Abstract
IL-1β and IL-18 are crucial mediators of inflammation, and a defective control of their release may cause serious diseases. Yet, the mechanisms regulating IL-1β and IL-18 secretion are partially undefined. Both cytokines are produced as inactive cytoplasmic precursors. Processing to the active form is mediated by caspase-1, which is in turn activated by the multiprotein complex inflammasome. Here, we show that in primary human monocytes microbial components acting on different pathogen-sensing receptors and the danger-associated molecule uric acid are all competent to induce maturation and secretion of IL-1β and IL-18 through a process that involves as a first event the extracellular release of endogenous ATP. ATP release is followed by autocrine stimulation of the purinergic receptors P2X7. Indeed, antagonists of the P2X7 receptor (P2X7R), or treatment with apyrase, prevent IL-1β and IL-18 maturation and secretion triggered by the different stimuli. At variance, blocking P2X7R activity has no effects on IL-1β secretion by monocytes carrying a mutated inflammasome that does not require exogenous ATP for activation. P2X7R engagement is followed by K+ efflux and activation of phospholipase A2. Both events are required for processing and secretion induced by all of the stimuli. Thus, stimuli acting on different pathogen-sensing receptors converge on a common pathway where ATP externalization is the first step in the cascade of events leading to inflammasome activation and IL-1β and IL-18 secretion.
Keywords: inflammation, nonclassical secretion, pathogen-associated molecular patterns, processing
Inflammation causes a great amount of human morbidity and mortality, caused in large part by infections, but also by noninfective pathologic conditions (1). Microbial components, named pathogen-associated molecular patterns (PAMPs), and molecules released by injured tissues, called danger-associated molecular patterns (DAMPs), trigger pattern recognition receptors (PRRs) resulting in infectious and sterile inflammation, respectively (2–4).
PRR activation leads to expression of many inflammatory genes, including IL-1β (5). This cytokine lacks a secretory signal peptide and is secreted through a nonclassical pathway (6). The production of active IL-1β is tightly controlled on several levels to avoid severe adverse effects (5). The posttranslational regulation is less understood and concerns the process of maturation of the inactive IL-1β precursor (pro-IL-1β) to the active 17-kDa IL-1β. This process requires the assembly of the cytoplasmic multiprotein complex inflammasome (7), responsible for the conversion of the zymogen procaspase-1 into the active caspase-1 that mediates IL-1β processing. As pro-IL-1β cleavage is immediately followed by the release of the mature cytokine, processing and secretion appear to be linked (6, 8, 9). Inflammasomes harboring diverse molecular components have been described (10), increasing the complexity of the system.
Also, IL-18, a pleiotropic cytokine involved in the early events of the defensive innate immune reactions (11), lacks a secretory signal peptide. Unlike IL-1β, IL-18 is constitutively produced by monocytes but, like IL-1β, requires cleavage by caspase-1 to be secreted in its active form (11). However, whether IL-18 processing and secretion are regulated by the same mechanisms that control IL-1β is unclear.
The process of IL-1β secretion can be split into two major steps (8, 9). First, gene expression and synthesis of the IL-1β precursor are induced by inflammatory signals such as PAMPs; then a second signal, namely exogenous ATP, induces inflammasome activation and secretion of mature IL-1β (12).
PRRs include Toll-like receptors (TLRs, at least 10 in humans) and nucleotide oligomerization domain (NOD) proteins (NOD1 and NOD2) (3, 13, 14) that sense specifically different PAMPs. LPS from Gram-negative bacteria is the best-studied ligand for TLR4 (3), whereas Gram-positive bacteria preferentially activate TLR2 through lipoteichoic acid (15). TLR2 and TLR6 are targets for the yeast wall component zymosan (15), whereas TLR5 is the extracellular receptor for bacterial flagellin (16). PAMPs able to gain access to the cytosol, like peptidoglycans, bind NOD proteins (2, 3, 14). Interestingly, muramyl dipeptide (MDP), a component of peptidoglycans, also binds the inflammasome components NALP3 (17) and NALP1 (18). The different PRRs generate signaling cascades converging on similar effects on host cells, including induction of genes related to the inflammatory response (3, 14) and inflammasome activation (19–21). Recently, it has been proposed that not only PAMPs, but also some DAMPs released by injured tissues, such as uric acid, are able to trigger IL-1β secretion (22). Whether DAMPs can provide both the first and the second signal for secretion of active IL-1β and how the different inflammasomes sense and are assembled in response to signals from the various PRRs is still debated.
In mouse macrophages, activation of the purinergic P2X7 receptor (P2X7R) by exogenous ATP is strictly required as second signal for IL-1β processing and secretion in response to extracellular stimuli but not to intracellular bacteria (23). Recent evidence indicates that exogenous ATP is also involved in the intracellular delivery of bacterial molecules through a pore formed by P2X7R and the hemichannel protein pannexin-1, resulting in caspase-1 activation (24).
Also, in LPS-activated primary human monocytes exogenous ATP strongly accelerates IL-1β processing and secretion through a series of events (25), including efflux of K+ from the cell (25, 26), Ca2+ influx, and activation of phospholipases (9, 25, 27). However, LPS alone is sufficient to induce secretion of IL-1β, although at a low extent and with slow kinetics (5, 28, 29). Moreover, it has been proposed that monosodium urate (MSU)-induced IL-1β production is P2X7R-independent (22). These observations raise the possibility that, at least in humans, exogenous ATP is not compulsory, and alternative mechanisms may modulate IL-1β secretion.
The molecular target of the events elicited by exogenous ATP appears to be NALP3, as mice defective in this gene do not secrete IL-1β in response to LPS and ATP (20). Conversely, monocytes from patients affected by autoinflammatory syndromes such as chronic infantile neurologic cutaneous articular (CINCA), bearing mutations on NALP3, secrete huge amounts of IL-1β in response to LPS but do not increase the secretion after ATP stimulation (30). Thus, mutations seem to provide a “gain of function” that releases NALP3 from the requirement of the second signal for activation.
The source of extracellular ATP for IL-1β release in vivo is still questioned. Conceivably, cells injured at the site of inflammation can passively release ATP in amounts sufficient to activate P2X7R. In addition, a pioneering study by Ferrari et al. (31) showed that in microglia and monocytic cells LPS induces the release of ATP, suggesting its involvement in LPS-driven IL-1β secretion. Here, we show that, in human monocytes, agonists of different PRRs trigger the release of endogenous ATP as a common response. The autocrine stimulation of P2X7R by the released ATP is then responsible for the cascade of events that leads to maturation and secretion of both IL-1β and IL-18.
Results
PAMPs and DAMPs Acting on Different TLRs and NLRs Induce IL-1β Secretion at Different Extents.
Unstimulated monocytes from >80% of healthy donors did not synthesize IL-1β during 3 h of incubation on plastic dishes (Fig. 1A). After 15 h, a very weak induction of IL-1β synthesis and secretion was detected in most cases (Fig. 1 A–C). Exposure to ligands specific for different PRRs, including LPS, Staphylococcus aureus, zymosan, flagellin, and MDP strongly induced intracellular pro-IL-1β (Fig. 1A) and different extents of IL-1β processing and secretion (Fig. 1B). In general, IL-1β secretion was detected after 3 h, increased at 6 h, and was still rising at 15 h. Although variable in the different donors (30), IL-1β secretion was induced at higher levels by zymosan and S. aureus. MSU was also able to induce both pro-IL-1β synthesis and IL-1β processing and secretion (Fig. 1 A and C). A synergism was observed after the simultaneous exposure of monocytes to LPS and MDP, flagellin, or MSU (Fig. 1C). Treatment with ATP for up to 30 min did not induce pro-IL-1β synthesis on unstimulated monocytes (data not shown). Longer exposures could not be carried out because of ATP toxicity. In agreement with previous studies (reviewed in ref. 25) a short (15 min) exposure of LPS-stimulated monocytes to exogenous ATP resulted in a strong enhancement of IL-1β release (Fig. 1C). The maximal response to ATP was observed after 6 h and decreased thereafter, being almost undetectable after 15 h of culture with LPS (Fig. 1C).
Fig. 1.
Kinetics of production and secretion of IL-1β in response to different PAMPs and DAMPs. Monocytes were cultured for different periods of time in the absence (CTR) or presence of LPS, MDP, zymosan (ZYM), S. aureus (STAPH A), flagellin (FLAG), or MSU, alone or in association as indicated. (A) Western blot of IL-1β in cell lysates at 3 or 15 h of exposure to stimuli. Migration of the 35-kDa pro-IL-1β molecular form is indicated. (B and C) ELISA of IL-1β released by monocytes cultured for 3, 6, or 15 h with the same PAMPs or DAMPs as in A. Note that LPS+ATP represents the amount of IL-1β released during 15 min of exposure of LPS-treated monocytes to exogenous ATP. (D) ELISA of IL-1β released by monocytes cultured for 6 h with PAMPs or DAMPs (empty columns) followed by 15 min of ATP (filled columns). Results are expressed as ng/ml/106 cells. Values are the mean ± SEM of five experiments performed on monocytes from different donors.
Exogenous ATP stimulation of monocytes activated 6 h with the various PAMPs or DAMPs triggered different levels of secreted IL-1β (Fig. 1D). Higher amounts of IL-1β were secreted by monocytes activated by weak stimuli, such as LPS, that induced lower levels of secretion during the 6 h of incubation, whereas smaller amounts were induced in monocytes activated by stronger stimuli (Fig. 1D).
Phospholipase A2 (PLA2) and Histone Deacetylase (HDAC) Inhibitors Prevent IL-1β Secretion.
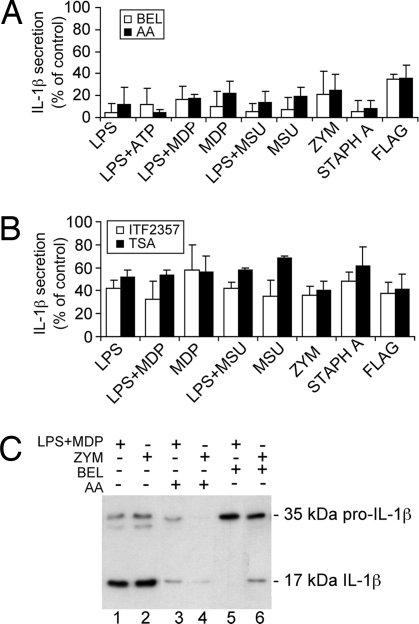
Calcium-independent (27) and calcium-dependent PLA2 (9) and HDAC are implicated in ATP-induced IL-1β processing and release by LPS-treated human monocytes (28). Their involvement in maturation and release of IL-1β induced by stimuli acting on different PRRs was then studied. As shown in Fig. 2A, the calcium-dependent PLA2 blocker arachidonyl trifluoromethylketone (AACOCF3) or the calcium-independent PLA2 inhibitor bromoenol lactone (BEL) (9, 27) significantly prevented IL-1β secretion induced by all of the stimuli tested, in the absence of any sign of cell toxicity (data not shown). A significant inhibition of IL-1β secretion was also obtained with the HDAC inhibitors trichostatin A or ITF2357 (Fig. 2B). As in the case of LPS stimulation (28), HDAC inhibitors required a longer exposure than PLA2 blockers to exert a full inhibitory effect. HDAC inhibitors and AACOCF3 prevented both processing and secretion, as indicated by the failure to detect mature IL-1β inside cells and mature or pro-IL-1β extracellularly (Fig. 2C). At variance, treatment with BEL inhibited processing but not secretion, as shown by the predominant secretion of pro-IL-1β with respect to mature IL-1β (Fig. 2C).
Fig. 2.
Inhibitors of PLA2 and HDAC prevent IL-1β secretion. (A and B) Monocytes were stimulated with the different PAMPs or DAMPs for 3 h in the absence or presence of the PLA2 inhibitors BEL (10 μM; empty columns) or AACOCF3 (AA, 40 μM; filled columns) (A) or for 15 h in the absence or presence of the HDAC inhibitors ITF2357 (100 nM; empty columns) or trichostatin A (TSA, 1 μM; filled columns) (B). Secreted IL-1β was quantified by ELISA. Data are expressed as percent of secretion with respect to untreated cells. Values are the mean ± SD of at least three experiments on monocytes from different donors (P < 0.001 and P < 0.01 in A and B, respectively). (C) Western blot analysis of supernatants from monocytes cultured for 3 h with LPS plus MDP (lanes 1, 3, and 5) or zymosan (ZYM; lanes 2, 4, and 6) in the absence (lanes 1 and 2) or presence of AACOCF3 (AA; lanes 3 and 4) or BEL (lanes 5 and 6). Migration of the 35-kDa pro-IL-1β and the 17-kDa mature IL-1β molecular forms is indicated.
IL-1β Processing and Secretion Is Modulated by K+ Concentration.
Monocytes were exposed to PAMPs or DAMPs in high K+ or in K+-free buffers that impede or induce K+ efflux, respectively (9, 29). As shown in Fig. 3 A and B, in all cases hindering K+ exit resulted in a significant inhibition of IL-1β processing and secretion without impairment of pro-IL-1β intracellular content. The inhibition was specific for IL-1β, as secretion of IL-8, induced by all PAMPs, was unaffected by the elevated extracellular K+ concentrations (Fig. 3C). In contrast, K+-free buffer strongly enhanced IL-1β secretion (Fig. 3B), whereas secretion of the classical secretory proteins IL-8 (Fig. 3C) or IL-6 (data not shown) was inhibited.
Fig. 3.
Role of K+ efflux in IL-1β production, processing, and secretion. (A) IL-1β in cell lysates (Left) and supernatants (Right) of monocytes incubated 3 h alone (lanes 1) or with LPS (lanes 2 and 5) or with LPS plus MDP (lanes 3 and 4) in control medium (5 mM KCl; lanes 1–3) or KCl buffer (150 mM KCl; lanes 4 and 5). Migration of the 35-kDa pro-IL-1β and the 17-kDa mature IL-1β molecular forms is indicated. (B and C) Effect of high K+ buffer or free K+ buffer on IL-1β (B) and IL-8 (C) secreted by monocytes stimulated 3 h with the different PAMPs and DAMPs. Values are the mean of at least four experiments on monocytes from different healthy donors ± SD (P < 0.05).
To investigate the role of cell lysis in K+ efflux, lactate dehydrogenase (LDH) and K+ release were measured at the end of the 3 h of incubation. In control medium, LDH release ranged from a minimum of 10% after LPS stimulation to a maximum of 20% after LPS plus MDP, whereas K+ release was consistently higher, ranging from 25% in culture exposed to LPS to 50% in cultures exposed to zymosan. In K+-free buffer, K+ release reached 60%, whereas the LDH values remained consistently ≤20%.
All Stimuli Induce Active Release of Endogenous ATP, Responsible for P2X7R Activation.
As K+ efflux occurs upon P2X7R activation by ATP (25, 26), we hypothesized that in the absence of exogenously added ATP, P2X7R triggering and the consequent K+ efflux are mediated by endogenous ATP released by activated monocytes. Luciferase assays revealed that indeed ATP is released by monocytes stimulated with the different PAMPs or DAMPs (Fig. 4A) in amounts correlating with the levels of secreted IL-1β (Fig. 4B). The extracellular accumulation of ATP reflects the balance between ATP release by cells and ATP hydrolysis by ectonucleotidases (32). Accordingly, when the different cultures were treated with the ecto-ATPase inhibitor ARL (33), the levels of ATP detected in the different supernatants increased up to 7-fold (Fig. 4A). This rise was not related to cell death, as LDH release was only slightly increased by ARL (up to 1.6-fold) and as similar levels of ATP were detected in cultures untreated or treated with the pancaspase inhibitor zVAD-fmk (Fig. 4A). In agreement with the increased release of ATP, IL-1β secretion was significantly enhanced by ARL treatment (Fig. 4B). Oxidized ATP (oATP) or KN-62, two compounds able to block ATP-induced P2X7R activation (34, 35), significantly inhibited IL-1β release induced by all stimuli (Fig. 5A), in the absence of any relevant decrease in the pro-IL-1β production (Fig. 5B). In contrast, no or little inhibition of IL-8 secretion was observed (Fig. 5C). The inhibitory effect of KN-62 was unrelated to its interference with calcium/calmodulin-dependent kinase pathway, because the calmodulin antagonist W-7, which does not bind P2X7R (36), did not inhibit IL-1β secretion (data not shown). Treatment of monocyte cultures with apyrase, which hydrolyzes ATP (37), similarly impaired secretion of IL-1β (Fig. 5A). The P2X7R inhibitors blocked both processing and secretion, as indicated by the lack of 17-kDa IL-1β in cell lysates (Fig. 5B).
Fig. 4.
All PAMPs and DAMPs induce endogenous ATP release by monocytes. (A) ATP levels in supernatants from monocytes cultured 3 h without (untreated) or with all of the different PAMPs or DAMPs in the absence (CTR) or presence of zVAD (40 μM) and ARL (200 μM) as indicated. Results are expressed as nM of ATP secreted per 106 cells. Values are the mean of at least four different experiments ± SD. (B) IL-1β secreted by monocytes from the same donors under the same experimental conditions, evaluated by ELISA (mean ± SD).
Fig. 5.
Role of P2X7R activation on IL-1β production, processing, and secretion. (A and C) IL-1β (A) and IL-8 (C) secreted by monocytes stimulated 3 h with the different PAMPs or DAMPs in the absence (CTR) or presence of oATP (300 μM), KN-62 (1 μM), and apyrase (2.5 units/ml) as indicated were quantified by ELISA. Values correspond to mean ± SEM of one representative experiment of five performed. IL-1β secretion is significantly inhibited by either oATP (P < 0.001) or KN-62 and apyrase (P < 0.01). (B) Aliquots of cell lysates were analyzed by Western blotting. Migration of the 35-kDa pro-IL-1β molecular forms is indicated. (D) IL-1β secreted by monocytes stimulated 3 h with LPS plus 15 min with ATP or nigericin (NIG), in the absence (CTR) or presence of oATP or KN-62 as indicated was quantified by ELISA. (E) IL-1β secreted by monocytes from one healthy control (HC) and one patient with CINCA syndrome was quantified by ELISA after 3 h of LPS stimulation or after additional 15 min with fresh medium supplemented with 1 mM ATP. oATP significantly inhibited secretion by HC monocytes (P < 0.001) but not by CINCA patient monocytes. Values correspond to four representative experiments ± SD.
The ionophore nigericin promotes K+ efflux, leading to efficient IL-1β processing and secretion (26). As shown in Fig. 5D, neither oATP nor KN-62 affected nigericin-induced IL-1β release, indicating that P2X7R activity is dispensable when K+ efflux is elicited bypassing PRR activation. The effect of oATP was also investigated on monocytes from a patient affected by CINCA syndrome, carrying a mutated NALP3, that upon LPS stimulation, secrete amounts of IL-1β much higher than healthy monocytes, but do not increase secretion in response to ATP (ref. 30 and Fig. 5E). Unlike in healthy monocytes, oATP did not affect IL-1β secretion by NALP3-mutated monocytes (Fig. 5E).
IL-18 Secretion by Activated Monocytes Is Induced by the Various PAMPs and Matches Secretion of IL-1β.
Human monocytes were exposed to PAMPs acting on different TLRs and NODs, and the secretion of IL-18 was investigated at 3 or 15 h. As shown in Fig. 6A, secretion of IL-18 was induced by all of the different PAMPs, indicating that, like for IL-1β, different signaling pathways converge on caspase-1 activation and IL-18 secretion. Notably, although the amount of IL-18 secreted was very low compared with IL-1β, the trend of secretion induced by the different PAMPs paralleled that of IL-1β: higher levels of secretion were indeed detected after stimulation with S. aureus and zymosan, or LPS plus MDP. Moreover, monocytes from the CINCA patient stimulated with LPS secreted higher levels of IL-18 than healthy controls (Fig. 6B).
Fig. 6.
Processing and secretion of IL-18 by monocytes stimulated with PAMPs or DAMPs. (A) Monocytes were cultured with the different stimuli as indicated for 3 or 15 h. Secreted IL-18 was determined by ELISA. Results are expressed as pg/ml/106 cells. Values are the mean ± SD of at least three of five experiments on monocytes from different donors. (B) Comparison of IL-18 secreted by monocytes from one representative healthy control (HC) and one patient with CINCA syndrome, cultured 3 h in the absence (CTR) or presence of LPS. Results are expressed as pg/ml per 106 cells ± SEM. (C) IL-18 secretion by monocytes cultured 3 h with the different stimuli in the presence of oATP. Data are expressed as percent of secretion with respect to untreated cells. Values represent the mean ± SD of at least three experiments on monocytes from different HC (P < 0.01), and the mean ± SEM of three experiments on monocytes from the CINCA patient. (D) Supernatants from monocytes cultured for 3 h with LPS plus MDP in the absence (lane 2) or presence of AACOCF3 (AA; lane 1) or BEL (lane 3) were analyzed by Western blotting with anti-IL-18. Migration of the 24-kDa pro-IL-18 and the 18-kDa IL-18 molecular forms is indicated.
Similarly to what was observed for IL-1β (Fig. 5), secretion of IL-18 was inhibited by oATP in a significant way in monocytes from healthy donors but not in the CINCA patient (Fig. 6C). In addition, in the case of IL-18, whereas the calcium-dependent PLA2 blocker AACOCF3 completely prevented secretion, the calcium-independent PLA2 inhibitor BEL prevented processing only, resulting in release of unprocessed pro-IL-18 by activated monocytes (Fig. 6D).
Discussion
Compelling evidence indicates the crucial role of TLRs in the induction of pro-IL-1β synthesis by extracellular noxia (12). However, whether TLR triggering is sufficient to induce processing and secretion of the cytokine is so far uncertain. Here, we have shown in human monocytes that single signals specific for different TLRs or NOD2 induce not only synthesis but also processing and secretion of IL-1β, although at variable extents. In all cases, PRR stimulation results in secretion of ATP that activates P2X7R through an autocrine loop and triggers a common series of events ending with secretion of mature IL-1β. In vitro, the amount of ATP released by monocytes will determine the amount of IL-1β secreted. In vivo, in the site of inflammation, it is conceivable that variable parameters including the abundance of additional DAMPs released by injured bystander cells and the physico-chemical features of the microenvironment (i.e., redox, levels of K+ or other ions) contribute to modulate the process of IL-1β maturation and release. An additional variable is represented by the levels of ecto-nucleotidases, which rapidly hydrolyze the released ATP (32, 33), thereby limiting IL-1β secretion, as confirmed by the strong increase in ATP and IL-1β in culture fluids when monocytes stimulations are carried out in the presence of ecto-ATPase inhibitors. Ecto-nucleotidases are expressed not only by monocytes (38), but also by parasites and bacteria (39), possibly representing an escape mechanism evolved by pathogens to limit the inflammatory response. Even in the presence of ATPase inhibitors, the ATP measured in monocytes culture fluids is well below the threshold required to stimulate P2X7R. This discrepancy has been found also in other experimental systems [astrocytes (32) and renal glomeruli (33)], where the ATP levels measured in extracellular media significantly underestimate the amount of ATP actually released at the cell surface, because of the fast diffusion and the rapid hydrolysis of the cell-derived ATP (32, 33).
Unlike in mouse macrophages, where flagellin triggers IL-1β processing only if delivered intracellularly (23), in human monocytes extracellular flagellin induces IL-1β synthesis, processing, and secretion. This difference is conceivably caused by the fact that mouse macrophages do not express the flagellin binding site TLR5, whereas human monocytes do (40), as also supported by the strong production of IL-8 induced by flagellin (see Figs. 3C and 5C), consistent with TLR5 activation (41). Not only PAMPs but also DAMPs, such as MSU, elicit a P2X7R-dependent IL-1β secretion, in contrast with a previous observation (22) that blocking P2X7R did not affect the MSU-induced release of IL-1β. This discrepancy may depend on the different cells analyzed (THP1 cell line) and the stimulation protocol used (phorbol esters and MSU), which might activate nonclassical pathways of IL-1β processing, leading to P2X7R-independent IL-1β secretion.
Consistent with previous observations on ATP-dependent IL-1β release (25), inhibition of P2X7R prevents both IL-1β processing and secretion by healthy monocytes. Conversely, blocking P2X7R in monocytes from a CINCA patient did not impair IL-1β secretion. This result supports our previous findings that in CINCA syndrome the first signal is sufficient to induce full activation of the mutated NALP3, and ATP is dispensable (30). Furthermore, this result corroborates the specificity of the oATP-mediated inhibition observed in healthy monocytes: if oATP blocked IL-1β secretion in a non-P2X7R-dependent way, inhibition also would likely be observed in CINCA monocytes.
The different PAMPs and DAMPs used in this study induced comparable pro-IL-1β synthesis but different degrees of ATP and IL-1β release. This finding implies that the single PRRs are differently efficient in inducing ATP externalization and that the amount of ATP released by monocytes is the limiting step in secretion of active IL-1β, unless exogenous ATP is supplied. On these bases, any PRR ligand is likely to elicit two main events: (i) production of pro-IL-1β and synthesis/activation of unknown factors, possibly belonging to inflammasome, required for caspase-1 activation and (ii) ATP release. IL-1β processing and secretion is then handled by the events that follow P2X7R triggering. Later, secreted IL-1β sustains autocrinally its own production and is finally downmodulated by the IL-1 receptor antagonist, also produced by monocytes (5).
In agreement with a previous study (42), the simultaneous stimulation of different PRRs provides addictive effects, thus allowing the innate immune system to modulate the intensity of its response according to characteristics of the inflammatory trigger (virulence and amounts of the pathogens, variety, and relative abundance of DAMPs). The entire set of PRRs is likely redundant, with extensive cross-talk and possibly positive or negative feedback controls (3). The idea of a large number of different sensors converging on relatively few signaling pathways, at least for a common inflammatory response such as IL-1β secretion, is supported by the observation that the same biochemical events previously identified as mediators of ATP-induced secretion [K+ efflux (26), activation of calcium-independent (27) and calcium-dependent (9) PLA2, deacetylation events (28)] are involved in the secretion of mature IL-1β driven by engagement of all of the PRRs tested.
Finally, our study shows that in human monocytes IL-18 processing and secretion is regulated similarly to IL-1β, being induced by all of the PAMPs/DAMPs that induce IL-1β and prevented by the same inhibitors that block its secretion. Moreover, not only IL-1β but also IL-18 secretion is dramatically increased in CINCA monocytes after TLR stimulation.
Like IL-1β, IL-18 has a part in inflammation and immune regulation (43). However, although unlike IL-1β, IL-18 is constitutively produced by cells of several tissues, some of which do not express all of the inflammasome components required for caspase-1 activation (11). On the one hand, the wide distribution of IL-18 may suggest that this cytokine has pleiotropic effects that go beyond its pure proinflammatory activity. On the other hand, it is conceivable that depending on the cell types, IL-18 secretion undergoes different regulation. Indeed, we have previously shown that IL-18 is secreted by dendritic cells, after interaction with other immune cells, mostly in form of precursor, without caspase-1 activation (44, 45). It is possible that the caspase-1 dependency of IL-18 secretion is restricted to monocytes, whereas other mechanisms are active in noninflammatory cells.
Materials and Methods
Chemicals.
ATP, oATP, trichostatin A, KN-62, apyrase, zymosan, and ARL were from Sigma/Aldrich. LPS TLRgrade, AACOCF3, BEL, flagellin, zVAD-fmk, and MSU were from Alexis Biochemicals. MDP, W-7, and nigericin were from Calbiochem. Heat-inactivated S. aureus was obtained from Invitrogen. ITF2357 was synthesized by Italfarmaco.
Cell Cultures.
Human monocytes isolated from buffy coats from healthy controls or heparinized blood from a CINCA patient (kindly provided by M. Gattorno, Giannina Gaslini Institute, after informed consent of the parents approved by the Ethical Board) were enriched by adherence and activated with different stimuli at 37°C in RPMI medium 1640 (Sigma/Aldrich) supplemented with 1% Nutridoma-SP (Roche Applied Science) as described (28, 30). The stimuli used were 1 μg/ml LPS, 3 μg/ml MDP (17), 107 heat-inactivated S. aureus per ml (46), 50 μg/ml zymosan (47), 0.1 μg/ml flagellin (41), and 5 μg/ml MSU (22). When indicated, after 3 h of LPS stimulation, supernatants were replaced with medium containing 1 mM ATP or 20 μM nigericin, and incubation was carried out for 15 min. K+ efflux was modulated by replacing the control medium with high K+ buffer [150 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM Hepes, 1 g/liter of LD-glucose, pH 7.4 (29)] or free K+ buffer [150 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM Hepes, 1 g/liter of LD-glucose, pH 7.4 (9, 29)].
Western Blot Analysis.
Triton X-100 cell lysates and trichloroacetic acid-concentrated supernatants were boiled in reducing Laemmli sample buffer, resolved on 12% SDS/PAGE, and electrotransferred (8, 9). Filters were probed with 3ZD anti-IL 1β mAb (IgG1; obtained from the National Cancer Institute Biological Resources Branch, Frederick, MD) or rabbit anti IL-18 (kind gift of C. A. Dinarello), followed by the relevant secondary Ab (DAKO) and developed with ECL-plus (GE Healthcare).
ELISA Analyses.
IL-1β, IL-8 (R&D Systems) and IL-18 (MBL) content in supernatants from monocyte cultures was determined by ELISA.
Determination of Cell Lysis.
The release of LDH was measured by the colorimetric assay from Sigma/Aldrich.
Measurement of ATP and K+.
Extracellular ATP concentration was determined with an ATP Determination Kit (Invitrogen). The concentration of K+ in supernatants and 0.5% Triton X-100 lysates was assayed in an atomic absorption spectrophotometer (28).
Statistical Analysis.
The data were statistically analyzed by using one-way ANOVA test, followed by Bonferroni posttest, using GraphPad software.
Acknowledgments.
We thank Dr. M. Gattorno for helpful discussion and blood samples from the CINCA patient; Dr. C. A. Dinarello and the National Cancer Institute Biological Resources Branch for the generous gift of Abs; Mr. G. Rossi for K+ measurement; and the Blood Center of San Martino Hospital (Genoa, Italy) for buffy coats. This work was supported in part by grants from Associazione Italiana per la Ricerca sul Cancro, Ministero Salute, Istituto Superiore Sanità, and Telethon. S.C. and S.T. were supported by Italfarmaco.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Kaczorowski DJ, Mollen KP, Edmonds R, Billiar TR. Early events in the recognition of danger signals after tissue injury. J Leukocyte Biol. 2008;83:546–552. doi: 10.1189/jlb.0607374. [DOI] [PubMed] [Google Scholar]
- 2.Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell JA, Paul-Clark MJ, Clarke GW, McMaster SK, Cartwright N. Critical role of toll-like receptors and nucleotide oligomerization domain in the regulation of health and disease. J Endocrinol. 2007;193:323–330. doi: 10.1677/JOE-07-0067. [DOI] [PubMed] [Google Scholar]
- 4.Rubartelli A, Lotze MT. Inside, outside, upside down: Damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28:429–436. doi: 10.1016/j.it.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Dinarello CA. Interleukin-1, interleukin-1 receptors, and interleukin-1 receptor antagonist. Int Rev Immunol. 1998;16:457–499. doi: 10.3109/08830189809043005. [DOI] [PubMed] [Google Scholar]
- 6.Rubartelli A, Cozzolino F, Talio M, Sitia R. A novel secretory pathway for interleukin-1β, a protein lacking a signal sequence. EMBO J. 1990;9:1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: Master switches of inflammation. Cell Death Differ. 2007;14:10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- 8.Andrei C, et al. The secretory route of the leaderless protein interleukin 1β involves exocytosis of endolysosome-related vesicles. Mol Biol Cell. 1999;10:1463–1475. doi: 10.1091/mbc.10.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrei C, et al. Phospholipases C and A2 control lysosome-mediated IL-1β secretion: Implications for inflammatory processes. Proc Natl Acad Sci USA. 2004;101:9745–9750. doi: 10.1073/pnas.0308558101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutterwala FS, Ogura Y, Flavell RA. The inflammasome in pathogen recognition and inflammation. J Leukocyte Biol. 2007;82:259–264. doi: 10.1189/jlb.1206755. [DOI] [PubMed] [Google Scholar]
- 11.Boraschi D, Dinarello CA. IL-18 in autoimmunity: Review. Eur Cytokine Network. 2006;17:224–252. [PubMed] [Google Scholar]
- 12.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: Intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 13.Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 14.Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. Nod-like proteins in immunity, inflammation, and disease. Nat Immunol. 2006;7:1250–1257. doi: 10.1038/ni1412. [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi O, Akira S. Toll-like receptors: Their physiological role and signal transduction system. Int Immunopharmacol. 2001;1:625–635. doi: 10.1016/s1567-5769(01)00010-8. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi F, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 17.Martinon F, Agostini L, Meylan E, Tschopp J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol. 2004;14:1929–1934. doi: 10.1016/j.cub.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 18.Faustin B, et al. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell. 2007;25:713–724. doi: 10.1016/j.molcel.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 19.Kanneganti TD, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;430:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 20.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 21.Sutterwala FS, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 23.Franchi L, Kanneganti TD, Dubyak GR, Nunez G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem. 2007;282:18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- 24.Kanneganti TD, et al. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Ferrari D, et al. The P2X7 receptor: A key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 26.Perregaux D, Gabel CA. Interleukin-1 maturation and release in response to ATP and nigericin: Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- 27.Walev I, et al. Potassium regulates IL-1β processing via calcium-independent phospholipase A2. J Immunol. 2000;164:5120–5124. doi: 10.4049/jimmunol.164.10.5120. [DOI] [PubMed] [Google Scholar]
- 28.Carta S, et al. Histone deacetylase inhibitors prevent exocytosis of interleukin-1β-containing secretory lysosomes: Role of microtubules. Blood. 2006;108:1618–1626. doi: 10.1182/blood-2006-03-014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walev I, Reske K, Palmer M, Valeva A, Bhakdi S. Potassium-inhibited processing of IL-1β in human monocytes. EMBO J. 1995;14:1607–1614. doi: 10.1002/j.1460-2075.1995.tb07149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gattorno M, et al. Pattern of interleukin-1β secretion in response to lipopolysaccharide and ATP before and after interleukin-1 blockade in patients with CIAS1 mutations. Arthritis Rheum. 2007;56:3138–3148. doi: 10.1002/art.22842. [DOI] [PubMed] [Google Scholar]
- 31.Ferrari D, Chiozzi P, Falzoni S, Hanau S, Di Virgilio F. Purinergic modulation of interleukin-1β release from microglial cells stimulated with bacterial endotoxin. J Exp Med. 1997;185:579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joseph SM, Buchakjian MR, Dubyak GR. Colocalization of ATP release sites and ecto-ATPase activity at the extracellular surface of human astrocytes. J Biol Chem. 2003;278:23331–23342. doi: 10.1074/jbc.M302680200. [DOI] [PubMed] [Google Scholar]
- 33.Karczewska J, Martyniec L, Dzierzko G, Stepinski J, Angielski S. The relationship between constitutive ATP release and its extracellular metabolism in isolated rat kidney glomeruli. J Physiol Pharmacol. 2007;58:321–333. [PubMed] [Google Scholar]
- 34.Murgia M, Hanau S, Pizzo P, Rippa M, Di Virgilio F. Oxidized ATP: An irreversible inhibitor of the macrophage purinergic P2Z receptor. J Biol Chem. 1993;268:8199–8203. [PubMed] [Google Scholar]
- 35.Humphreys BD, Virginio C, Surprenant A, Rice J, Dubyak GR. Isoquinolines as antagonists of the P2X7 nucleotide receptor: High selectivity for the human versus rat receptor homologues. Mol Pharmacol. 1998;54:22–32. doi: 10.1124/mol.54.1.22. [DOI] [PubMed] [Google Scholar]
- 36.Zhu W, et al. Ca2+/calmodulin-dependent kinase II and calcineurin play critical roles in endothelin-1-induced cardiomyocyte hypertrophy. J Biol Chem. 2000;275:15239–15245. doi: 10.1074/jbc.275.20.15239. [DOI] [PubMed] [Google Scholar]
- 37.Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1β processing and release. J Immunol. 2004;172:4987–4994. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- 38.Pulte ED, et al. CD39/NTPDase-1 activity and expression in normal leukocytes. Thromb Res. 2007;121:309–317. doi: 10.1016/j.thromres.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hopfe M, Henrich B. OppA, the substrate-binding subunit of the oligopeptide permease, is the major Ecto-ATPase of Mycoplasma hominis. J Bacteriol. 2004;186:1021–1028. doi: 10.1128/JB.186.4.1021-1028.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miao EA, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 41.Ivison SM, et al. Protein kinase D interaction with TLR5 is required for inflammatory signaling in response to bacterial flagellin. Immunol. 2007;178:5735–5743. doi: 10.4049/jimmunol.178.9.5735. [DOI] [PubMed] [Google Scholar]
- 42.Ferwerda G, et al. Engagement of NOD2 has a dual effect on pro-IL-1β mRNA transcription and secretion of bioactive IL-1β. Eur J Immunol. 2008;38:184–191. doi: 10.1002/eji.200737103. [DOI] [PubMed] [Google Scholar]
- 43.Dinarello CA. Interleukin-18 and the pathogenesis of inflammatory diseases. Semin Nephrol. 2007;27:98–114. doi: 10.1016/j.semnephrol.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 44.Gardella S, et al. Interleukin-18 synthesis and secretion by dendritic cells are modulated by interaction with antigen-specific T cells. J Leukocyte Biol. 1999;66:237–241. [PubMed] [Google Scholar]
- 45.Semino C, Angelini G, Poggi A, Rubartelli A. NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood. 2005;42:535–539. doi: 10.1182/blood-2004-10-3906. [DOI] [PubMed] [Google Scholar]
- 46.Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol. 2000;165:5392–5396. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- 47.Dillon S, et al. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J Clin Invest. 2006;116:916–928. doi: 10.1172/JCI27203. [DOI] [PMC free article] [PubMed] [Google Scholar]