of mRNA of LM-3A32 in the in the 1- to 7-day normal incisional wounds but not in the ulcer from the patient with diabetes. With the heterogeneity of the ulcer tissue specimens, it is difficult to make conclusions as to why there is an absence of LM-3A32 message in ulcer margins. Factors such as age of the ulcer or lack of debridement may play a role in reduction of LM-3A32 message. Studies in our laboratory in which acute incisional wounds were created in patients with diabetes showed presence of LM-3A32 in concurrence with the acute incisional wounds created in normal volunteers (data not shown). Because our data indicate that small wounds created in patients with diabetes express LM-3A32, therapeutic debridement (Hurvitz et al. 2004) of the ulcer edge may serve to reactivate LM 332 production by creating an acute wound.
In summary, keratinocytes at the margins of ulcers are highly proliferative, show an activated phenotype as indicated by presence of K16, have not yet initiated differentiation (K10 and K2 absent), and fail to migrate. We found a reduction in LM-3A32, a conformation necessary for keratinocyte migration. Insulin has been shown to regulate expression, deposition, and adhesion of LM-3A32 (Gil et al. 2002). Because patients with diabetes are insulin deficient or insulin resistant, the resultant reduction in production of LM-3A32 may be a factor in the failure of keratinocyte migration. A better understanding of molecular mechanisms involved in keratinocyte migration may lead to molecular targets for therapies for impaired wound healing.
Acknowledgments
This study was funded by National Institutes of Health (NIH) Grant DK-59221, NIH Grant CA-49259, National Science Foundation Grant EEC-959161, George F. Odland Endowed Research Funds, and Smith and Nephew/Advanced Tissue Sciences (ATS).
The technical support of Lara Muffley and generous donation of antibodies from Drs. Irwin McLean and Irene Leigh are gratefully acknowledged.
References
- Amano S, Scott IC, Takahara K, Koch M, Champliaud MF, Gerecke DR, Keene DR, et al. (2000) Bone morphogenetic protein 1 is an extracellular processing enzyme of the laminin 5 gamma 2 chain. J Biol Chem 275:22728–22735 [DOI] [PubMed] [Google Scholar]
- Andriessen MP, van Bergen BH, Spruijt KI, Go IH, Schalkwijk J, van de Kerkhof PC (1995) Epidermal proliferation is not impaired in chronic venous ulcers. Acta Derm Venereol 75:459–462 [DOI] [PubMed] [Google Scholar]
- Carter WG, Ryan MC, Gahr PJ (1991) Epiligrin, a new cell adhesion ligand for integrin alpha 3 beta 1 in epithelial basement membranes. Cell 65:599–610 [DOI] [PubMed] [Google Scholar]
- Coulombe PA (2003) Wound epithelialization: accelerating the pace of discovery. J Invest Dermatol 121:219–230 [DOI] [PubMed] [Google Scholar]
- Falanga V (2004) The chronic wound: impaired healing and solutions in the context of wound bed preparation. Blood Cells Mol Dis 32:88–94 [DOI] [PubMed] [Google Scholar]
- Falanga V (2005) Wound healing and its impairment in the diabetic foot. Lancet 366:1736–1743 [DOI] [PubMed] [Google Scholar]
- Freedberg IM, Tomic-Canic M, Komine M, Blumenberg M (2001) Keratins and the keratinocyte activation cycle. J Invest Dermatol 116:633–640 [DOI] [PubMed] [Google Scholar]
- Fuchs E (1995) Keratins and the skin. Annu Rev Cell Dev Biol 11:123–153 [DOI] [PubMed] [Google Scholar]
- Fuchs E, Weber K (1994) Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem 63:345–382 [DOI] [PubMed] [Google Scholar]
- Garlick JA, Taichman LB (1994) Fate of human keratinocytes during reepithelialization in an organotypic culture model. Lab Invest 70:916–924 [PubMed] [Google Scholar]
- Gil SG, Sigle RO, Carter WG (2002) Detection and purification of instructive extracellular matrix components with monoclonal antibody technologies. Methods Cell Biol 69:27–52 [DOI] [PubMed] [Google Scholar]
- Hamelers IH, Olivo C, Mertens AE, Pegtel DM, van der Kammen RA, Sonnenberg A, Collard JG (2005) The Rac activator Tiam1 is required for (alpha)3(beta)1-mediated laminin-5 deposition, cell spreading, and cell migration. J Cell Biol 171:871–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill KJ, McLean WH (2005) The alpha-3 polypeptide chain of laminin 5: insight into wound healing responses from the study of genodermatoses. Clin Exp Dermatol 30:398–404 [DOI] [PubMed] [Google Scholar]
- Hurvitz G, Zalavras C, Thordarson DB (2004) Debridement and primary closure of nonhealing foot wounds. Am J Orthop 33:507–509 [PubMed] [Google Scholar]
- Larjava H, Salo T, Haapasalmi K, Kramer RH, Heino J (1993) Expression of integrins and basement membrane components by wound keratinocytes. J Clin Invest 92:1425–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovich MP, Lunstrum GP, Burgeson RE (1992) The anchoring filament protein kalinin is synthesized and secreted as a high molecular weight precursor. J Biol Chem 267:17900–17906 [PubMed] [Google Scholar]
- Martin P (1997) Wound healing: aiming for perfect skin regeneration. Science 276:75–81 [DOI] [PubMed] [Google Scholar]
- Medina A, Scott PG, Ghahary A, Tredget EE (2005) Pathophysiology of chronic nonhealing wounds. J Burn Care Rehabil 26:306–319 [DOI] [PubMed] [Google Scholar]
- Mercurio AM, Rabinovitz I, Shaw LM (2001) The alpha 6 beta 4 integrin and epithelial cell migration. Curr Opin Cell Biol 13:541–545 [DOI] [PubMed] [Google Scholar]
- Mustoe T (2004) Understanding chronic wounds: a unifying hypothesis on their pathogenesis and implications for therapy. Am J Surg 187:65S–70S [DOI] [PubMed] [Google Scholar]
- Natarajan E, Omobono JD 2nd, Guo Z, Hopkinson S, Lazar AJ, Brenn T, Jones JC, et al. (2006) A keratinocyte hypermotility/growth-arrest response involving laminin 5 and p16INK4A activated in wound healing and senescence. Am J Pathol 168: 1821–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan E, Saeb M, Crum CP, Woo SB, McKee PH, Rheinwald JG (2003) Co-expression of p16(INK4A) and laminin 5 gamma2 by microinvasive and superficial squamous cell carcinomas in vivo and by migrating wound and senescent keratinocytes in culture. Am J Pathol 163:477–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olerud JE, Odland GF, Burgess EM, Wyss CR, Fisher LD, Matsen FA 3rd (1995) A model for the study of wounds in normal elderly adults and patients with peripheral vascular disease or diabetes mellitus. J Surg Res 59:349–360 [DOI] [PubMed] [Google Scholar]
- Olerud JE, Usui ML, Seckin D, Chiu DS, Haycox CL, Song IS, Ansel JC, et al. (1999) Neutral endopeptidase expression and distribution in human skin and wounds. J Invest Dermatol 112:873–881 [DOI] [PubMed] [Google Scholar]
- Paladini RD, Coulombe PA (1999) The functional diversity of epidermal keratins revealed by the partial rescue of the keratin 14 null phenotype by keratin 16. J Cell Biol 146:1185–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladini RD, Takahashi K, Bravo NS, Coulombe PA (1996) Onset of re-epithelialization after skin injury correlates with a reorganization of keratin filaments in wound edge keratinocytes: defining a potential role for keratin 16. J Cell Biol 132:381–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecoraro RE, Reiber GE, Burgess EM (1990) Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care 13:513–521 [DOI] [PubMed] [Google Scholar]
- Rousselle P, Lunstrum GP, Keene DR, Burgeson RE (1991) Kalinin: an epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J Cell Biol 114:567–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MC, Lee K, Miyashita Y, Carter WG (1999) Targeted disruption of the LAMA3 gene in mice reveals abnormalities in survival and late stage differentiation of epithelial cells. J Cell Biol 145:1309–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MC, Tizard R, VanDevanter DR, Carter WG (1994) Cloning of the LamA3 gene encoding the alpha 3 chain of the adhesive ligand epiligrin. Expression in wound repair. J Biol Chem 269:22779–22787 [PubMed] [Google Scholar]
- Sigle RO, Gil SG, Bhattacharya M, Ryan MC, Yang TM, Brown TA, Boutaud A, et al. (2004) Globular domains 4/5 of the laminin alpha3 chain mediate deposition of precursor laminin 5. J Cell Sci 117:4481–4494 [DOI] [PubMed] [Google Scholar]
- Singer AJ, Clark RA (1999) Cutaneous wound healing. N Engl J Med 341:738–746 [DOI] [PubMed] [Google Scholar]
- Stojadinovic O, Brem H, Vouthounis C, Lee B, Fallon J, Stallcup M, Merchant A, et al. (2005) Molecular pathogenesis of chronic wounds: the role of beta-catenin and c-myc in the inhibition of epithelialization and wound healing. Am J Pathol 167:59–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Folmer J, Coulombe PA (1994) Increased expression of keratin 16 causes anomalies in cytoarchitecture and keratinization in transgenic mouse skin. J Cell Biol 127:505–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomic-Canic M, Agren MS, Alvarez OM (2004) Epidermal Repair and the Chronic Wound. 1st ed. Boca Raton, FL, CRC Press
- Usui ML, Underwood RA, Mansbridge JN, Muffley LA, Carter WG, Olerud JE (2005) Morphological evidence for the role of suprabasal keratinocytes in wound reepithelialization. Wound Repair Regen 13:468–479 [DOI] [PubMed] [Google Scholar]
- Watt FM (2002) Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J 21:3919–3926 [DOI] [PMC free article] [PubMed] [Google Scholar]