Abstract
Leptin is a hormone that plays an important role in overall body energy homeostasis, and the obesity receptor, OB-R, is widely distributed in the organism. In the intestine, a multitude of leptin actions have been reported, but it is currently unclear to what extent the hormone affects the intestinal epithelial cells by an endocrine or exocrine signaling pathway. To elucidate this, the localization of endogenous porcine leptin and OB-R in enterocytes and colonocytes was studied. By immunofluorescence microscopy, both leptin and OB-R were mainly observed in the basolateral membrane of enterocytes and colonocytes but also in the apical microvillar membrane of the cells. By electron microscopy, coclustering of hormone and receptor in the plasma membrane and localization in endosomes was frequently detected at the basolateral surface of the epithelial cells, indicative of leptin signaling activity. In contrast, coclustering occurred less frequently at the apical cell surface, and subapical endosomal localization was hardly detectable. We conclude that leptin action in intestinal epithelial cells takes place at the basolateral plasma membrane, indicating that the hormone uses an endocrine pathway both in the jejunum and colon. In contrast, the data obtained did not provide evidence for an exocrine, lumenal action of the hormone in the intestine. (J Histochem Cytochem 56:677–685, 2008)
Keywords: leptin, OB-R, intestine, jejunum, colon, enterocyte, colonocyte
Leptin, the 16-kDa peptide hormone encoded by the ob gene, was originally described as a secretory protein of white adipose tissue acting on the hypothalamus to control energy storage (Zhang et al. 1994). It has since become apparent that a number of other tissues, including stomach, placenta, brain, skeletal muscle, and mammary epithelium, secrete leptin (Masuzaki et al. 1997; Bado et al. 1998; Wang et al. 1998; Ahima and Flier 2000; Wilkinson et al. 2000) and that the hormone also functions in a number of peripheral tissues and organs, as evidenced by the widespread tissue distribution of the obesity receptor (OB-R) (Baratta 2002; Fruhbeck 2006; Myers et al. 2008). Consequently, in addition to its role in body weight homeostasis, leptin signaling has been reported to play a role in a diverse range of physiological functions (Baratta 2002; Bjorbaek and Kahn 2004). As a member of the class I cytokine receptor superfamily, the OB-R signals by association with janus tyrosine kinases (Ihle 1995), but in addition, it may also participate in, and cross-talk with, other signaling pathways (Fruhbeck 2006). OB-R exists in several splice variants that share an extracellular ligand-binding domain and that are commonly classified into short, long, and secreted receptor forms. Of these, only the long form (OB-Rb) contains the intracellular motifs required for Janus kinase-signal transducer and activator of transcription signaling, and its expression is therefore considered crucial for leptin action (Fruhbeck 2006; Myers et al. 2008).
After the initial publication of leptin action in intestinal cells (Morton et al. 1998), many subsequent reports have described the localization of OB-R in enterocytes and colonocytes of the small intestine and colon, respectively (Hardwick et al. 2001; Buyse et al. 2001; Barrenetxe et al. 2002; Aparicio et al. 2004, 2005; Cammisotto et al. 2005). Functionally, leptin has been shown to affect a number of absorptive processes in the small intestine. Thus, leptin has been reported to lower levels of circulating apolipoprotein IV by suppressing synthesis in the small intestine (Morton et al. 1998; Doi et al. 2001), and the activity of PepT1, a peptide transporter in the brush border, was increased by leptin in Caco-2 cells, implying a role of leptin in controlling ingestion of dietary protein (Buyse et al. 2001). Furthermore, the brush border sodium-dependent glucose transporter SGLT-1 was shown to be rapidly downregulated by leptin (Lostao et al. 1998; Ducroc et al. 2005). Overall, these studies indicate that dietary uptake of all major classes of nutrients may be controlled by leptin. In the colon, leptin has been shown to stimulate mucin secretion from goblet cells (Plaisancie et al. 2006; El Homsi et al. 2007), but it has also been observed to act as a proinflammatory cytokine in inflammatory bowel disease (Sitaraman et al. 2004). In addition, leptin has been reported to promote invasiveness of colonic cells (Attoub et al. 2000) and to be overexpressed in colorectal cancer (Koda et al. 2007).
It is known that the gastric mucosa secretes leptin not only by an endocrine pathway but also by an exocrine pathway (Cammisotto et al. 2005). Furthermore, leptin secreted from the gastric chief cells binds to a protein corresponding to the extracellular domain of OB-R and thereby becomes resistant to the gastric juice (Cammisotto et al. 2006). Lumenal leptin may thus be enabled to interact with apically localized OB-Rs in intestinal epithelial cells and regulate nutrient assimilation, as described above, by an exocrine pathway. Alternatively, leptin, synthesized by the gastric mucosa or elsewhere in the body, may reach the intestinal epithelium through the blood in a classical endocrine fashion and interact with OB-Rs localized in the basolateral part of the plasma membrane.
Despite the large number of reports previously published on leptin action in the intestine, it is not yet fully clear to what extent the hormone functions by endocrine and/or exocrine pathways. In this study, we therefore performed a colocalization of endogeneous leptin and OB-R by high-resolution immunogold electron microscopy. Both hormone and receptor were detected apically and basolaterally in enterocytes and colonocytes, but only in the latter part of the plasma membrane was close colocalization and presence in endosomes frequently observed.
Materials and Methods
Reagents and Intestinal Tissues
Reagents were obtained from the following commercial suppliers: leptin (Sigma-Aldrich, St. Louis, MO; http://www.sigma-aldrich.com/); a rabbit antibody to leptin and a mouse antibody to the α-chain of Na+/K+-ATPase (cat. no. PA1-051 and MA3-928, respectively; Affinity Bioreagents, Golden, CO, http://www.bioreagents.com/); a rabbit antibody to the leptin receptor (OB-R) (cat. no. sc-8325; Santa Cruz, Santa Cruz, CA, http://www.scbt.com/); a rabbit antibody to intestinal alkaline phosphatase (IAP) (cat. no. 0300-1024; Biogenesis, Poole, UK, http://www.biogenesis.co.uk/); secondary Alexa 488/594-conjugated antibodies for immunofluorescence microscopy (Invitrogen, Carlsbad, CA; http://www.invitrogen.com/); secondary horseradish peroxidase–coupled antibodies for immunoblotting, secondary antibodies for immunogold electron microscopy, and antifade medium (Dako, Glostrup, Denmark; http://www.dako.dk/); and ECL immunoblotting detection reagents (GE Healthcare, Chalfont St. Giles, UK; http://www.gehealthcare.com/dkda/index.html). A rabbit antibody to aminopeptidase N (ApN) used in this study has been described previously elsewhere (Hansen et al. 1987).
Segments of adult porcine jejunum, taken ∼1.5 m from the pylorus and from the transverse part of the colon, were surgically obtained from anesthetized animals by licensed staff of the Department of Experimental Medicine, the Panum Institute, Copenhagen, Denmark.
Immunofluorescence Microscopy
Small segments of jejunum and colon were fixed immediately after excision from the animals in 4% paraformaldehyde in 0.1 M sodium phosphate, pH 7.2 (PB), for 2 hr at 4C. After a rinse in PB, the segments were frozen in precooled 2-methylbutane and mounted on a precooled cryostat table. Sections were cut in a Leica CM1850 cryostat at –20C, collected on glass slides, and labeled with antibodies to leptin, OB-R, and Na+/K+-ATPase, followed by labeling with Alexa 488/594-conjugated secondary antibodies. Control experiments were routinely performed in parallel by omission of the primary antibodies. Finally, sections were mounted in antifade medium and examined in a Leica DM 4000 B microscope equipped with a Leica DC 300 FX digital camera (Leica; Wetzlar, Germany).
Immunogold Electron Microscopy
For ultracryosectioning, intestinal segments were fixed in 4% paraformaldehyde and rinsed in PB as described above. The segments were immersed overnight in 2.3 M sucrose, containing 1% paraformaldehyde, and mounted on top of a metal pin and frozen in liquid nitrogen. Ultracryosections were cut in a RMC MT 6000-XL ultramicrotome (Tucson, AZ), collected with a sucrose droplet, and attached to formvar-coated nickel grids. Immunogold labeling was performed with primary antibodies to leptin and OB-R, followed by gold-conjugated secondary antibodies, as previously described (Hansen et al. 1999). The antibody to OB-R was raised against amino acids 541-840 mapping within an internal domain of the human OB-R. Among several commercial antibodies initially tested for this study, this antibody was selected because it recognized a single band of ∼150 kDa in immunoblotting in the membrane fractions of porcine jejunum and colon. This particular antibody has previously been used by others for immunodetection of OB-R in breast cancer cells and bone marrow stromal cells (Kim et al. 2003; Saxena et al. 2007).
Control experiments were routinely included in parallel by omission of the primary antibodies. Finally, the labeled ultracryosections were examined in a Zeiss EM 900 electron microscope equipped with a Mega View II digital camera (Zeiss; Oberhochen, Germany).
Morphometric Analysis
A morphometric analysis of double immunogold–labeled sections of jejunum and colon was performed as follows: 7- (OB-R) and 13-nm (leptin) gold particles were counted in images of apical and basolateral areas of both jejunum and colon. Only particles lining the plasma membrane were counted and scored either as non-colocalized (when the distance between small and large particles was >30 nm) or colocalized. About 300 μm of randomly selected plasma membranes was analyzed in each of the subcellular locations, using the Mega View II software system.
Subcellular Fractionation
Samples of ∼1 g of jejunal mucosa were fractionated by the divalent cation precipitation method (Booth and Kenny 1974). Briefly, the mucosa was homogenized in 5 ml 2 mM Tris-HCl and 50 mM mannitol, pH 7.1, containing 10 μg/ml aprotinin and leupeptin using a manually operated Potter-Elvehjem homogenizer. The homogenate was centrifuged at 120 × g for 5 min, and MgCl2 was added to the supernatant to a final concentration of 10 mM. After a 10-min incubation on ice, the preparation was centrifuged at 1100 × g for 10 min to pellet intracellular and basolateral membranes (Mg2+-precipitated fraction). The supernatant was centrifuged at 48,000 × g for 30 min to obtain a pellet of microvillar membranes and a supernatant of soluble proteins.
Samples of ∼1 g of colon were homogenized in 5 ml 25 mM HEPES-HCl and 150 mM NaCl, pH 7.1, containing 10 μg/ml aprotinin and leupeptin as described above. The homogenate was centrifuged at 120 × g for 5 min, and the supernatant was centrifuged at 48,000 × g for 30 min to obtain a pellet of total membranes and a supernatant of soluble proteins.
Analysis of Detergent-resistant Membranes (DRMs)
DRMs were prepared from jejunal Mg2+-precipitated membranes and resuspended in 1 ml of 25 mM HEPES-HCl and 150 mM NaCl, pH 7.1, containing 10 μg/ml aprotinin and leupeptin by extraction with 1% Triton X-100 for 10 min on ice. The DRMs were isolated by sucrose gradient ultracentrifugation as previously described (Brown and Rose 1992; Danielsen 1995), and after centrifugation, the gradients were fractionated for subsequent analysis by SDS/PAGE and immunoblotting.
Electrophoresis and Immunoblotting
SDS/PAGE in 10% or 15% gels was performed as previously described (Laemmli 1970). After electrophoresis and electrotransfer onto Immobilon polyvinylidene difluoride (PVDF) membranes, immunoblotting was performed using primary antibodies to leptin, OB-R, or IAP, followed by incubation with horseradish peroxidase–coupled secondary antibodies. The blots were developed using an electrochemiluminescence detection reagent according to the protocol supplied by the manufacturer. After immunoblotting, total protein was visualized by staining the membrane with Coomassie Brilliant Blue.
Results
Localization by Immunofluorescence Microscopy of OB-R and Leptin in the Small Intestine and Colon
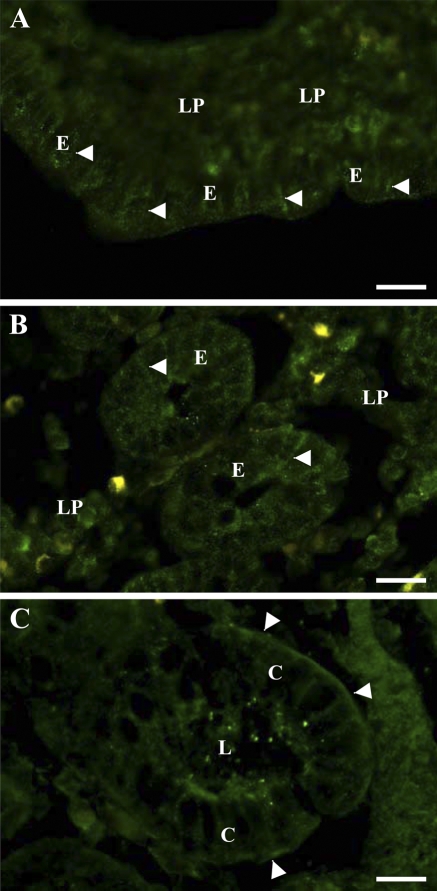
Figure 1A shows the localization of OB-R in the porcine jejunum. The strongest labeling was seen along the lateral sides of the enterocytes, which colocalized with that of the Na+/K+-ATPase, a basolateral marker of epithelial cells (Figures 1B and 1C). In addition, a fainter and more patchy labeling of the apical brush border was detected as well.
Figure 1.
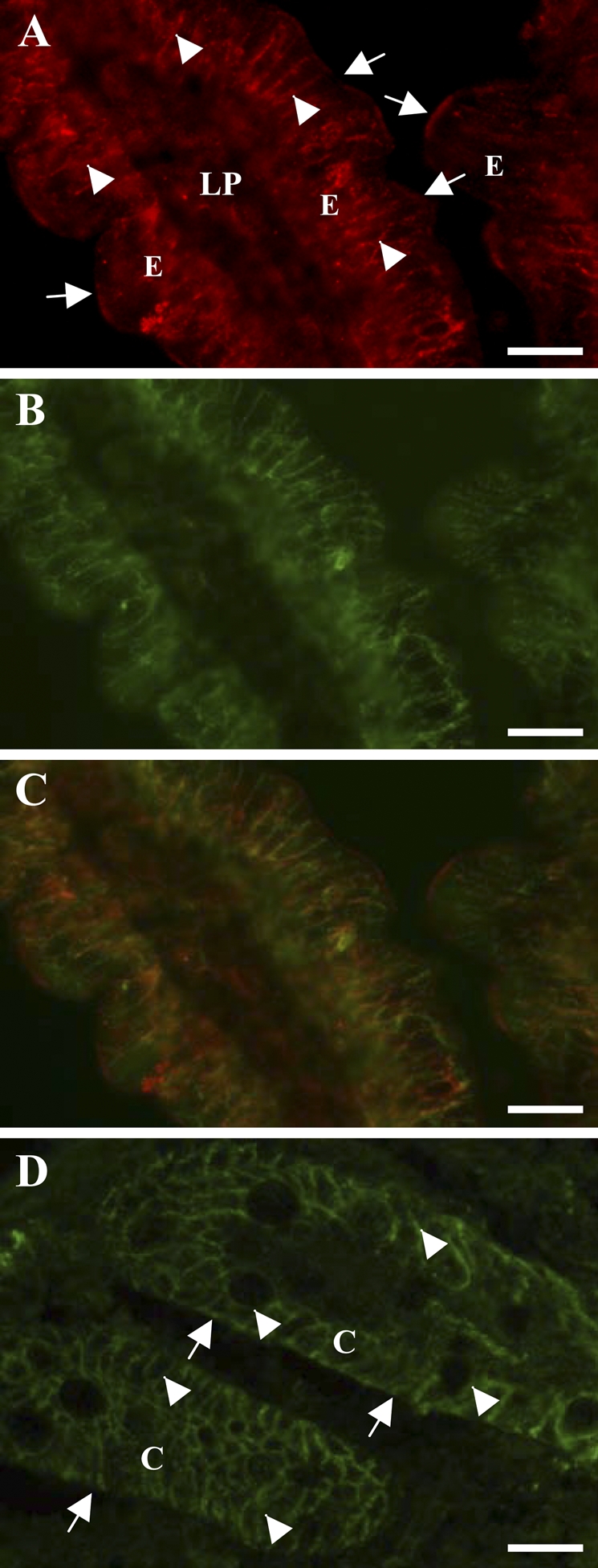
Localization by immunofluorescence microscopy of OB-R in jejunum and colon. Cryosections of jejunum (A–C) or colon (D) were labeled with an antibody to OB-R (A,D) or Na+/K+-ATPase (B). In the jejunum, OB-R labeling was most intense along the basolateral surface (arrowheads in A) of the enterocytes, as shown by its colocalization with the basolateral marker Na+/K+-ATPase (C), but patchy labeling at the apical brush border was also observed (arrows in A). In colonocytes, OB-R was mainly seen along the basolateral surface (arrowheads in D) and only very weakly at the apical membrane (arrows in D). C, colonocytes; E, enterocytes; LP, lamina propria. Bar = 10 μm.
In the colon, OB-R was clearly seen lining the basolateral sides of the colonocytes, whereas little labeling was observed along the apical surface (Figure 1D).
Figures 2A and 2B shows the distribution of leptin in the villus and crypt regions of the jejunum. In both areas, the leptin labeling of the enterocytes was punctate. The labeling mainly appeared along the lateral sides of the cells, but in addition, a weaker, diffuse labeling was present over the lamina propria.
Figure 2.
Localization by immunofluorescence microscopy of endogeneous leptin in jejunum and colon. Cryosections of jejunum (A,B) or colon (C) were labeled with an antibody to leptin. In enterocytes of both villus (A) and crypt (B) regions, the labeling was punctate and most intense along the basolateral surface (arrowheads). In colonocytes (C), the labeling was most distinct at the basal surface (arrowheads), and some amorphous labeling was present at the apical surface in the crypt lumen (L). In both tissues, labeling over the lamina propria was also observed. E, enterocytes; LP, lamina propria. Bar = 10 μm.
In the colon, leptin labeling was strongest and most distinct along the basal side of the colonocytes, but the hormone was also seen along the lateral sides. At the apical surface, some amorphous labeling was seen. In addition, a diffuse labeling was present over the lamina propria (Figure 2C).
Results of immunofluorescence microscopy showed that the OB-R is mainly localized at the cell surfaces of enterocytes and colonocytes. For both cell types, the most intense labeling was at the basolateral side of the plasma membrane, but distinct apical labeling was also detected, most notably in enterocytes. In addition, endogeneous leptin was detectable both in the jejunum and the colon. Again, labeling was mainly seen basolaterally, but the detectable apical labeling suggests that an immunoactive form of the hormone is present in the lumen of the gut.
Colocalization by Double Immunogold Electron Microscopy
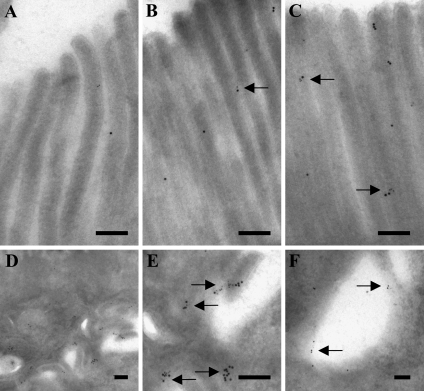
To study in closer detail the colocalization of OB-R and leptin, double immunogold labeling electron microscopy for the two proteins was performed on sections of both jejunum and colon. As shown in Figures 3A–3C, both OB-R and leptin were detected in the enterocyte brush border. The labeling for both proteins was less dense over the microvilli and rarely seen in “microcrypts” at the base of the microvilli. Furthermore, close colocalization of receptor and ligand, i.e., clusters of small and large gold particles, was detected less frequently.
Figure 3.
Double immunogold labeling for OB-R and leptin in jejunal enterocytes. Electron micrographs of the apical brush border (A–C) show a scattered labeling for both proteins along the microvilli. Colocalization of OB-R (large particles) and leptin (small particles) was only occasionally observed (arrows), but labeling at the bottom of the microvilli was not detected. By comparison, labeling along the basolateral plasma membrane was more intense (D). At a higher magnification of a section of the same view (E), most of the small and large gold particles were seen to be colocalized along the membrane (arrows). In addition, both OB-R and leptin were seen in endosomes in the vicinity of the basolateral plasma membrane (F), where they also often colocalized (arrows). Bar = 0.2 μm.
Both OB-R and leptin labeling was seen at a higher intensity at the lateral surfaces of the enterocytes (Figures 3D and 3E). Here, clusters of small and large gold particles were frequently observed, indicating the presence of receptor ligand complexes. Clusters of labeling for both proteins was also frequently seen in endosomes in the vicinity of the lateral surface (Figure 3F).
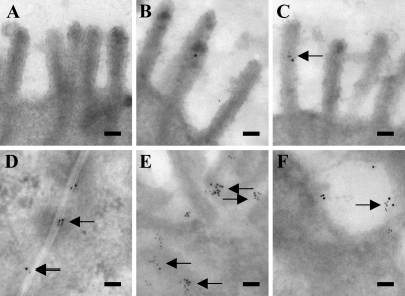
In the colon, relatively small amounts of leptin and OB-R labeling were observed at the apical microvillar surface of the colonocytes (Figures 4A–4C). At the basolateral surface, more immunogold particles, both large (leptin) and small (OB-R), were seen, showing the presence of both ligand and receptor (Figures 4D and 4E). Furthermore, close colocalization of small and large gold particles occurred more frequently than at the apical surface. In addition, both particles were also coclustered in endosomes along the basolateral membrane (Figure 4F). A morphometric analysis of the apical/basolateral labeling density along the plasma membrane of enterocytes and colonocytes is summarized in Table 1.
Figure 4.
Double immunogold labeling for OB-R and leptin in colonocytes. Electron micrographs of the apical surface (A–C) showed no (A) or little (B,C) labeling for both OB-R (large particles) and leptin (small particles) along the microvilli, and colocalization was only very rarely detected (arrow). Labeling at the flat, non-microvillar part of the apical plasma membrane was not observed. At the basolateral surface (D,E) and in nearby endosomes (F), labeling for both proteins was much more abundant, and colocalization was frequently observed (arrows). Bar = 0.1 μm.
Table 1.
Morphometric analysis of localization and colocalization of leptin and OB-R in jejunum and colon
| Membrane | Leptin, non-colocalized (particles/100 μm) | Leptin, colocalized (particles/100 μm) | OB-R, non-colocalized (particles/100 μm) | OB-R, colocalized (particles/100 μm) |
|---|---|---|---|---|
| Jejunum, apical | 13.5 | 5.3 | 23.1 | 12.9 |
| Jejunum, basolateral | 44.4 | 22.6 | 49.9 | 33.2 |
| Colon, apical | 13.8 | 9.5 | 31.4 | 13.2 |
| Colon, basolateral | 11.2 | 23.1 | 43.5 | 58.8 |
Seven- (OB-R) and 13-nm (leptin) gold particles lining the apical and basolateral membranes of enterocytes and colonocytes were counted in double immunogold–labeled sections of jejunum and colon, as described in Materials and Methods.
No labeling over nuclei or mitochondria was observed for either of the antibodies used, implying that nonspecific reactivity was absent or at a very low level.
In summary, leptin and OB-R were detected by immunofluorescence and immunogold microscopy both at the apical and basolateral surfaces of enterocytes and colonocytes. However, the basolateral plasma membrane was the predominant cellular location for receptor and ligand, and close colocalization, indicative of receptor–ligand complexes, was more frequently observed here than at the apical cell surface. Finally, the presence of leptin and OB-R in endosomes along the basolateral but not the apical cell surface suggests that this receptor–ligand system is functional only at the “blood side” of both enterocytes and colonocytes.
Leptin and OB-R in Subcellular Fractions
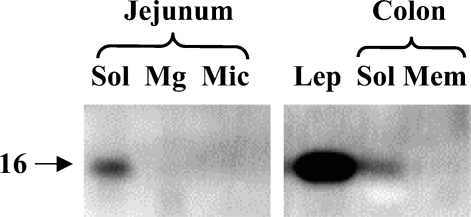
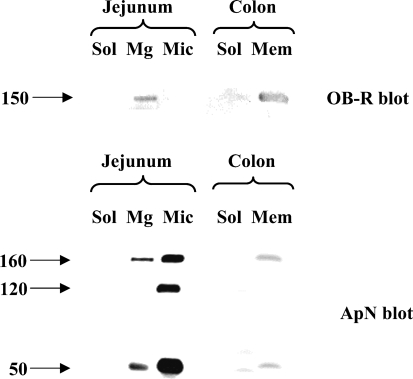
As shown in Figure 5, endogeneous leptin was detectable by immunoblotting in both jejunum and colon. In both intestinal tissues, leptin was present in the soluble fraction, as should be expected of a hormone. Figure 6 shows the subcellular distribution of the OB-R in jejunum and colon. In jejunum, a single ∼150-kDa band of the receptor was present predominantly in the Mg2+-precipitated fraction, which contains basolateral and intracellular membranes (Booth and Kenny 1974). In previous reports, the size of the long form (OB-Rb) of the receptor was reported to be 130–135 kDa, whereas the size of the short forms was in the range of 85–90 kDa (Buyse et al. 2001; Hardwick et al. 2001). The 150-kDa band seen in Figure 6 therefore most likely represents the porcine form of OB-Rb. By comparison, the amounts of OB-R in the microvillar fraction were barely at the level of detection, and no OB-R was detectable in the soluble fraction. Unlike OB-R, the brush border marker aminopeptidase N (ApN) was mainly present in the microvillar fraction (Figure 6). The subcellular distribution of Ob-R agrees well with the localization of the receptor observed by fluorescence and electron microscopy (Figures 1–4).
Figure 5.
Localization of leptin in subcellular fractions of jejunum and colon by immunoblotting. Samples of soluble (Sol), Mg2+-precipitated (Mg), and microvillar (Mic) fractions of jejunum and soluble (Sol) and total membrane (Mem) fractions of colon were subjected to SDS/PAGE in a 15% gel together with a sample of pure leptin (Lep, 1 μg). After transfer onto an Immobilon membrane, leptin was detected by immunoblotting. Molecular mass value of leptin is indicated by the arrow.
Figure 6.
Localization of OB-R and aminopeptidase N (ApN) in subcellular fractions. Samples of soluble (Sol), Mg2+-precipitated (Mg), and microvillar (Mic) fractions of jejunum and soluble (Sol) and total membrane (Mem) fractions of colon were subjected to SDS/PAGE in 10% gel. OB-R and ApN were detected by immunoblotting. Molecular mass values are indicated by arrows.
In the colon, OB-R (as well as ApN) was present in the total membrane fraction but not in the cytosol (Figure 6).
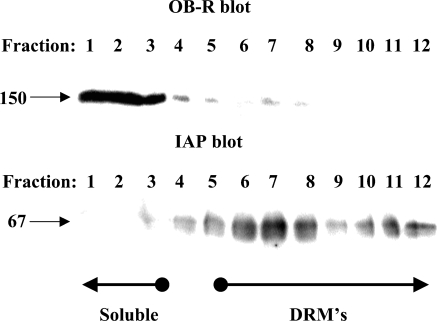
Many proteins destined for insertion in the apical plasma membrane of epithelial cells associate with lipid rafts during intracellular transport (Danielsen 1995; Simons and Ikonen 1997). Biochemically, this can be analyzed by their ability to resist solubilization with Triton X-100 at a low temperature and become part of DRMs that can be isolated by subsequent density gradient ultracentrifugation (Brown and Rose 1992). As shown in Figure 7, OB-R in enterocytes was largely soluble in cold Triton X-100, whereas the glycosylphosphatidylinositol-anchored and lipid raft–associated brush border enzyme IAP strongly associated with the DRMs. This experiment thus shows that OB-R is not actively sorted in the trans-Golgi network by lateral inclusion into lipid rafts, as is the case for many resident apical proteins of the enterocyte (Danielsen and Hansen 2003).
Figure 7.
Detergent-resistant membrane (DRM) analysis of OB-R and intestinal alkaline phosphatase (IAP). DRMs were prepared from the Mg2+-precipitated membrane fraction by extraction with Triton X-100 followed by sucrose gradient ultracentrifugation as described in Materials and Methods. The gradient fractions were subjected to SDS/PAGE in a 10% gel, and OB-R and IAP were detected by immunoblotting. Molecular mass values and the position in the gradient of soluble proteins and DRMs are indicated by arrows.
Discussion
This study was prompted by the fact that, despite the many reports published thus far on leptin action in intestinal cells, the question remains of whether the hormone uses an endocrine or exocrine pathway or both. At the level of light microscopy, several studies have previously localized the intestinal OB-R to the apical and/or basolateral side of enterocytes and colonocytes (Buyse et al. 2001; Hardwick et al. 2001; Barrenetxe et al. 2002; Aparicio et al. 2004,2005; Cammisotto et al. 2005), and in most of these studies, labeling at both aspects of the plasma membrane was detected, albeit at varying intensity. By immunofluorescence microscopy, we were able to detect OB-R at the basolateral plasma membrane both in enterocytes and colonocytes. Apically, we observed a patchy but distinct labeling of the enterocyte brush border and a relatively weak labeling at the lumenal membrane of colonocyte. Collectively, this large pool of published data therefore implies that the receptor is not sorted very discriminitatively to either the blood or lumenal side of the epithelial cells of the gut. This may be explained by the fact that, for many newly synthesized proteins that are truly apically destined, efficient sorting involves association with lipid rafts in the trans-Golgi network (Danielsen 1995; Ikonen and Simons 1998). Therefore, the absence of the OB-R in DRMs prepared from enterocyte membranes implies that the receptor lacks a recognition signal for lipid raft–mediated apical transport. This may not be surprising given the widespread distribution of OB-R in non-polarized cells of tissues where its ligand can only be encountered at the blood side, but in this context, it nevertheless suggests that the basolateral surface is the “true” destination of the OB-R. Signals for protein sorting to the basolateral plasma membrane are commonly known to be short tyrosine– or dileucine-based motifs residing in the cytoplasmic tail that interact with adaptor protein complexes and clathrin (Rodriguez-Boulan and Musch 2005; Ellis et al. 2006). Most of the proteins in this category are blood side cell surface receptors (including the LDL receptor, transferrin receptor, asialoglycoprotein receptor) that recycle to the endosomes, such as OB-R (Fruhbeck 2006).
In this study, we observed the highest labeling intensity for OB-R by fluorescence microscopy at the basolateral surface, whereas the apical labeling of both enterocytes and colonocytes was weaker and patchy. For leptin, the punctate labeling seen mainly along the basolateral surfaces of enterocytes and colonocytes, but also apically, indicates an approximately similar localization as OB-R. Altogether, this implies that receptor activation by ligand binding in principle may take place at both surfaces of intestinal epithelial cells. In search for a closer interaction between OB-R and leptin, we therefore performed immunogold double labeling electron microscopy to detect in situ protein–protein colocalization in the nanometer range.
At the basolateral surface, colocalization of OB-R and leptin by immunogold electron microscopy showed the presence of clusters of large and small gold particles, indicating that functional receptor–ligand complexes form at this part of the plasma membrane. Furthermore, as a member of the cytokine receptor family, OB-R is known to be internalized after ligand binding through clathrin-coated vesicles into early endosomes (Sweeney 2002; Fruhbeck 2006). In both enterocytes and colonocytes, coclusters of OB-R and leptin were frequently detected in endosomal vesicles along the basolateral cell surface, indicative of an ongoing endocytosis.
At the apical microvillar membrane, labeling for both OB-R and leptin was relatively weak, and clusters of large and small immunogold particles occurred less frequently. Furthermore, in the enterocyte, the labeling for both proteins was mainly seen up along the sides of the microvilli where the dense, actin-based microvillar cytoskeleton sterically prohibits coated pit formation and vesicle internalization. In fact, the enterocyte brush border is known to act effectively as a permeability barrier and does not normally engage in endocytosis after “closure” at the neonatal stage (Rodewald 1970). However, when triggered to do so, for instance by cholera toxin, the coated pits will form at the “microcrypts” situated between adjacent microvilli (Hansen et al. 2005). However, despite a thorough inspection of labeled sections, we failed to detect any immunogold localization of either OB-R or leptin, not to mention colocalization, in these microcrypts. A possible explanation for this could be that lumenal leptin may not be fully functional in the harsh environment of the gut. Any evidence of a functional interaction between the OB-R and leptin at the lumenal surface of intestinal epithelial cells therefore was not shown by this colocalization study.
In summary, the close colocalization of leptin and OB-R seen at the basolateral plasma membrane of enterocytes and colonocytes strongly argues for an endocrine leptin action in intestinal cells. In contrast, the results obtained do not support the notion of a functional interaction between hormone and receptor at the lumenal surface. A physiological role of lumenally secreted gastric leptin therefore remains to be clarified, but interestingly, a recent publication described transcytosis of gastric leptin through duodenal enterocytes to reach the blood circulation (Cammisotto et al. 2007). The receptor responsible for this apical-to-basolateral transcytosis, which occurred in fed but not fasted animals, was not identified, but the finding nevertheless implies that exocrine gastric leptin may have autocrine and endocrine functions in the intestine.
Acknowledgments
This work was supported by grants from the Danish Medical Research Council, the Novo-Nordic Foundation, the Augustinus Foundation, and the Beckett Foundation.
References
- Ahima RS, Flier JS (2000) Leptin. Annu Rev Physiol 62:413–437 [DOI] [PubMed] [Google Scholar]
- Aparicio T, Guilmeau S, Goiot H, Tsocas A, Laigneau JP, Bado A, Sobhani I, et al. (2004) Leptin reduces the development of the initial precancerous lesions induced by azoxymethane in the rat colonic mucosa. Gastroenterology 126:499–510 [DOI] [PubMed] [Google Scholar]
- Aparicio T, Kermorgant S, Darmoul D, Guilmeau S, Hormi K, Mahieu-Caputo D, Lehy T (2005) Leptin and Ob-Rb receptor isoform in the human digestive tract during fetal development. J Clin Endocrinol Metab 90:6177–6184 [DOI] [PubMed] [Google Scholar]
- Attoub S, Noe V, Pirola L, Bruyneel E, Chastre E, Mareel M, Wymann MP, et al. (2000) Leptin promotes invasiveness of kidney and colonic epithelial cells via phosphoinositide 3-kinase-, Rho-, and Rac-dependent signaling pathways. FASEB J 14:2329–2338 [DOI] [PubMed] [Google Scholar]
- Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, Moizo L, et al. (1998) The stomach is a source of leptin. Nature 394:790–793 [DOI] [PubMed] [Google Scholar]
- Baratta M (2002) Leptin: from a signal of adiposity to a hormonal mediator in peripheral tissues. Med Sci Monit 8:RA282–292 [PubMed] [Google Scholar]
- Barrenetxe J, Villaro AC, Guembe L, Pascual I, Munoz-Navas M, Barber A, Lostao MP (2002) Distribution of the long leptin receptor isoform in brush border, basolateral membrane, and cytoplasm of enterocytes. Gut 50:797–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorbaek C, Kahn BB (2004) Leptin signaling in the central nervous system and the periphery. Recent Prog Horm Res 59:305–331 [DOI] [PubMed] [Google Scholar]
- Booth AG, Kenny AJ (1974) A rapid method for the preparation of microvilli from rabbit kidney. Biochem J 142:575–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Rose JK (1992) Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68:533–544 [DOI] [PubMed] [Google Scholar]
- Buyse M, Berlioz F, Guilmeau S, Tsocas A, Voisin T, Peranzi G, Merlin D, et al. (2001) PepT1-mediated epithelial transport of dipeptides and cephalexin is enhanced by luminal leptin in the small intestine. J Clin Invest 108:1483–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammisotto PG, Gingras D, Bendayan M (2007) Transcytosis of gastric leptin through the rat duodenal mucosa. Am J Physiol Gastrointest Liver Physiol 293:G773–G779 [DOI] [PubMed] [Google Scholar]
- Cammisotto PG, Gingras D, Renaud C, Levy E, Bendayan M (2006) Secretion of soluble leptin receptors by exocrine and endocrine cells of the gastric mucosa. Am J Physiol Gastrointest Liver Physiol 290:G242–249 [DOI] [PubMed] [Google Scholar]
- Cammisotto PG, Renaud C, Gingras D, Delvin E, Levy E, Bendayan M (2005) Endocrine and exocrine secretion of leptin by the gastric mucosa. J Histochem Cytochem 53:851–860 [DOI] [PubMed] [Google Scholar]
- Danielsen EM (1995) Involvement of detergent-insoluble complexes in the intracellular transport of intestinal brush border enzymes. Biochemistry 34:1596–1605 [DOI] [PubMed] [Google Scholar]
- Danielsen EM, Hansen GH (2003) Lipid rafts in epithelial brush borders: atypical membrane microdomains with specialized functions. Biochim Biophys Acta 1617:1–9 [DOI] [PubMed] [Google Scholar]
- Doi T, Liu M, Seeley RJ, Woods SC, Tso P (2001) Effect of leptin on intestinal apolipoprotein AIV in response to lipid feeding. Am J Physiol Regul Integr Comp Physiol 281:R753–759 [DOI] [PubMed] [Google Scholar]
- Ducroc R, Guilmeau S, Akasbi K, Devaud H, Buyse M, Bado A (2005) Luminal leptin induces rapid inhibition of active intestinal absorption of glucose mediated by sodium-glucose cotransporter 1. Diabetes 54:348–354 [DOI] [PubMed] [Google Scholar]
- El Homsi M, Ducroc R, Claustre J, Jourdan G, Gertler A, Estienne M, Bado A, et al. (2007) Leptin modulates the expression of secreted and membrane-associated mucins in colonic epithelial cells by targeting PKC, PI3K, and MAPK pathways. Am J Physiol Gastrointest Liver Physiol 293:G365–373 [DOI] [PubMed] [Google Scholar]
- Ellis MA, Potter BA, Cresawn KO, Weisz OA (2006) Polarized biosynthetic traffic in renal epithelial cells: sorting, sorting, everywhere. Am J Physiol Renal Physiol 291:F707–713 [DOI] [PubMed] [Google Scholar]
- Fruhbeck G (2006) Intracellular signalling pathways activated by leptin. Biochem J 393:7–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen GH, Dalskov SM, Rasmussen CR, Immerdal L, Niels-Christiansen LL, Danielsen EM (2005) Cholera toxin entry into pig enterocytes occurs via a lipid raft- and clathrin-dependent mechanism. Biochemistry 44:873–882 [DOI] [PubMed] [Google Scholar]
- Hansen GH, Niels-Christiansen LL, Immerdal L, Hunziker W, Kenny AJ, Danielsen EM (1999) Transcytosis of immunoglobulin A in the mouse enterocyte occurs through glycolipid Raft- and Rab17-containing compartments. Gastroenterology 116:610–622 [DOI] [PubMed] [Google Scholar]
- Hansen GH, Sjostrom H, Noren O, Dabelsteen E (1987) Immunomicroscopic localization of aminopeptidase N in the pig enterocyte. Implications for the route of intracellular transport. Eur J Cell Biol 43:253–259 [PubMed] [Google Scholar]
- Hardwick JC, Van Den Brink GR, Offerhaus GJ, Van Deventer SJ, Peppelenbosch MP (2001) Leptin is a growth factor for colonic epithelial cells. Gastroenterology 121:79–90 [DOI] [PubMed] [Google Scholar]
- Ihle JN (1995) Cytokine receptor signalling. Nature 377:591–594 [DOI] [PubMed] [Google Scholar]
- Ikonen E, Simons K (1998) Protein and lipid sorting from the trans-Golgi network to the plasma membrane in polarized cells. Semin Cell Dev Biol 9:503–509 [DOI] [PubMed] [Google Scholar]
- Kim GS, Hong JS, Kim SW, Koh JM, An CS, Choi JY, Cheng SL (2003) Leptin induces apoptosis via ERK/CPLA2/cytochrome c pathway in human bone marrow stromal cells. J Biol Chem 278:21920–21929 [DOI] [PubMed] [Google Scholar]
- Koda M, Sulkowska M, Kanczuga-Koda L, Surmacz E, Sulkowski S (2007) Overexpression of the obesity hormone leptin in human colorectal cancer. J Clin Pathol 60:902–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- Lostao MP, Urdaneta E, Martinez-Anso E, Barber A, Martinez JA (1998) Presence of leptin receptors in rat small intestine and leptin effect on sugar absorption. FEBS Lett 423:302–306 [DOI] [PubMed] [Google Scholar]
- Masuzaki H, Ogawa Y, Sagawa N, Hosoda K, Matsumoto T, Mise H, Nishimura H, et al. (1997) Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat Med 3:1029–1033 [DOI] [PubMed] [Google Scholar]
- Morton NM, Emilsson V, Liu YL, Cawthorne MA (1998) Leptin action in intestinal cells. J Biol Chem 273:26194–26201 [DOI] [PubMed] [Google Scholar]
- Myers MG, Cowley MA, Munzberg H (2008) Mechanisms of leptin action and leptin resistance. Annu Rev Physiol 70:537–556 [DOI] [PubMed] [Google Scholar]
- Plaisancie P, Ducroc R, El Homsi M, Tsocas A, Guilmeau S, Zoghbi S, Thibaudeau O, et al. (2006) Luminal leptin activates mucin-secreting goblet cells in the large bowel. Am J Physiol Gastrointest Liver Physiol 290:G805–812 [DOI] [PubMed] [Google Scholar]
- Rodewald R (1970) Selective antibody transport in the proximal small intestine of the neonatal rat. J Cell Biol 45:635–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Musch A (2005) Protein sorting in the Golgi complex. Shifting paradigms. Biochim Biophys Acta 1744:455–464 [DOI] [PubMed] [Google Scholar]
- Saxena NK, Vertino PM, Anania FA, Sharma D (2007) Leptin-induced growth stimulation of breast cancer cells involves recruitment of histone acetyltransferases and mediator complex to CYCLIN D1 promoter via activation of Stat3. J Biol Chem 282:13316–13325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Ikonen E (1997) Functional rafts in cell membranes. Nature 387:569–572 [DOI] [PubMed] [Google Scholar]
- Sitaraman S, Liu X, Charrier L, Gu LH, Ziegler TR, Gewirtz A, Merlin D (2004) Colonic leptin: source of a novel proinflammatory cytokine involved in IBD. FASEB J 18:696–698 [DOI] [PubMed] [Google Scholar]
- Sweeney G (2002) Leptin signalling. Cell Signal 14:655–663 [DOI] [PubMed] [Google Scholar]
- Wang J, Liu R, Hawkins M, Barzilai N, Rossetti L (1998) A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature 393:684–688 [DOI] [PubMed] [Google Scholar]
- Wilkinson M, Morash B, Ur E (2000) The brain is a source of leptin. Front Horm Res 26:106–125 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432 [DOI] [PubMed] [Google Scholar]