Abstract
Purpose
NOD2 plays an important role in the recognition of intracellular bacteria through its ability to sense the components of bacterial peptidoglycan (PGN), namely muramyl dipeptide (MDP) and muramyl tripeptide (MTP). Specific mutations in the human NOD2 gene cause Blau syndrome, an autosomal dominant form of uveitis, arthritis, and dermatitis. As a first step toward understanding the role of NOD2 in the pathogenesis of uveitis, the authors developed a mouse model of MDP-dependent uveitis.
Methods
BALB/c mice and mice deficient in L-selectin or NOD2 received intravitreal injection of MDP, MTP, or PGN. The intravascular response within the iris and cellular infiltration was quantified by intravital microscopy and histologic assessment.
Results
MDP induced an acute, ocular inflammatory response, wherein rolling and adhering leukocytes within the vasculature were significantly increased within 6 hours after MDP treatment. A minor increase in cellular infiltration occurred at 12 hours after MDP treatment. The adhesion molecule L-selectin participated in MDP-induced vascular inflammation because L-selectin knockout mice showed a significant decrease in the number of rolling cells. Importantly, NOD2 plays an essential role in ocular inflammation induced by MDP, as indicated by the fact that uveitis did not develop in Nod2 knockout mice in response to MDP. Nod2 knockout mice also showed abolished ocular inflammation in response to MTP but not to PGN treatment.
Conclusions
These findings demonstrate a novel mouse model of uveitis, wherein NOD2 plays an essential role in inflammation induced by the minimal components of PGN. Thus, innate immune responses mediated by NOD2 may participate in the development of uveitis in response to bacterial products.
Innate immunity plays a fundamental role in host defense through its ability to rapidly respond to invading pathogens. The capacity of the innate immune system to initiate inflammatory responses is attributed to the presence of germ line-encoded receptors referred to as pattern recognition receptors (PRRs). Such PRRs are known to sense pathogen-associated molecular patterns (PAMPs), or invariant molecular structures present in pathogens, and endogenous danger signals (so-called DAMPs). Although the toll-like receptor (TLR) family has represented the major constituent of the PRRs, it is now apparent that the NOD-like receptor (NLR) family also plays an important role in the detection of bacterial products. The role of members of the NLR family in the eye has been relatively uncharacterized. However, it is likely that they may participate in the basic immune mechanisms involved in ocular inflammation, as has been previously demonstrated for TLRs such as TLR4 in endotoxin-induced uveitis.1–3
The discovery of a single nucleotide change that results in the development of uveitis is a seminal finding that clearly implicates the gene, NOD2, whose contribution to ocular inflammation must be clarified.4,5 NOD2 belongs to the NLR family and so shares conserved structural domains, including an N-terminal caspase recruitment domain (CARD), a central nucleotide-binding oligomerization domain (NOD), and C-terminal leucine-rich repeats (LRRs).6,7 NOD2 has been shown to sense muramyl dipeptide (MDP) and muramyl tripeptide (MTP), which are naturally occurring breakdown products of the bacterial cell wall component peptidoglycan (PGN).8,9 The capacity of NOD2 to sense MDP is dependent on its LRR domains.10 In response to MDP, NOD2 forms a multiprotein complex containing RICK/RIP2, which promotes the IKK complex to activate the transcription factor NF-κB.11,12 Signaling pathways other than NF-κB that may be activated by NOD2 are not well understood, although apoptosis,13,14 the MAP kinase pathway,15,16 and the inflammasome have all been implicated.17 NOD2 also appears to play a role in controlling different bacterial infections, including Listeria monocytogenes18 –20 and Salmonella typhimurium.21
The importance of NOD2 in regulating inflammatory responses is further underscored by the discovery that mutated forms of NOD2 are responsible for Blau syndrome. Blau syndrome (also referred to as Jabs syndrome) was first identified as an autosomal dominant disease characterized by multiorgan granulomatous inflammation of the eye, skin, and joint.22,23 The uveitis is usually bilateral, causes chorioretinal scarring, and can result in severe visual loss. It was subsequently discovered that patients with Blau syndrome had missense changes within the NOD domain affecting specific residues 334 and 469 and other coding region mutations,4,5,24,25 thereby definitively identifying NOD2 as the gene responsible for Blau syndrome. Intriguingly, additional polymorphisms in NOD2 have been associated with increased susceptibility to Crohn disease, a granulomatous inflammation of the bowel that can also involve the uvea and joints.26 Thus, this provides us with the unique opportunity to examine how the function of a particular gene such as NOD2, which is involved in innate immunity, may participate in the initiation of uveitis. Indeed it was recently demonstrated that Nod2 is expressed in mouse eye tissue27 and within human vascular endothelial cells from the iris, choroid, or retina28; little is known regarding the in vivo function of NOD2 within the eye.
As a first step toward understanding the pathogenesis of eye inflammation in Blau syndrome, we sought to investigate the role of NOD2 in a mouse model of uveitis. We tested the hypothesis that activation of NOD2 by MDP results in an ocular inflammatory response in mice. Given that the hallmark of ocular inflammation is the activation and recruitment of leukocytes through the vasculature of the eye, we used an established technique of intravital microscopy to visualize such a response to MDP. We demonstrated that an intravitreal injection of MDP elicited a transient increase in the number of rolling and adhering leukocytes within the iris microvasculature and a minor cellular infiltrate within the iris tissue. The process by which leukocytes interact with the vasculature and infiltrate inflamed tissue has been described and occurs in a stepwise fashion that involves specific adhesion molecules. We took advantage of intravital microscopy to visualize the functional role of L-selectin in MDP-induced inflammation and showed that deficiency in L-selectin significantly reduced the number of rolling cells in response to MDP. Importantly, such cellular inflammatory responses were mediated by NOD2 because we found that MDP-induced cellular inflammation was abolished in Nod2 knockout mice. NOD2 was also required for the ocular inflammatory response induced by MTP, another breakdown product of PGN. In contrast, uveitis induced by the administration of intact PGN, which is a TLR2 agonist, did not require NOD2. To our knowledge, these are the first findings to demonstrate a functional role for NOD2 in ocular inflammation in mice. We believe these studies provide the foundation for future evaluation of NOD2-mediated ocular effects.
Methods
Reagents
Synthetic stereoisoforms (L and D) of MDP and synthetic MTP were purchased from Bachem (Torrance, CA) and dissolved in sterile saline. MDP tested below the lower limit of detection of endotoxin activity by limulus amebocyte lysate assay. Purified PGN of Staphylococcus aureus was purchased from Fluka (Buchs, Switzerland). For MDP treatment, mice were given intravitreal injections (2 μL volume) using a Hamilton syringe with a 30½-gauge needle.
Mice
Age-matched (8 –10 weeks old) female BALB/c mice, tyrosinase knockout (albino) C57BL/6, L-selectin knockout mice, and their appropriate strain controls (nonobese diabetic background) were obtained from Jackson Laboratories (Bar Harbor, ME). Nod2 knockout mice were kindly provided by Richard Flavell of Yale University, which we then backcrossed 10 generations onto a BALB/c background. Mice were housed in a facility approved by the Association of Assessment and Accreditation of Laboratory Animal Care International. Procedures were carried out according to National Institutes of Health, the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and Oregon Health & Science University Institutional Animal Care and Use policies.
Intravital Microscopy
The leukocyte response within the vasculature and extravascular tissue of the iris was assessed by intravital microscopy according to a previously established method.29 Briefly, at the time of imaging, animals were injected with 35 mg/kg rhodamine 6G (Sigma, St. Louis, MO) and anesthetized with 1.7% isofluorane. Digital images of the iris vasculature were captured with a black-and-white video camera (Kappa Scientific, Gleichen, Germany) on an epifluorescence intravital microscope (modified Orthoplan; Leica, Wetzlar, Germany) in three independent regions. Diameter and length of each vessel segment or iris tissue and leukocyte phenotype (i.e., rolling, adhering, infiltrating) were quantified off-line with Image J analysis software, previously described.29
Histology
Mouse eyes were prepared for histologic assessment as previously described.30 Briefly, whole eyes were dissected, fixed, and embedded in paraffin for sectioning. Seven-micrometer tissue sections were stained with hematoxylin and eosin. An observer masked to treatment groups counted the number of leukocytes within the anterior chamber of seven sections for each eye (approximately every tenth slide of a whole eye sectioned completely). The mean number of leukocytes per section was then calculated for each mouse eye.
Statistical Analysis
Data are represented as mean ± SEM. Mean differences between treatment and genotype were analyzed using two-way and one-way analysis of variance with Bonferroni test or t-test post hoc analyses. Differences were considered statistically significant when P < 0.05.
Results
MDP Induces an Ocular Inflammatory Response in Mice
In this study, we sought to investigate whether NOD2 participates in uveitis resulting from innate immune responses in mice. Some previous studies in rabbit models of uveitis showed that systemic treatment with PGN complexes or MDP elicited acute, ocular inflammation.31,32 Since then, the discovery of TLRs and, more recently, NLRs has provided new insights into the mechanisms by which bacterial derived products (PAMPs) induce inflammation. Here we took advantage of the availability of chemically synthesized MDP, devoid of contamination of other bacterial products, to examine whether MDP could induce uveitis in mice. Because earlier studies involving MDP had been performed in rabbits, we started with a dose–response study, wherein mice were treated with intravitreal injection of MDP (dose range, 5–100 μg), and inflammation was assessed by intravital microscopy 6 hours later. Although we did observe a significant effect of systemically administered 100 μg MDP on rolling (320 ± 113 vs. saline, 10 ± 10 cells/mm2 vessel/min) and adhering (70 ± 24 vs. saline, 8 ± 4 cells/mm2 vessel; data not shown) leukocytes within the iris vasculature, we determined that an intravitreal injection of 100 μg MDP was most effective in consistently eliciting ocular inflammation. Shown as Supplementary Figures online at http://www.iovs.org/cgi/content/full/49/4/1518/DC1 are images taken of mice treated with intravitreal injections of saline or 100 μg MDP; their intravascular inflammatory responses were imaged 6 hours later.
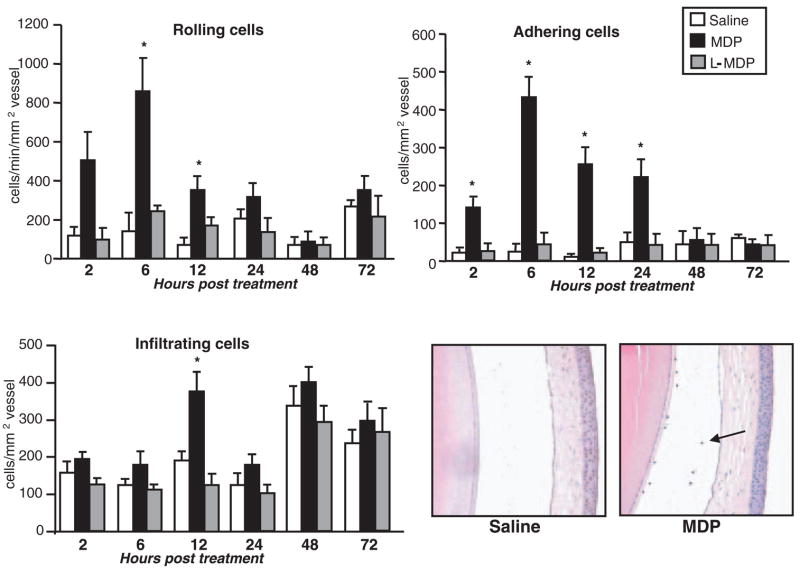
To determine the duration of the intravascular inflammatory response induced by MDP, mice were treated with intravitreal injections of 100 μg MDP or saline. Given that it had been reported that the biological activities of MDP are influenced by the stereo-configuration of MDP,33 wherein the second amino acid (D-Glx) is replaced with L-Glx enantiomer (L-MDP), we also investigated whether the chemically synthesized L-isoform of MDP (100 μg) could induce an ocular inflammatory response to the same extent as MDP. Cellular inflammation was then assessed by intravital microscopy at different time intervals after treatment (Fig. 1). We found that MDP-induced inflammation developed within 2 hours because there was a significant increase in the number of adherent cells and rolling cells that extended through 24 hours. Intravascular inflammation was completely resolved by 48 hours after treatment with MDP. We also observed by intravital microscopy that MDP resulted in a minor yet significant increase in cellular infiltration within the iris tissue at 12 hours after MDP treatment. The increase in cellular infiltration within the iris at 12 hours corresponded to a minor increase in the presence of leukocytes within the aqueous of the anterior chamber of the eye (Fig. 1). Based on their nuclear morphology, most appeared to be neutrophils. We did not observe any posterior inflammation, indicating that MDP elicits an inflammatory response predominantly within the iris vasculature. We did not observe any significant effect of the L-MDP within the same duration of time after treatment (Fig. 1), thereby indicating that the inflammatory capacity of MDP is dependent on the stereoisomeric configuration. Consistent with this conclusion, we did not observe a decrease in the amount of MDP-induced intravascular inflammation in the presence of threefold concentration of the L-MDP (data not shown). We have also found that the potential of MDP to elicit ocular inflammation occurs in the C57BL/6 mouse strain in addition to the BALB/c strain because albino C57BL/6 mice (tyrosinase knockout) also showed a significant increase in the number of rolling and adhering cells in response to MDP (data not shown). These data define the specific dose and timeframe of a novel model of MDP-induced uveitis in mice.
Figure 1.
MDP-induced uveitis in mice. Female BALB/c mice were treated with intravitreal injection of saline, 100 μg MDP, or 100 μg L-MDP. The leukocyte inflammatory response within the iris vasculature was then assessed by intravital microscopy at the indicated times after treatment. Lower right: representative eye sections stained with hematoxylin and eosin 12 hours after treatment. Data are the mean ± SEM (n = 7–10 mice/treatment/time point). *P < 0.05 versus saline-treated controls.
L-Selectin Participates in Leukocyte Rolling within the Vasculature in Response to MDP
Leukocyte diapedesis has been well described and is dependent on an array of adhesion molecules expressed on the leukocytes and the endothelium. Specific adhesion molecules mediate such interactions in a stepwise progression (i.e., rolling, adherence, and extravasation), and each of these steps has been visualized in vivo in several systems, including the eye.29,34 In the mouse endotoxin–induced uveitis model, several adhesion molecules have been identified that mediate the distinct processes of leukocyte rolling, sticking, and extravasation.34 We took advantage of the ability of intravital microscopy to assess specific leukocyte-endothelium interactions to test the functional role for L-selectin in MDP-induced inflammation.
Mice that lacked expression of L-selectin received intravitreal injections of MDP, and the intravascular inflammatory response within the eye was assessed by intravital microscopy 6 hours later (Fig. 2). As expected, the wild-type (WT) control mice treated with MDP showed significant increases in the number of rolling leukocytes; this was significantly reduced in the L-selectin knockout mice. There was an obvious trend, though it was not statistically significant, toward fewer adhering cells in response to MDP in the L-selectin knockout mice. This is consistent with the mechanisms involved in rolling preceding those involved in adherence and further suggests that MDP upregulates other adhesion molecules responsible for such downstream events. These data demonstrate a critical role for L-selectin in mediating the initiation of the intravascular inflammatory response to MDP.
Figure 2.
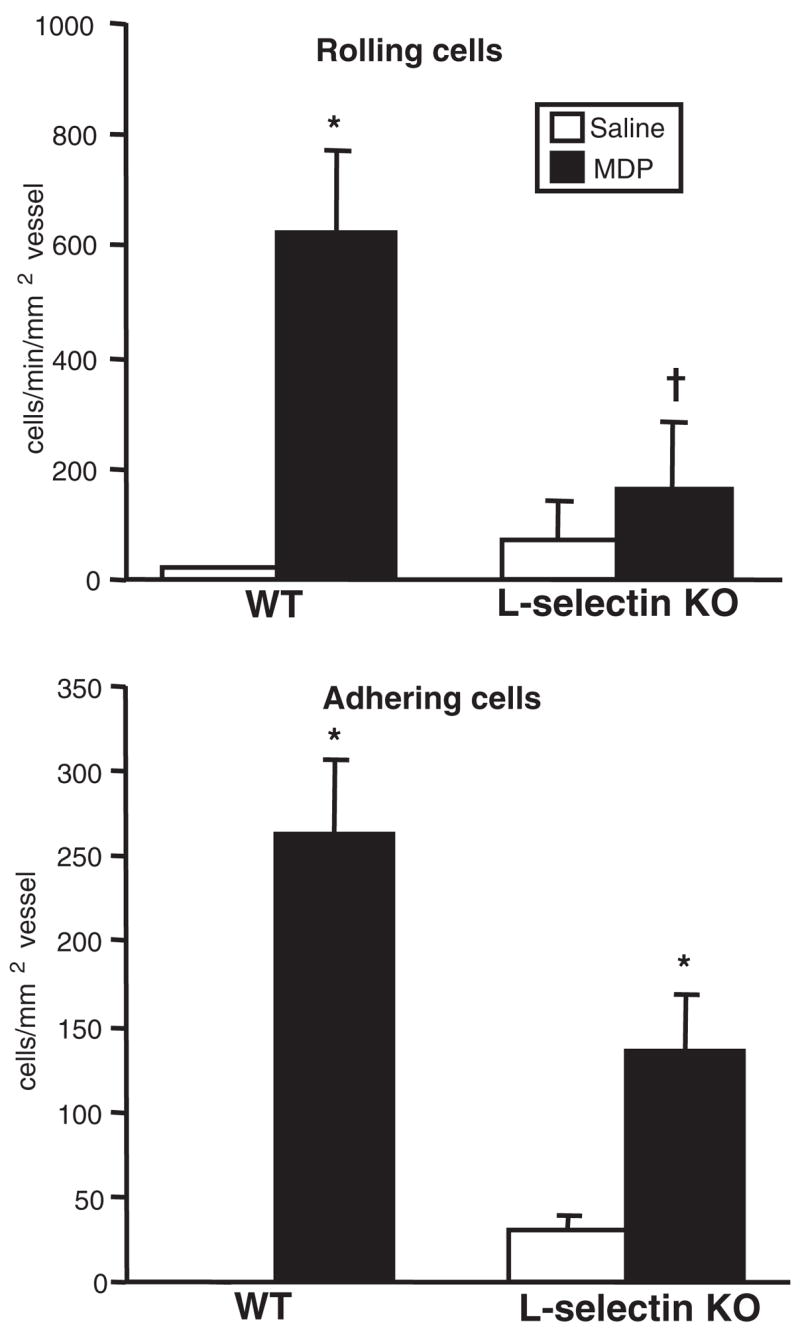
L-selectin participates in leukocyte rolling in response to MDP. L-selectin knockout mice or WT controls were treated with intravitreal injection of 100 μg MDP or saline. The intravascular response was assessed by intravital microscopy 6 hours after treatment. Data are the mean ± SEM (n = 6 –10 mice/treatment/genotype). *P < 0.05 versus saline-treated controls within a genotype. †P < 0.05 versus MDP-treated WT controls.
NOD2 Is Essential for MDP-Induced Uveitis in Mice
We went on to test directly whether the ocular inflammatory response to MDP was altered in Nod2 knockout mice. Nod2 knockout mice or their congenic controls received intravitreal injections of MDP, and the intravascular inflammatory response was assessed by intravital microscopy 6 hours later. As expected, the WT mice showed significantly increased numbers of rolling and adhering leukocytes in response to MDP. In contrast, the Nod2 knockout mice did not exhibit an increase in rolling or adhering cells in response to MDP (Fig. 3). Nod2 knockout mice also failed to show an increased intravascular inflammation or cellular infiltration in the iris tissue as assessed by intravital microscopy 12 hours after treatment (data not shown). This was consistent with a lack of leukocyte presence within the aqueous of the anterior chamber of the eye 12 hours after treatment (data not shown) and is the first direct evidence for a model in which uveitis is dependent on NOD2.
Figure 3.
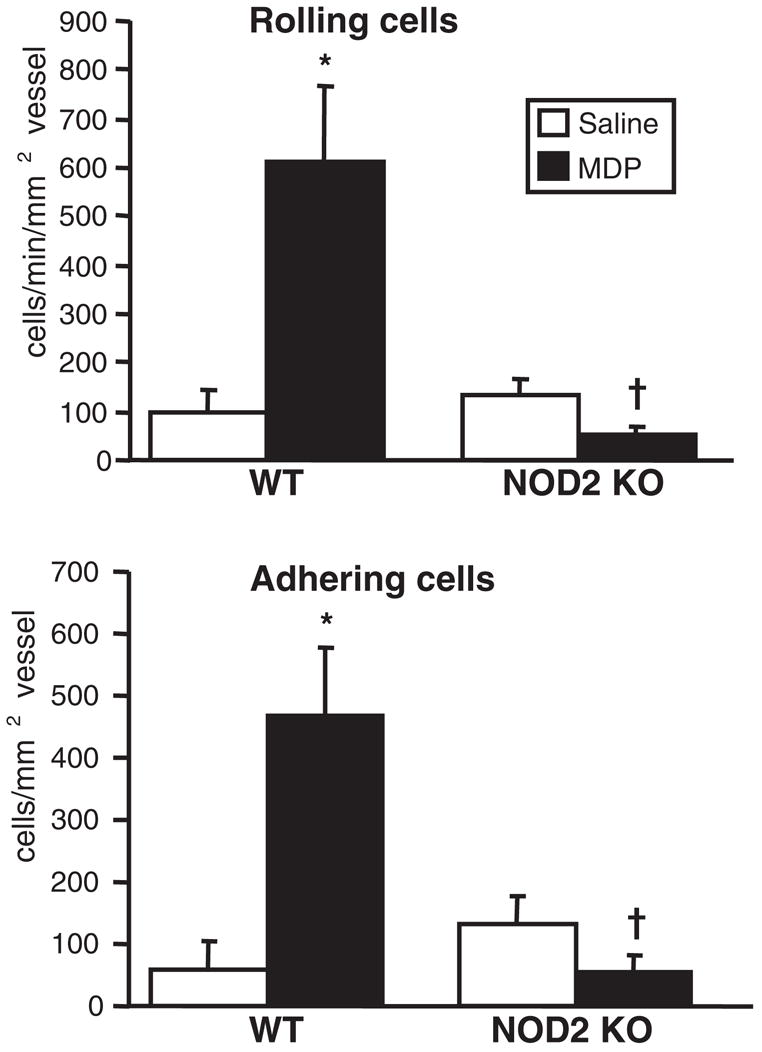
NOD2 is essential for MDP-induced uveitis in mice. Nod2 knockout mice or WT controls were treated with intravitreal injection of 100 μg MDP or saline. The intravascular response was assessed by intravital microscopy 6 hours after treatment. Data are the mean ± SEM (n = 7–9 mice/treatment/genotype). *P < 0.05 versus saline-treated controls within a genotype. †P < 0.05 versus MDP-treated WT controls.
NOD2 Is Essential for Ocular Inflammation Induced by the Muramic Acid MTP but Not by PGN
In vitro studies demonstrated that NOD2-expressing cell lines responded to PGN and that NOD2 requires intact muramyl peptides with two MDP or three MTP amino acids.33 These observations prompted us to further define the functional role of NOD2 in vivo within the eye. We first assessed the ability of synthesized MTP (wherein the third amino acid is a lysine residue) or purified PGN of Staphylococcus aureus to induce ocular inflammation in mice. Because this had not been previously demonstrated, the dose and time of the vascular inflammatory response induced by an intravitreal treatment of either PGN or MTP were optimized, as assessed by intravital microscopy (data not shown). We then went on to investigate the possible requirements for NOD2 in MTP- or PGN-induced ocular inflammation. Nod2 knockout mice were treated with intravitreal injections of MTP, and the ocular inflammatory response was assessed by intravital microscopy 6 hours after treatment (Fig. 4). We found that MTP elicits a significant increase in the number of rolling and adhering leukocytes within the eye of WT mice. In contrast, Nod2 knockout mice show an abolished response to MTP, indicating that this response is dependent on NOD2. We then tested the role for NOD2 in response to PGN. Although PGN is degraded into metabolites that include MDP and MTP, PGN itself is also recognized by TLR2.35 Mice were treated with intravitreal injections of PGN, and inflammation was assessed by intravital microscopy 6 hours later (Fig. 5). We found that though PGN induced a significant ocular inflammatory response, this response was not entirely dependent on NOD2 because the number of rolling cells was only partially reduced and no difference was detected in the number adhering cells. This finding suggests that NOD2 may contribute in part to PGN responses such as leukocyte rolling. Thus, NOD2 is required for ocular inflammation induced specifically by the minimal peptide fragments of PGN, and it demonstrates that Nod2 knockout mice are fully capable of mounting an intraocular inflammatory response to other stimuli of innate immune responses.
Figure 4.
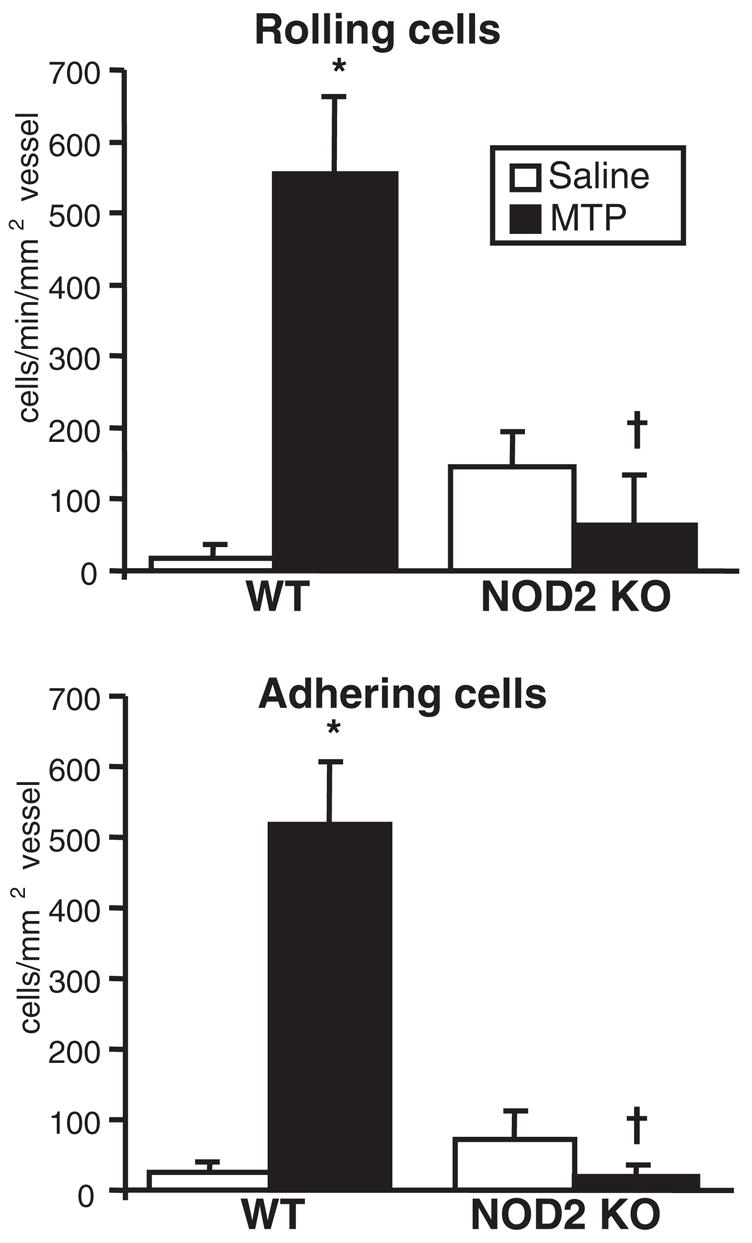
NOD2 is essential for inflammation induced by the muramic acid MTP. Nod2 knockout mice or WT controls were treated with intravitreal injection of 4 μg MTP or saline. The intravascular response was assessed by intravital microscopy 6 hours after treatment. Data are the mean ± SEM (n = 6 –10 mice/treatment/genotype). *P < 0.05 versus saline-treated controls within a genotype. †P < 0.05 versus MDP-treated WT controls.
Figure 5.
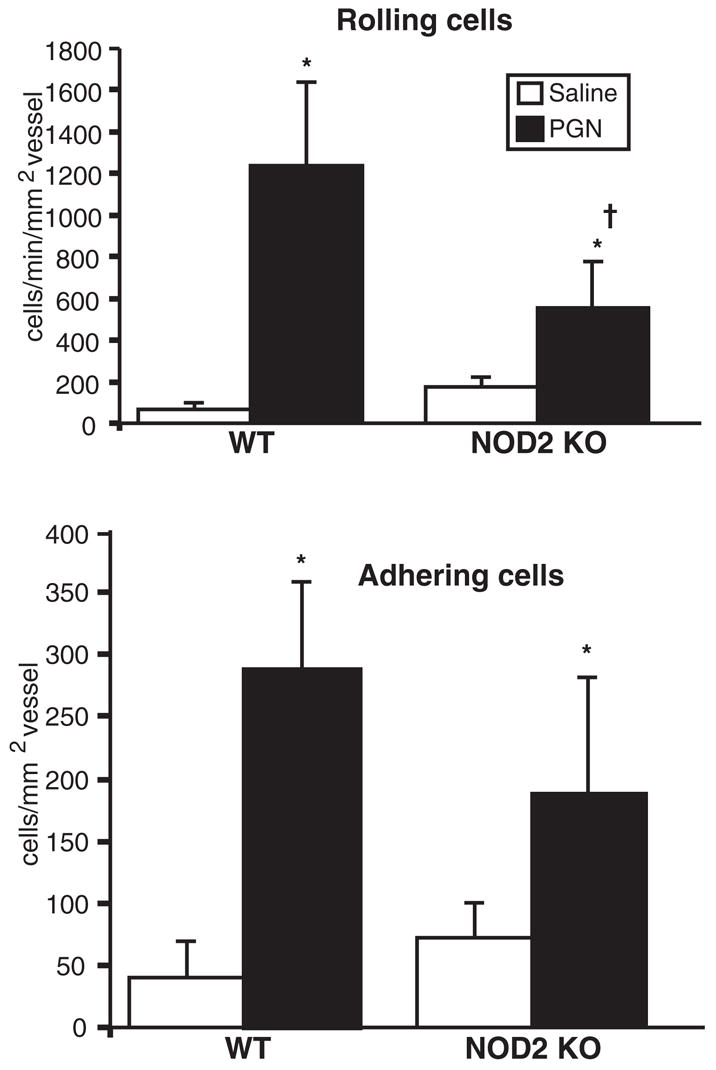
NOD2 is not essential for inflammation induced by PGN. Nod2 knockout mice or WT controls were treated with intravitreal injection of 1 μg PGN or saline. The intravascular response was assessed by intravital microscopy 6 hours after treatment. Data are the mean ± SEM (n = 6–10 mice/treatment/genotype). *P < 0.05 versus saline-treated controls within a genotype. †P < 0.05 versus PGN-treated WT controls.
Discussion
Uveitis, or intraocular inflammation, is an immune-mediated disease that occurs as recurrent episodes of inflammation or as chronic inflammation. The complications of uveitis can include cataracts, glaucoma and retinal detachment, and it is one of the leading causes of blindness. Although the characteristics of uveitis can be confined to the eye, it can be associated with systemic immune-mediated disease such as ankylosing spondylitis, juvenile idiopathic arthritis, reactive arthritis, Behçet syndrome, inflammatory bowl disease, and Blau syndrome.22,36,37 The heterogeneity of uveitis suggests that multiple pathways contribute to its pathogenesis. Microbial triggers are considered to be crucial players in some inflammatory eye diseases.3 Therefore, the innate immune recognition system and its pattern recognition receptors (PRRs) within the eye and their interactions with their microbial PAMPs may play important roles in the underlying mechanisms involved in uveitis. The discovery of a single nucleotide change that virtually guarantees the development of uveitis as a component of Blau syndrome is a seminal finding that clearly identifies a gene, NOD2, whose contribution to ocular inflammation must be clarified.
Here, we report the novel finding that NOD2 functions in ocular inflammation in mice. We show that activation of NOD2 by MDP elicits a transient increase in rolling and adhering leukocytes within the vasculature of the eye and a mild infiltration within the iris tissue. The capacity of MDP to induce ocular inflammation in mice was dependent on the stereoisomeric configuration of the isoglutamine moiety because the L-isomer did not induce significant ocular inflammation. This is consistent with an earlier study in vitro that showed stimulation of NOD2-transfected cell lines with L-MDP did not activate a NF-κB luciferase reporter.33 We demonstrated that the adhesion molecule L-selectin participates in the initiation of the MDP-induced inflammatory response within the vasculature because L-selectin knockout mice showed fewer rolling cells. Importantly, Nod2 knockout mice did not show an intravascular response to MDP, which indicates that NOD2 plays an essential role in MDP-induced uveitis. NOD2 knockout mice also failed to show a cellular inflammatory response to MTP treatment. These findings reveal an in vivo function for NOD2 in ocular inflammation and indicate that NOD2 specifically mediates ocular inflammatory responses to the minimal motifs of PGN, the muramic acid peptides MDP and MTP. It does not, however, mediate responses to the entire PGN molecule because Nod2 knockout mice retained their ability to elicit a cellular inflammatory response to PGN. Although the role of NOD2 has been studied indirectly in other mouse models of inflammation such as colitis, this work is the first, to our knowledge, to describe an in vivo model of ocular inflammation that depends on NOD2.
Treatment with bacterial cell wall components such as peptidoglycan complexes or MDP has been shown to elicit acute anterior uveitis in rabbits.31,32,38,39 In the present study, a direct function of NOD2 was shown for the first time to be essential for ocular inflammation induced by an intravitreal treatment of MDP or MTP in mice. Although intravitreal and systemic administration of MDP in the rabbit resulted in ocular inflammation, we found that intravitreal injection of MDP in mice was more effective than systemic treatment. PGN-induced uveitis did not completely require NOD2 because Nod2 knockout mice were still capable of increased rolling and sticking leukocytes within the iris vasculature. This finding is consistent with the observation that TLR2 mediates the recognition of PGN35 and that NOD2 is capable of being activated independently of TLRs.40,41 Our results also emphasize that it is unlikely that MDP or MTP is capable of inducing ocular inflammation independently of NOD2 (e.g., by TLRs) because MDP- and MTP-induced ocular inflammations were completely abolished in the Nod2 knockout mice. Given the few studies of the functional consequences of NOD2 activation on inflammatory cascades or adhesion molecules in the eye, this mouse model should help to clarify specific ocular activities of NOD2.
In addition to its role in host defense, the importance of NOD2 in regulating inflammation is further emphasized by the fact that mutations in NOD2 cause Blau syndrome, an autosomal dominant, multiorgan, granulomatous inflammation of the eye, skin, and joint.22,23 Distinct polymorphisms of NOD2 have also been strongly linked to the onset of Crohn disease,26,42 an inflammatory disorder of the intestine and that can involve the uvea. Mutations in other NLR family members also cause auto-inflammatory diseases, many of which are associated with uveitis. Notably, the three common residues affected by the Blau mutations correspond to the position of pathogenic mutations in the closely related and NLR family member Cryopyrin/Nalp3, which causes a triad of diseases: chronic infantile neurologic cutaneous and articular syndrome, familial cold autoinflammatory syndrome, and Muckle-Wells syndrome.14,43,44 Because of the functional and mutational similarities of NOD2 and NALP3, it has been hypothesized that the underlying mechanisms might be similar.
MDP-induced uveitis in the mouse results in a relatively mild nature of inflammation, wherein the inflammatory response occurs predominantly within the vasculature. MDP is a small molecule and is rapidly cleared, with less than 10% recoverable MDP in the urine 2 hours after intravenous or subcutaneous injection in mice.45 This might explain why MDP elicits such an acute and mild inflammation compared with that of the more robust endotoxin-induced uveitis. However, in both uveitis models, neutrophil response predominates. Repeated treatments or a slow-release depot of MDP might elicit a more robust or chronic ocular inflammatory response. It is possible that altered function of NOD2 attributed to the Blau mutations might also account for the more severe nature of uveitis than the transient inflammation we observed in response to activation of the endogenous form of NOD2. In vitro assays with transfected HEK cells have shown that Blau mutations result in increased basal NF-κB activity,46 which would suggest a gain of function in the mutant NOD2. It is intriguing to speculate whether chronic activation of NOD2 might drive perpetual production of inflammatory mediators that promote neutrophil recruitment and granuloma formation. The functional consequences of Blau mutations of NOD2 are a topic for further investigation.
In addition to the role of NOD2 in mediating MDP responsiveness, NOD2 appears to cooperate with other TLRs. This is best exemplified in the setting of “synergy,” wherein MDP, together with LPS, enhances cytokine production that is greater than the additive effects of either treatment alone.47 Such synergist effects result in increased sensitization of mice to LPS-induced lethality. The essential role of NOD2 in MDP synergy was recently demonstrated in Nod2 knockout mice, which showed increased resistance to LPS-induced lethality because of cotreatment with MDP.18 Although NOD2 is capable of enhancing TLR responses, in certain situations it also appears to regulate TLR responses negatively.48 Unpublished data indicate that treatment with MTP-phosphatidylethanolamine reduced LPS-induced ocular inflammation in rats (Kozak Y, et al. IOVS 2006;47:ARVO E-Abstract 2917). Taken together, these data support a possible function for NOD2 in ocular host defense and for modulation of subsequent vascular inflammatory responses in the eye to other PAMPs.
In conclusion, we demonstrate an essential role for NOD2 in uveitis in mice. There are a number of fundamental questions regarding the role of NOD2 in uveitis that must be explored, and these studies provide the foundation for future studies that will be critical to our understanding of how NOD2 contributes to the pathogenesis of uveitis.
Acknowledgments
The authors thank Kelley Goodwin, Hari Sawkar, Michelle Pengshung, and Jeff Jensen for their technical contributions.
Supported by National Eye Institute Grants F32-EY017254, EY015137, EY013093, and EY006484; a VA Merit Review grant (MPD); Research to Prevent Blindness (JTR, TMM, SRP); and the Casey Eye Institute.
Footnotes
Disclosure: H.L. Rosenzweig, None; T.M. Martin, None; M.M. Jann, None; S.R. Planck, None; M.P. Davey, None; K. Kobayashi, None; R.A. Flavell, None; J.T. Rosenbaum, None
References
- 1.Rosenbaum JT, McDevitt HO, Guss RB, Egbert PR. Endotoxin-induced uveitis in rats as a model for human disease. Nature. 1980;286:611–613. doi: 10.1038/286611a0. [DOI] [PubMed] [Google Scholar]
- 2.Li Q, Peng B, Whitcup SM, Jang SU, Chan C-C. Endotoxin induced uveitis in the mouse: susceptibility and genetic control. Exp Eye Res. 1995;61:629–632. doi: 10.1016/s0014-4835(05)80056-9. [DOI] [PubMed] [Google Scholar]
- 3.Chang JH, McCluskey PJ, Wakefield D. Toll-like receptors in ocular immunity and the immunopathogenesis of inflammatory eye disease. Br J Ophthalmol. 2006;90:103–108. doi: 10.1136/bjo.2005.072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miceli-Richard C, Lesage S, Rybojad M, et al. CARD15 mutations in Blau syndrome. Nat Genet. 2001;29:19–20. doi: 10.1038/ng720. [DOI] [PubMed] [Google Scholar]
- 5.Rose CD, Doyle TM, McIlvain-Simpson G, et al. Blau syndrome mutation of CARD15/NOD2 in sporadic early onset granulomatous arthritis. J Rheumatol. 2005;32:373–375. [PubMed] [Google Scholar]
- 6.Inohara N, Ogura Y, Nunez G. Nods: a family of cytosolic proteins that regulate the host response to pathogens. Curr Opin Microbiol. 2002;5:76–80. doi: 10.1016/s1369-5274(02)00289-8. [DOI] [PubMed] [Google Scholar]
- 7.Girardin SE, Tournebize R, Mavris M, et al. CARD4/Nod1 mediates NF-κB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2001;2:736–742. doi: 10.1093/embo-reports/kve155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inohara Chamaillard, McDonald C, Nunez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Ann Rev Biochem. 2005;74:355–383. doi: 10.1146/annurev.biochem.74.082803.133347. [DOI] [PubMed] [Google Scholar]
- 9.Girardin SE, Hugot JP, Sansonetti PJ. Lessons from Nod2 studies: towards a link between Crohn’s disease and bacterial sensing. Trends Immunol. 2003;24:652–658. doi: 10.1016/j.it.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Tanabe T, Chamaillard M, Ogura Y, et al. Regulatory regions and critical residues of NOD2 involved in muramyl dipeptide recognition. EMBO J. 2004;23:1587–1597. doi: 10.1038/sj.emboj.7600175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi K, Inohara N, Hernandez LD, et al. RICK/Rip2/CAR-DIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 2002;416:194–199. doi: 10.1038/416194a. [DOI] [PubMed] [Google Scholar]
- 12.Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-κB. J Biol Chem. 2001;276:4812–4818. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 13.Chen D, Duggan C, Reden TB, Kooragayala LM, Texada DE, Langford MP. Calreticulin is a binding protein for muramyl dipeptide and peptidoglycan in RK13 cells. Biochemistry. 2004;43:11796–11801. doi: 10.1021/bi0490789. [DOI] [PubMed] [Google Scholar]
- 14.Inohara N, Nunez G. NODs: intracellular proteins involved in inflammation and apoptosis. Nat Rev Immunol. 2003;3:371–382. doi: 10.1038/nri1086. [DOI] [PubMed] [Google Scholar]
- 15.Windheim M, Lang C, Peggie M, Plater LA, Cohen P. Molecular mechanisms involved in the regulation of cytokine production by muramyl dipeptide. Biochem J. 2007;404:179–190. doi: 10.1042/BJ20061704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu YM, Zhang Y, You Y, et al. The adaptor protein CARD9 is required for innate immune responses to intracellular pathogens. Nat Immunol. 2007;8:198–205. doi: 10.1038/ni1426. [DOI] [PubMed] [Google Scholar]
- 17.Pan Q, Mathison J, Fearns C, et al. MDP-induced interleukin-1β processing requires Nod2 and CIAS1/NALP3. J Leukoc Biol. 2007;82:177–183. doi: 10.1189/jlb.1006627. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi KS, Chamaillard M, Ogura Y, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 19.Herskovits AA, Auerbuch V, Portnoy DA. Bacterial ligands generated in a phagosome are targets of the cytosolic innate immune system. PLoS Pathogens. 2007;3:e51. doi: 10.1371/journal.ppat.0030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kufer TA, Banks DJ, Philpott DJ. Innate immune sensing of microbes by Nod proteins. Ann N Y Acad Sci. 2006;1072:19–27. doi: 10.1196/annals.1326.020. [DOI] [PubMed] [Google Scholar]
- 21.Hisamatsu T, Suzuki M, Reinecker HC, Nadeau WJ, McCormick BA, Podolsky DK. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology. 2003;124:993–1000. doi: 10.1053/gast.2003.50153. [DOI] [PubMed] [Google Scholar]
- 22.Blau EB. Familial granulomatous arthritis, iritis, and rash. J Pediatr. 1985;107:689–693. doi: 10.1016/s0022-3476(85)80394-2. [DOI] [PubMed] [Google Scholar]
- 23.Jabs DA, Houk JL, Bias WB, Arnett FC. Familial granulomatous synovitis, uveitis, and cranial neuropathies. Am J Med. 1985;78:801–804. doi: 10.1016/0002-9343(85)90286-4. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Kuivaniemi H, Bonavita G, et al. CARD15 mutations in familial granulomatosis syndromes: a study of the original Blau syndrome kindred and other families with large-vessel arteritis and cranial neuropathy. Arthritis Rheum. 2002;46:3041–3045. doi: 10.1002/art.10618. [DOI] [PubMed] [Google Scholar]
- 25.van Duist MM, Albrecht M, Podswiadek M, et al. A new CARD15 mutation in Blau syndrome. Eur J Hum Genet. 2005;13:742–747. doi: 10.1038/sj.ejhg.5201404. [DOI] [PubMed] [Google Scholar]
- 26.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Martinez S, Cancino-Diaz ME, Jimenez-Zamudio L, Garcia-Latorre E, Cancino-Diaz JC. TLRs and NODs mRNA expression pattern in healthy mouse eye. Br J Ophthalmol. 2005;89:904–910. doi: 10.1136/bjo.2004.056218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davey MP, Martin TM, Planck SR, Lee J, Zamora D, Rosenbaum JT. Human endothelial cells express NOD2/CARD15 and increase IL-6 secretion in response to muramyl dipeptide. Microvascular Res. 2006;71:103–107. doi: 10.1016/j.mvr.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Becker MD, Nobiling R, Planck SR, Rosenbaum JT. Digital video-imaging of leukocyte migration in the iris: intravital microscopy in a physiological model during the onset of endotoxin-induced uveitis. J Immunol Meth. 2000;240:23–37. doi: 10.1016/s0022-1759(00)00165-4. [DOI] [PubMed] [Google Scholar]
- 30.Planck SR, Han YB, Park JM, O’Rourke L, Gutierrez-Ramos J-C, Rosenbaum JT. The effect of genetic deficiency of adhesion molecules on the course of endotoxin-induced uveitis. Curr Eye Res. 1998;17:941–946. doi: 10.1076/ceyr.17.9.941.5139. [DOI] [PubMed] [Google Scholar]
- 31.Fox A, Hammer ME, Lill P, Burch TG, Burrish G. Experimental uveitis elicited by peptidoglycan-polysaccharide complexes, lipopolysaccharide, and muramyl dipeptide. Arch Ophthalmol. 1984;102:1063–1067. doi: 10.1001/archopht.1984.01040030857033. [DOI] [PubMed] [Google Scholar]
- 32.Kufoy EA, Fox K, Fox A, Parks C, Pakalnis VA. Modulation of the blood-aqueous barrier by gram positive and gram negative bacterial cell wall components in the rat and rabbit. Exp Eye Res. 1990;50:189–195. doi: 10.1016/0014-4835(90)90230-r. [DOI] [PubMed] [Google Scholar]
- 33.Girardin SE, Travassos LH, Herve M, et al. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J Biol Chem. 2003;278:41702–41708. doi: 10.1074/jbc.M307198200. [DOI] [PubMed] [Google Scholar]
- 34.Becker MD, O’Rourke LM, Blackman WS, Planck SR, Rosenbaum JT. Reduced leukocyte migration, but normal rolling and arrest, in interleukin-8 receptor homologue knockout mice. Invest Ophthalmol Vis Sci. 2000;41:1812–1817. [PubMed] [Google Scholar]
- 35.Dziarski R, Gupta D. Peptidoglycan recognition in innate immunity. J Endotoxin Res. 2005;11:304–310. doi: 10.1179/096805105X67256. [DOI] [PubMed] [Google Scholar]
- 36.Jimenez Martinez MC, Carrera I, Pedroza Seres M, Jaimes M, Schlaen A, Garfias Y. [Uveitis immunopathology: currents knowledge, clinical correlation and research perspectives in the immueocular area] Rev Alerg Mex. 2006;53:226–235. [PubMed] [Google Scholar]
- 37.Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14:303–308. doi: 10.1007/BF00163549. [DOI] [PubMed] [Google Scholar]
- 38.Waters RV, Terrell TG, Jones GH. Uveitis induction in the rabbit by muramyl dipeptides. Infect Immun. 1986;51:816–825. doi: 10.1128/iai.51.3.816-825.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li T, Fox K, Fox A, Pakalnis V. Recurrent anterior uveitis induced by multiple systemic injections of muramyl dipeptide. Exp Eye Res. 1993;57:79–87. doi: 10.1006/exer.1993.1101. [DOI] [PubMed] [Google Scholar]
- 40.Inohara N, Ogura Y, Chen FF, Muto A, Nunez G. Human Nod1 confers responsiveness to bacterial lipopolysaccharides. J Biol Chem. 2001;276:2551–2554. doi: 10.1074/jbc.M009728200. [DOI] [PubMed] [Google Scholar]
- 41.Park JH, Kim YG, McDonald C, et al. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J Immunol. 2007;178:2380–2386. doi: 10.4049/jimmunol.178.4.2380. [DOI] [PubMed] [Google Scholar]
- 42.Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 43.Tschopp J, Martinon F, Burns K. NALPs: a novel protein family involved in inflammation. Nat Rev. 2003;4:95–104. doi: 10.1038/nrm1019. [DOI] [PubMed] [Google Scholar]
- 44.Albrecht M, Lengauer T, Schreiber S. Disease-associated variants in PYPAF1 and NOD2 result in similar alterations of conserved sequence. Bioinformatics (Oxf) 2003;19:2171–2175. doi: 10.1093/bioinformatics/btg370. [DOI] [PubMed] [Google Scholar]
- 45.Parant M, Parant F, Chedid L, Yapo A, Petit JF, Lederer E. Fate of the synthetic immunoadjuvant, muramyl dipeptide (14C-labelled) in the mouse. Int J Immunopharmacol. 1979;1:35–41. doi: 10.1016/0192-0561(79)90028-6. [DOI] [PubMed] [Google Scholar]
- 46.Chamaillard M, Philpott D, Girardin SE, et al. Gene-environment interaction modulated by allelic heterogeneity in inflammatory diseases. Proc Natl Acad Sci U S A. 2003;100:3455–3460. doi: 10.1073/pnas.0530276100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beutler E, Gelbart T, West C. Synergy between TLR2 and TLR4: a safety mechanism. Blood Cell Mol Dis. 2001;27:728–730. doi: 10.1006/bcmd.2001.0441. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe T, Kitani A, Murray PJ, Strober W. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol. 2004;5:800–808. doi: 10.1038/ni1092. [DOI] [PubMed] [Google Scholar]