Abstract
Objectives. To evaluate the analytical performance of an ELISA for the detection of anti-RNA polymerase III antibody (ARA) and to assess IIF as a method for identifying this antibody.
Methods. A commercially available ELISA was used to assess the presence of ARA in sera from 1018 SSc patients. The sera had been divided into sub-populations based on the presence of specific autoantibodies, ANA pattern or the absence of both. Patients with ARA (n = 209) had been identified by characteristic ANA pattern by IIF on HEp-2 cell substrate [and additionally by radio-immunoprecipitation (IP) in 157/209 cases]. The remaining 809 SSc patients acted as a control group.
Results. Of 157 patients in whom ARA had been confirmed by IP, 150 were positive by ELISA providing a sensitivity of 96%. In the group where ARA had only been assessed by IIF, 100% (52/52) were ELISA positive. The ANA patterns indicating the presence of ARA were a fine-speckled nucleoplasmic stain with additional occasional bright dots, with or without concurrent punctate nucleolar staining. In the SSc control group, the ELISA attained a specificity of 98%, ARA being detected in 17/809 patients.
Conclusions. We report the outcome of a study on a large population of SSc patients that shows the ARA ELISA to be of high analytical sensitivity and specificity. We confirm that there is minimal overlap between ARA and other SSc-specific autoantibodies. Additionally, it is demonstrated that the presence of ARA correlates with identifiable patterns by IIF on HEp-2 cell substrate.
Keywords: Anti-ribonucleic acid polymerase antibody, Systemic sclerosis, Anti-nuclear antibody, Indirect immunofluorescence, Enzyme-linked immunosorbent assay, Autoantibodies
Introduction
Systemic sclerosis (SSc) is a heterogeneous autoimmune rheumatic disease characterized by fibrosis of the skin and other organs [1]. SSc patients have autoantibodies to specific cellular components that are mutually exclusive and correlate with distinct clinical subsets of disease [2]. The best documented of these, and the only ones routinely tested outside specialist centres, are ACA and anti-topoisomerase (anti-Scl-70) (ATA). However, these two specificities are only positive in ∼50% of patients with SSc [3]. A third major subset of SSc patients with distinct clinical features has antibodies to RNA polymerases (RNAPs). The prevalence of this antibody is influenced by ethnic origin and varies widely from 4% to 25% [4–6]. It has been suggested that at least 12% of UK patients are anti-RNAP antibody (ARA) positive [3]. These autoantibodies are highly specific and are predominantly present in patients with dcSSc [2, 7]. They are strongly associated with hypertensive renal crisis, a serious complication that may be the presenting feature of disease [8]. Additionally, a number of reports indicate that the antibody can be demonstrated before the onset of skin involvement [9–12]. It would therefore be desirable to be able to detect ARA in the routine clinical laboratory.
Until recently the only specific method for the identification of ARA was radio-immunoprecipitation (IP), a technique not well suited to routine use. Using this method the three subtypes of RNAP (I, II and III) can be precipitated by antibodies and there are three recognized patterns of reactivity specific for SSc: most ARA-positive SSc sera precipitate RNAP I and III in combination, some recognize all three polymerases and a small minority precipitate RNAP III alone [13]. Some patients with SSc have antibodies that precipitate only RNAP II, but these do not exhibit disease specificity [14, 15]. The recent identification of a major antigenic epitope on RNAP III recognized by almost all sera positive for ARA by IP [16] has led to the development of an ELISA for ARA [17]. Unlike IP, ELISA is used in the majority of diagnostic laboratories making routine detection of ARA feasible.
The technique of IIF is also widely employed for the detection of autoantibodies. Several studies have noted the association of ARA with positive staining by IIF on HEp-2 cell substrate. Interest in SSc-specific ARA by IIF originally focused on a punctate (speckled) nucleolar pattern consistent with the cellular location of RNAP I [18]. However, speckled nucleoplasmic staining patterns were subsequently described, this time consistent with the cellular location of RNAP III [13, 19]. As a consequence, there remains no consensus on the specific pattern produced by SSc-specific ARA on HEp-2 cell substrate.
In this study, we have assessed the utility of two techniques—IIF on HEp-2 cell substrate and a commercially available ELISA—to identify patients with ARA. We further evaluated the specificity of the ELISA method against a large control group of SSc patients.
Materials and methods
Patient samples
Serum samples were from 1018 SSc patients diagnosed by an experienced rheumatologist at the Royal Free Hospital, a major tertiary referral centre for SSc, according to the preliminary ACR criteria [20]. The local ethics committee gave the study ethical approval and informed consent was obtained from all patients. The sera had previously been tested for ANA by IIF on HEp-2 cell substrate (Bion Enterprises Ltd, Des Plaines, IL, USA) and antibodies to ENAs by counter-immunoelectrophoresis (CIE) or IP as previously described [3, 21, 22]. Sera were stored at −20°C prior to ARA testing by ELISA. Patients were divided into groups based on autoantibody profile. Two groups were formed of patients whose sera had produced ANA patterns characteristic of ARA: ARA-IP (n = 157) (patients in whom the antibody had been confirmed by IP) and ARA-IF (n = 52) (ARA not confirmed by IP due to the withdrawal of the radioisotope method from the laboratory). The remaining 809 sera formed antibody groups as follows: ACA, n = 197; ATA, n = 210; anti-fibrillarin (U3-RNP) (AFA), n = 48; anti-Pm-Scl, n = 49; anti-U1-RNP, n = 59; anti-Ro-60, n = 13; anti-AATS (aminoacyl tRNA synthetases) n = 6; and anti-Ku, n = 5. In addition, two further groups were formed from SSc patients with no defined antibody (NDA), n = 174 and those whose sera produced an ANA pattern of fine-speckled nucleoplasmic staining with additional homogeneous nucleolar staining (FSNU), n = 48. The latter group represented a heterogeneous population including patients with anti-Th-RNP. As a consequence of the selection process employed, the antibody frequencies represented in this study do not reflect the prevalence of each antibody type in the SSc patient population as a whole.
ARA ELISA
ARA was detected by a commercially available ELISA method employing a recombinant peptide of RNAP III [6, 16] (Quanta Lite RNAP III ELISA, INOVA Diagnostics, Inc., San Diego, CA, USA). The ELISA was performed in accordance with the manufacturer's instructions using the recommended cut-off of 20 U/ml.
IIF
ANA substrates from five additional manufacturers (HEp2000, ImmunoConcepts, Sacramento, CA, USA; HEp-2 from Bio-Diagnostics Ltd, Upton-upon-Severn, UK; Immco Diagnostics, Buffalo, NY, USA; INOVA Diagnostics Inc.; and The Binding Site Ltd, Birmingham, UK.) were used to test selected ARA-positive sera to assess the consistency of the described staining patterns. IIF was performed as previously described [22].
Statistical analysis
Statistical analysis was performed using SPSS v 11.0 software. The Mann–Whitney U-test was employed to compare median levels of ARA in the ARA-IP and ARA-IF groups. The association between qualitative variables (antibody group and the presence of ARA by ELISA) was evaluated by Fisher's exact test. A P-value of < 0.05 was considered to denote statistical significance in all cases.
Results
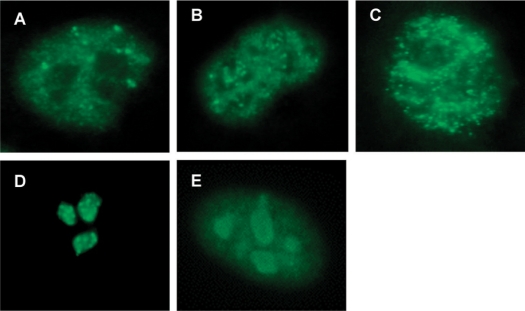
Sera from 209 patients with confirmed or suspected ARA were initially assessed by ELISA. Of the 157 patients in whom ARA had previously been demonstrated by IP (ARA-IP), 150 sera gave a positive result by ELISA with a median of 73 U/ml providing a sensitivity of 96%. An additional 52 patients had previously been identified by IIF as having one of two staining patterns characteristic of ARA but had not had the presence of the antibody confirmed by IP (ARA-IF). ARA was detected by the ELISA method in sera from all 52 patients and the median ARA level in these sera was 90.5 U/ml. The staining patterns indicating the presence of ARA were either a fine-speckled nucleoplasmic stain with additional occasional bright dots (Fig. 1A), or this same pattern accompanied by varying intensities of punctate (speckled) nucleolar staining (Fig. 1B). The fine-speckled nucleoplasmic pattern typical of anti-Ro and the characteristic patterns of AFA and anti-Pm-Scl showing clumpy and homogeneous nucleolar staining respectively are shown for comparison (Fig. 1C–E). In sera from consecutive ARA-positive SSc patients, 70% produced the nucleoplasmic only stain and 30% the pattern with additional punctate nucleolar staining. These two patterns were indistinguishable on all six substrate preparations used. There was no significant difference between the levels of ARA in the ARA-IP and ARA-IF groups (P = 0.297 by Mann–Whitney U-test) and for the second part of the study these were considered as one group, ARA, n = 209. None of the other described antibodies was demonstrated in any of the 209 sera.
Fig. 1.
ANA patterns by IIF on HEp-2 cell substrate. (A) Fine-speckled nucleoplasmic stain with additional occasional bright dots: ARA. (B) Fine-speckled nucleoplasmic stain with additional occasional bright dots accompanied by punctate (speckled) nucleolar staining: ARA. (C) Fine-speckled nucleoplasmic stain: anti-Ro. (D) Clumpy nucleolar stain: AFA. (E) Homogenous (very fine speckled) nucleoplasmic with homogenous nucleolar stain: anti-PmScl.
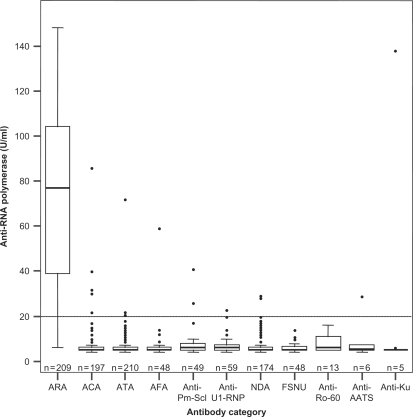
Eight hundred and nine disease control sera (SSc patient sera characterized for autoantibody and not exhibiting either of the characteristic staining patterns of ARA) were subsequently tested for the presence of ARA by ELISA. Out of these, 792 were negative for ARA, providing a specificity of 98%. The median level of ARA in each of the control antibody groups was between 5 and 6 U/ml compared with the overall median for the combined ARA group, 77 U/ml (Fig. 2). Statistically significant negative associations with the presence of ARA were obtained for the ACA, ATA, AFA, anti-Pm-Scl, anti-U1-RNP, NDA, and FSNU groups (P < 0.001 by Fisher's exact test). ARA was detected in 17 of 809 control samples with 10 having only low levels of reactivity (20–30 U/ml). None of these showed any evidence of the characteristic ANA patterns associated with ARA. IP results were available for the single ELISA positives in the anti-AATS and anti-Ku groups. Neither was positive for ARA by this method.
Fig. 2.
ARA detected by ELISA in 1018 SSc patients divided into groups based on autoantibody profile. Boxes show interquartile ranges, lines within the boxes indicate median values and lines outside the boxes indicate maximum and minimum values excluding outliers (represented by dots). Numbers in each group are shown on the figure. The line represents the 20 U/ml cut-off suggested by the manufacturer.
Discussion
Despite being described in up to 25% of patients with SSc [5] and having an important role in disease classification and prediction of organ involvement [2, 7], detection of ARA has been confined to a small number of specialist centres and time-consuming IP assays. The lack of consensus for a specific ANA pattern associated with these antibodies has previously ruled out IIF as a screening test. The recent development of an ELISA for ARA has provided a more accessible test for the detection of these antibodies [6, 17, 23]. In this study, we have assessed both the analytical performance of an ELISA method and the reliability of IIF using HEp-2 cell substrate for the detection of ARA in a large cohort of SSc patients.
Initially, 157 sera that showed characteristic staining patterns in which ARA had additionally been confirmed by the gold standard method of IP were tested. The ELISA performed with 96% sensitivity, which represents a favourable comparison with IP and is similar to sensitivities of 91% and 100% achieved in other studies employing ELISA [17, 23].
The literature suggests that ARA do not produce a specific pattern by IIF on HEp-2 cell substrate [3, 6, 13, 19, 24]; however, we contend that discrete patterns of fluorescence can be used to identify this antibody. There was 100% concordance between IIF and the ELISA method when testing 52 sera with suspected ARA. This demonstrates that the patterns can be reliably identified by experienced observers and suggests that recognition should be within the capability of most laboratory scientists with appropriate training.
To confirm that the distinct patterns observed on HEp-2 cell substrate were not specific to the slides routinely used in our laboratory we tested ARA-positive sera on substrate cells from six different manufacturers. Staining was found to be consistent on all slides tested. The fine-speckled staining produced by ARA is different to that produced by Ro antibodies and the punctate nucleolar staining can be readily distinguished from other patterns of nucleolar fluorescence such as the homogeneous nucleolar staining seen with anti-PmScl antibodies and the clumpy nucleolar staining associated with AFA. Recently, two groups have assessed IIF as a means of detecting ARA, but principally with a focus on nucleolar patterns of fluorescence [6, 24]. They both concluded that although present in a subset of patients, nucleolar IIF was not a sensitive marker for ARA and that it could not be used to screen for these antibodies. Our results are entirely consistent with these conclusions and in line with previous findings we estimate nucleolar fluorescence to be present in only 30% of ARA-positive sera [6, 19]. It is not the presence of nucleolar staining but that of the typical fine-speckled nucleoplasmic pattern that is the distinctive finding on IIF. Consistent with our observation, Yamasaki et al.[24] reported the presence of the nucleoplasmic pattern in all of their tested ARA-positive patients.
The availability of an ELISA method has made it possible to quantify ARA for the first time, an advance that may provide further correlations to clinical manifestations of disease. Preliminary evidence has suggested that ARA levels may change over the course of disease [17]. The majority of patients in the ARA-IP group had long-standing disease whilst those identified by IIF (and subsequently confirmed by ELISA) were predominantly newly diagnosed. Although we found no statistically significant difference in antibody levels for the two ARA-positive groups, the median titre of ARA in the ARA-IF group was observed to be higher than that for the ARA-IP group (median 90.5 U/ml vs 73 U/ml). Further work with serial samples is needed to identify any relationship between ARA titre and both disease progression and organ involvement.
In the final part of the study, we found the specificity of the ELISA to be 98% when tested on a rigorous disease control group of 809 SSc patients not displaying the characteristic IIF patterns of ARA. This specificity is similar to those quoted in previous studies but is markedly better than that seen by another group who used sera from SSc patients as controls and found 10% (7/72) gave false-positive results by ELISA [17]. Of the 17 samples in the current study that gave positive results most had low levels of reactivity. Two with higher ARA levels by ELISA had previously been tested by IP; both were negative for ARA by this method and can be considered false positive by ELISA. Without IP results for the remaining 15 patients we could not rule out the possibility that they had low levels of ARA.
It is a characteristic feature of SSc that each patient will typically have only one of a series of hallmark autoantibodies [2, 7, 19]. It was therefore expected that patients with defined SSc-specific antibodies would not exhibit overlap with ARA and vice versa. Indeed, there was a statistically significant negative association between ARA and all the SSc-specific autoantibodies. The NDA and FSNU groups in this study were over-represented with respect to the SSc population as a whole as it was felt that these were the most likely sera to contain previously undetected ARA, there being no other defining antibody other than anti-Th-RNP. None of the sera in the FSNU group and only 2/174 (1%) in the NDA group were positive for ARA. These results support the hypothesis that in the absence of the characteristic staining patterns described, the presence of ARA is highly unlikely.
In conclusion, we report that the ELISA is both highly sensitive and specific for the detection of ARA and as such is comparable with IP as a test for this antibody. In addition, we confirm that there is minimal overlap between ARA and other SSc-specific autoantibodies. Finally, we suggest that characteristic patterns of staining by IIF on HEp-2 cell substrate can reliably indicate the presence of ARA. Together these advances infer that the detection of clinically important ARA need not be confined to specialist centres.

Disclosure statement: R.W.B. and T.T.W. are employees of INOVA Diagnostics Inc. All other authors have declared no conflicts of interest.
References
- 1.Denton CP, Black CM. Scleroderma – clinical and pathological advances. Best Pract Res Clin Rheumatol. 2004;18:271–90. doi: 10.1016/j.berh.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Steen VD. Autoantibodies in systemic sclerosis. Semin Arthritis Rheum. 2005;35:35–42. doi: 10.1016/j.semarthrit.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Bunn CC, Denton CP, Shi-Wen X, Knight C, Black CM. Anti-RNA polymerases and other autoantibody specificities in systemic sclerosis. Br J Rheumatol. 1998;37:15–20. doi: 10.1093/rheumatology/37.1.15. [DOI] [PubMed] [Google Scholar]
- 4.Kuwana M, Okano Y, Junichi K, Takeshi T, Medsger TA. Racial differences in the distribution of systemic sclerosis-related serum antinuclear antibodies. Arthritis Rheum. 1994;37:902–6. doi: 10.1002/art.1780370619. [DOI] [PubMed] [Google Scholar]
- 5.Meyer OC, Fertig N, Lucas M, Somogyi N, Medsger TA. Disease subsets, antinuclear antibody profile, and clinical features in 127 French and 247 US adult patients with systemic sclerosis. J Rheumatol. 2007;34:104–9. [PubMed] [Google Scholar]
- 6.Santiago M, Baron M, Hudson M, Burlingame RW, Fritzler MJ. Antibodies to RNA polymerase III in systemic sclerosis detected by ELISA. J Rheumatol. 2007;34:1528–34. [PubMed] [Google Scholar]
- 7.Kuwana M, Kaburaki J, Okano Y, Takeshi T, Homma M. Clinical and prognostic associations based on serum antinuclear antibodies in Japanese patients with systemic sclerosis. Arthritis Rheum. 1994;37:75–83. doi: 10.1002/art.1780370111. [DOI] [PubMed] [Google Scholar]
- 8.Penn H, Howie AJ, Kingdon EJ, et al. Scleroderma renal crisis: patient characteristics and long term outcomes. Q J Med. 2007;100:485–94. doi: 10.1093/qjmed/hcm052. [DOI] [PubMed] [Google Scholar]
- 9.Sanders PW, Herrera GA, Ball GV. Acute renal failure without fibrotic skin changes in progressive systemic sclerosis. Nephron. 1988;48:121–5. doi: 10.1159/000184889. [DOI] [PubMed] [Google Scholar]
- 10.Molina JF, Anaya JM, Cabrera GE, Hoffman E, Espinoza LR. Systemic sclerosis sine scleroderma: an unusual presentation in scleroderma renal crisis. J Rheumatol. 1995;22:557–60. [PubMed] [Google Scholar]
- 11.Phan TG, Cass A, Gillin A, Trew P, Fertig A, Sturgess A. Anti-RNA polymerase III antibodies in the diagnosis of scleroderma renal crisis sine scleroderma. J Rheumatol. 1999;26:2489–92. [PubMed] [Google Scholar]
- 12.Laing CM, Roberts R, Lightstone L, et al. A patient with suspected miscarriage is found to have hypertension, renal failure, and thrombocytopenia: case outcome. Br Med J. 2007;335:205–08. doi: 10.1136/bmj.39239.479560.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuwana M, Kaburaki J, Mimori T, Tojo T, Homma M. Autoantibody reactive with three classes of RNA polymerases in sera from patients with systemic sclerosis. J Clin Invest. 1993;91:1399–404. doi: 10.1172/JCI116343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satoh M, Ajmani AK, Ogasawara T, et al. Autoantibodies to RNA polymerase II are common in systemic lupus erythematosus and overlap syndrome. J Clin Invest. 1994;94:1981–9. doi: 10.1172/JCI117550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Satoh M, Kuwana M, Ogasawara T, et al. Association of autoantibodies to topoisomerase I and the phosphorylated (IIO) form of RNA polymerase II in Japanese scleroderma patients. J Immunol. 1994;153:5838–48. [PubMed] [Google Scholar]
- 16.Kuwana M, Kimura K, Kawakami Y. Identification of an immunodominant epitope on RNA polymerase III recognised by systemic sclerosis sera – application to enzyme-linked immunosorbent assay. Arthritis Rheum. 2002;46:2742–7. doi: 10.1002/art.10521. [DOI] [PubMed] [Google Scholar]
- 17.Kuwana M, Okano Y, Pandey JP, Silver RM, Fertig N, Medsger TA. Enzyme-linked immunosorbent assay for the detection of Anti-RNA polymerase III antibody. Arthritis Rheum. 2005;52:2425–32. doi: 10.1002/art.21232. [DOI] [PubMed] [Google Scholar]
- 18.Reimer G, Rose KM, Scheer U, Tan EM. Autoantibody to RNA polymerase I in scleroderma sera. J Clin Invest. 1987;79:65–72. doi: 10.1172/JCI112809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okano Y, Steen VD, Medsger TA. Autoantibody reactive with RNA polymerase III in systemic sclerosis. Ann Intern Med. 1993;119:1005–13. doi: 10.7326/0003-4819-119-10-199311150-00007. [DOI] [PubMed] [Google Scholar]
- 20.Subcommittee for scleroderma criteria of the American Rheumatism Association diagnostic and therapeutic criteria committee: preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 21.Bunn CC, Kveder T. Counterimmunoelectrophoresis and immunodiffusion for the detection of antibodies to soluble cellular antigens. In: van Venrooij WJ, Maini RN, editors. Manual of biological markers of disease. Dordrecht, The Netherlands: Kluwer Academic; 1993. pp. A3.1–12. [Google Scholar]
- 22.Humbel RL. Detection of antinuclear antibodies by indirect immunofluorescence. In: van Venrooij WJ, Maini RN, editors. Manual of biological markers of disease. Dordrecht, The Netherlands: Kluwer Academic; 1993:. pp. A2.1–16. [Google Scholar]
- 23.Codullo V, Morozzi G, Bardoni A, et al. Validation of a new immunoenzymatic method to detect antibodies to RNA polymerase III in systemic sclerosis. Clin Exp Rheum. 2007;25:373–7. [PubMed] [Google Scholar]
- 24.Yamasaki Y, Honkanen-Scott M, Hernandez L, et al. Nucleolar staining cannot be used as a screening test for the scleroderma marker anti-RNA polymerase I/III antibodies. Arthritis Rheum. 2006;54:3051–6. doi: 10.1002/art.22043. [DOI] [PubMed] [Google Scholar]