Abstract
Canine brucellosis is rare in Canada. This report describes an outbreak of Brucella canis infection within a kennel, emphasizing diagnostic and pathologic findings. Gender differences are described. The progestational, nongravid uterus, female spleen, and prostate gland are consistent sites of bacterial isolation.
Abstract
Brucellose canine dans un chenil de Saskatchewan. La brucellose canine est rare au Canada. Ce rapport fait part d’une éclosion d’infection à Brucella canis dans un chenil et décrit les observations diagnostiques et pathologiques. Les différences de genre sont décrites. Les sites constants d’isolement bactérien comprennent l’utérus progestatif non gravide, la rate des chiennes et la prostate.
(Traduit par Docteur André Blouin)
A newly established breeding kennel in Saskatchewan began to experience intermittent abortions during its 1st year of operation (2002). The kennel population consisted of 33 dogs of various ages: 30 Chihuahuas, 1 Lhasa Apso, 1 Pomeranian, and 1 Shih Tzu. During the kennel’s 1st year of operation, the kennel owners recorded 15 matings, resulting in 3 abortions, 2 premature litters, and 2 failures of conception. The attending veterinarian submitted serum from an aborting dam, as well as placental and fetal tissues from the same dog, to the Prairie Diagnostic Services (PDS) laboratory located at the Western College of Veterinary Medicine (WCVM), University of Saskatchewan.
Case description
Serum from the aborting dam was tested by indirect fluorescence (IFA), using fluorescent-labeled, anti-canine immunoglobulin (Ig)G directed against antibodies to Brucella canis (VRMD, Pullman, Washington, USA) according to the manufacturer’s instructions. Placental and fetal tissues were cultured routinely, with isolates identified as Brucella spp. on the basis of colonial morphology and Gram staining (small translucent colonies and Gram-negative coccobacilli), positive Koster staining, and a positive urease test within 30 min. Confirmation of canine brucellosis occurring within the kennel prompted submission of sera from all 33 dogs to the PDS laboratory for serological testing by indirect fluorescence antibody (IFA): 20 dogs were positive for anti-Brucella IgG, with fluorescence detected at titers of 1:100. Of these, 8 female and 5 male dogs were submitted to PDS for blood-culture, euthanasia, and postmortem examination.
From each of these 13 dogs, approximately 5 mL of blood was collected and cultured at 37°C for 7 d in a blood culture medium (Oxoid SIGNAL medium, Basingstoke, Hampshire, UK) prior to the inoculum being transferred to blood agar plates and cultured routinely. The dogs were euthanized, whereupon tissues were sampled for light microscopic examination and bacterial culture. Tissues selected for culture were those considered likely to harbor bacteria (1). Tissues selected for light microscopic examination, including submitted placental and fetal tissue, were routinely fixed in 10% buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin-eosin. Samples of the cultured isolate were sent for confirmation of species to the United States Department of Agriculture (USDA), National Veterinary Services Laboratory, Ames, Iowa, USA; the National Microbiology Laboratory of the Canadian Science Centre for Human and Animal Health (CSCHAH), Health Canada, Winnipeg; the Canadian Food Inspection Agency (CFIA), Brucellosis Centre of Expertise, Ottawa, and from there, to the Department for Environment, Food and Rural Affairs, Veterinary Laboratories Agency (VLA), Surrey, UK.
Serum from all seropositive dogs (20/33) was pooled and diluted with a solution of 1% ovalbumin in neutral phosphate buffered saline to a concentration of 1:200. Following the method of Haines and Chelack (2), this pooled serum was used as a source of primary antibody for avidin-biotin complex immunoenzyme staining of the paraffin-embedded tissues. Negative and omission controls were achieved by the application of normal (uninfected) dog serum prepared and applied in identical manner and by the omission of any primary antibody, respectively.
Isolates submitted to the USDA and the CSCHAH were interpreted as B. canis, whereas those tested by the CFIA and the VLA were interpreted as B. suis biovar 3.
Brucella spp. isolates were recovered in tissues from 12 of the 13 dogs (Table 1), as well as from the placenta, fetal lung, and fetal liver. Of the 8 female dogs, isolates were most frequently obtained from the spleen (6/8) and uterus (6/8); less frequently in the sublumbar lymph nodes (4/7) and blood (4/8), and uncommonly in both mammary gland (1/7) and ovary (2/8). Of the 5 male dogs, only the prostate was consistently positive (4/4), followed by the sublumbar lymph nodes (2/5) and epididymis (1/5). Bacteria were not recovered from the splenic tissue or blood of males. One male dog was culture-negative in all tissues submitted; however, the prostate from this dog was not tested.
Table 1.
Serologic and tissue-culture results from dogs with canine brucellosis
| Dog # | Sex | IFAa | Blood | Lymph nodeb | Spleen | Tonsil | Mammary gland | Ovary | Uterus | Epididymis | Prostate gland |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | + | - | - | +c | ns | - | - | + | ||
| 2 | F | + | + | + | + | ns | + | - | + | ||
| 3 | F | + | - | - | + | ns | - | + | + | ||
| 4 | F | + | - | ns | - | + | - | - | - | ||
| 5 | F | + | + | 3+ | + | ns | - | - | + | ||
| 6 | F | + | - | + | + | ns | ns | - | - | ||
| 7 | F | + | + | + | + | ns | - | + | + | ||
| 8 | F | + | + | - | - | ns | - | - | - | ||
| 9 | M | + | - | + | - | ns | - | 4+ | |||
| 10 | M | + | - | + | - | ns | + | + | |||
| 11 | M | + | - | - | - | ns | - | + | |||
| 12 | M | + | - | - | - | ns | - | + | |||
| 13 | M | + | - | - | - | ns | - | ns |
IFA — Indirect fluorescence using fluorescent-labeled, anti-canine IgG directed against antibodies to Brucella canis
Sublumbar lymph node
+ — Isolate reported as ‘few’ or 1+ unless otherwise indicated
- — No isolate recovered
ns — Not sampled
3+ — Moderate numbers of bacteria isolated
4+ — Large numbers of bacteria isolated
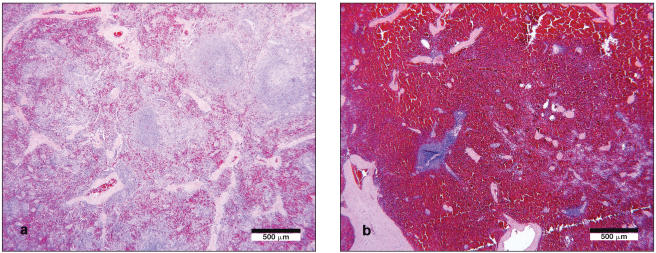
Tissues from 5 females and 5 males were examined histologically. With the exception of the spleen, lymphatic tissues (Peyer’s patches, tonsil, and sublumbar lymph node) of both sexes revealed a consistent lymphocytic to lymphohistiocytic follicular hyperplasia of varying severity. In female dogs, there was an expansion of the splenic white pulp in marked excess of that observed in males (Figure 1).
Figure 1.
a) Lymphohistiocytic proliferation of splenic white pulp was a consistent lesion in females. Hematoxylin and eosin. Bar = 500 μm. b) In male dogs, the splenic white pulp was unremarkable; Hematoxylin and eosin. Bar = 500 μm.
In females, other lesions included lymphohistiocytic endometritis, occurring in the aborting female (1/3); lymphohistiocytic and neutrophilic endophthalmitis, which included a mild lymphoplasmacytic retinitis (1/5); and meningitis (4/5). Meningeal lesions were very mild and multifocal, consisting of rare perivascular clusters of a few lymphocytes and histiocytes. One female dog also had a mild neutrophilic, lymphocytic, and histiocytic infiltrate of the choroid plexus.
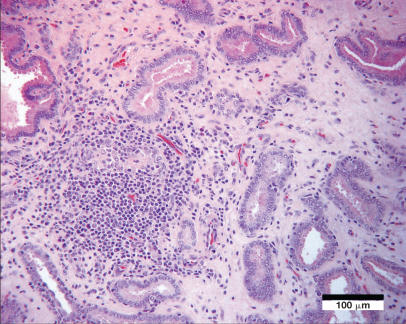
Of the male reproductive tissues, lesions included chronic lymphohistiocytic orchitis, with testicular fibrosis and atrophy (1/5); lymphohistiocytic interstitial epididymitis (3/5), with evidence of intratubular neutrophils and spermatophagic macrophages (1/5); lymphohistiocytic funiculitis (spermatic cord) in 3/4 dogs; and lymphohistiocytic interstitial prostatitis in 4/4 dogs (Figure 2). The prostate gland of 1 dog contained a spermatic granuloma adjacent to an eroded urethral lining. In contrast to the female dogs, only 1 male had mild edema of the choroid plexus. Significant lesions were not present in the other tissues examined from males.
Figure 2.
Interstitial lymphoplasmacytic and histiocytic infiltration of the prostate gland with fibrosis and occasional disruption of glandular profiles; Hematoxylin and eosin. Bar = 100 μm.
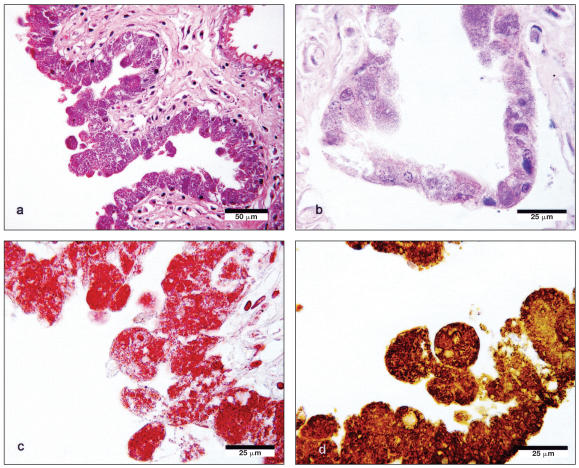
Small numbers of squamous epithelial cells were present in the pulmonary airways of the fetus. Placental trophoblasts were markedly distended by large numbers of Gram-negative bacteria and stained strongly when exposed to antibodies derived from the serum of infected dogs (Figure 3). Staining was not present within infected trophoblasts when they were exposed to normal dog serum or when the addition of a primary antibody was omitted. Positive controls were unavailable. Histochemical (Gram and Giemsa) and immunohistochemical stains did not demonstrate organisms within any of the lesions from adult dogs from which bacteria had been isolated.
Figure 3.
a) Chorioallantoic membrane lined by distended, bacteria-laden trophoblasts. Hematoxylin and eosin. Bar = 50 μm. b) Higher magnification of trophoblasts; Hematoxylin and eosin. Bar = 25 μm. c) Gram-negative bacteria within trophoblasts. Bar = 25 μm. d) Avidin-biotin complex immunoenzyme staining of trophoblasts, using pooled canine sera from infected dogs diluted in PBS/1% ovalbumin to a concentration of 1:200 as primary antibody. Bar = 25 μm.
Discussion
Brucella canis is a potential zoonotic agent that infects dogs and wild canidae almost exclusively. Infection by B. canis is suspected in male dogs that have epididymitis or are infertile, and in female dogs that abort in late gestation or fail to conceive. Occasionally, infection may manifest as systemic disease in various organs, including bone, eyes, and, rarely, brain. Brucella canis is spread via contact with the genitourinary secretions from infected dogs, including aborted placental and fetal material, vaginal secretions from infected females that are in heat, prostatic and seminal fluid, and urine. Following abortion, females may continue to shed bacteria intermittently for weeks or months (3).
The dogs in this case were initially diagnosed with B. canis infection on the basis of clinical history, serological testing, and bacterial culture. Novel findings included differences between male and female dogs in regards to both lesion distribution and tissues most likely to yield bacteria. Further efforts to characterize the recovered isolates by specialized laboratories yielded conflicting interpretations (B. canis and B. suis biovar 3). Currently, 6 species of the genus Brucella are recognized (4). That the distinction between B. canis and B. suis is difficult should not be surprising; B. canis was once considered a biotype of B. suis, with considerable genetic and phenotypic overlap between them (5), emphasizing the complexity and necessary experience associated with speciating some members of such a closely related genus. Currently, Canada is considered free of B. suis biovar 3, the presence of which represents a significant concern to the swine industry, whereas B. canis is considered endemic (6). The origins of the dogs in this case could not be firmly established; all were purchased from Canadian sources. The kennel owners in this case had no knowledge of importation or of exposure to swine or swine offal, potential sources of exposure to B. suis (7–9). Although there is rationale for the diagnosis of B. suis biovar 3 in this case, the sum of evidence suggests infection by B. canis as more likely. Commercially available polymerase chain reaction (PCR) tests typically identify Brucella spp. only to the genus level and were not used here; however, some assays in use at specialized laboratories are able to distinguish between certain species of Brucella (10).
Although frequently suspected in the differential diagnosis of canine abortion, infection by B. canis is infrequently confirmed and rarely reported in Canada. We are aware of only 2 previous cases that were supported by bacteriological confirmation (11). In both cases, infection was ultimately traced to dogs imported to Canada from either Mexico or the southern USA. Serological surveys of dogs in Ontario and Quebec estimate a prevalence of 0.3% and 1.6%, respectively (12,13). Serosurveys from western Canadian populations have not been performed. All of the dogs from this kennel were purchased from other breeders within Saskatchewan, Alberta, and Ontario. It was not possible to investigate the origin of these dogs further, as many of the foundation kennels were no longer in operation. Excluding this case, a search of the PDS database from 1990 to 2004 showed that canine brucellosis was tested for serologically on 29 separate occasions: 1 dog tested positive by IFA, with no bacteria being isolated from a single blood culture. Between 2000 and 2004, prostatitis and epididymitis were diagnosed 9 and 4 times, respectively, from approximately 14 000 canine necropsy and surgical submissions, with none confirmed as brucellosis.
Antemortem diagnosis of B. canis infection in dogs typically relies on serological testing, supported by confirmatory blood culture. Diagnosis can be challenging, with serological false-positives ranging between less than 10% and 75%, depending on the method used (14). In this case, the diagnosis was established first by indirect immunoflourescence. Sensitivity and specificity levels have not been established for this particular test, although, in general, indirect immunoflourescence may have a lower sensitivity than some of the other available serological tests that employ tube agglutination or enzyme immunoassay-based methodologies (3). All of the 13 dogs were seropositive, and confirmatory isolates were collected from all but 1 male. In that dog, the prostate gland, the organ from which bacteria were most reliably isolated in the male group, was not cultured. Lesions consistent with brucellosis were present, suggesting that this dog was not a false seropositive. It is unknown if any of the seronegative dogs that were not examined post mortem might have been false negatives.
Blood culture remains the definitive test for antemortem diagnosis. None of the male and only half of the female group had positive blood cultures, despite evidence of infection. These results underscore the observation that bacteremia in infected dogs is inconsistent and that distinguishing a true from false seropositive result cannot be confirmed on the basis of a single blood culture. Repeat blood cultures of seropositive dogs every 4–6 mo have been recommended to help determine whether or not a serologically positive test is true or false (3).
Although no clinical signs other than abortion were reported, light microscopic lesions were described in a wide variety of tissues. In both sexes, histological lesions were consistent with published reports (1,15,16), with lymphohistiocytic proliferation of the sampled lymphoid tissues typically seen in dogs of both sexes. In addition, differences between males and females were noted in the spleen and other organs.
In the female group, the spleen and uterus were the most consistent sources of bacterial isolation. One dog had recently aborted, and in this dog there was also an endometritis. The other females were not gravid; however, all but 1 of the females were histologically progestational, with the remaining dog containing ovarian follicles in various stages of development, indicative of active cycling. Although this represents a small sample, these findings contrast with those of other reports that cite the nongravid, diestrual uterus as an unfavored site of bacterial isolation (3,16). Descriptively, comparison of the gravid and nongravid uterus through the course of the estrous cycle has not been fully explored in reference to the pathogenesis of B. canis infection in the bitch. Hyperplasia of the splenic white pulp was prominent in females rather than males, correspondent with consistent recovery of bacteria from the spleen of females. The meninges of the females had more frequently occurring and more cellular lesions relative to the males, although they were mild with no history suggestive of any clinical significance.
The lesion distribution in males appears more restricted, with consistent lesions being noted in the prostate gland and epididymis. Correspondingly, the prostate also appears to be the most reliable tissue for bacterial isolation. Differences in the lesion distribution between males and females have not been well characterized, and it is possible that these differences relate to the differences in blood-culture results seen between the male and female dogs in this group. Fetal lesions were unremarkable, despite isolation of B. canis from the liver and lung.
With the exception of placental trophoblasts, efforts to identify the organism within these lesions by histochemical or immunohistochemical staining were unsuccessful, despite bacterial isolation from a variety of tissues. Immunohistochemical staining has been used to identify B. abortus in fresh and formalin-fixed tissues from cattle, using a commercially available, polyclonal primary antibody (17). The application of similar techniques for detection of B. canis antigen is hampered by the lack of a commercially available antibody. In-house production is restricted to level 3 biosecure facilities; a level to which veterinary diagnostic laboratories are not routinely approved (6). In this case, serum from the infected dogs was used as a source of primary antibody for immunohistochemical staining. Placental trophoblasts observed under light microscopy to be clearly distended by bacteria showed clear immunostaining relative to negative controls. However, under these conditions, antigen could not be detected in other tissues from which bacteria were recovered. The use of other antibodies against which B. canis may cross react remains a further avenue for immunohistochemical development. Such cross reactivity has been exploited in the development of an indirect enzyme immunoassay using lipopolysaccaharide (LPS) antigen derived from a variant strain of B. abortus that binds with antibodies from B. canis-infected dogs (18).
Currently, control of canine brucellosis within a kennel typically relies on prevention of infection and euthanasia of infected dogs (3). Diagnosis can be frustrating, with quarantine of suspect animals and repeated cycles of serological testing and hemoculture. In kennels that do elect to treat infected dogs, repeated post-treatment testing is necessary before a dog can be considered to be free of infection. Recrudescence, as well as permanent declines in fertility of sexually intact dogs, especially males, can occur. Despite these caveats, in a recent clinical trial in which the use of enrofloxacin within an infected kennel population was examined, it was found that antibiotic therapy may permit maintenance of fertility, prevent abortions, and stop transmission of the disease to pups, even with persistent seropositivity in some breeding dogs (19). Treatment is usually reserved for pet animals early in the course of infection, typically involving castration or ovariohysterectomy in combination with antibiotic therapy. Details of these measures, as well as their respective pitfalls, have been well presented elsewhere (3).
Of the remaining dogs within the kennel that were not submitted to the WCVM, 1 seropositive dog underwent ovariohysterectomy, was treated by the referring veterinarian, and was kept as a pet within the home. The other 6 seropositive dogs were euthanized. The remaining 13 dogs that were seronegative when initially screened were treated for B. canis infection and retested by IFA on at least 2 separate occasions of unknown interval. All IFA test results from these dogs were negative, following which they were permitted to resume their reproductive careers within the kennel. Blood cultures were not performed. To the time of writing, the breeder has reported no abortions within the kennel subsequent to this outbreak.
Acknowledgments
We thank the staff of the Prairie Diagnostic Services, Inc. immunology section for assistance with immunohistochemical testing and interpretation, and Jennifer Cowell of the WCVM for assistance in the preparation of the figures. CVJ
Footnotes
Reprints will not be available from the authors.
Dr. Brennan was supported by a Western College of Veterinary Medicine Interprovincial Graduate Student Fellowship. Funding for this report was provided by the Western College of Veterinary Medicine Companion Animal Health Fund.
Authors’ contributions
Drs. Brennan and Allen interviewed the breeder and attending veterinarian. Dr. Brennan conducted the histologic evaluation of the formalin-fixed tissues, participated in the immunohistochemical testing of tissues, reviewed the literature, and contributed significantly to the writing of the manuscript and the production of figures. Dr. Ngeleka conducted or supervised the serologic tests, isolated and identified the bacteria and interpreted the results. Dr. Philibert was involved with the postmortem examinations and interpretation of the histologic sections. Dr. Forbes provided guidance regarding the testing of the bacterial isolates and the interpretation of the results. Drs. Ngeleka, Philibert, and Forbes provided input on the content and style of the manuscript. Dr. Allen provided oversight on many aspects of the investigation: review and interpretation of histologic sections and guidance on the immunohistochemical testing of tissues, contributed significantly to the writing and revision of the manuscript, and refinement of the figures.
References
- 1.Carmichael LE. Brucella canis. In: Nielsen K, Duncan JR, editors. Animal Brucellosis. Boca Raton: CRC Pr; 1990. pp. 335–350. [Google Scholar]
- 2.Haines DM, Chelack BJ. Technical considerations for developing enzyme immunohistochemical staining procedures on formalin-fixed paraffin-embedded tissues for diagnostic pathology. J Vet Diagn Invest. 1991;3:101–112. doi: 10.1177/104063879100300128. [DOI] [PubMed] [Google Scholar]
- 3.Carmichael LE, Greene CE. Canine brucellosis. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3. Philadelphia: Elsevier Saunders; 2006. pp. 369–381. [Google Scholar]
- 4.International Committee on Systematics of Prokaryotes [page on the Internet] Taxonomic subcommittees, Genus Brucella. [Last Accessed: 25 February 2008];Subcommittee on the Taxonomy of Brucella. Available at: http://www.the-icsp.org/subcoms/Brucella.htm Last modified: 12 February 2008.
- 5.Moreno E, Cloeckaert A, Moriyon I. Brucella evolution and taxonomy. Vet Microbiol. 2002;90:209–227. doi: 10.1016/s0378-1135(02)00210-9. [DOI] [PubMed] [Google Scholar]
- 6.Canadian Food Inspection Agency [page on the Internet] Animals, Animal Pathogens Biohazard Containment and Safety, Food Animals Disease Agents. [Last Accessed: 25 February 2008];Pathogen Safety Data Sheet: Brucellosis. Available at: http://www.inspection.gc.ca/english/sci/bio/anima/disemala/brucelle.shtml. Last modified: 26 May 2005.
- 7.Kormendy B, Nagy G. The supposed involvement of dogs carrying Brucella suis in the spread of swine brucellosis. Acta Vet Acad Sci Hung. 1982;30:1–7. [PubMed] [Google Scholar]
- 8.Neiland KA, Miller LG. Experimental Brucella suis type 4 infections in domestic and wild Alaskan carnivores. J Wildl Dis. 1981;17:183–89. doi: 10.7589/0090-3558-17.2.183. [DOI] [PubMed] [Google Scholar]
- 9.Barr SC, Eilts BE, Roy AF, Miller R. Brucella suis biotype 1 infection in a dog. J Am Vet Med Assoc. 1986;189:686–687. [PubMed] [Google Scholar]
- 10.Bricker BJ. PCR as a diagnostic tool for brucellosis. Vet Microbiol. 2002;90:435–446. doi: 10.1016/s0378-1135(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 11.Forbes LB, Pantekoek JF. Brucella canis isolates from Canadian dogs. Can Vet J. 1988;29:149–152. [PMC free article] [PubMed] [Google Scholar]
- 12.Bosu WTK, Prescott JF. A serological survey of dogs for Brucella canis in southwestern Ontario. Can Vet J. 1980;21:198–200. [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins H, Hoquet R, Bourque R, Gosselin Y. A serological survey for Brucella canis in dogs in the province of Quebec. Can Vet J. 1979;20:315–317. [PMC free article] [PubMed] [Google Scholar]
- 14.Carmichael LE, Shin SJ. Canine brucellosis: A diagnostician’s dilemma. Semin Vet Med Surg (Small Anim) 1996;11:161–165. doi: 10.1016/s1096-2867(96)80028-4. [DOI] [PubMed] [Google Scholar]
- 15.Gordon JC, Pue HL, Rutgers HC. Canine brucellosis in a household. J Am Vet Med Assoc. 1985;186:695–698. [PubMed] [Google Scholar]
- 16.Carmichael LE, Kenney RM. Canine brucellosis: The clinical disease, pathogenesis, and immune response. J Am Vet Med Assoc. 1970;156:1726–1734. [PubMed] [Google Scholar]
- 17.Perez J, Quezada M, Lopez J, Casquet O, Sierra MA, Martin de las Mulas J. Immunohistochemical detection of Brucella abortus antigens in tissues from aborted bovine fetuses using a commercially available polyclonal antibody. J Vet Diagn Invest. 1998;10:17–21. doi: 10.1177/104063879801000104. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen K, Smith P, Conde S, et al. Rough lipopolysaccharide of Brucella abortus RB51 as a common antigen for serological detection of B. ovis, B. canis, and B. abortus RB51 exposure using indirect enzyme immunoassay and fluorescence polarization assay. J Immunoassay Immunochem. 2004;25:171–182. doi: 10.1081/ias-120030526. [DOI] [PubMed] [Google Scholar]
- 19.Wanke M, Delpino M, Baldi P. Use of enrofloxacin in the treatment of canine brucellosis in a dog kennel (clinical trial) Theriogenology. 2006;66:1573–1578. doi: 10.1016/j.theriogenology.2006.01.034. [DOI] [PubMed] [Google Scholar]