Abstract
Background
Animal research suggests that antenatal stress exposure and postnatal rearing style act in concert to shape offspring biobehavioral outcomes. However, the combination of these maternally–mediated influences has not been studied in human infants.
Aims
To examine antenatal psychiatric status and maternal sensitivity in relation to 4–month–olds’ autonomic regulation, HPA–axis functioning, and behavior.
Study design
Prospective study of 47 pregnant women recruited from an urban hospital who completed questionnaire measures of anxiety and depression and underwent a psychiatric interview in the 2nd trimester. At four months postpartum, women again completed mood questionnaires and the mother–infant dyads participated in a 10-minute free–play session evaluated for maternal sensitivity.
Outcome measures
Baseline infant salivary cortisol and electrocardiogram (EKG) collected at the start of the 4–month sessions. Infant responsiveness and maternal report of temperament also were evaluated.
Results
Maternal sensitivity, but not antenatal psychiatric diagnosis, predicted greater levels of infant high frequency heart rate variability, after controlling for birth weight and age. Maternal sensitivity, but not psychiatric status, also predicted infant responsiveness. Maternal sensitivity modulated the effects of psychiatric illness on infant cortisol such that cortisol was low regardless of sensitivity for children of healthy women yet higher if the infant had insensitive versus sensitive caregiving when the mother had had an antenatal diagnosis.
Conclusions
Biobehavioral adaptation, even that initiated in utero, is influenced by interactions with the social world. These findings support the compatibility of fetal programming and social–context models of infant biobehavioral development and have promising implications for pre and postnatal clinical intervention.
The prevalence of antenatal mood and anxiety disorders and their inadequate treatment contribute to growing concerns about mental illnesses’ impact on fetal and infant development. Current research suggests that antenatal depression and anxiety are associated with alterations in physiology and behavior during infancy and beyond. Compared to infants born to non-depressed women, neonates whose mothers were depressed during pregnancy have higher cortisol levels [1], greater relative right frontal EEG activity [2], and lower vagal tone [3]. Antenatal anxiety predicts behavioral and emotional problems in preschoolers [4] and in 8–9 year–old children [5], as well as alterations in HPA axis activation among 10-year-olds [6]. Taken together, such findings support the prenatal programming hypothesis [7], which asserts that characteristics of the in utero environment, such as mood–based changes in women’s HPA–axis functioning, sculpt the uniquely plastic fetal brain, resulting in long–term maladaptive patterns of behavior and physiology [8–10].
Studies on the effects of antenatal mood and anxiety on child outcomes have controlled for the influences of postnatal depression and anxiety [5, 6]. However, there is a critical gap in our understanding with respect to the potential influence of parenting style on the relationship between antenatal diagnosis and infant physiological and behavioral outcomes. Maternal depression and anxiety have been associated with disturbances in the quality of caregiving, specifically in parental sensitivity, which is the extent to which the parent can consistently read and respond to the child’s cues. Parental sensitivity is an important aspect of caregiving and has been shown to play an integral role in adaptive self-regulatory behaviors [11, 12], and more recently, in infant fearfulness and right frontal EEG asymmetry [13, 14]. Depressed and anxious mothers tend to be less emotionally available to their children, often displaying signs of withdrawal and disinterest or behaving in an intrusive and hostile manner [15–17]. Because antenatal mood disturbance predicts postpartum depression [18, 19], and because maternal mood dysregulation has a negative effect on parenting, it is unclear the degree to which the quality of parenting may explain the infant and child outcomes that have been attributed to antenatal depression and anxiety.
In the present study, we aimed to provide additional empirical support for the effect of parental sensitivity on infant physiology and behavior, and to determine how pre-and postnatal maternal factors interact to influence infant biobehavioral development. Specifically, we examined women’s antenatal diagnostic status and postnatal maternal sensitivity in relation to the following variables in 4-month–old infants: HPA–axis functioning (salivary cortisol), autonomic regulation (high frequency heart rate variability), and behavioral style (emotional responsiveness and temperament). Based on the findings of the studies described above [9, 20] [3], we expected that infants of mothers with an antenatal psychiatric diagnosis would demonstrate decreased HRV and increased baseline salivary cortisol. We also hypothesized that lower maternal sensitivity would be associated with this same pattern of infant physiology and that infants whose mothers had antenatal mood dysregulation and who were also insensitive caregivers would show the lowest HRV and highest cortisol levels. We hypothesized that infant emotional responsiveness would be attributed largely to maternal sensitivity, rather than antenatal diagnostic status, in line with the current relationship–based approach to socialization [11, 21]. We also examined an alternative hypothesis that mothers’ caregiving depends on their perception of their infant’s temperament so that infant temperament qualities “lead” the dyadic interaction, thereby eliciting different levels of maternal sensitivity and bringing about the associated influences on infant development. Specifically, we examined the hypothesis that women who describe their children as difficult are less sensitive with them.
Methods
Participants
As part of a larger study, 314 medication-free, non-smoking pregnant women with singleton fetuses were recruited at the Columbia University Medical Center (CUMC). Women were excluded from the study if there were any maternal or fetal complications including hypertension, diabetes mellitus, suspected fetal growth restriction, or a fetal structural anomaly on ultrasound. The attrition rate from the first session to the final session at four months post-partum was approximately 62%. Forty-seven women and their 4-month old infants were ultimately included in the study.1 Infant demographic information is shown in Table 1. Women’s mean age was 28 (range = 19–40) years. Sixty-five percent of the sample was Latina, 23% Caucasian, and 12% African American. Ninety-three percent of women in the study had received at least a high school degree. This study was approved by the New York State Psychiatric Institute Institutional Review Board. Informed consent was obtained from each subject.
Table 1.
Infant Demographics.
| Measure | Mean | SD | Range | |
|---|---|---|---|---|
| Birthweight (g) | ||||
| NC | 3278.0 | 354.6 | 2840 – 4055 | |
| DX | 3514.4 | 494.2 | 2560 – 4335 | |
| Both | 3376.0 | 428.8 | 2560 – 4335 | |
| Age (weeks) at | ||||
| 4-month assessment | NC | 17.6 | 1.65 | 14 – 21 |
| DX | 19.5 | 2.4 | 16 – 25 | |
| Both | 18.4 | 2.18 | 14 –25 | |
| Gestational Age | ||||
| (weeks) | NC | 39.3 | 1.06 | 38 – 41.3 |
| DX | 39.5 | 1.22 | 37 – 41.5 | |
| Both | 39.4 | 1.12 | 37 – 41.5 | |
| Sex (% Female) | ||||
| NC | 52.2 | |||
| DX | 76.5 | |||
| Both | 61 | |||
| Ethnicity (% Latina) | ||||
| NC | 66.7 | |||
| DX | 70.6 | |||
| Both | 68.3 | |||
Antenatal Psychiatric Assessment
During the second trimester, subjects came to the laboratory at CUMC and underwent a psychiatric interview with a licensed psychologist using the Axis I module of the Structured Clinical Interview for DSM-IV (SCID) [22]. Based on the assessment, women were either rated as free of Axis I diagnoses or assigned a diagnosis. Every woman in the study who met diagnostic criteria for a psychiatric disorder was referred to treatment, but only about 15% of these women received psychotherapeutic intervention at some point in the perinatal period. None of the women in the study took psychotropic medication during pregnancy. In the 3rd trimester, they made a second trip to the laboratory for a psychophysiology session, reported elsewhere [23]. Women completed questionnaires assessing current feelings of depression (CES-D) [24] and state and trait anxiety (STAI) [25]. The CES–D is a 20–item questionnaire with a possible score range from 0–60. A score of 16 or more is considered an indication of depression. The STAI is a 40-item, self-report instrument that measures a predisposition to feel “generally” anxious (trait anxiety) or the current experience of it (state anxiety). Anxiety scores on the trait and state categories range from 20–80, with higher scores reflecting higher levels of anxiety. Reported average scores of state and trait for nonpregnant women from two samples ages 19 to 39 are 35.20 ± 10.61 and 36.17 ± 10.96 (state) and 35 ± 9 and 36 ± 10 (trait) [26].
4-Month Assessment
At four months post–partum, women and their infants returned to the laboratory and participated in an interactive session. All sessions began between 1:00–2:00 pm to control for diurnal variations in hormone levels. At the start of the session, infants were seated in an infant seat on a child–friendly carpet on the laboratory floor. Prior to the initiation of the protocol, infant baseline salivary cortisol was collected with a cotton roll. Infant electrocardiogram (EKG) was collected for one minute during the baseline period of a temperament assessment paradigm, reported elsewhere [27], while women again completed the CES-D and STAI. Lastly, the mother-infant dyad engaged in a 10–minute minute free-play session. Children’s toys were made available and women were instructed to play with their children as they would at home. The pair was left alone in the room and the interaction was videotaped. After the play session, women completed the Infant Behavior Questionnaire (IBQ), a caregiver report of infant behavior [28].
Heart-rate variability
Infant electrode leads were attached to a heart rate monitor (HP 78202A), and analog EKG waveforms were digitized at 500 Hz and collected by a microcomputer. Specially written software was used to detect R-waves, resulting in a time series of RR intervals. Errors in R-wave detection were corrected interactively by a trained researcher. Outliers were recognized as RR intervals more than three times the interquartile range below the 25th and above the 75th percentile of the RR interval in the surrounding ten-second window. Waveforms with fewer than 97.5% acceptable RR intervals were excluded. Spectral power was calculated in a high frequency (HF) band (0.15-0.5Hz) using an interval method for computing Fourier transforms [29]. High frequency heart-rate variability (HRV) is commonly used as an index of parasympathetic control of the heart, and the selected bandwidth is used to assess HRV in adults as well as infants [30, 31]. The high frequency component of HRV was log transformed to reduce the skewness of the data.
Salivary cortisol
Cortisol was measured by radioimmunoassay using a standard radioimmunoassay test tube pre-coated with antibody specific for cortisol and I-125 labeled cortisol-3-derivatives as a ligand (Micromedic RIA kit: Micromedic Systems, Inc.) Cortisol was analyzed by the Analytical Psychopharmacology Laboratories at the Nathan Kline Institute. All samples were assayed in duplicate in the same assay and mean values for each sample are reported here. The intra-assay coefficient of variation (C.V.) was 2.95% and the inter-assay C.V. was 6.0%.
Infant temperament
The IBQ includes 94 items evaluated on a 7–point scale reflecting the relative frequency of specified infant reactions in certain situations in the last week (e.g., when put into the bath water how often did the baby wave his her arm, squirm or try to roll away?) that are then tabulated under six subscales (ranging from 1–7): activity level, distress to limitations, latency to approach novel situations (fear), duration of orienting, smiling and laughter, and soothability. Studies have demonstrated the IBQ to be a highly reliable measure of infant temperament [32].
Maternal sensitivity & child responsiveness
Maternal sensitivity and child responsiveness observed in the 10-minute free-play session were assessed using the Emotional Availability (EA) Scales, 3rd Edition [33]. The EA scales integrate concepts from attachment theory [34] and emotional availability [35]. In contrast to other rating schemes, EA is highly global, emphasizing the emotional quality of an interaction and behavioral style, rather than discrete quantifiable behaviors. The EA scales have been validated for very young infants [36], and ratings are highly reliable throughout infancy and childhood [37].
The present study focused on the EA dimension of parental sensitivity, which is rated on a scale from 9 (highly sensitive) to 1 (highly insensitive). Parental sensitivity reflects the quality of the dyad’s emotional connection, which is believed to depend largely on the parent’s ability to attend to and successfully read the infant’s cues. A sensitive parent is highly perceptive of and responds promptly to the infant’s needs for excitatory or calming sensory input and exhibits genuine enjoyment in the interaction with the child. In contrast, an insensitive parent may demonstrate some positive affect, but overall can be characterized as either harsh and overbearing or passive and disinterested in the child. For example, if an infant puts in his or her mouth a toy that has been on the floor, according to this rating system, an insensitive caregiver would harshly prohibit this behavior and grab the toy away, whereas a sensitive caregiver would gently remove the toy and either provide a more appropriate way to meet the child’s sensory needs or smoothly transition to an alternate means of playing with the toy.
During the mother–child play session, child responsiveness also was assessed using the EA scales. For infants, responsiveness ratings are based on the extent of positive or negative affect during the interaction and evidence of joint attention and eye contact. Responsiveness is rated from 7 (highly responsive) to 1 (unresponsive). For a complete description of the EA Scales, see Biringen [33].
The videotaped interactions were reviewed by two independent and blind raters, who had achieved reliability on the established coding construct with both the EA scale developer Zeynep Biringen, and with each other. Videos were coded after all participants completed the study, allowing for the two raters to overlap on 17 randomly chosen dyads. Inter-rater reliability (Cronbach’s α) was above 0.9 for all EA dimensions.
Results
Maternal Pre- and Postnatal Mood
Based on the 2nd trimester SCID assessment, 19 women met diagnostic criteria for a DSM–IV mood or anxiety disorder, comprising the patient (DX) group. The distribution of specific diagnoses was as follows: Generalized Anxiety Disorder (n=9), major unipolar depression (n=3), or both (n=7). Twenty–eight women free of Axis I pathology constituted the normal control group (NC). Although a structured clinical interview was only conducted prenatally, there were high correlations between pre– and postnatal CES-D (r =0.72) and STAI scores (r =0.84) across all subjects. However, the average postnatal CES–D depression score of DX (and NC) women was below the standard cut–off for an indication of depression (13.9 versus 16). Descriptive statistics for the pre and postnatal mood ratings are reported in Table 2.
Table 2.
Self-reported Antenatal (A) and Postnatal (P) Mood and Anxiety in Healthy Controls (NC) and in Women with Clinical Diagnosis (DX).
| Measure | N* | Mean | SD | |
|---|---|---|---|---|
| CES-D (A) | ||||
| NC | 27 | 9.2 | 7.1 | |
| DX | 14 | 19.0 | 10.7 | |
| CES-D (P) | ||||
| NC | 27 | 8.2 | 7.9 | |
| DX | 9 | 13.9 | 11.3 | |
| STAI-Trait (A) | ||||
| NC | 28 | 32.3 | 9.2 | |
| DX | 18 | 41.1 | 9.1 | |
| STAI-Trait (P) | ||||
| NC | 28 | 32.2 | 8.7 | |
| DX | 16 | 39.3 | 10.1 | |
| STAI-State (A) | ||||
| NC | 28 | 28.8 | 8.0 | |
| DX | 18 | 35.2 | 12.0 | |
| STAI-State (P) | ||||
| NC | 28 | 28.9 | 8.6 | |
| DX | 16 | 30.8 | 11.4 | |
Ns vary as sometimes subjects failed to complete questionnaires.
Sensitivity Groupings
Participants with sensitivity ratings above the sample mean (M = 5.49, SD = 1.45) were classified as high in sensitivity (HS) and those with ratings equal to or below the mean were classified as low in sensitivity (LS). This dichotomy also corresponds to a natural shift in the coding scheme, as ratings below 6 are considered inconsistently sensitive or insensitive. The averages sensitivity rating for women in the LS group was 4.41 (SD = 1.1) and was 6.68 (SD = 0.63) for women in the HS group. Maternal sensitivity ratings were similar in the DX (5.1 ± 0.42) and NC (5.7 ± 0. 25) groups (t =1.3, p > 0.2). Similarly, women diagnosed with an antenatal mood or anxiety disorder were represented equally in the HS and LS groups (χ2 =0.04, p =0.84). Independent t-tests indicated that postnatal depression and anxiety symptoms did not significantly differ between HS and LS mothers (see Table 3).
Table 3.
Self-reported Postnatal Mood and Anxiety in Low Sensitive (LS) and High Sensitive (HS) Mothers.
| Measure | N | Mean | SD | t | p | |
|---|---|---|---|---|---|---|
| STAI State | ||||||
| LS | 23 | 31.1 | 11.4 | 1.12 | 0.27 | |
| HS | 21 | 27.9 | 7.1 | |||
| STAI Trait | ||||||
| LS | 23 | 36.3 | 11.2 | 1.06 | 0.30 | |
| HS | 21 | 33.1 | 7.9 | |||
| CES-D | ||||||
| LS | 18 | 11.4 | 10.4 | 1.20 | 0.24 | |
| HS | 18 | 7.8 | 7.3 | |||
Heart Rate Variability
Thirty-nine of the 47 dyads in our sample were included in the HRV analysis. Five infants were excluded because their raw EKG waveforms had fewer than 97.5% acceptable RR intervals due to signal drop-out and noise. Three infants with missing birth weight information were also excluded, because birth weight, as well as age, can significantly influence HRV during infancy [38, 39], and these measures were included in the model as covariates. No maternal variables of postnatal mood or anxiety correlated with infant HRV, and thus were not included in the model. Antenatal diagnostic status and maternal sensitivity were entered into a 2 × 2 ANCOVA, controlling for infant birth weight and age at 4-month assessment. Neither of these covariates significantly predicted infant HRV (p > 0.1). Infants of HS mothers had significantly higher HRV than infants of LS mothers (F(1,35) = 4.34, p = .045; Cohen’s d = 0.73) (Figure 1). There was neither a significant main effect of diagnostic status on HRV (F = 0.64, p = 0.43; Cohen’s d = 0.28), nor an interaction between diagnostic status and sensitivity (F = 0.01, p = 0.92). Table 4 shows the descriptive statistics for infant HRV, split by sensitivity and diagnostic groups.
Figure 1.

Resting infant high frequency heart rate variability (HF HRV) at 4-months differs for mothers with low sensitivity (LS) and high sensitivity (HS). Compared to infants receiving LS parenting (n=20), infants receiving HS parenting (n=19) had significantly higher HF HRV (F = 4.34, p < 0.05). Group means are adjusted for infant birth weight and age at assessment. HRV reflects the power spectral density and is expressed in absolute units (ms2).
Table 4.
Summary Statistics for Infant High Frequency HRV (log transformed).
| Diagnosis | Sensitivity | N | Mean | SD |
|---|---|---|---|---|
| NC | ||||
| LS | 13 | 3.63 | 0.99 | |
| HS | 13 | 4.29 | 0.85 | |
| DX | ||||
| LS | 7 | 3.83 | 1.13 | |
| HS | 6 | 4.45 | 1.18 | |
Cortisol
Thirty-three of the 47 dyads in our sample were included in the salivary cortisol analysis. Eleven infants did not have a sufficient sample volume to determine cortisol level and three infants were excluded from the analysis because they were identified as extreme outliers, with cortisol values more than 1.5 times the interquartile range above the 75th percentile of the sample. As a result, 20 infants from the NC group and 13 infants from the DX group were included in this analysis. When diagnostic status and sensitivity were combined in a 2 × 2 ANOVA, the main effects approached significance for both diagnostic group (F (1,29) = 3.9, p = 0.10; Cohen’s d = 0.51) and sensitivity (F(1,29) = 3.5, p = 0.07; Cohen’s d=0.61), and there was a significant interaction between diagnostic group and sensitivity (F(1,29) = 5.1, p = 0.03). Figure 2 illustrates this interaction where infant cortisol levels are low regardless of sensitivity if their mothers belong to the NC group, whereas for infants of mothers in the DX group, cortisol is significantly higher if they have insensitive compared to sensitive parenting.
Figure 2.
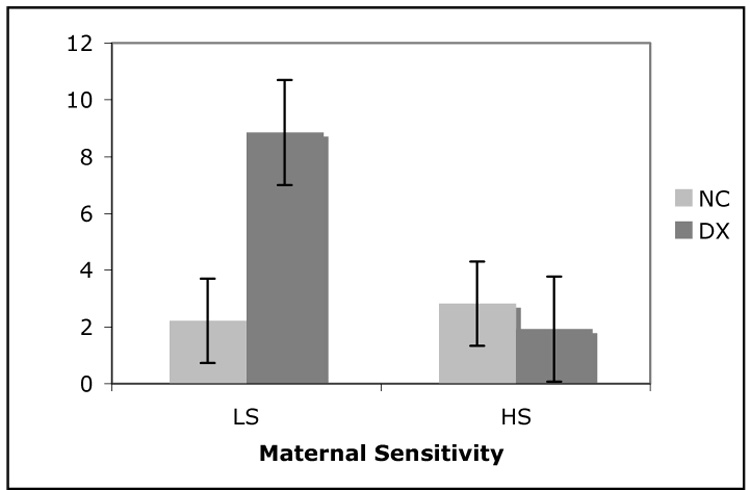
Interaction between diagnostic status and maternal sensitivity on infant salivary cortisol. Cortisol units (ng/mL) reflect the concentration of cortisol in saliva. Infants from the NC group had low cortisol levels regardless of whether they received high sensitivity (HS) parenting (n=10) or low sensitivity (LS) parenting (n=10). However, infants in the DX group had significantly higher cortisol levels if they received LS parenting (n=6), but not if they received HS parenting (n=7) (F = 5.05, p < 0.05).
Infant Responsiveness
EA–assessed child responsiveness was entered into a 2 × 2 ANOVA with antenatal diagnosis and maternal sensitivity. The average responsiveness rating for infants in the DX group was 5.0 (SD = 0.39) and the average rating for infants in the NC group was 4.9 (SD = 0.34). There was no significant main effect of antenatal diagnostic status (F (1,39) = 0.30, p = 0.59; Cohen’s d = 0.12) or sensitivity–by–diagnosis interaction (F (1,39) = 0.56, p =0.46). However, independent of women’s antenatal diagnosis, infants of HS mothers were rated significantly higher in child responsiveness, demonstrating less negative affect and more interest in the interaction than infants of LS mothers (F (1,39) = 29.303, p < 0.001; Cohen’s d = 1.63)(See Figure 3).
Figure 3.

Infant emotional responsiveness differs by maternal sensitivity. Infants receiving high sensitivity (HS) parenting (n = 22) received higher ratings of emotional responsiveness (i.e. more positive affect and less negative affect or disinterest in the parent) than did infants receiving low sensitivity (LS) parenting (n = 25) (F = 34.3, p < 0.0005).
Infant Temperament
Scores from each dimension of the IBQ (e.g. soothability) were analyzed in separate 2 × 2 ANOVAs. There were no main effects of maternal sensitivity or diagnostic status, nor any significant interactions, on any of the IBQ dimensions (all p > 0.1).
Discussion
Consistent with previous studies [13, 14], we found that infant physiology and behavior was significantly influenced by caregiving behavior. Higher levels of HRV have been associated with more adaptive emotion regulation in children [40] and adults [41]. We found that infants of highly sensitive mothers had higher resting HRV than did infants of less sensitive mothers. These results are consistent with developmental [42] and transactional models [43] of emotional health, which assert that biobehavioral adaptation is shaped, in part, by interactions with the social environment. The perception of control and social support during mild stress has been shown to predict decreased heart rate reactivity [44, 45], and increased HRV [46] in both adults and children. Thus, differences in HRV among infants of HS and LS mothers may reflect the larger amount of stress experienced in the unfamiliar laboratory environment by infants whose mothers do not consistently attend to the child’s needs.
Surprisingly, we did not find reduced HRV in infants of antenatally depressed/anxious mothers, as we had hypothesized. However, this inconsistency with previous research [3] could be explained by another unexpected finding. Although other studies report depressed and anxious mothers tend to be less sensitive in their parenting [15–17], we observed no difference in sensitivity ratings for depressed and anxious women in our sample2. As a result, our sample allowed for the dissociation between prenatal influences (e.g. antenatal depression/anxiety) and postnatal influences (e.g. sensitivity) on infant autonomic regulation.
Neither antenatal diagnosis nor maternal sensitivity alone predicted infant baseline cortisol level, but the interaction between the two factors was significant. Infants of women without an antenatal diagnosis had comparatively low baseline cortisol levels regardless of their mother’s sensitivity. However, infants of women with an antenatal diagnosis had significantly higher cortisol levels if their mothers were less sensitive, but were indistinguishable from infants of control women if they received more sensitive parenting. Consistent with the fetal programming hypothesis [8–10], these findings suggest that antenatal diagnostic status influences infant stress–related physiology, potentially mediated by genetic transmission and/or mood–based alterations in women’s HPA–axis that affect fetal physiological development. However, this infant HPA–axis programming appears to be modulated by maternal sensitivity, indicating that a developmental programming model can be complemented by other theories of development [9], such as those focusing on the social context of biopsychological adaptation.
Maternal sensitivity, but not antenatal psychiatric status, was associated with EA assessment of child responsiveness during the free-play session. Infants whose mothers were rated as more sensitive showed greater positive affect and engagement in the free–play session compared to infants whose mothers were rated as less sensitive. These results are consistent with previous work showing the significant influence of caregiving characteristics in child socialization and self–regulation [47, 48]. Our findings highlight the role of maternal caregiving behavior in shaping infant biobehavioral development. However, we also investigated the alternative hypothesis that infant temperament constrains the quality of mothers’ caregiving so that temperament qualities in the infant “lead” the dyadic interaction and the associated effects on infant development. Specifically, we examined the hypothesis that compared to sensitive caregivers, women who are less sensitive with their infants describe them as more difficult. This hypothesis was not supported. Mothers’ subjective reports of infant temperament did not differ as a function of sensitivity group. Our results are consistent with those from Hane & Fox [13], who found that the quality of maternal care did not differ with objective classifications of temperament.
Although the interaction between antenatal stress and postnatal caregiving behavior has been studied extensively in the animal literature [49, 50] [51], until recently, no studies had addressed the interaction between similar prenatal and postnatal influences on physiology and behavior in human infants. In 2006, Diego et al. [14] found that right frontal EEG asymmetry was most pronounced for infants of depressed women who were also characterized as withdrawn or intrusive, suggesting that insensitive caregiving may exacerbate the adverse effects of prenatal depression. In the current study, we present evidence that maternal sensitivity modulates the effect of antenatal depression and anxiety on infant cortisol, suggesting that the epigenetic processes leading to associations between antenatal mood and stress-related physiology in the perinate can be over–ridden by postnatal environmental factors such as the quality of parenting.
There are some limitations to this study. Our measures of HRV and cortisol were not taken in response to a stressor or stimulus, and thus cannot inform how parental sensitivity interacts with antenatal psychiatric status to influence infant stress reactivity. HRV data was based on a brief assessment period and may be more susceptible to minor variations in the RR intervals than if it were based on a longer EKG sample. Because we did not find a main effect of antenatal psychiatric status on HRV or on cortisol, these data contrast somewhat with previous work showing that antenatal depression and anxiety is associated with decreased HRV in newborns and infants [3] and with elevated levels of awakening cortisol in 10-year-olds [6]. This discrepancy may be due, in part, to differences between the metrics used to assess HRV in the present study and in previous studies. Furthermore, the size of our patient group may have limited our statistical power to detect an effect of antenatal diagnostic status on these infant outcome variables. The patient group included in the present study may represent pregnant women with only mild to moderate depression/anxiety, and thus the current findings may not be generalizeable to populations with more severe depression and anxiety.
Collectively, the results of this study illustrate the significant influence of maternal sensitivity on infant physiology and emotional responsiveness. Sensitive parenting may provide the infant with repeated instances of appropriate support and successful coping, which may ultimately contribute to the shaping of physiologic regulation to future stressors. In addition, maternal sensitivity may promote appropriate behavioral responses from the child, which may subsequently influence others’ responses to the child throughout development. Interestingly, it appears that the in utero programming of autonomic and HPA–axis regulation may be open to postnatal shaping of brain–behavior development, a finding that supports the compatibility of fetal programming and social–context models of infant biobehavioral development. As such, these findings, and those from similar studies, have promising implications for pre and postnatal clinical intervention.
Acknowledgements
This research was supported by the March of Dimes, the National Alliance for Research on Schizophrenia and Depression, the Sackler Institute, and by a Career Development Award MH01928 to Catherine Monk. We would like to thank the many women and children who participated in this research, Yixin Fang for statistical support, as well as Liz Werner, Sylvia Cabral, and Katelyn Beaudette for their contributions in the collection of this data. We also extend thanks to Michael Meyers, Daniel Pine, and Richard Sloan for reviewing early drafts of this manuscript.
Footnotes
Only 47 of the 117 women were included in this study due to the following: (a) data acquisition procedures changed midway through the sample and those collected by the earlier method were excluded for these analyses; (b) dyads were unable to finish the session (e.g. baby fell asleep or was too distressed to continue); (c) computer or experimenter error resulted in loss or damage to data.
Women who had higher CES–D scores during pregnancy were less likely to return for the 4–month session. Thus, the lack of an association between sensitivity and postnatal depression may reflect a constricted range of depression severity.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Field T, Diego M, Dieter J, Hernandez-Reif M, Schanberg S, Kuhn C, et al. Prenatal depression effects on the fetus and the newborn. Infant Behavior & Development. 2004;27:216–229. [Google Scholar]
- 2.Jones NA, Field T, Fox NA, et al. EEG asymmetry in one-month old infants of depressed mothers. Dev Psychopathol. 1997;9:491–505. doi: 10.1017/s0954579497001260. [DOI] [PubMed] [Google Scholar]
- 3.Field T, Pickens J, Fox NA, Nawrocki T, Gonzalez J. Vagal tone in infants of depressed mothers. Development and Psychopathology. 1995;7:227–231. [Google Scholar]
- 4.O'Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children's behavioral/emotional problems at 4 years. British Journal of Psychiatry. 2002;180:502–508. doi: 10.1192/bjp.180.6.502. [DOI] [PubMed] [Google Scholar]
- 5.Van den Bergh BRH, Marcoen A. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-olds. Child Dev. 2004;75(4):1085–1097. doi: 10.1111/j.1467-8624.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- 6.O'Connor TG, Ben-Shlomo Y, Heron J, Golding J, Adams D, Glover V. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biol Psychiatry. 2005;58:211–217. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 7.Barker DJ. The Wellcome Foundation Lecture, 1994. The fetal origins of adult disease. Proceedings of the Royal Society of London B. 1995;262(1363):37–43. doi: 10.1098/rspb.1995.0173. [DOI] [PubMed] [Google Scholar]
- 8.Wadhwa PD. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005 Sep;30(8):724–743. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 9.O'Connor TG, Heron J, Golding J, Glover V. Maternal antenatal anxiety and behavioural/emotional problems in children: a test of a programming hypothesis. J Child Psychol Psychiatry. 2003 Oct;44(7):1025–1036. doi: 10.1111/1469-7610.00187. [DOI] [PubMed] [Google Scholar]
- 10.Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. Journal of Neuroendocrinology. 2001;13:113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- 11.Crockenberg SC, Leerkes EM. Infant and maternal behaviors regulate infant reactivity to novelty at 6 months. Dev Psychol. 2004 Nov;40(6):1123–1132. doi: 10.1037/0012-1649.40.6.1123. [DOI] [PubMed] [Google Scholar]
- 12.Parpal M, Maccoby EE. Maternal responsiveness and subsequent child compliance. Child Development. 1985;56(5):1326–1334. [Google Scholar]
- 13.Hane AA, Fox NA. Ordinary variations in maternal caregiving influence human infants' stress reactivity. Psychological Science. 2006;17(6):550–556. doi: 10.1111/j.1467-9280.2006.01742.x. [DOI] [PubMed] [Google Scholar]
- 14.Diego MA, Field T, Jones NA, Hernandez-Reif M. Withdrawn and intrusive maternal interaction style and infant frontal EEG asymmetry shifts in infants of depressed and non-depressed mothers. Infant Behav Dev. 2006 Apr;29(2):220–229. doi: 10.1016/j.infbeh.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frankel KA, Harmon RJ. Depressed mothers: They don't always look as bad as they feel. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35(3):289–298. doi: 10.1097/00004583-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Tronick EZ, Weinberg MK. Depressed mothers and infants: Failure to form dyadic states of consciousness. In: Murray L, Cooper PJ, editors. Postpartum Depression and Child Development. New York: The Guilford Press; 1997. pp. 54–81. [Google Scholar]
- 17.Zeanah CH, Boris NW, Larrieu JA. Infant development and developmental risk: A review of the past 10 years. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(2):165–178. doi: 10.1097/00004583-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Heron J, O'Connor TG, Evans J, Golding J, Glover V. The course of anxiety and depression through pregnancy and the postpartum in a community sample. J Affect Disord. 2004 May;80(1):65–73. doi: 10.1016/j.jad.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Honey KL, Bennett P, Morgan M. Predicting postnatal depression. J Affect Disord. 2003 Sep;76(1–30):201–210. doi: 10.1016/s0165-0327(02)00085-x. [DOI] [PubMed] [Google Scholar]
- 20.O'Connor TG, Ben-Shlomo Y, Heron J, Golding J, Adams D, Glover V. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biol Psychiatry. 2005 Aug 1;58(3):211–217. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 21.Kochanska G, Aksan N. Development of mutual responsiveness between parents and their young children. Child Dev. 2004 Nov-Dec;75(6):1657–1676. doi: 10.1111/j.1467-8624.2004.00808.x. [DOI] [PubMed] [Google Scholar]
- 22.First M, Spitzer R, Gibbon M, Williams J. Structured clinicacl interview diagnostic (SCID) for DSM-IV axis I Disorders 3/4 Clinical Version (SCID-CV) Washington: American Psychiatric Press; 1997. [Google Scholar]
- 23.Monk C, Sloan RP, Myers MM, Ellman L, Werner E, Jeon J, et al. Fetal Heart Rate Reactivity Differs by Women's Psychiatric Status: An Early Marker for Developmental Risk? Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(3):283–290. doi: 10.1097/00004583-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. J Appli Psychol Meas. 1977;1:385–401. [Google Scholar]
- 25.Spielberger CD. Manual for the State-Trait Anxiety Inventory (STAI) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 26.Spielberger CD, Sydeman SJ. State-Trait anxiety inventory and State-Trait anger expression inventory. In: Maruish ME, editor. The Use of Psychological Tests for Treatment Planning and Outcome Assessment. Hillsdale, NJ: LEA; 1994. [Google Scholar]
- 27.Werner EA, Myers MM, Fifer WP, Monk D. Prenatal predictors of infant temperament. Developmental Psychobiology. doi: 10.1002/dev.20232. Submitted. [DOI] [PubMed] [Google Scholar]
- 28.Rothbart MK. Measurement of temperament in infancy. Child Development. 1981;52:569–578. [Google Scholar]
- 29.deBoer RW, Karemaker JM, Strackee J. Comparing spectra of a series of point events, particularly for heart rate variability spectra. IEEE Trans Biomed Eng. 1984;31:384–387. doi: 10.1109/TBME.1984.325351. [DOI] [PubMed] [Google Scholar]
- 30.Massin M, von Bernuth G. Normal ranges of heart rate variability during infancy and childhood. Pediatric cardiology. 1997 Jul-Aug;18(4):297–302. doi: 10.1007/s002469900178. [DOI] [PubMed] [Google Scholar]
- 31.Mehta SK, Super DM, Connuck D, Salvator A, Singer L, Fradley LG, et al. Heart rate variability in healthy newborn infants. The American journal of cardiology. 2002 Jan 1;89(1):50–53. doi: 10.1016/s0002-9149(01)02162-2. [DOI] [PubMed] [Google Scholar]
- 32.Rothbart MK. Longitudinal observation of infant temperament. Developmental Psychology. 1986;22:356–365. [Google Scholar]
- 33.Biringen Z. Emotional availability: conceptualization and research findings. Am J Orthopsychiatry. 2000;70(1):104–114. doi: 10.1037/h0087711. [DOI] [PubMed] [Google Scholar]
- 34.Ainsworth MDS, Blehar MC, Waters E, Wall S. Patterns of attachment: A psychological study of the strange situation. Hillsdale, NJ: Lawrence Erlbaum; 1978. [Google Scholar]
- 35.Emde RN. Emotional availability: A reciprocal reward system for infants and parents with implications for prevention of psychosocial disorders. In: Taylor PM, editor. Parent-infant relationships. Orlando, FL: Grune & Stratton; 1980. [Google Scholar]
- 36.Carter AS, Little C, Garrity-Rokous FE. Adapting the emotional availability scales for 4-month old infant-parent dyads: Associations with molecular coding and parental psychopathology. International Conference on Infant Studies; 1998; Atlanta: 1998. [Google Scholar]
- 37.Bornstein MH, Gini M, Putnick DL, Haynes OM, Painter KM, Suwalsky JTD. Short-term reliability and continuity of emotional availability in mother-child dyads across contexts of observation. Infancy. 2006;10(1):1–16. doi: 10.1207/s15327078in1001_1. [DOI] [PubMed] [Google Scholar]
- 38.Finley JP, Nugent ST. Heart rate variability in infants, children and young adults. Journal of the Autonomic Nervous System. 1995;51:103–108. doi: 10.1016/0165-1838(94)00117-3. [DOI] [PubMed] [Google Scholar]
- 39.Massin MM, Withofs N, Maeyns K, Ravet F. The influence of fetal and postnatal growth on heart rate variability in young infants. Cardiology. 2001;95(2):80–83. doi: 10.1159/000047350. [DOI] [PubMed] [Google Scholar]
- 40.Porges SW, Doussard-Roosevelt JA, Maiti AK. Vagal tone and the physiological regulation of emotion. Monogr Soc Res Child Dev. 1994;59(2–3):167–186. [PubMed] [Google Scholar]
- 41.Sloan RP, Shapiro PA, Bagiella E, Boni SM, Paik M, Bigger JT, Jr, et al. Effect of mental stress throughout the day on cardiac autonomic control. Biol Psychol. 1994 Mar;37(2):89–99. doi: 10.1016/0301-0511(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 42.Sroufe LA. Psychopathology as an outcome of development. Development and Psychopathology. 1997;9:251–268. doi: 10.1017/s0954579497002046. [DOI] [PubMed] [Google Scholar]
- 43.Smith TW. Hostility and health: current status of a psychosomatic hypothesis. Health Psychol. 1992;11(3):139–150. doi: 10.1037//0278-6133.11.3.139. [DOI] [PubMed] [Google Scholar]
- 44.Gallo LC, Smith TW, Kircher JC. Cardiovascular and electrodermal responses to support anad provocation: Interpersonal methods in the study of psychophysiological reactivity. Psychophysiology. 2000;37:289–301. [PubMed] [Google Scholar]
- 45.Chen E, Matthews KA, Salomon K, Ewart CK. Cardiovascular reactivity during social and nonsocial stressors: Do children's personal goals and expressive skills matter? Health Psychology. 2002;21(1):16–24. [PubMed] [Google Scholar]
- 46.Horsten M, Ericson M, Perski A, Wamala SP, Schenck-Gustafsson K, Orth-Gomer K. Psychosocial factors and heart rate variability in healthy women. Psychosomatic Medicine. 1999;61:49–57. doi: 10.1097/00006842-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 47.Crockenberg SB, Leerkes EM. Infant and maternal behaviors regulate infant reactivity to novelty at 6 months. Developmental Psychology. 2004;40(6):1123–1132. doi: 10.1037/0012-1649.40.6.1123. [DOI] [PubMed] [Google Scholar]
- 48.Kochanska G, Aksan N. Development of mutual responsiveness between parents and their young children. Child Dev. 2004;75(6):1657–1676. doi: 10.1111/j.1467-8624.2004.00808.x. [DOI] [PubMed] [Google Scholar]
- 49.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 50.Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating hte expression of fearfulness in the rat. Proc Natl Acad Sci. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of matneral behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]


