Abstract
Enhanced green fluorescent protein (GFP) irreversibly loses not only fluorescence but also antigenicity recognized with conventional anti-GFP antibodies by heat denaturation. This hinders combinatory applications of the GFP immunodetection technique with heat-requiring procedures, such as in situ hybridization histochemistry, antigen retrieval, and Western blot. Here we produced new rabbit and guinea pig antibodies against heat-denatured GFP. The polyclonal antibodies affinity-purified with the antigen column detected a single band corresponding to the molecular size of GFP in Western blot analysis, with mouse brain expressing GFP from the GAD67 locus. By immunofluorescence labeling, the new antibodies detected GFP molecules in heat (≥70C)-treated sections but not in untreated sections of the mouse brain. When the sections were incubated at ≥37C with in situ hybridization buffer containing 50% formamide, a denaturing reagent, the sections lost immunoreactivity with the conventional anti-GFP antibodies but acquired immunoreactivity with the new antibodies to heat-denatured GFP. Finally, GFP immunofluorescence was successfully visualized with the new antibodies in sections of the GFP-expressing mice labeled by fluorescence in situ hybridization histochemistry against GAD67 mRNA. Thus, the antibodies produced in this study may provide an opportunity to combine GFP immunodetection with procedures requiring heat treatment. This manuscript contains online supplemental material at http://www.jhc.org. Please visit this article online to view these materials. (J Histochem Cytochem 56:647–657, 2008)
Keywords: immunofluorescence, in situ hybridization, fluorescence microscopy, antigen retrieval, Western blot
Heat treatment of tissue sections and cultured cells is often required in cytochemical and histochemical detection of nucleic acids and proteins. In situ hybridization histochemistry usually requires heat treatment and denaturing reagents for effective hybridization of nucleic acid probes with tissue mRNA (for review, see Darby et al. 2006). In immunohistochemistry, heat treatment as an antigen retrieval technique is sometimes needed before immunolabeling (Shi et al. 1991). For instance, the detection of 5-bromo-2-deoxyuridine (BrdU), which is administered for labeling of newly synthesized DNA in many developmental studies, requires heat treatment (Moran et al. 1985; Beisker et al. 1987; Van Furth and Van Zwet 1988). Presumably, the epitope highly packed in the nuclear DNA is inaccessible to the antibodies and only exposed by heat denaturation of DNA and nuclear proteins. Detection of some nuclear proteins, such as estrogen receptor, proliferative cell nuclear antigen (PCNA), and Ki-67, also requires heat treatment for unmasking of their epitopes (Shi et al. 1995). Furthermore, immunoreactivity for some non-nuclear proteins is known to be enhanced by heat treatment (Jiao et al. 1999; Ino 2003; Nakamura et al. 2005), because the epitopes would be better exposed by heat denaturation of the protein than in the native form.
Enhanced green fluorescent protein (GFP) is a derivative of green fluorescent protein derived from the jellyfish Aequorea victoria (Cormack et al. 1996; Zhang et al. 1996). Because GFP emits bright green fluorescence without any exogenous substrates or cofactors (Cormack et al. 1996), it has widely been used in biological studies to monitor gene expression and protein localization in many kinds of model organisms, including mice (Okabe et al. 1997; for review, see Chalfie and Kain 2005). However, those GFP-based technologies have a pitfall in that they are incompatible with the histological methods requiring heat treatment, such as antigen retrieval and in situ hybridization histochemistry. In fact, heat treatment irreversibly denatures GFP to obliterate its fluorescence (Bokman and Ward 1981; Ward 1981; Ward and Bokman 1982) and antigenicity recognized by conventional anti-GFP antibodies (unpublished data). In particular, studies on neurogenesis often require visualization of BrdU or Ki-67, and antigen retrieval by heat treatment is indispensable for BrdU and Ki-67 immunolabeling. There are also increasing demands for in situ characterization of mRNAs expressed in GFP-labeled cells, because many transgenic animals expressing GFP have been produced. However, these analyses have been hindered by the loss of antigenicity caused by heat denaturation of GFP. Thus, a new antibody that recognizes heat-denatured GFP is desired to circumvent this problem.
In this study, we immunized rabbits and guinea pigs with heat-denatured GFP. The newly obtained polyclonal antibodies affinity-purified with the antigen column were characterized by Western blot analysis and preabsorption tests of immunofluorescence labeling using the brain tissue of GFP-expressing mice in comparison with the polyclonal antibodies raised against native GFP (Tamamaki et al. 2000). We further examined temperature dependence of GFP epitopes in the tissue with the antibodies to heat-denatured GFP and native GFP. Finally, the new antibodies were applied to combined labeling of GFP-immunoreactive neurons in the tissue with fluorescence in situ hybridization histochemistry against endogenous mRNA.
Materials and Methods
Animals
The experiments were conducted in accordance with the rules of animal care by the Institute of Laboratory Animals, Graduate School of Medicine, Kyoto University (Kyoto, Japan). For production of antibodies, two female rabbits (Japanese White, 2-kg body weight; SLC, Shizuoka, Japan) and four female guinea pigs (200-g body weight; SLC) were used. For Western blot analysis and histochemical characterization of antibodies, seven adult male ΔNeo strain (a strain lacking the Neomycin resistance gene) of GAD67-GFP knock-in mice, whose GABAergic neurons express GFP (Tamamaki et al. 2003), and two adult male C57BL6/J mice (SLC) were used in this study. All efforts were made to minimize animal suffering and the number of animals used.
Affinity-purified Polyclonal Antibodies to GFP
For the production of antibodies to heat-denatured GFP, the full-length coding sequence of GFP was amplified by PCR using the plasmid pEGFP-N2 (GenBank accession number U57608.1; Clontech Laboratories, Mountain View, CA) as a template. Primer sequences used for the PCR were 5′–TTACTTGTACAGCTCGTCCATG–3′ and 5′–ATGGTGAGCAAGGGCGAGGA–3′. The blunt-ended PCR product was cloned into pGEX-4T2 (GenBank accession number U13854; GE Healthcare Bio-Sciences, Piscataway, NJ) at the SmaI site. Glutathione-S-transferase(GST)–GFP fusion protein was induced in Escherichia coli by adding isopropyl-β-d-thiogalactopyranoside (IPTG) to the medium. After GST was cleaved by thrombin protease, GFP was purified according to the protocol by the manufacturer (GE Healthcare Bio-Sciences). The purified GFP was treated at 100C for 30 min in 5 mM PBS 0.9% (w/v), pH 7.4, emulsified with Freund's complete adjuvant (Difco; BD Biosciences, San Jose, CA), and injected intradermally into female rabbits (0.8 mg/animal) and guinea pigs (0.2 mg/animal). Four weeks after immunization, the same amount of GFP emulsified with Freund's incomplete adjuvant was injected into the animals. The sera were recovered 9 (for rabbits) or 16 days (for guinea pigs) after the second immunization.
The sera were further affinity-purified by column chromatography because they showed nonspecific binding activity to the brain tissue. GFP (2 mg) was conjugated with a mixture of 0.5 ml Affi-Gel 10 and 0.5 ml Affi-Gel 15 (Bio-Rad; Hercules, CA). The GFP–Affi-Gel conjugate was treated at 100C for 30 min in PBS. After 25 mg of crude IgG fractions of the guinea pig or rabbit antibodies, which were prepared by salt precipitation (Johnstone and Thorpe 1987), was applied to 1 ml of the antigen column, antibody fractions were eluted with 0.1 M glycine-HCl, pH 2.5, and then with 0.1 M triethylamine, pH 11.8. In the following studies, only the triethylamine eluates were used as anti–heat-denatured GFP antibodies.
Anti-native GFP antibodies raised in rabbits and guinea pigs were described previously (Tamamaki et al. 2000). In short, these antibodies were produced as described above, except that the native GFP molecule was used as the immunogen. After being applied to the GFP–Affi-Gel conjugate column, affinity-purified IgG fractions were eluted with 0.1 M glycine-HCl, pH 2.5.
Western Blot
Approximately 0.3 g of GAD67-GFP knock-in mouse brain was homogenized with 2.7 ml of 50 mM Tris-HCl, pH 6.8, 10 mM EDTA, and 0.1 mg/ml phenylmethylsulfonyl fluoride (nacalai tesque; Kyoto, Japan). The homogenate was centrifuged at 15,000 rpm for 30 min at 4C, and the supernatant was stored at −80C until use. The supernatant was reduced by heating at 100C for 10 min with 0.7% (v/v) 2-mercaptoethanol and 1% (w/v) SDS and electrophoresed in 12% polyacrylamide gel in the presence of 0.1% (w/v) SDS. Electrophoresed proteins were further transferred onto a polyvinylidene difluoride membrane (ATTO; Tokyo, Japan) for Western blotting. After blocking with Block-Ace (Dainippon Sumitomo Pharma; Osaka, Japan), the membranes were incubated overnight at room temperature with 1 μg/ml of rabbit or guinea pig antibody to heat-denatured or native GFP and then for 1 hr with alkaline phosphatase–conjugated goat antibody to rabbit IgG (0.03 μg/ml, AP156A; Chemicon, Temecula, CA) or to guinea pig IgG (0.05 μg/ml, AP108A; Chemicon). The antibodies were diluted with PBS containing 10% (v/v) Block-Ace and 0.2% (v/v) Tween 20. The membranes were finally developed with 0.003% (w/v) nitroblue tetrazolium and 0.017% (w/v) bromochloroindolyl phosphate in 0.1 M NaCl, 5 mM MgCl2, and 0.1 M Tris-HCl, pH 9.5.
Tissue Sections
Mice were anesthetized with 7% (w/v) chloral hydrate and were perfused transcardially with PBS and then with 4% (w/v) formaldehyde [diluted from formalin, 37% (w/v) formaldehyde solution] in 0.1 M sodium phosphate buffer, pH 7.4. Brains were removed and postfixed overnight at 4C in the same fixative. After cryoprotection in 30% (w/v) sucrose in PBS at 4C, the brains were cut into 50-μm-thick coronal sections with a freezing microtome for the following immunofluorescence and in situ hybridization analyses.
Immunofluorescence Labeling
To evaluate the effect of heat treatment on immunolabeling of GFP, we incubated sections in PBS at 25C, 37C, 50C, 60C, 70C, and 80C for 20 hr before the antigen–antibody reaction. The heat treatment was omitted for control sections.
The heat-treated and untreated sections were immunofluorescence-labeled with Cy3 (Table 1). The sections were reacted with the primary antibody in PBS containing 0.3% (v/v) Triton X-100, 0.25% (w/v) λ-carrageenan, and 1% (v/v) donkey serum (together, referred to as PBS-XCD), followed by two washes with PBS containing 0.3% (v/v) Triton X-100 (PBS-X). The sections were incubated with the Cy3-conjugated secondary antibody in PBS-XCD, followed by two washes in PBS-X. Sections were mounted on aminopropyltriethoxysilane-coated glass slides (Matsunami; Kishiwada, Japan) and coverslipped with 90% (v/v) glycerol, 2.5% (w/v) triethylene diamine (an antifading reagent), and 20 mM Tris-HCl, pH 7.4. The sealed sections were stored at −20C until observation.
Table 1.
Summary of histological labeling methods
| Methods | Step | |
|---|---|---|
| Cy3 labeling | ||
| r/h | 1ry Ab | 1 μg/ml affinity-purified rabbit antibody to heat-denatured GFP, overnight |
| 2ry Ab | 10 μg/ml Cy3-conjugated donkey antibody to rabbit IgG (AP182C; Chemicon), 2 hr | |
| g/h | 1ry Ab | 1 μg/ml affinity-purified guinea pig antibody to heat-denatured GFP, overnight |
| 2ry Ab | 10 μg/ml Cy3-conjugated donkey antibody to guinea pig IgG (AP193C; Chemicon), 2 hr | |
| r/n | 1ry Ab | 1 μg/ml affinity-purified rabbit antibody to native GFP, overnight |
| 2ry Ab | 10 μg/ml Cy3-conjugated donkey antibody to rabbit IgG (AP182C; Chemicon), 2 hr | |
| g/n | 1ry Ab | 1 μg/ml affinity-purified guinea pig antibody to native GFP, overnight |
| 2ry Ab | 10 μg/ml Cy3-conjugated donkey antibody to guinea pig IgG (AP193C; Chemicon), 2 hr | |
| Double labeling | ||
| Hybridization | 1 μg/ml sense or antisense DIG-labeled riboprobe for GAD67 in hybridization buffer A (Table 2), 70C, 20 hr | |
| 1ry Ab | 1/1000 alkaline phosphatase–conjugated anti-DIG sheep antibody Fab fragment (1093274; Roche) and 1.0 μg/ml affinity-purified guinea pig antibody to heat-denatured GFP, overnight | |
| 2ry Ab | 10 μg/ml Alexa Fluor 488–conjugated goat antibody to guinea pig IgG (A-11073; Invitrogen, Carlsbad, CA), 2 hr | |
| Reaction | HNPP/Fast Red TR (1758888; Roche), 1 hr | |
Unless otherwise stated, all incubations above were carried out at room temperature. Ab, antibody; GFP, green fluorescent protein.
To check specificity of anti-GFP antibodies on mouse brain sections, some heat-treated (at 80C for 20 hr in PBS) or untreated sections were subjected to immunolabeling with each of the antibodies preabsorbed for 1 hr at room temperature with 10 μg/ml native GFP or 30 μg/ml heat-denatured GFP diluted in PBS containing 1% (v/v) donkey serum and 0.3% (v/v) Triton X-100, followed by the labeling method described above.
Double Labeling of GFP Immunofluorescence and Fluorescence In Situ Hybridization Histochemistry for GAD67 mRNA
cDNA fragments corresponding to a region of the GAD67 cDNA (nucleotides 276–894; GenBank accession number NM_008077.3) cloned into a vector pBluescript II KS(+) (Stratagene; La Jolla, CA) were described previously (Tamamaki et al. 2003). Using the linearized plasmids as templates, we synthesized sense and antisense single-strand RNA probes with a DIG labeling kit (Roche; Basel, Switzerland).
The procedures for non-radioactive in situ hybridization are described elsewhere (Liang et al. 2000; Tochitani et al. 2001; Nakamura et al. 2007). Free-floating sections were incubated for 20 min in 0.3% (v/v) Triton X-100 and 0.1 M PB, pH 7.0, and acetylated in freshly prepared 0.25% (v/v) acetic anhydride in 0.1 M triethanolamine for 10 min by vigorous shaking at room temperature. After rinsing, sections were hybridized for 20 hr at 70C with 1 μg/ml sense or antisense DIG-labeled riboprobe in hybridization buffer A (Table 2). After two washes at 70C in 50% (v/v) formamide, 2× saline sodium citrate (SSC), and 0.2% (w/v) N-lauroylsarcosine (NLS) for 20 min, hybridized sections were treated with 20 μg/ml RNase A in a mixture of 10 mM Tris-HCl, pH 8.0, 1 mM EDTA, and 0.5 M NaCl at 37C for 30 min. Sections were washed at 37C twice in 2× SSC and 0.1% (w/v) NLS for 20 min and twice in 0.2× SSC and 0.1% (w/v) NLS for 20 min.
Table 2.
Buffers used for heat treatment of tissue sections
| Hybridization buffer Aa | Hybridization buffer Ba | ||
|---|---|---|---|
| 50% (v/v) | Formamide (16345-65; nacalai tesque) | 50% (v/v) | Formamide |
| 5× | SSC (1× SSC = 150 mM NaCl, 15 mM sodium citrate, pH 7.0) | 5× | SSC |
| 2% (w/v) | Blocking reagentb (1096176; Roche) | 5× | Denhardt's solution (10727-74; nacalai tesque) |
| 0.1% (w/v) | N-lauroylsarcosine (20116-22; nacalai tesque) | 250 μg/ml | Yeast tRNA (15401-011; Invitrogen) |
| 0.1% (w/v) | SDS (31607-65; nacalai tesque) | 500 μg/ml | Salmon sperm DNA (15632-011; Invitrogen) |
Hybridization buffer A (Liang et al. 2000; Tochitani et al. 2001; Nakamura et al. 2007) or B (Tamamaki et al. 2003; Furuta et al. 2004) has been used for in situ hybridization histochemistry in previous studies.
The blocking reagent was made of a casein fraction that is prepared from non-fat dry milk by precipitation in acetic acid (pH 4.6) followed by solubilization in 0.1 M NaOH (manufacturer's information).
SSC, saline sodium citrate.
Subsequently, the hybridized sections were incubated with a mixture of alkaline phosphatase–conjugated anti-DIG antibody (Table 1) and guinea pig anti–heat-denatured GFP antibody in 0.1 M Tris-HCl, pH 7.5, and 0.15 M NaCl (TBS) with 1% (w/v) blocking reagent. After two washes with 0.05% (v/v) Tween 20 in TBS (TBS-T), the sections were incubated with Alexa Fluor 488–conjugated secondary antibody (Table 1) in TBS with 1% (w/v) blocking reagent. After two washes in TBS-T, sections were incubated with a HNPP Fluorescent Detection kit (HNPP/Fast Red TR; Table 1). The sections were immediately mounted on aminopropyltriethoxysilane-coated slides (Matsunami), air-dried, and cover slipped with an aqueous mounting medium Permafluor (Beckman Coulter; Fullerton, CA). Sections hybridized with the sense probe showed no mRNA signals in the cortex and thalamus.
Image Acquisition
Images of sections were obtained with a confocal laser-scanning microscope, LSM5 PASCAL (Carl Zeiss; Oberkochen, Germany), with a pinhole opened entirely for ×5 (Plan-Neofluar, NA = 0.15; Carl Zeiss) or ×10 objective lens (Plan-Neofluar, NA = 0.30; Carl Zeiss) and with a pinhole size of 1.0 Airy unit for ×20 (Plan-Neofluar, NA = 0.5; Carl Zeiss) or ×40 objective lens (Plan-Neofluar, NA = 0.75; Carl Zeiss). GFP or Alexa Fluor 488 was excited with 488-nm laser beam and observed through a 510- to 530-nm emission filter, whereas Cy3 or HNPP/Fast Red TR was excited with a 543-nm laser beam and observed through a ≥560-nm emission filter.
Results
Characterization of Antibodies
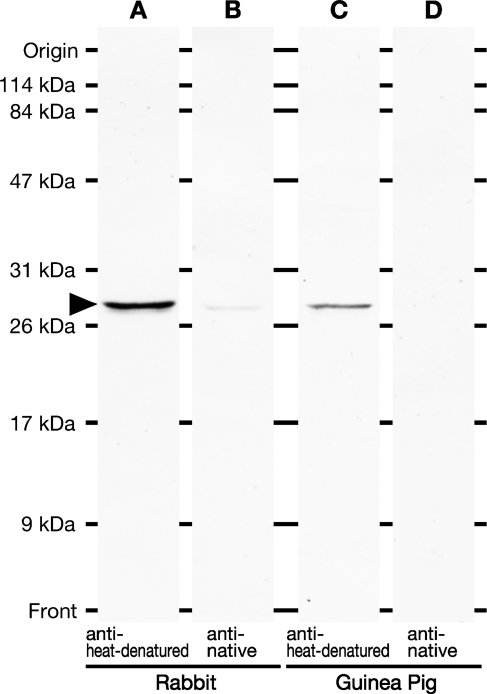
Antibodies against heat-denatured GFP were raised in rabbits and guinea pigs and affinity-purified with the antigen-conjugated column. In Western blot tests with the supernatant from the brain homogenate of GAD67-GFP knock-in mice, we examined specificity of the new antibodies in comparison with rabbit and guinea pig anti-native GFP antibodies (Figure 1). Except anti-native GFP antibody produced in guinea pigs (Figure 1D), the other antibodies readily detected a single protein band at the position of 27,000 Da on the membrane (Figures 1A–1C). Rabbit anti–heat-denatured GFP antibody showed much more intense immunoreactivity than the rabbit anti-native GFP antibody.
Figure 1.
Characterization of anti-green fluorescent protein (GFP) antibodies by Western blot analysis. The supernatant of brain extract prepared from GAD67-GFP knock-in mice was electrophoresed in 12% polyacrylamide gel in the presence of SDS. The proteins were blotted onto a polyvinylidene difluoride membrane, and immunostained with the affinity-purified rabbit anti-heat-denatured GFP (A), rabbit anti-native GFP (B), guinea pig anti–heat-denatured GFP (C), or guinea pig anti-native GFP (D) antibody. Arrowhead indicates the positive bands of ∼27,000 Da. Note that clear single bands were detected with anti–heat-denatured GFP antibodies, whereas only a weak ban and no band were observed with rabbit and guinea pig anti-native GFP antibodies, respectively.
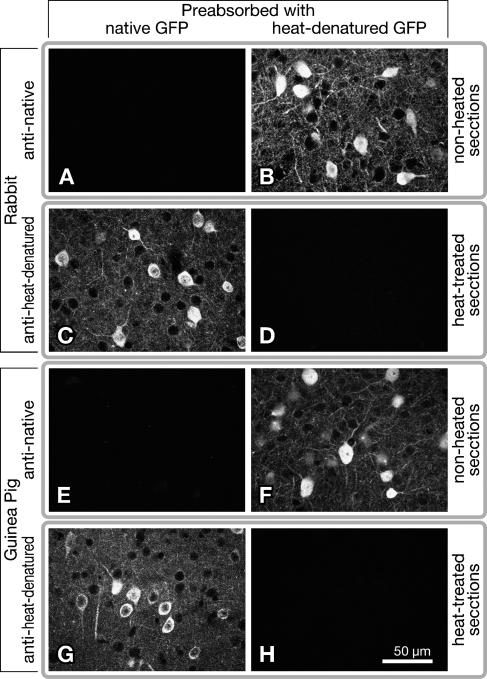
To further characterize the anti–heat-denatured GFP antibodies, we immunostained heat-treated or non-heated sections of GAD67-GFP knock-in mice with the antibodies (Figure 2). The non-heated sections showing bright native fluorescence of GFP (Figure 2A) were immunolabeled for GFP with antibodies to native GFP (Figures 2C and 2G) but not with those to heat-denatured GFP (Figures 2E and 2I). In sharp contrast, native fluorescence of GFP (Figure 2B) and GFP immunofluorescence detected with the anti-native GFP antibodies (Figures 2D and 2H) were lost on the heat-treated sections. However, both rabbit and guinea pig antibodies against heat-denatured GFP visualized cell bodies and neuropil of GFP-immunoreactive neurons clearly in the heat-treated sections (Figures 2F and 2J). In addition, we noticed that homogenous autofluorescence of tissue elevated after heat treatment, and it was observed through an emission filter for GFP but not through that for Cy3 dye (Figure 2B). Neither antibodies to heat-denatured GFP nor native GFP detected anything more than background in heat-treated or untreated sections of the wild-type mouse brain (data not shown), indicating little cross-reactivity to endogenous proteins in the wild-type mouse brain.
Figure 2.
Immunofluorescence labeling of brain sections from GAD67-GFP knock-in mice with antibodies to heat-denatured GFP (E,F,I,J) or native GFP (C,D,G,H). Some sections of GAD67-GFP knock-in mice were heat-treated at 80C for 20 hr in PBS (B,D,F,H,J), whereas the others remained untreated (A,C,E,G,I). After being reacted with 1 μg/ml each of the primary antibodies, GFP immunoreactivity was visualized with Cy3-conjugated secondary antibodies (C,D, stained by r/n; E,F, stained by r/h; G,H, stained by g/n; I,J, stained by g/h; staining methods described in Table 1). Fluorescence images of layer IV in the somatosensory cortex are shown. (A,B) Native fluorescence of GFP, which is bright in untreated sections (A) but absent in heat-treated sections (B). Note that homogenous autofluorescence of tissue was elevated after heat treatment (B).
Some heat-treated or untreated sections were subjected to immunolabeling with the anti-GFP antibodies preabsorbed with native GFP or GFP denatured at 100C for 30 min in PBS (Figure 3). GFP immunoreactivity on heat-treated sections was lost when anti–heat-denatured GFP antibodies were preincubated with heat-denatured GFP (Figures 3D and 3H) but not with native GFP (Figures 3C and 3G). By contrast, GFP immunoreactivity on non-heated sections disappeared when anti-native GFP antibodies were preabsorbed with native GFP (Figures 3A and 3E) but not with heat-denatured GFP (Figures 3B and 3F). Taken together, these data indicate that anti–heat-denatured GFP and anti-native GFP antibodies preferentially recognize heat-denatured and native forms of GFP, respectively.
Figure 3.
Immunofluorescence labeling of GAD67-GFP knock-in mouse brain sections with anti-GFP antibodies preabsorbed with native or heat-denatured GFP. Some sections were heat-treated at 80C for 20 hr in PBS, before immunolabeling with anti-heat-denatured GFP antibodies (C,D,G,H), whereas the other sections remained non-heated for labeling with anti-native GFP antibodies (A,B,E,F). After the sections were reacted with 1 μg/ml each of the primary antibodies preincubated with native or heat-denatured GFP, GFP immunoreactivity was visualized with Cy3-conjugated secondary antibodies (A,B, stained by r/n; C,D, stained by r/h; E,F, stained by g/n; G,H, stained by g/h; staining methods described in Table 1). Fluorescence images of layer IV in the somatosensory cortex are shown. Note that preabsorption of anti–heat-denatured GFP antibodies with heat-denatured GFP resulted in loss of GFP immunoreactivity on the sections (D,H), but that with native GFP did not (C,G). By contrast, preabsorption of anti-native GFP antibodies with native GFP eliminated GFP immunoreactivity on the sections (A,E) but that with heat-denatured GFP did not (B,F).
Effect of Heat Treatment on GFP Immunolabeling of Tissue Sections
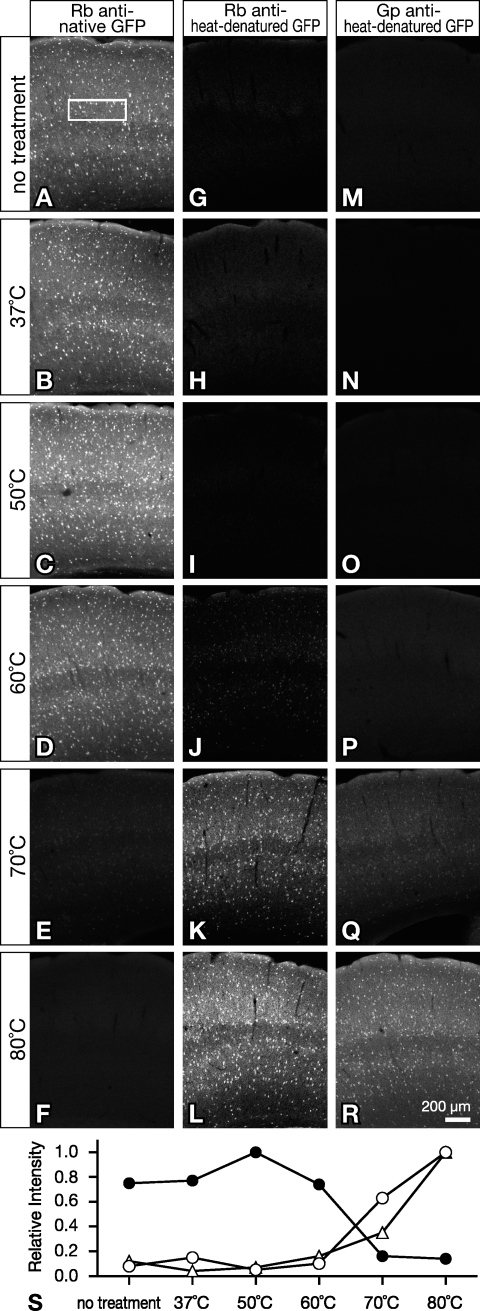
We addressed the question of how the temperature of heat treatment affects immunolabeling of GFP using tissue specimens. Brain sections of GAD67-GFP knock-in mice were thus incubated in PBS for 20 hr at room temperature (25C, data not shown), 37C, 50C, 60C, 70C, or 80C before immunofluorescence labeling of GFP (Figure 4). For each labeling method, relative fluorescence intensity in layer IV was measured from images that were taken under the same condition (Figure 4S).
Figure 4.
Temperature dependence of immunolabeling with anti-GFP antibodies. Brain sections from GAD67-GFP knock-in mice were incubated for 20 hr in PBS at 25C (data not shown), 37C, 50C, 60C, 70C, and 80C. Control sections were omitted from this treatment and directly processed for immunolabeling. The sections were reacted with 1 μg/ml antibody to native GFP or heat-denatured GFP, followed by Cy3-conjugated secondary antibodies. Panels show micrographs of Cy3-labeled GFP immunoreactivity detected with rabbit anti-native GFP antibody (A–F, stained by r/n described in Table 1), rabbit anti-heat-denatured GFP antibody (G–L, stained by r/h), or guinea pig anti–heat-denatured GFP antibody (M–R, stained by g/h) in the somatosensory cortex. Images aligned in each column were taken under the same conditions of the confocal microscope for comparison of relative fluorescence intensity. Average fluorescence intensity in a rectangle placed in layer IV (150 μm in height and 450 μm in width, shown in A) was measured for each panel. Relative fluorescence intensity in each column was normalized to 1 and plotted in S (solid circles, A–F; open circles, G–L; open triangles, M–R).
Rabbit antibody to heat-denatured GFP did not react with GFP on sections heat-treated at 60C or lower temperatures (Figures 4H–4J and 4S). GFP immunoreactivity detected with the rabbit antibody was intense in those treated at 70C and 80C (Figures 4K, 4L, and 4S). Likewise, with anti–heat-denatured GFP antibody raised in guinea pigs, GFP immunoreactivity was not detected on sections treated at 60C or lower temperatures (Figures 4N–4P and 4S), but was weak and intense on those treated at 70C and 80C, respectively (Figures 4Q–4S).
On the contrary, with rabbit antibody to native GFP, GFP immunofluorescence was readily detected on sections heat treated at 60C and lower temperatures (Figures 4B–4D and 4S), but heat treatment at 70C and higher temperatures led to loss of the immunofluorescence signal (Figures 4E, 4F, and 4S). Results obtained from sections reacted with guinea pig antibody to native GFP were similar to those with the rabbit antibody.
Thus, the emergence of immunoreactivity for heat-denatured GFP was accompanied by the loss of that for native GFP (Figure 4S). This implies that GFP might have two stable conformational states that could be selectively recognized by either anti-native GFP antibodies or anti–heat-denatured GFP antibodies.
Double Labeling of GFP Immunofluorescence and Fluorescence In Situ Hybridization to GAD67 mRNA
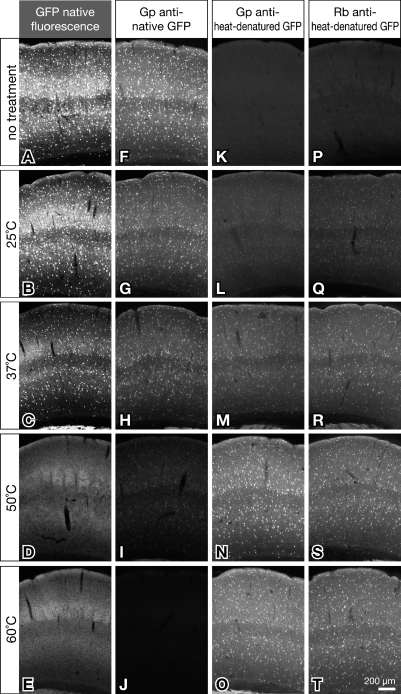
The new antibodies to heat-denatured GFP could be most advantageous when used in combination with in situ hybridization histochemistry. In advance of the combinatory labeling, we examined whether heat denaturation of GFP in hybridization buffers A and B (Table 2) differs from that in PBS by immunofluorescence labeling of sections from GAD67-GFP knock-in mice, because those buffers contained denaturing reagents, such as formamide. We incubated sections for 20 hr in hybridization buffer A or B at 25C, 37C, 50C, 60C, and 70C (data not shown). The heat treatment was omitted for control sections. The heat-treated and untreated sections were subjected to immunofluorescence labeling with Cy3 (Table 1). Figure 5 shows the results for hybridization buffer B, which is more routinely used than buffer A. Native fluorescence of GFP was clear on untreated sections (Figure 5A), decreased in sections heat treated at 37C in hybridization buffer B (Figure 5C), and almost absent in sections incubated at 50C and higher temperatures, despite elevated autofluorescence (Figures 5D and 5E). The similar decline was observed in GFP immunoreactivity with rabbit and guinea pig antibodies to native GFP (for guinea pig antibody, see Figures 5F–5J). Incubation of sections in hybridization buffer B even at 25C decreased the immunofluorescence (Figures 5F and 5G). On the other hand, GFP immunoreactivity labeled with guinea pig and rabbit antibodies to heat-denatured GFP was absent in untreated sections (Figures 5K and 5P), weak in the sections treated in hybridization buffer B at 25C (Figures 5L and 5Q), and moderate on those at 37C (Figures 5M and 5R). Heating at 50C and higher temperatures resulted in intense immunofluorescence signals to GFP (Figures 5N, 5S, 5O, and 5T). We obtained virtually the same results with hybridization buffer A.
Figure 5.
Temperature dependence of immunolabeling with anti-GFP antibodies in sections treated with hybridization buffer. Brain sections of GAD67-GFP knock-in mice were incubated for 20 hr in hybridization buffer B (Table 2) at 25C, 37C, 50C, 60C, and 70C (data not shown). Control sections were omitted this treatment and directly processed for immunolabeling. The sections were reacted with 1 μg/ml anti-native GFP antibody or anti–heat-denatured GFP antibody, and then with Cy3-conjugated secondary antibodies. Panels show micrographs of native green fluorescence of GFP (A–E) and Cy3-labeled GFP immunoreactivity detected with guinea pig anti-native GFP antibody (F–J, stained by g/n described in Table 1), guinea pig anti–heat-denatured GFP antibody (K–O, stained by g/h), or rabbit anti-heat-denatured GFP antibody (P–T, stained by r/h) in the somatosensory cortex. Images aligned in each column were taken under the same conditions of the confocal microscope for comparison of relative fluorescence intensity.
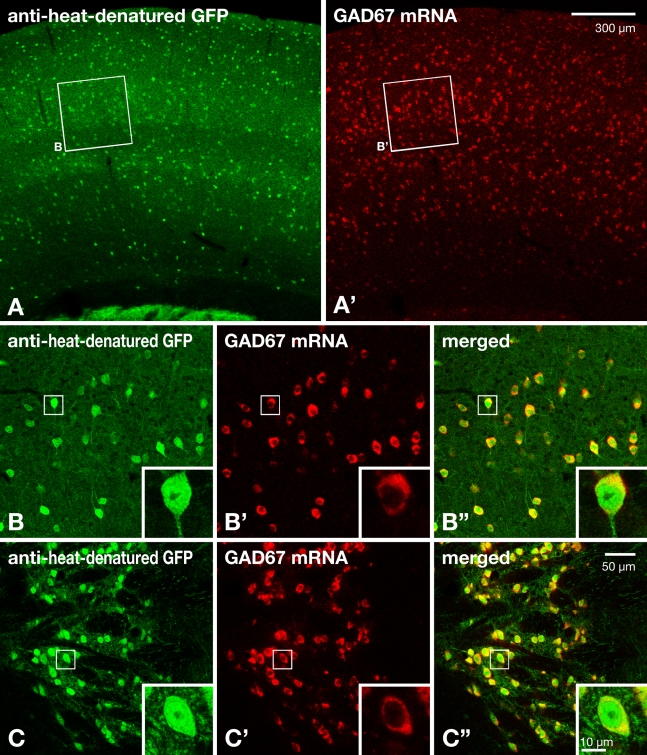
To exemplify an application of the anti–heat-denatured GFP antibodies, we finally double labeled brain sections of GAD67-GFP knock-in mice for GFP immunoreactivity and GAD67 mRNA signals by using guinea pig antibody to heat-denatured GFP in combination with fluorescence in situ hybridization histochemistry to GAD67 mRNA (Figure 6; Table 1). After hybridization at 70C for 20 hr in hybridization buffer A (Table 2), virtually all the neuronal cell bodies positive for GAD67 mRNA signal (shown as HNPP/Fast Red TR reaction product; Figures 6A′–6C′) displayed Alexa Fluor 488 fluorescence signal for GFP both in the neocortex and thalamic reticular nucleus (Figures 6B″ and 6C″), indicating that the new anti–heat-denatured GFP antibody successfully detected GFP in the sections after in situ hybridization. The complete colocalization of GFP immunoreactivity and mRNA signals for GAD67 further indicates that the new antibody has sufficient sensitivity to detect the GFP molecule.
Figure 6.
Double labeling of immunofluorescence for GFP (green) and fluorescence in situ hybridization histochemistry for GAD67 mRNA (red) in brain sections of GAD67-GFP knock-in mice. After hybridization at 70C for 20 hr in hybridization buffer A (Table 2) with a DIG-labeled riboprobe, the sections were reacted with anti–heat-denatured GFP antibody raised in guinea pig, followed by visualization with Alexa Fluor 488–conjugated secondary antibody (A–C). The hybridization signal for GAD67 mRNA (A′–C′) was visualized with HNPP/Fast Red TR reaction after application of alkaline phosphatase–conjugated anti-DIG antibody (Table 1). (A–B″) Images of the somatosensory area of neocortex. Boxed regions of layers IV–V in A and A′ are shown in B–B″. (C–C″) Images of the thalamic reticular nucleus. Boxed regions in B–C″ are shown at higher magnification in insets. Note that virtually all the cell bodies positive for GAD67 mRNA signals showed GFP immunoreactivity (yellow; B″,C″). Bars in A′,C″, and inset in C″ apply to A–A′, B–C″, and insets in B–C″, respectively.
It was noted that heat treatment increased green autofluorescence (Figures 2B, 5D, and 5E) but that the autofluorescence did not interfere with green immunofluorescence detection with the antibody to heat-denatured GFP (online Supplementary Material, Figure SF1A–B′). Actually, autofluorescence in Figures 5D and 5E was fairly enhanced by the imaging conditions that were optimized to native GFP fluorescence. Furthermore, if the autofluorescence was a problem, it could be attenuated by CuSO4 treatment (online Supplementary Material, Figure SF1C–C′; Schnell et al. 1999). Thus, we could practically circumvent the problem of heat-induced autofluorescence.
Discussion
The new affinity-purified polyclonal antibodies to heat-denatured GFP were shown to be highly specific to GFP by Western blot analysis and immunofluorescence labeling of the brain tissue of mice expressing GFP. Immunofluorescence labeling of the heat-treated and untreated sections of the mice as well as the preabsorption tests indicated that the new antibodies selectively bind to the heat-denatured form of GFP. Pretreatment of the sections at various temperatures showed the temperature dependence of epitopes recognized by the new antibodies. We finally showed that the new antibodies work compatibly with the fluorescence in situ hybridization histochemical technique.
Specificity of Antibodies
In Western blot analysis, both rabbit and guinea pig antibodies to heat-denatured GFP detected a single band at the position of 27,000 Da (Figures 1A and 1C), which was in register with the molecular mass of GFP deduced from its amino acid sequence (26,940 Da). The antibodies clearly showed GFP immunoreactivity both in neuronal cell bodies and neuropil in heat-treated sections of mouse brain expressing GFP but not in untreated sections, suggesting that they preferentially recognize the heat-denatured form of GFP. No immunolabeling was seen in sections reacted with the anti–heat-denatured GFP antibodies preabsorbed with heat-denatured GFP (Figures 3D and 3H) or in sections of wild-type mice (data not shown), further confirming their specificity to heat-denatured GFP.
On the contrary, the antibodies to native GFP clearly labeled GFP-expressing cells on the tissue specimens without heat treatment (Tamamaki et al. 2000; Furuta et al. 2001) (Figures 2C and 2G). No labeling was seen in sections reacted with the anti-native GFP antibodies preabsorbed with native GFP, verifying that the labeling was specific to GFP. Moreover, the GFP immunolabeling remained intense when the antibodies were preincubated with heat-denatured GFP, but was lost in heat-treated sections, indicating their preferential recognition of the native form of GFP. In Western blot analysis, for which proteins were subjected to denaturation by heat and SDS treatments, rabbit anti-native GFP antibody showed a weak band corresponding to GFP, whereas that made in guinea pig failed to display any signal. The antibodies against native GFP were thus clearly less sensitive to GFP in the Western blot test than those against heat-denatured GFP, further indicating their selectivity to native form of GFP.
Heat Denaturation of GFP and Possible Epitopes
Aequorea victoria GFP and its derivatives require precise folding for the functional conformation of fluorophores. The fluorophore of wild-type Aequorea GFP is reportedly to be rather stable, even at pH of 5–12 and to temperatures as high as 60C (Bokman and Ward 1981; Ward 1981). Fluorescence of wild-type GFP showed 90% recovery in a few minutes simply by pH neutralization after acid or base denaturation or by dilution after guanidine denaturation (Ward and Bokman 1982), further indicating the highly stabilized structure of the fluorophore. Accordingly, analyses on the crystal structures of wild-type GFP (Yang et al. 1996) and that of the Ser65Thr mutant (Ormö et al. 1996) have shown that the outside of the protein fold is formed by the β-can structure that makes up a single α-helix and 11-stranded β-barrel and seems to protect the central fluorophore from the external environment. After heat denaturation, however, GFP never renatures effectively or recovers fluorescence (Ward 1998; this study). This irreversibility of heat denaturation suggests that the free energy level of the heat-denatured form of GFP is much lower than that of the native form and that this conformational change might need high activation energy.
The virtually complementary labeling of GFP by the antibodies to heat-denatured and native GFP suggest that they bind to two distinct epitopes whose exposure is mutually exclusive. Epitopes for antibodies to heat-denatured GFP are normally hidden in native GFP but exposed when the irreversible denaturation has occurred by heat treatment. In this study, the antibodies to heat-denatured GFP recognized a denatured form of GFP in heat-treated tissue sections (Figure 2) or in those treated under hybridization conditions with a denaturing reagent (Figure 5), as well as those on the membrane blotted with SDS-treated and heat-denatured proteins (Figure 1). These results imply that the same epitopes might be exposed in those conditions. It therefore seems plausible that antibodies to heat-denatured GFP may recognize a portion of the primary structure, rather than the secondary or tertiary structure, of GFP, because it would be exposed after severe denaturation of the protein such as heat treatment and SDS treatment.
On the other hand, epitopes for the anti-native GFP antibodies are normally exposed but probably disrupted by heat or SDS treatment. The epitopes might thus lie in the secondary or tertiary structure of GFP. Presumably, they could be a portion of the β-can structure that forms the outside of the protein folding and may protect the central fluorophore (Ormö et al. 1996; Yang et al. 1996). Unfolding of the β-can by heat treatment might therefore be a critical step that causes loss of antigenicity for anti-native GFP antibodies in parallel with fluorophore disruption that leads to loss of native fluorescence of GFP.
Heat treatment in the hybridization buffer unmasked epitopes for antibodies to heat-denatured GFP at a much lower temperature than that in PBS (Figures 4 and 5). Although melting temperature, at which one half of the native fluorescence is lost, is reportedly 76C for GFP (Bokman and Ward 1981; Ward 1981), incubation of sections even at 25C in the hybridization buffer allowed anti–heat-denatured GFP antibodies to recognize GFP. The buffer contained formamide for effective denaturation and hybridization of nucleic acids at low temperature (Bonner et al. 1967). Formamide in the buffer might also facilitate heat denaturation of proteins, although this study does not intend to thoroughly exclude contributions of the other chemical components in the buffer.
Applications of Antibodies to Heat-denatured GFP
This study showed that the new antibodies to heat-denatured GFP are suitable for Western blotting (Figure 1) and combined fluorescence labeling of GFP immunoreactivity with in situ hybridization histochemistry (Figure 6). The new antibodies may also be compatible with the simultaneous immunofluorescence detection of GFP and the antigens requiring heat-induced antigen retrieval. Epitopes of those antigens, including BrdU, Ki-67, PCNA, estrogen receptor, glial fibrillary acidic protein, enkephalin, tyrosine hydroxylase, NeuN, and vesicular glutamate transporters 1 and 2, are better unmasked by heat treatment before immunolabeling, leading to enhancement of their immunoreactivity (Moran et al. 1985; Shi et al. 1991,1995; Jiao et al. 1999; Ino 2003; Nakamura et al. 2005,2007). In addition, availability of both rabbit and guinea pig antibodies to heat-denatured GFP enables us to use various combinations of antibodies for multiple labeling in wider applications. Thus, the new antibodies to heat-denatured GFP may open an opportunity to combine GFP immunodetection with those histological procedures that require heat treatment. This may advance the identification of proteins and mRNAs coexpressed with GFP in various transgenic organisms.
Supplementary Material
Acknowledgments
This work was supported by Ministry of Education, Science, Sports and Culture of Japan Grants 16200025, 17022020, 17650100, 18019007, 18300102, and 19700317.
References
- Beisker W, Dolbeare F, Gray JW (1987) An improved immunocytochemical procedure for high-sensitivity detection of incorporated bromodeoxyuridine. Cytometry 8:235–239 [DOI] [PubMed] [Google Scholar]
- Bokman SH, Ward WW (1981) Renaturation of Aequorea green-fluorescent protein. Biochem Biophys Res Commun 101:1372–1380 [DOI] [PubMed] [Google Scholar]
- Bonner J, Kung G, Bekhor I (1967) A method for the hybridization of nucleic acid molecules at low temperature. Biochemistry 6:3650–3653 [DOI] [PubMed] [Google Scholar]
- Chalfie M, Kain SR (2005) Green Fluorescent Protein: Properties, Applications, and Protocols. 2nd ed. Hoboken, NJ, Wiley
- Cormack BP, Valdivia RH, Falkow S (1996) FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33–38 [DOI] [PubMed] [Google Scholar]
- Darby IA, Bisucci T, Desmouliere A, Hewitson TD (2006) In situ hybridization using cRNA probes: isotopic and nonisotopic detection methods. Methods Mol Biol 326:17–31 [DOI] [PubMed] [Google Scholar]
- Furuta T, Koyano K, Tomioka R, Yanagawa Y, Kaneko T (2004) GABAergic basal forebrain neurons that express receptor for neurokinin B and send axons to the cerebral cortex. J Comp Neurol 473:43–58 [DOI] [PubMed] [Google Scholar]
- Furuta T, Tomioka R, Taki K, Nakamura K, Tamamaki N, Kaneko T (2001) In vivo transduction of central neurons using recombinant Sindbis virus: Golgi-like labeling of dendrites and axons with membrane-targeted fluorescent proteins. J Histochem Cytochem 49:1497–1508 [DOI] [PubMed] [Google Scholar]
- Ino H (2003) Antigen retrieval by heating en bloc for pre-fixed frozen material. J Histochem Cytochem 51:995–1003 [DOI] [PubMed] [Google Scholar]
- Jiao Y, Sun Z, Lee T, Fusco FR, Kimble TD, Meade CA, Cuthbertson S, et al. (1999) A simple and sensitive antigen retrieval method for free-floating and slide-mounted tissue sections. J Neurosci Methods 93:149–162 [DOI] [PubMed] [Google Scholar]
- Johnstone A, Thorpe R (1987) Immunochemistry in Practice. 2nd ed. Oxford, Blackwell Scientific
- Liang F, Hatanaka Y, Saito H, Yamamori T, Hashikawa T (2000) Differential expression of gamma-aminobutyric acid type B receptor-1a and -1b mRNA variants in GABA and non-GABAergic neurons of the rat brain. J Comp Neurol 416:475–495 [PubMed] [Google Scholar]
- Moran R, Darzynkiewicz Z, Staiano-Coico L, Melamed MR (1985) Detection of 5-bromodeoxyuridine (BrdUrd) incorporation by monoclonal antibodies: role of the DNA denaturation step. J Histochem Cytochem 33:821–827 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Hioki H, Fujiyama F, Kaneko T (2005) Postnatal changes of vesicular glutamate transporter (VGluT)1 and VGluT2 immunoreactivities and their colocalization in the mouse forebrain. J Comp Neurol 492:263–288 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Watakabe A, Hioki H, Fujiyama F, Tanaka Y, Yamamori T, Kaneko T (2007) Transiently increased colocalization of vesicular glutamate transporters 1 and 2 at single axon terminals during postnatal development of mouse neocortex: a quantitative analysis with correlation coefficient. Eur J Neurosci 26:3054–3067 [DOI] [PubMed] [Google Scholar]
- Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y (1997) ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett 407:313–319 [DOI] [PubMed] [Google Scholar]
- Ormö M, Cubitt AB, Kallio K, Gross LA, Tsien RY, Remington SJ (1996) Crystal structure of the Aequorea victoria green fluorescent protein. Science 273:1392–1395 [DOI] [PubMed] [Google Scholar]
- Schnell SA, Staines WA, Wessendorf MW (1999) Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J Histochem Cytochem 47:719–730 [DOI] [PubMed] [Google Scholar]
- Shi SR, Imam SA, Young L, Cote RJ, Taylor CR (1995) Antigen retrieval immunohistochemistry under the influence of pH using monoclonal antibodies. J Histochem Cytochem 43:193–201 [DOI] [PubMed] [Google Scholar]
- Shi SR, Key ME, Kalra KL (1991) Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem 39:741–748 [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Nakamura K, Furuta T, Asamoto K, Kaneko T (2000) Neurons in Golgi-stain-like images revealed by GFP-adenovirus infection in vivo. Neurosci Res 38:231–236 [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T (2003) Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol 467:60–79 [DOI] [PubMed] [Google Scholar]
- Tochitani S, Liang F, Watakabe A, Hashikawa T, Yamamori T (2001) The occ1 gene is preferentially expressed in the primary visual cortex in an activity-dependent manner: a pattern of gene expression related to the cytoarchitectonic area in adult macaque neocortex. Eur J Neurosci 13:297–307 [DOI] [PubMed] [Google Scholar]
- Van Furth R, Van Zwet TL (1988) Immunocytochemical detection of 5-bromo-2-deoxyuridine incorporation in individual cells. J Immunol Methods 108:45–51 [DOI] [PubMed] [Google Scholar]
- Ward WW (1981) Properties of the coelenterate green-fluorescent protein. In DeLuca M, McElroy SH, eds. Bioluminescence and Chemiluminescence: Basic Chemistry and Analytical Applications. New York, Academic Press, 235–242
- Ward WW (1998) Biochemical and physical properties of green fluorescent protein. In Chalfie M, Kain S, eds. Green Fluorescent Protein: Properties, Applications, and Protocols. 1st ed. New York, Wiley-Liss, 45–75
- Ward WW, Bokman SH (1982) Reversible denaturation of Aequorea green-fluorescent protein: physical separation and characterization of the renatured protein. Biochemistry 21:4535–4540 [DOI] [PubMed] [Google Scholar]
- Yang F, Moss LG, Phillips GN Jr (1996) The molecular structure of green fluorescent protein. Nat Biotechnol 14:1246–1251 [DOI] [PubMed] [Google Scholar]
- Zhang G, Gurtu V, Kain SR (1996) An enhanced green fluorescent protein allows sensitive detection of gene transfer in mammalian cells. Biochem Biophys Res Commun 227:707–711 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.