Abstract
Phosphatidylinositol lipids play diverse physiological roles, and their concentrations are tightly regulated by various kinases and phosphatases. The enzymatic activity of Ciona intestinalis voltage sensor-containing phosphatase (Ci-VSP), recently identified as a member of the PTEN (phosphatase and tensin homolog deleted on chromosome 10) family of phosphatidylinositol phosphatases, is regulated by its own voltage-sensor domain in a voltage-dependent manner. However, a detailed mechanism of Ci-VSP regulation and its substrate specificity remain unknown. Here we determined the in vitro substrate specificity of Ci-VSP by measuring the phosphoinositide phosphatase activity of the Ci-VSP cytoplasmic phosphatase domain. Despite the high degree of identity shared between the active sites of PTEN and Ci-VSP, Ci-VSP dephosphorylates not only the PTEN substrate, phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3], but also, unlike PTEN, phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2]. Enzymatic action on PI(4,5)P2 removes the phosphate at position 5 of the inositol ring, resulting in the production of phosphatidylinositol 4-phosphate [PI(4)P]. The active site Cys-X5-Arg (CX5R) sequence of Ci-VSP differs with that of PTEN only at amino acid 365 where a glycine residue in Ci-VSP is replaced by an alanine in PTEN. Ci-VSP with a G365A mutation no longer dephosphorylates PI(4,5)P2 and is not capable of inducing depolarization-dependent rundown of a PI(4,5)P2-dependent potassium channel. These results indicate that Ci-VSP is a PI(3,4,5)P3/PI(4,5)P2 phosphatase that uniquely functions in the voltage-dependent regulation of ion channels through regulation of PI(4,5)P2 levels.
Keywords: phosphoinositide; PI(4,5)P2; voltage sensor; PI(3,4,5)P3; substrate specificity
Phosphatidylinositol (PI) lipids serve structural roles in biological membranes as well as playing important roles as signaling molecules. Phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] regulates cell motility, cell shape, vesicle turnover, and membrane excitability either through directly binding to target proteins (1–3) or by mediating calcium signaling via its cleavage by phospholipase C into inositol 1,4,5-trisphosphate and diacylglycerol (4). Phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3] regulates cell proliferation, survival, and morphology (5). To exert physiological roles, the concentrations of these phosphoinositides in membranes are strictly regulated by multiple kinases and phosphatases. Recently, we identified a phosphoinositide phosphatase, Ciona intestinalis voltage sensor-containing phosphatase (Ci-VSP), consisting of an ion channel-like transmembrane domain followed by a phosphatase domain which shares sequence identity to the phosphatase and tensin homolog deleted on chromosome 10q23 (PTEN). PTEN is a well characterized PI phosphatase that dephosphorylates the phosphate on the 3′ position of PI(3,4,5)P3, resulting in generation of PI(4,5)P2 (6, 7). Ci-VSP shares overlapping substrate specificity with PTEN in that it was also shown to dephosphorylate PI(3,4,5)P3 (8).
A voltage-gated ion channel commonly consists of two parts: the N terminus (S1 to S4) functions as a voltage sensor while the C terminus (S5 to S6) functions as an ion-permeable pore (9). The transmembrane region of Ci-VSP shows significant sequence homology to the voltage-sensor domains of the conventional voltage-gated channels but does not contain the pore domain. Basic amino acids spaced periodically in the fourth transmembrane segments (S4) are known to be essential for sensing changes in the membrane potential in voltage-gated ion channels (8, 9). This pattern of basic amino acids is also conserved in the fourth transmembrane segment (S4) of Ci-VSP. Indeed, Xenopus oocytes injected with Ci-VSP cRNA showed transient “gating” currents as the readouts of the movement of S4 across the membrane in response to voltage change, demonstrating that the N terminus of Ci-VSP functions as a voltage sensor (8).
We hypothesized that the voltage-sensor domain of Ci-VSP could potentially regulate the activity of the phosphatase domain. Along these lines we were able to demonstrate that the activity of the PI(4,5)P2-sensitive potassium channel coexpressed with Ci-VSP increases at hyperpolarization and decreases at depolarization (8). This result cannot be reconciled with the known enzymatic activity of Ci-VSP, which would result in increased PI(4,5)P2 levels, thereby resulting only in activation of the potassium channel. In addition, confocal imaging of pleckstrin homology domains (PHDs) fused to GFP as detectors of PI(4,5)P2 and PI(3,4,5)P3, as well as electrophysiological measurements of potassium currents in Xenopus oocytes, showed that the concentrations of both PI(4,5)P2 and PI(3,4,5)P3 decrease during depolarization (10). Thus, we speculated that Ci-VSP may be able to dephosphorylate PI(4,5)P2 in addition to PI(3,4,5)P3.
In this study, we examine the substrate specificity of the cytoplasmic phosphatase domain of Ci-VSP and find that unlike PTEN, it can dephosphorylate a number of phospholipids including PI(4,5)P2. These in vitro results validate our in vivo channel activity and confocal imaging data described above. Furthermore, we demonstrate that Ci-VSP removes the 5′ phosphate of PI(4,5)P2, resulting in the production of phosphatidylinositol 4-phosphate [PI(4)P]. A closer examination of the Cys-X5-Arg (CX5R) sequence present at the active site of this phosphatase family shows that Ci-VSP differs from PTEN in only one position, a glycine (Gly) at position 365 replaces an alanine (Ala) in PTEN. Interestingly, when a Gly to Ala mutation is made in Ci-VSP, its phosphatase activity against PI(4,5)P2 is abrogated and this mutant, expressed in Xenopus oocytes, does not exhibit depolarization-dependent decrease of PI(4,5)P2 as reported by the activities of GIRK2 (Kir3.2) potassium channels and translocation of PHD-GFP. Therefore, we can demonstrate that changing one amino acid at the active site produces a physiologically relevant change in substrate specificity for this family of phosphatases.
Results
Comparison of the Substrate Specificities Between Ci-VSP and PTEN.
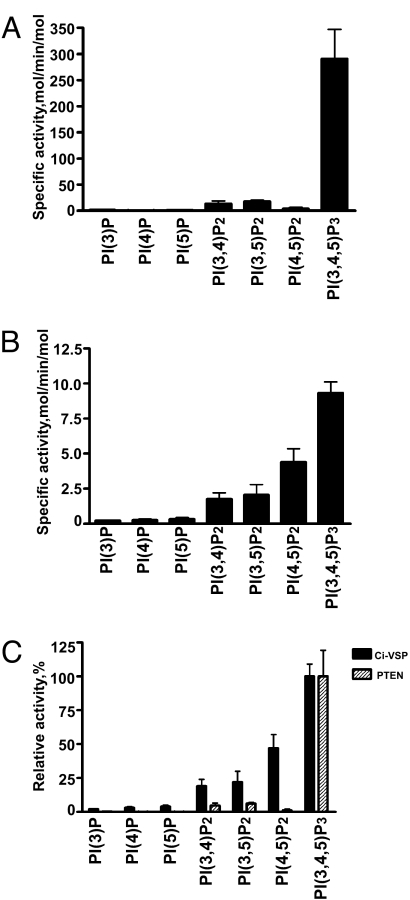
The substrate specificity of Ci-VSP was compared to that of PTEN by incubating the GST-tagged cytoplasmic domain of Ci-VSP or His-tagged PTEN with vesicles containing dipalmitoylated phosphoinositides (11). Release of phosphate was measured by malachite green analyses. As can be seen in Fig. 1, PTEN prefers PI(3,4,5)P3 as a substrate (7, 12). PTEN displays limited activity toward the other bis-phosphorylated phosphoinositides that contain a 3′ phosphate and no activity toward PI(4,5,)P2. In addition, monophosphorylated phosphoinositides do not serve as substrates for PTEN (Fig. 1A). In contrast, Ci-VSP has a broader substrate specificity. Like PTEN, Ci-VSP also preferentially dephosphorylates PI(3,4,5)P3, but unlike PTEN it harbors significant activity against the bis-phosphorylated phosphoinositides, including PI(4,5)P2 (Fig. 1B). Although Ci-VSP does dephosphorylate phosphoinositides, it is noteworthy that the specific activity of Ci-VSP is almost 10-fold lower than that of PTEN. This is probably due to our removal of the amino terminal transmembrane domains of Ci-VSP in efforts to produce soluble-tagged protein. Clearly, appropriate positioning in the membrane may be critical for optimal enzymatic activity. When the PI(3,4,5)P3 activity for both PTEN and Ci-VSP is set to 100%, it is clear that the largest difference in substrate specificity is seen for PI(4,5)P2 (Fig. 1C). Given the much higher concentration of PI(4,5)P2 in the plasma membrane, this result suggests that Ci-VSP's activity against PI(4,5)P2 may be important for its in vivo function.
Fig. 1.
Determination of Ci-VSP- and PTEN-specific activities. The specific activities of bacterial recombinant PTEN (A) and Ci-VSP (B) toward a panel of synthetic di-C16-phosphoinositides were determined by using a malachite green assay (see Materials and Methods). Reactions were carried out in triplicate, and the specific activities are represented as moles of phosphate released per minute per mole of enzyme ± SD. (C) The relative activities of Ci-VSP and PTEN for the panel of phospholipids were calculated from their specific activities divided by the specific activities against PI(3,4,5)P3 (9.33 ± 0.79 mol/min/mol for Ci-VSP and 291 ± 56 mol/min/mol for PTEN).
Ci-VSP Dephosphorylates the 5′ Phosphate of PI(4,5)P2.
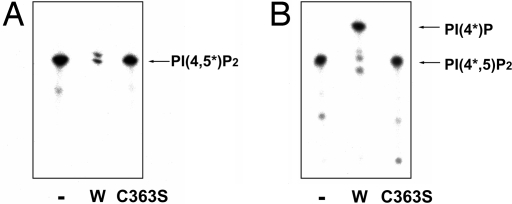
Because the malachite green assays indicate that PI(4,5)P2 is dephosphorylated by Ci-VSP, we sought to determine which phosphate on the inositol ring of PI(4,5)P2 is removed. This was accomplished by using PI(4,5)P2 labeled with 32P either on the 4′ or 5′ phosphate as a substrate for Ci-VSP. To label the 5′ position of PI(4,5)P2, we used the activity of phosphatidylinositol-4-phosphate 5-kinase (PIP5Kβ), a kinase that phosphorylates the 5′ position of PI(4)P generating PI(4,5)P2 (13). If the phosphate at the 5′ position on the inositol ring is dephosphorylated by Ci-VSP, radioactive phosphate will be released from the inositol ring and the intensity of PI(4,5*)P2, as visualized by autoradiography of the TLC analysis, will be reduced. To the contrary, if the phosphate on the 4′ position of phosphate is dephosphorylated, the intensity of the band will not be reduced but will be shifted upward corresponding to the position of PI(5*)P. The control lanes representing the input level of PI(4,5*)P2 contain no enzyme or enzyme rendered inactive by mutation of the catalytic cysteine present in the active site (Ci-VSP-C363S). Because no signal representing PI(5*)P was obtained in the presence of catalytically active Ci-VSP by using PI(4,5*)P2 as the substrate (Fig. 2A), we conclude that the 5′ position of the inositol ring of PI(4,5)P2 is dephosphorylated by Ci-VSP.
Fig. 2.
Ci-VSP dephosphorylates the 5′ position of PI(4,5)P2. (A) PI(4,5)P2 labeled with 32P on the 5′ position of the inositol ring was used as a substrate for Ci-VSP. 32P was incorporated into PI(4)P by incubating with [γ-32P]ATP and PIP5K. The intensity of the band in the lane incubated with wild-type Ci-VSP (W) was greatly reduced whereas it was not reduced in the lanes incubated with buffer (−) or Ci-VSPC363S, which does not have any phosphatase activity. (B) PI(4,5)P2 labeled on the 4′ position of the inositol ring with 32P was used as a substrate for Ci-VSP. The intensity of the band in the lane incubated with Ci-VSP (W) was not reduced compared to both the lane incubated with buffer (−) and the lane incubated with Ci-VSPC363S, but the band in the lane treated with Ci-VSP (W) migrated faster than the other lanes, showing that PI(4,5)P2 was dephosphorylated to become PI(4)P in the lane incubated with Ci-VSP.
The complementary experiment was also performed by using PI(4*,5)P2 (as the substrate) in which the phosphate at the 4′ position of the inositol ring was labeled with 32P by using type II PIP kinase (13). Using PI(4*,5)P2, the intensity of the band in the TLC analysis was not reduced but its mobility was shifted to the position expected for PI(4*)P (Fig. 2B). This provides conclusive evidence that the 5′ phosphate of PI(4,5)P2 was removed by Ci-VSP.
Active Site Gly-365 of Ci-VSP Is Critical for PI(4,5)P2 Substrate Specificity.
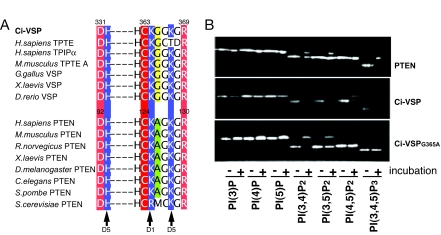
The substrate specificity of Ci-VSP is distinct from that of PTEN. The active site sequences of Ci-VSP and PTEN are very similar with the notable difference that Gly-365 of Ci-VSP (yellow) is replaced by Ala in PTEN (green) (Fig. 3A). To determine whether this Gly is responsible for the distinct substrate specificity of Ci-VSP, Gly-365 was mutated to Ala in Ci-VSP and the phosphatase activity of this mutant (Ci-VSPG365A) was compared to that of wild-type Ci-VSP and PTEN by TLC analyses. Similar to PTEN, Ci-VSPG365A dephosphorylated PI(3,4)P2 and PI(3,5)P2 and did not appreciably dephosphorylate PI(4,5)P2 (Fig. 3B). Unlike PTEN, the mutant enzyme's activity against PI(3,4,5)P3 was also reduced. This can be explained if Ci-VSP preferentially removes the 5′ phosphate from PI(3,4,5)P3, an activity which is curtailed by the Gly-to-Ala substitution, but retains its ability to dephosphorylate the 3′ phosphate. Indeed, we have evidence that Ci-VSP can remove both the 3′ and the 5′ phosphate of PI(3,4,5)P3 (data not shown). Therefore, we conclude that the Gly-365-to-Ala mutation effectively removed Ci-VSP's ability to dephosphorylate PI(4,5)P2, thereby converting the substrate specificity of Ci-VSP to one resembling PTEN.
Fig. 3.
Glycine-365 is important for the substrate specificity of Ci-VSP. (A) Alignment of PTEN and VSP active site motifs from various species. The critical catalytic residues (red or pink) and conserved invariant basic residues (blue) are highlighted. The positions of the invariant basic residues that interact with the phosphate groups on the inositol ring of phosphoinositides are indicated by arrows. The residue at 365 of Ci-VSP is Gly (yellow) whereas the corresponding site in PTEN is Ala (green). (B) Substrate specificity of PTEN (Top), Ci-VSP (Middle), and Ci-VSPG365A (Bottom). Ci-VSPG365A displays reduced activity against PI(4,5)P2 and PI(3,4,5)P3 while retaining activity against PI(3,4)P2 and PI(3,5)P2. The panels are representative of three independent experiments.
Glycine at 365 Is Critical for Ci-VSP's Capability of Regulating Activities of PI(4,5)P2-Sensitive Ion Channel.
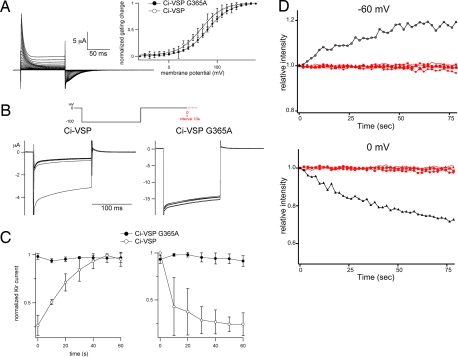
The localization of Ci-VSP to the plasma membrane, its voltage-sensing capability, and its ability to dephosphorylate PI(4,5)P2 uniquely position this phosphatase to regulate local phospholipid concentrations and therefore phospholipid-dependent channel activities. PI(4,5)P2 levels are particularly important in the regulation of several ion channels including the GIRK2 (Kir3.2) potassium channel (14–16). Therefore, one possible functional role of Ci-VSP is to regulate ion channel activities in response to membrane potential change (17). In our previous studies (8), GIRK2 activity, a potassium channel whose activity requires PI(4,5)P2, was modulated by the voltage-dependent activity of Ci-VSP in Xenopus oocytes. Depolarization-induced voltage-sensor movement results in run-down of GIRK2 channel activities (10), indicating that PI(4,5)P2 phosphatase activity is induced by depolarization. To test whether Gly-365 contributes to the modulation activities of ion channels by Ci-VSP, Ci-VSPG365A was coexpressed with GIRK2 in Xenopus oocytes, and the magnitude of the GIRK2 current was measured. The GIRK2 current did not show any significant decrease in amplitude at depolarization when Ci-VSPG365A was coexpressed (Fig. 4B Right and C), whereas the wild-type Ci-VSP induced depolarization-dependent rundown of GIRK2 channel activities (Fig. 4 B Left and C). The “gating” current was observed to a similar level in Ci-VSPG365A-expressing oocytes as those expressing wild-type Ci-VSP (Fig. 4A), ruling out the possibility that the lack of a voltage-dependent change of GIRK2 activities in the presence of Ci-VSPG365A was due to the altered expression level of the protein.
Fig. 4.
G365A mutant does not exhibit voltage-dependent modification of ion channels. (A) “Gating” currents of G365A mutant. ON and OFF “gating” currents are elicited upon depolarizing step and hyperpolarizing step, respectively. Inset shows the Q-V relationships of wild-type (n = 4) and G365A (n = 7). (B) Change of the amplitude of currents derived from GIRK2 potassium channels coexpressed with Ci-VSP in oocyte expressing wild-type Ci-VSP or the G365A mutant. Note that current amplitude does not significantly change in G365A. (C) Time-dependent change of GIRK2 current (Kir) amplitude while cells were held at −60 (Left) or 0 (Right) mV. Amplitude divided by the maximum amplitude during experiments is plotted. Values were averaged from three wild-type cells and five Ci-VSP-G365A-injected cells. (D) PI(4,5)P2 level is not significantly altered by Ci-VSP-G365A as indicated by the confocal imaging of PHD-GFP from voltage clamped oocyte coexpressing Ci-VSP-G365A (n = 4). Fluorescence intensity of GFP in a fixed area containing the contour of oocyte in each image was measured and plotted against time from the timing of initiation of voltage step either from −60 mV or 0 mV or from 0 mV to −60 mV. Different red symbols represent data from individual oocytes. Black plots are from an oocyte expressing wild-type Ci-VSP that were done in our previous work (10).
To verify that the amount of PI(4,5)P2 does not change in the presence of Ci-VSPG365A, PI(4,5)P2 levels in the plasma membrane were visualized by the cell surface translocation of the PI(4,5)P2-specific PHD fused with green fluorescence protein (GFP) under the two-microelectrode voltage clamp as previously described (10). Wild-type Ci-VSP dephosphorylates PI(4,5)P2, leading to reduction of PHD-GFP translocation to the plasma membrane (10). In contrast, Ci-VSPG365A did not exhibit such activity, and fluorescent signal was constant with time either by depolarization or hyperpolarization (Fig. 4D, red). This contrasts with wild-type Ci-VSP which reduces PI(4,5)P2 level upon depolarization (Fig. 4D, black) (10). These in vivo experiments confirm that the dephosphorylation of PI(4,5)P2 by Ci-VSP requires Gly-365.
Discussion
Despite the high degree of identity between the amino acids at the active site of PTEN and Ci-VSP, Ci-VSP showed a different substrate specificity than PTEN in that it dephosphorylates PI(4,5)P2 producing PI (4)P. Dephosphorylation of PI(4,5)P2 by Ci-VSP is consistent with the recent findings of electrophysiological studies and optical imaging studies (10). Imaging of the PHD of phospholipase C-δ tagged with GFP that is specific for binding to PI(4,5)P2 (18) indicated that PI(4,5)P2 concentration increased at hyperpolarization and decreased at depolarization. By using a PHD-GFP specific to PI(3,4,5)P3, the concentration of PI(3,4,5)P3 showed a similar change dependent on membrane potential (18). The finding that Ci-VSP dephosphorylates both PI(3,4,5)P3 and PI(4,5)P2 strongly supports the idea that Ci-VSP phosphatase activity is activated upon depolarization, as suggested by previous electrophysiological studies (10). Because Ci-VSP dephosphorylates both PI(3,4,5)P3 and PI(4,5)P2, it is possible that the activity against PI(3,4,5)P3 could also play a role in regulating channel activity. To address this, we tested the PI3 kinase inhibitor, LY294002, on potassium channel activity and found that it had no effect (data not shown). Therefore, we conclude that dephosphorylation of PI(4,5)P2 is the mechanism by which Ci-VSP regulates the activity of potassium channels.
Experiments using PI(4,5)P2 labeled with radioisotope showed that Ci-VSP dephosphorylates the phosphate at position 5 of the inositol ring of PI(4,5)P2. This activity raises the possibility that Ci-VSP removes the 5′ phosphate from PI(3,4,5)P3 to produce the signaling molecule, PI(3,4)P2. This phospholipid has been shown to activate a molecular cascade for production of reactive oxygen species by activating the p47phox subunit of NADPH oxidase (19) and plays some role in recruiting membrane proteins to subcellular compartments (20). Conversely, analogous to PTEN, Ci-VSP could remove the phosphate from the 3′ site of the inositol ring of PI(3,4,5)P3 producing its other substrate, PI(4,5)P2. Because the amount of PI(3,4,5)P3 at resting state is much smaller than that of PI(4,5)P2, the enzymatic activity of Ci-VSP would ultimately result in a decrease in the total amount of PI(4,5)P2 present during depolarization.
The difference in substrate specificity between PTEN and Ci-VSP may be attributable to a single residue, Gly-365, because mutation of glycine to alanine ablated Ci-VSP's activity against PI(4,5)P2. One interesting observation is that the inositol ring of phosphoinositides is nearly symmetrical down the axis of the ring spanning position 1 to position 4. The Ci-VSP active site lacks the alanine's methyl group and would be predicted to have a wider space for substrate binding or insertion, thus allowing more flexibility for recognizing or accommodating phosphoinositides. Given the rotational freedom of the inositol ring in PIP molecules (21) and the near symmetry of the inositol ring of the substrate along the axis spanning from position 1 to position 4, the substrate might be able to “flip over”(i.e., position 3 moves to position 5, while position 4 is unchanged). Subsequently, the substrate could be dephosphorylated at position 5 in addition to position 3. The presence of glycine at position 365 might thus be expected to alter the specificity of the phosphate preference by relaxing the restriction upon the substrate orientation, i.e., by allowing a 180° rotation, rather than by precisely shifting the specificity to a different phosphate of the inositol ring. Indeed, experimental evidence that the substrate specificity of Ci-VSP is broader than that of PTEN is presented in Fig. 1.
Mammalian VSP homologues have already been reported as TPTE (human) (22), TPIP (human) (23), and PTEN2 (mouse) (24). In the previous reports, their substrate specificities were identical to that of PTEN (23, 24). However, Gly-365 in Ci-VSP is conserved in all mammalian VSPs, suggesting that mammalian VSPs may also dephosphorylate PI(4,5)P2. A recent study indicated that PI(4,5)P2 is dephosphorylated by the teleost VSP (25). Further studies will be necessary to address whether substrate specificity of Ci-VSP is conserved in mammals.
The finding that phosphatase activity of Ci-VSP is activated at depolarization will provide a basis for us to gain physiological insights into the roles of Ci-VSP. We have reported that Ci-VSP is expressed in testis, especially in the plasma membrane of ascidian sperm flagella (8). PI(4,5)P2 is known to regulate many types of ion channels and transporters that could be abundantly expressed in sperm (23). Upon depolarization, Ci-VSP may tonically silence the activities of many ion channel species through its PI(4,5)P2 phosphatase activity (24). Alternatively, the primary role of Ci-VSP in sperm may be to increase the amount of PI(4)P. PI(4)P is known to be most abundant in Golgi membranes and possibly functions in maintaining Golgi structure both directly and/or through conversion to PI(4,5)P2 (26). PI(4)P recruits the cytoskeleton to Golgi membranes and maintains the flux of membranes moving through the organella (26). In sperm, the acrosome is generated by the Golgi and its reorganization is triggered by membrane depolarization in sea urchin (27) and mammalian sperms (28). Measurements of phosphoinositides in native sperm under distinct levels of membrane potential should address the in vivo roles of Ci-VSP.
We have uncovered a catalytic activity for Ci-VSP, i.e., the dephosphorylation of PI(4,5)P2, which can ultimately result in the regulation of PI(4,5)P2-sensitive ion channels. To our knowledge, a phosphatase whose activity is regulated by an internal voltage sensor and whose phosphatase activity would then regulate the activity of neighboring PI-sensitive ion channels has not been previously described. The mechanism by which the voltage-sensor domain transmits signals to the phosphatase domain are of interest and will undoubtedly give us insights into the structure/function relationships of this unique class of phosphatases.
Materials and Methods
Materials.
Construction of GST-Ci-VSP and PTEN-His were reported previously (8, 29). Site-directed mutagenesis was performed by using QuikChange kit (Stratagene). Primers for introduction of mutation of G365A into Ci-VSP were ACTGTAAAGCCGGGAAGGGA as the forward primer and TCCCTTCCCGGCTTTACAGT as the reverse primer.
His-tagged type II PIP kinase was provided by Robin Irvine and Jon Clarke (University of Cambridge, Cambridge, United Kingdom), myc-tagged phosphatidylinositol-4-phosphate kinase (PIP5Kβ) was provided by Yasunori Kanaho and Takeaki Yokozeki (University of Tsukuba, Japan). di-C6-NBD6-labeled phosphoinositides were purchased from Echelon Biosciences. dC16-PI(3,4,5)P3 and phosphatidylserine were from Cayman Chemicals and Avanti Polar Lipids. [γ-32P]di-C16-ATP was from GE Healthcare Bioscience.
Purification of Recombinant Proteins.
GST-tagged proteins were expressed in Escherichia coli, JM109. His-tagged PTEN and type II PIP kinase were expressed in BL21(DE3). Protein expression was induced by the addition of 1 mM isopropyl β-d-thiogalactoside at 0.4–0.8 A600 followed by overnight incubation at 16°C. Bacteria were lysed with lysozyme in 20 mM Tris·HCl (pH 8.0), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, and protease inhibitors. GST-fused proteins were purified by using Gluthatione Sepharose 4B (GE Healthcare Bioscience). His-tagged enzymes were purified by using Ni-NTA resin (Qiagen).
Thin-Layer Chromatography (TLC) Assay.
Because the mobility of each phosphoinositide on TLC is different and is determined by the number of phosphates in the inositol ring, we can evaluate phosphatase activity on each phosphoinositide by the shift of mobility in TLC analyses. Nonradioisotope experiments were done according to the previous reports (10, 11). di-C6-NBD6-phosphoinositides (1.5 μg; Echelon) were incubated with GST-fused proteins (1 μg per reaction) in the phosphatase assay buffer (100 mM Tris·HCl, pH 8.0/10 mM DTT) at 23°C for 1 h. The length of carbon chain of these phosphoinositides was shorter than that in malachite green assay (6 vs. 16). The reactions were terminated by centrifugation and supernatants were added to acetone and dried in a SpeedVac. The dried fluorescent products were resuspended in CHCl3/2-propanol/glacial acetic acid (5:5:2) and spotted onto the TLC plate (Silica Gel 60; Merck). The silica plates were treated with 1.2% solution of potassium oxalate and heated at 180°C for 1 h before use. The spotted plate was developed in a solvent system consisting of CHCl3/MeOH/acetone/glacial acetic acid/dH2O (70:50:20:20:20) and was placed on a UV illuminator to visualize the fluorescent phosphoinositide bands.
For labeling the 5′ position of PI(4,5)P2, 1,2-dipalmitoyl PI(4)P was dried with phosphatidic acid and was sonicated in the kinase buffer [50 mM Tris·HCl (pH 7.5)/10 mM Hepes-NaOH (pH 7.4)/1 mM EGTA/10 mM MgCl2] with PIP5Kβ and incubated at 37°C for 2 h (13). For labeling the 4′ position of PI(4,5)P2, 1,2-dipalmitoyl PI(5)P was incubated with type II PIP kinase in double-strength buffer [100 mM Tris·HCl (pH 7.5)/20 mM MgCl2,/160 mM KCl/4 mM EGTA] supplemented with ATP solution (1 mM ATP/200 mCi [γ-32P]ATP) at 30°C for 2 h (13). Radiolabeled phosphoinositides were extracted by the Bligh-Dyer method and checked by TLC (14). The solvent system was identical to that previously described. After development, the silica plate was dried and visualized by a BAS2000 bioimaging analyzer (Hitachi).
Malachite Green Phosphatase Assay.
di-C16 phosphoinositides (Cayman Chemicals and Echelon Biosciences) and 1-palmitoyl-2-oleoyl-phosphatidylserine (Avanti Polar Lipids) were dried together in a SpeedVac and resuspended via vortexing for 15 min and sonication for 2 min in a sonifier in 19 μl of assay buffer [100 mM Tris (pH 8.0)/10 mM DTT] to final concentrations of 200 and 1000 μM, respectively. After sonication, vesicles were passed 17 times through a Liposofast microextruder containing a 100-nm polycarbonate filter. The reactions were initiated by the addition of 100-1000 ng of GST-Ci-VSP (248–576) or 1–500 ng of PTEN-His diluted in assay buffer after prewarming at 37°C for 5 min. Reactions were quenched after 5–30 min by the addition of 20 μl of 0.1 M N-ethylmaleimide and spun at 18,000 × g for 15 min to sediment the lipid aggregates. The supernatant (25 μl) was added to 50 μl of malachite green reagent and vortexed. Samples were allowed to sit for 40 min for color development before measuring absorbance at 620 nm. The catalytically inactive mutants, C363S for Ci-VSP and C124S for PTEN, were used for the blanks of the absorbance measurements. Inorganic phosphate release was quantified by comparison to a standard curve of KH2PO4 in distilled H2O.
Electrophysiology of Xenopus Oocytes.
Surgery for isolating gonadal tissue from Xenopus laevis was performed following the guidelines of the National Institute for Physiological Sciences. Before surgery, the frogs were anesthetized in water containing 0.15% tricaine. Oocytes were removed with collagenase (1 mg/ml, S-1; Nitta Gelatin) and injected with 50 nl of cRNA solution. All of the GIRK2 cRNAs were coinjected with Gβ1 and γ1. The injected oocytes were incubated for 2–4 d at 18°C in ND96 solution (30). Measurements were done by using a “bath-clamp” amplifier (OC-725C-HV; Warner). Stimulation, data acquisition, and analysis were done by using ITC-16 AD/DA converter and Pulse software (HEKA Electronic). Intracellular microelectrodes were filled with 3 M KCl. The electrode resistance ranged from 0.1 to 0.6 MΩ. For GIRK2 currents, the bath solution contained 92 mM KCl, 3 mM MgCl2, 4 mM KOH, and 5 mM Hepes (pH 7.4), and leak subtraction by P over N protocol was not performed. “Gating” current was measured in ND96 solution. Holding potential was set to −60 mV. Moved charge was calculated from OFF-current after current components of cell capacitance were subtracted with the P/−10 protocol using Pulse+Plusefit software (HEKA Electronic). Whereas ON-current is superimposed by the endogenous outward current at high depolarization such as over 100 mV, OFF-current upon hyperpolarization gave indistinguishable Q-V relationship from that obtained with cut-open oocyte method over a wide range of voltage, as previously reported (8).
Imaging of Phosphoinositides Using Confocal Microscopy in Xenopus Oocyte.
Imaging of PI(4,5)P2 was done by following the method previously described (10). cRNAs of PHD-GFP (PHD derived from phospholipase C tagged with GFP) coinjected either with or without Ci-VSP. The oocytes were imaged by using FV300 confocal microscope system (Olympus) under the two-electrode voltage clamp using AxoClamp2B (Molecular Devices) in ND96 solution. The oocytes were imaged using a magnification ×20 objective. For each episode, 80 images were collected for 2 sec each by using Fluoview Tiempo software (Olympus). Fluorescence intensity in the parts of cell membrane was calculated for each image and plotted against time. All experiments were carried out at 22–25°C.
Acknowledgments.
We thank Drs. Robin Irvine and Jon Clarke for providing type II PIP kinase cDNA, Drs. Yasunori Kanaho and Takeaki Yokozeki for PIP5K cDNA, Dr. Ken-ichi Watanabe for help in experiments of radioisotope labeling of phosphoinositides, and Dr. Tomohiko Maehama for reading the manuscript and for helpful advice. This work was supported by grants from the Japan Ministry of Education, Culture, Sports, Science and Technology (to Y.O. and H.I.), the Japan Society for the Promotion of Science (to Y.O., H.I., and Y.M.), the Uehara Memorial Foundation (to Y.O.), and Human Frontier Science Program (to Y.O. and J.E.D.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Janetopoulos C, Devreotes P. Phosphoinositide signaling plays a key role in cytokinesis. J Cell Biol. 2006;174:485–490. doi: 10.1083/jcb.200603156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Logan MR, Mandato CA. Regulation of the actin cytoskeleton by PIP2 in cytokinesis. Biol Cell. 2006;98:377–388. doi: 10.1042/BC20050081. [DOI] [PubMed] [Google Scholar]
- 3.Ling K, Schill NJ, Wagoner MP, Sun Y, Anderson RA. Movin' on up: The role of PtdIns(4,5)P(2) in cell migration. Trends Cell Biol. 2006;16:276–284. doi: 10.1016/j.tcb.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Patterson RL, Boehning D, Snyder SH. Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu Rev Biochem. 2004;73:437–465. doi: 10.1146/annurev.biochem.73.071403.161303. [DOI] [PubMed] [Google Scholar]
- 5.Cantrell D. Phosphoinositide 3-kinase signalling pathways. J Cell Sci. 2001;114:1439–1445. doi: 10.1242/jcs.114.8.1439. [DOI] [PubMed] [Google Scholar]
- 6.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 7.Maehama T, Taylor GS, Dixon JE. PTEN and myotubularin: novel phosphoinositide phosphatases. Annu Rev Biochem. 2001;70:247–279. doi: 10.1146/annurev.biochem.70.1.247. [DOI] [PubMed] [Google Scholar]
- 8.Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. doi: 10.1038/nature03650. [DOI] [PubMed] [Google Scholar]
- 9.Bezanilla F. The voltage-sensor structure in a voltage-gated channel. Trends Biochem Sci. 2005;30:166–168. doi: 10.1016/j.tibs.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Murata Y, Okamura Y. Depolarization activates the phosphoinositide phosphatase Ci-VSP, as detected in Xenopus oocytes coexpressing sensors of PIP2. J Phys. 2007;583:875–889. doi: 10.1113/jphysiol.2007.134775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maehama T, Taylor GS, Slama JT, Dixon JE. A sensitive assay for phosphoinositide phosphatases. Anal Biochem. 2000;279:248–250. doi: 10.1006/abio.2000.4497. [DOI] [PubMed] [Google Scholar]
- 12.Maehama T, Dixon JE. PTEN: A tumour suppressor that functions as a phospholipid phosphatase. Trends Cell Biol. 1999;9:125–128. doi: 10.1016/s0962-8924(99)01519-6. [DOI] [PubMed] [Google Scholar]
- 13.Morris JB, Hinchliffe KA, Ciruela A, Letcher AJ, Irvine RF. Thrombin stimulation of platelets causes an increase in phosphatidylinositol 5-phosphate revealed by mass assay. FEBS Lett. 2000;475:57–60. doi: 10.1016/s0014-5793(00)01625-2. [DOI] [PubMed] [Google Scholar]
- 14.Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbetagamma. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, et al. PIP(2) activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron. 2003;37:963–975. doi: 10.1016/s0896-6273(03)00125-9. [DOI] [PubMed] [Google Scholar]
- 16.Suh BC, Hille B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr Opin Neurobiol. 2005;15:370–378. doi: 10.1016/j.conb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Okamura Y. Biodiversity of voltage sensor domain proteins. Pflugers Arch. 2007;454:361–371. doi: 10.1007/s00424-007-0222-6. [DOI] [PubMed] [Google Scholar]
- 18.Varnai P, Balla T. Visualization of phosphoinositides that bind pleckstrin homology domains: Calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J Cell Biol. 1998;143:501–510. doi: 10.1083/jcb.143.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrews S, Stephens LR, Hawkins PT. PI3K class IB pathway in neutrophils. Sci STKE. 2007;2007:cm3. doi: 10.1126/stke.4072007cm3. [DOI] [PubMed] [Google Scholar]
- 20.Cheung SM, Kornelson JC, Al-Alwan M, Marshall AJ. Regulation of phosphoinositide 3-kinase signaling by oxidants: Hydrogen peroxide selectively enhances immunoreceptor-induced recruitment of phosphatidylinositol (3,4) bisphosphate-binding PH domain proteins. Cell Signal. 2007;19:902–912. doi: 10.1016/j.cellsig.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Kishore AI, Prestegard JH. Molecular orientation and conformation of phosphatidylinositides in membrane mimetics using variable angle sample spinning (VASS) NMR. Biophys J. 2003;85:3848–3857. doi: 10.1016/S0006-3495(03)74799-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H, et al. A testis-specific gene, TPTE, encodes a putative transmembrane tyrosine phosphatase and maps to the pericentromeric region of human chromosomes 21 and 13, and to chromosomes 15, 22, and Y. Hum Genet. 1999;105:399–409. doi: 10.1007/s004390051122. [DOI] [PubMed] [Google Scholar]
- 23.Walker SM, Downes PC, Leslie NR. TPIP: A novel phosphoinositide 3-phosphatase. Biochem J. 2001;360:277–283. doi: 10.1042/0264-6021:3600277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Y, et al. PTEN 2, a Golgi-associated testis-specific homologue of the PTEN tumor suppressor lipid phosphatase. J Biol Chem. 2001;276:21745–21753. doi: 10.1074/jbc.M101480200. [DOI] [PubMed] [Google Scholar]
- 25.Hossain MI, et al. Enzyme domain affects the movement of the voltage sensor in ascidian and zebrafish VSPs. J Biol Chem. 2008 doi: 10.1074/jbc.M706184200. [DOI] [PubMed] [Google Scholar]
- 26.Roth MG. Phosphoinositides in constitutive membrane traffic. Physiol Rev. 2004;84:699–730. doi: 10.1152/physrev.00033.2003. [DOI] [PubMed] [Google Scholar]
- 27.Schackmann RW, Christen R, Shapiro BM. Membrane potential depolarization and increased intracellular pH accompany the acrosome reaction of sea urchin sperm. Proc Natl Acad Sci USA. 1981;78:6066–6070. doi: 10.1073/pnas.78.10.6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Florman HM, Corron ME, Kim TD-H, Babcock DF. Activation of voltage-dependent calcium channels of mammalian sperm is required for zona pellucida-induced acrosomal exocytosis. Dev Biol. 1992;152:304–314. doi: 10.1016/0012-1606(92)90137-6. [DOI] [PubMed] [Google Scholar]
- 29.Taylor GS, Dixon JE. Assaying phosphoinositide phosphatases. Methods Mol Biol. 2004;284:217–227. doi: 10.1385/1-59259-816-1:217. [DOI] [PubMed] [Google Scholar]
- 30.Goldin AL. Maintenance of Xenopus laevis and oocyte injection. Methods Enzymol. 1992;207:266–279. doi: 10.1016/0076-6879(92)07017-i. [DOI] [PubMed] [Google Scholar]