Abstract
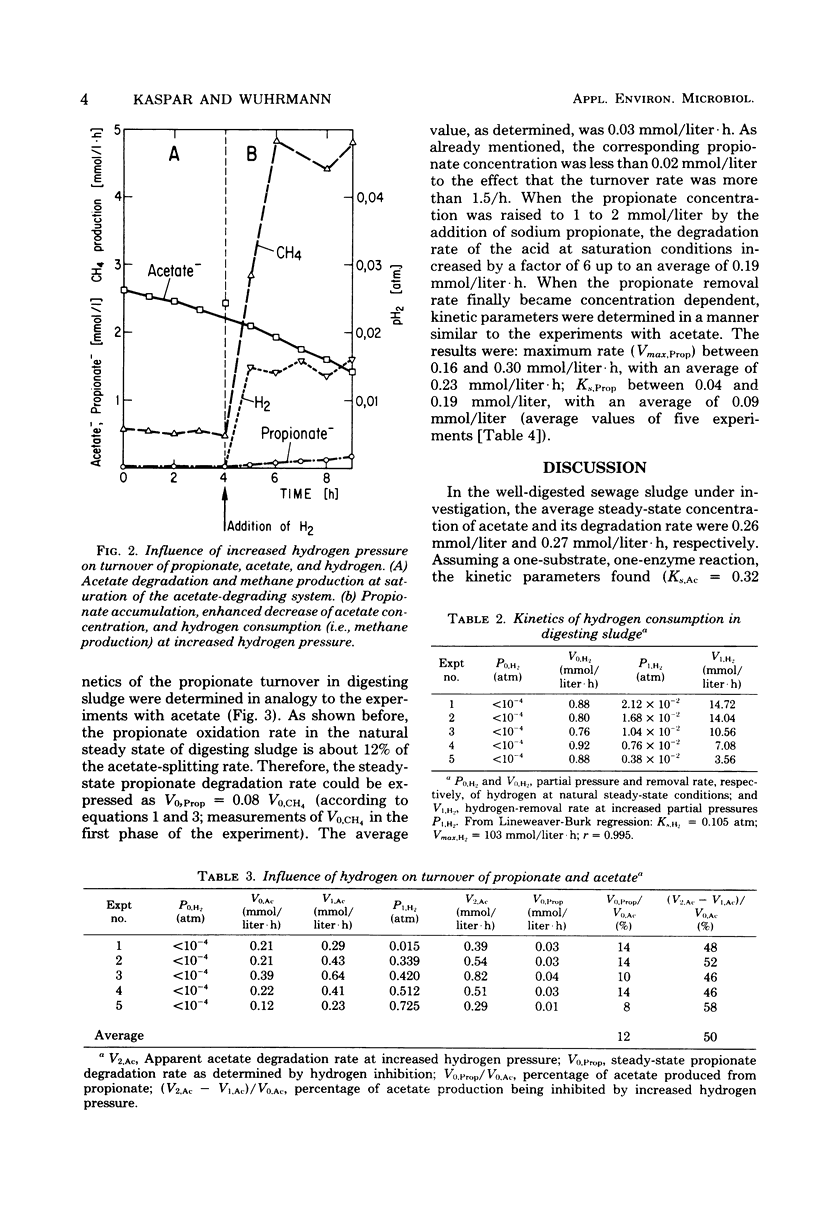
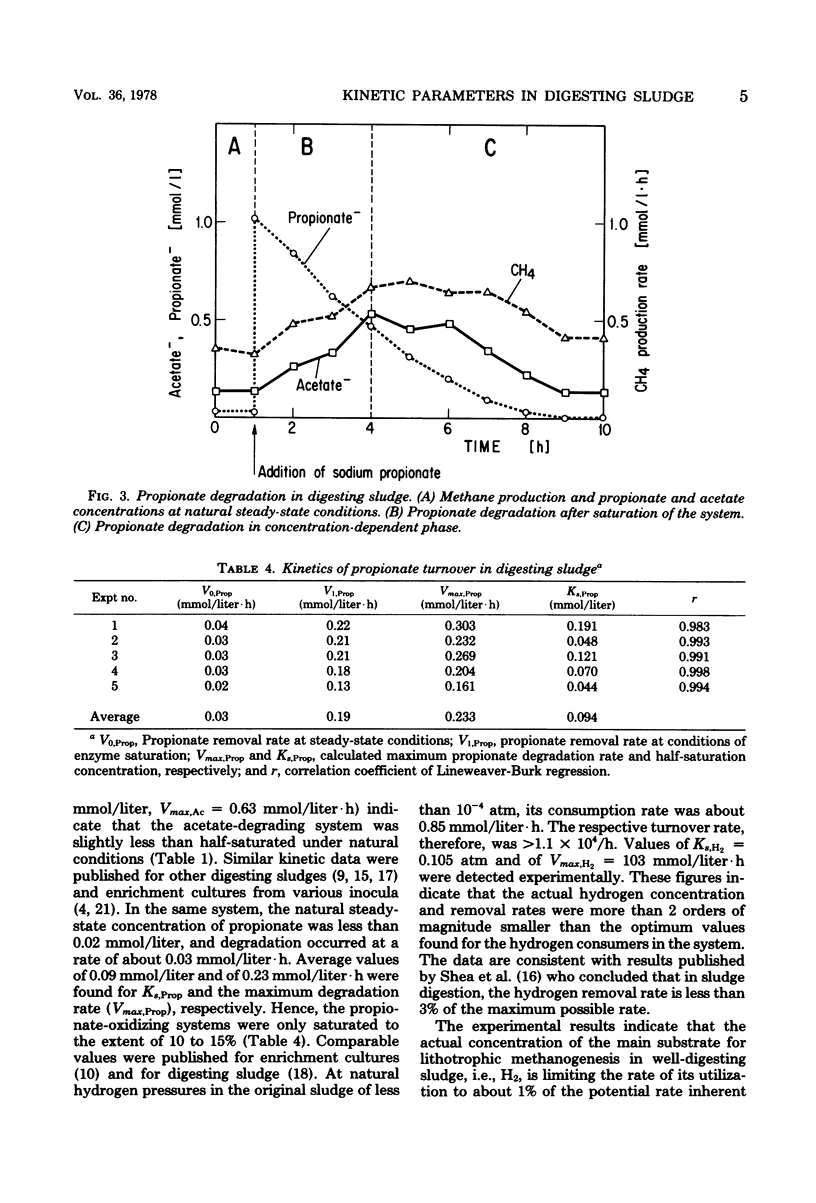
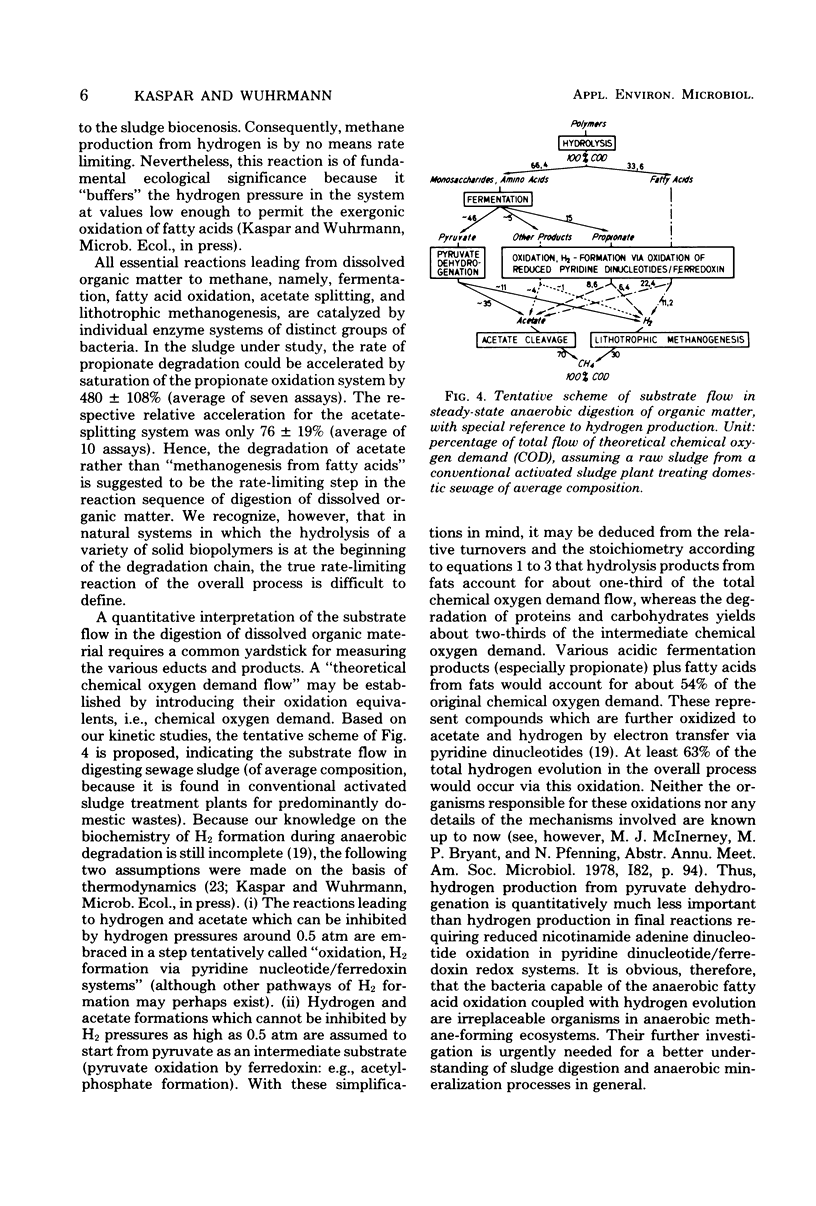
The kinetics of propionate degradation, acetate splitting, and hydrogen consumption in digesting sludge were investigated in a lab-scale digester. At natural steady-state conditions, the acetate-splitting systems in well-digested sludge were about half saturated. Propionate-degrading systems were saturated to only 10 to 15%, and hydrogen removal was less than 1% of the maximum possible rate. It was concluded that acetate splitting rather than "methanogenesis from fatty acids" is the rate-limiting reaction in the anaerobic degradation of dissolved organic matter and that a methoanogenic anaerobic ecosystem is stabilized by its large unused capacity of hydrogen consumption which is "buffering" the partial pressure of dissolved hydrogen in the system at sufficiently low values to permit rapid fatty acid oxidation. A tentative scheme of the substrate flow in sludge digestion is presented. It suggests that acid formation coupled with hydrogen formation via pyridine dinucleotide oxidation yields the immediate substrates, namely acetate and hydrogen, for about 54% of the total methanogenesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryant M. P., Wolin E. A., Wolin M. J., Wolfe R. S. Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch Mikrobiol. 1967;59(1):20–31. doi: 10.1007/BF00406313. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Conrad J. R., Klass D. L. Anaerobic acidogenesis of wastewater sludge. J Water Pollut Control Fed. 1975 Jan;47(1):30–45. [PubMed] [Google Scholar]
- Smith P. H., Mah R. A. Kinetics of acetate metabolism during sludge digestion. Appl Microbiol. 1966 May;14(3):368–371. doi: 10.1128/am.14.3.368-371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe R. S. Microbial formation of methane. Adv Microb Physiol. 1971;6:107–146. doi: 10.1016/s0065-2911(08)60068-5. [DOI] [PubMed] [Google Scholar]
- van den Berg L., Patel G. B., Clark D. S., Lentz C. P. Factors affecting rate of methane formation from acetic acid by enriched methanogenic cultures. Can J Microbiol. 1976 Sep;22(9):1312–1319. doi: 10.1139/m76-194. [DOI] [PubMed] [Google Scholar]


