Abstract
CCA-adding enzymes are specialized polymerases that add a specific sequence (C-C-A) to tRNA 3′ ends without requiring a nucleic acid template. In some organisms, CCA synthesis is accomplished by the collaboration of evolutionary closely related enzymes with partial activities (CC and A addition). These enzymes carry all known motifs of the catalytic core found in CCA-adding enzymes. Therefore, it is a mystery why these polymerases are restricted in their activity and do not synthesize a complete CCA terminus. Here, a region located outside of the conserved motifs was identified that is missing in CC-adding enzymes. When recombinantly introduced from a CCA-adding enzyme, the region restores full CCA-adding activity in the resulting chimera. Correspondingly, deleting the region in a CCA-adding enzyme abolishes the A-incorporating activity, also leading to CC addition. The presence of the deletion was used to predict the CC-adding activity of putative bacterial tRNA nucleotidyltransferases. Indeed, two such enzymes were experimentally identified as CC-adding enzymes, indicating that the existence of the deletion is a hallmark for this activity. Furthermore, phylogenetic analysis of identified and putative CC-adding enzymes indicates that this type of tRNA nucleotidyltransferases emerged several times during evolution. Obviously, these enzymes descend from CCA-adding enzymes, where the occurrence of the deletion led to the restricted activity of CC addition. A-adding enzymes, however, seem to represent a monophyletic group that might also be ancestral to CCA-adding enzymes. Yet, experimental data indicate that it is possible that A-adding activities also evolved from CCA-adding enzymes by the occurrence of individual point mutations.
Keywords: tRNA maturation, CCA-adding enzyme, flexible loop
Nucleotidyltransferases are a family of enzymes that add nucleotides to substrates like nucleic acids, proteins, antibiotics, and others (1, 2). Prominent members are tRNA nucleotidyltransferases (CCA-adding enzymes) with a unique template-independent mechanism of polymerization. These enzymes are responsible for generating and maintaining the nucleotide triplet CCA at the tRNA 3′ end. This sequence is an essential prerequisite for tRNAs to be charged with the corresponding amino acid and to participate in translation (3). CCA-adding enzymes can be divided into two subclasses with a common signature motif (4). The catalytic core of class II enzymes (representing eubacterial and eukaryotic CCA-adding enzymes) consists of several highly conserved motifs that determine nucleotide specificity and catalysis (Fig. 1A) (4–7). Whereas motif A (common signature motif for all nucleotidyltransferases) includes two carboxylates involved in catalysis and the binding site of the triphosphate moiety of incoming NTPs, motif B is involved in ribose recognition. Nucleotide selection is accomplished by motif D, carrying a set of amino acids (EDxxR) which form Watson–Crick-like hydrogen bonds with the incoming base, acting thereby as an amino acid template (6). Motifs C and E have connecting and stabilizing functions.
Fig. 1.
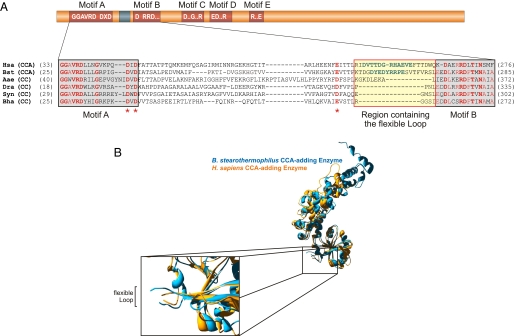
A flexible region is present in CCA-adding enzymes but not in CC-adding enzymes. (A) Alignment of CC-adding enzymes with CCA-adding enzymes of known crystal structures. (Upper) The catalytic core of class II nucleotidyltransferases consists of five conserved motifs (red boxes). (Lower) Alignment of the region between motifs A and B. Conserved positions are indicated in red, and the asterisks designate the three highly conserved carboxylates involved in catalysis. The flexible loop is indicated in green [CCA-adding enzymes (CCA)]. CC-adding enzymes (CC) share a deletion in the corresponding region, whereas other elements, including the conserved carboxylates, are present. Hsa, H. sapiens; Bst, B. stearothermophilus; Aae, A. aeolicus; Dra, D. radiodurans; Syn, Synechocystis sp.; Bha, B. halodurans. (B) Overlay of crystal structures of CCA-adding enzymes. In the N-terminal domains, the position of the flexible loop highly disordered in the crystals is indicated as a gap. Structure overlays were done by using Protein Data Bank files 1OU5 and 1MIV and Swiss Pdb Viewer 3.7. Optimization of the graphical presentation was done by using POV-Ray 3.6.
Surprisingly, these motifs for CCA addition are also present in a subset of bacterial tRNA nucleotidyltransferases that exert only partial activities (CC and A addition) and collaborate to synthesize a complete CCA end (8, 9). This indicates that there must be other, not yet identified features responsible for the restricted nucleotide incorporation of these closely related enzymes. Here, it is shown that CC-adding enzymes share a deletion of a flexible region close to the catalytic core. When introduced from a CCA-adding enzyme, the region restores complete CCA-adding activity in the chimeric protein. Correspondingly, deleting the region converts a CCA-adding enzyme into a CC-adding enzyme. In addition, the presence of this deletion allowed us to anticipate the activity of unidentified nucleotidyltransferases in Thermus thermophilus and Bacillus clausii as new CC-adding enzymes. Alignments and phylogenetic analyses predict further CC-adding enzymes, and biochemical data suggest that these proteins descend from ancestral CCA-adding enzymes.
Results
CC-Adding Enzymes Share the Deletion of a Short Flexible Loop Region.
In Aquifex aeolicus, Deinococcus radiodurans, Bacillus halodurans, and Synechocystis sp., CCA synthesis is catalyzed by two nucleotidyltransferases (CC-adding and A-adding enzyme) in a stepwise addition of two C and one A residues (8–10). Most CC-adding enzymes show some deviation in the amino acid template EDxxR, where the first position (E) is replaced by R, A, or D, respectively. Yet, this discrepancy is not responsible for the restriction to CC addition, because restoration of the position in the A. aeolicus protein did not lead to an enzyme capable of complete CCA addition (data not shown). Furthermore, the CC-adding enzyme of B. halodurans carries the complete EDxxR template, still its activity is restricted to CC addition (10). Therefore, there must be additional deviations from the CCA-enzyme consensus sequence leading to such a restriction in activity.
One such element was found in the Escherichia coli CCA-adding enzyme, where a point mutation at position 70, replacing G by D, dramatically reduced AMP incorporation but did not affect addition of C residues, leading to an activity that corresponds to that of CC-adding enzymes (11). G70 is located in a region that seems to be a highly flexible loop, because it is not resolved in crystal structures of the enzymes of Homo sapiens and Bacillus stearothermophilus (Fig. 1B) (6, 12). Furthermore, additional mutations in corresponding regions of human as well as E. coli CCA-adding enzymes also interfere dramatically with A addition (data not shown). Such a flexible loop was also identified in the A-adding enzyme of A. aeolicus, where point mutations show a similar effect (13). Because this region is obviously essential for A incorporation, a sequence alignment of the two CCA-adding enzymes of known crystal structure (H. sapiens, B. stearothermophilus) and identified CC-adding enzymes was performed (Fig. 1A). Because former whole sequence alignments did not identify any features specific for CC-adding enzymes (6, 9), the alignment was restricted to the region surrounding the loop position. Carrying motifs A and B as well as the conserved putative third carboxylate involved in catalysis (6, 12), this specific region allows a refined and improved alignment of individual sequences. As shown in Fig. 1A, the new alignment revealed that CC-adding enzymes carry a deletion of 12–17 aa at a position corresponding to the flexible loop found in CCA-adding enzymes, indicating that this deletion is a conserved feature of these polymerases.
Restoration of CCA-Adding Activity in a CC-Adding Enzyme.
Because mutations within the flexible loop interfere with A addition, it was assumed that the deletion identified in CC-adding enzymes is responsible for the inability to incorporate AMP. To address this question, the corresponding region of the CCA-adding enzyme of Bacillus subtilis (14) was introduced into the CC-adding enzyme of B. halodurans. These two enzymes were chosen because they show a high sequence similarity (10), which increases the probability of catalytically active chimeric constructs, as it was successfully shown for other nucleotidyltransferase chimeras (5). For insertion, two highly conserved amino acid residues present in CC- as well as CCA-adding enzymes (E79, representing the postulated third catalytic carboxylate, and E89 in the B. halodurans enzyme) flanking the loop region were chosen as fusion positions. Furthermore, to reduce the risk of incompatible loop insertions leading to inactive or insoluble proteins, additional chimeras with different fusion positions were generated (Fig. 2). Recombinantly expressed and purified proteins were tested by using radioactively labeled tRNA lacking a CCA terminus (substrate for a complete CCA addition) or ending with two C residues (tRNA-CC; substrate for addition of the terminal A). Reaction products were separated by PAGE and visualized by autoradiography. Whereas parental enzymes showed the expected activities (CCA and CC addition, respectively), all chimeric enzymes exhibited a new reaction (for reasons of clarity, only reaction products of chimera 1 are shown; Fig. 3A). Depending on the tRNA substrate, the chimeras added either three residues (Fig. 3A Left) or specifically incorporated a single A to complete the CCA terminus (Fig. 3A Right). After isolation, 3′ ends of the reaction products were ligated to an oligonucleotide, amplified by RT-PCR, and cloned. Sequence analysis of clones representing individual reaction products confirmed the correct addition of complete CCA triplets with varying efficiencies, depending on the individual chimeras (50–93%; data not shown). Therefore, the chimeras carrying the flexible loop of the CCA-adding enzyme in the context of a CC-adding enzyme showed an activity and accuracy identical to that of a bona fide CCA-adding enzyme, with a specific addition of CTP and ATP, whereas UTP and GTP were not accepted. The fact that the various chimeras show different efficiencies in the catalyzed reaction can be explained by differences of the transplanted loop regions. Furthermore, many chimeric proteins have lower catalytic activity compared to wild-type enzymes (5), because the transplanted elements were not optimized for the context of the new enzyme during evolution. Therefore, a comparative kinetic analysis with an original CCA-adding enzyme would not be informative. Nevertheless, the observed gain of function indicates that the loop region is an essential element for complete CCA addition.
Fig. 2.
Construction of enzyme chimeras carrying the flexible loop of a CCA-adding enzyme. The flexible region (green) of the B. subtilis CCA-adding enzyme [Bsu(CCA)] was transplanted into the corresponding position of the B. halodurans CC-adding enzyme [Bha(CC)]. For compatibility reasons, conserved amino acids (underlined) located before and after the deletion were used as fusion positions in chimera 1. To further reduce the risk of generating insoluble chimeras, additional fusion positions were chosen, leading to chimeras 2–4. Orange helps to distinguish between CC-adding enzyme (gray bar) and CCA-adding enzyme (orange bar, also shown in Fig. 1). Red and pink bars represent the motifs of the catalytic core.
Fig. 3.
The flexible loop is required for the A-adding activity in tRNA nucleotidyltransferases. (A) The chimeric protein displays a bona fide CCA-adding activity. As a representative example, reactions catalyzed by chimera 1 are shown. Activities of chimeras 2–4 are identical to the presented reaction. (Left) CCA addition. The chimera incorporated three nucleotides into a tRNA without a CCA end, leading to a reduced electrophoretic mobility of the tRNA identical to the reaction product of the CCA-adding enzyme. The original CC-adding enzyme, however, incorporated only two C residues to the tRNA substrate, documented by a lower migration position of the product. (Right) Addition of the terminal A residue. When the tRNA carried two C residues of the CCA end (tRNA-CC), the chimera specifically added the terminal A residue, whereas other nucleotides were not accepted. This highly selective reaction is comparable to the activity of the CCA-adding enzyme, whereas the CC-adding enzyme could not incorporate any additional nucleotide. Bsu, B. subtilis; Bha, B. halodurans; M, mock incubation of tRNA without enzyme. (B) Enzymatic activity of the E. coli (Eco) tRNA nucleotidyltransferase with a deletion of the flexible loop. Whereas the wild-type CCA-adding enzyme displays a complete CCA synthesis, the deletion variant [lacking amino acids 65–74 in the loop region (Δ Loop)] adds only two nucleotides. Analysis of the reaction products revealed that exclusively two C residues were added, whereas incorporation of the terminal A was not observed. Therefore, the presence of a flexible loop is essential for the incorporation of the terminal A residue. M, mock incubation.
To further analyze whether the loop deletion is sufficient to restrict the enzymatic activity to CC addition, a corresponding region (positions 65–74) was deleted in the E. coli CCA-adding enzyme, while all conserved elements remained intact. When tested on tRNA substrates, the resulting deletion variant catalyzed the addition of two C residues, whereas A addition was not observed (Fig. 3B). On a substrate tRNA ending with CC, this enzyme variant did not incorporate any nucleotide (data not shown). Therefore, this loss of function due to the loop removal is consistent with the observed gain of function in the loop insertion chimeras, corroborating the observation that such a simple deletion is responsible for the restricted catalytic properties of CC-adding enzymes.
A Hallmark for CC-Adding Enzymes.
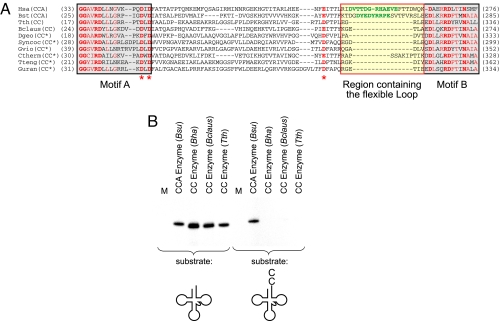
If the deletion is indeed a hallmark of CC-adding enzymes, it should be possible to predict the activity of unknown nucleotidyltransferases and to identify new CC-adding enzymes. To test this idea, the region between motifs A and B of a series of putative bacterial nucleotidyltransferases was aligned with the corresponding region of tRNA nucleotidyltransferases of known activity, according to the alignment procedure mentioned above. Eight sequences showed a deletion similar to those identified in the known CC-adding enzymes, whereas all other elements (motifs A and B, conserved carboxylates) were present in these proteins (Fig. 4A). As representative members of this group, the enzymes of T. thermophilus and B. clausii were further characterized. In the SwissProt database, both proteins are described as CCA-adding enzyme/poly(A) polymerase (T. thermophilus; accession no. Q72K91) or CCA-adding enzyme (B. clausii; accession no. Q5WGA1). The corresponding ORFs were cloned and recombinantly expressed in E. coli. Resulting proteins were purified and incubated with unlabeled substrates for either complete CCA addition (tRNA without CCA end) or addition of the terminal A (tRNA ending with CC). The offered NTP mix contained radioactively labeled CTP (to detect C incorporation) or ATP (to detect A incorporation), respectively. Indeed, both enzymes showed the typical incorporation of two C residues but failed to add the terminal A to the tRNA substrates (Fig. 4B). Therefore, these proteins represent two previously unrecognized CC-adding enzymes. According to these results, the existence of the loop deletion is a characteristic feature of CC-adding enzymes and can be used to predict their activity.
Fig. 4.
An alignment predicts further CC-adding enzymes. (A) Sequence alignment with CCA-adding enzymes reveals the existence of a loop deletion in several unidentified nucleotidyltransferases. The flexible loop located between motifs A and B (invariant positions in bold red characters; semiconserved positions in red characters; highly conserved catalytic carboxylates labeled by the red asterisks) is indicated by the yellow box. Flexible loops of CCA-adding enzymes of known crystal structure [H. sapiens (Hsa) and B. stearothermophilus (Bst)] are presented in green. Unidentified nucleotidyltransferases carry deletions that correspond to the observed situation in CC-adding enzymes, suggesting that these proteins are also CC-adding enzymes [asterisks indicate putative CC-adding enzymes; CC-adding activity of T. thermophilus (Tth) and B. clausii (Bclaus) enzymes was verified in B]. Dgeo, Deinococcus geothermalis; Syncoc, Synechococcus sp.; Gvio, Gloeobacter violaceus; Ctherm, C. thermocellum; Tteng, Thermoanaerobacter tengcongensis; Guran, G. uraniumreducens. (B) Nucleotidyltransferases from T. thermophilus and B. clausii are CC-adding enzymes. Unlabeled tRNA transcripts (tRNA, Left; tRNA-CC, Right) and an NTP mix with labeled CTP (Left) or ATP (Right) were offered as substrates. The T. thermophilus (Tth) and B. clausii (Bclaus) enzymes carrying similar deletions incorporate two C residues into a tRNA without CCA end (as the CC-adding enzyme from B. halodurans does), whereas the CCA-adding enzyme from B. subtilis (Bsu) synthesizes a complete CCA terminus (Left). On a tRNA ending with CC, only the CCA-adding enzyme incorporated the terminal A residue, whereas the CC-adding enzymes did not incorporate any nucleotide (Right). Bha, B. halodurans; M, mock incubation without enzyme. This result demonstrates that the existence of a loop deletion correlates with CC-adding activity.
Phylogenetic Distribution of tRNA Nucleotidyltransferases.
To clarify the evolutionary relation of the analyzed proteins, a phylogenetic analysis was performed, based on complete protein sequences of identified and putative CC- and A-adding enzymes as well as CCA-adding enzymes (Fig. 5). Five putative CC-adding enzymes are found in a single branch of the tree, where they cluster with identified CC-adding enzymes. However, one predicted (Clostridium thermocellum) as well as two identified CC-adding enzymes (B. clausii, B. halodurans) cluster in a different branch with CCA-adding enzymes, indicating a second, independent evolutionary origin of this type of enzymes.
Fig. 5.
Phylogenetic analysis of tRNA nucleotidyltransferases. Neighbor-joining tree based on a whole sequence alignment. Numbers indicate bootstrap values. CCA, identified CCA-adding enzymes; CC, identified CC-adding enzymes; A, identified A-adding enzymes; CC*, putative CC-adding enzymes with loop deletion; A*, putative A-adding enzymes found in the genomes of organisms carrying putative CC-adding enzymes (CC*). Syn, Synechocystis sp.; Aae, A. aeolicus; Dra, D. radiodurans. Abbreviations for other organisms are indicated in the legend of Fig. 4. All known and putative A-adding enzymes are found in one branch of the tree (blue), indicating a monophyletic origin. CC-adding enzymes, however, are found in two independent branches. One branch (red) exclusively carries identified and putative CC-adding enzymes, whereas a second branch (green) shows CCA- as well as some (identified and putative) CC-adding enzymes. This suggests that CC-adding enzymes arose in two independent events during evolution. The most likely mechanism responsible for this event is the occurrence of a deletion in the flexible loop region of ancestral CCA-adding enzymes, leading to the emergence of CC-adding enzymes.
Furthermore, in organisms where putative CC-adding enzymes were identified, a second type of nucleotidyltransferase sequence was found. These enzymes carry a specific element common to poly(A) polymerases and A-adding enzymes (downstream motif sRxxxExxxhh in helix J; s, small residue, h, hydrophobic; x, any amino acid) (15). Indeed, when tested as a recombinant enzyme in vitro, the corresponding protein of T. thermophilus could be identified as a genuine A-adding enzyme, corroborating the prediction (data not shown). Accordingly, this organism is a further species with two nucleotidyltransferases collaborating in CCA addition. Interestingly, and in contrast to the CC-adding enzymes, the phylogenetic analysis of A-adding enzymes (seven putative and five identified) shows that these proteins are summarized in one branch of the tree, indicating a monophyletic origin (Fig. 5).
Discussion
The Absence of the Flexible Loop Region Is a Common Element in CC-Adding Enzymes.
The data shown here indicate that the deletion of a flexible loop in the N-terminal domain is a shared feature in CC-adding enzymes. Because mutations in corresponding regions of CCA- and A-adding enzymes also interfere with AMP incorporation, the presence of this flexible loop seems to be an essential prerequisite for A addition. This is supported by the observation that a corresponding deletion converts a CCA-adding enzyme into a nucleotidyltransferase with restricted activity identical to that of CC-adding enzymes. Despite its functional importance, this region shows high sequence variation and does not display a consensus sequence in CCA-adding enzymes as other important motifs. Consequently, it is highly unlikely that the loop is required for ATP binding, as it was discussed for the E. coli enzyme, where the region shows similarity to nucleotide-binding P-loops (11, 16, 17). This is corroborated by the observation that the G70D mutation in the E. coli enzyme (11) has no influence on Km for ATP (18). Rather, the function of the loop might be an indirect one. Because the switch from C to A addition requires a structural rearrangement of the amino acid template (6, 13, 19), it is conceivable that such a flexible region is required as a hinge for reorganization of the NTP binding pocket during CCA synthesis. Similar loops connecting individual movable regions are described as essential elements of many proteins interacting with specific ligands (20, 21).
Moreover, the insertion of the loop region into a CC-adding enzyme regenerated a further important element involved in A addition: Replacements of R79 and E81 in the A-adding enzyme of A. aeolicus interfere with enzymatic activity (13). In the corresponding crystal structure, R79 forms a hydrogen bond to the penultimate C residue (C75) of the tRNA, which might assist a proper positioning of the tRNA 3′ end for A addition (13). Therefore, a movement of the loop could influence the correct positioning of these residues and affect their contribution to A addition. Interestingly, these residues are presumably also conserved in CCA-adding enzymes (G. Martin, personal communication), and replacements by alanine restrict the activity of the E. coli as well as the human CCA-adding enzyme to CC-addition, documenting their functional importance for A incorporation (data not shown). The loop regions inserted into the CC-adding enzyme of B. halodurans restored the equivalent residues R84 and E86 in the resulting chimeras, contributing to the observed gain of function in A addition. Yet, the presence of these residues alone, without a flexible loop, is not sufficient to promote A addition, as the newly identified CC-adding enzyme of B. clausii carries corresponding residues (K83 and E85; furthermore, the putative CC-adding enzyme of Geobacter uraniumreducens also carries similar positions R99 and E101; Fig. 4). This is further demonstrated by the activity of the loop deletion variant of the E. coli CCA-adding enzyme. The analogous positions R62 and E64 are present in this variant, yet it is not able to incorporate the terminal A residue, underscoring the functional importance of the flexible loop. Obviously, the gain of function identified in the described chimeric enzymes is the result of the combined introduction of both flexible loop as well as residues R84 and E86 from the B. subtilis CCA-adding enzyme.
Evolutionary Considerations.
The phylogenetic analysis in Fig. 5 shows that some of the CC-adding enzymes are more closely related to CCA-adding enzymes than other CC-adding enzymes. Accordingly, the nucleotidyltransferase sequence found in B. clausii is described as a CCA-adding enzyme in the SwissProt database, whereas biochemical characterization identified it as a CC-adding enzyme. These facts indicate that CC-adding enzymes evolved in at least two independent events. Furthermore, the close relation to CCA-adding enzymes supports the idea that the deletion of the loop region converted an ancestral CCA-adding enzyme into a CC-adding enzyme. This is corroborated by the deletion variant of the E. coli enzyme and by the fact that transplantation of the loop region from the B. subtilis CCA-adding enzyme into the CC-adding enzyme of B. halodurans resulted in an enzyme capable of complete CCA addition. Nevertheless, it is possible that not all modern CC-adding enzymes can be converted into a CCA-adding enzyme by such insertions, because it is likely that some of these enzymes accumulated further mutations during evolution that also might interfere with A addition.
Although it is also discussed that CC- and A-adding enzymes represent ancestral enzymatic activities that evolved into CCA-adding enzymes (8), the observations presented by Cho et al. (22) are more consistent with a CCA-adding enzyme as the ancestral protein. If the primordial form was indeed a CC-adding enzyme, the evolution of CCA-adding activities would have required a position-specific insertion of the loop region that led to a gain of function. In contrast, the emergence of a deletion causing a loss of function and restricting an ancestral CCA-adding activity to CC addition is much more likely and is supported by the general observation that deletions are much more frequent than insertions (23–25). Therefore, the biochemical data presented here support the evolutionary scenario of ancestral CCA-adding enzymes that evolved into CC-adding enzymes by the occurrence of the described deletion.
The A-adding enzymes, on the other hand, seem to be of monophyletic origin. Whether these enzymes represent progenitors of modern CCA-adding enzymes or whether they descend from those is not clear yet. A common deletion comparable to that found in CC-adding enzymes could not be identified. However, experimental data support the possibility that a CCA-adding enzyme could have accumulated point mutations that abolish C incorporation but are compatible with an A-adding activity. In the human CCA-adding enzyme, replacements at position 81 and 165 led to activities with impairments in the addition of C residues, whereas A addition was not affected (data not shown). Whereas replacement T81V led to an enzyme with impaired addition of the first C residue (only the two terminal positions CA were incorporated into a tRNA-C substrate), mutation D165A, located in the amino acid template, interfered with the incorporation of the second C (only one C residue was incorporated on tRNA without CCA end, and the terminal A residue was added to tRNA ending with CC). Therefore, it is possible that similar events led to the occurrence of A-adding enzymes. On the other hand, one cannot rule out that A-adding enzymes came first and had a different or additional unknown function in the cell. Accumulation of certain mutations then led to the appearance of CCA-adding enzymes that in some cases further evolved into CC-adding enzymes.
The fact that prokaryotic as well as eukaryotic CCA-adding enzymes can be converted into proteins with restricted activities indicates that such an evolution of tRNA nucleotidyltransferases might be a universal mechanism that could also take place in organisms other than bacteria. Accordingly, a CC-adding enzyme was observed in mammals, resulting from alternative mRNA splicing (19). Although it is conceivable that in some bacteria, a selectively neutral combination of CC- and A-adding enzymes might have replaced the single CCA-adding enzymes, the coexistence of a CC- and a CCA-adding enzyme in mammals in not understood yet.
Taken together, the restored CCA-adding activity in a CC-adding enzyme and the phylogenetic analysis are a step forward to understand the mysterious evolution of CC-adding enzymes. Although criteria common for CCA- and CC-adding enzymes were already described (15), the deletion of the flexible loop seems to be a unique identity element for bacterial CC-adding enzymes. Furthermore, phylogenetic analysis indicates that collaborative CCA addition catalyzed by CC- and A-adding enzymes seems to be more widespread in bacteria than previously expected. In addition, the data not only support the idea of an ancestral CCA-adding enzyme but also indicate that nucleotidyltransferases are composed of individual modules that can be replaced by corresponding elements of related enzymes (5). Such combinations lead to proteins with new and often unexpected activities, ranging from partial to complete CCA addition.
Materials and Methods
Construction of Recombinant Clones.
Nucleotidyltransferase genes of E. coli, B. subtilis (DSM 10), B. halodurans (DSM 497), B. clausii (DSM 8716), and T. thermophilus HB27 (DSM 7039) were amplified and cloned into pET-30 Ek/LIC (Novagen) or pTrcHis vectors (Invitrogen).
For chimeras, indicated regions of the B. subtilis CCA-adding enzyme were inserted at the corresponding position of the B. halodurans CC-adding enzyme according to the QuikChange mutagenesis protocol (Stratagene). This procedure was also used to delete the flexible loop region in the E. coli enzyme. Correct assembly was confirmed by sequence analysis (ABI Prism 3730; Amersham Biosciences).
Protein Expression and Purification.
Enzymes were overexpressed in E. coli BL21(DE3) or E. coli ArcticExpress (Stratagene). Transformed BL21(DE3) cells were grown at 37°C (30°C for T. thermophilus enzymes) in LB containing 30 μg/ml kanamycin. Expression was induced by addition of isopropyl β-d-thiogalactoside (IPTG) to a final concentration of 1 mM. After 3–14 h incubation, cells were harvested and lysed by sonication in ice-cold 50 mM Tris·HCl (pH 7.8)/10 mM MgCl2/0.5 M KCl/6 mM 2-mercaptoethanol/5% glycerol or 50 mM Tris·HCl (pH 7.9)/5 mM MgCl2/150 mM NaCl/1 mM DTT/30 mM imidazole. ArcticExpress cells were grown for 24–48 h at 12°C after IPTG induction and then harvested and lysed by sonication in ice-cold 20 mM Tris·HCl (pH 7.6)/0.5 M NaCl/5 mM MgCl2/5 mM imidazole/100 mg/ml lysozyme.
Soluble recombinant proteins were purified by FPLC on 1 ml HisTrap HP columns (GE Healthcare) and eluted with 500 mM imidazole. Purified enzymes were dialyzed against buffer 1 [50 mM Tris·HCl (pH 7.8)/10 mM MgCl2/200 mM KCl/6 mM 2-mercaptoethanol/10% glycerol], buffer 2 [60 mM Hepes/KOH (pH 7.6)/30 mM KCl/6 mM MgCl2], or buffer 3 [for E. coli enzyme; 20 mM Tris·HCl (pH 7.9)/5 mM MgCl2/20% glycerol/1 mM DTT].
Preparation of tRNA Substrates.
Yeast tRNAPhe lacking CCA terminus or ending with two C residues (tRNA-CC) was prepared as described (26, 27). In some cases, [α-33P]UTP was added to the transcription to obtain labeled transcripts.
Enzyme Activity Assays.
33P-labeled tRNA (5 pmol) were incubated for up to 2 h at 30°C or 65°C (T. thermophilus A-adding enzyme) with up to 200 ng of recombinant enzyme in the presence of all four (1 mM each) or individual NTPs (1 mM) in 20 μl of 30 mM Hepes/KOH (pH 7.6)/6 mM MgCl2/30 mM KCl/2 mM DTT. In the case of unlabeled tRNA substrates, the NTP mix was spiked with either [α-32P]ATP (34 nM) or [α-32P]CTP (34 nM), depending on the transcript (tRNA without CCA end or tRNA ending with CC). Reaction products were separated by denaturing PAGE and visualized by autoradiography.
Sequence Analysis of Reaction Products.
tRNA 3′ ends were analyzed as described (5).
Phylogenetic Analysis.
Protein sequences were aligned by using ClustalW (www.ebi.ac.uk/Tools/clustalw/) with default parameters. Sequences of human and B. stearothermophilus enzymes were further adjusted by hand according to the crystal structure overlays. Phylogenetic analysis of whole sequence alignment was performed by using PHYLIP 3.6a3 (28, 29). Bootstrapping was replicated 1,000 times with randomized input. Phylogenetic trees were calculated by using NEIGHBOR. DRAWTREE was used to draw the consensus tree.
Acknowledgments.
We thank P. Braun, F. Butter, J. M. Good, and J. Kelso for valuable discussions and S. Bonin for expert technical assistance. This work was supported by Deutsche Forschungsgemeinschaft Grants Mo 634/2-2 and Mo 634/3-2.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Aravind L, Koonin EV. DNA polymerase beta-like nucleotidyltransferase superfamily: Identification of three new families, classification and evolutionary history. Nucleic Acids Res. 1999;27:1609–1618. doi: 10.1093/nar/27.7.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holm L, Sander C. DNA polymerase beta belongs to an ancient nucleotidyltransferase superfamily. Trends Biochem Sci. 1995;20:345–347. doi: 10.1016/s0968-0004(00)89071-4. [DOI] [PubMed] [Google Scholar]
- 3.Sprinzl M, Cramer F. The -C-C-A end of tRNA and its role in protein biosynthesis. Prog Nucleic Acid Res Mol Biol. 1979;22:1–69. doi: 10.1016/s0079-6603(08)60798-9. [DOI] [PubMed] [Google Scholar]
- 4.Yue D, Maizels N, Weiner AM. CCA-adding enzymes and poly(A) polymerases are all members of the same nucleotidyltransferase superfamily: Characterization of the CCA-adding enzyme from the archaeal hyperthermophile Sulfolobus shibatae. RNA. 1996;2:895–908. [PMC free article] [PubMed] [Google Scholar]
- 5.Betat H, Rammelt C, Martin G, Mörl M. Exchange of regions between bacterial poly(A) polymerase and CCA adding enzyme generates altered specificities. Mol Cell. 2004;15:389–398. doi: 10.1016/j.molcel.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 6.Li F, et al. Crystal structures of the Bacillus stearothermophilus CCA-adding enzyme and its complexes with ATP or CTP. Cell. 2002;111:815–824. doi: 10.1016/s0092-8674(02)01115-7. [DOI] [PubMed] [Google Scholar]
- 7.Yue D, Weiner AM, Maizels N. The CCA-adding enzyme has a single active site. J Biol Chem. 1998;273:29693–29700. doi: 10.1074/jbc.273.45.29693. [DOI] [PubMed] [Google Scholar]
- 8.Tomita K, Weiner AM. Collaboration between CC- and A-adding enzymes to build and repair the 3′-terminal CCA of tRNA in Aquifex aeolicus. Science. 2001;294:1334–1336. doi: 10.1126/science.1063816. [DOI] [PubMed] [Google Scholar]
- 9.Tomita K, Weiner AM. Closely related CC- and A-adding enzymes collaborate to construct and repair the 3′-terminal CCA of tRNA in Synechocystis sp. and Deinococcus radiodurans. J Biol Chem. 2002;277:48192–48198. doi: 10.1074/jbc.M207527200. [DOI] [PubMed] [Google Scholar]
- 10.Bralley P, Chang SA, Jones GH. A phylogeny of bacterial RNA nucleotidyltransferases: Bacillus halodurans contains two tRNA nucleotidyltransferases. J Bacteriol. 2005;187:5927–5936. doi: 10.1128/JB.187.17.5927-5936.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu LQ, Cudny H, Deutscher MP. A mutation in Escherichia coli tRNA nucleotidyltransferase that affects only AMP incorporation is in a sequence often associated with nucleotide-binding proteins. J Biol Chem. 1986;261:14875–14877. [PubMed] [Google Scholar]
- 12.Augustin MA, et al. Crystal structure of the human CCA-adding enzyme: Insights into template-independent polymerization. J Mol Biol. 2003;328:985–994. doi: 10.1016/s0022-2836(03)00381-4. [DOI] [PubMed] [Google Scholar]
- 13.Tomita K, et al. Structural basis for template-independent RNA polymerization. Nature. 2004;430:700–704. doi: 10.1038/nature02712. [DOI] [PubMed] [Google Scholar]
- 14.Raynal LC, Krisch HM, Carpousis AJ. The Bacillus subtilis nucleotidyltransferase is a tRNA CCA-adding enzyme. J Bacteriol. 1998;180:6276–6282. doi: 10.1128/jb.180.23.6276-6282.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin G, Keller W. Sequence motifs that distinguish ATP(CTP):tRNA nucleotidyl transferases from eubacterial poly(A) polymerases. RNA. 2004;10:899–906. doi: 10.1261/rna.5242304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prasad GS. Glycine rich P-loop motif in deoxyuridine pyrophosphatase. Curr Protein Pept Sci. 2001;2:301–311. doi: 10.2174/1389203013381017. [DOI] [PubMed] [Google Scholar]
- 17.Saraste M, Sibbald PR, Wittinghofer A. The P-loop—a common motif in ATP- and GTP-binding proteins. Trends Biochem Sci. 1990;15:430–434. doi: 10.1016/0968-0004(90)90281-f. [DOI] [PubMed] [Google Scholar]
- 18.McGann RG, Deutscher MP. Purification and characterization of a mutant tRNA nucleotidyltransferase. Eur J Biochem. 1980;106:321–328. doi: 10.1111/j.1432-1033.1980.tb06026.x. [DOI] [PubMed] [Google Scholar]
- 19.Lizano E, Schuster J, Müller M, Kelso J, Mörl M. A splice variant of the human CCA-adding enzyme with modified activity. J Mol Biol. 2007;366:1258–1265. doi: 10.1016/j.jmb.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Kato H, et al. Flexible loop that is novel catalytic machinery in a ligase. Atomic structure and function of the loopless glutathione synthetase. Biochemistry. 1994;33:4995–4999. doi: 10.1021/bi00183a001. [DOI] [PubMed] [Google Scholar]
- 21.Kempner ES. Movable lobes and flexible loops in proteins. Structural deformations that control biochemical activity. FEBS Lett. 1993;326:4–10. doi: 10.1016/0014-5793(93)81749-p. [DOI] [PubMed] [Google Scholar]
- 22.Cho HD, Verlinde CL, Weiner AM. Reengineering CCA-adding enzymes to function as (U,G)- or dCdCdA-adding enzymes or poly(C,A) and poly(U,G) polymerases. Proc Natl Acad Sci USA. 2007;104:54–59. doi: 10.1073/pnas.0606961104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Jong WW, Ryden L. Causes of more frequent deletions than insertions in mutations and protein evolution. Nature. 1981;290:157–159. doi: 10.1038/290157a0. [DOI] [PubMed] [Google Scholar]
- 24.Petrov DA. Mutational equilibrium model of genome size evolution. Theor Pop Biol. 2002;61:531–544. doi: 10.1006/tpbi.2002.1605. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Gerstein M. Patterns of nucleotide substitution, insertion and deletion in the human genome inferred from pseudogenes. Nucleic Acids Res. 2003;31:5338–5348. doi: 10.1093/nar/gkg745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schürer H, Lang K, Schuster J, Mörl M. A universal method to produce in vitro transcripts with homogeneous 3′ ends. Nucleic Acids Res. 2002;30:e56. doi: 10.1093/nar/gnf055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mörl M, Lizano E, Willkomm DK, Hartmann RK. Production of RNA with homogeneous 5′- and 3′ -ends. In: Hartmann RK, Bindereif A, Schön A, Westhof E, editors. Handbook of RNA Biochemistry. Weinheim, Germany: Wiley-VCH; 2005. pp. 22–35. [Google Scholar]
- 28.Felsenstein J. PHYLIP—Phylogeny Inference Package (version 3.2) Cladistics. 1989;5:164–166. [Google Scholar]
- 29.Felsenstein J. PHYLIP, Phylogeny Inference Package. Seattle: Department of Genetics, Univ of Washington; 1993. Version 3.5c. [Google Scholar]