Abstract
IQGAP1 is a multifunctional junction molecule that is involved in cell migration, proliferation, differentiation, cell polarity, and cell–cell adhesion. It is highly expressed in the kidney and has recently been identified in the glomerular basement membrane as a nephrin-associated protein. However, the distribution of IQGAP1 in renal tubular epithelial cells is unknown. We performed confocal microscopic studies to localize IQGAP1 in each nephron segment using dual immunofluorescence staining with various antibodies against segment-specific markers. We found that IQGAP1 was strongly expressed in the distal convoluted tubule (DCT), collecting duct, and macula densa and moderately in the thick ascending limb and proximal tubule. In the DCT, the IQGAP1–F-actin complex forms a comb-like structure with multiple parallel spikes sitting on the basal membrane. In the macula densa cells, IQGAP1 is strongly expressed in the apical membrane, whereas in type A intercalated cells, IQGAP1 is expressed in the basolateral membrane, where it colocalizes with anion exchanger 1, and in principal cells, it is diffusely expressed. In conclusion, we showed the expression and subcellular localization of IQGAP1 in various nephron segments. The site-specific expression pattern of this potent modulator of multiple biological pathways in the renal tubules suggests that IQGAP1 may have multiple important roles in various renal functions. (J Histochem Cytochem 56:659–666, 2008)
Keywords: distal convoluted tubule, collecting duct, macula densa, thick ascending limb, F-actin, cyclooxygenase 2, anion exchanger 1, calbindinD28k
IQGAP1 is a potent modulator involved in cross-talk among diverse biological pathways. It was first cloned in 1994 (Weissbach et al. 1994) and named after the discovery that the N-terminal half of IQGAP1 contains four IQ motifs, a sequence that mediates interactions with calmodulin and calmodulin-related proteins (Cheney and Mooseker 1992), and that it contains a sequence similar to the Ras GTPase-activating proteins. IQGAP1 is widely expressed in multiple organs with the highest levels of expression in kidney, lung, and placenta (Weissbach et al. 1994). It has been implicated in many pathways including modulation of the actin cytoskeleton through Rac1 and Cdc42, organization of microtubules through the Rac1/Cdc42/CLIP-170 complex, and cell–cell adhesion through E-cadherin and β-catenin [see Brown and Sacks (2006) for review]. These data suggest that IQGAP1 functions as a junction molecule by receiving and transmitting signals for diversified cellular functions such as cell migration, cell–cell adhesion, cell polarity, proliferation, and differentiation.
In the kidney, IQGAP1 was recently identified as a nephrin-associated protein by nephrin pull-downs in glomeruli (Lehtonen et al. 2005; Liu et al. 2005). The presence of IQGAP1 in slit diaphragms and its association with nephrin suggests that IQGAP1 and nephrin may form a scaffolding protein complex in the podocyte slit diaphragm and contribute to the regulation of ultrafiltration by binding slit membrane proteins and establishing their cytosolic connections. The expression pattern of IQGAP1 in renal tubular epithelial cells has not been reported.
Because the actin cytoskeleton plays an important role in regulation of renal transporters and IQGAP1 has been shown to bind actin directly and modulate actin cytoskeleton dynamics (Bashour et al. 1997; Erickson et al. 1997; Fukata et al. 1997), it is likely that IQGAP1 may play a major role in regulation of renal transporters by directing trafficking, directly or indirectly through downstream signaling. Up to now, the expression and subcellular location of IQGAP1 and its relationship to the actin cytoskeleton in renal tubules are unknown. In this study, we performed confocal microscopic analysis of dual immunofluorescence staining of IQGAP1 and F-actin to illustrate the relationship between the two molecules. We were intrigued by the striking difference in the expression of the two molecules and their colocalization along different segments of renal tubules. To identify the different types of tubules, we also performed dual immunofluorescence staining of IQGAP1 with Tamm-Horsfall protein (THP), Calbindin D-28K (CB28K) (Lee et al. 2004), anion exchanger 1 (AE1) (Alper et al. 1989), aquaporin 2 (AQ2) (Nielsen et al. 1993); and cyclooxygenase 2 (COX2) (Harris et al. 1994), which are markers for thick ascending limb (TAL), distal convoluted tubule (DCT), intercalated cells, principal cells of collecting duct (CD), and macula densa cells, respectively. Our results characterize site-specific expression of IQGAP1 and its colocalization with the actin cytoskeleton in renal tubules.
Materials and Methods
Animals and Procedures
Male National Institutes of Health (NIH) Swiss mice (20–25 g) were purchased from the National Cancer Institute and allowed free access to water and standard mouse chow. All procedures involving animals were approved by the animal care committee of the University of Arizona and adhere to the NIH Guide for the Care and Use of Laboratory Animals.
The mice were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg body weight) and xylazine (5 mg/kg body weight). After a midline abdominal incision, the kidneys were excised, decapsulated, and embedded in OCT embedding medium (Tissue-Tek; Sakura Finetechnical Co., Torrance, CA) under liquid nitrogen.
Immunofluorescent Staining
The sources of primary and secondary antibodies were anti-IQGAP1, COX2, CB28K, anti-AQ2 (Santa Cruz Biotechnology; Santa Cruz, CA), anti-THP (Biomedical Technologies; Stoughton, MA), anti-AE1 (Alpha Diagnostic; San Antonio, TX), far-red fluorescence nuclear counterstain TO-PRO-3 iodide, FITC-labeled phalloidin, and Alexa Flour-488 or 594 conjugated secondary antibodies (Molecular Probes; Eugene, OR). Immunofluorescent staining was carried out as described previously (Lien et al. 2006). Briefly, frozen kidney samples were cut into 5-μm sections in a cryostat, fixed with 4% paraformaldehyde for 15 min, and incubated with primary antibodies for 1 hr and then with Alexa Fluor-conjugated secondary antibodies and nuclear counterstain TO-PRO-3 iodide for 30 min, all at room temperature. Negative controls were routinely performed using non-immunized IgG to exclude any nonspecific cross-reactivity from the primary antibodies or secondary antibodies. Images were captured with a confocal laser-scanning microscopy system (BioRad MRC-500; Hercules, CA) attached to a Nikon TMD-300 microscope (Melville, NY)with an oil-immersed × 100 magnified objective lens.
Western Blot Analysis
Western blot analysis was performed as described previously (Lee et al. 2002) with minor modifications. Briefly, kidney tissue was homogenized in protein lysis buffer (1% of SDS, 1.0 mM sodium Orth-Vanadate, 10 mM Tris at pH 7.4, and protease inhibitors cocktail including 4-(2-Aminoethyl) benzenesulfonyl fluoride hydrochloride, aprotinin, bestatin hydrochloride, E-64-systeine proteases, leupeptin hemisulfate salt, pepstain A; Sigma, St. Louis, MO). Samples were resolved by 4-12% NuPAGE Bis-Tris gel and transferred to polyvinylidene difluoride membranes. After blocking with 5% non-fat milk, the membrane was incubated with anti-IQGAP1 antibodies (1:1000; Santa Cruz Biotechnology) for 1 hr. After washing in 0.5% TBS-T for 5 min three times, the membrane was incubated with secondary antibodies conjugated with horseradish peroxidase (1:50,000; Sigma) for 30 min. Signal emission was detected by chemiluminescence method using ECL PLUS reagent (GE Healthcare Bioscience; Piscataway, NJ).
Results
The distribution of IQGAP1 in the mouse renal tubule is segment specific as depicted in Table 1, shown in Figures 1–4, and described as follows.
Table 1.
Expression of IQGAP1 in mouse renal tubules
| Nephron segments | Intensity | Location |
|---|---|---|
| Proximal tubule | Moderate | Apical |
| Thick ascending limb | Moderate | Diffuse, apical > basolateral and cytosolic |
| Thin limb | Minimal | — |
| Macula densa | Strong | Apical > basolateral |
| Distal convoluted tubule | Strong | Diffuse |
| Collecting duct | Strong | Principal cells: diffuse |
| Intercalated cells: basolateral > apical |
Figure 1.
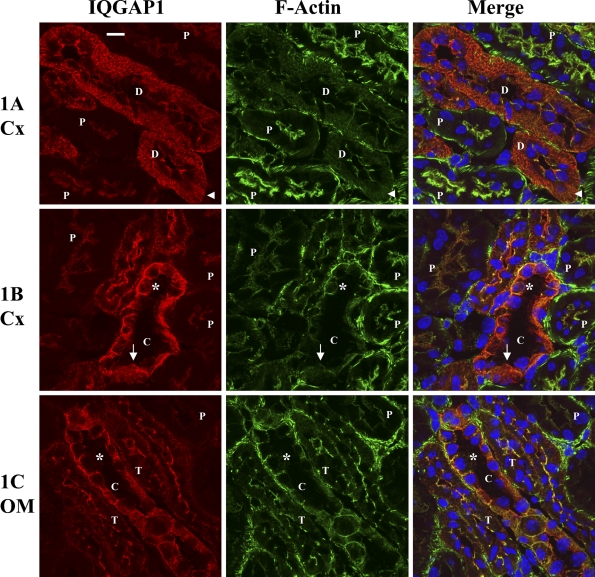
Immunolocalization of IQGAP1 in mouse renal tubules. Double staining with antibodies against IQGAP1 (red fluorescence) and phalloidin, which labels F-actin (green fluorescence). (A) IQGAP1 staining in proximal tubules (labeled with P) and distal convoluted tubules (DCT; labeled with D). Arrowhead indicates a comblike structure in DCT. (B) Collecting duct (CD; labeled with C) in the cortex (Cx). The intercalated cells (asterisks) show basolateral staining, whereas the principal cells (arrows) show diffuse staining. (C) Medullary thick ascending limb (mTAL; labeled with T) and CD in the outer medulla (OM). Notice the colocalization of IQGAP1 with F-actin. Bar = 10 μm.
Figure 2.
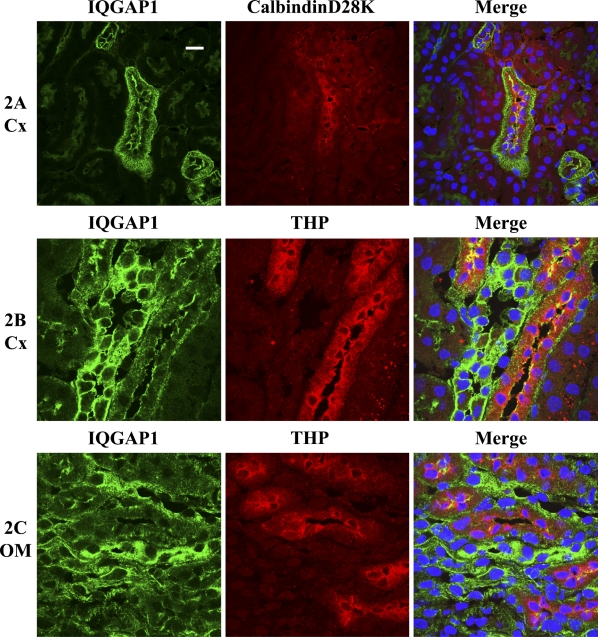
Immunolocalization of IQGAP1 in mouse DCT (A), cortical TAL (B), and mTAL (C) using antibodies against specific markers for each segment: DCT, calbindin D28k (red); TAL, Tamm-Horsfall protein (THP; red). IQGAP1 is green. Bar = 10 μm.
Figure 3.
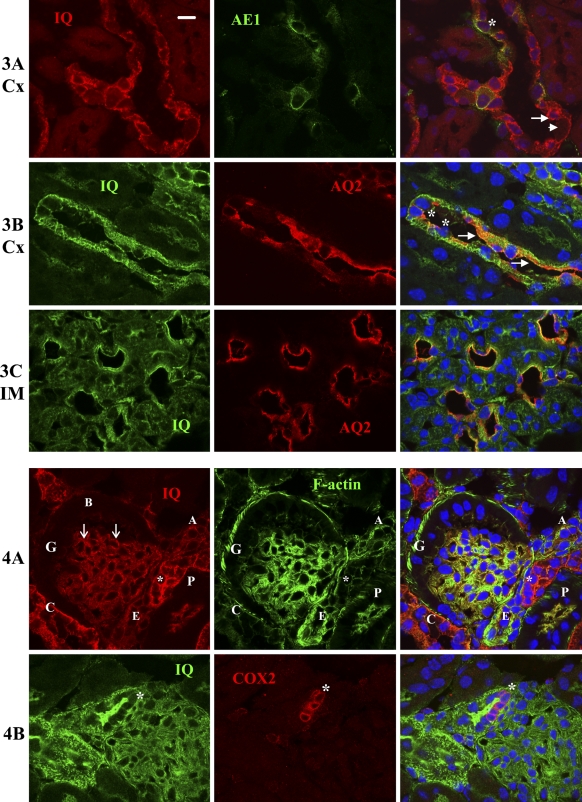
Immunolocalization of IQGAP1 in mouse cortical and medullary CD (cCD, A,B; mCD, C) using antibodies against anion exchanger 1 (AE1, green) for type A intercalated cells and aquaporin 2 (AQ2, red) for principal cells. IQGAP1 is green in A and red in B and C. IQGAP1 in the AE1-positive cells (type A intercalated cells) shows basolateral staining (asterisks), whereas the principal cells (arrows) show diffuse staining. Some AE1 negative cells, presumably type B or non-A, non-B intercalated cells, also showed the membranous staining pattern (arrowhead). IM, inner medula. Bar = 10 μm.
Figure 4.
Immunolocalization of IQGAP1 in macula densa cells (asterisks). (A) Dual staining with antibodies against IQGAP1 (red fluorescence) and phalloidin (green fluorescence). Macula densa cells (asterisks) show strong apical staining of IQGAP1 and weak basolateral staining, both colocalized with F-actin. Afferent arteriole (labeled as A), and efferent arteriole (labeled as E) show strong F-actin staining and weak IQGAP1 staining. Glomerulus (G) also shows strong IQGAP1 and F-actin stain, especially in the glomerular basement membrane (arrows). Bowman's capsule (labeled with B) showed weak IQGAP1 staining. Proximal (P) and collecting duct (C) are also indicated. (B) Dual staining with antibodies against IQGAP1 (green fluorescence) and anti-COX2 (red fluorescence). Macula densa cells are identified with perinuclear staining of COX 2. Bar = 10 μm.
Proximal Tubules
IQGAP1 was expressed moderately in the proximal convoluted tubule (PCT) and was mainly colocalized with F-actin in the apical membrane as shown in Figures 1A and 1B. The staining pattern of proximal straight tubule in the outer medulla (Figure 1C, labeled with P) was similar to the PCT in the cortex (Figures 1A and 1B, labeled with P).
Distal Convoluted Tubules
In contrast to the PCT, IQGAP1 was highly expressed in the DCT (Figure 1A, labeled with D) with a distinct staining pattern. IQGAP1 was abundant in the apical and basolateral membrane as well as in the cytosol. The cytosolic IQGAP1 staining appeared as short parallel linear spikes perpendicular to the base of the cell, and in certain areas, showing a comb-like structure (Figure 1A, labeled with an arrowhead). To verify that these tubules are indeed DCT, we performed dual staining using antibodies against CB28K, a calcium-binding protein specifically expressed in the mouse DCT. As shown in Figure 2A, this unique IQGAP1 staining pattern and intensity were only present in CB28K-positive cells, indicating that they are specific for DCT cells.
Thick Ascending Limbs
Dual staining using antibodies against IQGAP1 and THP identified the cortical TAL (cTAL) and showed that the IQGAP1 pattern staining in the cTAL was similar to but less intense than that of the DCT and appeared in the apical and basolateral membrane and in the cytosol (Figure 2B). The IQGAP1 staining pattern in the medullary TAL (mTAL) was similar to that in the cTAL; however, the intensity decreased as the TAL moved from cortex to outer medulla (Figure 2C).
Collecting Ducts
IQGAP1 was strongly expressed in both cortical CD (cCD; Figure 1B, labeled with C) and medullary CD (mCD; Figure 1C, labeled with C). There were at least two distinct IQGAP1 staining patterns in the CD: one with membranous staining, particularly in the basolateral membrane (Figures 1B and 1C, labeled with asterisks), and another with weaker staining throughout the cell (Figure 1B, labeled with an arrow). To determine cell-specific expression in the CD, we did dual staining using antibodies against IQGAP1 and AE1, which is expressed in the type A intercalated cells (Alper et al. 1989). The AE1-positive cells or type A intercalated cells showed colocalization of AE1 with IQGAP1 in the basolateral membrane (Figure 3A, labeled with asterisks). The diffuse expression pattern of IQGAP1 was found only in AE1-negative cells (Figure 3A, labeled with arrows). Using dual labeling with anti-AQ2 and anti-IQGAP1 antibodies, we confirmed that these cells with the diffuse expression pattern are indeed principal cells (Figure 3B, labeled with arrows). Some AE1-negative cells also showed the membranous staining pattern. They are presumably type B or non-A, non-B intercalated cells (Figure 3A, labeled with arrowheads) (Teng-umnuay et al. 1996). In the inner medulla, IQGAP1 staining was seen diffusely in mCD cells, with the strongest expression in the apical membrane, where IQGAP1 colocalizes with AQ2 (Figure 3C).
Macula Densa
IQGAP1 was strongly expressed in the macula densa, with staining mainly in the cell membrane and with the apical membrane staining much stronger than basolateral membrane (Figure 4A, labeled with asterisks). IQGAP1 was detected in glomeruli and was mostly concentrated in the glomerular basement membrane (Figure 4A, labeled with arrows), consistent with a previous report (Lehtonen et al. 2005). Mesangial cells, which had strong F-actin staining, showed weak to moderate IQGAP1 staining. Bowman's capsule also showed very weak IQGAP1 staining (Figure 4A, labeled with B). Both afferent (Figure 4A, labeled with A) and efferent (Figure 4A, labeled with E) arterioles were moderately positive for IQGAP1 with a membranous pattern. The F-actin staining was much stronger in the efferent than afferent arteriole. The presence of IQGAP1 in macula densa was confirmed with dual staining for both COX2 and IQGAP1. Although COX2 was expressed in the perinuclear area, IQGAP1 was strongly expressed in the apical membrane and lightly expressed in the basolateral membrane (Figure 4B, labeled with asterisks).
Topotropical Relationship Between IQGAP1 and F-actin
Because IQGAP1 is closely associated with the actin cytoskeleton, we further analyzed the relationship between IQGAP1 and F-actin in various nephron segments. In PCT cells, IQGAP1 and F-actin colocalized in the apical microvillar region (Figures 1A–1C, labeled with P). In DCT, IQGAP1 was strongly expressed throughout the cell and also colocalized with F-actin at pericellular cytoskeleton structures, especially the lateral cell–cell junctions where F-actin is normally expressed (Figure 1A, labeled with D). In CDs, F-actin colocalized with IQGAP1 in both intercalated and principal cells (Figure 1B, labeled with C). In the macula densa, although IQGAP1 was still associated with F-actin, IQGAP1 staining was mainly in the apical membrane, whereas F-actin staining was stronger in the basolateral membrane (Figure 4A).
Western Blot Analyses
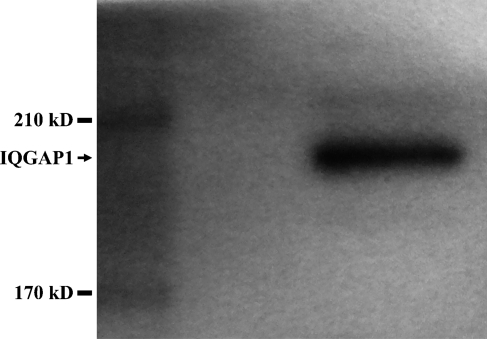
The anti-IQGAP1 antibodies used in this study recognize the N terminus of human IQGAP1. Because the predicted cross-reactivity of this antibody to mouse IQGAP1, IQGAP2, and IQGAP3 is 85%, 36%, and 48%, respectively (data provided from the manufacturer), it is highly specific to IQGAP1. To confirm this, we performed Western blot analyses to determine whether the anti-IQGAP1 antibodies have any cross-reactions to other isoforms. As predicted, there is only one band, with an estimated molecular mass of 190 kDa (Figure 5), consistent with IQGAP1.
Figure 5.
Western blot of IQGAP1. Molecular mass markers for 210 and 170 kDa are shown in the left lane. A single band of ∼190 kDa is shown in the right lane.
Discussion
We report for the first time the immunolocalization of IQGAP1 in renal tubules. We have shown that IQGAP1 is abundantly expressed in normal renal tubules, and its cellular localization is highly segment specific. In addition, IQGAP1 is strongly expressed in the macula densa where tubuloglomerular feedback is regulated. The subcellular location of IQGAP1 is closely related to the location of F-actin, indicating that IQGAP1 may play an important role in the regulation of tubular cytoskeleton.
The expression of IQGAP1 in nephron segments is site specific in terms of both intensity and location (Table 1). For intensity, nephron segments can be divided into three groups: (a) strong expression of IQGAP1: DCT, cCD, mCD, and macula densa; (b) moderate intensity: cTAL, mTAL and PCT; and (c) minimal intensity: thin limbs. As for the location of IQGAP1, there are three distinct patterns: (a) predominantly apical staining: PCT and macula densa; (b) predominantly basolateral staining: intercalated cells of CDs; and (c) diffuse staining: cTAL, DCT, and principal cells of CDs. The physiological meanings of the differential staining of IQGAP1 in each type of renal tubular epithelial cell are unclear. From IQGAP1 and F-actin dual staining experiments, it seems that when IQGAP1 is expressed in the renal epithelial cells, it is always coexpressed with F-actin. This finding is consistent with other investigators' finding that IQGAP1 binds to F-actin and plays an important role in maintaining cell polarity and cytoskeleton (Bashour et al. 1997; Fukata et al. 1997; Brown and Sacks 2006). However, because all renal epithelial cells are polarized cells, the distinct subcellular distribution of IQGAP1 suggests that IQGAP1 may have additional functions, probably related to the specific IQGAP1 functions in respective renal tubular segments.
The most striking finding is the strong diffuse staining in the DCT. The most unique finding is the cytosolic staining pattern that manifested as a comb-like structure with multiple parallel spikes perpendicular to the basal membrane. These comb-like cytosolic IQGAP1 patterns are colocalized with F-actin. The DCT is characterized by extensive invaginations of basal plasma membrane and interdigitations between adjacent cells. These structures extend perpendicularly over roughly two thirds of the epithelial height (Madsen and Tisher 1986), a pattern very similar to our IQGAP1/F-actin staining. It is possible that IQGAP1 may be important for stabilization of basolateral membrane expansion, which is critical for solute reabsorption in the DCT.
The macula densa cells, however, have strong IQGAP1 staining in the apical membrane. Morphologically, the apical membrane of macula densa cells is studded with slender microvilli (Kaissling et al. 1977). These microvilli are rich in furosemide-sensitive Na+, K+, 2Cl− cotransporters, which serve as sensors of luminal NaCl concentration for regulation of tubuloglomerular feedback (TGF) (Lapointe et al. 1998; Bell et al. 2003). The presence of IQGAP1 in this region suggests that IQGAP1 may participate in the TGF process. Further studies are needed to examine this hypothesis.
Nearly all renal epithelial cells with positive IQGAP1 staining appear to show apical staining of various intensities. The major function of renal epithelial cells is reabsorption of solutes and water through the apical membrane. To fulfill this function, the apical transporters constantly undergo a recycling process that involves dynamic changes of the cytoskeleton. It is possible that IQGAP1 may participate in the trafficking of apical transporters in multiple nephron segments (Noda and Sasaki 2005). The strong expression of IQGAP1 in the principal cells also suggests that IQGAP1 may be involved in the trafficking of AQ2, which is actively placed and removed from the apical membrane depending on the presence or absence of vasopressin and other regulators (Noda and Sasaki 2005). As for the colocalization of IQGAP1 and AE1 in type A intercalated cells, the significance is unknown. Interestingly, IQGAP1 has been located in the basolateral membrane in the chief and mucous neck cells of the isolated gastric gland. On stimulation by cholinergic agonists or phobol ester, IQGAP1 is translocated to the apical membrane. These findings suggest that IQGAP1 may be involved in hormonal regulation of exocytosis in gastric mucosa (Chew et al. 2005). Whether IQGAP1 has a similar function in renal epithelial cells remains to be determined.
The IQGAP1 knockout (KO) mice generated by Li et al. (2000) showed no significant phenotypes other than gastric mucosa dysplasia, and no apparent abnormalities were found in kidney sections stained for AQP2, megalin, or the H+-ATPase (Li et al. 2000). The data from IQGAP1 KO mice suggest that IQGAP1 may not play essential non-redundant roles in renal physiology. However, it is possible that the loss of IQGAP1 is compensated by other IQGAP family members. These are IQGAP2, expressed in the liver and gastric parietal cells (Brill et al. 1996; Zhou et al. 2003), and a third putative member, IQGAP3 (Briggs and Sacks 2003). Because IQGAP1 has unique expression patterns in each nephron segment, it would be interesting to challenge IQGAP1 KO mice with various stimuli to see whether renal adaptation is affected.
In conclusion, we report here for the first time the distribution of IQGAP1 in the renal tubule and differential subcellular location of IQGAP1 among nephron segments.
Acknowledgments
This work was supported by a grant from the Dialysis Clinic, a non-profit organization.
We thank Suzu Igarashi for excellent technical assistance.
References
- Alper SL, Natale J, Gluck S, Lodish HF, Brown D (1989) Subtypes of intercalated cells in rat kidney collecting duct defined by antibodies against erythroid band 3 and renal vacuolar H+-ATPase. Proc Natl Acad Sci USA 86:5429–5433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashour AM, Fullerton AT, Hart MJ, Bloom GS (1997) IQGAP1, a Rac- and Cdc42-binding protein, directly binds and cross-links microfilaments. J Cell Biol 137:1555–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell PD, Lapointe JY, Peti-Peterdi J (2003) Macula densa cell signaling. Annu Rev Physiol 65:481–500 [DOI] [PubMed] [Google Scholar]
- Briggs MW, Sacks DB (2003) IQGAP proteins are integral components of cytoskeletal regulation. EMBO Rep 4:571–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill S, Li SH, Lyman CW, Church DM, Wasmuth JJ, Weissbach L, Bernards A, et al. (1996) The Ras GTPase-activating-protein-related human protein IQGAP2 harbors a potential actin binding domain and interacts with calmodulin and Rho family GTPases. Mol Cell Biol 16:4869–4878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MD, Sacks DB (2006) IQGAP1 in cellular signaling: bridging the GAP. Trends Cell Biol 16:242–249 [DOI] [PubMed] [Google Scholar]
- Cheney RE, Mooseker MS (1992) Unconventional myosins. Curr Opin Cell Biol 4:27–35 [DOI] [PubMed] [Google Scholar]
- Chew CS, Okamoto CT, Chen XS, Qin HY (2005) IQGAPs are differentially expressed and regulated in polarized gastric epithelial cells. Am J Physiol Gastrointest Liver Physiol 288:G376–387 [DOI] [PubMed] [Google Scholar]
- Erickson JW, Cerione RA, Hart MJ (1997) Identification of an actin cytoskeletal complex that includes IQGAP and the Cdc42 GTPase. J Biol Chem 272:24443–24447 [DOI] [PubMed] [Google Scholar]
- Fukata M, Kuroda S, Fujii K, Nakamura T, Shoji I, Matsuura Y, Okawa K, et al. (1997) Regulation of cross-linking of actin filament by IQGAP1, a target for Cdc42. J Biol Chem 272:29579–29583 [DOI] [PubMed] [Google Scholar]
- Harris RC, McKanna JA, Akai Y, Jacobson HR, Dubois RN, Breyer MD (1994) Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest 94:2504–2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaissling B, Peter S, Kriz W (1977) The transition of the thick ascending limb of Henle's loop into the distal convoluted tubule in the nephron of the rat kidney. Cell Tissue Res 182:111–118 [DOI] [PubMed] [Google Scholar]
- Lapointe JY, Laamarti A, Bell PD (1998) Ionic transport in macula densa cells. Kidney Int 67(suppl):S58–64 [DOI] [PubMed] [Google Scholar]
- Lee CT, Huynh VM, Lai LW, Lien YH (2002) Cyclosporine A-induced hypercalciuria in calbindin-D28k knockout and wild-type mice. Kidney Int 62:2055–2061 [DOI] [PubMed] [Google Scholar]
- Lee CT, Shang S, Lai LW, Yong KC, Lien YH (2004) Effect of thiazide on renal gene expression of apical calcium channels and calbindins. Am J Physiol Renal Physiol 287:F1164–1170 [DOI] [PubMed] [Google Scholar]
- Lehtonen S, Ryan JJ, Kudlicka K, Iino N, Zhou HL, Farquhar MG (2005) Cell junction-associated proteins IQGAP1, MAGI-2, CASK, spectrins, and alpha-actinin are components of the nephrin multiprotein complex. Proc Natl Acad Sci USA 102:9814–9819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SH, Wang QJ, Chakladar A, Bronson RT, Bernards A (2000) Gastric hyperplasia in mice lacking the putative Cdc42 effector IQGAP1. Mol Cell Biol 20:697–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien YH, Yong KC, Cho C, Igarashi S, Lai LW (2006) S1P(1)-selective agonist, SEW2871, ameliorates ischemic acute renal failure. Kidney Int 69:1601–1608 [DOI] [PubMed] [Google Scholar]
- Liu XL, Kilpelainen P, Hellman U, Sun Y, Wartiovaara J, Morgunova E, Pikkarainen T, et al. (2005) Characterization of the interactions of the nephrin intracellular domain: evidence that the scaffolding protein IQGAP1 associates with nephrin. FEBS J 272:228–243 [DOI] [PubMed] [Google Scholar]
- Madsen KM, Tisher CC (1986) Structural-functional relationship along the distal nephron. Am J Physiol 250:F1–15 [DOI] [PubMed] [Google Scholar]
- Nielsen S, DiGiovanni SR, Christensen EI, Knepper MA, Harris HW (1993) Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc Natl Acad Sci USA 90:11663–11667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda Y, Sasaki S (2005) Trafficking mechanism of water channel aquaporin-2. Biol Cell 97:885–892 [DOI] [PubMed] [Google Scholar]
- Teng-umnuay P, Verlander JW, Yuan W, Tisher CC, Madsen KM (1996) Identification of distinct subpopulations of intercalated cells in the mouse collecting duct. J Am Soc Nephrol 7:260–274 [DOI] [PubMed] [Google Scholar]
- Weissbach L, Settleman J, Kalady MF, Snijders AJ, Murthy AE, Yan YX, Bernards A (1994) Identification of a human Rasgap-related protein containing calmodulin-binding motifs. J Biol Chem 269:20517–20521 [PubMed] [Google Scholar]
- Zhou RH, Guo Z, Watson C, Chen E, Kong R, Wang WX, Yao XB (2003) Polarized distribution of IQGAP proteins in gastric parietal cells and their roles in regulated epithelial cell secretion. Mol Biol Cell 14:1097–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]