Abstract
An increasing amount of evidence indicates that a small extracellular chondroitin/dermatan sulfate proteoglycan, decorin, is indirectly involved in angiogenesis. Given that angiogenesis is a sine qua non for tumor growth and progression, we attempted to examine whether human malignant vascular tumors differ from human benign vascular tumors in terms of their decorin expression and synthesis. CD31 immunostaining demonstrated that the human malignant vascular tumors Kaposi's sarcoma and angiosarcoma were filled with capillary-like structures, whereas in benign cavernous and capillary hemangiomas, blood vessels were not as abundantly present. By utilizing in situ hybridization and immunocytochemical assays for decorin, we showed that there was no detectable decorin mRNA expression or immunoreactivity within the tumor mass in the Kaposi's sarcoma or angiosarcoma group. Instead, decorin was expressed in the connective tissue stroma lining the sarcoma tissue. In contrast to sarcomas, in hemangiomas, decorin mRNA expression and immunoreactivity were observed also within the tumor mass, particularly in the connective tissue stroma surrounding the clusters of intratumoral blood vessels. Finally, distribution of type I collagen was found to be similar to that of decorin in these tumor tissues. Our findings can be explained with different states of angiogenesis in dissimilar growths. In sarcomas, angiogenesis is extremely powerful, whereas in hemangiomas, angiogenesis has ceased. Thus, decorin is likely to possess a suppressive effect on human tumor angiogenesis in vivo, as previously described by studies using different experimental models. Decorin certainly provides a usable biomarker for distinguishing between benign and malignant vascular tumors in patients. (J Histochem Cytochem 56:639–646, 2008)
Keywords: decorin, type I collagen, sarcoma, hemangioma, angiogenesis
Angiogenesis involves degradation of the extracellular matrix (ECM), proliferation, migration, and capillary tube formation of endothelial cells, followed by matrix remodeling (Carmeliet 2003). Molecular markers that can be used to distinguish physiological and pathological angiogenesis in normal tissues and in various disease states are required for both diagnostic purposes and to formulate new therapeutic approaches (Seaman et al. 2007). Recently it has become evident that normal and tumor-associated endothelium are extremely different with relation to their gene expression profile. Furthermore, it has been found that several of the differentially expressed genes between normal and tumor-derived endothelial cells belong to genes encoding ECM proteins such as α 1 chains of types I and III collagen (St. Croix et al. 2000). An ECM molecule that has also been shown to be involved in angiogenesis is decorin, a multifunctional small chondroitin/dermatan sulfate proteoglycan of the leucine-rich proteoglycan gene family (Iozzo 1997). Originally, decorin was connected to angiogenesis in vitro by demonstrating that cultured bovine aortic endothelial cells exhibiting a spontaneous sprouting phenotype initiate the synthesis of decorin during their morphological transition from a polygonal monolayer to an angiogenic phenotype (Järveläinen et al. 1992). Subsequently, decorin's involvement in angiogenesis, particularly in association with inflammation, has been demonstrated in several studies (e.g., Schönherr et al. 1999; Nelimarkka et al. 2001; Burke et al. 2004). Currently, it is thought that decorin is primarily an inhibitor of angiogenesis (De Lange Davies et al. 2001; Sulochana et al. 2005; Järveläinen et al. 2006), although opposing views have also been presented (Schönherr et al. 2005). There is also evidence available suggesting that the role of decorin in tumor angiogenesis is suppressive (Grant et al. 2002).
The molecular mechanisms governing decorin's involvement in various forms of angiogenesis are still speculative. However, it is most likely that decorin's role in the regulation of angiogenesis is indirect. This statement can be based, for example, on the fact that decorin is capable of interacting with and regulating the activity of a number of growth factors (e.g., Hildebrand et al. 1994; Penc et al. 1998; Nili et al. 2003) and growth factor receptors involved in angiogenesis (Reed et al. 2005; De Luca et al. 2008). Moreover, decorin can reduce the expression of endogenous vascular endothelial growth factor mRNA and protein, thereby influencing angiogenesis (Grant et al. 2002). Furthermore, decorin modulates the formation of fibrillar pericellular matrix in a way that stabilizes the differentiated endothelial phenotype during angiogenesis (Kinsella et al. 2000).
In this study we have continued to further investigate the role of decorin in angiogenesis, particularly in tumor angiogenesis in patients. Using tissue samples of the human vascular malignancies Kaposi's sarcoma and angiosarcoma, and tissue samples of human benign vascular tumors, hemangiomas, we have specifically aimed to examine whether there is a difference in the expression of decorin between malignant and benign vascular growths in vivo. In addition, by applying in situ hybridization (ISH), we have also focused our attention on the spatial location of decorin mRNA expression within human vascular tumors and their surrounding tissue. Furthermore, because decorin is known to interact with collagens such as type I collagen (Brown and Vogel 1989) and growth factor receptors, particularly epidermal growth factor receptor (EGFR) (Iozzo et al. 1999), we have examined the distribution of both of the above molecules in the same tumor tissues using immunocytochemistry (ICC).
Materials and Methods
Tissue Specimens
In this study, human malignant (Kaposi's sarcoma and angiosarcoma) and benign (capillary and cavernous hemangiomas) vascular tumor specimens were used. Kaposi's sarcoma specimens were derived from skin of seven patients (five men, two women, mean age 75 years, range 72 to 83 years at the time of diagnosis). Similarly, angiosarcoma specimens of seven patients were used (six women and one man, mean age 70 years, range 28 to 86 years at the time of diagnosis). Hemangiomas were collected from skin of eight patients. Four of the hemangioma specimens were cavernous hemangiomas and four were capillary hemangiomas. All sarcoma and hemangioma specimens were obtained from the archives of Turku University Central Hospital, Department of Pathology, Turku, Finland. The specimens were classified according to the standard criteria of Weedon (2002), as recommended by the International Academy of Pathology. All specimens were fixed in 10% neutral-buffered formalin and, following paraffin-embedding, 5-μm transverse sections were cut and used for ICC and ISH analyses.
ICC
ICC analyses were performed as previously described (Nelimarkka et al. 2001). Briefly, 5-μm transverse sections of paraffin-embedded tissue specimens were dewaxed in xylene and rehydrated in a descending concentration of ethanol, then washed in PBS. Next, the sections were treated with 0.3% H2O2 in deionized water for 15 min to block endogenous peroxidase activity. Nonspecific antibody binding was blocked by treatment with 2% BSA and 2% normal goat serum (Vector Laboratories; Burlingame, CA) in PBS for 30 min. Incubations with primary antibodies were performed overnight at 4C, then slides were washed with PBS and incubated for 1 hr with a biotinylated secondary antibody (dilution 1:200). After rinses with PBS, the sections were treated with avidin-peroxidase complex solution (Vector Laboratories) for 35 min. Visualization of the signal was achieved with DAB (Vector Laboratories), and the sections were counterstained with Papanicolaou hematoxylin and mounted using Aquamount (BDH Laboratory Supplies; Dorset, England). In type I collagen immunostaining, unspecific binding of the primary antibody was blocked by treatment with 2% BSA and 2% normal goat serum (Vector Laboratories) in PBS overnight, and incubation with the primary antibody was performed for 1 hr at room temperature. PBS used after the primary and secondary antibody contained 0.2% Tween-20. All procedures were carried out at room temperature unless otherwise specified. Control stainings of the tissue sections were performed as described above, but no primary antibody was used. Primary antibodies used in this study were as follows: a polyclonal rabbit antiserum LF-136 and LF-67 to human decorin and type I collagen, respectively (kindly provided by Dr. Larry Fisher, National Institute of Dental Research, Bethesda, MD; dilution 1:400 for decorin and 1:100 for type I collagen) (Fisher et al. 1995), a polyclonal rabbit antiserum against EGFR (1005): sc-03 (Santa Cruz Biotechnology Inc., Santa Cruz, CA; dilution 1:50), and a monoclonal mouse antibody to human CD31 (BioGenex, San Ramon, CA; dilution 1:20). For decorin immunostaining, epitope retrieval was performed with 0.125 U/ml chondroitin ABC lyase (ICN Biomedicals Inc.; Aurora, OH) in 3 M Tris containing 0.6 mg/ml BSA and 18 mM sodium acetate, for 1 hr at 37C in a humid chamber. For EGFR immunostaining, epitope retrieval was achieved by incubating the slides in preheated sodium citrate buffer (pH 6.0) for 20 min.
ISH
ISH was performed on 5-μm tissue sections by probing with human decorin antisense and sense single-stranded RNA riboprobes. A 533-bp fragment containing human decorin cDNA was cloned into the Eco RI/Hind III site of pGEM-4Z transcription vector (kindly provided by Dr. Liliana Schaefer, University of Frankfurt, Frankfurt am Main, Germany). Linearized plasmid DNA was purified with QIAquick PCR Purification Kit (Qiagen; Hilden, Germany) and digoxigenin (DIG)-labeled sense and antisense RNA probes were synthesized by in vitro transcription with SP6 and T7 polymerases, respectively, by using a DIG RNA Labeling Kit (Roche Applied Science; Mannheim, Germany). Probe quantification was carried out with a DIG Nucleic Acid Detection Kit (Roche Applied Science) and ISH was performed as described (Laine et al. 1999), with some minor modifications. Paraffin-embedded tissue sections were deparaffinized and rehydrated in decreasing concentrations of ethanol. Air-dried sections were treated with 0.2 M HCl to improve the signal-to-noise ratio and then washed twice with 2 × SSC for 3 min. Cell membranes were permeabilized by digestion for 15 min at 37C with 20 μg/ml proteinase K in 10 mM Tris, pH 7.4, containing 2 mM CaCl2. The protease treatment was stopped with 2 g/liter glycine in PBS, and the specimens were acetylated with 0.1 M triethanolamine/0.40% acetic anhydride and dehydrated with rising ethanol series. Subsequently, air-dried slides were pretreated by ficin (Digest-All; Zymed Lab Inc, South San Francisco, CA) for 20 min at 37C before incubation with prehybridization mixture (50% deionized formamide, 10% dextran sulfate in 4 × SSC, 1 × Denhardt's solution, 2 mM EDTA, and 0.5 mg/ml denatured salmon sperm DNA) in a moist chamber at 42C for 2 hr. The hybridization solution contained the anti-sense and sense RNA probes at a final concentration of 60 ng/ml in prehybridization mixture. Hybridization was carried out overnight at 42C in a humid chamber. Posthybridization washes were performed for 1 min and 5 min with 2 × SSC, three times in 60% formamide in 0.2 × SSC at 37C, and twice with 2 × SSC for 5 min each to remove unbound probes. Tissue sections were blocked with 3% BSA in TBS for 30 min, followed by DIG label detection with alkaline phosphatase-conjugated anti-DIG Fab fragments (Roche Applied Science; Penzberg, Germany) for 2 hr, at a dilution of 1:2000 anti-DIG Fab in blocking buffer. Both procedures were carried out at room temperature in a moist chamber, then slides were washed twice with 0.05% Triton X-100 in TBS and once with TBS for 5 min each. Slides were treated for 2 min with 100 mM Tris, pH 9.5, containing 100 mM NaCl and 50 mM MgCl2 (detection buffer) before colorimetric detection of DIG-labeled probe. Visualization of the signal was achieved with 0.34 mg/ml nitroblue tetrazolium and 0.18 mg/ml 5-bromo-4-chloro-3-indolyl phosphate in detection buffer. One mM levamisole was added to the substrate solution to block endogenous alkaline phosphatase, and the slides were incubated in a humid chamber for 3 hr at room temperature, overnight at 4C, and again for 2 hr at room temperature. The reaction was stopped with washing twice in 10 mM Tris, pH 8.0, containing 1 mM EDTA. The slides were counterstained with Mayer's hematoxylin and mounted using Aquamount (BDH Laboratory Supplies).
Imaging
The dotslide (.slide) System (Soft Imaging System, An Olympus Company; Münster, Germany) was used to create images of tissue sections analyzed by ICC and ISH as described above.
Results
Highly Vascularized Kaposi's Sarcomas Lack Decorin
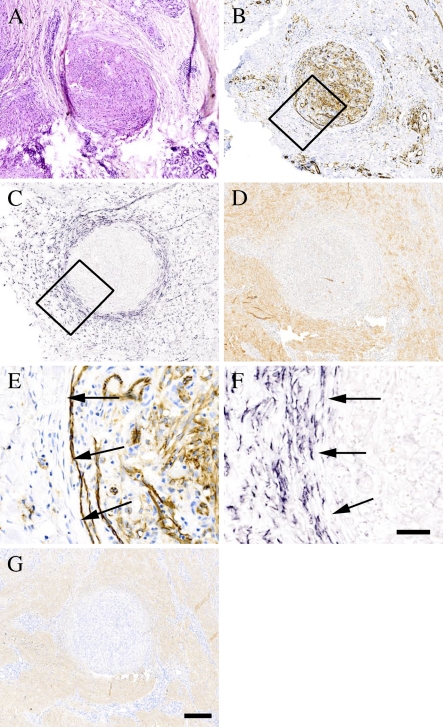
Kaposi's sarcoma is a malignant neoplasm derived from vascular or lymphatic endothelium. Hematoxylin and eosin (HE) staining of Kaposi's sarcoma tissue specimen revealed a round tumor mass in the center of the tissue section (Figure 1A). The tumor was highly vascularized, as indicated by immunostaining with an antibody to the endothelial cell marker CD31 (Figures 1B and 1E). ISH and immunostaining of serial sections of the specimen for decorin demonstrated that the tumor, including all capillary blood vessel–like structures within it, was completely devoid of decorin mRNA expression (Figures 1C and 1F) and immunoreactivity (Figure 1D). In contrast, decorin mRNA was readily detected around the tumor mass, the most intense decorin expression residing in the connective tissue stroma surrounding the tumor mass (Figures 1C and 1F). Interestingly, the type I collagen staining pattern followed that of decorin (compare Figure 1G to 1D). The tumors of Kaposi's sarcoma were devoid of detectable immunoreactivity for EGFR (data not shown). The possibility that the lack of staining was due to methodological reasons was excluded by demonstrating a clear immunoreactivity for EGFR of the healthy epidermis and numerous glands residing outside the tumor tissue (data not shown).
Figure 1.
Lack of decorin expression by highly vascularized Kaposi's sarcoma. ICC and ISH analyses of serial sections of a representative Kaposi's sarcoma tissue specimen. (A) Hematoxylin and eosin (HE) staining. (B) Staining with an antibody to the endothelial cell marker CD31. (C) ISH for human decorin. (D) Distribution of decorin identified with LF-136 antiserum for human decorin. Boxed regions shown in B and C represent corresponding areas in serial tissue sections of the same specimen, and magnified illustrations of these regions are shown in E and F, respectively. (G) Distribution of type I collagen identified with LF-67 antiserum for human type I collagen. ICC reactions are brown and, counterstain for nuclei by hematoxylin is blue. Positive digoxigenin (DIG) reaction in ISH assay can be seen in purple. Arrows (E,F) indicate the border between Kaposi's sarcoma and surrounding tissue. Bars: A–D,G = 200 μm; E,F = 50 μm.
Angiosarcomas Are Also Devoid of Decorin Expression and Immunoreactivity
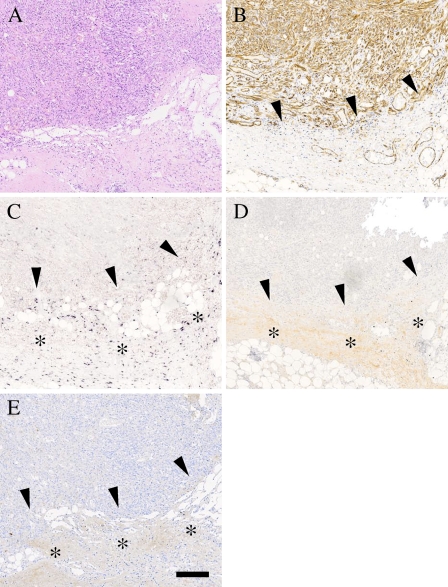
Malignant proliferation of blood vessels is called angiosarcoma. Immunostaining of the angiosarcoma tissue specimen (Figure 2A) with an antibody to endothelial cell marker CD31 demonstrated, as expected, that the tumor was enriched with capillary blood vessels (Figure 2B). ISH and immunostaining assays for decorin indicated that the tumor mass of angiosarcoma, similarly to Kaposi's sarcoma, was completely negative for both decorin mRNA expression and immunoreactivity (Figures 2C and 2D). Instead, decorin was expressed in detectable amounts in the connective tissue stroma next to the sarcoma tumor (Figure 2C). The same area was also positive for decorin immunoreaction (Figure 2D). As in Kaposi's sarcomas, in angiosarcomas, the type I collagen staining pattern followed that of decorin (Figure 2E). No immunoreactivity for EGFR was detected within angiosarcomas (data not shown).
Figure 2.
Angiosarcomas are also devoid of decorin. ICC and ISH analyses of serial sections of a representative specimen of angiosarcoma. (A) HE staining. (B) Staining with an antibody to the endothelial cell marker CD31. (C) ISH for human decorin. (D) Distribution of decorin identified with LF-136 antiserum for human decorin. (E) Distribution of type I collagen identified with LF-67 antiserum for human type I collagen. ICC reactions are brown and counterstain for nuclei by hematoxylin is blue. Positive DIG reaction in ISH assay can be seen in purple. Arrowheads (B–E) indicate the border between the angiosarcoma and its surrounding tissue. Asterisks in C show an area positive for decorin mRNA expression around the tumor. Asterisks in D,E show the location of positive immunoreactivity for decorin and type I collagen. Bar = 200 μm.
Hemangiomas, Unlike Kaposi's Sarcoma and Angiosarcoma, Contain Detectable Amounts of Decorin mRNA and Are Positive for Decorin and Type I Collagen Immunostaining
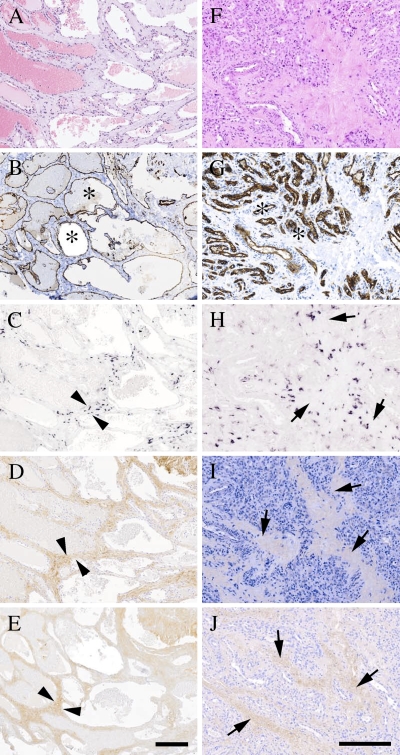
The vast majority of vascular lesions are represented by benign hemangiomas (Hunt and Santa Cruz 2004). Histological sections of cavernous as well as capillary hemangioma tissue specimens were used to examine whether decorin is detected in cases where the growth of vascular neoplasm has ceased. HE staining (Figures 3A and 3F) in combination with ICC using endothelial cell marker CD31 (Figures 3B and 3G) revealed blood vessel–rich morphology for cavernous and capillary hemangiomas, respectively. When serial sections of these hemangioma specimens were analyzed for decorin by ISH and immunostaining, decorin mRNA expression and immunoreactivity could be detected within the hemangiomas. The expression and immunoreactivity of decorin within the hemangiomas were most prominent in the connective tissue stroma surrounding the blood vessels (Figures 3C, 3D, 3H, and 3I). A similar observation was made for type I collagen (Figures 3E and 3J). Similarly to Kaposi's sarcomas and angiosarcomas, hemagiomas did not show detectable immunoreactivity for EGFR (data not shown).
Figure 3.
Cavernous and capillary hemangiomas show positivity for decorin mRNA and also have immunoreactivity for decorin. ICC and ISH analyses of serial sections of representative cutaneous cavernous (A–E) and capillary (F–J) hemangioma tissue specimens. (A,F) HE staining. (B,G) Staining with an antibody to the endothelial cell marker CD31. (C,H) ISH for human decorin. (D,I) Distribution of decorin identified with LF-136 antiserum for human decorin. (E,J) Distribution of type I collagen indentified with LF-67 antiserum for human type I collagen. Both types of hemangiomas contain numerous blood vessel structures (asterisks in B,G). Arrowheads (C–E) indicate border of the connective tissue stroma surrounding selected intratumoral blood vessels in cavernous hemangioma. Arrows (H–J) indicate connective tissue stroma surrounding intratum oral blood vessels in capillary hemangioma. ICC reactions are brown and counterstain for nuclei by hematoxylin is blue. Positive DIG reaction in ISH assay can be seen in purple. Bar (A–E and F–J) = 200 μm.
Discussion
A fundamental finding of this study has been the demonstration of the fact that Kaposi's sarcoma and angiosarcoma, both of which represent malignant vascular neoplasms, completely lack decorin mRNA expression and immunoreactivity, whereas benign vascular tumors, namely hemangiomas, express decorin in readily detectable amounts. This differential expression pattern of decorin suggests that decorin is a useful biomarker for distinguishing between benign and malignant vascular growths in patients. In addition, because angiogenesis in vascular sarcomas is frequent, contrary to angiogenesis in hemangiomas, the differential expression pattern of decorin within Kaposi's sarcoma and angiosarcoma tissues in comparison with hemangiomas supports the possibility that decorin displays an inhibitory effect on human tumor angiogenesis in vivo. Earlier studies utilizing cell culture and animal models have been consistent with this possibility (Grant et al. 2002). The study results are also consistent with previous findings indicating that decorin is rarely expressed by actively proliferating or transformed cells (Iozzo 1997), but its expression is upregulated in cases such as quiescence of vascular smooth muscle cells (Asundi and Dreher 1992). It has also been demonstrated that ectopic expression or overexpression of decorin in cancer cells of various histogenic origins causes increased expression of cdk-inhibitor p21 WAP/CIP (De Luca et al. 1996; Santra et al. 1997; Moscatello et al. 1998; Schönherr et al. 2001), an inhibitor of cell proliferation (Santra et al. 1997; Reed et al. 2002). Moreover, regarding decorin's involvement in tumor growth in vivo, it has been shown that decorin is differentially expressed in many tumors, including human colon cancer (Adany et al. 1990), basal cell carcinoma (Hunzelmann et al. 1995), pancreatic tumor (Köninger et al. 2004), and thyroid tumor (Arnaldi et al. 2005). Furthermore, in a recent study, a reduced expression of decorin was associated with poor prognosis of soft tissue tumors (Matsumine et al. 2007), a finding that is also applicable to this study. Breast cancer research has also produced these findings (Troup et al. 2003). The possibility that the lack of decorin immunoreactivity within malignant vascular tumors is due to the fixative (10% neutral buffered formalin) is dismissed because fixation protocol was equal for both malignant and benign tissues. Consequently, the lack of decorin mRNA detection by ISH in malignant tumors is in line with the unobserved decorin immunoreactivity.
In addition to demonstrating that decorin is not expressed within Kaposi's sarcoma or angiosarcoma tissue, we have shown in this study that decorin is expressed in abundant amounts in the areas lining these human malignant vascular tumors. Thus, because defining the borders of vascular sarcomas reliably is not always possible in clinical practice (e.g., whether the sarcoma tissue has been completely excised during surgery), the determination of the spatial location of decorin expression in sarcoma is a highly useful tool. In addition, the strong expression of decorin surrounding the sarcoma mass can be considered as an indication of excessive ECM production, probably representing a protective reaction of the healthy tissue against the invading tumor (Köninger et al. 2004; Matsumine et al. 2007). Indeed, it has been shown that the invading tumor cells induce a specific stromal reaction (desmoplastic reaction) in the host cells, resulting in a stroma being composed of several different interstitial connective tissue molecules, including collagens, proteoglycans, and glycosaminoglycans (Desmoulière et al. 2004). Many of them, including type I collagen and decorin, have been linked to such essential steps in tumor growth as angiogenesis, tumor cell migration, and invasion (Grant et al. 2002; Honma et al. 2007).
High expression of EGFR by tumor cells and/or tumor endothelial cells has been correlated with increased angiogenesis (Moon et al. 2006). On the other hand, decorin has been shown to interact with EGFR and to activate specific signaling pathways leading to e.g., cell cycle arrest (Moscatello et al. 1998). Therefore, we attempted to determine whether the mechanism whereby decorin contributes to the growth of vascular tumors is mediated through EGFR. By utilizing ICC, we examined the existence of EGFR in malignant and benign vascular tumors. Our results showed that EGFR is not present in detectable amounts in either type of tumor. Thus, it is unlikely that decorin's contribution to the growth of vascular tumors is mediated through EGFR. Consequently, it is also unlikely that EGFR plays a role in vascular tumorigenesis.
In conclusion, we have shown that there is a striking difference in decorin expression between human malignant and benign vascular tumors; i.e., within Kaposi's sarcoma and angiosarcoma the expression of decorin is completely lacking, whereas within hemangiomas, decorin is expressed in detectable amounts. This suggests that decorin is a usable biomarker for distinguishing between malignant and benign vascular neoplasms. We have also shown that in Kaposi's sarcoma and angiosarcoma, decorin expression is upregulated in the areas surrounding these human vascular tumors. Thus, the determination of decorin expression in tissue specimens of human vascular neoplasms provides an applicable tool for localizing the border of healthy and diseased tissue. Immunostaining results of type I collagen indicate the same conclusion to be true for this collagen type as well. Finally, our findings provide opportunities for developing a new strategy for the future treatment of human vascular malignancies.
Acknowledgments
This study was supported by the Finnish Foundation for Cardiovascular Research, Medical Research Fund (EVO) of Turku University Central Hospital, and Turku University Foundation. The authors disclose that there is no financial or personal conflict of interest amongst themselves and with industry.
We thank Marja Nykänen and Heidi Pakarinen for excellent technical assistance.
References
- Adany R, Heimer R, Caterson B, Sorrell JM, Iozzo RV (1990) Altered expression of chondroitin sulphate proteoglycan in the stroma of human colon carcinoma. Hypomethylation of PG-40 gene correlates with increased PG-40 content and mRNA levels. J Biol Chem 265:11389–11396 [PubMed] [Google Scholar]
- Arnaldi LA, Borra RC, Maciel RM, Cerutti JM (2005) Gene expression profiles reveal that DCN, DIO1, and DIO2 are underexpressed in benign and malignant thyroid tumors. Thyroid 15:210–221 [DOI] [PubMed] [Google Scholar]
- Asundi VK, Dreher KL (1992) Molecular characterization of vascular smooth muscle decorin: deduced core protein structure and regulation of gene expression. Eur J Cell Biol 59:314–321 [PubMed] [Google Scholar]
- Brown DC, Vogel KG (1989) Characteristics of the in vitro interaction of a small proteoglycan (PG II) of bovine tendon with type I collagen. Matrix 9:468–478 [DOI] [PubMed] [Google Scholar]
- Burke AP, Järveläinen H, Kolodgie FD, Goel A, Wight TN, Virmani R (2004) Superficial pseudoanerysms: clinicopathologic aspects and involvement of extracellular matrix proteoglycans. Mod Pathol 17:482–488 [DOI] [PubMed] [Google Scholar]
- Carmeliet P (2003) Angiogenesis in health and disease. Nat Med 9:653–660 [DOI] [PubMed] [Google Scholar]
- De Lange Davies C, Melder RJ, Munn LL, Mouta-Carreira C, Jain RK, Boucher Y (2001) Decorin inhibits endothelial migration and tube-like structure formation: role of thrombospondin-1. Microvasc Res 62:26–42 [DOI] [PubMed] [Google Scholar]
- De Luca A, Carotenuto A, Rachiglio A, Gallo M, Maiello MR, Aldinucci D, Pinto A, et al. (2008) The role of the EGFR signaling in tumor microenvironment. J Cell Physiol 214:559–567 [DOI] [PubMed] [Google Scholar]
- De Luca A, Santra M, Baldi A, Giordano A, Iozzo RV (1996) Decorin-induced growth suppression is associated with up-regulation of p21, an inhibitor of cyclin-dependent kinases. J Biol Chem 271:18961–18965 [DOI] [PubMed] [Google Scholar]
- Desmoulière A, Guyot C, Gabbiani G (2004) The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol 48:509–517 [DOI] [PubMed] [Google Scholar]
- Fisher LW, Stubbs JT 3rd, Young MF (1995) Antisera and cDNA probes to human and certain animal model bone matrix noncollagenous proteins. Acta Orthop Scand 66(suppl 266):61–65 [PubMed] [Google Scholar]
- Grant DS, Yenisey C, Rose RW, Tootell M, Santra M, Iozzo RV (2002) Decorin suppresses tumor cell-mediated angiogenesis. Oncogene 21:4765–4777 [DOI] [PubMed] [Google Scholar]
- Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, Ruoslahti E (1994) Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J 302:527–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma K, Miyata T, Ochiya T (2007) Type I collagen gene suppresses tumor growth and invasion of malignant human glioma cells. Cancer Cell Int 7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SJ, Santa Cruz DJ (2004) Vascular tumors of the skin: a selective review. Semin Diagn Pathol 21:166–218 [DOI] [PubMed] [Google Scholar]
- Hunzelmann N, Schönherr E, Bonnekoh B, Hartmann C, Kresse H, Krieg T (1995) Altered immunohistochemical expression of small proteoglycans in the tumor tissue and stroma of basal cell carcinoma. J Invest Dermatol 104:509–513 [DOI] [PubMed] [Google Scholar]
- Iozzo RV (1997) The family of the small leucine-rich proteoglycans: key regulators of matrix assembly and cellular growth. Crit Rev Biochem Mol Biol 32:141–174 [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Moscatello KG, McQuillan DJ, Eichstetter I (1999) Decorin is a biological ligand for the epidermal growth factor receptor. J Biol Chem 274:4489–4492 [DOI] [PubMed] [Google Scholar]
- Järveläinen H, Puolakkainen P, Pakkanen S, Brown EL, Höök M, Iozzo RV, Sage EH, et al. (2006) A role for decorin in cutaneous wound healing and angiogenesis. Wound Repair Regen 14:443–452 [DOI] [PubMed] [Google Scholar]
- Järveläinen HT, Iruela-Arispe ML, Kinsella MG, Sandell LJ, Sage EH, Wight TN (1992) Expression of decorin by sprouting bovine aortic endothelial cells exhibiting angiogenesis in vitro. Exp Cell Res 203:395–401 [DOI] [PubMed] [Google Scholar]
- Kinsella MG, Fischer JW, Mason DP, Wight TN (2000) Retrovirally mediated expression of decorin by macrovascular endothelial cells. Effects on cellular migration and fibronectin fibrillogenesis in vitro. J Biol Chem 275:13924–13932 [DOI] [PubMed] [Google Scholar]
- Köninger J, Giese T, di Mola FF, Wente MN, Esposito I, Bachem MG, Giese NA, et al. (2004) Pancreatic tumor cells influence the composition of the extracellular matrix. Biochem Biophys Res Commun 322:943–949 [DOI] [PubMed] [Google Scholar]
- Laine VJ, Grass DS, Nevalainen TJ (1999) Protection by group II phospholipase A2 against Staphylococcus aureus. J Immunol 162:7402–7408 [PubMed] [Google Scholar]
- Matsumine A, Shintani K, Kusuzaki K, Matsubara T, Satonaka H, Wakabayashi T, Iino T, et al. (2007) Expression of decorin, a small leucine-rich proteoglycan, as a prognostic factor in soft tissue tumors. J Surg Oncol 96:411–418 [DOI] [PubMed] [Google Scholar]
- Moon WS, Park HS, Yu KH, Park MY, Kim KR, Jang KY, Kim JS, et al. (2006) Expression of betacellulin and epidermal growth factor receptor in hepatocellular carcinoma: implications for angiogenesis. Hum Pathol 37:1324–1332 [DOI] [PubMed] [Google Scholar]
- Moscatello DK, Santra M, Mann DM, McQuillan DJ, Wong AJ, Iozzo RV (1998) Decorin suppresses tumor cell growth by activating the epidermal growth factor receptor. J Clin Invest 101:406–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelimarkka L, Salminen H, Kuopio T, Nikkari S, Ekfors T, Laine J, Pelliniemi L, et al. (2001) Decorin is produced by capillary endothelial cells in inflammation-associated angiogenesis. Am J Pathol 158:345–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nili N, Cheema AN, Giordano FJ, Barolet AW, Babaei S, Hickey R, Eskandarian MR, et al. (2003) Decorin inhibition of PDGF-stimulated vascular smooth muscle cells function: potential mechanism for inhibition of intimal hyperplasia after balloon angioplasty. Am J Pathol 163:869–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penc SF, Pomahac B, Winkler T, Dorschner RA, Eriksson E, Herndon M, Gallo RL (1998) Dermatan sulphate released after injury is a potent promoter of fibroblast growth factor-2 function. J Biol Chem 273:28116–28121 [DOI] [PubMed] [Google Scholar]
- Reed CC, Gauldie J, Iozzo RV (2002) Suppression of tumorigenicity by adenovirus-mediated gene transfer of decorin. Oncogene 21:3688–3695 [DOI] [PubMed] [Google Scholar]
- Reed CC, Waterhouse A, Kirby S, Kay P, Owens RT, McQuillan DJ, Iozzo RV (2005) Decorin prevents metastatic spreading of breast cancer. Oncogene 24:1104–1110 [DOI] [PubMed] [Google Scholar]
- Santra M, Mann DM, Mercer EW, Skorski T, Calabretta B, Iozzo RV (1997) Ectopic expression of decorin protein core causes a generalised growth suppression in neoplastic cells of various histogenic origin and requires endogenous p21, an inhibitor of of cyclin-dependent kinases. J Clin Invest 100:149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönherr E, Levkau B, Schaefer L, Kresse H, Walsh K (2001) Decorin-mediated signal transduction in endothelial cells. Involvement of Akt/protein kinase B in up-regulation of p21(WAF1/CIP1) but not p27(KIP1). J Biol Chem 276:40687–40692 [DOI] [PubMed] [Google Scholar]
- Schönherr E, O'Connell BC, Schittny J, Robenek H, Fastermann D, Fisher LW, Plenz G, et al. (1999) Paracrine or virus-mediated induction of decorin expression by endothelial cells contributes to tube formation and prevention of apoptosis in collagen lattices. Eur J Cell Biol 78:44–55 [DOI] [PubMed] [Google Scholar]
- Schönherr E, Sunderkötter C, Iozzo RV, Schaefer L (2005) Decorin, a novel player in the insulin-like growth factor system. J Biol Chem 280:15767–15772 [DOI] [PubMed] [Google Scholar]
- Seaman S, Stevens J, Yang MY, Logsdon D, Graff-Cherry C, St. Croix B (2007) Genes that distinguish physiological and pathological angiogenesis. Cancer Cell 11:539–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, et al. (2000) Genes expressed in human tumor endothelium. Science 289:1197–1202 [DOI] [PubMed] [Google Scholar]
- Sulochana KN, Fan H, Jois S, Subramanian V, Sun F, Kini RM, Ge R (2005) Peptides derived from human decorin leucine-rich repeat 5 inhibit angiogenesis. J Biol Chem 280:27935–27948 [DOI] [PubMed] [Google Scholar]
- Troup S, Njue C, Kliewer EV, Parisien M, Roskelley C, Chakravarti S, Roughley PJ, et al. (2003) Reduced expression of the small leucine-rich proteoglycans, lumican, and decorin is associated with poor outcome in node-negative invasive breast cancer. Clin Cancer Res 9:207–214 [PubMed] [Google Scholar]
- Weedon D (2002) Vascular tumors. In Houston MJ, ed. Skin Pathology. 2nd ed. London, Churchill-Livingstone, 1001–1043