Abstract
Protein 4.1R (4.1R) is a multifunctional component of the red cell membrane. It forms a ternary complex with actin and spectrin, which defines the nodal junctions of the membrane-skeletal network, and its attachment to the transmembrane protein glycophorin C creates a bridge between the protein network and the membrane bilayer. We now show that deletion of 4.1R in mouse red cells leads to a large diminution of actin accompanied by extensive loss of cytoskeletal lattice structure, with formation of bare areas of membrane. Whereas band 3, the preponderant transmembrane constituent, and proteins known to be associated with it are present in normal or increased amounts, glycophorin C is missing and XK, Duffy, and Rh are much reduced in the 4.1R-deficient cells. The inference that these are associated with 4.1R was borne out by the results of in vitro pull-down assays. Furthermore, whereas Western blot analysis showed normal levels of band 3 and Kell, flow cytometric analysis using an antibody against the extracellular region of band 3 or Kell revealed reduction of these two proteins, suggesting a conformational change of band 3 and Kell epitopes. Taken together, we suggest that 4.1R organizes a macromolecular complex of skeletal and transmembrane proteins at the junctional node and that perturbation of this macromolecular complex not only is responsible for the well characterized membrane instability but may also remodel the red cell surface.
Keywords: macromolecular complex, cytoskeleton
An essential attribute of the red cell is its ability to undergo extensive and repeated deformations while maintaining structural integrity. The cell owes this mechanical resilience to the membrane-associated protein skeleton (1, 2). This has the form of a lattice, made up of spectrin tetramers, formed by self-association of αβ spectrin heterodimers (3). The tetramers are attached at their ends to predominantly sixfold junctions consisting of short F-actin filaments (protofilaments) and several actin-binding proteins, including 4.1R, protein 4.9 (dematin), adducin, tropomyosin, and tropomodulin (4). Defects or deficiency of components of the junctional complexes, and especially of 4.1R, lead to instability of the network and consequently of the cell. This reveals itself in progressive fragmentation in vivo (5).
In addition to the above-mentioned cytoskeletal proteins, a number of transmembrane proteins that specify blood group antigens have also been purified and characterized biochemically. These include band 3, glycophorin A, glycophorin B, glycophorin C, RhAG, Rh, Duffy, Lu, LW, CD44, CD47, Kell, and XK (6). These transmembrane proteins exhibit diverse functions. For example, band 3 functions as an anion exchanger. Rh/RhAG are probably gas transporters although there is some controversy regarding whether they transport ammonia or carbon dioxide (7, 8). Duffy serves as a chemokine receptor and is also a receptor for the malarial parasite Plasmodium vivax (9, 10). Lu, LW, and CD44 are proteins that are involved in adhesive interactions (11). CD47 can function as a marker of self on erythrocytes by binding to the inhibitory receptor SIRPα (12). Kell possess endothin-3-converting enzyme activity (13), but the function of XK remains to be defined.
The membrane-skeletal network is coupled to the lipid bilayer through transmembrane proteins. One such linkage is generated by ankyrin, which forms a bridge between spectrin and band 3 tetramers (14–17). Band 3 also contains a binding site for carbonic anhydrase II at its C-terminal cytoplasmic domain (18) and binding sites for glycolytic enzymes, hemoglobin, and protein 4.2 at its N-terminal cytoplasmic domain (19). In addition, there is a clear interaction between glycophorin A (GPA) and band 3 (20–23). The association of these proteins with band 3 forms the band-3-based complex. In addition to the band 3 complex, studies using human Rh-null erythrocytes suggested the existence of the Rh protein complex comprising RhAG, Rh, CD47, LW, and GPB (24, 25). More recently, the finding that components of both the band 3 complex and the Rh complex are absent or reduced in band-3-deficient erythrocytes led to the concept of a band-3-based macromolecular complex (26).
A second membrane skeleton–bilayer link, consisting of a nexus among 4.1R, p55, and the transmembrane glycophorin C (GPC), is located at the network junctions (27–29). GPC and p55 are missing from 4.1R−/− mouse red cells (30) and are much reduced in human 4.1R-deficient red cells (31, 32). These proteins, as well as some transmembrane blood group proteins, Duffy, Lu, and CD44, and the glucose transporter GLUT1 are found in normal or elevated amounts in band-3-deficient red cells (26). The work described here was undertaken to examine whether and to what extent 4.1R plays a part in the formation of membrane structures other than the network junctions. The results, based on the study of 4.1R−/− mouse red cells, have allowed us to identify a 4.1R-based macromolecular complex and to develop a more refined model of red cell membrane organization.
Results
Specificity of Various Anti-Mouse Antibodies.
To compare the expression of red cell membrane proteins between wild-type and 4.1R−/− cells, we first needed to generate a panel of various antibodies against mouse transmembrane and cytoskeletal proteins. For transmembrane proteins we usually generate two antibodies, one against the extracellular region and one against the cytoplasmic part. The antigens used for antibody production are listed in supporting information (SI) Table S1. All of the antibodies are raised in rabbit with the exception of monoclonal anti-Kell antibody, which was generated in mice using red cell as antigen. The specificity of our antibodies was confirmed by Western blot analysis using corresponding knockout mice as negative controls. All antibodies generated recognize the corresponding mouse proteins, and some also recognize the cognate human proteins. Fig. S1 demonstrates the specificity of a representative set of antibodies against mouse red cell proteins.
Analysis of Cytoskeletal Protein Components of 4.1R−/− Red Cells by Western Blot.
We have previously shown that the membranes of mouse red cells lacking 4.1R have greatly impaired shear resistance (30). The same is true of human 4.1R-deficient cells (5). Although it is assumed that this is probably because of weakened interaction between spectrin and actin in the absence of 4.1R, the detailed molecular basis is not clear. Thus, we first examined the expression of skeletal proteins of red cells from 4.1R+/+ and 4.1R−/− mice by Western blotting. Protein loadings were standardized by applying to the gels equal amounts of membranes as measured in terms of cholesterol concentration. Fig. 1 shows that, in addition to the absence of 4.1R and p55, the amount of actin in the membrane was unexpectedly reduced by ≈50% in 4.1R-deficient red cells. When the entire protein complement of whole red cells was examined, an ≈50% deficit of actin was again recorded (data not shown), showing that the missing actin was not in the cytosol. Whereas two junction-associated proteins (tropomyosin and adducin) were increased, spectrin, tropomodulin, and dematin were unchanged. The increase of tropomyosin and adducin is probably due to reticulocytosis in 4.1R−/− mice because an increase is also noted in other anemias with increased reticulocytosis such as sickle cell disease and thalassemia (data not shown). Another major skeleton protein, ankyrin, was unchanged.
Fig. 1.
Immunoblots of membrane skeletal proteins in red cells of 4.1R+/+ and 4.1R−/− mice. Blots of SDS/PAGE of total membrane protein were probed with antibodies against the indicated proteins. Note the absence of 4.1R, as well as p55 in the 4.1R-deficient cells, the reduced actin concentration, and the elevated tropomyosin and adducin.
Structural Consequences of 4.1R Deletion.
Having discovered the significant reduction of actin in 4.1R-deficient red cells, we then examined the structural consequences of 4.1R deletion. Staining of fixed red cells with fluorescent phalloidin demonstrates that F-actin is indeed much sparser on the 4.1R−/− cell membranes (Fig. S2). To determine whether F-actin is reduced or redistributed, we quantitated the fluorescence levels (pixel intensity/unit area) and found an ≈30% reduction in phalloidin levels in 4.1R-null red cells when compared with wild-type erythrocytes [knockout, 17,958 ± 1,783; wild type, 25,479 ± 1,858 (n = 8, P = 0.0000004)]. To establish how the actin deficiency affects the structure of the network, we examined the membranes by electron microscopy under negative stain. Fig. 2 demonstrates that the regularity of the lattice is grossly disrupted, with large bare regions. It thus appears that many of the junctions that are normally present are missing in the mutant membranes.
Fig. 2.
Electron micrographs of membrane skeletons of red cells of 4.1R+/+ and 4.1R−/− mice. (Left) Membrane skeleton of wild-type cells. (Right) Membrane skeleton of 4.1R-deficient cells. Note deficient membrane junctions in the mutant cells and large bare areas.
Integral Membrane Proteins in 4.1R−/− Cells.
It has been shown that in both mouse and human band-3-deficient red cells the known or surmised band-3-associated proteins, namely GPA, GPB, RhAG, Rh, CD47, and LW, are missing or greatly reduced. GPC, 4.1R, and p55, which are confined to the network junctions, are present in normal amounts, and so also are other transmembrane proteins (Duffy, Lu, GLUT1, LFA-3, and CD44) with no known cytoskeleton interactions (26). We have used Western blots to compare the abundance of all of these proteins in 4.1R+/+ and 4.1R−/− mouse red cells. The outcome was that the amounts of band 3, GPA, RhAG, CD47, LW, and NHE1 (Na+–H+ exchanger) were either unchanged or increased, and GPC was missing entirely, whereas XK, Duffy, and Rh were significantly reduced in the mutant cells (Fig. 3). Once again, the increase of GPA, LW, and NHE1 is probably due to reticulocytosis in 4.1R−/− mice because these proteins are also increased in other anemias such as sickle cell disease and thalassemia (data not shown). The implication is that not only GPC and p55, but also XK, Duffy, and Rh, are associated directly or indirectly with the 4.1R.
Fig. 3.
Immunoblots of transmembrane proteins in red cells of 4.1R+/+ and 4.1R−/− mice. Blots of SDS/PAGE of total membrane protein were probed with antibodies against the indicated proteins. Note the disappearance of GPC and diminution of Rh, XK, and Duffy proteins in the 4.1R-deficient mice and the increase of GPA, LW, and NHE1 expression.
Surface Expression of Transmembrane Proteins in 4.1R−/− Cells.
We have used flow cytometry with antibodies against extracellular epitopes of transmembrane proteins to refine our estimations of their relative concentrations in 4.1R−/− cells by Western blotting. As Fig. 4 shows, no GPC could be detected, and the concentration of Duffy was reduced, whereas that of GPA was normal, in accord with the results from the immunoblots. By striking contrast, the proportions of band 3 and of Kell protein registered by flow cytometry were much lower than by Western blotting. The low surface expression of band 3 was also confirmed by labeling with eosin-5′-maleimide, a reagent commonly used for the diagnosis of band 3 deficiency in human red cells (33). The results from age- and sex-matched mice are summarized in the Table 1. These results suggest that a fraction of band 3 and Kell may undergo a conformational change that masks the extracellular epitope in 4.1R-deficient red cells.
Fig. 4.
Flow cytometric analysis of red cell membrane proteins of 4.1R+/+ and 4.1R−/− mice. The ordinate measures the number of cells displaying the fluorescent intensity given by the abscissa. Black lines, 4.1R+/+; gray lines, 4.1R−/−; dotted lines, negative control.
Table 1.
Expression of blood group antigen-carrying proteins as assessed by flow cytometry
| Protein | Mean fluorescence |
Knockout % of wild type | P value | |
|---|---|---|---|---|
| Wild type (n) | Knockout (n) | |||
| TER119 | 2,791 ± 161 (6) | 2,703 ± 355 (6) | 97 | 0.601 |
| GPC | 2,800 ± 137 (10) | 250 ± 147 (10) | 8 | 0.00023 |
| Duffy | 1,746 ± 207 (11) | 1,183 ± 295 (12) | 68 | 0.00003 |
| Kell | 2,405 ± 243 (14) | 2,020 ± 265 (14) | 84 | 0.00049 |
| Band 3 | 1,108 ± 260 (8) | 534 ± 200 (9) | 48 | 0.00026 |
| EOM | 8,721 ± 224 (5) | 5,970 ± 1,178 (5) | 68 | 0.002 |
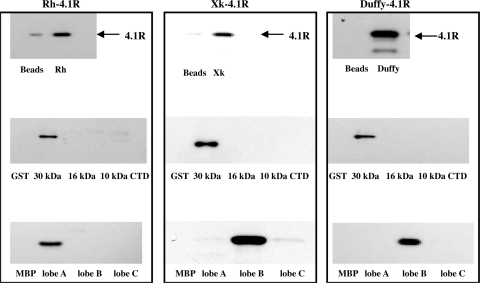
Binding of XK, Duffy, and Rh to 4.1R in Vitro.
The finding that XK, Duffy, and Rh are reduced in 4.1R−/− red cells strongly implies their association with 4.1R. We used a pull-down assay to examine whether they directly bind to 4.1R. As shown in Fig. 5, 4.1R binds to the cytoplasmic domain of XK, Duffy, or Rh. As controls, we also examined the binding of 4.1R to cytoplasmic domains of GPA, CD47, and Kell that are not reduced in 4.1R-deficient red cells and found no binding under the same experimental condition (data not shown). Furthermore, the cytoplasmic domain of XK, Duffy, or Rh binds specifically to the 30-kDa membrane-binding domain of 4.1R but to neither of the other domains. The 30-kDa domain is composed of three lobes that bind to different transmembrane proteins (34). By examining subfragments of the 30-kDa domain we found that the binding site for Rh is contained in lobe A and that the binding sites for XK and Duffy are both located in lobe B.
Fig. 5.
Direct interaction of 4.1R with Rh, XK, and Duffy. The binding of 4.1R 80-kDa and its functional domains to cytoplasmic tails of XK, Duffy, and Rh was assessed by streptavidin pull-down assay. The binding of 4.1R, GST-tagged domains of 4.1R, and MBP-tagged subdomains of 30-kDa to the cytoplasmic domains of XK, Duffy, and Rh was detected by using anti-4.1R antibody, anti-GST antibody, and anti-MBP antibody, respectively. Note the binding of all three proteins to 30-kDa, the binding of Rh to lobe A, and the binding of XK and Duffy to lobe B.
Discussion
It has become clear that the simple model of red cell membrane organization, which endured for so long based on an irreversibly assembled membrane skeleton and a population of predominantly free-floating transmembrane proteins, is inadequate. It was discovered, for instance, that a number of transmembrane and cytosolic proteins are associated with the abundant anion channel protein band 3, thereby forming a metabolon (26). We can now define two types of multiprotein complexes surrounding the connections between the membrane skeleton and the bilayer. Fig. 6 summarizes in schematic form the probable interactions that go to make up what we shall term the band-3-based macromolecular complex and the 4.1R-based macromolecular complex. The band-3-based macromolecular complex is attached through ankyrin to spectrin tetramers at a point near their center. The band 3 in this complex is thought to be a tetramer (35). The remaining band 3 is dimeric (35), and some or all of this fraction is surmised to bind to 4.1R and to another junction component, adducin (36), to generate the 4.1R-based macromolecular complex, as depicted in Fig. 6 Right. According to our evidence from present study, the 4.1R macromolecular complex comprises, besides the ternary spectrin–actin–4.1R nexus, p55 and the transmembrane proteins GPC, Rh, Duffy, Kell, and XK. The last four are present in much smaller numbers than GPC and 4.1R and are presumably associated with certain 4.1R–GPC complexes.
Fig. 6.
Schematic representation of two types of multiprotein complexes in the red cell membrane. (Left) Protein complex attached to spectrin near the center of the tetramer (dimer–dimer interaction site). Tetrameric band 3 is bound to ankyrin, which is bound to spectrin. The membrane skeletal protein 4.2 has binding sites for band 3 and for ankyrin. Transmembrane glycoproteins GPA, Rh, and RhAG bind to band 3, and CD47 and LW associate with Rh/RhAG. The two cytoplasmic domains of band 3 contain binding sites for soluble proteins, the short C-terminal domain for CA II, the large N-terminal domain for deoxyhemoglobin and for glycolytic enzymes, aldolase, phosphofructokinase (PFK), and glyceraldehyde 3′-phosphate dehydrogenase (GAPDH). (Right) Protein complex at membrane skeletal junctions. The junctions contain the ternary complex of spectrin, F-actin, and 4.1R, as well as the actin-binding proteins tropomyosin, tropomodulin, adducin, and dematin. 4.1R enters into an additional ternary interaction with the transmembrane protein GPC and p55 and is taken also to bind to band 3, in the form of a dimer, which also carries GPA. Rh, Kell, and XK also have binding sites on 4.1R. Note, however, that the copy numbers of all transmembrane proteins except GPA and GPC are low and therefore will not be present on all complexes.
It appears unlikely that any of the known integral membrane proteins normally exist as free-floating monomeric chains. The evolutionary advantage of sequestering these blood group proteins as captives within large complexes may be to prevent them from clustering upon encountering an adhesive surface and thereby forming tight, possibly irreversible, attachments.
The breakdown of membrane-skeletal organization in 4.1R-deficient mouse red cells is striking, although not necessarily unexpected. The stability of the ternary complex of spectrin, F-actin, and 4.1R is high (Kd ≈ 10−15 M) (2) whereas the binary complex of spectrin and F-actin is weak (Kd ≈ 10−5 M) (37). Therefore, one may expect that the actin protofilaments might dissociate or disproportionate into longer filaments and cause the junctions to disintegrate. This must be assumed to apply to human 4.1R-deficient red cells, which are associated with elliptocytosis and anemia (38–40). Indeed the skeleton network was also found to be markedly disrupted in human 4.1R-deficient red cells (41). It is interesting to note that the 4.1R-deficient mice exhibited a much more severe hemolytic anemia than 4.1R-deficient human patients. One possible reason is that the nature of 4.1 deficiency between mice and humans is different. Whereas in humans the high-molecular-mass (135 kDa) isoform of 4.1R appears early in erythropoiesis (42), in both the normal and the 4.1R-deficient case, to be replaced by the mature (80 kDa) isoform in normals, the 4.1R knockout mice produce neither isotype (30). The 135-kDa form may suffice to ensure normal assembly of the protofilaments, which are then at least partially stabilized by tropomyosin (43, 44), spectrin, and actin-capping proteins. The mechanism(s) by which lack of 4.1R results in reduced actin is not clear, but one possibility is that a part of the actin in the protofilaments dissociates and is proteolytically degraded in the cytosol because of failure of appropriate actin assembly in the immature cells.
The finding of normal levels of band 3 and Kell by Western blot analysis but reduced levels by flow cytometric analysis suggests possible conformational change of band 3 and Kell epitopes in intact membranes. Because band 3 tetramers are present at the band 3–ankrin-based macrocomplex, we speculate that the conformation of band 3 dimers that are present at the 4.1R-based macrocomplex is probably affected by the absence of 4.1R. The conformation of Kell is probably due to reduction of XK in the absence of 4.1R. Another interesting finding is that RhAG levels remain constant while Rh levels decline in 4.1R−/− cells. This finding may seem contradictory with the well accepted notion that Rh and RhAG exist as tetramers in mature red cells. However, it should be noted that we recently found that Rh and RhAG traffic differently in the erythroblasts (our unpublished data) and as such that these two proteins may exist independent of each other during erythroid development.
In summary, our results throw light on the basis of red cell membrane skeleton stability and enlarge our understanding of the organization of the membrane as a whole.
Materials and Methods
Materials.
A SulfoLink kit was purchased from Pierce. A Cholesterol Quantification Kit was from BioVision. pMAL vector, MBP resin, monoclonal anti-MBP antibody, T4 ligase, and restriction enzymes were obtained from New England BioLabs. Top Pfu polymerase and BL21 (DE3) bacteria were from Stratagene, reduced-form glutathione and isopropyl β-d-thiogalactopyranoside were from Sigma, proteinase inhibitor mixture set II was from Calbiochem, a DC Protein Assay Kit, SDS/PAGE, and electrophoresis reagents were from Bio-Rad, and a SuperSignal West Pico chemiluminescence detection kit reagent was from Pierce. Alexa Fluor 633-conjugated phalloidin was purchased from Invitrogen–Molecular Probes. HRP-conjugated anti-mouse IgG and HRP-conjugated anti-rabbit IgG were from Jackson ImmunoResearch Laboratories. Biotin-labeled synthetic peptides corresponding to the cytoplasmic tail of XK, Duffy, or Rh were from Genemed.
Mice.
The generation of 4.1R knockout mice has been described previously (30). The mice were backcrossed onto a C57BL/6 background and have been inbred for >20 generations. All of the mice were maintained at the animal facility of the New York Blood Center under specific pathogen-free conditions according to institutional guidelines. Animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee. Band 3 knockout mice and Duffy knockout mice were kindly provided by Luanne Peters (The Jackson Laboratory) and Asok Chaudhuri (the New York Blood Center), respectively. Blood samples were obtained by tail or cardiac puncture of anesthetized mice.
Generation of Antibodies.
Antibodies against mouse transmembrane proteins GPC, band 3, Rh, RhAG, XK, Kell, Duffy, LW, and CD47 were raised in rabbit by using synthetic peptides as antigens. With the exception of LW and Duffy, two antibodies (one against the extracellular region and one against the cytoplasmic region) against each protein were made. The antibodies were affinity-purified by using specific peptides immobilized to SulfoLink Coupling Gel, and the specificity of the antibodies was examined by Western blotting using the corresponding knockout mouse red cells as negative controls.
Phalloidin Staining of Red Cells.
Freshly drawn blood was washed three times in PBG buffer (PBS/0.1% BSA/10 mM glucose). A total of 4 × 107 cells were fixed in 100 μl of 0.5% acrolein–PBG for 5 min at room temperature and resuspended in PBG buffer to a final concentration of 107 cells per milliliter. The cells were plated on poly-l-lysine-treated coverslips, permeabilized with 0.05% Triton X-100–PBS for 10 seconds, and washed three times with 0.1 M glycine–PBS. Staining with 1 unit of Alexa Fluor 633–phalloidin was carried out at room temperature for 40 min. Coverslips were washed four times with PBS, and slides were mounted. Images were obtained with a Zeiss LSM META 510 Confocal Microscope.
Electron Microscopy.
Mouse erythrocytes were attached to polyl-lysine-coated coverslips by centrifugation at 200 × g for 5 min at room temperature. Erythrocytes were permeabilized by using 0.5% Triton X-100 in PHEM buffer (60 mM PHEM/25 mM Hepes/2 mM MgCl2/10 mM EGTA/1 μM phallacidin) containing 0.05% glutaraldehyde for 2 min. Membrane skeletons were rapidly washed with PHEM buffer without fixative and then fixed with 1% glutaraldehyde in PHEM buffer for 10 min at 37°C. They were washed into distilled water and rapidly frozen, freeze-dried at −90°C, and metal-cast with 1.4 nm of platinum at 45°C with rotation and 5 nm of carbon at 90°C without rotation (Cressington CFE-60 Freeze Fracture Machine). Metal casts were separated from the coverslips by using 25% hydrofluoric acid, washed with distilled water, picked up with 200-mesh formvar-coated copper grids, and photographed at 100 kV in a JEOL 1200-EX electron microscope at 80-kV accelerating voltage.
Preparation of Red Cell Membranes.
RBCs were washed three times in PBS. White ghosts were prepared by lysis of RBCs in ice-cold 5T5K buffer (5 mM KCl/5 mM Tris, pH 7.4/0.1 mM DFP) in the presence of 1 mM MgCl2 followed by three washes in 35 volumes of the same buffer. Membrane cholesterol content was measured by using a Cholesterol Quantification Kit according to the manufacturer's protocol. Protein concentrations were measured with the Bradford method using the DC Protein Assay Kit.
SDS/PAGE and Western Blot Analysis.
Aliquots of RBC ghosts, matched for cholesterol content, were separated by 10% SDS/PAGE. The proteins were transferred to a nitrocellulose membrane. After blocking for 1 h in blocking buffer (10 mM Tris, pH 7.4/150 mM NaCl/0.5% Tween 20/5% nonfat dried milk powder), the blot was probed for 1 h with the desired primary antibodies. After several washes, the blot was incubated with anti-rabbit or anti-mouse IgG coupled to HRP and developed with the SuperSignal West Pico chemiluminescence detection kit. All steps were performed at room temperature.
Flow Cytometry Analysis.
RBCs from wild-type and 4.1R knockout mice were washed three times in PBS supplemented with 0.1% BSA (PBS-BSA). A total of 1 × 106 cells were incubated for 20 min on ice with the appropriate amount of antibodies against the extracellular region of transmembrane proteins GPC, band 3, GPA, Kell, and Duffy. After washing two times in PBS-BSA, the cells were incubated with Alexa Fluor 488-conjugated secondary antibody (Molecular Probes) for 20 min on ice. Cells were washed three times in cold PBS-BSA before analysis on a Becton Dickinson FACSCanto. Preimmune IgGs were used as negative control. The mean fluorescence intensity was used as a measure of antibody binding. Eosin-5′-maleimide staining was performed as described previously (45) with few modifications. Briefly, 5 μl of whole blood was incubated with 25 μl of PBS containing 0.5 mg/ml EMA for 1 h at room temperature. Cells were washed four times with PBS-BSA before flow cytometry analysis.
Preparation of Recombinant Proteins.
The full-length 4.1R 80-kDa, GST-tagged 30-kDa, 16-kDa, 10-kDa, and 22/24-kDa domains of 4.1R were constructed and purified as described previously (46, 47). MBP-tagged lobe A, lobe B, and lobe C of the 30-kDa domain were subcloned into pMAL-p2x vector using EcoRI and SalI upstream and downstream, respectively. cDNA encoding the desired sequences was transformed into the BL21 bacterial strain. Expression was induced by 0.1 mM isopropyl β-d-thiogalactopyranoside at 4°C overnight. MBP-tagged recombinant proteins were purified on an amylose resin affinity column. Proteins were dialyzed against binding buffer (10 mM Tris·HCl, pH 7.4/150 mM NaCl). Protein concentrations were determined spectrophotometrically using extinction coefficients calculated from the tryptophan and tyrosine contents (48).
Pull-Down Assay.
To measure the binding of 4.1R 80-kDa and its functional domains and subdomains of the 30-kDa domain to cytoplasmic tails of XK, Duffy, and Rh, 4.1R or its domains were incubated with the biotin-labeled synthetic peptide at room temperature for 1 h. Streptavidin beads were added to the reaction mixture, incubated for 10 min, pelleted, washed, and eluted with 10% SDS. The pellet was analyzed by SDS/PAGE, followed by transfer to nitrocellulose membrane and the subsequent Western blotting. The binding of 4.1R to the cytoplasmic domains of XK, Duffy, and Rh was detected by using anti-4.1R antibody. Similarly, the binding of GST-tagged 30-kDa, 16-kDa, 10-kDa and 22/24-kDa domains of 4.1R was detected by using anti-GST antibody, and the binding of MBP-tagged lobe A, lobe b, and lobe C was detected by using anti-MBP antibody.
Supplementary Material
Acknowledgments.
This work was supported in part by National Institutes of Health Grants DK26263, DK32094, HL31579, HL78826, and HL075716.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803225105/DCSupplemental.
References
- 1.Mohandas N, Evans E. Mechanical properties of the red cell membrane in relation to molecular structure and genetic defects. Annu Rev Biophys Biomol Struct. 1994;23:787–818. doi: 10.1146/annurev.bb.23.060194.004035. [DOI] [PubMed] [Google Scholar]
- 2.Discher DE, Carl P. New insights into red cell network structure, elasticity, and spectrin unfolding—a current review. Cell Mol Biol Lett. 2001;6:593–606. [PubMed] [Google Scholar]
- 3.Shotton DM, Burke BE, Branton D. The molecular structure of human erythrocyte spectrinBiophysical and electron microscopic studies. J Mol Biol. 1979;131:303–329. doi: 10.1016/0022-2836(79)90078-0. [DOI] [PubMed] [Google Scholar]
- 4.Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: Metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81:1353–1392. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- 5.Takakuwa Y, Tchernia G, Rossi M, Benabadji M, Mohandas N. Restoration of normal membrane stability to unstable protein 4.1-deficient erythrocyte membranes by incorporation of purified protein 4.1. J Clin Invest. 1986;78:80–85. doi: 10.1172/JCI112577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reid ME, Mohandas N. Red blood cell blood group antigens: Structure and function. Semin Hematol. 2004;41:93–117. doi: 10.1053/j.seminhematol.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Soupene E, et al. Rhesus expression in a green alga is regulated by CO2. Proc Natl Acad Sci USA. 2002;99:7769–7773. doi: 10.1073/pnas.112225599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soupene E, Lee H, Kustu S. Ammonium/methylammonium transport (Amt) proteins facilitate diffusion of NH3 bidirectionally. Proc Natl Acad Sci USA. 2002;99:3926–3931. doi: 10.1073/pnas.062043799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhuri A, et al. Expression of the Duffy antigen in K562 cells. Evidence that it is the human erythrocyte chemokine receptor. J Biol Chem. 1994;269:7835–7838. [PubMed] [Google Scholar]
- 10.Chaudhuri A, et al. Purification and characterization of an erythrocyte membrane protein complex carrying Duffy blood group antigenicity. Possible receptor for Plasmodium vivax and Plasmodium knowlesi malaria parasite. J Biol Chem. 1989;264:13770–13774. [PubMed] [Google Scholar]
- 11.Parsons SF, Spring FA, Chasis JA, Anstee DJ. Erythroid cell adhesion molecules Lutheran and LW in health and disease. Baillieres Best Pract Res Clin Haematol. 1999;12:729–745. doi: 10.1053/beha.1999.0050. [DOI] [PubMed] [Google Scholar]
- 12.Oldenborg PA, et al. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 13.Lee S, et al. Proteolytic processing of big endothelin-3 by the kell blood group protein. Blood. 1999;94:1440–1450. [PubMed] [Google Scholar]
- 14.Thevenin BJ, Willardson BM, Low PS. The redox state of cysteines 201 and 317 of the erythrocyte anion exchanger is critical for ankyrin binding. J Biol Chem. 1989;264:15886–15892. [PubMed] [Google Scholar]
- 15.Davis L, Lux SE, Bennett V. Mapping the ankyrin-binding site of the human erythrocyte anion exchanger. J Biol Chem. 1989;264:9665–9672. [PubMed] [Google Scholar]
- 16.Willardson BM, et al. Localization of the ankyrin-binding site on erythrocyte membrane protein, band 3. J Biol Chem. 1989;264:15893–15899. [PubMed] [Google Scholar]
- 17.Michaely P, Bennett V. The ANK repeats of erythrocyte ankyrin form two distinct but cooperative binding sites for the erythrocyte anion exchanger. J Biol Chem. 1995;270:22050–22057. doi: 10.1074/jbc.270.37.22050. [DOI] [PubMed] [Google Scholar]
- 18.Sterling D, Reithmeier RAF, Casey JR. A transport metabolon: Functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers. J Biol Chem. 2001;276:47886–47894. doi: 10.1074/jbc.M105959200. [DOI] [PubMed] [Google Scholar]
- 19.Zhang D, Kiyatkin A, Bolin JT, Low PS. Crystallographic structure and functional interpretation of the cytoplasmic domain of erythrocyte membrane band 3. Blood. 2000;96:2925–2933. [PubMed] [Google Scholar]
- 20.Telen MJ, Chasis JA. Relationship of the human erythrocyte Wrb antigen to an interaction between glycophorin A and band 3. Blood. 1990;76:842–848. [PubMed] [Google Scholar]
- 21.Knowles DW, Chasis JA, Evans EA, Mohandas N. Cooperative action between band 3 and glycophorin A in human erythrocytes: Immobilization of band 3 induced by antibodies to glycophorin A. Biophys J. 1994;66:1726–1732. doi: 10.1016/S0006-3495(94)80965-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hassoun H, et al. Complete deficiency of glycophorin A in red blood cells from mice with targeted inactivation of the band 3 (AE1) gene. Blood. 1998;91:2146–2151. [PubMed] [Google Scholar]
- 23.Auffray I, et al. Glycophorin A dimerization and band 3 interaction during erythroid membrane biogenesis: In vivo studies in human glycophorin A transgenic mice. Blood. 2001;97:2872–2878. doi: 10.1182/blood.v97.9.2872. [DOI] [PubMed] [Google Scholar]
- 24.Daniels GL, et al. Terminology for red cell surface antigens. ISBT Working Party Oslo Report. International Society of Blood Transfusion. Vox Sang. 1999;77:52–57. doi: 10.1159/000031074. [DOI] [PubMed] [Google Scholar]
- 25.Avent ND, Reid ME. The Rh blood group system: A review. Blood. 2000;95:375–387. [PubMed] [Google Scholar]
- 26.Bruce LJ, et al. A band 3-based macrocomplex of integral and peripheral proteins in the RBC membrane. Blood. 2003;101:4180–4188. doi: 10.1182/blood-2002-09-2824. [DOI] [PubMed] [Google Scholar]
- 27.Marfatia SM, Leu RA, Branton D, Chishti AH. Identification of the protein 4.1 binding interface on glycophorin C and p55, a homologue of the Drosophila discs-large tumor suppressor protein. J Biol Chem. 1995;270:715–719. doi: 10.1074/jbc.270.2.715. [DOI] [PubMed] [Google Scholar]
- 28.Marfatia SM, Morais-Cabral JH, Kim AC, Byron O, Chishti AH. The PDZ domain of human erythrocyte p55 mediates its binding to the cytoplasmic carboxyl terminus of glycophorin C. Analysis of the binding interface by in vitro mutagenesis. J Biol Chem. 1997;272:24191–24197. doi: 10.1074/jbc.272.39.24191. [DOI] [PubMed] [Google Scholar]
- 29.Nunomura W, Takakuwa Y, Parra M, Conboy J, Mohandas N. Regulation of protein 4.1R, p55, and glycophorin C ternary complex in human erythrocyte membrane. J Biol Chem. 2000;275:24540–24546. doi: 10.1074/jbc.M002492200. [DOI] [PubMed] [Google Scholar]
- 30.Shi ZT, et al. Protein 4.1R-deficient mice are viable but have erythroid membrane skeleton abnormalities. J Clin Invest. 1999;103:331–340. doi: 10.1172/JCI3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reid ME, Takakuwa Y, Conboy J, Tchernia G, Mohandas N. Glycophorin C content of human erythrocyte membrane is regulated by protein 4.1. Blood. 1990;75:2229–2234. [PubMed] [Google Scholar]
- 32.Lorenzo F, et al. Severe poikilocytosis associated with a de novo alpha 28 Arg→Cys mutation in spectrin. Br J Haematol. 1993;83:152–157. doi: 10.1111/j.1365-2141.1993.tb04646.x. [DOI] [PubMed] [Google Scholar]
- 33.Stoya G, Baumann E, Junker U, Hermann J, Linss W. Flow cytometric analysis of band 3 protein of human erythrocytes. Acta Histochem. 1997;99:29–36. doi: 10.1016/S0065-1281(97)80005-0. [DOI] [PubMed] [Google Scholar]
- 34.Han BG, Nunomura W, Takakuwa Y, Mohandas N, Jap BK. Protein 4.1R core domain structure and insights into regulation of cytoskeletal organization. Nat Struct Biol. 2000;7:871–875. doi: 10.1038/82819. [DOI] [PubMed] [Google Scholar]
- 35.Yi SJ, et al. Red cell membranes of ankyrin-deficient nb/nb mice lack band 3 tetramers but contain normal membrane skeletons. Biochemistry. 1997;36:9596–9604. doi: 10.1021/bi9704966. [DOI] [PubMed] [Google Scholar]
- 36.Allenspach EJ, et al. ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity. 2001;15:739–750. doi: 10.1016/s1074-7613(01)00224-2. [DOI] [PubMed] [Google Scholar]
- 37.Morris MB, Lux SE. Characterization of the binary interaction between human erythrocyte protein 4.1 and actin. Eur J Biochem. 1995;231:644–650. doi: 10.1111/j.1432-1033.1995.tb20743.x. [DOI] [PubMed] [Google Scholar]
- 38.Tchernia G, Mohandas N, Shohet S. Deficiency of skeletal membrane protein band 4.1 in homozygous hereditary elliptocytosis. Implications for erythrocyte membrane stability. J Clin Invest. 1981;68:454–460. doi: 10.1172/JCI110275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palek J, Lux SE. Red cell membrane skeletal defects in hereditary and acquired hemolytic anemias. Semin Hematol. 1983;20:189–224. [PubMed] [Google Scholar]
- 40.Conboy J, Mohandas N, Tchernia G, Kan YW. Molecular basis of hereditary elliptocytosis due to protein 4.1 deficiency. N Engl J Med. 1986;315:680–685. doi: 10.1056/NEJM198609113151105. [DOI] [PubMed] [Google Scholar]
- 41.Yawata A, Kanzaki A, Gilsanz F, Delaunay J, Yawata Y. A markedly disrupted skeletal network with abnormally distributed intramembrane particles in complete protein 4.1-deficient red blood cells (allele 4.1 Madrid): Implications regarding a critical role of protein 4.1 in maintenance of the integrity of the red blood cell membrane. Blood. 1997;90:2471–2481. [PubMed] [Google Scholar]
- 42.Chasis JA, et al. Differential use of protein 4.1 translation initiation sites during erythropoiesis: Implications for a mutation-induced stage-specific deficiency of protein 4.1 during erythroid development. Blood. 1996;87:5324–5331. [PubMed] [Google Scholar]
- 43.Fowler VM. Regulation of actin filament length in erythrocytes and striated muscle. Curr Opin Cell Biol. 1996;8:86–96. doi: 10.1016/s0955-0674(96)80052-4. [DOI] [PubMed] [Google Scholar]
- 44.An X, Salomao M, Guo X, Gratzer W, Mohandas N. Tropomyosin modulates erythrocyte membrane stability. Blood. 2007;109:1284–1288. doi: 10.1182/blood-2006-07-036954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.King MJ, et al. Rapid flow cytometric test for the diagnosis of membrane cytoskeleton-associated haemolytic anaemia. Br J Haematol. 2000;111:924–933. [PubMed] [Google Scholar]
- 46.An XL, et al. Structural and functional characterization of protein 4.1R-phosphatidylserine interaction: potential role in 4.1R sorting within cells. J Biol Chem. 2001;276:35778–35785. doi: 10.1074/jbc.M101364200. [DOI] [PubMed] [Google Scholar]
- 47.Nunomura W, et al. Regulation of CD44-protein 4.1 interaction by Ca2+ and calmodulin. Implications for modulation of CD44-ankyrin interaction. J Biol Chem. 1997;272:30322–30328. doi: 10.1074/jbc.272.48.30322. [DOI] [PubMed] [Google Scholar]
- 48.Perkins SJ. Protein volumes and hydration effects. The calculations of partial specific volumes, neutron scattering matchpoints and 280-nm absorption coefficients for proteins and glycoproteins from amino acid sequences. Eur J Biochem. 1986;157:169–180. doi: 10.1111/j.1432-1033.1986.tb09653.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.