Abstract
Objectives. There are few data on the treatment patterns and associated cost of treating children with inflammatory arthritis including juvenile idiopathic arthritis (JIA), in the short or long term. The aim of this study was to obtain patient-based costs for treating children with JIA in the UK, in the first year from diagnosis and from the secondary health care payer perspective.
Methods. The Childhood Arthritis Prospective Study (CAPS) is an ongoing longitudinal study recruiting children with inflammatory arthritis from four UK hospital centres. Included children are newly diagnosed, ≤16 years old with inflammatory arthritis of one or more joints, which has persisted for at least 2 weeks. Health service resource use data were collected as part of routine clinical care at study entry, 6 months and 1 year. Reference unit costs were applied to these data and the cost of treatment per child calculated for the first year from diagnosis.
Results. A total of 297 children attended a 12-month follow-up visit. The mean annual total cost per child was £1649 (s.d. £1093, range £401–£6967). The highest cost component was for appointments with paediatric rheumatologists. Mean total costs were highest for children with enthesitis-related, systemic JIA or extended oligoarthritis.
Conclusions. In the first 12 months after diagnosis, children with all JIA disease subtypes consume large, but highly variable quantities of health service resources. Individual patient costs are required to reflect the wide variation in cost between patients and allow appropriate recouping of costs for contracted services and for assessing the economic impact of interventions.
Keywords: Inflammatory arthritis, Juvenile idiopathic arthritis, Economics, Costing study
Introduction
Juvenile idiopathic arthritis (JIA) is a debilitating chronic disease in children. Early, intensive, multi-disciplinary management is needed to reduce joint and other damage from the disease and achieve the best outcomes for these children [1]. Chronic treatment into adulthood is often necessary, placing a long-term economic burden on health care providers. However, many bodies with purchasing responsibility have limited knowledge of the costs of treating such patients. Recent pharmaceutical advances, such as anti-TNF therapy, may be useful for improving the health of these children but are associated with increased costs [2]. Individual patient cost data are required to assess the economic impact of these interventions. There are few data on the cost of treating children with JIA. Seven studies have reported costs of management and suggest a substantial economic impact but do not provide sufficient data to describe the long-term resource use requirements of children with the different subtypes of JIA [2–8]. Therefore, the aim of this study was to obtain accurate patient-based costs for the cost of treating children with JIA in the UK, in the first year after diagnosis.
Patients and methods
The patients
The Childhood Arthritis Prospective Study (CAPS) is an ongoing UK longitudinal study conducted by the Arthritis Research Campaign (ARC) Epidemiology Unit, the University of Manchester. The aim of CAPS is to identify predictors of outcome, both short- and long-term, following presentation with childhood-onset inflammatory arthritis and to identify the relative contributions of socio-demographic, clinical, psychological, laboratory and genetic factors in explaining outcome. In total, children are recruited from five paediatric rheumatology centres; this analysis used data from four centres (Booth Hall Children's Hospital in Manchester, Royal Liverpool Children's Hospital, Royal Victoria Infirmary in Newcastle and Royal Hospital for Sick Children in Glasgow). The inclusion criteria are children ≤16 years old with newly-diagnosed inflammatory arthritis of one or more joints, which has persisted for at least 2 weeks. JIA is subsequently classified according to the ILAR criteria [9–11]. Exclusion criteria include arthritis subsequently diagnosed to be caused by infection, trauma, foreign body or haematological/oncological conditions, and connective tissue disorders. Data are collected as part of routine clinical care at first presentation and study entry (baseline), 6 months and at 1–5 years. At each time-point, children undergo a rheumatological examination by the consultant, a comprehensive case notes review is undertaken and the nurse completes an assessment form together with the child and parents.
Design of costing study
This costing study aimed to describe the resource use and associated costs in children with JIA recruited to CAPS, from the perspective of the secondary care provider. Although CAPS data were collected as a study examining outcomes, the database also provided detailed information on NHS treatment patterns. The categories of resource use obtained from the CAPS database were: consultant paediatric rheumatologist appointments and referrals to other specialists or care; medication for inflammatory arthritis; laboratory investigations and clinical imaging; and surgery.
Consultant paediatric rheumatologist appointments and referrals to other specialists or care
The number of appointments with paediatric rheumatologists during the follow-up period was recorded for each child. The database recorded routine referrals to ophthalmologists, specialist paediatric rheumatology nurses, physiotherapists, occupational therapists, podiatrists, and requirements for splinting and orthotics. However, the number of appointments with each specialist was not recorded, so these data were estimated based on known treatment approaches, or through consultation with relevant health care providers (Table 1). National and local reference unit costs (using the mean cost) were attached to the resource use data.
Table 1.
Unit costs of appointments with health care professionals
| Number of sessions: actual or estimated | Source | Cost (£) | |
|---|---|---|---|
| Appointment/referral | |||
| Paediatric rheumatologist appointment | CAPS database | Department of Health Reference Costs 2004 Paediatric clinic follow-up appointment | 133.00 |
| Ophthalmologist appointment | 2 | Department of Health Reference Costs 2004 Adult follow-up appointment | 55.00 |
| Specialist nurse visit | 3 | Personal Social Services Research Unit Costs of Health and Social Care 2005 Half an hour of patient contact | 16.50 |
| Physiotherapist visit | 6 | Personal Social Services Research Unit Costs of Health and Social Care 2005 Half an hour of patient contact | 21.00 |
| Occupational therapy | 2 | Personal Social Services Research Unit Costs of Health and Social Care 2005 Half an hour of patient contact | 22.50 |
| Splinting | 2 | Personal Social Services Research Unit Costs of Health and Social Care 2005 Half an hour of patient contact | 22.50 |
| Podiatry | 3 | Department of Health Reference Costs 2004 Adult follow-up appointment | 45.00 |
| Orthotics | 3 | Department of Health Reference Costs 2004 Adult follow-up appointment Used podiatry costs. | 45.00 |
| Dietician visit | 1 | Personal Social Services Research Unit Costs of Health and Social Care 2005 Half an hour of patient contact | 17.50 |
| Endocrinologist appointment | 1 | Department of Health Reference Costs 2004 Adult follow-up appointment | 121.00 |
| Dermatologist appointment | 1 | Department of Health Reference Costs 2004 Adult follow-up appointment | 62.00 |
| Cardiologist appointment | 1 | Department of Health Reference Costs 2004 Adult follow-up appointment | 95.00 |
| Psychologist appointment | 1 | Personal Social Services Research Unit Costs of Health and Social Care 2005 Half an hour of patient contact | 38.50 |
| Hydrotherapya | 24 | Personal Social Services Research Unit Costs of Health and Social Care 2005, Epps et al. [3] | 61.10 |
| Investigations/laboratory tests | |||
| Blood and biochemistry | CAPS database | Department of Health Reference Costs 2004 | Haematology 32.28 |
| Immunology 8.16 | |||
| Biochemistry 1.88 | |||
| Other 8.44 | |||
| X-rays | CAPS database | Local costs | 195.92 |
| MRI | CAPS database | Department of Health Reference Costs 2004 | 224.00 |
| Ultrasound | CAPS database | Department of Health Reference Costs 2004 | 32.00 |
| Bone scan | CAPS database | Department of Health Reference Costs 2004 | 142.00 |
Sources of data: Department of Health Reference Costs 2004 (www.dh.gov.uk/PolicyAndGuidance/OrganisationPolicy/FinanceAndPlanning/NHSReferenceCosts/fs/en). Personal Social Services Research Unit Costs of Health and Social Care 2005, University of Kent [17].
aBased on information and data in a study of hydrotherapy in children with JIA [3]. Two physiotherapists were needed for an appointment lasting 29 min. The costs for the physiotherapists were taken from the PSSR Unit Costs of Health and Social Care 2005, per hour of patient contact. The fixed costs of a hydrotherapy appointment were obtained from Epps et al. and inflated to 2005 costs using Hospital and Community Health services pay and price inflation [17]. Fixed costs and staff costs were added to give the total cost.
Medication for JIA
Type of medication prescribed was recorded on CAPS but not doses. The recommended doses of drugs were taken from the British National Formulary for Children [12] and the British National Formulary [13], December 2005, as appropriate; consultation with paediatric rheumatologists ensured that they reflected local practice. Individual doses were then calculated for each child in the study from the weight (kg) and age of each child. For MTX dosage, body surface area (BSA) was calculated from height and weight using the equation of Mosteller: BSA = √[height (cm) × weight (kg)/3600] [14, 15]. Where heights and weights were missing for follow-up visits, regression of the weights from all children estimated a mean increase in weight or height per child per year, which was added to the baseline value to supply a missing follow-up value. For children in whom weight or height was not recorded either at baseline or follow-up, the weights were applied from national children's mean values recorded in the Health Survey for England (http://www.ic.nhs.uk/pubs/hlthsvyeng2004upd). The duration of treatment for each drug for each child was calculated from the recorded dates of starting and stopping treatment for each follow-up visit.
The costs of drugs were obtained from the British National Formulary for Children [12] and the British National Formulary [13], December 2005. Costs of generic drugs were used throughout. For drugs where different strength tablets were available, the cost of the lowest strength tablet was used to calculate the total cost. Where drugs were available as liquid formulation or tablets, it was assumed that children aged <12 years old would receive syrup, suspension or soluble tablets whereas older children would be able to take tablets. For calculating the cost of eye drops, it was assumed that there were 200 drops in one 10 ml bottle and that patients would need two drops twice a day in both eyes and the bottle would have a 28-day expiry.
For IA corticosteroid injections, it was expected that all children treated were booked into the hospital on a day-case basis. After discussion with the paediatric rheumatologists, a general guideline was applied, in that children under 8 years old would need a general anaesthetic but older children could have nitrous oxide, although in practice this would vary depending on the number of joints to be treated and the individual child. The cost of an episode of day-case surgery for children was obtained from a previous study [16]. The mean total cost per child per surgical incident was £89.30 for year 2000 and was inflated to 2005 costs [17]. Only this single cost was available for day-case surgery in children and so it was assumed that there was no difference in cost used for children having general anaesthetic or nitrous oxide. As children would only require one session of anaesthesia regardless of the number of injections required, the analysis assumed a single injection for a large joint, in a single anaesthetic session. The cost of day-case surgery was added to the cost of the corticosteroid injection in the analysis.
Laboratory investigations and clinical imaging
Resource data for haemaglobin, platelets, white blood cell count, lymphocyte count, neutrophils, ESR, CRP, ANA, B27 and immunoglobulin estimation were collected. Referral for clinical imaging procedures (X-ray, ultrasound, MRI and bone scans) was recorded. As it is unlikely that many children would have had more than one of each imaging procedure in each follow-up period, it was assumed that they had just one investigation. Mean costs per test were taken from the NHS Reference Costs 2004. None of the reference costs were specific to children. The local cost for X-rays in children from Booth Hall Children's Hospital, Manchester was used as these costs were not available elsewhere.
Surgery
Referrals for surgery were also collected but by 12 months, none were JIA related.
Cost analysis
Costs of appointments and referrals, drug treatment, laboratory tests, clinical imaging and total costs were estimated for all patients who remained in the study for 1 year. Analysis was undertaken using STATA version 8.
The objective of the statistical analysis was to test whether there were statistically significant differences in costs between JIA subtypes. The small sample size in this study, with a highly skewed distribution, indicated non-normality. Standard parametric-based approaches would not be robust and non-parametric statistical tests were considered inappropriate because they do not test differences in arithmetic means [18, 19]. The arithmetic mean is considered to be the most relevant measure for health care policy decisions, which should be based on information about the distribution of the costs of treatment as well as the average cost. Data transformation to achieve approximate normality does not result in a comparison of arithmetic means. Therefore, non-parametric bootstrapping was used to compare arithmetic means of cost data [19]. Analysis of variance (ANOVA) was applied to the data generated by bootstrapping (using Excel), for multiple group comparisons.
Research ethics committee approval
CAPS was approved by the North West Multicentre Research Ethics Committee. Parent(s) or guardians were given an information sheet and children an age-adjusted information sheet. Parent(s)/guardians provided informed consent for recruitment and children, if considered able, provided consent.
Results
At the time of analysis, 358 children were expected to have attended both a baseline visit and a 12-month follow-up visit. One year of data were available for 297 of these children; the remaining children who did not attend the 12-month appointment included non-attenders, those who had moved provider or those in whom JIA had resolved. For the purpose of this analysis, these children have not been included. However, although children who were lost to follow-up were slightly older than those who were included in the analysis (9.2 and 7.9 years, respectively), distributions of sex and ILAR subtype were similar between the two groups.
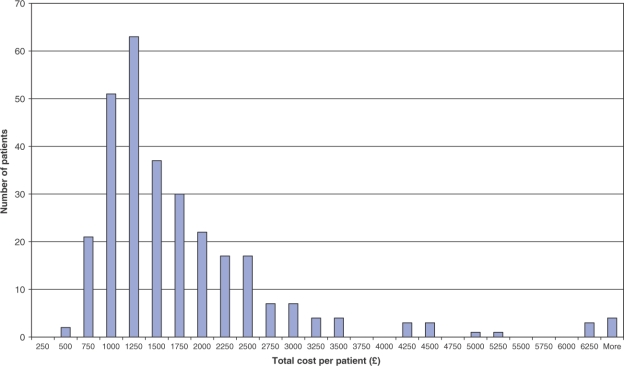
Table 2 summarizes baseline characteristics, total costs and cost of each component of care. For all children with inflammatory arthritis, the mean annual total cost of management per child was £1649 (s.d. £1093, range £401–£6967), which equates to US$ 3347 (2219, 814–1423) and 2391 euros (1585, 581–10 102) (source: Reuters). The distribution of total costs for the children was truncated with a highly skewed distribution, indicating non-normality (Fig. 1).
Table 2.
Baseline characteristics and 1-yr costs of treatment after diagnosis of JIA
| All | Systemic | Oligoarthritis | Extended oligoarthritis | Polyarthritis RF-negative | Polyarthritis RF-positive | Enthesitis- related arthritis | Psoriatic arthritis | Undifferentiateda | Other inflammatory arthritisb | |
|---|---|---|---|---|---|---|---|---|---|---|
| N | 297 | 17 | 139 | 17 | 41 | 9 | 24 | 17 | 12 | 17 |
| Male/female | 106/191 | 2/15 | 45/94 | 6/11 | 7/34 | 1/8 | 2/22 | 5/17 | 6/6 | 9/8 |
| Age at baseline in years, mean (s.d.) | 8.2 (4.3) | 7.8 (4.0) | 7.3 (4.3) | 5.8 (3.7) | 8.0 (3.7) | 13.8 (2.8) | 12.3 (2.3) | 9.9 (4.5) | 8.4 (4.3) | 8.2 (4.1) |
| Cost of treatment: mean cost per child (s.d.) and range | ||||||||||
| Consultant paediatric rheumatologist appointments | £742 (479) 266–3990 | £1072 (780) 399–3591 | £689 (398) 266–3325 | £782 (420) 399–1862 | £834 (379) 399–1862 | £680 (458) 399–1862 | £848 (611) 399–3192 | £563 (197) 266–1064 | £964 (980) 399–3990 | £579 (282) 399–1463 |
| Referrals to other specialists/care | £385 (332) 0–1954 | £262 (399) 0–1592 | £365 (271) 0–1122 | £594 (385) 126–1702 | £511 (416) 0–1938 | £494 (301) 126–978 | £357 (435) 0–1954 | £352 (215) 0–700 | £240 (326) 0–1032 | £321 (291) 0–992 |
| Clinical imaging | £309 (861) 0–5345 | £227 (249) 0–1035 | £350 (1019) 0–5345 | £142 (192) 0–708 | £291 (828) 0–5345 | £125 (160) 0–392 | £483 (1097) 0–4198 | £118 (155) 0–452 | £421 (493) 0–1278 | £305 (906) 0–3781 |
| Laboratory tests: blood and biochemistry | £37 (32) 0–277 | £49 (58) 0–223 | £30 (21) 0–98 | £58 (39) 12–154 | £44 (25) 7–121 | £62 (20) 30–92 | £44 (53) 0–277 | £37 (21) 7–65 | £24 (23) 0–63 | £27 (24) 0–78 |
| Drugs | £175 (272) 0–2705 | £319 (383) 16–1288 | £144 (168) 0–1122 | £336 (498) 0–2073 | £163 (142) 0–622 | £248 (410) 0–1288 | £253 (569) 0–2704 | £147 (132) 0–2704 | £128 (217) 0–780 | £62 (86) 0–262 |
| Total cost £c | £1649 (1093) 401–6967 | £1929 (925) 560–4053 | £1579 (1163) 490–6967 | £1912 (730) 686–3368 | £1843 (982) 867–6519 | £1608 (740) 875–2745 | £1981 (1395) 730–5003 | £1217 (369) 791–2260 | £1778 (1478) 702–6032 | £1290 (958) 401–4324 |
| Total cost US$ | 3347 (2219) 814–1423 | 3916 (1878) 1137–8228 | 3205 (2361) 995–14 143 | 3881 (1482) 1393–6837 | 3741 (1993) 1760–13 234 | 3264 (1502) 1776–5572 | 4021 (2832) 1482–10 156 | 2471 (749) 1606–4588 | 3609 (3000) 1425–12245 | 2619 (1945) 814–8778 |
| Total cost in euros | 2391 (1585) 581–10, 102 | 2797 (1341) 812–5877 | 2290 (1686) 711–10 102 | 2772 (1059) 995–4884 | 2672 (1424) 1257–9453 | 2332 (1073) 1268–3980 | 2872 (2023) 1059–7254 | 1765 (535) 1147–3277 | 2578 (2143) 1018–8746 | 1871 (1389) 581–6139 |
aFailure of diagnosis to comply with one of the other JIA subtypes or because of the fulfilment of more than one set of criteria. bInflammatory arthritis but not classifiable as JIA. cANOVA: F (8, 1882.47); P < 0.0001; Tukey's honest significance difference test, P < 0.0001. All cost groups are significantly different at P < 0.01 level, except for systemic arthritis and extended oligoarthritis.
Fig. 1.
Distribution of individual total costs of management of children with JIA for the first year after diagnosis.
The 297 children had a total of 1658 appointments with paediatric rheumatologists during the 1-yr study period, in addition to 2094 appointments with physiotherapists (including hydrotherapy), 798 with ophthalmologists for screening and management of uveitis, 438 with specialist rheumatology nurses, 192 with occupational therapists and 180 with podiatrists. The cost of appointments with paediatric rheumatologists comprised the major portion of the cost of management for each of the JIA subtypes and overall (Table 1) (Fig. 2).
Fig. 2.
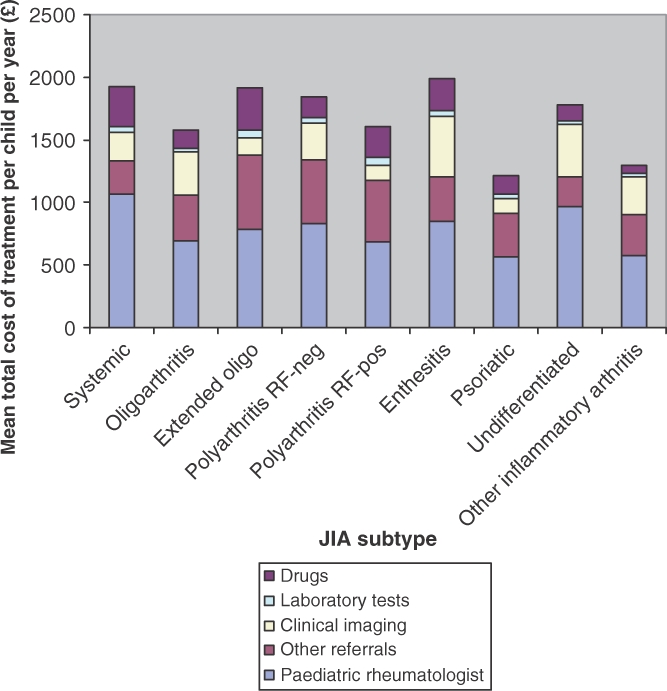
Mean total cost of treatment per child according to JIA subtype.
Statistically significant differences in total costs were found between nearly all of the disease subtypes [ANOVA: F(8, 1882.47); P < 0.0001; Tukey's honest significance difference test, P < 0.0001] (Fig. 2). Mean (s.d.) total costs were highest for children with enthesitis-related arthritis £1981 (£1395), P < 0.01. The costs of managing systemic JIA £1929 (£925) and extended oligoarthritis £1912 (£730) were not significantly different from one another. Costs for all other JIA subtypes were significantly different from one another (P < 0.01).
Discussion
This cohort study demonstrated that, in the first 12 months after diagnosis, children with inflammatory arthritis consume large, but highly variable quantities of health service resources. It is not known whether this consumption pattern persists for individual patients as their disease progresses beyond the first year from diagnosis. The largest component of health provider costs was consultant rheumatology appointments, followed in order of magnitude by referrals to other specialists, clinical imaging, drugs and laboratory tests. The right-skewed distribution of costs suggests that a few high-cost outliers increased the mean costs for the group overall, and within individual disease subgroups [18, 19]. This is a typical pattern for cost data. These data also appear to suggest that different disease subgroups may be associated with different levels of resource consumption. For example, the higher costs for enthesitis-related arthritis appear to be a result of an increased need for imaging. Bernatsky et al. [8] also found that systemic and polyarthritis JIA subtypes predicted higher cost of treatment compared with the other subtypes. However, numbers in some of the subgroups were very small in our study. Data from a larger cohort, over a longer period of time are required to further substantiate these results. For this analysis, children were allocated into the categories early on in the disease course and the classification of the child can be changed with time; it would be expected that some children would move from the oligoarthritis category into the extended oligoarthritis category.
There were limitations of our analysis. The CAPS study database was not designed to reflect all NHS contact and the routine data may not be identifying all the interventions involved, so we had to make informed assumptions about treatment from the data that were available. For example, the length and number of appointments was estimated, dose of drugs estimated, one session of general anaesthetic was assumed to be sufficient for any number of joint injections and we assumed only one clinical X-ray image per follow-up and we had limited data on inpatient management. Thus, it is likely that our costs are conservative estimates of the true costs. Data collection can be refined for future analyses. Furthermore, anecdotal evidence suggests that costs of interventions such as joint injections and MRI scans can vary hugely between individual children, such that our costs underestimate true variability. It was not possible to calculate the loss to follow-up because the reasons for non-attendance are not clear yet. It may be that patients with milder disease are lost from the database because of resolution of disease, such that our data reflects more of the severely ill children only, thus overestimating the costs. However, children may have persisting disease but may have moved to a different geographical area. There are no data on resource use before the child reaches paediatric rheumatology care; retrospective data collection may be needed to better understand the range and quantity of testing carried out in children with suspected JIA. Furthermore, this study does not provide information on the cost of JIA in primary care or the wider impact on personal social services. Despite these limitations, these data provide a valuable insight into the management of children with JIA during the first year from diagnosis. This is the first time longitudinal treatment practice and cost data have been assessed in this disease group, presenting essential information for contracting and research purposes.
A review of the published literature confirmed the lack of data on the cost of treating children with JIA [2–8]. Only two previous studies were relevant to the UK healthcare perspective [2, 3]. Cummins et al. [2] undertook a health technology assessment of etanercept in JIA, from a UK perspective. The authors adapted an adult cost–utility model for evaluating the outcomes and costs of treatment and had to make many assumptions in order to apply it to children. In a UK 6-month randomized controlled trial, Epps et al. [3] compared the costs of standard physiotherapy with physiotherapy plus hydrotherapy in children with JIA for >3 months. All treatment costs from the perspectives of the NHS and the children's families were included. The most recent and comprehensive study was conducted from the Canadian healthcare perspective [8]. Bernatsky et al. [8] estimated the mean direct medical costs for children with JIA as Canadian $3002 compared with $1315 for outpatient control children without chronic disease. The higher cost for children with JIA was mainly because of higher drug costs although these children also had higher costs related to appointments with healthcare professionals and diagnostic tests. One study evaluated the costs of 3 and 12 months of treatment in the United States [5]. The mean annualized direct cost per child was $7905 (inpatient, $1717; outpatient, $5700; and non-medical, $488). Family costs averaged $1524/yr (out of pocket medical and non-medical, $1196; lost salary, $328), which represented 5% of mean family income. A second US study compared the costs and health-related quality of life of children with JIA treated with MTX, etanercept or a combination of the two agents [7]. A German study evaluated the burden and cost of illness in JIA but only estimated short-term adult treatment costs [4]. Haapasaari et al. [6] evaluated the costs of adding etanercept to existing treatment in Finland.
Unfortunately, these previous JIA costing studies vary greatly in methodological quality. Many studies report costs only for individual courses of treatment, report costs in adults only and so are not relevant to children, or use approximate charge information rather than patient-based information. In this study, great effort was made to use as standardized an approach as possible. Because of the detailed patient-based data available, we have been able to identify cost patterns that may not have been apparent if top-down or other more approximate estimation methods had been used. A drawback to this study, as in many costing studies, was the retrospective nature of the cost component of the data collection, so we had no control over quality of routinely collected data.
The National Institute for Health and Clinical Excellence (NICE) has issued clear guidelines on the minimum requirements for costs that are to be used to inform NHS decision-making (‘NICE reference case’) [20]. Cost data should have a perspective that includes both the NHS and personal social services (PSS), which covers both social work and ‘social care’, services to people which fall outside the remit of health services. A study evaluating the specific cost of a hydrotherapy programme in JIA included the costs of primary care and PSS, as well as time away from work for parents [3]. Future studies of costing of treatment should consider all these aspects of JIA management. As we discovered when deriving our total costs, current reference costs for JIA are not specific enough to the paediatric status of the patient nor to the interventions involved in their care.
Our study has presented the costs of 1 yr of treatment and future analyses will examine costs of longer-term management after 2 or 3 yrs of treatment. Ongoing developments in treatment should also be considered. More children are now being treated with biologic agents such as etanercept. These are more expensive drugs but may, for example, reduce the incidence of disability and poor bone health, or cardiac risk from uncontrolled inflammation and poor physical fitness, thus reducing the overall cost of management. These longer-term costs and benefits should be considered in future studies.
This costing study demonstrates the importance of patient-based cost data to allow characterization of inter-patient variation. Individual patient costs, or a proxy measure sensitive to treatment intensity, are required to reflect the wide variation in cost between patients, and thus allow appropriate recouping of costs for contracted services and for assessing the economic impact of interventions. Our study has shown that the key cost-generating events in the hospital setting in JIA are specialist referrals. Early input from paediatric rheumatologists is crucial for these children and may spare them from both unnecessary invasive investigations, for example, arthroscopy and synovial biopsy, and also facilitate access to appropriate and prompt management [21]. In a second analysis of data from 507 children in CAPS, the median symptom duration at first paediatric rheumatology assessment was 4.6 months and was shortest for children presenting with systemic arthritis (1.6 months). Rheumatology units assessing cost for both practice and research purposes should concentrate on factors affecting referrals, admissions, imaging and staff input.

Acknowledgements
The authors would like to thank the CAPS specialist rheumatology nurses (Carol Lydon, Joanne Buckley, Sharon Bradshaw, Alexandra Meijer, Vicki Price and Mick Eltringham) who collected the data and Professor Alan Silman, Medical Director of the ARC, for his help in establishing the study.
Funding: The project wishes to acknowledge that this analysis was funded by the UK Department of Health through its Health Technology Assessment Programme. The opinions and conclusions expressed here are those of the authors and do not necessarily reflect those of the UK National Health Service or the Department of Health. The CAPS is funded by a programme grant to the arc Epidemiology Unit by the ARC. Funding to pay the Open Access publication charges for this article was provided by the ARC.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Hashkes PJ, Laxer RM. Medical treatment of juvenile idiopathic arthritis. J Am Med Assoc. 2005;294:1671–84. doi: 10.1001/jama.294.13.1671. [DOI] [PubMed] [Google Scholar]
- 2.Cummins C, Connock M, Fry-Smith A, Burls A. A systematic review of effectiveness and economic evaluation of new drug treatments for juvenile idiopathic arthritis: etanercept. Health Technol Assess. 2002;6:1–43. doi: 10.3310/hta6170. [DOI] [PubMed] [Google Scholar]
- 3.Epps H, Ginnelly L, Utley M, et al. Is hydrotherapy cost-effective? A randomised controlled trial of combined hydrotherapy programmes compared with physiotherapy land techniques in children with juvenile idiopathic arthritis. Health Technol Assess. 2005;9:iii–iiv. doi: 10.3310/hta9390. [DOI] [PubMed] [Google Scholar]
- 4.Minden K, Niewerth M, Listing J, Biedermann T, Schontube M, Zink A. Burden and cost of illness in patients with juvenile idiopathic arthritis. Ann Rheum Dis. 2004;63:836–42. doi: 10.1136/ard.2003.008516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allaire SH, DeNardo BS, Szer IS, Meenan RF, Schaller JG. The economic impacts of juvenile rheumatoid arthritis. J Rheumatol. 1992;19:952–5. [PubMed] [Google Scholar]
- 6.Haapasaari J, Kautiainen HJ, Isomaki HA, Hakala M. Etanercept does not essentially increase the total costs of the treatment of refractory juvenile idiopathic arthritis. J Rheumatol. 2004;31:2286–9. [PubMed] [Google Scholar]
- 7.Brunner HI, Barron AC, Graham TB, et al. Effects of treatment on costs & health-related quality of life (HRQL) of children with polyarticular course juvenile rheumatoid arthritis (JRA) Arthritis Rheum. 2004;50:S686. [Google Scholar]
- 8.Bernatsky S, Duffy C, Malleson P, Feldman DE, St PierreY, Clarke AE. Economic impact of juvenile idiopathic arthritis. Arthritis Rheum. 2007;57:44–8. doi: 10.1002/art.22463. [DOI] [PubMed] [Google Scholar]
- 9.Fink CW. Proposal for the development of classification criteria for idiopathic arthritides of childhood. J Rheumatol. 1995;22:1566–9. [PubMed] [Google Scholar]
- 10.Petty RE, Southwood TR, Baum J, et al. Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J Rheumatol. 1998;25:1991–4. [PubMed] [Google Scholar]
- 11.Petty RE, Southwood TR, Manners P, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–2. [PubMed] [Google Scholar]
- 12.BMJ Publishing Group Ltd and Royal Pharmaceutical Society of Great Britain. British National Formulary for Children December 2005. London, UK: Pharmaceutical Press; 2005. [Google Scholar]
- 13.BMJ Publishing Group Ltd and Royal Pharmaceutical Society of Great Britain. British National Formulary December 2005. London, UK: Pharmaceutical Press; 2005. [Google Scholar]
- 14.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 15.Lam TK, Leung DT. More on simplified calculation of body-surface area. N Engl J Med. 1988;318:1130. doi: 10.1056/NEJM198804283181718. [DOI] [PubMed] [Google Scholar]
- 16.Elliott RA, Davies LM, Payne K, Moore JK, Harper NJ. Costing day case anesthesia: obtaining accurate patient-based costs for adults and children. Int J Technol Assess Health Care. 2004;20:552–61. doi: 10.1017/s0266462304001497. [DOI] [PubMed] [Google Scholar]
- 17.Curtis L, Netten A. Unit costs of health and social care. Canterbury: PSSRU University of Kent; 2005. [Google Scholar]
- 18.Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med. 2000;19:3219–36. doi: 10.1002/1097-0258(20001215)19:23<3219::aid-sim623>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 19.Briggs A, Gray A. The distribution of health care costs and their statistical analysis for economic evaluation. J Health Serv Res Policy. 1998;3:233–45. doi: 10.1177/135581969800300410. [DOI] [PubMed] [Google Scholar]
- 20.National Institute for Clinical Excellence. Guide to the methods of technology appraisal. [(1 May 2007, date last accessed)];2004 http://www.nice.org.uk/download.aspx?o=201973. [PubMed]
- 21.Foster HE, Eltringham MS, Kay LJ, Friswell MS, Abinun M, Myers A. Delay in access to appropriate care for children presenting with musculoskeletal complaints and ultimately diagnosed as juvenile idiopathic arthritis. Arthritis Care Res. 2007;57:921–7. doi: 10.1002/art.22882. [DOI] [PubMed] [Google Scholar]