Abstract
Background
Ozone exposure induces airway neutrophilia and modifies innate immune monocytic cell-surface phenotypes in healthy individuals. High-dose inhaled corticosteroids can reduce O3-induced airway inflammation, but their effect on innate immune activation is unknown.
Objectives
We used a human O3 inhalation challenge model to examine the effectiveness of clinically relevant doses of inhaled corticosteroids on airway inflammation and markers of innate immune activation in healthy volunteers.
Methods
Seventeen O3-responsive subjects [> 10% increase in the percentage of polymorphonuclear leukocytes (PMNs) in sputum, PMNs per milligram vs. baseline sputum] received placebo, or either a single therapeutic dose (0.5 mg) or a high dose (2 mg) of inhaled fluticasone proprionate (FP) 1 hr before a 3-hr O3 challenge (0.25 ppm) on three separate occasions at least 2 weeks apart. Lung function, exhaled nitric oxide, sputum, and systemic biomarkers were assessed 1–5 hr after the O3 challenge. To determine the effect of FP on cellular function, we assessed sputum cells from seven subjects by flow cytometry for cell-surface marker activation.
Results
FP had no effect on O3-induced lung function decline. Compared with placebo, 0.5 mg and 2 mg FP reduced O3-induced sputum neutrophilia by 18% and 35%, respectively. A similar effect was observed on the airway-specific serum biomarker Clara cell protein 16 (CCP16). Furthermore, FP pretreatment significantly reduced O3-induced modification of CD11b, mCD14, CD64, CD16, HLA-DR, and CD86 on sputum monocytes in a dose-dependent manner.
Conclusions
This study confirmed and extended data demonstrating the protective effect of FP against O3-induced airway inflammation and immune cell activation.
Keywords: inhaled corticosteroids, innate immune markers, ozone, sputum neutrophils
Ozone is a commonly encountered environmental air pollutant. In epidemiologic investigations, exposure to increased levels of ambient air O3 has been associated with exacerbations of asthma, chronic obstructive pulmonary disease (COPD), and pneumonia, generally 24–48 hr after exposure occurs (Bernstein et al. 2004; Peden 2001). Controlled chamber exposures to O3 cause an influx of neutrophils to the airway and a decrease in lung function, although these two effects do not correlate with each other, indicating that separate mechanisms account for these effects (Bernstein et al. 2004). O3 exposure also causes increased responsiveness to allergen in allergic asthmatics (Peden 2001). We have recently observed that O3 exposure can also result in increased expression of CD11b, CD14, CD16, CD80, CD86, and HLA-DR on airway dendritic cells (DCs), monocytes, and macrophages (Alexis et al. 2004b). It has been suggested that the action of O3 on airway neutrophils, monocytes, and macrophages accounts for much of the disease outcomes associated with O3 exposure. These inflammatory events also mimic the type of inflammation that occurs with acute viral and bacterial infection and exacerbations of asthma and COPD (Maneechotesuwan et al. 2007; Pauwels 2004).
Together, these observations suggest that O3 challenge may be a useful controlled human disease model for screening novel anti-inflammatory pharmaceutical agents in phase I proof-of-concept trials. Holz et al. (2005) tested the utility of a 0.25-ppm O3 challenge as a drug efficacy screen, using a single pre-treatment dose of the established anti-inflammatory agents fluticasone propionate (FP) and oral prednisolone as test agents in a randomized three-arm crossover study in 18 healthy subjects comparing the effect of these two treatments with that of placebo on O3-induced airway inflammation. Holz et al. (2005) reported that, compared with placebo, pretreatment with 2 mg inhaled FP and 50 mg oral prednisolone resulted in a significant reduction in post-O3 sputum neutrophils per milliliter (by 62% and 64%, respectively) and myeloperoxidase (MPO; by 55% and 42%, respectively). These results demonstrated that corticosteroids do inhibit the proinflammatory actions of O3.
In the present study, we sought to extend these observations by comparing the effect of a single administration of a high dose of inhaled FP (2 mg) with a dose that is employed in clinical practice for asthma and COPD (0.5 mg) and placebo. Given the importance that monocytes, macrophages, and DCs likely have in the pathophysiology of O3-induced exacerbations of disease, we also examined the effect of these treatments on expression of CD11b/CR3, mCD14, CD16/FcγRIII, CD64/FcγRI, CD86/B7, and HLA-DR on monocytes, macrophages, and DCs recovered from airway sputum. Clara cell protein 16 (CCP16) and surfactant protein D (SP-D) are innate immune molecules and products of airway epithelial cells (Haczku 2006) that can be released to the circulation during lung injury (Holz et al. 2005). CCP16 is induced in the serum of subjects exposed to O3 challenge (Blomberg et al. 2003). We have previously shown that SP-D levels in the lung are significantly altered after O3 inhalation in mice (Kierstein et al. 2006), but whether similar changes can be detected in the human serum is not known. Thus, we evaluated CCP16 and SP-D for their potential utility as serum biomarkers for assessing the effects of inhaled corticosteroids on O3 injury in the respiratory tract.
Materials and Methods
Subjects
Seventeen (nine male and eight female) nonsmoking healthy volunteers (10 from the Center for Environmental Medicine, Asthma and Lung Biology; 7 from Rancho Los Amigos National Rehabilitation Center) between 18 and 50 years of age (age, 26.4 ± 7.4 years, mean ± SD; body mass index, 20–30 kg/m2) were recruited for this study. All subjects underwent a thorough physical examination and had no history of cardiovascular or chronic respiratory disease and were free of upper or lower respiratory tract infection at least 4 weeks before study participation. All subjects had a forced expiratory volume in 1 sec (FEV1) of at least 80% predicted for a normal population of similar weight and height. A positive urine pregnancy test resulted in exclusion of female subjects from the study. The use of prescription drugs, over-the-counter medication (e.g., aspirin and nonsteroidal anti-inflammatory drugs), vitamins, antioxidants, and dietary supplements was not permitted for the duration of the study. All study participants were able to produce an adequate sputum sample (≥ 1 × 106 total cells, ≥ 50% cell viability, ≤ 20% squamous epithelial cells) as measured on their first baseline visit (sputum with no O3 exposure), and all were responsive to O3 (defined as ≥ 10% increase in total and percent sputum neutrophils) (Holz et al. 2005) after exposure to 0.25 ppm O3 for 3 hr with intermittent moderate exercise (ventilationexpiratory = 12.5 L/min/m2 body surface area) as measured on the second study visit. The study was approved by the Committee on the Protection of the Rights of Human Subjects, School of Medicine, University of North Carolina at Chapel Hill, and by the Institutional Review Board at the Rancho Los Amigos National Rehabilitation Center. Informed written consent was obtained from all subjects before their participation in the study.
Study design
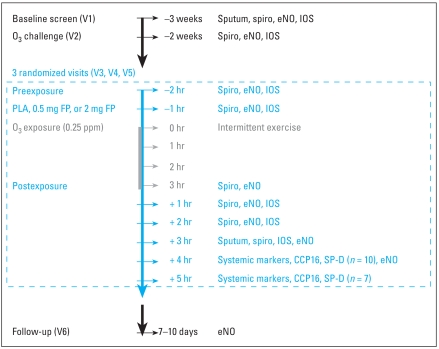
This was a double-blind, placebo-controlled, single-dose, randomized, three-period crossover study conducted at two sites. Controlled O3 exposures were performed in comparable chamber setups at both the University of North Carolina, Chapel Hill and the Rancho Los Amigos facility (Alexis et al. 2004a; Gong et al. 1998). All subjects underwent 3-hr exposures to 0.25 ppm O3 with intermittent moderate exercise (15 min rest, 15 min exercise at 12.5 L/min/m2 body surface area) at screening visit 2 and each study session thereafter (visits 3–5). Based on FP half-life and washout of sputum neutrophils after O3 exposure (Holz et al. 2005), O3 exposures were separated by a minimum of 2 weeks to avoid carryover effects. FEV1 and forced vital capacity (FVC) were also measured for the purpose of assessing subject safety. Sputum induction was performed at screening visits and at 3 hr after the conclusion of each O3 exposure (i.e., post-exposure). Sputum was analyzed for total and differential leukocyte count and fluid-phase components and in a subset of subjects (n = 7) for cell-surface phenotypes and cell function by flow cytometry. The study design, including measurement time points, is depicted in Figure 1. FP was provided as a metered dry powder inhaler (Diskus; GlaxoSmithKline, Research Triangle Park, NC). Each Diskus device contained 60 × 0.5 mg doses of FP. A matching placebo Diskus was also provided. Subjects were randomized to receive one of the following treatment regimens: a) 0.5 mg FP (one inhalation of 0.5 mg FP plus three inhalations of placebo); b) 2 mg FP (one inhalation of 0.5 mg FP plus three inhalations of 0.5 mg FP); and c) placebo (one inhalation of placebo plus three inhalations of placebo). The study staff observed each subject using the Diskus during clinic visits to ensure that the device was used correctly.
Figure 1.
Schematic of the study design. Abbreviations: PLA, placebo; Spiro, spirometry; V, visit. The study was a double-blinded, randomized, cross-over design with a 2-week washout period between visits. Except for the first visit (screen) and last visit (follow-up), all visits included an O3 exposure (0.25 ppm, 3 hr).
Pulmonary function
We used both spirometry and impulse oscillometry (IOS) to assess lung function status in subjects. Spirometry was assessed at preexposure, immediately postexposure, and then at 1-hr intervals for 3 hr. IOS was assessed at pre-exposure, and then hourly for 3 hr beginning 1 hr postexposure. Airway resistance and airway reactance were determined by IOS (Jaeger MS-IOS and LAB Manager Software, version 4.53.2; Jaeger, Hoechberg, Germany) using the recommended techniques of the manufacturer and as previously described (Singh et al. 2006). Real-time recordings of mouth pressure and flow signals pulsed through 5- to 35-Hz spectrum were superimposed on tracings of tidal breathing and displayed on a computer screen. Measurements of total respiratory resistance, resonant frequency (Fres), reactance at 5 Hz, and low-frequency reactance area (area of reactance integrated from 5 Hz up to Fres) were recorded at the 5−, 10−, 15−, and 20-min time points after the IOS test challenge. Spirometry was performed according to current American Thoracic Society spirometry standards (Enright 2003).
Sputum induction and processing and fluid-phase analyses
Subjects provided an induced sputum sample during the screening visit and at 3 hr post-O3 exposure. The sputum induction and processing methods have been previously described in detail (Alexis et al. 2003, 2006). In brief, three 7-min inhalation periods of nebulized hypertonic saline (3%, 4%, 5%; Devilbiss UltraNeb 99 ultrasonic nebulizer; Sunrise Medical, Somerset, Somerset, PA) were followed by expectoration of sputum into a sterile specimen cup. Sputum cell aggregates (cellular mucus plugs) were macroscopically identified and manually selected from their surrounding fluid and treated with 0.1% dithiothreitol (DTT; Sputolysin, Calbiochem, San Diego, CA). Total cell counts and cell viability were determined using a Neubauer hemacytometer and trypan blue (Sigma Chemical Co., St. Louis, MO) exclusion staining. Differential cell counts were analyzed using the Hema-Stain-3 kit (Fisher Diagnostics, Middletown, VA). Aliquots of DTT-treated sputum supernatant were immediately frozen and stored at −80°C for later analysis of MPO and total protein by multiplex assay (Pierce Biotechnology, Rockford, IL). All soluble factors (cytokines and chemokines) in sputum (MPO, total protein) were analyzed by a contract laboratory (HFL, Fordham, UK) using validated commercial enzyme-linked immunosorbent assay (ELISA) kits. All compounds were validated in the presence of DTT. The limits of detection after dilution (to minimize potential effects of DTT and to achieve sufficient volume for measurements) were 40 μg/mL for total protein (Dojindo Molecular Technologies, Inc., Gaithersburg, MD) and 36 ng/mL for MPO (Immundiagnostik, Bensheim, Germany).
For a subset of samples, remaining cells were resuspended in Hank’s balanced salt solution and kept on ice for immediate use in flow cytometric assays for selected cell-surface molecules and phagocytosis.
Systemic biomarkers
Venipuncture was performed at 4 or 5 hr after O3 exposures to obtain serum for Multiplex systemic biomarker analysis of tumor necrosis factor-α (TNF-α), interferon-γ (INF-γ), interleukin-6 (IL-6), IL-1β, IL-1Ra, IL-17, eotaxin, and IL-12P40 using fluorometric custom-designed validated Multiplex kits (Pathway Diagnostics, Malibu, CA). CCP16 and SP-D were assayed using commercially available ELISA kits (Biovendor, Candler, NC) according to the manufacturer’s instructions.
Flow cytometry and immunofluorescent staining
All flow cytometry acquisitions and analyses (surface markers, phagocytosis) were performed as previously described (Alexis et al. 2000a) using a FACSort flow cyto-meter (Becton Dickinson, Franklin Lakes, NJ) and CellQuest Pro v5.3 software (Becton Dickinson).
Cell-surface phenotypes
Immuno-fluorescent staining and flow-cytometry methodology have been described in detail in previous publications (Alexis et al. 2003, 2006). In brief, cells (100 μL, 1 × 106/mL) were incubated with 10 μL fluorochrome-labeled monoclonal antibodies, washed in Dulbecco’s phosphate-buffered saline (DPBS), fixed with 0.5% paraformaldehyde in DPBS, and analyzed by flow cytometry within 48 hr of fixation. Viable macrophages, monocytes, neutrophils, lymphocytes, and DCs in sputum were initially identified and gated on the basis of light-scatter properties and positive expression for CD45 (pan-leukocyte marker). Cell populations were then confirmed by positive staining with CD16 (neutrophils), mCD14 (monocytes), HLA-DR (macrophages), HLA-DR/CD86 (DCs), and CD3 (lymphocytes). The acquired data were analyzed using CellQuest Pro v5.3 software, and results were expressed as a rightward shift from control in mean fluorescence intensity (MFI) on histogram analysis. Control cells were incubated with appropriately labeled isotypic control antibodies. Surface markers analyzed included markers of innate (CD11b/CR3, mCD14/LPS receptor, CD16/FcγRIII, CD64/FcγRI) and adaptive (HLA-DR/MHC class II, and CD86/B7.2 co-receptor) immune function. All monoclonal antibodies were purchased from Beckman Coulter Corporation (Miami, FL).
Phagocytosis
We analyzed phagocytosis using fluorescein isothiocyanate–labeled IgG-opsonized Saccharomyces cerevisiae zymosan-A BioParticles (Molecular Probes, Eugene, OR) as previously described (Alexis et al. 2003, 2006). All samples were analyzed by flow cytometry within 24–48 hr of fixation in 1% paraformaldehyde. Particle uptake was displayed on histograms and identified as a rightward shift in MFI of the phagocytic population versus autofluorescence of the unlabeled control cells.
Exhaled nitric oxide
We measured exhaled NO (eNO) levels preexposure, immediately after exposure, and then at 1-hr intervals for 4 hr according to standardized procedures jointly recommended by the American Thoracic Society and the European Respiratory Society (2005) using a NIOX NO analyzer (Aerocrine AB, Solna, Sweden).
Statistical analysis
To determine the total number of neutrophils and fluid-phase markers (MPO, protein) in induced sputum 6 hr postchallenge, we analyzed data following a natural logarithmic transformation using a mixed effects model, with period and treatment fitted as fixed effects and subject as a random effect. The suitability of the transformation was assessed by examining the model residuals. Treatment effects were evaluated in terms of treatment ratios and were calculated as the antilog for the differences between the least squares means; 95% confidence intervals (CIs) were determined using pooled estimates of variance for the least squares means difference and then antilogged.
For assessment of differences between specific treatment conditions (postscreen O3 challenge vs. placebo vs. both doses of FP) for CCP16, SP-D, systemic cytokines, and cell-surface marker expression in the subset (n = 7) of volunteers studied at University of North Carolina, Chapel Hill, we used nonparametric one-way analysis of variance for repeated measures (Friedman test) and Dunn’s post hoc analysis of specific pairs of variables. An overall significance level of p < 0.05 was considered to be significant. All values are expressed as mean ± SE. We used GraphPad Prism 3.1 software (GraphPad Software, Inc., San Diego, CA) for statistical analysis.
Results
Patient demographics and overall safety
Seventeen volunteers participated in the study; patient demographics are outlined in Table 1. No serious adverse events were reported during this study.
Table 1.
Subject demographics (n = 17).
| Characteristic | Mean ± SE |
|---|---|
| Age (years) | 26.4 ± 1.8 |
| Sex | |
| Female | 9 |
| Male | 8 |
| Race | |
| Caucasian | 10 |
| African American | 3 |
| American Hispanic | 2 |
| Asian | 1 |
| Other | 1 |
| Height (cm) | 170 ± 2.6 |
| Weight (kg) | 78 ± 3.9 |
Effects of FP on 0.25 ppm O3-induced changes in pulmonary function
O3 exposure caused decreases in FVC and FEV1 during all exposures. Decrements in FVC and FEV1 were evident immediately after O3 exposure during placebo, 0.5 mg, and 2 mg FP treatments but were subsiding by 1 hr post-exposure for each treatment condition (Table 2). Decrements in FVC and FEV1 were minimal by 3 hr postexposure (Table 2). Neither dose of FP had a statistically significant effect on O3-induced lung function changes compared with placebo. No consistent O3-induced changes were observed in IOS end points at any postexposure time point (Table 2).
Table 2.
Mean (± SE) pulmonary function, eNO, and IOS.
| IOS
|
||||||
|---|---|---|---|---|---|---|
| FEV1 (L) | FVC (L) | eNO (ppb) | R5 | X5 | Fres (Hz) | |
| Baseline | ||||||
| Pretreatment | 3.76 ± 0.18 | 4.69 ± 0.21 | 12.54 ± 1.55 | 0.376 ± 0.008 | −0.112 ± 0.003 | 12.27 ± 0.30 |
| Placebo | ||||||
| Preexposure (0 hr) | 3.84 ± 0.01 | 4.70 ± 0.02 | 11.49 ± 1.26 | 0.409 ± 0.007 | −0.161 ± 0.002 | 12.03 ± 0.28 |
| Immediately after exposure | 3.52 ± 0.07 | 4.39 ± 0.08 | 13.51 ± 1.39 | |||
| 1 hr postexposure | 3.71 ± 0.05 | 4.60 ± 0.06 | 14.24 ± 1.49 | 0.379 ± 0.008 | −0.099 ± 0.002 | 11.85 ± 0.27 |
| 2 hr postexposure | 13.76 ± 1.43 | 0.404 ± 0.04 | −0.102 ± 0.01 | 11.87 ± 1.14 | ||
| 3 hr postexposure | 8.94 ± 1.15 | 0.414 ± 0.04 | −0.221 ± 0.11 | 12.60 ± 1.38 | ||
| 0.5 mg FP | ||||||
| Preexposure (0 hr) | 3.84 ± 0.02 | 4.75 ± 0.03 | 11.65 ± 1.91 | 0.383 ± 0.007 | −0.112 ± 0.002 | 12.10 ± 0.24 |
| Immediately after exposure | 3.51 ± 0.05 | 4.43 ± 0.05 | 14.80 ± 1.68 | |||
| 1 hr postexposure | 3.69 ± 0.04 | 4.60 ± 0.05 | 15.53 ± 1.70 | 0.356 ± 0.007 | −0.106 ± 0.002 | 12.01 ± 0.25 |
| 2 hr postexposure | 15.85 ± 1.60 | 0.382 ± 0.05 | −0.101 ± 0.01 | 12.26 ± 1.43 | ||
| 3 hr postexposure | 12.16 ± 1.2 | 0.393 ± 0.05 | −0.196 ± 0.08 | 12.99 ± 1.76 | ||
| 2.0 mg FP | ||||||
| Preexposure (0 hr) | 3.73 ± 0.02 | 4.61 ± 0.02 | 10.81 ± 1.75 | 0.382 ± 0.008 | −0.113 ± 0.003 | 12.23 ± 0.25 |
| Immediately after exposure | 3.51 ± 0.07 | 4.35 ± 0.08 | 13.84 ± 1.32 | |||
| 1 hr postexposure | 3.60 ± 0.04 | 4.41 ± 0.05 | 14.87 ± 1.57 | 0.367 ± 0.007 | −0.109 ± 0.003 | 11.70 ± 0.24 |
| 2 hr postexposure | 13.65 ± 1.18 | 0.365 ± 0.04 | −0.099 ± 0.01 | 11.47 ± 1.07 | ||
| 3 hr postexposure | 11.88 ± 1.45 | 0.373 ± 0.04 | −0.188 ± 0.08 | 11.45 ± 1.07 | ||
Abbreviations: R5, total respiratory resistance (cm H2O/L/sec); X5, reactance (cm H2O/L/sec).
Effects of FP on 0.25 ppm O3-induced changes in sputum neutrophils and fluid-phase markers of neutrophil activation (MPO, total protein)
Analysis of percent neutrophil levels post-O3 challenge yielded evidence of a statistically significant difference for both active treatments (0.5 mg and 2 mg FP) relative to placebo. Mean ± SE levels of percent polymorphonuclear leukocytes (PMNs) for placebo and 0.5 mg and 2 mg FP were 54 ± 5.4%, 44 ± 4.5%, and 35 ± 3.6%, respectively (Figure 2), which reflect an 18% and 35% reduction in sputum percent PMNs for 0.5 mg and 2 mg FP, respectively. The data indicate a dose–response pattern.
Figure 2.
The percent sputum neutrophils after O3 exposure for each pretreatment dose of FP (0.5 or 2 mg) or placebo.
*p < 0.05 compared with placebo.
FP also affected the relatively more variable total number of neutrophils/mL. The mean (95% CI) numbers of PMNs/mL were 66.05 × 104 cells/mL (34.78–125.41 cells/mL), 56.87 × 104 cells/mL (30.15–107.27 cells/mL), and 37.49 × 104 cells/mL (19.89–70.68 cells/mL) for placebo, 0.5 mg FP, and 2 mg FP, respectively, 3 hr post-O3 exposure. These values reflected 14% fewer neutrophils in sputum when subjects were pretreated with 0.5 mg FP and statistically significantly (p < 0.05) fewer neutrophils (43%) when pretreated with 2 mg FP, indicating a dose–response effect on the total number of neutrophils per milliliter. In terms of variability, the neutrophil responses on the O3/placebo visit versus the O3-only visit were very similar for both percent neutrophils (mean ± SE, 54 ± 5% vs. 55 ± 5%) and the absolute number of neutrophils per milligram sputum [mean (95% CI), 66.05 × 104 cells/mL (34.78 to 125.41 cells/mL) vs. 62.20 × 104 cells/mL (−10.17 to 312.97 cells/mL), respectively]. Other than percent macrophages, FP exerted no statistically significant effect on total leukocytes per milliliter or total and percent eosinophils, lymphocytes, and bronchial epithelial cells. Relative to placebo, we observed a 24% and 48% increase in percent macrophages with 0.5 mg and 2 mg FP, respectively.
We observed no statistically significant treatment effect of 0.5 mg or 2 mg FP on MPO or total protein levels in sputum. There was, however, borderline evidence of a difference in levels of the MPO/total protein ratio relative to placebo for 2 mg FP. We observed, on average, reductions of 18% and 43% in the MPO/total protein ratio for 0.5 mg and 2 mg FP, respectively, suggesting a dose–response relationship.
Effects of FP on 0.25 ppm O3-induced changes in surface marker expression and phagocytosis on sputum monocytes, macrophages, DCs, and neutrophils
Figure 3 shows the effect of 0.5 and 2 mg pretreatments with FP on O3-induced changes in the cell-surface markers CD11b, mCD14, CD64, CD16, HLA-DR, and CD86 on monocytes, macrophages, and DCs. Baseline (i.e., no O3 exposure) sputum cell-surface marker values (MFI; mean ± SE) from a different cohort of healthy volunteers (n = 15) were as follows: for CD11b, 21 ± 8 macrophages, 16 ± 3 DCs; for mCD14, 65 ± 16 macrophages, 59 ± 14 monocytes, 61 ± 11 DCs; for CD64, 5 ± 1 monocytes; for CD16, 238 ± 44, macrophages, 195 ± 28 DCs; for HLA-DR: 31 ± 5 monocytes; and for CD86, 22 ± 4 monocytes (Lay et al. 2007). Compared with the O3-only condition in this study (data not shown), baseline expression of these surface markers was significantly (p < 0.05) lower, indicating that O3 causes an up-regulation of these cell-surface phenotypes.
Figure 3.
Expression (MFI; mean ± SE) of cell-surface phenotypes on sputum monocytic cells and DCs after O3 exposure with 0.5 mg FP, 2 mg FP, or placebo pretreatment. (A) CD11b/CR3. (B) mCD14. (C) CD64/FcγRI. (D) CD16/FcγRIII. (E) HLA-DR. (F) CD86. Only results in which at least one dose of FP resulted in a change in surface marker expression compared with placebo are shown.
*p < 0.05 for CD11b, mCD14, CD64, CD16, and CD86 compared with 2 mg FP and for HLA-DR and CD86 with compared with 0.5 mg FP.
In general, 2 mg FP exerted a statistically significant effect on post-O3 surface marker expression relative to placebo treatment. There was also a similar trend after the 0.5 mg dose, which suggests a dose–response effect of FP on O3-induced changes in monocytic cell-surface markers. We also observed a significant decrease in CD16/FcγRIII expression on neutrophils after 2.0 mg FP compared with placebo (MFI, 406 ± 64 vs. 515 ± 72; p < 0.05). We observed no significant drug effect of 0.5 mg or 2 mg FP versus placebo on sputum cells as measured by MFI (mean ± SE): for phagocytosis for macrophages, 478 ± 76 and 606 ± 102 versus 400 ± 50; for monocytes, 348 ± 46 and 365 ± 55 versus 292 ± 39; and for neutrophils, 296 ± 48 and 418 ± 87 versus 270 ± 29.
Effects of FP on 0.25 ppm O3-induced changes in serum CCP16, SP-D, eNO, and other systemic biomarkers
To determine whether serum levels of the airway epithelial cell products SP-D and CCP16 would reflect inflammatory airway changes after O3 exposure, we measured the concentration of these molecules at baseline and after each O3 inhalation session in a subset of seven subjects 5 hr after O3 exposure. Our results showed that serum CCP16 levels were statistically significantly increased after O3 inhalation and that pretreatment with 2 mg FP statistically significantly inhibited this effect compared with placebo (Figure 4). The effects of FP on CCP16 were dose dependent. SP-D levels were not statistically significantly altered pre-versus post-O3 exposure (mean ± SE, 61 ± 6 ng/mL vs. 55 ± 5.4 ng/mL) and were not significantly affected by 0.5 mg FP (53 ± 5 ng/mL) or 2 mg FP (64 ± 5 ng/mL) compared with placebo (55 ± 5 ng/mL).
Figure 4.
CCP16 levels (mean ± SE) in serum pre-O3 and 8 hr post-O3 for placebo and 0.5 and 2 mg FP
*p < 0.05 compared with placebo. #p < 0.05 for post-O3 compared with pre-O3.
Statistical analysis of other systemic bio-markers or eNO yielded no clear changes induced by O3 exposure. No significant effects on systemic cytokines (IL-6, IL-12P40, IL-15, IL-17, IL-1β, IL-1Ra, INF-γ, TNF-α), mediators (MPO, eotaxin), or eNO (Table 2) were observed after 0.5 mg or 2 mg FP versus placebo. For eNO, levels at 1, 2, and 3 hr (Table 2) postexposure were not statistically significantly different from one another.
Discussion
Numerous laboratory studies of healthy young individuals exposed to O3 at a dose comparable to that used in the present study have demonstrated decrements in spirometric lung function (Holz et al. 1999, 2005; McDonnell et al. 1997; Nightingale et al. 2000). A study similar to this one in terms of the cohort characteristics, O3 concentration, and ventilation rate also reported similar postexposure decrements in FVC and FEV1 (Holz et al. 1999). In the present study we found that pre-treatment with therapeutic doses of FP had no significant protective effect on spirometric response, which is in agreement with the finding of Nightingale et al. (2000). FP did, however, inhibit inflammatory cell (neutrophils, PMNs) influx to the airways induced by a 3-hr exposure to 0.25 ppm O3 in a dose-dependent manner. The lack of correlation between spirometry and airway inflammation after O3 has been well documented (Blomberg et al. 1999; Hazucha et al. 1996), so our finding with FP in this regard was not surprising.
We observed a significant inhibition of the percent PMNs present in airway sputum after O3 challenge with either 0.5 mg or 2 mg FP pretreatment, and a significant reduction and a trend for reduction in the number of sputum neutrophils per milliliter post-O3 with 2 mg and 0.5 mg FP, respectively. Furthermore, we showed that serum CCP16 is a valuable systemic marker of the inflammatory state of the lung and is responsive to the effects of inhaled FP. We also observed evidence of diminished neutrophil activation with 2 mg FP, as it decreased the expression of CD16, a marker of neutrophil activation, compared with placebo. This observation coincided with a reduced MPO/total protein ratio with 2 mg FP, supporting the notion of reduced neutrophil activation. Taken together with previously published results (Holz et al. 2005), our results indicate that O3 challenge in healthy individuals is a useful model for screening novel anti-inflammatory agents designed for treatment of airway diseases that have elevated neutrophils as a principal component of their airway inflammation. These include a subtype of severe asthma with minimal airway eosinophils (Louis et al. 2000; Wenzel 2003; Wenzel et al. 1999), as well as COPD during an exacerbation (Hill et al. 1999; Stockley 1998).
An important feature of our study design was that we limited volunteer recruitment to persons with documented responsiveness to O3, defined as a minimum of a 10% increase in air-way PMNs after a screening O3 challenge, to enable the assessment of FP. Nightingale et al. (2000) failed to observe an effect when they examined the effect of 2 weeks of treatment with 800 μg inhaled budesonide twice daily on O3-induced neutrophilia in normal volunteers. In our study, although there was a significant effect of O3 alone on percent PMNs, a substantial number of persons examined failed to have an absolute neutrophil response (using neutrophils per milligram sputum as a measure) after placebo treatment. Thus, it is possible that Nightingale et al.’s (2000) results were influenced by a study population that included a high proportion of O3 “non-responders.” In contrast, Vagaggini et al. (2001) examined the effect of 4 weeks of pre-treatment with 400 μg inhaled budesonide twice daily on O3-induced neutrophilia in asthmatics, and reported a significant decrease in airway neutrophils present 6 hr after 0.27 ppm O3 challenge compared with placebo pretreatment; most volunteers in the Vagaggini et al. (2001) study appeared to be O3 responsive. Furthermore, the objective of the present study was not to test the efficacy of the O3 model, but rather to determine whether clinically relevant doses of FP could be assessed to support subsequent larger studies in subjects with preexisting airway disease.
In addition to its effects on airway neutrophilia, we have recently reported that O3 challenge (0.4 ppm, 2 hr) causes an increase in expression of cell-surface phenotypes CD11b, mCD14, CD16, CD86, and HLA-DR on sputum monocytes recovered from normal volunteers (Alexis et al. 2004a). We also reported an increase in the numbers of sputum monocytes (in addition to neutrophils), suggesting that O3 exposure resulted in an influx of activated monocytes. These data are supported by a recent animal study showing that O3 enhanced the expression of interstitial lung cell-surface molecules associated with antigen presentation and increased the number of antigen-presenting cells in the lung (Koike and Kobayashi 2004). In the present study, we found that 2 mg inhaled FP decreased the expression of CD11b, mCD14, CD16, CD64, CD86, and HLA-DR on sputum monocytic cells after O3 challenge compared with placebo treatment. The 0.5 mg dose of FP decreased the expression of CD86 and HLA-DR on sputum monocytes after O3 challenge compared with placebo. Given that these surface molecules are involved with mediating innate immune responses (CD11b and mCD14), acquired immune responses (CD16, CD64), and antigen presentation (CD86, HLA-DR), we speculate that O3 exposure may play a role in modifying how airway cells respond to a number of pathologic agents in the airborne environment. It is unclear whether the effect of FP on O3-induced changes in monocytic cell populations is due to effects on monocytes present in the airway when exposure began, or on the subsequent influx of monocytes that are activated from the circulation. Decreased monocytic cell influx could be mediated by an effect of FP on production of monocyte-associated chemotactic factors or decreased adhesion molecule expression on endothelial cells lining the postcapillary venules.
The use of CCP16 as a systemic marker for injury of the epithelium has been examined by several investigators (Blomberg et al. 2003; Helleday et al. 2006). O3 exposure is associated with increased serum levels of CCP16 (Blomberg et al. 2003). We likewise observed that O3 exposure caused an increase in serum CCP16 and further showed that pretreatment with either 0.5 mg or 2 mg inhaled FP prevented the O3-induced increase in CCP16. We compared CCP16 with SP-D, an innate immune molecule produced by type II alveolar epithelial cells and Clara cells. We previously showed that SP-D plays a protective role in O3-induced injury of the lung (Kierstein et al. 2006), but whether release of this protein into the circulation could parallel the inflammatory airway changes was unclear. Our study showed that CCP16 is a superior serum marker for injury of the airway epithelium and is a more sensitive biomarker for the effect of inhaled FP on airway inflammation compared with SP-D or, indeed, compared with the wide range of cytokines, chemokines, and inflammatory mediators we investigated.
The apparent discrepancy we observed between serum SP-D and CCP16 was likely influenced by many factors, including changes in lung concentrations. We previously showed that intracellular SP-D mRNA and protein expression are very sensitive to corticosteroids, cAMP, and cytokine levels (Cao et al. 2004) and that SP-D in broncho-alveolar lavage fluid is subject to rapid break-down after O3 exposure of mice (Kierstein et al. 2006). Although no formal comparisons have been made between local (pulmonary) expression of SP-D and CCP16, we speculate that the CCP16 molecule is more resistant to O3-induced breakdown than is SP-D. This is supported by the fact that CCP16 serum levels in a number of animal and human studies accurately reflected the extent of increases in capillary permeability after acute exposure to lipopolysaccharide, chlorine, or O3 (Lakind et al. 2007; Michel et al. 2005). Thus, the discrepancy we observed between serum levels of SP-D and CCP16 after O3 exposure may be due to different structure, regulation of expression, and sensitivity to O3-induced molecular changes. This discrepancy highlights the specific importance of CCP16 as a biomarker for lung injury and treatment effectiveness.
Overall, our observations are consistent with the hypothesis that FP will inhibit acute airway inflammation due to O3 exposure in a dose-dependent fashion that includes the therapeutic dose of 0.5 mg FP. Apart from percent PMNs, however, several of our find-ings with 0.5 mg FP did not reach statistical significance. This was likely due to an insufficient number of subjects examined at this dose. Subsequent studies using the 0.5 mg dose of FP will require a larger sample size. It is also possible that a more prolonged pre-treatment with FP before O3 challenge would have resulted in a more pronounced effect of 0.5 mg FP on airway inflammation, but our single-use administration of FP provided adequate drug exposure over the challenge time. We also note that this study was conducted in normal healthy volunteers. We chose healthy volunteers to avoid the potentially high variation in baseline inflammation associated with subjects with preexisting airway disease. Lower variability in healthy subjects would reduce the need to examine a large cohort of subjects in this study and allow us to attain an initial proof of pharmacology for new anti-inflammatory chemical entities. One cannot rule out, however, that because asthmatics have been reported to have an increased pulmonary sensitivity to O3 exposure (Alexis et al. 2000b; Scannell et al. 1996), although we did not observe a statistically significant effect using a single administration of 0.5 mg FP on airway inflammation, this might have been observed in a cohort of asthmatics. As noted above, Vagaggini et al. (2001) reported a significant inhibition of O3-induced inflammation in asthmatics with treatment of 400 μg budesonide administered twice daily. Using endotoxin as an inflammatory stimulus, we previously observed that 440 μg FP for 2 weeks delivered via a metered dose inhaler twice daily inhibited the effect of endotoxin on neutrophilic airway inflammation in allergic asthmatics (Alexis and Peden 2001). Thus, implementation of a longer treatment period in healthy individuals may have resulted in demonstration of anti-inflammatory efficacy of a single 500 μg dose of inhaled FP.
Conclusion
We confirmed original observations that O3-induced airway neutrophilic inflammation was inhibited by a single administration of 2 mg FP and extended the findings by demonstrating decreased neutrophilic inflammation with the 0.5 mg FP treatment, as well. We also observed that both doses of FP inhibited the up-regulatory effect of O3 on airway monocytic cell-surface phenotypes and that 2 mg FP inhibited serum levels of CCP16. Taken together, these observations suggest that brief treatments with inhaled corticosteroids by persons in anticipation of exposure to air pollution may offer protection against the inflammatory effects of ambient air O3, particularly for those individuals with preexisting airway disease. However, it is important to note that inhaled corticosteroids had no protective effect on the spirometric decrements induced by O3, suggesting this component of airway function, particularly in individuals with preexisting airway disease, remains susceptible to the modifying effects of O3 exposure. A second conclusion is that O3 challenge with subsequent analysis of airway sputum and serum CCP16 is a good acute disease model for phase I screening of novel anti-inflammatory agents intended for use in asthma and COPD.
Correction
In the original manuscript published online, Brad Harris was not included as an author. His name has been added here.
Footnotes
We thank M. Almond, M. Herbst, H. Wells, F. Dimeo, and L. Newlin-Clapp for their technical assistance with this study.
This work was supported by GlaxoSmithKline and by the National Institutes of Health (grant ES012706).
REFERENCES
- Alexis NE, Becker S, Bromberg PA, Devlin R, Peden DB. Circulating CD11b expression correlates with the neutrophil response and airway mCD14 expression is enhanced following ozone exposure in humans. Clin Immunol. 2004a;111(1):126–131. doi: 10.1016/j.clim.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Alexis NE, Eldridge MW, Peden DB. Effect of inhaled endotoxin on airway and circulating inflammatory cell phagocytosis and CD11b expression in atopic asthmatic subjects. J Allergy Clin Immunol. 2003;112(2):353–361. doi: 10.1067/mai.2003.1651. [DOI] [PubMed] [Google Scholar]
- Alexis NE, Eldridge MW, Peden DB. Effect of inhaled endotoxin on airway and circulating inflammatory cell phagocytosis and CD11b expression in atopic asthmatic subjects. J Allergy Clin Immunol. 2004b;12:353–361. doi: 10.1067/mai.2003.1651. [DOI] [PubMed] [Google Scholar]
- Alexis NE, Lay JC, Zeman K, Bennett WE, Peden DB, Soukup JM, et al. Biological material on inhaled coarse fraction particulate matter activates airway phagocytes in vivo in healthy volunteers. J Allergy Clin Immunol. 2006;117:1396–1403. doi: 10.1016/j.jaci.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Alexis NE, Peden DB. Blunting airway eosinophilic inflammation results in a decreased airway neutrophil response to inhaled LPS in patients with atopic asthma: a role for CD14. J Allergy Clin Immunol. 2001;108:577–580. doi: 10.1067/mai.2001.118511. [DOI] [PubMed] [Google Scholar]
- Alexis N, Soukup J, Ghio A, Becker S. Sputum phagocytes from healthy individuals are functional and activated: a flow cytometric comparison with cells in bronchoalveolar lavage and peripheral blood. Clin Immunol. 2000a;97:21–32. doi: 10.1006/clim.2000.4911. [DOI] [PubMed] [Google Scholar]
- Alexis N, Urch B, Tarlo S, Corey P, Pengelly D, O’Byrne P, et al. Cyclooxygenase metabolites play a different role in ozone-induced pulmonary function decline in asthmatics compared to normals. Inhal Toxicol. 2000b;12(12):1205–1224. doi: 10.1080/08958370050198548. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society and European Respiratory Society. Recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am J Respir Crit Care Med. 2005;171(8):912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- Bernstein JA, Alexis N, Barnes C, Bernstein IL, Nel A, Peden D, et al. Health effects of air pollution. J Allergy Clin Immunol. 2004;114:1116–1123. doi: 10.1016/j.jaci.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Blomberg A, Mudway IS, Nordenhall C, Hedenstrom H, Kelly FJ, Frew AJ, et al. Ozone-induced lung function decrements do not correlate with early airway inflammatory or antioxidant responses. Eur Respir J. 1999;13(6):1418–1428. doi: 10.1183/09031936.99.13614299. [DOI] [PubMed] [Google Scholar]
- Blomberg A, Mudway I, Svensson M, Hagenbjork-Gustafsson A, Thomasson L, Helleday R, et al. Clara cell protein as a biomarker for ozone-induced lung injury in humans. Eur Respir J. 2003;22:883–888. doi: 10.1183/09031936.03.00048203. [DOI] [PubMed] [Google Scholar]
- Cao Y, Tao JQ, Bates SR, Beers MF, Haczku A. IL-4 induces production of the lung collectin surfactant protein-D. J Allergy Clin Immunol. 2004;113(3):439–444. doi: 10.1016/j.jaci.2003.11.031. [DOI] [PubMed] [Google Scholar]
- Enright PL. How to make sure your spirometry tests are of good quality. Respir Care. 2003;48:773–776. [PubMed] [Google Scholar]
- Gong H, Wong R, Sarma RJ, Linn WS, Sullivan ED, Shamoo DA, et al. Am J Respir Crit Care Med. 1998;158(2):538–546. doi: 10.1164/ajrccm.158.2.9709034. [DOI] [PubMed] [Google Scholar]
- Haczku A. Role and regulation of lung collectins in allergic airway sensitization. Pharmacol Ther. 2006;110(1):14–34. doi: 10.1016/j.pharmthera.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Hazucha MJ, Madden M, Pape G, Becker S, Devlin R, Koren HS, et al. Effects of cyclo-oxygenase inhibition on ozone-induced respiratory inflammation and lung function changes. Eur J Appl Physiol Occup Physiol. 1996;73(1–2):17–27. doi: 10.1007/BF00262805. [DOI] [PubMed] [Google Scholar]
- Helleday R, Segerstedt B, Forsberg B, Mudway I, Nordberg G, Bernard A, et al. Exploring the time dependence of serum Clara cell protein as a biomarker of pulmonary injury in humans. Chest. 2006;130:672–675. doi: 10.1378/chest.130.3.672. [DOI] [PubMed] [Google Scholar]
- Hill AT, Campbell EJ, Bayley DL, Hill SL, Stockley RA. Evidence for excessive bronchial inflammation during an acute exacerbation of chronic obstructive pulmonary disease in patients with α1-antitrypsin deficiency. J Respir Crit Care Med. 1999;160:1968–1975. doi: 10.1164/ajrccm.160.6.9904097. [DOI] [PubMed] [Google Scholar]
- Holz O, Jorres FA, Timm P, Mucke M, Richter K, Koschyk S, et al. Ozone-induced airway inflammatory changes differ between individuals and are reproducible. Am J Respir Crit Care Med. 1999;159:776–784. doi: 10.1164/ajrccm.159.3.9806098. [DOI] [PubMed] [Google Scholar]
- Holz O, Tal-Singer R, Kanniess F, Simpson KJ, Gibson A, Vessey RS, et al. Validation of the human ozone challenge model as a tool for assessing anti-inflammatory drugs in early development. J Clin Pharmacol. 2005;45:498–503. doi: 10.1177/0091270004273527. [DOI] [PubMed] [Google Scholar]
- Kierstein S, Poulain FR, Cao Y, Grous M, Mathias R, Kierstein G, et al. Susceptibility to ozone-induced airway inflammation is associated with decreased levels of surfactant protein D. Respir Res. 2006;7:85. doi: 10.1186/1465-9921-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike E, Kobayashi T. Ozone exposure enhances antigen-presenting activity of interstitial lung cells in rats. Toxicology. 2004;196:217–227. doi: 10.1016/j.tox.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Lakind JS, Holgate ST, Ownby DR, Mansur AH, Helms PJ, Pyatt D, et al. A critical review of the use of Clara cell secretory protein (CC16) as a biomarker of acute or chronic pulmonary effects. Biomarkers. 2007;12(5):445–467. doi: 10.1080/13547500701359327. [DOI] [PubMed] [Google Scholar]
- Lay JC, Alexis NE, Kleeberger SR, Roubey RAS, Harris BD, Bromberg PA, et al. Ozone exposure enhances expression of surface markers of innate immunity and antigen presentation on airway monocytes in healthy individuals. J Allergy Clin Immunol. 2007;120(3):719–722. doi: 10.1016/j.jaci.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Louis R, Lau LCK, Bron AO, Roldaan AC, Radermecker M, Djukanovi R. The relationship between airways inflammation and asthma severity. Am J Respir Crit Care Med. 2000;161:9–16. doi: 10.1164/ajrccm.161.1.9802048. [DOI] [PubMed] [Google Scholar]
- Maneechotesuwan K, Essilfie-Quaye S, Kharitonov SA, Adcock IM, Barnes PJ. Loss of control of asthma following inhaled corticosteroid withdrawal is associated with increased sputum interleukin-8 and neutrophils. Chest. 2007;132(1):98–105. doi: 10.1378/chest.06-2982. [DOI] [PubMed] [Google Scholar]
- McDonnell WF, Stewart PW, Andreoni S, Seal E, Jr, Kehrl HR, Horstman DH, et al. Prediction of ozone-induced FEV1 changes. Effects of concentration, duration, and ventilation. Am J Respir Crit Care Med. 1997;156:715–722. doi: 10.1164/ajrccm.156.3.9611044. [DOI] [PubMed] [Google Scholar]
- Michel O, Murdoch R, Bernard A. Inhaled LPS induces blood release of Clara cell specific protein (CC16) in human beings. J Allergy Clin Immunol. 2005;115:1143–1147. doi: 10.1016/j.jaci.2005.01.067. [DOI] [PubMed] [Google Scholar]
- Nightingale JA, Rogers DF, Fan CK, Barnes PJ. No effect of inhaled budesonide on the response to inhaled ozone in normal subjects. Am J Respir Crit Care Med. 2000;161:479–486. doi: 10.1164/ajrccm.161.2.9905031. [DOI] [PubMed] [Google Scholar]
- Pauwels RA. Similarities and differences in asthma and chronic obstructive pulmonary disease exacerbations. Proc Am Thorac Soc. 2004;1(2):73–76. doi: 10.1513/pats.2306024. [DOI] [PubMed] [Google Scholar]
- Peden DB. Air pollution in asthma: effect of pollutants on airway inflammation. Ann Allergy Asthma Immunol. 2001;87:12–17. doi: 10.1016/s1081-1206(10)62334-4. [DOI] [PubMed] [Google Scholar]
- Scannell C, Chen L, Aris RM, Tager I, Christian D, Ferrando R, et al. Greater ozone-induced inflammatory responses in subjects with asthma. Am J Respir Crit Care Med. 1996;154(1):24–29. doi: 10.1164/ajrccm.154.1.8680687. [DOI] [PubMed] [Google Scholar]
- Singh R, Tal-Singer R, Faiferman I, Lasenby S, Henderson A, Wessels D, et al. Plethysmography and impulse oscillometry assessment of tiotropium and ipratropium bromide; a randomised, double blind, placebo controlled, crossover study in healthy subjects. Br J Clin Pharm. 2006;61(4):398–404. doi: 10.1111/j.1365-2125.2006.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockley RA. Role of bacteria in the pathogenesis and progression of acute and chronic lung infection. Thorax. 1998;53:58–62. doi: 10.1136/thx.53.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagaggini B, Taccola M, Conti I, Carnevali S, Cianchetti S, Bartoli ML, et al. Budesonide reduces neutrophilic but not functional airway response to ozone in mild asthmatics. Am J Respir Crit Care Med. 2001;164:2172–2176. doi: 10.1164/ajrccm.164.12.2009090. [DOI] [PubMed] [Google Scholar]
- Wenzel S. Severe/fatal asthma. Chest. 2003;123:405S–410S. doi: 10.1378/chest.123.3_suppl.405s-a. [DOI] [PubMed] [Google Scholar]
- Wenzel SE, Schwartz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999;160:1001–1008. doi: 10.1164/ajrccm.160.3.9812110. [DOI] [PubMed] [Google Scholar]