Abstract
Multiple congenital ocular anomalies in purebred and crossbred Rocky and Kentucky Mountain horses in Canada are frequently diagnosed with biomicroscopic and indirect ophthalmoscopic examination. In order of frequency detected, these include temporal ciliary epithelial cysts; iridal hypoplasia; prominent corneas; focal temporal retinal degeneration related to ciliary cysts; and, rarely, retinal detachment. A pedigree analysis confirms a dominant mode of inheritance with incomplete penetrance and with a linkage to coat color.
Abstract
Anomalie oculaires congénitales chez des chevaux Rocky et Kentucky Mountain de race pure et croisée au Canada. Des anomalies oculaires congénitales multiples chez des chevaux Rocky et Kentucky Mountain de race pure et croisée au Canada sont souvent diagnostiquées par examens biomicroscopiques et ophtalmoscopiques indirects. Par ordre de fréquence, les anomalies détectées comprennent les kystes épithéliaux ciliaires temporaux, l’hypoplasie iridienne, la cornée proéminente, la dégénération focale de la rétine temporale reliée aux kystes ciliaires et, rarement, le détachement de la rétine. Une analyse du pedigree confirme un mode dominant de transmission génétique avec pénétrance incomplète et liaison à la couleur de la robe.
(Traduit par Docteur André Blouin)
Introduction
Inherited congenital multiple ocular anomalies in Rocky Mountain horses were originally reported by Ramsey et al (1). There are 2 phenotypes of ocular manifestation of these anomalies: temporal ciliary cysts and temporal ciliary cysts with other anomalies, including iridal hypoplasia, megalocornea, retinal dysplasia, focal cataracts, and megaloblepharon (1). The ocular anomalies reported in order of frequency detected are temporal posterior iris, ciliary and retinal cysts, temporal curvilinear streaks of retinal pigment epithelial pigmentation and proliferation, retinal dysplasia, iridal hypoplasia, megalocornea, megaloblepharon, pectinate ligament hypoplasia, and focal goniosynechiae with absence of angle structures (1). Although the corneal shape appears abnormal and gives the perception of megalocornea and anterior megalophthalmos, both may have been misinterpreted initially (1). A follow-up study by the same authors reported that there was not a significant difference in corneal size; intraocular pressures; or the thickness of the dorsal, ventral, or medial peripheral parts of the cornea of affected horses compared with control horses (2). Only the central and temporal parts of the cornea were significantly thicker, and these were unlikely to create the allusion of megalocornea or anterior megalophthalmos, leading to the conclusion that the corneal anomaly is corneal globosa, an abnormally shaped cornea (2).
Multiple ocular anomalies in these horses are inherited; the mode of inheritance proposed was codominance with expression of cysts in heterozygotes and multiple anterior segment anomalies in homozygotes, and incomplete penetrance was suggested (3). Autosomal codominant inheritance is defined as dominant expression of an allele with phenotypic differences noted between confirmed heterozygotes and homozygotes. However, an autosomal recessive mode of inheritance could not be excluded statistically, and examination of out-crosses that evaluated phenotypic ocular disease in heterozygotes and homozygotes was not reported (3).
Based on PubMed, CAB, and Google Scholar searches for the last decade (1997–2007), we did not identify reports of multiple congenital anomalies in Rocky and Kentucky Mountain horses in other countries. The objectives of this study were to determine the prevalence of congenital ocular anomalies in purebred and crossbred Rocky Mountain and Kentucky Mountain horses in Canada, to report the expression and types of anomalies present, and to provide a pedigree of related horses that identifies the mode of inheritance and determines the relationship of the ocular anomalies to coat color.
Materials and methods
The eyes of purebred or crossbred Rocky Mountain horses, Kentucky Mountain horses, and unrelated breeds were examined by a veterinary ophthalmologist with a transilluminator, a biomicroscope (Osram 64222; Carl Zeiss Canada, Don Mills, Ontario), and an indirect ophthalmoscope (Heine Omega 200; Heine Instruments Canada, Kitchener, Ontario) after application of topical 1% tropicamide (Mydriacyl; Sandoz Canada, Boucherville, Quebec) to dilate the pupils.
A pedigree was constructed with related horses to investigate the mode of inheritance of the multiple ocular anomalies and their relationship to coat color. Coat color was assessed by a coat color expert and photographs of all horses were archived for their examination.
Results
A total of 134 horses were included in this study, 97 purebred and F1 and F2 Rocky Mountain crosses, and 37 Kentucky Mountain horses: 48 and 12 horses of each of these groups, respectively, had ocular anomalies (ciliary epithelial cysts, iridal hypoplasia, and prominent corneas) (Table 1). The most common anomalies diagnosed in these horses were bilateral and, rarely, unilateral temporal ciliary epithelial cysts (Figure 1). Other less common bilateral ocular anomalies observed were temporal fundic curvilinear streaks (Figure 2), iridal hypoplasia (Figure 3), and a prominent corneal shape. Multiple anomalies and temporal ciliary epithelial cysts were noted in 7 affected Rocky Mountain horses and 2 Kentucky Mountain horses (Figure 4). All Rocky Mountain horses with iridal hypoplasia and prominent corneas had ophthalmoscopically detectable ciliary epithelial cysts. Cataracts were uncommon: they were noted in only 1 Rocky Mountain and 2 Kentucky Mountain horses affected with cysts and multiple ocular anomalies, but they were present in 14 Rocky Mountain and 5 Kentucky Mountain horses without cysts and other ocular anomalies. Focal retinal degeneration, demarcated by curvilinear streaks presumably related to peripheral ciliary epithelial and retinal cysts, focal retinal detachments, and retinal pigment epithelial hypertrophy and pigmentation were noted frequently in 22 Rocky Mountain horses affected with these anomalies (Figure 2). Retinal tears, vitreous degeneration, and rhegmatogenous retinal detachment were uncommon and noted in only 2 Rocky Mountain horses affected with ciliary cysts and multiple ocular anomalies.
Table 1.
Ocular anomalies noted in the Rocky Mountain and Kentucky Mountain horses in Canada
| Breed | Rocky Mountain horses and (F1 and F2 crosses) n = 97 | Kentucky Mountain horse n = 37 |
|---|---|---|
| No abnormalities except (incipient cataracts)a | 49 (14)a | 25 (5)a |
| Temporal ciliary cysts (with incipient cataracts)a | 41 (1)a | 10 |
| Multiple ocular anomalies (with incipient cataracts)a | 7a | 2 (2)a |
With incipient cataracts
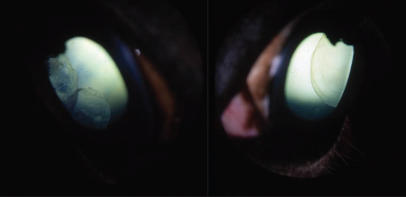
Figure 1.
The most common lesions in Rocky and Kentucky Mountain horses in Canada were large temporal ciliary cysts. Most were bilateral as noted in this Rocky Mountain filly.
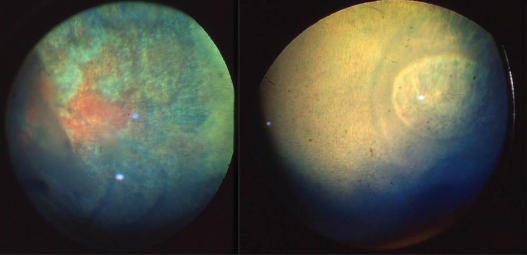
Figure 2.
Other common lesions in this study were focal retinal degeneration with curvilinear streaks related to cysts and focal retinal detachment. Complete retinal detachments were uncommon. Note the hyperreflectivity in the temporal fundi of these 2 Rocky Mountain mares and the multiple curvilinear streaks.
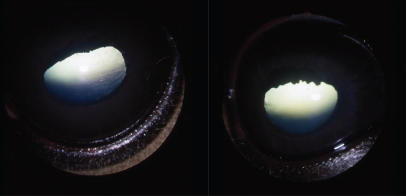
Figure 3.
Iridal hypoplasia was confirmed by a lack of any substantial dilatation after 1% tropicamide was applied for approximately 30 minutes and by small pupils, encircling hypoplastic granula iridica, and thin irides.
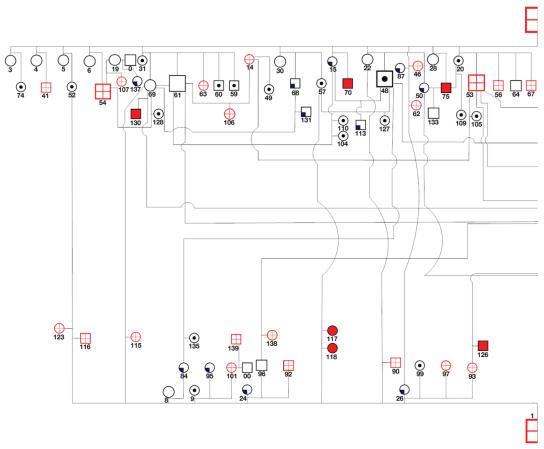
Figure 4.
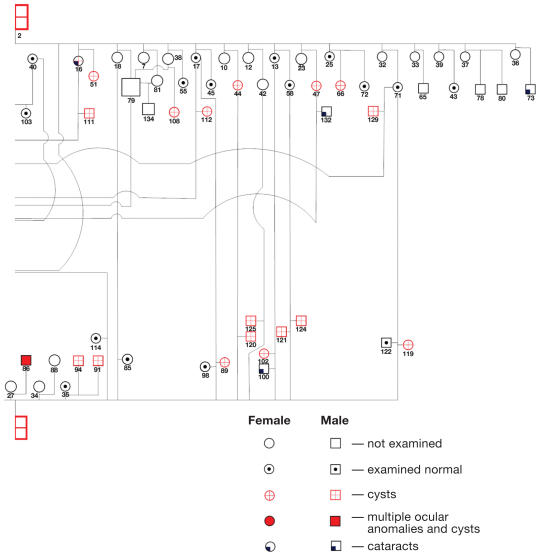
Pedigree of horses affected with Rocky Mountain horse eye anomaly in Canada. Note that cysts were detected in the F1 crossbred generation sired by a Rocky Mountain stallion with cysts (all but 2 dams were gaited breeds). Note also that the F2 generation failed to express ciliary cysts, expressed ciliary cysts alone, expressed ciliary cysts and multiple ocular anomali es, when sired by either a Rocky Mountain stallion with ciliary cysts or a Rocky Mountain stallion with ciliary cysts and multiple ocular anomalies.
The pedigree of related Rocky Mountain horses is complex and precludes sex-linked, autosomal recessive, and codominant modes of inheritance (Figure 4). Both fillies and colts in both the F1 and the F2 generations were affected with both phenotypes of the disease, ruling out sex-linked modes of inheritance. There were multiple F1 foals with temporal ciliary cysts alone, and with multiple ocular anomalies and ciliary cysts born to foundation mares that were not related to Rocky or Kentucky Mountain horses (Figure 4, Table 3). This finding rules out autosomal recessive and codominant modes of inheritance. The F2 generation sired by both foundation Rocky Mountain stallions with temporal ciliary cysts (horse #1) and cysts with multiple ocular anomalies (horse #2) produced foals with no abnormalities, ciliary cysts, or ciliary cysts and multiple anomalies (Figure 4, Table 4). This finding also rules out a codominant mode of inheritance. This pedigree confirms incomplete penetrance of a dominant inherited trait. It appears that ciliary cysts and multiple ocular anomalies are expressed together or not at all in the F1 and F2 generations, and from repeated breedings of the same stallions and mares.
Table 3.
F1 generation of Rocky Mountain horses with coat color and ocular phenotype
| Normal ocular examination | Incipient cataracts only | Temporal ciliary cysts only | Multiple ocular anomalies + cysts | Unknown ocular examination |
|---|---|---|---|---|
| 43. Black | 50. Black | 41. Silver black | 51. Silver black | 42. Silver black |
| 45. Black | 54. Silver black | 44. Silver bay | 56. Chocolate tobiano | 61. Black |
| 48. Black | 57. Silver bay | 46. Silver black | 60. Black | 65. Silver black |
| 49. Black | 58. Silver black | 47. Silver black | 62. Silver black | 69. Silver black |
| 52. Black tobiano | 68. Bay | 53. Chocolate tobiano | 67. Chocolate tobiano | 76. Silver black |
| 55. Dark chestnut | 73. Dark chestnut | 63. Silver black | 70. Silver bay | 77. Silver black |
| 59. Black | 84. Chestnut | 66. Silver black | 75. Silver black | 78. Bay |
| 64. Black | 87. Chestnut | 89. Silver bay | 86. Chestnut | 80. Black |
| 71. Bay | 95. Palomino | 93. Silver black | 90. Silver black | |
| 72. Black | 137. Brown roan | 97. Silver bay | 91. Silver bay | |
| 74. Black tobiano | 101. Chestnut | 92. Silver bay | ||
| 85. Black | 102. Chestnut | 94. Silver bay | ||
| 99. Chestnut | 107. Chestnut | 90. Chestnut | ||
| 109. Black | 108. Silver bay | 116. Silver bay | ||
| 110. Chestnut | 111. Silver bay | 126. Silver black | ||
| 113. Bay | 112. Chestnut tobiano | |||
| 114. Dark Bay | 115. Silver black | |||
| 122. Bay | 120. Chestnut |
Table 4.
F2 generation of Rocky Mountain horses with coat color and ocular phenotype
| Normal ocular examination | Incipient cataracts only | Temporal uveal cysts only | Multiple ocular anomalies + cysts | Unknown — no ocular examination |
|---|---|---|---|---|
| 103. Dark bay | 113. Bay | 106. Silver black | 104. Chestnut | 133. Silver black |
| 105. Chestnut tobiano | 131. Chestnut | 111. Silver black | 108. Silver bay | 134. Silver black |
| 109. Black | 132. Dark bay | 112. Chestnut tobiano | 116. Silver bay | |
| 110. Chestnut | 120. Chestnut | 117. Dark palomino | ||
| 127. Silver bay | 139. Silver bay | 118. Dark palomino | ||
| 128. Red roan | 119. Silver bay | |||
| 131. Chestnut | 121. Chestnut | |||
| 132. Dark bay | 123. Silver bay | |||
| 135. Black | 124. Silver bay
125. Black silver mane and tail 138. Silver bay |
Coat color does appear to be linked to the expression of the ocular anomalies. Specifically, the silver black and silver bay colors were commonly associated with these anomalies (Tables 2–4).
Table 2.
Foundation mares and stallions, breed, number, coat color, and ocular phenotype
| Normal ocular examination | Incipient cataracts only | Temporal ciliary cysts only | Multiple ocular anomalies + ciliary cysts | Unknown — no ocular examination |
|---|---|---|---|---|
| 13. Tennessee walker, chestnut | 15. Kentucky × Rocky Mountain, palomino | 1. Rocky Mountain, silver black | 2. Rocky Mountain, silver black | 0. Tennessee walker, color unknown |
| 17. Saddlebred, chestnut | 24. Saddlebred, chestnut | 14. Rocky × Kentucky Mountain, silver black | 3. Saddlebred, pinto tobiano | |
| 20. Tennessee walker, chestnut tobiano | 25. Tennessee walker × standard bred, black | 16. Rocky Mountain horse, silver black | 4. Standardbred, bay | |
| 31. Tennessee walker, chestnut | 26. Tennessee walker, black | 5. Morgan, black | ||
| 35. Arabian, grey | 6. Tennessee walker, chestnut sabino | |||
| 36. Saddlebred, palomino | 7. Tennessee walker, chestnut sabino | |||
| 37. Standardbred, chestnut | 8. Saddlebred, palomino | |||
| 39. Morgan, chestnut | 9. Pony, palomino | |||
| 40. Standardbred, bay | 10. Welsh pony, bay
12. Tennessee walker, bay 18. Tennessee walker, black 19. Standardbred, bay 22. Tennessee walker, black 23. Tennessee walker, bay 27. Morgan, chestnut 28. Saddlebred, palomino 30. Saddlebred, chestnut 32. Standardbred, bay 34. Tennessee walker, chestnut sabino |
Discussion
The prevalence of multiple ocular anomalies in purebred and crossbred Rocky Mountain and Kentucky Mountain horses in Canada is approximately 50%, which is similar to the prevalence reported in the USA (1). The most common anomalies were temporal uveal cysts, as previously reported by Ramsey et al (1). Focal retinal degeneration and curvilinear streaks were commonly noted and were likely related to previous focal detachments and peripheral retinal cysts. Although we do not have histological confirmation of retinal pigment epithelial pigmentation and retinal degeneration, their ophthalmoscopic appearance was very similar to those described and confirmed with light microscopy by Ramsey et al (1).
In contrast to the study by Ramsey et al (1), we did not document any retinal folds or dysplasia with indirect ophthalmoscopy in our population of horses. This may indicate that in our population of horses, the lesions were either too small to identify with the ophthalmoscope (we did not have ocular tissues for light microscopic examination) or this population does not manifest with retinal dysplasia. Further investigations with light microscopic examination of these anomalies in these horses are required. Similarly, we did not document any filtration angle anomalies in the horses we examined. Although the irides were hypoplastic, as documented by a lack of dilatation after application of a mydriatic and by the thin iris leaflets with small encircling granula iridica, the trabecular meshwork appeared normal on biomicroscopic examination in all horses in the visible temporal and nasal areas.
Focal anterior and posterior cortical cataracts were noted only occasionally in horses affected with ciliary cysts and uveal hypoplasia, but quite frequently in horses without any of these anomalies; thus, they are not likely inherited as part of this syndrome. We also noted more horses with apparent megaloblepharon than were recorded by Ramsey et al (1) (data not shown as eyelid fissure measurements were incomplete). Megaloblepharon may contribute to the perception of increased corneal size and scleral exposure, similar to that seen in brachycephalic dogs with euryblepharon and shallow orbits (4). Further evaluations with caliper and ultrasonographic measurements and advanced imaging (computed tomography, etc.) of the eyelid fissure and orbital structures of affected and normal horses are warranted.
We noted multiple coat colors in the horses affected with ciliary cysts and multiple ocular anomalies (Tables 2–4). In common with observations in previous studies, horses that appear to carry the silver dapple mutation (silver black and silver bay) are those that express most, but not all, of the uveal cysts and multiple ocular anomalies (1–3). The silver dapple allele dilutes black eumelanin pigment and a causative mutation has been identified (5,6). Many of the horses with ocular anomalies that we studied were chestnuts (pheomelanin production only), and it is unknown if these horses also carry the silver dapple mutation. However, 1 black horse (horse #60, Table 3) not suspected to be carrying the silver dapple mutation had an apparent increased eyelid fissure length but no other ocular anomalies and 1 silver bay horse (horse #127, Table 4) suspected to have the silver mutation had a normal ocular examination. This may indicate that these anomalies are linked to coat color; however, they are not likely due to the silver mutation.
One of the herds we examined was unique in that 2 Rocky Mountain stallions were crossbred with multiple unrelated mares of various breeds and coat colors. Only 2 of these foundation mares were Rocky Mountain horses and expressed ciliary cysts. All the other foundation mares were varied cross breeds, including saddlebreds, standardbreds, Morgans, Tennessee walkers, Welsh ponies, and Arabians — all breeds that have not been reported with temporal retinal cysts. Although not all these mares were examined during the study period, 6 were examined and no ocular anomalies were present. These factors make it very unlikely that these foundation mares would be carriers. In addition, the F2 generation (horses #117, 118), sired by foundation stallion (horse #1), which had only temporal ciliary cysts out of F1 mare (horse #57), a phenotypically normal mother, had multiple ocular anomalies (Figure 4). This would not be possible if this condition was inherited as an autosomal recessive trait, unless the mare was a carrier. In contrast to Ewart’s report (3), our pedigree involved crossing Rocky Mountain stallions affected with ciliary cysts or cysts and multiple anomalies with non Rocky Mountain horses. Our pedigree confirms that codominant inheritance is not present, as mare #57, though phenotypically normal, produced offspring with multiple ocular anomalies. Rather, this disorder is inherited with a dominant mode with expression of ciliary cysts, cysts plus multiple ocular anomalies, or normal phenotype, as evidenced by the breeding of F1 mares with 2 foundation stallions (Figure 4). The expression of these anomalies inconsistently in the F1 and F2 generations confirms the incomplete expression of this dominant trait.
Ramsey et al (1,2) and Ewart et al (3) reported that multiple ocular anomalies in the Rocky Mountain horse develop as an anterior segment dysgenesis. Inherited anterior segment dysgenesis in humans and mouse models encompasses a wide range of anomalies, including microphthalmia, Peter’s anomaly, Axenfeld-Reiger syndrome, Sturge-Weber syndrome, posterior polymorphous syndrome, etc. (7–12). Anterior segment dysgenesis has been reported in animals, including aniridia in horses (13), keratolenticular dysgenesis in a kitten (14), congenital anterior staphyloma with rudimentary lens in a calf (15), and microphthalmia in dogs (16). Congenital glaucoma in the horse (17) and the llama (18) also appears to develop secondary to anterior segment dysgenesis. Congenital glaucoma is manifested with buphthalmos and microphakia, and the anterior uvea and filtration angles are always hypoplastic (17,18). The corneal endothelium plays an essential role in anterior segment development and its absence leads to anterior segment dysgenesis (19).
It is possible that Rocky Mountain horse eye anomalies develop partially as anterior segment dysgenesis, given the apparent corneal and eyelid anomalies described by Ramsey et al (1) and Cook (20). However, the lack of inner corneal and lenticular anomalies is considered very unusual in anterior segment dysgenesis. In addition, in subsequent studies, Ramsey et al (2) have confirmed a lack of corneal enlargement and thickness. We also did not document abnormal shapes, internal corneal abnormalities, filtration angle abnormalities, microphakia or congenital cataracts, or microphthalmia or buphthalmia, which have been described previously in inherited anterior segment dysgenesis syndromes (21). We noted only incipient anterior and posterior cortical cataracts in several horses without multiple ocular anomalies, and only occasionally, in horses with ciliary cysts and iridal hypoplasia. The light microscopic sections of the hypoplastic uvea described by Ramsey et al (1) reveals a lack of iris dilator muscle. This muscle and the corpora iridica are derived from the neuroectoderm. Neuroectoderm may play a role in the development of the ocular anomalies of the Rocky Mountain horse: the pathogenesis of inherited multiple ocular anomalies in horses warrants further investigation.
In conclusion, multiple ocular anomalies are prevalent in purebred and crossbred Rocky and Kentucky Mountain horses in Canada. This syndrome is inherited and the mode of inheritance is dominant with incomplete penetrance and it appears to be linked to coat color. The pathogenesis and mutation responsible for this syndrome are unknown.
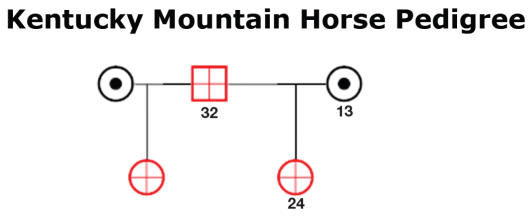
Figure 5.
Pedigree of some of the Kentucky Mountain horses that were related and when examined found to be affected with uveal cysts.
Acknowledgments
The authors thank Jennifer Cowell and Claudia Pequin for their assistance in the development of the pedigree. CVJ
Footnotes
This research was funded by a Dube-Ryan Equine research grant.
Authors’ contributions
Dr. Grahn was the major contributor of the clinical analysis, the pedigree development, and the writing of the manuscript. Dr. Pinard was responsible for collecting the clinical data on the Kentucky Mountain Horse herd, and collaborating with Dr. Sandemeyer in regards to the congenital ocular anomalies in the Rocky Mountain horses in western Canada. Drs. Bellone, Forsyth, and Sheila Archer, were part of our genetics investigation team and their expertise was used in regards to the pedigree development and the coat color association with the multiple ocular anomalies noted in the Rocky Mountain herd during the preparation of the manuscript.
References
- 1.Ramsey DT, Ewart SL, Render JA, Cooke CS, Latimer CA. Congenital ocular abnormalities of Rocky Mountain horses. Vet Ophthalmol. 1999;2:47–59. doi: 10.1046/j.1463-5224.1999.00050.x. [DOI] [PubMed] [Google Scholar]
- 2.Ramsey DT, Hauptman JG, Petersen-Jones SM. Corneal thickness, intraocular pressure, and optical corneal diameter in Rocky Mountain horses with cornea globosa or clinically normal corneas. Am J Vet Res. 1999;60:1317–1321. [PubMed] [Google Scholar]
- 3.Ewart SL, Ramsey DR, Xu J, Meyers D. The horse homolog of congenital aniridia conforms to codominant inheritance. J Hered. 2000;91:93–98. doi: 10.1093/jhered/91.2.93. [DOI] [PubMed] [Google Scholar]
- 4.Bedford PGC. Diseases and surgery of the canine eyelid. In: Gelatt KN, editor. Veterinary Ophthalmology. 3. Philadelphia: Lippincott Williams and Wilkins; 1999. pp. 535–568. [Google Scholar]
- 5.Brunberg E, Andersson L, Cothran G, Sandberg K, Mikko S, Lindgren G. A missense mutation in PMEL17 is associated with the silver coat color in the horse. BMC Genet. 2006;7:46. doi: 10.1186/1471-2156-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reissmann M, Bierwolf J, Brockmann GA. Two SNPs in the SILV gene are associated with silver coat colour in ponies. Anim Genet. 2007;38:1–6. doi: 10.1111/j.1365-2052.2006.01553.x. [DOI] [PubMed] [Google Scholar]
- 7.Gotz W. Transgenetic models of eye malformations. Ophthalmic Genet. 1995;16:85–104. doi: 10.3109/13816819509059967. [DOI] [PubMed] [Google Scholar]
- 8.Yang LL, Lambert SR. Peter’s anomaly: A synopsis of surgical management and outcome. Ophthalmol Clin North Am. 2001;14:467–477. doi: 10.1016/s0896-1549(05)70245-5. [DOI] [PubMed] [Google Scholar]
- 9.Alward WL. Axenfeld-Reiger syndrome in the age of molecular genetics. Am J Ophthalmol. 2000;130:107–115. doi: 10.1016/s0002-9394(00)00525-0. [DOI] [PubMed] [Google Scholar]
- 10.Lines MA, Kozlowski K, Walter MA. Molecular genetics of Axenfeld-Reiger malformation. Hum Mol Gen. 2002;11:1177–1184. doi: 10.1093/hmg/11.10.1177. [DOI] [PubMed] [Google Scholar]
- 11.Williams DL. A comparative approach to anterior segment dysgenesis. Eye. 1993;7:607–616. doi: 10.1038/eye.1993.142. [DOI] [PubMed] [Google Scholar]
- 12.Churchill A, Booth A. Genetics of aniridia and anterior segment dysgenesis. Br J Ophthalmol. 1996;80:669–673. doi: 10.1136/bjo.80.7.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irby NL, Aguirre GD. Congenital aniridia in a pony. J Am Vet Med Assoc. 1985;186:281–283. [PubMed] [Google Scholar]
- 14.Peiffer RL, Belkin P. Keratolenticular dysgenesis in a kitten. J Am Vet Med Assoc. 1983;182:1242–1243. [PubMed] [Google Scholar]
- 15.Rebhun W. Congenital anterior staphyloma with rudimentary lens in a calf. J Am Vet Med Assoc. 1977;171:440–442. [PubMed] [Google Scholar]
- 16.Lewis D, Kelly D, Sansom J. Congenital microphthalmia and other developmental ocular anomalies in the Doberman. J Small Anim Pract. 1986;27:559–566. [Google Scholar]
- 17.Halenda RM, Grahn BH, Sorden SD, Collier LL. Equine congenital glaucoma: Clinical and light microscopic findings in two cases. J Vet Comp Ophthalmol. 1997;7:105–109. [Google Scholar]
- 18.Cullen CL, Grahn BH. Congenital glaucoma in a llama (Llama glama) J Vet Comp Ophthalmol. 1997;7:253–257. [Google Scholar]
- 19.Renker LW, Silversides DW, Xu Li, Overbeek PA. Formation of corneal endothelium is essential for anterior segment development — a transgenic mouse model of anterior segment dysgenesis. Development. 2000;127:533–542. doi: 10.1242/dev.127.3.533. [DOI] [PubMed] [Google Scholar]
- 20.Cook CS. Ocular embryology and congenital malformations. In: Gelatt KN, editor. Veterinary Ophthalmology. 3. Philadelphia: Lippincott Williams and Wilkins; 1999. pp. 3–30. [Google Scholar]
- 21.van Heyeningen V. Developmental eye disease-a genome era paradigm. Clin Genet. 1998;54:272–282. doi: 10.1034/j.1399-0004.1998.5440403.x. [DOI] [PubMed] [Google Scholar]