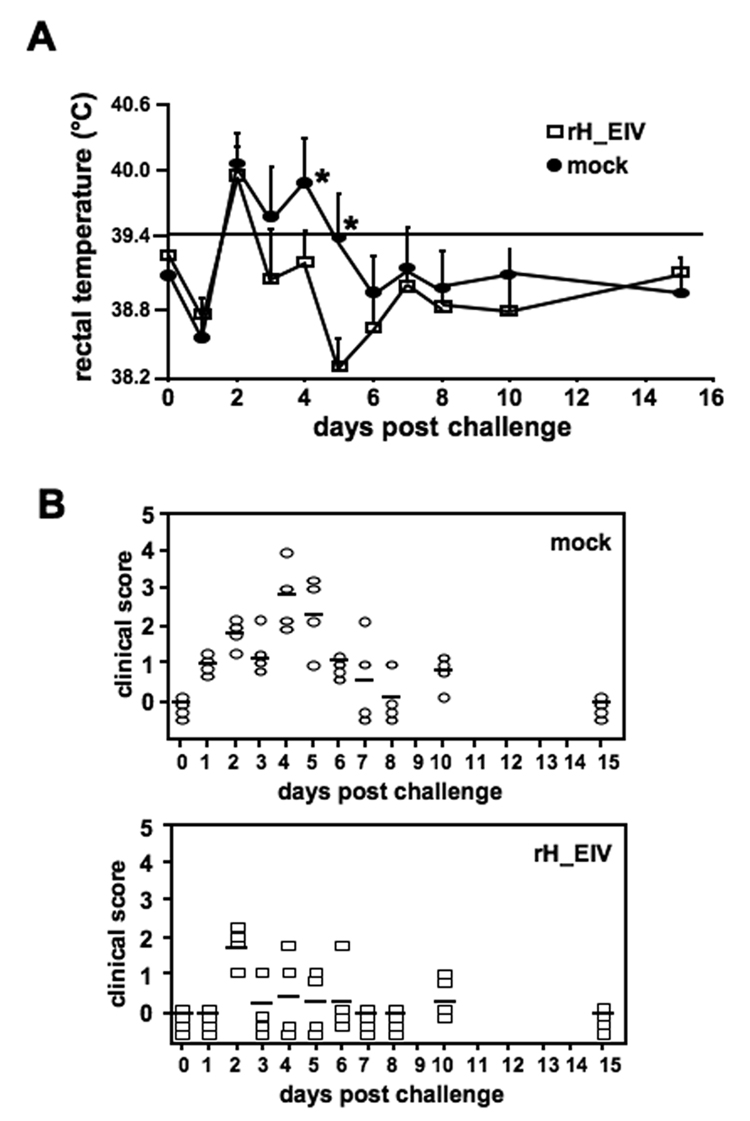
Figure 5. Immunization of dogs with rH_EIV provides partial clinical protection against challenge with virulent CIV.
Two groups of four dogs were vaccinated s.c. twice with rH_EIV or mock-vaccinated. All dogs were challenged 3 weeks after booster vaccination with 1×106 PFU A/canine/PA/10915-07 and physical exams were performed on days 0–8, 10, and 15. The clinical score given to each individual was based on several clinical signs, as described under Mat. & Meth (A). Body temperatures were also measured in these dogs and fever was defined as a rectal temperature of ≥ 39.4°C (B). Asterisks indicate statistically significant differences.