Abstract
The carboxyl tail of G protein-coupled receptors contains motifs that regulate receptor interactions with intracellular partners. Activation of the human neutrophil complement fragment C5a receptor (C5aR) is terminated by phosphorylation of the carboxyl tail followed by receptor internalization. In this study, we demonstrated that bulky hydrophobic residues in the membrane proximal region of the C5aR carboxyl tail play an important role in proper structure and function of the receptor: Substitution of leucine 319 with alanine (L319A) resulted in receptor retention in the endoplasmic reticulum, whereas a L318A substitution allowed receptor transport to the cell surface, but showed slow internalization upon activation, presumably due to a defect in phosphorylation by both PKC and GRK. Normal agonist-induced activation of ERK1/2 and intracellular calcium release suggested that the L318A mutation did not affect receptor signaling. Binding of GRK2 and PKCβII to intracellular loop 3 of C5aR in vitro indicated that mutagenesis of L318 did not affect kinase binding. Limited proteolysis with trypsin revealed a conformational difference between wild type and mutant receptor. Our studies support a model in which the L318/L319 stabilizes an amphipathic helix (Q305–R320) in the membrane proximal region of C5aR.
Keywords: Chemoattractant receptor, endocytosis, protein folding, cytoplasmic helix 8
Introduction
Neutrophil activation with the complement fragment C5a is involved in the regulation of numerous immunological processes, including host defense and inflammation [1]. The C5a receptor (C5aR) is a classical chemoattractant receptor that is primarily expressed in neutrophils. C5a binding to C5aR initiates a signal transduction cascade, which results in cell activation and movement from circulation into the tissue along the gradient of agonist.
C5aR belongs to a family of seven transmembrane helix G protein-coupled receptors (GPCRs) and preferentially activates members of the Gi family of heterotrimeric G proteins. Binding of agonist initiates conformational changes in the receptor such that the receptor can interact with and activate specific heterotrimeric G proteins. Activated G proteins stimulate or inhibit specific effector proteins, which eventually result in specialized functional outcomes. Receptor phosphorylation is an important physiological mechanism that protects cells against harmful overstimulation. Activated C5aR undergoes phosphorylation by both PKC, a second messenger activated kinase, and by G protein-coupled receptor kinases (GRKs) [1]. Seven members of three GRK subfamilies are known to date. They display different tissue distribution, physical properties and substrate specificity for GPCRs (for review, see [2]). For instance, GRK2 and 6 are expressed at high levels in immune cells including neutrophils [3]. The role of GRK2 in C5aR phosphorylation is inconclusive: Overexpression of GRK2 in COS cells enhanced phosphorylation of C5aR [4], while no translocation of GRK2 was detected to membranes of C5a-activated leukocytes [5].
Internalization and trafficking of the receptor through the endosomal system is regulated by many interactions providing specificity for protein targeting. Endocytosed receptors accumulate initially in the early (sorting) endosomes from which they are targeted to lysosomes for degradation or transported back to the cell surface, either directly or via a recycling compartment. Numerous studies of GPCR endocytosis have described multiple internalization signals as well as cognate interactive partners that determine the final destination of the receptor. Many of the signals consist of short linear amino acid sequences that were classified as tyrosine- or di-leucine based motifs [6]. In addition to classical di-leucine motifs that require the presence of acidic residues upstream of di-leucine ([D/E] X2-3 L [L/I]), a few unconventional di-leucine signals have been involved in protein endocytosis. Some of them have one or several serines that become phosphorylated upstream of di-leucine [7]. Classical di-leucine motifs are usually part of nonstructured loops, while a serine-dependent motif is likely to adopt a coil structure [8].
In our previous study we analyzed trafficking pathways of C5aR in CHO cells and neutrophils [9]. We found that ligand activation resulted in degradation of a significant percentage of the receptor during each round of internalization. A search of the amino acid sequence of the putative intracellular domains of C5aR did not reveal any classical tyrosine- or di-leucine based motifs for internalization. Nevertheless, C5aR contains di-leucine in the membrane proximal region of the carboxyl tail that is accompanied by phosphogroup acceptors, S317 and S314, at -1 and -4 positions. We decided to examine the role of the di-leucine L318/L319 in C5aR internalization.
Materials and Methods
Reagents and antibodies
Human recombinant C5a was purchased from Sigma-Aldrich (St.Louis, MO) or Biovision Research Products (Mountain View, CA). 125I-labeled recombinant C5a was from Perkin Elmer (Boston, MA). Trypsin Gold, Mass spectrometry grade was obtained from Promega Corporation (Madison, WI). Pertussis toxin was purchased from Sigma-Aldrich (St. Louis, MO). Endoglycosidase H was obtained from New England Biolabs Inc. (Ipswich, MA). Affinity purified rabbit polyclonal anti-GRK2 (c-15), anti-RhoA (119), and mouse monoclonal anti-Arf6 (3A-1) antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Rabbit polyclonal anti-phospho-PKC(pan) (βIISer660) and rabbit monoclonal anti-phospho-p44/42 MAPK (Thr202/Tyr204) (197G2) antibodies were obtained from Cell Signaling Technology Inc. (Danvers, MA). Rabbit polyclonal anti-p44/42 MAPK antibody was from Upstate Cell Signaling Solutions (Lake Placid, NY). The polyclonal anti-C5aR serum [9] raised against the C-terminal 12 amino acids of the receptor was affinity purified using recombinant GST-C5aR carboxyl tail as an antigen. Monoclonal antibody 32-G1 against C5aR phosphorylated on Ser-334 was described previously [10]. Horseradish-peroxidase-conjugated goat anti-mouse and anti-rabbit antibodies were purchased from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA). Alexa™488-conjugated goat anti-rabbit antibody was obtained from Molecular Probes Inc. (Eugene, OR).
Oligonucleotide-directed mutagenesis of C5aR and CHO cell transfection
Point mutations of human C5aR were generated by oligonucleotide-directed mutagenesis using single-stranded DNA template and confirmed by DNA sequencing (Nevada Genomics Center, University of Nevada – Reno). cDNA with mutation was inserted into pBGSA expression vector [11]. Constructs were transfected into Chinese hamster ovary (CHO) cells using Lipofectamine™2000 transfection reagent (Invitrogen Life Technologies) according to the manufacturer's recommendations. CHO cells were cultured in α-modified Eagle's medium (α-MEM; Sigma) supplemented with 5% fetal bovine serum (FBS), 50 units/ml penicillin and 50 μg/ml streptomycin. Stable transfectants were selected with 0.5 mg/ml of G-418. Twelve to sixteen single colonies were tested for C5aRs expression and cellular localization by immunofluorescence microscopy. A few clones with expression levels similar to the wild type receptor were chosen for further study. Wild type C5aR and C5aR L318A mutant showed similar binding affinity for 125I-C5a (Perkin Elmer).
Indirect immunofluorescence microscopy
CHO cells expressing C5aRs were grown on a glass cover slip. After the experiment, cells were rinsed with PBS, fixed with 2.5% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) for 1 h and permeabilized with 0.01% saponin in PBS/0.2% gelatin solution. Antibody labeling was performed with rabbit anti-C5aR diluted in PBS/saponin/gelatin solution. Secondary Alexa™488-conjugated goat anti-rabbit antibody was used at 1:1000 dilution. The coverslips were mounted in 2.5 mg/ml DABCO/Glycergel (Sigma) and viewed using a 63× objective fitted to a Axioskop 2 plus microscope (Carl Zeiss Inc., Thornwood, NY). The digital images were cropped, assembled, and labeled in Adobe Photoshop.
Ca2+ mobilization assay
CHO cells expressing C5aRs were induced overnight with 6 mM Na butyrate, then incubated with 1 mM EDTA, pH 8.0 in Ca2+/Mg2+ - free PBS to remove cells from plates. Release of intracellular Ca2+ in response to C5a or ATP stimulation was detected using fluorescent marker Fura 2-AM (Molecular Probes) as previously described [12]. To determine EC50 values, the relative amount of C5a-induced calcium release was calculated by subtracting the base line from the C5a peak value. Data were analyzed by non-linear regression analysis using Prism 3.0 software (GraphPad Software, San Diego, CA).
ERK1/2 activation assay
CHO cells expressing C5aRs were induced overnight with 6 mM Na butyrate and pre-incubated in serum-free medium for 4 h to reduce background ERK1/2 activation caused by growth factors in the serum. Cells were stimulated with C5a in a time- or dose-dependent manner, rinsed with cold PBS and collected in Laemmli sample buffer [13]. Cell lysates were analyzed in western blots using rabbit anti-ERK1/2 and anti-phospho-ERK1/2 antibodies.
Radioligand internalization assay
Monolayers of CHO cells expressing C5aR or C5aR L318A were stimulated for 3, 10 and 30 min with 100 nM C5a and 125I-C5a (Perkin Elmer, Boston, MA) at a 100:1 ratio. After incubation cells were rinsed with cold PBS. To remove a surface bound ligand half of the dishes representing each time point were rinsed with acidic PBS pH 2.8. Cells were lysed in 1% Triton X-100, collected with cotton swabs and counted in the Apex™ Series 10/200 Plus gamma-counter (ICN Biomedical Inc., Irvine, CA). Non-specific binding was determined using non-transfected CHO cells. The specific radioligand uptake was quantified essentially as previously described by Pollok-Kopp et al. [10].
Pulse-chase dephosphorylation assay
Stably transfected CHO cells were placed in fresh complete medium for 15 min, and then stimulated with 50 nM C5a for 10 min (pulse). Cells were rinsed twice with cold PBS to remove ligand. After adjustment to original incubation conditions with warm complete medium, cells were further incubated for indicated times (chase). For a chase time longer than 1 h, 1 mM cycloheximide was included in the medium to prevent synthesis of new proteins. After the experiment, cells were briefly washed with ice-cold PBS, collected in PBS with protease inhibitor cocktail III (Calbiochem) and sonicated briefly. The total membranes were collected by centrifugation at 21,000 × g for 20 min and solubilized in Laemmli sample buffer.
Western blotting
Protein samples were lysed in Laemmli sample buffer, heated at 60°C for 15 min, separated on 8% or 10% SDS-polyacrylamide gel, and transferred to a 0.45 μm nitrocellulose membrane using a semi-wet unit (Bio-Rad). To confirm equal loading and efficient transfer, the membrane was briefly stained in a water-soluble protein dye, 0.1% Ponceau S in 3% glacial acetic acid. The membrane was blocked in 10% non-fat dry milk in Tris buffered saline with 0.5% Tween-20 (TBST) for at least 1 h. After extensive washes with PBS, the membrane was incubated with a primary antibody for 1 h at room temperature or overnight at 4°C. Primary antibodies were diluted in TBST containing 5% BSA. The following dilutions were used: rabbit anti-C5aR, 1:1000; anti-ERK1/2, 1:4000; anti-phospho-ERK1/2, 1:1000; anti-GRK2, 1:200; anti-phospho-PKCβII, 1:1000; anti-RhoA, 1:1000; mouse 32-G1, 40 μg/ml; and anti-Arf6, 1:500.
Recombinant protein design, expression and in vitro binding assay
Three constructs, GST-C5aR il1 (T62-W74), GST-C5aR il2 (R134-L152) and GST- C5aR il3 (I225-L241) were obtained by annealing multiple oligonucleotides. Wild type and mutant cytoplasmic tails (N296-V350) were amplified by PCR and cloned into pGEM T-easy vector (Promega) followed by sub-cloning into pGEX T4-1 expression vector (Amersham Biosciences). The integrity of all constructs was verified by nucleotide sequencing (Nevada Genomics Center, Reno, NV). Recombinant proteins were expressed in E. coli and affinity purified according to the manufacturer's protocol (Amersham Biosciences). Due to the relatively high content of hydrophobic residues in the intracellular domains of C5aR, cells expressing GST-tagged proteins were induced with 100 μM IPTG for 16 h at low temperature (18°C). Initial lysis was performed in the buffer containing 1% Triton X-100.
For in vitro binding assay, 25μg of GST fusion protein was mixed with 150 μl of neutrophil cytosol (an equivalent of 108 cells/ml) in binding buffer (100 mM Tris, pH 7.0, 1% octylglucoside, 10% sorbitol, 100 mM NaCl, 1mM EDTA and protease inhibitor cocktail). Binding was allowed for 4-5 h at 4°C with rotation. BSA-blocked Glutathione-Sepharose beads (15 μl) were added for an additional 30 min incubation. Beads were washed twice with binding buffer. Complexes were eluted with 20 mM reduced glutathione (pH 7.4), mixed with Laemmli sample buffer and separated on 10% SDS-polyacrylamide gel. Interacting proteins were detected by western blotting, as described above.
Preparation of neutrophil cytosol
Neutrophils were purified by gelatin sedimentation from human venous blood of healthy donors. Cell activation and lysis were performed as previously described [14]. Cytosol was frozen in aliquots at -70°C.
Limited proteolysis with trypsin
CHO cells expressing C5aR or C5aR L318A were collected in PBS with protease inhibitor cocktail. After a brief sonication, membranes were sedimented at 21,000 × g for 20 min at 4°C and then solubilized with 1% Triton X-100 in sterile PBS on ice for 1 h. Clarified membrane extract was used in the experiments. Trypsin stock solution and dilutions were made according to the manufacturer's recommendation (Promega). To select a trypsin dilution, CHO membranes were treated with increasing concentrations of trypsin for 1 h at room temperature. Proteolysis was stopped by placing samples on ice for 10 min, adding Laemmli sample buffer and heating at 60°C for 5 min. A time-course digest was performed on duplicate membrane aliquots using 1.25 μg/ml trypsin. C5aR proteolysis was detected by western blotting using anti-C5aR antibody.
Results
Mutation of L319 results in retention of C5aR in the endoplasmic reticulum
We have previously demonstrated that about 50% of C5aR undergoes degradation during 3 hours of continuous agonist stimulation, both in neutrophils and stably transfected CHO cells [9]. Based on pulse-chase-pulse studies of receptor phosphorylation, we were also able to determine that about 60-70% of the receptor recycled back to the cell surface [9]. To determine whether di-leucine (L318/L319) located in the membrane-proximal region of the C5aR cytoplasmic tail is part of an internalization motif, we generated single and double leucine to alanine mutations and expressed the mutants in CHO cells. Immunofluorescence analysis showed that C5aR L318A was expressed on the cell surface, whereas the C5aR L318A/L319A and the C5aR L319A mutants localized in the perinuclear region with a staining pattern characteristic of the endoplasmic reticulum (ER) (Figure 1A).
Figure 1. Exchange of L319 to alanine resulted in retention of C5aR in the ER.
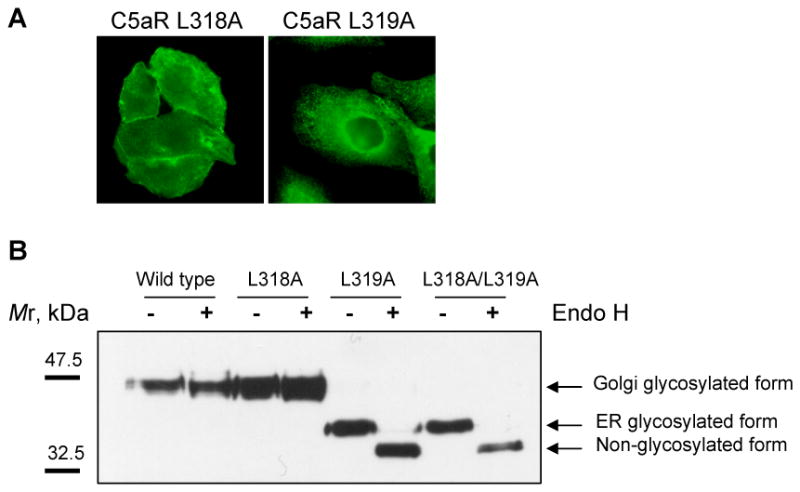
A) Immunofluorescence microscopy of stably transfected CHO cells expressing either C5aR L318A or C5aR L319A. Immunostaining with anti-C5aR antibody showed plasma membrane localization of C5aR L318A and intracellular retention of C5aR L319A. The images are representative of four different clones for each mutant receptor. B) Analysis of susceptibility of wild type C5aR and di-leucine mutants to Endo H treatment. Membranes of CHO cells expressing either wild type or mutant C5aR were denatured for 10 min at 60°C and treated with Endo H according to the manufacturer's recommendations (New England Biolabs Inc.). Non-treated (-) and Endo H treated (+) membranes were separated in 10% SDS-polyacrylamide gel and analyzed by western blotting using anti-C5aR antibody. Arrows indicate Golgi-glycosylated, ER-glycosylated and non-glycosylated forms of C5aR. One representative image is shown.
Previous studies have shown that certain conformational changes in transmembrane proteins lead to ER retention [15]. The localization of N-glycosylated proteins in the ER can be verified by removal of their characteristic high-mannose glycans with endoglycosidase H (Endo H). Upon entry to Golgi, the proteins become terminally glycosylated and are no longer Endo H sensitive [16]. We treated crude membranes of the CHO transfectants with Endo H and detected the receptors by western blotting. We found that the vast majority of the non-treated mutant C5aR L319A and C5aR L318A/L319A migrated faster than C5aR and C5aR L318A in SDS-polyacrylamide gel, indicating different post-translational modifications (Figure 1B). Upon Endo H treatment these fast migrating mutants were successfully trimmed, further increasing their mobility. The observed sensitivity of C5aR L319A and C5aR L318A/L319A to Endo H suggested that the receptors had not been transported beyond ER. In contrast, C5aR and C5aR L318A were resistant to Endo H digestion, implying their advanced processing in the Golgi. Therefore, we conclude that mutation of L319 in the carboxyl tail of C5aR resulted in ER retention, whereas mutation of the neighboring L318 did not interfere with trafficking through the secretory pathway.
Mutant receptor C5aR L318A is characterized by attenuated internalization
To examine the effect of the L318A mutation on ligand-induced endocytosis, we carried out immunofluorescence microscopy. We found that unlike wild type receptor, a significant proportion of the mutant was retained on the cell surface after a 3 h stimulation with 100 nM C5a (Figure 2A). To better visualize internalized receptor, we stimulated cells in the presence of ammonium chloride, a weak base that inhibits ligand dissociation and receptor recycling due to the reduction of endosome acidification [17]. Indeed, inhibition of the recycling step increased the intracellular retention of C5aR L318A (Figure 2) where it colocalized with early endosome antigen protein 1 (data not shown).
Figure 2. Internalization of C5aR L318A occurs at a reduced rate.
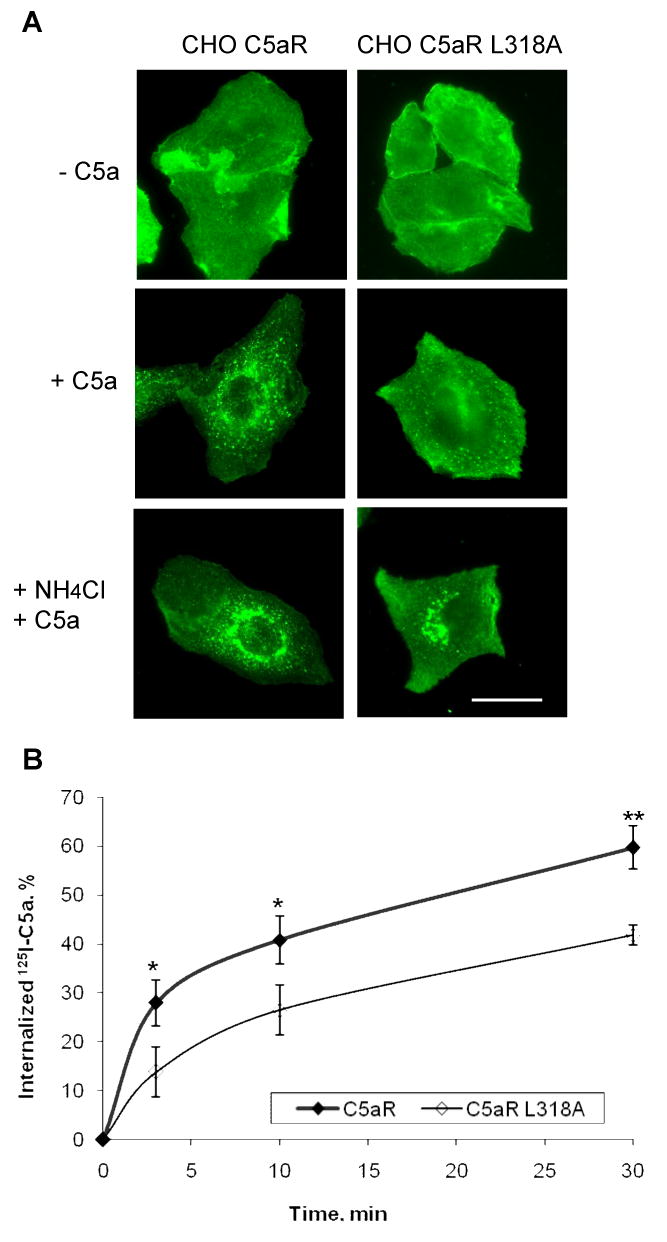
A) Stable CHO transfectants were incubated for 3 h with or without 100 nM C5a. To accumulate internalized receptor, cells were incubated with ligand and 50 mM NH4Cl. Receptors were visualized using rabbit anti-C5aR antibody. Representative images of two independent experiments are shown. Bar 50 μm. B) CHO transfectants were incubated at 37°C with 125I-C5a for the indicated times. Surface bound ligand was removed by washing cells with cold PBS, pH 2.8. Internalization was calculated as the percentage of the acid resistant radioactivity out of total cell-associated radioactivity. Results are mean ± S.D. of three independent experiments. (*, p < 0.05; **, p < 0.01).
To quantitatively analyze the internalization of wild type C5aR and C5aR L318A, we measured the uptake of 125I-C5a. Stimulation of the wild type receptor with 100 nM C5a mixed with a trace amount of 125I-C5a resulted in significant internalization of C5a (∼60%) within 30 min (Figure 2B). Internalization of the mutant was less efficient at every time point, corroborating the results from the immunofluorescence microscopy analysis. About 40% of C5a was internalized by C5aR L318A during 30 min of continuous stimulation, a significant difference compared to wild type receptor (p < 0.01). Together these results suggest that L318 is involved in regulating sequestration of the activated receptor.
Since agonist-dependent phosphorylation of C5aR is a prerequisite for efficient internalization, and dephosphorylation occurs after endocytosis (1), we examined the time course of receptor phosphorylation/dephosphorylation. The phosphorylation state of C5aR can be visualized in pulse-chase experiments as a shift in protein mobility in western blots [9,18]. Wild type C5aR showed complete receptor phosphorylation after a 10 min incubation with C5a (0 min chase) and a complete dephosphorylation after a 60 min chase, whereas C5aR L318A was not fully phosphorylated after the 10 min pulse and remained largely phosphorylated after the 60 min chase (Figure 3). This provided the third piece of evidence that the L318A mutation affects receptor endocytosis.
Figure 3. C5aR L318A becomes dephosporylated at a slower rate than wild-type C5aR.
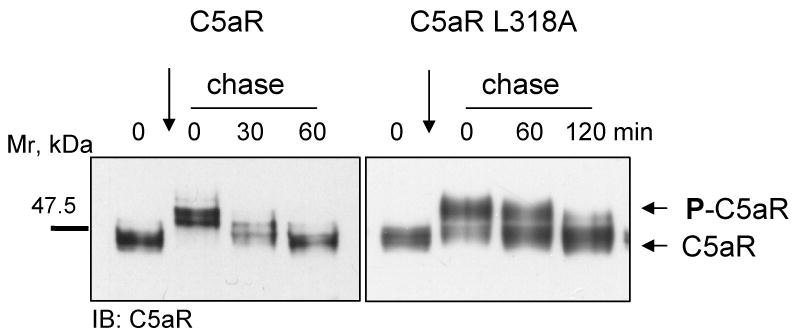
Cells were incubated for 10 min at 37°C with 50 nM C5a followed by a 0, 30 or 60 min chase in warm medium containing 1 mM cycloheximide (to inhibit protein synthesis). Wild type C5aR reached complete dephosphorylation after 60 min, whereas C5aR L318A remained partly phosphorylated up to 120 min.
The L318A mutation does not affect the signaling properties of C5aR
Several studies have demonstrated the importance of the membrane-proximal region of GPCR in the interaction with G protein and for proper signaling [19-21]. To determine whether the L318A mutation affects receptor signaling, we analyzed time- and ligand concentration-dependence on activation of C5aR signaling pathways. Two of the downstream effectors of C5aR activation are extracellular signal-regulated kinases 1/2 (ERK1/2). CHO cells expressing either wild type C5aR or the mutant receptor were stimulated with 100 nM C5a for 0, 2, 5, 10 and 30 min. Activation of ERK1/2 was detected using phospho-specific anti-ERK1/2 antibody. Both wild type and mutant receptors showed maximal ERK1/2 activation at 2 to 5 min of stimulation and saturation at 2 nM C5a (Figure 4A and B). Since pre-incubation with pertussis toxin (PTX), a Gi inhibitor, blocked ERK1/2 activation, we confirmed that both C5aR and C5aR L318A mainly used Gi to activate ERK1/2 (Figure 4A).
Figure 4. Wild type and mutant C5aR L318A showed similar activation of ERK1/2.

A) CHO transfectants were pre-incubated overnight with or without 200 ng/ml PTX and activated with 100 nM C5a for the indicated times. ERK1/2 phosphorylation was detected in the cell lysates by immunoblotting using anti-phospho-ERK1/2 antibody. Samples were tested for equal loading and expression of ERK1/2 by immunoblotting with anti-ERK1/2 antibody. B) CHO transfectants were exposed for 5 min to the indicated concentrations of C5a. Anti-phospho-ERK1/2 antibody was used to detect phosphorylated ERK1/2. Equal loading was tested by staining the filters with Ponceau S prior to antibody incubation (data not shown).
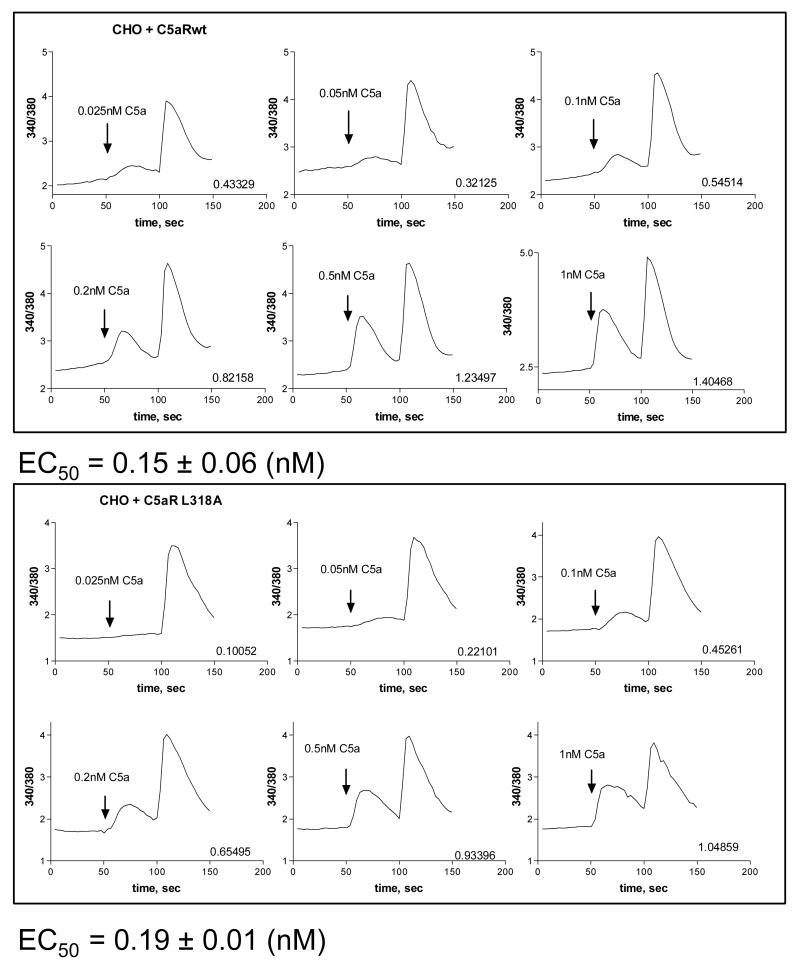
Another C5a-initiated pathway results in intracellular Ca2+ release from the ER [1]. To quantify the EC50 for calcium release, we stimulated transfected CHO cells with different concentrations of the ligand. Calculated EC50 values were similar for both receptors, 0.15 nM versus 0.19 nM for C5aR and C5aR L318A, respectively (Figure 5). Together these results indicated that the L318 mutation did not affect Gi-mediated signaling.
Figure 5. Wild type and C5aR L318A mutant induced similar C5a-mediated mobilization of intracellular Ca2+.
CHO transfectants were loaded with Fura 2-AM and calcium release was measured in dual wavelength excitation in response to addition of various concentrations of C5a (added at 50 sec). 10 μM ATP was added at 100 sec as a positive control stimulus. Numbers on each graph shows the difference between the base line value and the C5a-induced peak value. EC50 was calculated from three independent experiments by non-linear regression analysis.
L318A mutation causes a delay in C5aR phosphorylation
To examine whether the apparent reduction in endocytosis of the C5aR L318A mutant correlates with a reduction in receptor phosphorylation, we took advantage of two different antibodies against C5aR: 1) 32-G1, a recently developed and characterized monoclonal antibody that binds specifically to C5aR phosphorylated on S334. This residue has been shown to be rapidly and transiently phosphorylated by PKC βI/II at subnanomolar to nanomolar concentrations of C5a in myeloid cells [10]; 2) Rabbit anti-C5aR polyclonal antibody produced against the 12 C-terminal amino acids of C5aR (also used in Figure 3) detects both nonphosphorylated and phosphorylated receptor. A visible shift in C5aR is not detectable upon phosphorylation of S334 alone, but requires phosphorylation of multiple residues characteristic of GRK activity. [9,18]. Thus, these two antibodies can be used to follow receptor phosphorylation by PKC and GRK, respectively. We compared the kinetics of phosphorylation of the wild type and L318A receptors using 5 nM C5a. As previously shown in myeloid cells, wild type C5aR was phosphorylated on S334 in a time-dependent manner reaching maximal phosphorylation at 3 min, whereas C5aR L318A showed less efficient phosphorylation at early time points (1 and 3 min) (Figure 6). Similarly, western blots of the same samples using the polyclonal anti-C5aR antibody suggested that GRK-mediated phosphorylation of C5aR L318A is delayed at 3-10 min compared to wild type C5aR (Figure 6).
Figure 6. Mutation of L318 led to attenuated phosphorylation of C5aR.
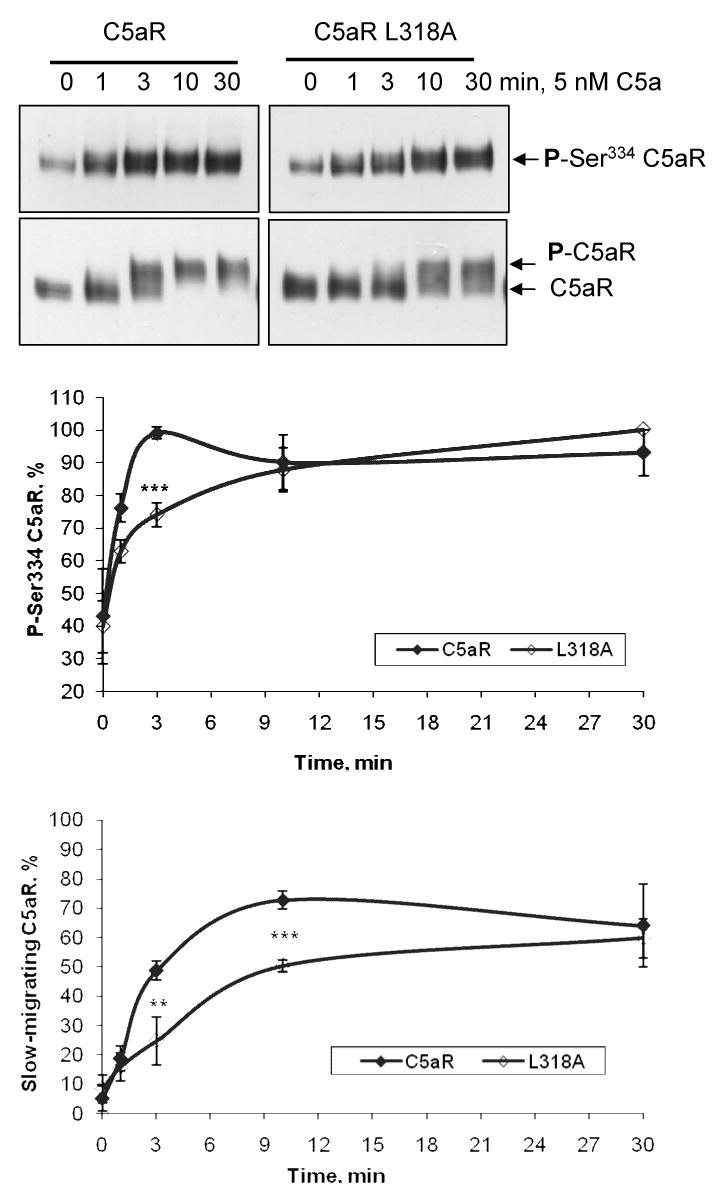
CHO cells stably expressing C5aR or C5aR L318A were stimulated with 5 nM C5a for the indicated times. Dynamics of the S334 phosphorylation were analyzed by western blotting of the total membranes using anti-phosphoS334-C5aR monoclonal antibody 32-G1 (upper panel). GRK-mediated phosphorylation was analyzed from the same samples by western blotting using polyclonal C5aR antibody (lower panel). The graphs show the relative amounts of phosphorylated C5aR. In the upper graph, the data are expressed as the percentage of S334 phosphorylation with the strongest signal set at 100%. In the lower graph, the relative amount of phosphorylated C5aR is shown as the percentage of slow-migrating C5aR out of total C5aR. Since these density measurements are difficult to carry out with complete accuracy, the results should not be viewed as absolute, but indicative of the phosphorylation trend. Each data point represents the mean value ± S.D. of three independent experiments. (**, p < 0.01; ***, p < 0.001)
GRK2 translocates to the cell membrane in response to C5a
GKR 2, 5 and 6 are putative kinases engaged in the homologous desensitization of C5aR [4]. Because GRK2 is particularly abundant in peripheral blood leukocytes and myeloid cell lines, we searched the sequence of the C5aR carboxyl tail for putative GRK2 phosphorylation sites [5]. The presence of the acidic residues E325, E326 and E331 upstream of S327 and S332, respectively, suggested that these serines are possible targets for phosphorylation by GRK2 [22]. Unlike GRK4, and GRK5/6 that are constitutively associated with membranes due to their lipid modification, membrane recruitment of GRK2/3 is entirely dependent on interaction with uncoupled G protein subunits [2]. This provides a tool to examine GRK2/3 activation upon receptor stimulation. CHO cells expressing either wild type C5aR or L318A mutant were briefly stimulated with different concentrations of C5a. To investigate the membrane translocation of GRK2, total membranes were analyzed by western blotting using anti-GRK2 antibody. We found that some GRK2 was associated with membranes even at steady-state (Figure 7A). However, activation of both wild type and mutant receptors with increasing amounts of C5a resulted in the gradual increase of GRK2 on the membranes. The results from four independent experiments on CHO C5aR cells showed a 3.2-fold (±1.3) increase in GRK2 on the membranes upon exposure to 100 nM C5a; for CHO C5aR L318A cells, the increase was 2.9-fold (±1.1), suggesting the involvement of GRK2 in agonist-dependent phosphorylation of C5aR.
Figure 7.
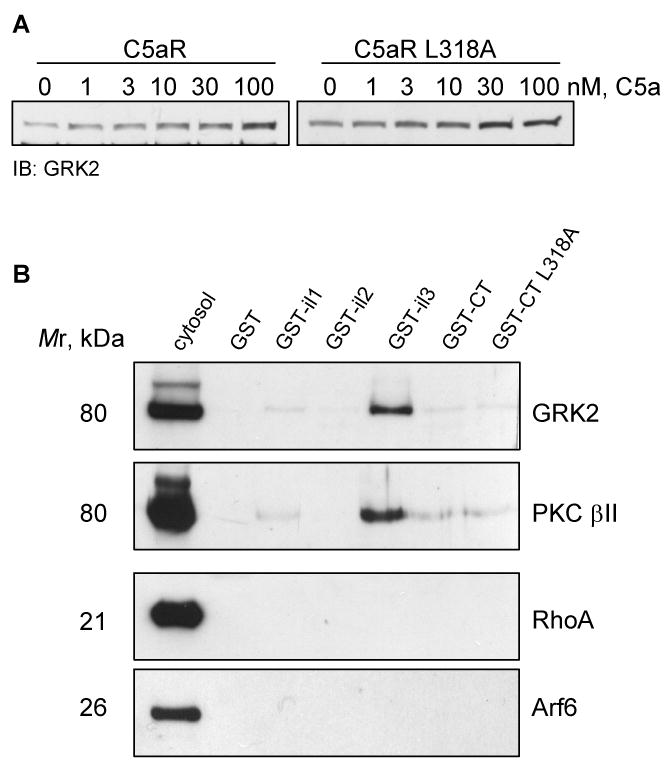
A) GRK2 translocates to the cell membrane upon activation with C5a. CHO transfectants were stimulated for 10 min with the indicated amounts of C5a, and GRK2 was detected from membrane fractions by western blotting. One representative image from three experiments is shown. B) PKCβII and GRK2 bind preferentially to the third intracellular loop of C5aR in in vitro binding assay. Affinity purified GST-tagged cytoplasmic domains of C5aR and the carboxyl tail of C5aR L318A were incubated with cytosol from activated neutrophils. Eluates were analyzed by immunoblotting with anti-GRK2, anti-phospho-PKCβII, anti-RhoA, and anti-Arf6 antibody. 2% of the cytosol versus 25% of the eluate was loaded. Abbreviation: il - intracellular loop, CT – carboxyl tail.
The carboxyl tail of C5aR may not be a major domain of physical interaction with kinases involved in receptor phosphorylation
One of the possible explanations for the slow phosphorylation of C5aR L318A is that the mutation reduces the binding affinity for kinases. To determine if L318 is part of a PKC or GRK2 binding domain, we cloned the putative cytoplasmic domains of C5aR as GST-tagged proteins. Recombinant proteins, GST-intracellular loop 1 (GST-il1), -il2, -il3, -cytoplasmic tail (-CT), CT L318A and GST alone, were tested for their capacity to bind cytosolic proteins from activated neutrophils. While no significant binding was detected in the control mixture (GST alone), we found a relatively strong interaction of C5aR il3 with GRK2 and PKCβII (Figure 7B). However, the carboxyl tails of wild type and mutant receptors showed a similar, but weaker interaction with kinases. This indicated that the C-tail of C5aR may not be a major binding domain for GRK or PKC, although we cannot exclude the possibility that these kinases may show different binding preferences to the native C5aR. Our result coincides with previous reports implicating a role for several basic residues in the second and third intracellular loops of GPCRs in GRK activation [23-25]. The putative third intracellular loop of C5aR contains four arginines that could potentially participate in the interaction.
L318A mutation affects the tryptic cleavage of C5aR suggesting a structural change in the mutant
Since the cytoplasmic tail of C5aR may not be involved in binding of GRK2 and PKCβII, we hypothesized that the L318A mutation may affect receptor phosphorylation by introducing changes in the receptor conformation. The putative hydrophobic folding motif HXXXHXXHH (H represents a hydrophobic residue, and X is any amino acid residue) was recently identified in the carboxyl tail of several GPCRs [26]. Studies with mutants revealed that the stretch of amino acid residues (VSSELRSLL) in the membrane proximal region of vasopressin 2 receptor (V2R) does not function as a linear transport signal, but rather is required for competent folding of the receptor [27]. C5aR contains a sequence resembling the described folding motif (311LRKSLPSLL319) located a short distance from the 7th transmembrane domain. If this sequence functions as a folding motif for C5aR, the L318A and L319A mutations would cause changes in the receptor structure. The fact that C5aR L319A and C5aR L318A/L319A mutants were retained in the ER favors our hypothesis, because an improper folding has been suggested to be the main reason for protein retention in the ER [28]. To determine whether there are differences in the folding of the wild type receptor and the L318A mutant, we compared their relative susceptibility to the protease trypsin. Membranes of CHO cells expressing either C5aR or C5aR L318A were solubilized in Triton X-100 and treated with diluted trypsin. Digestion efficiency was estimated by western blotting using polyclonal anti-C5aR antibody. Figure 8 shows the disappearance of the receptor band during a 1 h incubation. After 10 min of treatment, 90% of C5aR, but only 60% of the mutant was digested by trypsin. Therefore, wild type C5aR was relatively more susceptible to digestion than the mutant, implying a difference in receptor folding.
Figure 8. Limited proteolysis of C5aR and C5aR L318A in CHO membranes.

Triton X-100 solubilized membranes of CHO cells expressing C5aR or C5aR L318A were treated with trypsin. Samples were analyzed in western blots using anti-C5aR antibody. Images are representative of three independent experiments. The graph shows relative receptor degradation during 1 h incubation. Dynamics of the receptor disappearance on the western blots was analyzed by scanning densitometry using Scion Image software. Signal from non-treated membranes (0 min) was set at 100%. Each data point is the mean ± S.D. of three independent experiments.
Discussion
Proteins containing mutations that cause structural and functional changes are frequently recognized and retained by the quality control-system in the ER. Various retention signals have been characterized such as an arginine-based ER-retention motif that becomes exposed in a deletion mutant of cystic fibrosis transmembrane conductance regulator (CFTR) [28,29]. We found a correctly situated Arg-rich sequence in the cytoplasmic tail of C5aR that matches an ER-retention signal (308QGRLRKS314). An attempt by Matsumoto and co-workers to express selected C5aR cytosolic tail mutants created by random saturation mutagenesis in HEK293 or COS7 cells failed due to receptor retention in the ER [30]. Interestingly, analysis of these and other C5aR cytoplasmic tail mutants in yeast identified R310 as a “preserved” critical residue in the first half of the C5aR C-tail, favoring our assumption that the 308QGRLRKS314 sequence could function as an ER-retention signal for C5aR [31]. While masked in the properly folded C5aR, this stretch of residues could become exposed in certain mutants with altered conformation. This is a possibility for the C5aR L319A and C5aR L318A/L319A mutants which were shown to be retained in the ER of CHO cells.
The crystal structure of rhodopsin indicated a short amphipathic helix, helix 8, in the membrane proximal region of the C-tail [32]. This finding was recently confirmed from the crystal structure of another GPCR, the β2-adrenergic receptor [33]. Similar structures have been identified in many other GPCRs, either by computer modeling or peptide structure analysis [19-21,34,35]. Sequence alignment of C5aR and rhodopsin revealed a similar pattern of alteration of hydrophobic and hydrophilic residues, suggesting the presence of a similar helix in the carboxyl tail of C5aR (Figure 9A). From the rhodopsin structure, it appears that helix 8 is located in a hydrophobic environment, which could induce an α-helical structure. Helix 8 of rhodopsin ends at the membrane insertion of the palmitoyl groups attached to C322 and C323. The majority of GPCRs that belong to class A of rhodopsin-like family receptors also have single or multiple cysteines in the corresponding area of the cytoplasmic tail. Other GPCRs, such as receptors for angiotensin, fMLF, chemokines, somatostatin and C5a, have a helix-breaking proline instead of cysteines at the end of the putative helix. However, proline does not always behave as a strong helix breaker. Five out of seven C5aR transmembrane helices have prolines that introduce a kink in the helix. CD spectra of model amphipathic peptides revealed that the central proline kept α-helical structures in membrane-mimetic environments and broke an α-helix in aquatic buffer [36]. We suggested an alternative folding of the membrane-proximal region of C5aR, where P316 does not break, but rather induces a kink in the prolonged amphipathic α-helix (Figure 9B). Our suggestion is based on the continued regular occurrence of hydrophobic amino acids after P316. The bulky hydrophobic side chain of L318 and L319 extend a hydrophobic patch and, thereby, may support an α-helical structure of the amphipathic helix. In our model, the kink induced by P316 brings the bulky side chain of L319 closer to P316, which could be crucial for proper folding and/or stabilization of the structure. Our prediction coincides with the results of the PSIPRED computational analysis of C5aR (http://bioinf.cs.ucl.ac.uk/psipred/) that placed the P316 in the cytoplasmic helix with the highest possible confidence rate of 9 [37].
Figure 9. A structural model of the membrane-proximal domain of C5aR.
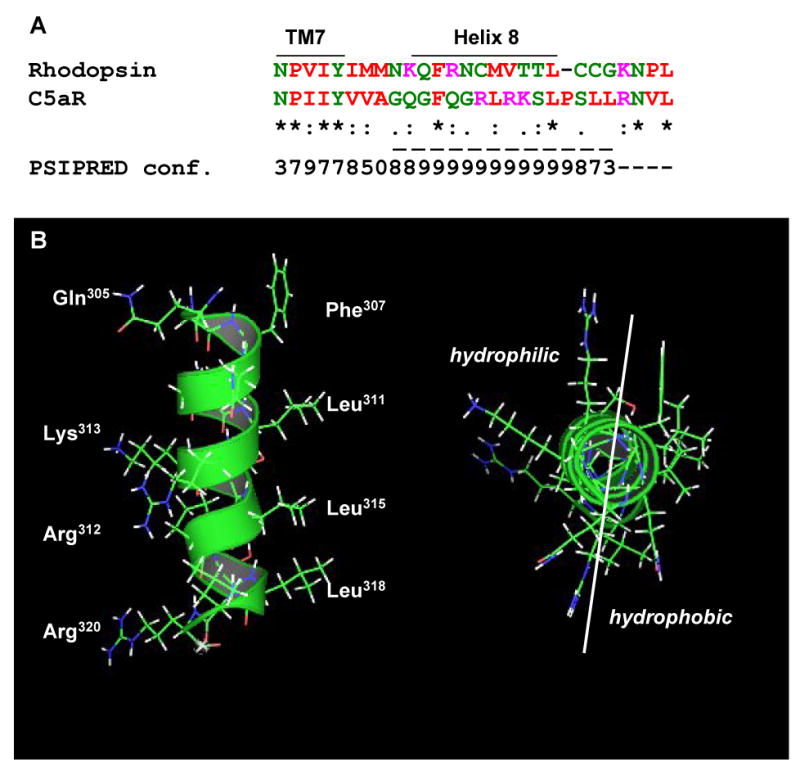
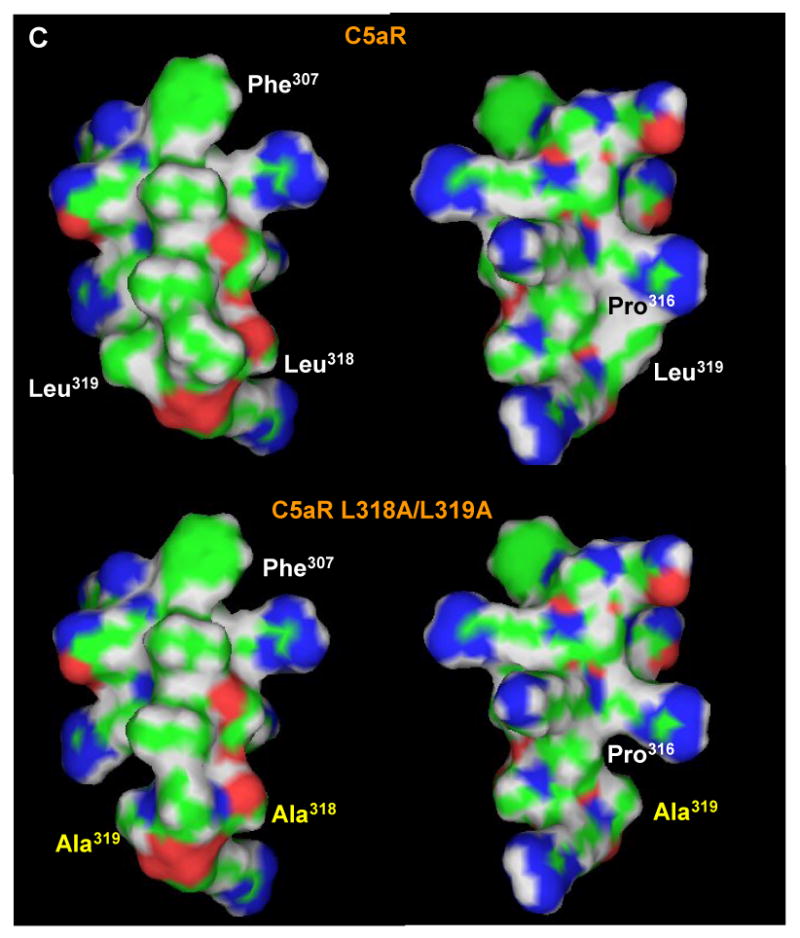
A) Sequence alignment of the putative helix 8 of bovine rhodopsin and human C5aR. PSIPRED prediction numbers represent the confidence rate for a helical secondary structure (0 = low, 9 = high). Dashed line underlines a cytoplasmic helix predicted by PSIPRED (http://bioinf.cs.ucl.ac.uk/psipred/). Color code: red - small and hydrophobic residues, magenta - basic residues, blue - acidic, green - hydroxyl, amine, basic residues and glutamine. B) Side view (left) and projection across the extended amphipathic helix 8 (right) of C5aR. C) Mutation of di-leucine L318/L319 to alanines significantly reduces the hydrophobicity around the COOH-terminus of the helix. Left and right panels show the hydrophobic and hydrophilic surfaces, respectively, of the C5aR (upper panel) and C5aR L318A/L319A (lower panel) helix. Leucine to alanine mutation is shown in yellow.
It is unlikely that the exchange of L318 and/or L319 to alanine would affect the helicity of the structure in our mutant receptors because hydrogen bonding within the main chain is unchanged. Instead, a major effect comes from the loss of the bulky hydrophobic side chains (Figure 9C). P316 would be more exposed to solvent, which may result in either a more pronounced kink or even a disordered helix. Indeed, the conformation of C5aR L319A is presumably significantly altered since that mutant is retained in the ER. The L318A mutation caused a less dramatic, but detectable structural change based on limited proteolysis with trypsin. Thus the leucine residues with their bulky side chains appear to play an important role in stabilizing the α-helix in the cytoplasmic tail of C5aR.
Other data that support the prolonged α-helix were obtained from recent structure-function mapping of C5aR in a yeast model system. Screening of the mutants obtained by RSM identified few residues with a preserved hydrophobicity in the first half of the C5aR carboxyl tail [31]. L311, L315, and L318 (L319 was excluded from the screen) tolerated only conservative substitutions, emphasizing the importance of elongation of the hydrophobic surface after P316 in the predicted amphipathic helix. On the other hand, previously reported mutations of the polar residues S314 and S317 located in the same area did not affect export of C5aR to the cell surface, implicating a hydrophilic face of the helix in functions other than receptor folding [38].
We hypothesized that the loss of bulky hydrophobic side chains of leucines at the COOH-terminus of helix 8 led to conformational changes in C5aR. To test this, we used limited proteolysis to study protein conformation and structural alterations based on cleavage of the folded polypeptide chain at the exposed flexible regions [39]. As expected, C5aR and the L318A mutant exhibited different relative susceptibility to trypsin: C5aR L318A was more resistant to proteolysis than the wild type receptor (Figure 8). This finding together with the observed ER retention of the L319A mutant supported our idea of the involvement of these hydrophobic residues in C5aR folding.
Interaction with G protein and control of receptor signaling are the most prominent roles of GPCRs' helix 8. Mutation of the basic residues in helix 8 of certain GPCRs including rhodopsin, β1-adrenergic receptor (β1-AR), and rat melanin-concentrating hormone receptor 1 affected their coupling to cognate G proteins [19-21]. The hydrophobic surface of helix 8 of C5aR may not be involved in interaction with G proteins, since no significant changes in intracellular signaling by the mutant receptor were detected (Figure 4 and 5). Bulky hydrophobic side chains may be required for membrane attachment of the carboxyl tail, orienting the polar side chains toward the cytoplasm. A similar orientation was predicted for an amphipathic helix of the proteins involved in intracellular trafficking [40]. Insertion of the hydrophobic face of the α-helix into the lipid bilayer is thought to increase the positive membrane curvature, which is essential for initiation of membrane vesicle budding. It is tempting to suggest that a similar orientation of C5aR helix 8 would facilitate receptor internalization, considering that certain GPCRs including C5aR are sequestered during desensitization. Nevertheless, another possible orientation of the helix should also be considered where positively charged side chains interact with negatively charged lipid head groups, leaving the hydrophobic surface of the helix for protein-protein interactions.
A growing body of evidence strengthens the role of helix 8 in internalization of agonist-activated GPCRs. Mutation of hydrophobic residues of the cytoplasmic helix of angiotensin II receptor and a bradykinin receptor chimera led to attenuated and/or inefficient endocytosis [34,35]. Similarly, we demonstrated that the mutation of L318 in the C5aR carboxyl tail resulted in attenuation of agonist-dependent internalization. Detailed analysis of the C5aR L318A mutant revealed one of the reasons for the slow internalization of the receptor. Disturbance of the hydrophobic surface of the putative cytoplasmic helix of C5aR led to defects in receptor phosphorylation by both PKC and GRK, which, in turn, affected receptor sequestration. This conclusion is based on the combination of our experimental data and previously published evidence of severely impaired internalization of certain phosphorylation deficient mutants of C5aR [10,18,41]. In addition, a direct connection between phosphorylation and internalization machineries was recently discovered through identification of the clathrin binding domain in the carboxyl terminal region of GRK2. The functionality of the domain was verified by inhibition of the β2-AR phosphorylation in cells overexpressing GRK2 using the siRNA for the clathrin heavy chain [42].
The specific interaction between GPCRs and kinases remains poorly characterized. We detected translocation of GRK2 to the membranes of C5a-activated CHO cells expressing either C5aR or C5aR L318A, which implicated this particular GRK in C5aR phosphorylation (Figure 7A). Experimental evidence suggested that ionic interactions between basic residues of GPCR and negatively charged amino acids of GRK2 regulate kinase binding to receptor [2,23,25]. Similarly to α2A AR and metabotropic glutamate receptor 1 (mGluR1), the third intracellular loop of C5aR showed interaction with GRK2 in our GST pull down assay (Figure 7B). Previous mapping of PKC-mediated phosphorylation sites of C5aR restricted them to serines in the carboxyl tail [43]. We found a preferential binding of PKCβII to the third loop of C5aR which is in agreement with the idea of distinct domains for PKC binding versus phosphorylation [44]. It has been suggested that the binding site situates the kinase into the most favorable position for phosphorylation. Similarly to GRK, the ionic interaction involving basic residues in the membrane proximal region of glutamate receptors participated in the binding of PKC [44,45]. C5aR has the polybasic sequence (229RTWSRR234) in the third cytoplasmic loop that resembles the PKCγ binding sites of GluR1 (RSESKR) and GluR4 (RAEAKR) and, thus, may regulate a sequential binding of PKC and GRK.
In summary, this study demonstrated that the bulky hydrophobic amino acid residues L318 and L319 in the membrane proximal region of the C5aR carboxyl tail play an important role in receptor structure and function. Mutation of leucine 319 to alanine caused retention of the receptor in the ER, presumably due to misfolding. Mutation of leucine 318 to alanine resulted in reduced receptor phosphorylation and endocytosis, apparently due to a structural change, as evidenced by differential sensitivity to tryptic cleavage.
Acknowledgments
We thank Dr. Ross M. Taylor (Montana State University, Bozeman, MT) for his advice on the analysis of protein structure. This study was supported by NIH grant R01 AI51726 (HMM) and American Heart Association Grant-in-Aid 060153Z (HMM). The DNA sequencing was supported by NIH grant P20 RR-016464 from the INBRE Program of the National Center for Research Recourses (University of Nevada, Reno, NV).
Abbreviations used in this paper
- C5aR
C5a rececptor
- GPCR
G protein-coupled receptor
- GRK
G protein-coupled receptor kinase
- ERK1/2
extracellular signal-regulated kinase 1/2
- CHO
Chinese hamster ovary
- EndoH
endoglycosidase H
- AR
adrenergic receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rabiet MJ, Huet E, Boulay F. The N-formyl peptide receptors and the anaphylatoxin C5a receptors: an overview. Biochimie. 2007;89:1089. doi: 10.1016/j.biochi.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reiter E, Lefkowitz RJ. GRKs and β-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrin Metab. 2006;17:159. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Loudon RP, Perussia B, Benovic JL. Differentially regulated expression of the G-protein-coupled receptor kinases, betaARK and GRK6, during myelomonocytic cell development in vitro. Blood. 1996;88:4547. [PubMed] [Google Scholar]
- 4.Langkabel P, Zwirner J, Oppermann M. Ligand-induced phosphorylation of anaphylatoxin receptors C3aR and C5aR is mediated by G protein-coupled receptor kinases. Eur J Immunol. 1999;29:3035. doi: 10.1002/(SICI)1521-4141(199909)29:09<3035::AID-IMMU3035>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 5.Chuang TT, Sallese M, Ambrosini G, Parruti G, De Blasi A. High expression of β-adrenergic receptor kinase in human peripheral blood leukocytes. Isoproterenol and platelet activating factor can induce kinase translocation. J Biol Chem. 1992;267:6886. [PubMed] [Google Scholar]
- 6.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 7.Dittrich E, Haft CR, Muys L, Heinrich PC, Graeve L. A di-leucine motif and an upstream serine in the interleukin-6 (IL-6) signal transducer gp130 mediate ligand-induced endocytosis and down-regulation of the IL-6 receptor. J Biol Chem. 1996;271:5487. doi: 10.1074/jbc.271.10.5487. [DOI] [PubMed] [Google Scholar]
- 8.Sandoval IV, Martinez-Arca S, Valdueza J, Palacios S, Holman GD. Distinct reading of different structural determinants modulates the dileucine-mediated transport steps of the lysosomal membrane protein LIMPII and the insulin-sensitive glucose transporter GLUT4. J Biol Chem. 2000;275:39874. doi: 10.1074/jbc.M006261200. [DOI] [PubMed] [Google Scholar]
- 9.Suvorova ES, Gripentrog JM, Miettinen HM. Different endocytosis pathways of the C5a receptor and the N-formyl peptide receptor. Traffic. 2005;6:100. doi: 10.1111/j.1600-0854.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- 10.Pollok-Kopp B, Hüttenrauch F, Rethorn S, Oppermann M. Dynamics of protein kinase C mediated phosphorylation of the complement C5a receptor on serine-334. J Biol Chem. 2006;282:4345. doi: 10.1074/jbc.M601317200. [DOI] [PubMed] [Google Scholar]
- 11.Uthayakumar S, Granger BL. Cell surface accumulation of overexpressed hamster lysosomal membrane glycoproteins. Cell Mol Biol Res. 1995;41:405. [PubMed] [Google Scholar]
- 12.Gripentrog JM, Miettinen HM. Activation and nuclear translocation of ERK1/2 by the formyl peptide receptor is regulated by G protein and is not dependent on β-arrestin translocation or receptor endocytosis. Cell Signal. 2005;17:1300. doi: 10.1016/j.cellsig.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Cleveland DW, Fischer SG, Kirschner MW, Laemmli UK. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977;252:1102. [PubMed] [Google Scholar]
- 14.Parkos CA, Delp C, Arnaout MA, Madara JL. Neutrophil migration across a cultured intestinal epithelium: Dependence on a CD11b/CD18-mediated event and enhanced efficiency in physiological direction. J Clin Invest. 1991;88:1605. doi: 10.1172/JCI115473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moyer BD, Balch WE. A new frontier in pharmacology: the endoplasmic reticulum as a regulated export pathway in health and disease. Expert Opin Ther Targets. 2001;5:165. doi: 10.1517/14728222.5.2.165. [DOI] [PubMed] [Google Scholar]
- 16.Freeze HH. Use of glycosidases to study protein trafficking. In: Bonifacino JS, Dasso M, Harford JB, Lippincott-Schwartz J, Yamada KM, editors. Current protocols in cell biology. John Wiley and Sons Inc.; Philadelphia, PA: 1999. p. 15.2.1. [Google Scholar]
- 17.Krueger KM, Daaka Y, Pitcher JA, Lefkowitz RJ. The role of sequestration in G protein-coupled receptor resensitization: Regulation of β2-adrenergic receptor dephosphorylation by vesicular acidification. J Biol Chem. 1997;272:5. doi: 10.1074/jbc.272.1.5. [DOI] [PubMed] [Google Scholar]
- 18.Naik N, Giannini E, Brouchon L, Boulay F. Internalization and recycling of the C5a anaphylatoxin receptor: evidence that the agonist-mediated internalization is modulated by phosphorylation of the C-terminal domain. J Cell Sci. 1997;110:2381. doi: 10.1242/jcs.110.19.2381. [DOI] [PubMed] [Google Scholar]
- 19.Marin EP, Krishna AG, Zvyaga TA, Isele J, Siebert F, Sakmar TP. The amino terminus of the fourth cytoplasmic loop of rhodopsin modulates rhodopsin-transducin interaction. J Biol Chem. 2000;275:1930. doi: 10.1074/jbc.275.3.1930. [DOI] [PubMed] [Google Scholar]
- 20.Tetsuka M, Saito Y, Imai K, Doi H, Maruyama K. The basic residues in the membrane-proximal C-terminal tail of the rat melanin-concentrating hormone receptor 1 are required for receptor function. Endocrinology. 2004;145:3712. doi: 10.1210/en.2003-1638. [DOI] [PubMed] [Google Scholar]
- 21.Delos Santos NM, Gardner LA, White SW, Bahouth SW. Characterization of the residues in helix 8 of the human β1-adrenergic receptor that are involved in coupling the receptor to G proteins. J Biol Chem. 2006;281:12896. doi: 10.1074/jbc.M508500200. [DOI] [PubMed] [Google Scholar]
- 22.Chen CY, Dion SB, Kim CM, Benovic JL. β-Adrenergic receptor kinase. Agonist-dependent receptor binding promotes kinase activation. J Biol Chem. 1993;268:7825. [PubMed] [Google Scholar]
- 23.Pao CS, Benovic JL. Structure/function analysis of alpha2A-adrenergic receptor interaction with G protein-coupled receptor kinase 2. J Biol Chem. 2005;280:11052. doi: 10.1074/jbc.M412996200. [DOI] [PubMed] [Google Scholar]
- 24.Kelleher DJ, Johnson GL. Characterization of rhodopsin kinase purified from bovine rod outer segments. J Biol Chem. 1990;265:2632. [PubMed] [Google Scholar]
- 25.Dhami GK, Anborgh PH, Dale LB, Sterne-Marr R, Ferguson SSG. Phosphorylation-independent regulation of metabotropic glutamate receptor signaling by G protein-coupled receptor kinase 2. J Biol Chem. 2002;277:25266. doi: 10.1074/jbc.M203593200. [DOI] [PubMed] [Google Scholar]
- 26.Pankevych H, Korkhov V, Freissmuth M, Nanoff C. Truncation of the A1 adenosine receptor reveals distinct roles of the membrane-proximal carboxyl terminus in receptor folding and G protein coupling. J Biol Chem. 2003;278:30283. doi: 10.1074/jbc.M212918200. [DOI] [PubMed] [Google Scholar]
- 27.Thielen A, Oueslati M, Hermosilla R, Krause G, Oksche A, Rosenthal W, Schulein R. The hydrophobic amino acid residues in the membrane-proximal C tail of the G protein-coupled vasopressin V2 receptor are necessary for transport-competent receptor folding. FEBS Lett. 2005;579:5227. doi: 10.1016/j.febslet.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 28.Michelsen K, Yuan H, Schwappach B. Hide and run. Arginine-based endoplasmic-reticulum-sorting motifs in the assembly of heteromultimeric membrane proteins. EMBO Rep. 2005;6:717. doi: 10.1038/sj.embor.7400480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang XB, Cui L, Hou YX, Jensen TJ, Aleksandrov AA, Mengos A, Riordan JR. Removal of multiple arginine-framed trafficking signals overcomes misprocessing of delta F508 CFTR present in most patients with cystic fibrosis. Mol Cell. 1999;4:137. doi: 10.1016/s1097-2765(00)80196-3. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto ML, Narzinski K, Nikiforovich GV, Baranski TJ. A comprehensive structure-function map of the intracellular surface of the human C5a receptor. II. Elucidation of G protein specificity determinants. J Biol Chem. 2007;282:3122. doi: 10.1074/jbc.M607683200. [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto ML, Narzinski K, Kiser PD, Nikiforovich GV, Baranski TJ. A comprehensive structure-function map of the intracellular surface of the human C5a receptor. I. Identification of critical residues. J Biol Chem. 2007;282:3105. doi: 10.1074/jbc.M607679200. [DOI] [PubMed] [Google Scholar]
- 32.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 33.Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC. High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science. 2007;318:1258. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas WG, Baker KM, Motel TJ, Thekkumkara TJ. Angiotensin II receptor endocytosis involves two distinct regions of the cytoplasmic tail: A role for residues on the hydrophobic face of a putative amphipathic helix. J Biol Chem. 1995;270:22153. doi: 10.1074/jbc.270.38.22153. [DOI] [PubMed] [Google Scholar]
- 35.Faussner A, Bauer A, Kalatskaya I, Schussler S, Seidl C, Proud D, Jochum M. The role of helix 8 and of the cytosolic C-termini in the internalization and signal transduction of B(1) and B(2) bradykinin receptors. FEBS J. 2005;272:129. doi: 10.1111/j.1432-1033.2004.04390.x. [DOI] [PubMed] [Google Scholar]
- 36.Yang ST, Lee JY, Kim HJ, Eu YJ, Shin SY, Hahm KS, Kim JI. Contribution of a central proline in model amphipathic alpha-helical peptides to self-association, interaction with phospholipids, and antimicrobial mode of action. FEBS J. 2006;273:4040. doi: 10.1111/j.1742-4658.2006.05407.x. [DOI] [PubMed] [Google Scholar]
- 37.McGuffin LJ, Bryson K, Jones DT. The PSIPRED protein structure prediction server. Bioinformatics. 2000;16:404. doi: 10.1093/bioinformatics/16.4.404. [DOI] [PubMed] [Google Scholar]
- 38.Christophe T, Rabiet MJ, Tardif M, Milcent MD, Boulay F. Human complement 5a (C5a) anaphylatoxin receptor (CD88) phosphorylation sites and their specific role in receptor phosphorylation and attenuation of G protein-mediated responses - Desensitization of C5a receptor controls superoxide production but not receptor sequestration in HL-60 cells. J Biol Chem. 2000;275:1656. doi: 10.1074/jbc.275.3.1656. [DOI] [PubMed] [Google Scholar]
- 39.Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, Gottesman MM. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315:525. doi: 10.1126/science.1135308. [DOI] [PubMed] [Google Scholar]
- 40.McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- 41.Braun L, Christophe T, Boulay F. Phosphorylation of key serine residues is required for internalization of the complement 5a (C5a) anaphylatoxin receptor via a β-arrestin, dynamin, and clathrin-dependent pathway. J Biol Chem. 2003;278:4277. doi: 10.1074/jbc.M210120200. [DOI] [PubMed] [Google Scholar]
- 42.Mangmool S, Haga T, Kobayashi H, Kim KM, Nakata H, Nishida M, Kurose H. Clathrin required for phosphorylation and internalization of β2-adrenergic receptor by G protein-coupled receptor kinase 2 (GRK2) J Biol Chem. 2006;281:31940. doi: 10.1074/jbc.M602832200. [DOI] [PubMed] [Google Scholar]
- 43.Giannini E, Boulay F. Phosphorylation, dephosphorylation, and recycling of the C5a receptor in differentiated HL60 cells. J Immunol. 1995;154:4055. [PubMed] [Google Scholar]
- 44.Gomes AR, Correia SS, Esteban JA, Duarte CB, Carvalho AL. PKC anchoring to GluR4 AMPA receptor subunit modulates PKC-driven receptor phosphorylation and surface expression. Traffic. 2007;8:259. doi: 10.1111/j.1600-0854.2006.00521.x. [DOI] [PubMed] [Google Scholar]
- 45.Correia SS, Duarte CB, Faro CJ, Pires EV, Carvalho AL. Protein kinase C gamma associates directly with the GluR4 alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor subunit. Effect on receptor phosphorylation. J Biol Chem. 2003;278:6307. doi: 10.1074/jbc.M205587200. [DOI] [PubMed] [Google Scholar]