Abstract
A growing body of epidemiological, clinical, and experimental evidence has underscored both the pharmacological potential and the nutritional value of dietary fish oil enriched in very long chain n-3 PUFAs such as docosahexaenoic acid (DHA, 22:6, n-3) and eicosapentaenoic acid (EPA, 20:5, n-3). The broad health benefits of very long chain n-3 PUFAs and the pleiotropic effects of dietary fish oil and DHA have been proposed to involve alterations in membrane structure and function, eicosanoid metabolism, gene expression and the formation of lipid peroxidation products, although a comprehensive understanding of the mechanisms of action has yet to be elucidated. In this review, we present data demonstrating that DHA selectively modulates the subcellular localization of lipidated signaling proteins depending on their transport pathway, which may be universally applied to other lipidated protein trafficking. An interesting possibility raised by the current observations is that lipidated proteins may exhibit different subcellular distribution profiles in various tissues, which contain a distinct membrane lipid composition. In addition, the current findings clearly indicate that subcellular localization of proteins with a certain trafficking pathway can be subjected to selective regulation by dietary manipulation. This form of regulated plasma membrane targeting of a select subset of upstream signaling proteins may provide cells with the flexibility to coordinate the arrangement of signaling translators on the cell surface. Ultimately, this may allow organ systems such as the colon to optimally decode, respond, and adapt to the vagaries of an ever-changing extracellular environment.
Keywords: protein targeting, trafficking, fish oil, chemoprevention, colon cancer
Introduction
A growing body of literature supports the contention that bioactive food components containing n-3 polyunsaturated fatty acids (PUFA) are important in suppressing colon cancer. For example, there is substantial experimental, epidemiological and clinical evidence indicating that fish oil-containing diets rich in docosahexaenoic acid (DHA, 22:6Δ4,7,10,13,16,19) and eicosapentaenoic acid (EPA, 20:5Δ5,8,11,14,17) are protective against colon tumorigenesis [Anti 1992, 1994; Bartrum, 1993; Caygill, 1996; Chang, 1997, 1998; Fernandez, 1999; Rao, 2001; Cheng, 2003; Tavani, 2003; Reddy, 2005; Phillips, 2006; Courtney, 2007]. In contrast, several systematic reviews have challenged the premise that dietary fish oil reduces colon cancer risk, fueling a debate regarding the role of n-3 PUFA as chemoprotective nutrients [Lupton, 2004; MacLean, 2006]. It has been estimated that the mean dietary intake of EPA and DHA in North America is approximately 100 mg/day [Arterburn, 2006; Hibbeln, 2006]. This corresponds to a fasting blood level of ~100 µM EPA and ~280 µM DHA. Upon supplementation, EPA and DHA levels can exceed 700 µM and 500 µM, respectively [Conquer, 1998]. These changes in circulating n-3 PUFA result in the corresponding enrichment of colonocyte membrane lipids [Anti, 1992; Bartram, 1993; Hong, 2002; Chapkin, 2002].
Although the mechanism of EPA and DHA action is still not fully defined in molecular terms, it is becoming increasingly clear that these fatty acids are pleiotropic. The challenge for the future is to determine precisely how n-3 PUFA alter membrane microdomain structure and function, eicosanoid metabolism, and the formation and mobilization of reactive oxygen products and intracellular Ca2+. This review will focus of recent research efforts which contribute to the development of unifying molecular mechanisms which underlie the pleiotropic protective effects of n-3 PUFA with respect to colon cancer and inflammatory diseases of the intestine.
I. Suppressive effect of n-3 PUFA on colon tumor development
The balance between proliferation and apoptosis is critical to the maintenance of steady-state number for cell populations in the colon. In general, dysregulation of this mechanism can disrupt homeostasis, resulting in clonal expansion, with the resultant over production of affected cells [Bedi, 1995; Siniscope, 1995]. The programmed induction of cell death also represents a mechanism by which colonocytes possessing DNA damage can be deleted. This “targeted” immediate apoptotic response to DNA damage is p53-dependent in contrast to “spontaneous” apoptosis which is largely a p53-independent process [Chapkin, 1999]. These protective processes can occur anywhere along the crypt axis [Bedi, 1995; Chang, 1997; Hong, 2000]. It has now been clearly established that the transformation of colonic epithelium to carcinoma is in part associated with a progressive inhibition of apoptosis [Siniscope, 1995; Bedi, 1995; Chang, 1997; Hanahan, 2000]. Importantly, we have demonstrated that measurements of apoptosis have greater prognostic value to detect dietary effects on colon tumor incidence than do measurements of cell proliferation [Chang, 1997; Hong, 2000].
In a major step toward understanding why n-3 PUFA suppress colon cancer, we determined the effect of fish oil feeding on genetic signatures during colon cancer initiation and progression. Specifically, we contrasted the biological properties of three types of commonly consumed dietary fats, i.e., corn oil/n-6 PUFA, fish oil/n-3 PUFA and olive oil/n-9 monounsaturated fat, following carcinogen treatment at both the initiation and post-initiation stages of colon tumor development. We found that only the consumption of n-3 PUFA exerted a protective effect at the initiation (DNA adduct formation) and promotional (apoptosis and aberrant crypt foci levels) stages (Table 1) [Davidson, 2004]
Table 1. Effect of dietary lipid source on apoptotic index in the top third (upper tertile) of the colonic crypt.
Rats fed diets containing either n-3, n-6 or n-9 fatty acid enriched oils and injected with a colon specific carcinogen, azoxymethane or saline were terminated 10 wk later. Apoptosis was assessed by TUNEL assay on 10 rats per group. Aberrant crypt foci (ACF) were scored following methylene blue staining and high multiplicity ACF (>3 aberrant crypts per foci) were scored. n=5 rats per diet group with 20 crypts per animal scored. Means with different letters are significantly different (p<0.05). Adapted from Davidson et al (2004).
| Dietary Lipid Source | |||
|---|---|---|---|
| n-3 | n-6 | n-9 | |
| Apoptotic Index (%) | 3.08 ± 0.52b | 1.28 ± 0.40 a | 1.51 ± 0.31a |
| # of High Multiplicity ACF | 45.42 ± 5.9a | 65.69 ± 7.48b | 67.32 ± 7.13b |
A very exciting outcome of our studies was the demonstration that fish oil (containing both DHA and EPA) fed rats have higher levels of colonic apoptosis, conferring resistance to alkylation and oxidation-induced DNA damage [Hong, 2000, 2005; Bancroft, 2003; Sanders, 2004; Leyk, 2005]. The protective effect of n-3 PUFA was exerted at both the initiation and post-initiation stages of carcinogenesis [Chang, 1997, 1998; Davidson, 2004].This important observation identifies a clear mechanism by which dietary n-3 PUFA exert a protective modulatory effect on colonocyte deletion in the whole animal. These data are consistent with recent observations indicating that the intake of n-3 PUFA (EPA 100 mg/d and DHA 400 mg/d for 2 years) promotes apoptosis of colonic mucosa in humans [Cheng, 2003]; and supplementation with EPA (2 g/day for 3 months) significantly increased apoptosis in normal colonic mucosa in subjects with a history of colorectal adenomas [Courtney, 2007].
From a mechanistic standpoint, we hypothesized that fish oil-induced up-regulation of apoptosis would be coincident with a down-regulation of bcl-2, a well known anti-apoptotic mediator. This is based on the fact that PGE2 is known to inhibit apoptosis in part by induction of bcl-2 [Sheng, 1998] and we have previously demonstrated that fish oil downregulates PGE2 and COX-II in colonic mucosa [Lee, 1993; Lupton 1999]. Tissues were collected from rats consuming diets containing either corn oil or fish oil at 3–12 h after carcinogen (azoxymethane) injection. Fish oil decreased bcl-2 expression (p<0.05) and increased apoptosis (p < 0.05) in the top third of the distal colon (Table 2).
Table 2. Fish oil doubles the apoptotic index (percentage of cells undergoing apoptosis) compared to corn oil in the top 1/3 of the colonic crypt following carcinogen exposure.
The induction of apoptosis is associated with a decrease in bcl-2 in the top 1/3 of the crypt. Mean ± SE, n=15 rats/diet. Adapted from Hong et al (2003).
| Dietary Lipid Source | ||
|---|---|---|
| n-3 | n-6 | |
| Apoptotic Index (%) | 1.22 ± 0.44 b | 0.61 ± 0.23a |
| Bcl-2 (staining intensity) | 26.01 ± 1.62a | 30.34 ± 1.53b |
Finally, we have demonstrated that the pleiotropic bioactive components generated by fermentable fiber (butyrate) and fish oil (DHA and perhaps EPA) work coordinately to protect against colon tumorigenesis, primarily by increasing apoptosis rather than decreasing cell proliferation in vivo [Chang, 1998; Davidson, 2000; Hong, 2000]. Although it has been well documented that butyrate is an inhibitor of histone deacetylases and can activate the Fas receptor mediated “extrinsic” death pathway [Smith, 1998; Fan, 1999], the role of these mechanisms in the induction of colonocyte apoptosis may be a secondary consequence of its ability to promote cellular oxidation [Archer, 1998; Mariadason, 2000]. For example, serving as the primary energy source for colonic epithelial cells, butyrate induces cellular reactive oxygen species (ROS) generation [Benard, 1997; Giardina, 1998; Smith, 1998]. This is relevant because DHA is an oxidatively susceptible lipid due to its high degree of unsaturation [Gardner, 1989; Chapkin, 2002; Hong, 2002].
II. Molecular mechanisms by which DHA modulates apoptosis
The “intrinsic” apoptotic pathway channels cell death signals through the mitochondrion which serves as a damage/oxidative stress sensor and monitor of metabolic status [Costantini, 2000; Fulda, 2001]. Consistent with previous reports linking oxidative stress and apoptosis, there is mounting evidence that cis-unsaturated fatty acids, particularly DHA, induce this pathway via the generation of ROS such as superoxide/hydrogen peroxide, and in particular, phospholipid hydroperoxides (PLOOH), which disrupt the mitochondrial permeability transition pore (mtPTP) and trigger the release of soluble intermembrane proteins [Nomura, 1999; Wang, 2000; Kokoszka, 2001; Koumura, 2005; Engel, 2006]. For example, we have recently demonstrated that dietary DHA is incorporated into mitochondrial membrane phospholipids, thereby enhancing oxidative stress induced by butyrate metabolism [Hong, 2002; Chapkin, 2002]. In complimentary experiments, in order to elucidate the subcellular origin of oxidation induced by DHA and butyrate, immortalized mouse colonocytes (YAMC) were treated with 0–200 µM DHA or linoleic acid (LA, n-6 PUFA control) for 72 h with or without 5 mM butyrate for the final 24 h [Ng, 2005]. Cytosolic reactive oxygen species (ROS, measured using CMH2-DCFDA), membrane lipid oxidation (PLOOH, measured using diphenyl-1-pyrenylphosphine, DPPP), and mitochondrial membrane potential (MP, measured using rhodamine 123), were assayed by live-cell fluorescence microscopy. After 24 h of butyrate treatment, DHA primed cells exhibited a 151% increase in lipid oxidation (p<0.01), compared to no butyrate treatment, which could be blocked by a mitochondria-specific antioxidant, MitoQ (p<0.05). LA treatment did not show any significant effect. In the absence of butyrate, DHA treatment, compared to LA, increased resting MP by 120% (p<0.01). In addition, butyrate-induced MP dissipation was 21% greater in DHA primed cells as compared to LA at 6 h. This effect was reversed by preincubation with inhibitors of the mitochondrial permeability transition (MPT) pore, cyclosporin A or bongkrekic acid. The functional importance of these events is supported by the demonstration that DHA and butyrate-induced apoptosis is blocked by MitoQ. These data indicate that DHA and butyrate potentiate mitochondrial lipid oxidation and the dissipation of MP which contributes to the induction of apoptosis (Figure 1).
Figure 1. Induction of apoptosis in fatty acid and butyrate-treated colonocytes.
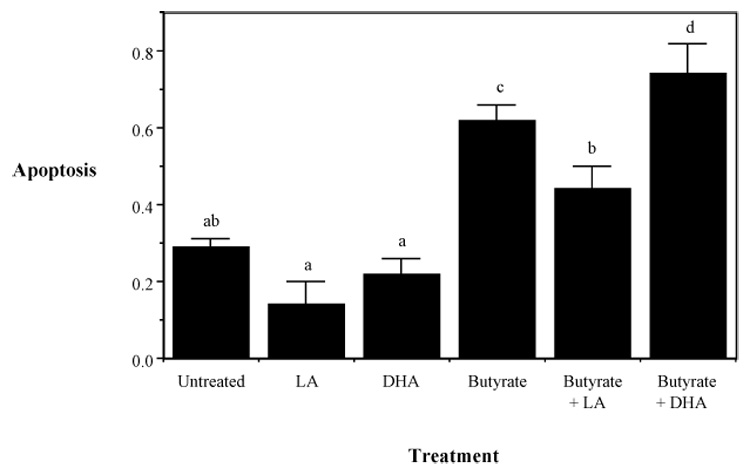
Cultures contained 5 mM butyrate and 50 µM DHA, LA or no fatty acid treatment. Apoptosis was measured by DNA fragmentation ELISA. Data represent mean absorbance at 405 nm ± SE divided by the total number of adherent cells per dish, n=4–6 separate wells from 1 of 4 representative experiments. Values not sharing the same letters are significantly different (P < 0.05). Adapted from Ng et al (2005).
We have recently demonstrated a significant enrichment of EPA and DHA in mitochondrial phospholipids, e.g., cardiolipin, isolated from (i) colonic mucosa from rats fed fish oil or EPA/DHA ethyl esters, and (ii) colonocytes cultured with DHA, [Collett, 2001; Chapkin, 2002; Hong, 2002]. It is possible, therefore, that cardiolipin incorporating substantial quantities of EPA and DHA, containing 5 and 6 double bonds, respectively, would be highly susceptible to lipid peroxidation [Gardner, 1989; Chapkin, 2002]. This is noteworthy, because the accumulation of cardiolipin hydroperoxides (CL-OOH) directly triggers the release of proapoptotic factors from mitochondria [Kagan, 2005, Bayir, 2006]. Along these lines, the above mentioned observations are particularly relevant in that a mitochondria-specific antioxidant, MitoQ, blocks lipid oxidation and apoptosis induced by butyrate. Interestingly, oxidative stress regulates a broad array of signal transduction pathways that regulate mitochondrial function and apoptosis [Storz, 2005]. For example, the production of ROS/PLOOH in mitochondria is strictly regulated by L-form mitochondrial phospholipid hydroperoxide glutathione peroxidase (PHGPx), classical glutathione peroxidase (cGPx), and Mn-dependent superoxide dismutase (SOD2). Among these mitochondrial antioxidant enzymes, the L-form of PHGPx is unique because it directly reduces peroxidized phospholipids in membranes [Weitzel, 1990; Arai, 1999]. However, the precise mechanisms regulating dietary oxidative stress-induced apoptosis in the colon have not been clearly defined.
With respect to molecular triggers for apoptosis, Ca2+ is one of the most versatile and universal signaling mediators in cells and is required for the activation of many cellular processes. Increasing evidence indicates that alterations in the finely tuned intracellular homeostasis and compartmentalization of Ca2+ can lead to cell death either through apoptosis or necrosis [Berridge, 2000]. Eukaryotic cells can increase their cytosolic Ca2+ levels via 2 mechanisms: release of Ca2+ from intracellular stores or influx via plasma membrane channels. Channels located in the plasma membrane, e.g., store operated Ca2+ channels (SOC), receptor operated channels, and voltage operated channels, regulate the influx of Ca2+ into the cell. Although the importance of the endoplasmic reticulum (ER) as the major storage organelle is indisputable, growing evidence indicates that functional compartmentalization of Ca2+ exists within the various cellular organelles. Recent studies have identified the contributions of the mitochondria in maintaining intracellular Ca2+ homeostasis and cellular physiologic function [Orrenius, 2007]. In fact, it is now recognized that mitochondria play a key role in both apoptosis and necrosis by regulating energy metabolism, intracellular Ca2+ homeostasis, activation of caspases and the release of reactive oxygen species (ROS) [Jacobson, 2002; Fariss, 2005].
Given the central role of mitochondria in the commitment to apoptosis, we hypothesized that DHA and butyrate can interactively promote apoptosis by triggering changes in mitochondrial Ca2+ levels that contribute to caspase activation and colonocyte cell death. We used isogenic p53 wild type and deficient human colon tumor (HCT 116) cell lines as well as an immortalized mouse colonocyte (YAMC) cell line to determine whether or not chemoprotective nutrients modulate intracellular Ca2+ compartmentalization and store-operated channel (SOC) entry to induce colonocyte apoptosis [Kolar, 2007a]. The results confirm and extend our previous observations [Ng, 2005] and demonstrate that DHA and butyrate synergistically enhance both mitochondrial Ca2+ accumulation and lipid peroxidation which serve as triggers for apoptosis in a p53-independent manner [Kolar, 2007b].
Based on the findings described above, we propose a pathway for the induction of apoptosis in colonic epithelium that involves the synergistic action of two bioactive molecules, DHA and butyrate, on enhanced mitochondrial ROS/PLOOH and Ca2+ accumulation (Figure 2). Our results indicate that the effects of individual chemoprotective nutrients (DHA and butyrate) may not be as important as the nutritional combination. We propose that the failure to address the interaction between fat and fiber may explain why the chemoprotective effects of n-3 PUFA and butyrate may be partly obscured in human studies [Alberts, 2000; Peters, 2003; Park, 2005; MacLean, 2006].
Figure 2. Proposed molecular model of DHA and butyrate-induced apoptosis.
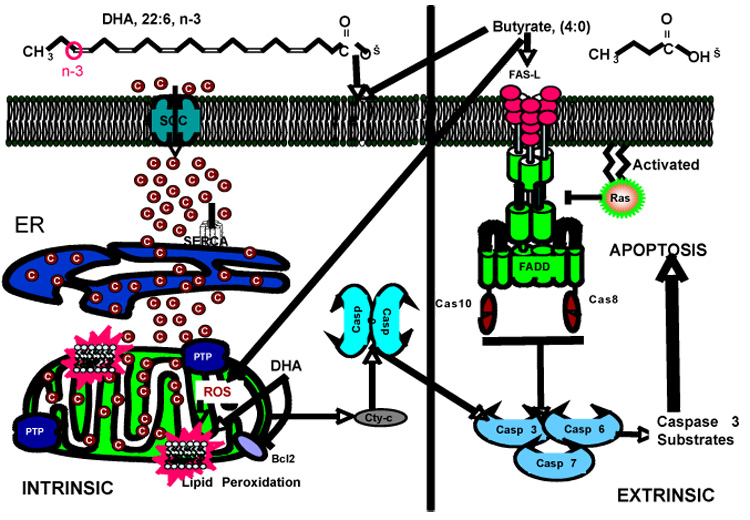
We have previously demonstrated that butyrate induces colonocyte apoptosis via a non-mitochondrial, Fas-mediated, extrinsic pathway which is antagonized by activated Ras [Fan, 1999]. DHA and butyrate work coordinately in the colon to initiate a distinct intrinsic proapoptotic cycle involving the activation of store-operated channels (SOC), leading to rapid entry of Ca2+ through the plasma membrane and mitochondrial Ca2+ loading. This directly or indirectly increases mitochondrial phospholipid hydroperoxides (PLOOH) and triggers the opening of the permeability transition pore (PTP) and release of pro-apoptotic molecules like cytochrome C. These effects culminate in the induction of apoptosis.
III. Effects of DHA and EPA on membrane structure and function, and membrane-associated proteins
EPA and DHA affect diverse physiological processes including cell membrane structure/function and eicosanoid-dependent signaling [Smith, 2005; Chapkin, 2007a,b], thereby providing significant protection against a variety of apparently unrelated human diseases [Stulnig, 2003; Lupton, 2004; Chapkin, 2007a]. n-3 PUFA are rapidly incorporated into cells, primarily into membrane phospholipids at the sn-2 position [Chapkin, 1991; Stillwell, 2003], and in general, the cellular level is readily influenced by diet [Katan, 1997]. Increasing evidence suggests that DHA is a unique fatty acid because it significantly alters basic properties of cell membranes, including acyl chain order and fluidity, phase behavior, elastic compressibility, ion permeability, fusion, rapid flip-flop and resident protein function [Stillwell, 2003; Shaikh, 2003]. The presence of long chain n-3 PUFA in membrane phospholipids imparts unique physicochemical properties to cellular membranes and DHA-induced alterations in membrane structure and function have been proposed to underlie its pleiotropic salutary effects [Ehringer, 1990; Hashimoto, 2001, 2006; Ma, 2004a,b; Seo, 2006; Stillwell, 2006; Chapkin, 2007a,b].
A. Effects on membrane microdomains and protein compartmentalization
The plasma membranes of all eukaryotic cells contain specific detergent-resistant domains in which key signal transduction proteins are localized. These regions are classified as “lipid rafts”, which are composed mostly of cholesterol and sphingolipids and therefore do not integrate well into the fluid phospholipid bilayers causing them to form microdomains [Hancock, 2006]. In addition, caveolae represent a subtype of lipid raft that form flask-shaped membrane invaginations containing the structural protein caveolin-1 [Anderson, 1998]. Because of its polyunsaturation, DHA and possibly EPA are sterically incompatible with sphingolipid and cholesterol and, therefore, appear to alter lipid raft behavior and protein function [Stulnig, 2003; Shaikh, 2004; Chen, 2007]. Many cytosolic proteins covalently modified with saturated palmitate (16:0) preferentially partition into liquid-ordered microdomains such as lipid rafts and caveolae, which is critical for their function (Figure 3). PUFA have been shown to selectively displace acylated signaling proteins from lipid rafts and caveolae (liquid ordered phases in the plasma membrane), influencing downstream signaling events [Stulnig, 1998, 2001; Zeyda, 2002, 2005; Webb, 2000: Liang, 2001]. Since dietary n-3 PUFA are incorporated into colonocytes [Hong, 2002], we have demonstrated that PUFA classes (n-6 vs n-3) differentially modulate colonocyte membrane microdomains [Ma, 2004a,b; Chapkin, 2005]. Specifically, dietary n-3 PUFA-induced perturbations in colonocyte membrane microdomain (caveolae) lipid composition can alter signaling protein microdomain localization and function in the colon [Ma, 2004a,b; Chapkin, 2005; Stillwell, 2006]. Of relevance to colonic apoptosis and tumor development, we have recently demonstrated that DHA suppresses the activation of Ras, a 21 kDa guanine nucleotide binding protein, by limiting its microlocalization to the lipid raft/caveolae [Ma, 2004a,b]. This is significant because the high frequency of Ras mutations/overexpression in human and animal model adenomas and adenocarcinomas directly drives colonic tumor development [Hoshino, 1999; Sebolt-Leopold, 1999]. Furthermore, since oncogenic Ras generates a powerful anti-apoptotic signal in the colon [Bissonnette, 2000; Sears, 2002; Liu, 2005, 2006], capable of blocking extrinsic death (Fas) receptor mediated signaling [Peli, 1999], its antagonism will promote apoptosis and have therapeutic value. Our findings highlight a novel modality by which n-3 PUFA influence membrane micro-organization and biochemical makeup, thereby modulating biological responses.
Figure 3. Modulation of protein-membrane localization by dietary DHA.

Potential effects of dietary PUFA on post translational lipidation (fatty acylation and/or prenylation) and membrane composition. These effects may culminate in a shift in the localization of intracellular proteins.
B. Effects on protein cytosol-to-membrane translocation
In some cases, cytosolic signaling proteins are transiently recruited to and released from the cytoplasmic surface of cellular membranes. Regulation of dynamic membrane translocation of these proteins is critical for their function, and has been shown to be affected by n-3 PUFA. For example in Con A-stimulated human peripheral blood mononuclear cells, DHA dose-dependently promoted membrane translocation of ADP-ribosylation factor (ARF), but only to the detergent-soluble non-raft membranes. This was associated with the dose-dependent activation of phospholipase D (PLD) by DHA [Diaz, 2002]. In addition, DHA increased membrane translocation of protein kinase C (PKC) α, β, and ξ in unstimulated cells, while dose-dependently suppressing membrane translocation in stimulated cells.
We examined the separate effects of dietary corn oil (devoid on n-3 PUFA), fish oil, containing EPA and DHA, and highly purified DHA ethyl ester on the membrane subdomain distribution of critical signal transducing molecules (PKCθ, LAT, and Fas/CD95) in mouse splenic T lymphocytes [Fan, 2004]. Fish oil and DHA ethyl ester feeding increased phospholipid n-3 fatty acyl content while reducing sphingomyelin content in T cell lipid rafts. In addition, both DHA-containing diets inhibited raft recruitment and downstream signaling of PKCθ in stimulated T cells, decreasing lymphoproliferation. In complimentary studies, the effects of EPA and DHA (vs untreated control) on membrane translocation and downstream signaling of PKC isoforms in Jurkat T cells were examined [Denys, 2005]. EPA and DHA inhibited phorbol ester-induced membrane translocation of PKCα and PKCε, but not PKCδ, which was associated with the inhibition of downstream signaling. This may in part be attributed to the ability of certain long chain PUFA to alter ER-Golgi protein trafficking [Shaikh, 2007]. Collectively, these data support the hypothesis that PUFA classes (n-6 vs n-3) differentially modulate the dynamic translocation of signaling proteins. The mechanisms by which n-3 fatty acids alter plasma membrane translocation remain to be elucidated.
C. Effects on lipidated protein targeting via vesicular transport
Many signaling proteins must associate with membranes to function properly. For this purpose, cytosolic proteins that are initially synthesized a soluble proteins are co- or post-translationally modified with specific lipid anchors or contain special domains with high affinity for membranes (Figure 3). Since membrane localization of these otherwise soluble proteins is mediated by interacations between lipid anchors of proteins and cell membranes, it is conceivable that their membrane localization and hence function are sensitive to n-3 PUFA-induced changes in the cellular lipid environment. However, while the importance of post-translational protein lipidation to provide membrane anchors and targeting signals is well established and our understanding of protein transport mechanisms has substantially progressed, the potential regulatory role of the cellular lipid environment in the subcellular targeting of lipidated proteins has not been well appreciated.
Recently, our laboratory has demonstrated that DHA significantly inhibited plasma membrane targeting of Ras isoforms and Src-related tyrosine kinases using green fluorescent protein (GFP) fusion chimeras and quantitative fluorescence imaging of living colonic epithelial cells [Seo, 2006]. DHA selectively decreased the plasma membrane targeting of cytoplasmic lipidated protein cargo of the exocytic pathway, which involves constant budding and fusion of transport vesicles. However, the vesicular transport-independent trafficking of lipidated proteins as well as the general secretory vesicular traffic in colonic epithelial cells was unaffected DHA. Interestingly, this DHA effect appears to be only dependent on the protein trafficking route, irrespective of the types of membrane anchors as well as the functional status of lipidated proteins. It is noteworthy that DHA enrichment in cellular membranes was essential to elicit this inhibitory effects. These data are significant because overactivation of Ras proteins either by mutation, protooncogene overexpression, or chronic up-regulation of their upstream receptors contributes to deleterious cellular malfunction including oncogenesis and inflammatory diseases of the colon [Bos, 1989; Egan, 1989; Mangues, 1992; Kim, 1997]. For example, Ras effectors induce cyclin D1 and COX-2 resulting in hyperproliferation and apoptotic resistance. In addition, Src-related nonreceptor tyrosine kinases such as Lck play a key role in T cell-mediated immune responses and inflammatory diseases [Lowell, 2004]. Therefore, the selective suppression of plasma membrane targeting of lipidated signaling proteins by n-3 PUFA may constitute a novel modality of the mechanisms responsible for the long appreciated chemoprotective and immunomodulatory effects of dietary fish oil and DHA.
IV. Mechanisms by which DHA affects lipidated protein targeting
The exact mechanism(s) whereby DHA suppresses the plasma membrane targeting of lipidated protein via the exocytic pathway remains elusive. Although this DHA effect seems universal to lipidated proteins traveling through the secretory pathway, DHA does not appear to alter the bulk flow of secretory vesicular traffic since the plasma membrane delivery of the conventional transmembrane protein cargo remained unaffected [Seo, 2006] (Figure 4). Furthermore, it is unlikely that DHA induces recycling from the cell surface via endocytosis since the selectivity of DHA effects cannot be accounted for in this manner. However, potential explanations can be proposed based on previous findings from our laboratory and other groups as discussed below.
Figure 4.

DHA-induced modulation of vesicular protein transport
A. Alterations in protein palmitoylation
The role of palmitate (16:0) in protein plasma membrane targeting seems to be more than just increasing membrane binding affinity but rather operating as an actual targeting signal directing proteins to the plasma membrane [Greaves, 2007]. Recent work revealed that a dynamic de/repalmitoylation cycle is essential for maintaining the plasma membrane localization of Ras [Rocks, 2005, 2006]. We have demonstrated that Ras palmitoylation is unaffected by DHA in colonocytes [Collett, 2001]. Given that palmitoylation is the last step of Ras posttranslational modification, requiring the prior processing of the CAAX motif including farnesylation, this further indicates that DHA does not compromise the overall posttranslational processing of Ras proteins. This is supported by the lack of a DHA effect on farnesyltransferase activity, HMG-CoA reductase expression and Ras farnesylation state [Collett, 2001; Davidson, 1999]. However, DHA-induced changes in de/repalmitoylation kinetics may have not been detected by measurements of steady-state palmitoylation status in previous studies [Resh, 2006a,b,c]. Clearly, further progress in the identification and characterization of protein palmitoyltransferases and acyl protein thioesterases is required to clarify whether DHA influences the dynamics of de/repalmitoylation [Fernandez-Hernando, 2006; Mitchell, 2006; Linder, 2007].
An alternative possibility concerning protein lipidation is that DHA may be directly acylated to proteins in place of palmitate, thereby altering the membrane binding properties and subcellular localization of normally palmitoylated proteins. Although palmitate is the predominant fatty acid linked to cysteine residues of proteins, it appears that some proteins can be S-acylated with other acyl chains with different chain lengths and a varying degree of unsaturation [Bizzozero, 1986; Casey, 1994; Fujimoto, 1993; Hallak, 1994; Muszbek, 1993, 1999; O'Brien, 1987]. However, the efficiency of protein acylation with PUFAs appears extremely low relative to palmitate [DeMar, 1997; O'Brien, 1987; Stone, 1979], even in the PUFA-enriched environment such as cells incubated with PUFAs [Liang, 2001] and the biological significance and in vivo consequences of heterogeneous S-acylation of proteins remain under debate [Stulnig, 2001]. Our findings that the palmitoylation state of Ras is unaffected by DHA in colonocytes indicates that proteins modified with DHA, if any, must constitute only a minor fraction [Collett, 2001; Davidson, 1999], arguing that neither dramatic alterations in protein localization profile nor the differential effects on palmitoylated proteins observed can be accounted for by S-acylation of proteins with DHA [Seo, 2006]. More detailed exploration of the potential alterations in protein palmitoylation by DHA, either inhibition or substitution with DHA, will require further advances in the characterization of protein palmitoyl transferases.
B. Alteration of lipid-lipid and lipid-protein interactions
Given that alterations in protein modification are unlikely to provide sufficient explanations for the selectivity and magnitude of DHA effects, it seems reasonable to conclude that the unique properties of the DHA-enriched cellular lipid environment, i.e., lipid-lipid and lipid-protein interactions, are primarily responsible for the effects of DHA on lipidated protein localization.
We propose that DHA-enriched membranes do not provide an environment conducive to forward transport of newly synthesized lipidated proteins. Since the vesicular transport-independent targeting of lipidated proteins and the bulk flow of secretory membrane traffic are unaffected by DHA, it is most likely that lipidated cytoplasmic cargos are discriminated against in DHA-enriched secretory apparatus during sorting into transport vesicles (Figure 4). The unique membrane properties of DHA may involve subtle changes in the profile of heterogeneous transport vesicle/carrier populations in terms of lipid composition, size, curvature, and budding efficiency. Reduced plasma membrane delivery of lipidated proteins but not transmembrane proteins may result from the facilitated formation of certain types of transport vesicles in which packaging of membrane-embedded cargo is unaffected but lipidated peripheral proteins are not efficiently anchored on the cytoplasmic surface. Our finding that Ras plasma membrane targeting was inhibited only when DHA was sufficiently enriched in membranes to substantially increase membrane unsaturation [Seo, 2006] supports our hypothesis that altrerations in membrane physical properties are involved. However, further studies are required to establish 1) differential membrane binding affinities of lipid-anchored proteins as a function of membrane fatty acyl composition, 2) heterogeneous lateral lipid-protein organization in DHA-enriched ER and Golgi/trans Golgi network (TGN) membranes allowing for the formation of discrete microdomains, and 3) DHA-induced alterations in the transport vesicle/carrier population in relation to lipid composition and lipidated protein partitioning.
The unique properties of DHA-enriched membranes have been well studied using both model and biological membranes in view of the broad health benefits of dietary fish oil and DHA [Stillwell, 2000, 2003]. The presence of six cis-double bonds confers DHA exceptional conformational flexibility of the acyl chain to bend, tilt, and back-fold within membrane bilayers [Feller, 2002; Gawrisch, 2003]. Consequently, membranes containing bulky, disorderd DHA have looser lipid packing relative to saturated membranes [Eldho, 2003; Holte, 1995; Huster, 1997, 1998; Mitchell, 1998a,b]. The less cohesive acyl chain packing in DHA-enriched membranes will likely weaken van der Waals chain-chain interactions between lipid anchors of proteins and membrane phospholipids, thereby lowering binding affinity of lipidated proteins relative to the more tightly packed saturated membranes. In addition, the presence of DHA renders membranes easily deformable elastically [Koenig, 1997; Smaby, 1997], prompting speculation that the local concentration of DHA may influence vesicle budding and fusion efficiency. In fact, the fusogenic effect of DHA has been documented [Ehringer, 1990]. Collectively, the formation of DHA-enriched transport vesicles that are relatively depleted in lipidated peripheral proteins but bud and fuse more efficiently than saturated vesicles may underlie the reduced plasma membrane delivery of lipidated proteins.
C. Examples of DHA-enriched membranes and altered protein trafficking
As discussed below, several observations support the primary hypothesis that a DHA-enriched cellular lipid environment attenuates anterograde (forward) transport of lipidated proteins.
Polarized DHA distribution in cell membranes has been well documented in certain cell types naturally enriched in DHA, such as retinal rod photoreceptor cells, brain nerve cells, and sperm [Breckenridge, 1973; Connor, 1998; Cotman, 1969; Fleischer, 1965; Poulos, 1973; Stone, 1979]. DHA is particularly concentrated (up to 60 mol%) in the specialized and functionally most active regions such as rod outer segments and synaptic vesicles in the brain, and sperm tails. Studies of photoreceptor membrane biogenesis provide evidence suggesting that there exist different local concentrations of DHA in TGN membranes as well as distinct protein partitioning therein, allowing for active sorting into discrete post-Golgi vesicles en route to specific destinations. Rodriquez de Turco and co-workers demonstrated that newly synthesized docosahexaenoyl phospholipids are sequestered and co-transported by rhodopsin-bearing post-Golgi vesicles upon transport from the ER of inner segments, where their biosynthesis takes place, to rod outer segments [Rodriguez de Turco, 1997]. Most notably, the segregation and association of newly synthesized docosahexaenoyl phospholipids and rhodopsin appeared to occur on the TGN membranes prior to their exit and subsequent vectorial co-transport on post-Golgi vesicles to rod outer segments.
In adrenocortical cells, in which docosapentaenoic acid (22:5, n-3) and DHA only account for 6.9 and 0.1 mol% of total cellular lipids, respectively, distinct types of coated vesicles, presumably originating from the Golgi, have been isolated. These coated vesicles differed in their size, density, and notably 22:5 and DHA profile, indicating the presence of heterogeneous intracellular vesicles with different DHA distribution [de Paillerets, 1987].
Analysis of lipid composition and membrane molecular order of isolated small exfoliated vesicles (50–200 nm in diameter) from T27A leukemia cells revealed that DHA enrichment significantly mitigated or reversed the characteristic differences between the parent plasma membrane and shed microdomain vesicles [Williams, 1998, 1999]. Given that exfoliated vesicles normally arise from non-random regions of the cell surface [Armstrong, 1988; Beaudoin, 1991; Black, 1980; Taylor, 1988; van Blitterswijk, 1979], these data clearly indicate that cellular lipid environment can influence the lipid composition and structure of vesicles formed therein.
Interesting effects of DHA have been observed on the localization and activation of regulators of vesicular traffic, ARF and PLD [Diaz, 2002]. ARF, a Ras-related small GTPase, regulates coated vesicle biogenesis by controlling assembly of coat proteins and by activating its effector, PLD, which also participates in regulation of membrane traffic and actin remodeling via phosphatidic acid and indirectly via phosphatidylinositol 4,5-bisphosphate [Brown, 1993; Cockcroft, 2001; Roth, 1999]. In human peripheral blood mononuclear cells, DHA enrichment of cell membranes dose-dependently increased ARF membrane translocation and PLD activation only in the disordered nonraft membrane fractions. In view of the role of ARF and PLD in vesicle formation, these findings of increased ARF translocation and PLD activation only in specific regions of DHA-enriched membranes indicate that vesicle budding efficiency depends on the local lipid composition in relation to DHA content.
Unsaturated fatty acids are known to promote fusion of natural membranes as well as liposomes [Ahkong, 1973; Lavoie, 1991; Meers, 1988] and this has been related to the curvature stress provided to membranes by inverted phase-forming lipids [Ellens, 1989]. In particular, this fusogenic effect was clearly correlated with the number of double bonds in acyl chains and was much more pronounced with DHA than α-linolenic acid (18:3, n-3) [Ehringer, 1990; Talbot, 1997]. These findings imply that local DHA concentrations in the donor and/or acceptor membranes may affect fusion rates of transport vesicles.
In conclusion, the health benefits of DHA are diverse and a plethora of nutritional studies continue to demonstrate important benefits from the consumption of omega-3 enriched oils. Recently, the U.S. Food and Drug Administration (FDA) has approved the use of a health claim on labels for foods containing DHA. As part of an ongoing commitment to provide consumers with innovative-healthy products, food companies are now scrambling to incorporate omega-3 fatty acids into a range of novel commercial foods in order to provide for the wider public consumption of DHA. It is both appropriate and timely, therefore, to precisely determine how DHA modulates cell signaling networks and reduces the risk of developing colon cancer and intestinal inflammatory disorders.
Acknowledgments
Supported in part by NIH grants CA59034, CA129444, DK71707, and P30ES09106.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Ahkong QF, Fisher D, Tampion W, Lucy JA. The fusion of erythrocytes by fatty acids, esters, retinol and alpha-tocopherol. Biochem. J. 1973;136:147–155. doi: 10.1042/bj1360147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts DS, Martinez ME, Roe DJ, Guillen-Rodriguez JM, Marshall JR, et al. Lack of effect of high-fiber cereal supplement on the recurrence of colorectal adenomas. N. Engl. J. Med. 2000;342:1156–1162. doi: 10.1056/NEJM200004203421602. [DOI] [PubMed] [Google Scholar]
- Anderson RGW. The caveolae membrane system. Annu. Rev. Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- Anti M, Giancarlo M, Armelao F, Bartoli GM, Ficarelli R, Percesepe A, De Vitis I, Maria G, Sofo L, Rapaccini GL, Gentiloni N, Piccioni E, Miggiano G. Effect of ω-3 Fatty acids on Rectal Mucosal Cell Proliferation in Subjects at Risk for Colon Cancer. Gastroenterology. 1992;103:883–891. doi: 10.1016/0016-5085(92)90021-p. [DOI] [PubMed] [Google Scholar]
- Anti M, Armelao F, Marra G, Percesepe A, Bartoli GM, Palozza P, Parrella P, Canetta C, Gentiloni N, De Vitis I, Gasbarrini G. Effects of Different doses of fish Oil on Rectal Cell Proliferation in Patients with Sporadic Colonic Adenomas. Gastroenterology. 1994;107:1709–1718. doi: 10.1016/0016-5085(94)90811-7. [DOI] [PubMed] [Google Scholar]
- Archer SY, Meng S, Shei A, Hodin RA. p21WAF1 is required for butyrate-mediated growth inhibition of human colon cancer cells. Proc. Natl. Acad. Sci. 1998;95:6791–6796. doi: 10.1073/pnas.95.12.6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai M, Imai H, Koumura T, Yoshida M, Emoto K, Umeda M, Chiba N, Nakagawa Y. Mitochondrial phospholipid hydroperoxide glutathione peroxidase plays a major role in preventing oxidative injury to cells. J. Biol. Chem. 1999;274:4924–4933. doi: 10.1074/jbc.274.8.4924. [DOI] [PubMed] [Google Scholar]
- Armstrong MJ, Storch J, Dainiak N. Structurally distinct plasma membrane regions give rise to extracellular membrane vesicles in normal and transformed lymphocytes. Biochim. Biophys. Acta. 1988;946:106–112. doi: 10.1016/0005-2736(88)90462-2. [DOI] [PubMed] [Google Scholar]
- Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006;83:1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- Bancroft LK, Lupton JR, Davidson LA, Taddeo SS, Murphy ME, Carroll RJ, Chapkin RS. Dietary fish oil reduces oxidative DNA damage in rat colonocytes. Free Radical Biol. Med. 2003;35:149–159. doi: 10.1016/s0891-5849(03)00240-5. [DOI] [PubMed] [Google Scholar]
- Bartram HP, Gostner A, Scheppach W, Reddy BS, Rao CV, Dusel G, Richter F, Richter A, Kasper H. Effects of Fish Oil on Rectal Cell Proliferation, Mucosal Fatty Acids, and Prostaglandin E2 Release in Healthy Subjects. Gastroenterology. 1993;105:1317–1322. doi: 10.1016/0016-5085(93)90135-y. [DOI] [PubMed] [Google Scholar]
- Bayir H, Fadeel B, Palladino MJ, Witasp E, Kurnikov IV, Tyrurina YY, Tyurin VA, Amoscato AA, Jiang J, Kochanek PM, DeKosky ST, Greenberger JS, Shvedova AA, Kagan VE. Apoptotic interactions of cytochrome c: Redox flirting with anionic phospholipids within and outside of mitochondria. Biochim. Biophys. Acta. 2006;1757:648–659. doi: 10.1016/j.bbabio.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Beaudoin AR, Grondin G. Shedding of vesicular material from the cell surface of eukaryotic cells: different cellular phenomena. Biochim. Biophys. Acta. 1991;1071:203–219. doi: 10.1016/0304-4157(91)90014-n. [DOI] [PubMed] [Google Scholar]
- Bedi A, Pasricha PJ, Alchtar AJ, Barber JP, Bedi GC, Giairdiello FM, Zehnbauer BA, Hamiltion SR, Jones RJ. Inhibition of apoptosis during development of colorectal cancer. Cancer Res. 1995;55:1811–1816. [PubMed] [Google Scholar]
- Benard O, Balasubramanian KA. Modulation of glutathione level during butyrate-induced differentiation in human colon derived HT-29 cells. Mol. Cell. Biochem. 1997;170:109. doi: 10.1023/a:1006892929652. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signaling. Nat. Rev. Mol. Cell. Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Bissonnette M, Khare S, von Lintig FC, Wali RK, Nguyen L, Zhang Y, Hart J, Skarosi S, Varki N, Boss GR, Brasitus TA. Mutational and nonmutational activation of p21ras in rat colonic azoxymethane-induced tumors: Effects on mitogen-activated protein kinase, cyclooxygenase-2, and cyclin D1. Cancer Res. 2000;60:4602–2609. [PubMed] [Google Scholar]
- Bizzozero OA, McGarry JF, Lees MB. Acylation of rat brain myelin proteolipid protein with different fatty acids. J. Neurochem. 1986;47:772–778. doi: 10.1111/j.1471-4159.1986.tb00678.x. [DOI] [PubMed] [Google Scholar]
- Black PH. Shedding from the cell surface of normal and cancer cells. Adv. Cancer Res. 1980;32:75–199. doi: 10.1016/s0065-230x(08)60361-9. [DOI] [PubMed] [Google Scholar]
- Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- Breckenridge WC, Morgan IG, Zanetta JP, Vincendon G. Adult rat brain synaptic vesicles. II. Lipid composition. Biochim. Biophys. Acta. 1973;320:681–686. doi: 10.1016/0304-4165(73)90148-7. [DOI] [PubMed] [Google Scholar]
- Brown D. The tyrosine kinase connection: how GPI-anchored proteins activate T cells. Curr. Opin. Immunol. 1993;5:349–354. doi: 10.1016/0952-7915(93)90052-t. [DOI] [PubMed] [Google Scholar]
- Casey WM, Gibson KJ, Parks LW. Covalent attachment of palmitoleic acid (C16:1 delta 9) to proteins in Saccharomyces cerevisiae. Evidence for a third class of acylated proteins. J. Biol. Chem. 1994;269:2082–2085. [PubMed] [Google Scholar]
- Caygill CP, Charlett A, Hill MJ. Fat, Fish, fish Oil and Cancer. Br. J. Cancer. 1996;74:159–164. doi: 10.1038/bjc.1996.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W-CL, Chapkin RS, Lupton JR. Predictive value of proliferation, differentiation and apoptosis as intermediate markers for colon tumorigenesis. Carcinogenesis. 1997;18:721–730. doi: 10.1093/carcin/18.4.721. [DOI] [PubMed] [Google Scholar]
- Chang WC, Chapkin RS, Lupton JR. Fish oil blocks azoxymethane-induced tumorigenesis by increased cell Differentiation and apoptosis rather than decreased cell proliferation. J. Nutr. 1998;18:351–357. doi: 10.1093/jn/128.3.491. [DOI] [PubMed] [Google Scholar]
- Chapkin RS, Akoh CC, Miller CC. Influence of dietary n-3 fatty acids on macrophage glycerophospholipid molecular species and peptido leukotriene synthesis. J. Lipid Res. 1991;32:1205–1213. [PubMed] [Google Scholar]
- Chapkin RS, Lupton JR. Colonic cell proliferation and apoptosis in rodent species: Modulation by diet. In: Colon Cancer Prevention: Dietary Modulation of Cellular and Molecular Mechanisms. Advances in Experimental Medicine and Biology. 1999;470:105–118. doi: 10.1007/978-1-4615-4149-3_12. [DOI] [PubMed] [Google Scholar]
- Chapkin RS, Hong MY, Fan YY, Davidson LA, Sanders LM, Henderson CE, Barhoumi R, Burghardt RC, Turner ND, Lupton JR. Dietary n-3 PUFA alter colonocyte mitochondrial membrane composition and function. Lipids. 2002;37:193–199. doi: 10.1007/s11745-002-0880-8. [DOI] [PubMed] [Google Scholar]
- Chapkin RS, Wang N, Lupton JR, Prior IA. American Society for Cell Biology Meeting. San Francisco, CA: 2005. Dec 13, Docosahexaeneic acid alters the size and distribution of lipid rafts. [Google Scholar]
- Chapkin RS, McMurray DN, Lupton JR. Colon cancer, fatty acids and anti-inflammatory compounds. Current Opinions in Gastroenterology. 2007a;23:48–54. doi: 10.1097/MOG.0b013e32801145d7. [DOI] [PubMed] [Google Scholar]
- Chapkin RS, Davidson LA, Ly L, Weeks BR, Lupton JR, McMurray DN. Immunomodulatory effects of omega-3 fatty acids: Putative link to inflammation and colon cancer. J. Nutr. 2007b;137:200S–204S. doi: 10.1093/jn/137.1.200S. [DOI] [PubMed] [Google Scholar]
- Chen W, Jump DB, Esselman WJ, Busik JV. Inhibition of cytokine signaling in human retinal endothelial cells through modification of caveolae/lipid rafts by docosahexaenoic acid. Invest. Opthalmol. Vis. Sci. 2007;48:18–26. doi: 10.1167/iovs.06-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Ogawa K, Kuriki K, Yokoyama Y, Kamiya T, Seno K, Okuyama H, Wang J, Luo C, Fujii T, Ickikawa H, Shirai T, Tokudome S. Increased intake of n-3 polyunsaturated fatty acids elevates the level of apoptosis in the normal sigmoid colon of patients polypectomized for adenomas/tumors. Cancer Lett. 2003;193:17–24. doi: 10.1016/s0304383502007176. [DOI] [PubMed] [Google Scholar]
- Cockcroft S. Signalling roles of mammalian phospholipase D1 and D2. Cell. Mol. Life Sci. 2001;58:1674–1687. doi: 10.1007/PL00000805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett ED, Davidson LA, Lupton JR, Chapkin RS. n-6 and n-3 polyunsaturated fatty acids differentially modulate oncogenic ras activation in colonocytes. Am. J. Physiol: Cell Physiology. 2001;280:C1066–C1075. doi: 10.1152/ajpcell.2001.280.5.C1066. [DOI] [PubMed] [Google Scholar]
- Connor WE, Lin DS, Wolf DP, Alexander M. Uneven distribution of desmosterol and docosahexaenoic acid in the heads and tails of monkey sperm. J. Lipid Res. 1998;39:1404–1411. [PubMed] [Google Scholar]
- Conquer JA, Holub BJ. Effect of supplementation with different doses of DHA on the levels of circulating DHA as non-esterified fatty acid in subjects of Asian Indian background. J. Lipid Res. 1998;39:286–292. [PubMed] [Google Scholar]
- Costantini P, Jacotot E, Decaudin D, Kroemer G. Mitochondrion as a novel target of anticancer chemotherapy. J. Natl. Cancer Inst. 2000;92:1042–1053. doi: 10.1093/jnci/92.13.1042. [DOI] [PubMed] [Google Scholar]
- Cotman C, Blank ML, Moehl A, Snyder F. Lipid composition of synaptic plasma membranes isolated from rat brain by zonal centrifugation. Biochemistry. 1969;8:4606–4612. doi: 10.1021/bi00839a056. [DOI] [PubMed] [Google Scholar]
- Courtney ED, Matthews S, Finlayson C, Di Pierro D, Belluzzi A, Roda E, Kang JY, Leicester RJ. Eicosapentaenoic acid (EPA) reduces crypt cell proliferation and increases apoptosis in normal colonic mucosa in subjects with a history of colorectal adenomas. Int. J. Colorectal Dis. 2007 Jan 10; doi: 10.1007/s00384-006-0240-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Lupton JR, Jiang YH, Chapkin RS. Carcinogen and dietary lipid regulate ras expression and localization in rat colon without affecting farnesylation kinetics. Carcinogenesis. 1999;20:785–791. doi: 10.1093/carcin/20.5.785. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Brown RE, Chang WC, Lupton JR, Morris JS, Wang N, Carroll RJ, Turner ND, Chapkin RS. Morphodensitometric analysis of protein kinase CβII expression in rat colon: modulation by diet and relation to in situ cell proliferation and apoptosis. Carcinogenesis. 2000;21:1512–1519. [PubMed] [Google Scholar]
- Davidson LA, Nguyen DV, Hokanson RM, Callaway ES, Isett RB, Turner ND, Dougherty ER, Lupton JR, Carroll RJ, Chapkin RS. Chemopreventive n-3 polyunsaturated fatty acids reprogram genetic signatures during colon cancer initiation and progression in the rat. Cancer Res. 2004;64:6797–6804. doi: 10.1158/0008-5472.CAN-04-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMar JC, Jr, Anderson RE. Identification and quantitation of the fatty acids composing the CoA ester pool of bovine retina, heart, and liver. J. Biol. Chem. 1997;272:31362–31368. doi: 10.1074/jbc.272.50.31362. [DOI] [PubMed] [Google Scholar]
- Denys A, Hichami A, Khan NA. n-3 PUFAs modulate T cell activation via protein kinase C-alpha and -epsilon and the NF-kappaB signaling pathway. J. Lipid Res. 2005;46:752–758. doi: 10.1194/jlr.M400444-JLR200. [DOI] [PubMed] [Google Scholar]
- de Paillerets C, Bomsel M, Weintraub H, Pepin D, Alfsen A. Clustering in coated vesicles of polyunsaturated phospholipids segregated from plasma and Golgi membranes of adrenocortical cells. FEBS Lett. 1987;219:113–118. doi: 10.1016/0014-5793(87)81201-2. [DOI] [PubMed] [Google Scholar]
- Diaz O, Berquand A, Dubois M, Di Agostino S, Sette C, Bourgoin S, Lagarde M, Nemoz G, Prigent AF. The mechanism of docosahexaenoic acid-induced phospholipase D activation in human lymphocytes involves exclusion of the enzyme from lipid rafts. J. Biol. Chem. 2002;277:39368–39378. doi: 10.1074/jbc.M202376200. [DOI] [PubMed] [Google Scholar]
- Egan SE, Broere JJ, Jarolim L, Wright JA, Greenberg AH. Co-regulation of metastatic and transforming activity of normal and mutant ras genes. Int. J. Cancer. 1989;43:443–448. doi: 10.1002/ijc.2910430317. [DOI] [PubMed] [Google Scholar]
- Ehringer W, Belcher D, Wassall SR, Stillwell W. A comparison of the effects of linolenic acid (18:3 omega 3) and docosahexaenoic (22:6 omega 3) acids on phospholipid bilayers. Chem. Phys. Lipids. 1990;54:79–88. doi: 10.1016/0009-3084(90)90063-w. [DOI] [PubMed] [Google Scholar]
- Eldho NV, Feller SE, Tristram-Nagle S, Polozov IV, Gawrisch K. Polyunsaturated docosahexaenoic vs docosapentaenoic acid-differences in lipid matrix properties from the loss of one double bond. J. Am. Chem. Soc. 2003;125:6409–6421. doi: 10.1021/ja029029o. [DOI] [PubMed] [Google Scholar]
- Ellens H, Siegel DP, Alford D, Yeagle PL, Boni L, Lis LJ, Quinn PJ, Bentz J. Membrane fusion and inverted phases. Biochemistry. 1989;28:3692–3703. doi: 10.1021/bi00435a011. [DOI] [PubMed] [Google Scholar]
- Engel RH, Evens AM. Oxidative stress and apoptosis: a new treatment paradigm in cancer. Front. Biosci. 2006;11:300–312. doi: 10.2741/1798. [DOI] [PubMed] [Google Scholar]
- Fan YY, Turner N, Zhang J, Barhoumi R, Burghardt RC, Lupton JR, Chapkin RS. Antagonism of CD95 (APO-1/Fas) signaling blocks butyrate induction of apoptosis in young adult mouse colonic (YAMC) cells. American Journal of Physiology. 1999;277:C310–C319. doi: 10.1152/ajpcell.1999.277.2.C310. (Cell Physiology 46) [DOI] [PubMed] [Google Scholar]
- Fan YY, Ly LH, Barhoumi R, McMurray DN, Chapkin RS. Dietary docosahexaenoic acid suppresses T cell protein kinase Cθ lipid raft recruitment and IL-2 recruitment. J. Immunol. 2004;173:6151–6160. doi: 10.4049/jimmunol.173.10.6151. [DOI] [PubMed] [Google Scholar]
- Fariss MW, Chan CB, Patel M, Van Houten BV, Orrenius S. Role mitochondria in toxic oxidative stress. Mol. Interventions. 2005;5:94–111. doi: 10.1124/mi.5.2.7. [DOI] [PubMed] [Google Scholar]
- Feller SE, Gawrisch K, MacKerell AD., Jr Polyunsaturated fatty acids in lipid bilayers: intrinsic and environmental contributions to their unique physical properties. J. Am. Chem. Soc. 2002;124:318–326. doi: 10.1021/ja0118340. [DOI] [PubMed] [Google Scholar]
- Fernandez E, Chatenoud L, La Vecchia C, Negri E, Franceschi S. Fish consumption and cancer risk. Am. J. Clin. Nutr. 1999;70:85–90. doi: 10.1093/ajcn/70.1.85. [DOI] [PubMed] [Google Scholar]
- Fernandez-Hernando C, Fukata M, Bernatchez PN, Fukata Y, Lin MI, Bredt DS, Sessa WC. Identification of golgi-localized acyl transferases that palmitoylate and regulate endothelail nitric oxide synthase. J. Cell Biol. 2006;174:369–377. doi: 10.1083/jcb.200601051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer S, Rouser G. Lipids of Subcellular Particles. J. Am. Oil. Chem. Soc. 1965;42:588–607. doi: 10.1007/BF02541295. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Stroud E, Whatley RE, Prescott SM, Muszbek L, Laposata M, McEver RP. P-selectin is acylated with palmitic acid and stearic acid at cysteine 766 through a thioester linkage. J. Biol. Chem. 1993;268:11394–11400. [PubMed] [Google Scholar]
- Fulda S, Meyer E, Friesen C, Susin SA, Kroemer G, Debatin KM. Cell type specific involvement of death receptor and mitochondrial pathways in drug-induced apoptosis. Oncogene. 2001;20:1063–1075. doi: 10.1038/sj.onc.1204141. [DOI] [PubMed] [Google Scholar]
- Gardner HW. Oxygen radical chemistry of polyunsaturated fatty acids. Free Rad. Biol. Med. 1989;7:65–86. doi: 10.1016/0891-5849(89)90102-0. [DOI] [PubMed] [Google Scholar]
- Gawrisch K, Eldho NV, Holte LL. The structure of DHA in phospholipid membranes. Lipids. 2003;38:445–452. doi: 10.1007/s11745-003-1082-0. [DOI] [PubMed] [Google Scholar]
- Giardina C, Inan MS. Nonsteroidal anti-inflammatory drugs, short-chain fatty acids, and reactive oxygen metabolism in human colorectal cancer cells. Biochim. Biophys. Acta. 1998;1401:277–288. doi: 10.1016/s0167-4889(97)00140-7. [DOI] [PubMed] [Google Scholar]
- Greaves J, Chamberlain LH. Palmitoylation-dependent protein sorting. J. Cell Biol. 2007;176:249–254. doi: 10.1083/jcb.200610151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallak H, Muszbek L, Laposata M, Belmonte E, Brass LF, Manning DR. Covalent binding of arachidonate to G protein alpha subunits of human platelets. J. Biol. Chem. 1994;269:4713–4716. [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hancock JF. Lipid rafts: contentious only from simplistic standpoints. Nature Rev. Mol. Cell Biol. 2006;7:456–462. doi: 10.1038/nrm1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Hosain MS, Shimada T, Yamasaki H, Fujii Y, Shido O. Effects of docosahexaenoic acid on annular lipid fluidity of the rat bile canalicular plasma membrane. J. Lipid Res. 2001;42:1160–1168. [PubMed] [Google Scholar]
- Hashimoto M, Hossain S, Shido O. Docosahexaenoic acid but not eicosapentaenoic acid withstands dietary cholesterol-induced decreases in platelet membrane fluidity. Mol. Cell. Biochem. 2006;293:1–8. doi: 10.1007/s11010-006-0164-x. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR, Nieminsen LR, Blasbalg TL, Riggs JA, Lands WE. Healthy intakes of n-3 and n-6 fatty acids: estimations considering worldwide diversity. Am. J. Clin. Nutr. 2006;83:1483S–1493S. doi: 10.1093/ajcn/83.6.1483S. [DOI] [PubMed] [Google Scholar]
- Holte LL, Peter SA, Sinnwell TM, Gawrisch K. 2H nuclear magnetic resonance order parameter profiles suggest a change of molecular shape for phosphatidylcholines containing a polyunsaturated acyl chain. Biophys. J. 1995;68:2396–2403. doi: 10.1016/S0006-3495(95)80422-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong MY, Lupton JR, Morris JS, Wang N, Carroll R, Davidson L, Elder R, Chapkin R. Dietary fish oil reduces DNA adduct levels in rat colon in part by increasing apoptosis during tumor initiation. Cancer Epidemiol. Biomark. Prev. 2000;9:819–826. [PubMed] [Google Scholar]
- Hong MY, Chapkin RS, Barhoumi R, Burghardt RC, Turner ND, Henderson CE, Sanders LM, Fan YY, Davidson LA, Murphy ME, Spinka CM, Carroll RJ, Lupton JR. Fish oil increases mitochondrial phospholipid unsaturation, upregulating reactive oxygen species and apoptosis in rat colonocytes. Carcinogenesis. 2002;23:1919–1925. doi: 10.1093/carcin/23.11.1919. [DOI] [PubMed] [Google Scholar]
- Hong MH, Chapkin RS, Davidson LA, Turner ND, Morris JS, Carroll RJ, Lupton JR. Fish oil enhances targeted apoptosis during colon tumor initiation in part by down regulating BCL-2. Nutrition & Cancer. 2003;46:44–51. doi: 10.1207/S15327914NC4601_06. [DOI] [PubMed] [Google Scholar]
- Hong MY, Turner ND, Carroll RJ, Chapkin RS, Lupton JR. Differential response to oxidative DNA damage may explain aspects of the cancer susceptibility between small and large intestine. Exp. Biol. Med. 2005;230:464–471. doi: 10.1177/153537020523000704. [DOI] [PubMed] [Google Scholar]
- Hoshino R, Chatani Y, Yamori T, Tsuruo T, Oka H, Yoshida O, Shimada R, Ari-I S, Wada H, Fujimoto J, Kohno M. Constitutive activation of the 41-/43-kDa mitogen-activated protein kinase signaling pathway in human tumors. Oncogene. 1999;18:813–822. doi: 10.1038/sj.onc.1202367. [DOI] [PubMed] [Google Scholar]
- Huster D, Jin AJ, Arnold K, Gawrisch K. Water permeability of polyunsaturated lipid membranes measured by 17O NMR. Biophys. J. 1997;73:855–864. doi: 10.1016/S0006-3495(97)78118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huster D, Arnold K, Gawrisch K. Influence of docosahexaenoic acid and cholesterol on lateral lipid organization in phospholipid mixtures. Biochemistry. 1998;37:17299–17308. doi: 10.1021/bi980078g. [DOI] [PubMed] [Google Scholar]
- Jacobson J, Duchen MR. Mitochondrial oxidative stress and cell death in astrocytes-requirement of stored Ca2+ and sustained opening of the permeability transition pore. J. Cell Sci. 2002;115:1175–1188. doi: 10.1242/jcs.115.6.1175. [DOI] [PubMed] [Google Scholar]
- Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nature Chem. Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- Katan MB, Deslypere JP, van Birgelen AP, et al. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J. Lipid Res. 1997;38:2012–2022. [PubMed] [Google Scholar]
- Kim K, Kuo T, Cai J, Shuja S, Murnane MJ. N-ras protein: Frequent quantitative and qualitative changes occur in human colorectal carcinomas. Int. J. Cancer. 1997;71:767–775. doi: 10.1002/(sici)1097-0215(19970529)71:5<767::aid-ijc13>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Koenig BW, Strey HH, Gawrisch K. Membrane lateral compressibility determined by NMR and x-ray diffraction: effect of acyl chain polyunsaturation. Biophys. J. 1997;73:1954–1966. doi: 10.1016/S0006-3495(97)78226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoszka JE, Coskun P, Esposito LA, Wallace DC. Increased mitochondrial oxidative stress in the Sod2 (+/−) mouse results in the age-related decline of mitochondrial function culminating in increased apoptosis. Proc. Natl. Acad. Sci. 2001;98:2278–2283. doi: 10.1073/pnas.051627098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolar SS, Barhoumi R, Lupton JR, Chapkin RS. Docosahexaenoic acid and butyrate synergistically induce colonocyte apoptosis by enhancing mitochondrial Ca2+ accumulation. 2007a doi: 10.1158/0008-5472.CAN-06-4716. (Submitted for publication) [DOI] [PubMed] [Google Scholar]
- Kolar SS, Barhoumi R, Lupton JR, Chapkin RS. To be presented at Experimental Biology. Washington D.C.: 2007b. Apr 28, Chemoprotective nutrients modulate intracellular calcium compartmentalization to induce colonocyte apoptosis via a p53 independent pathway. [Google Scholar]
- Koumura T, Nakamura C, Nakagawa Y. Involvement of hydroperoxides in mitochondria in the induction of apoptosis by the eicosapentaenoic acid. Free Radical Res. 2005;39:225–235. doi: 10.1080/10715760500043587. [DOI] [PubMed] [Google Scholar]
- Lavoie C, Jolicoeur M, Paiement J. Accumulation of polyunsaturated free fatty acids coincident with the fusion of rough endoplasmic reticulum membranes. Biochim. Biophys. Acta. 1991;1070:274–278. doi: 10.1016/0005-2736(91)90175-8. [DOI] [PubMed] [Google Scholar]
- Lee DY, Lupton JR, Aukema HM, Chapkin RS. Dietary fat and fiber alter rat colonic mucosal lipid mediators and cell proliferation. J. Nutr. 1993;123:1808–1917. doi: 10.1093/jn/123.11.1808. [DOI] [PubMed] [Google Scholar]
- Leyk M, Nguyen DV, Atoor SN, Dougherty ER, Turner ND, Bancroft LK, Chapkin RS, Lupton JR, Carroll RJ. Comparing automatic and manual image processing in FLARE assay analysis for colon carcinogenesis. Statistical Applications in Genetics and Molecular Biology. 2005;4:1–18. doi: 10.2202/1544-6115.1102. Article 5, http://www.bepress.com/sagmb/vol4/iss1/art5. [DOI] [PubMed]
- Liang X, Nazarian A, Erdjument-Bromage H, Bornmann W, Tempst P, Resh MD. Heterogeneous fatty acylation of Src family kinases with polyunsaturated fatty acids regulates raft localization and signal transduction. J. Biol. Chem. 2001;276:30987–30994. doi: 10.1074/jbc.M104018200. [DOI] [PubMed] [Google Scholar]
- Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nat. Rev. Mol. Cell Biol. 2007;8:74–84. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- Liu Z, Li H, Derouet M, Filmus J, LaCasse EC, Koneluk RG, Kerbel RS, Rosen KV. Ras oncogene triggers up-regulation of cIAP2 and XIAP in intestinal epithelial cells. J. Biol. Chem. 2005;45:37383–37392. doi: 10.1074/jbc.M503724200. [DOI] [PubMed] [Google Scholar]
- Liu Z, Li H, Wu X, Yoo BH, Yan SR, Stadnyk AW, Sasazuki T, Shirasawa S, LaCasse EC, Korneluk RG, Rosen KV. Detachment-induced upregulation of XIAP and cIAP2 delays anoikis of intestinal epithelial cells. Oncogene. 2006;25:7680–7690. doi: 10.1038/sj.onc.1209753. [DOI] [PubMed] [Google Scholar]
- Lowell CA. Src-family kinases: rheostats of immune cell signaling. Mol. Immunol. 2004;41:631–643. doi: 10.1016/j.molimm.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Lupton JR, Chang WC, Hong MY, Chapkin RS. Fat/fiber interaction on colonic cytokinetics: relationship to colon cancer. Asia Pacific J Clin Nutr. 1999;8:S37–S40. [Google Scholar]
- Lupton JR, Chapkin RS. Chemopreventive effects of Omega-3 fatty acids. In: Cancer Chemo prevention; Vol I. In: Kelloff GJ, Hawk ET, Sigman CC, editors. Promising Cancer Chemopreventive Agents. Totowa, NJ: Humana Press; 2004. pp. 591–608. [Google Scholar]
- Ma DW, Seo J, Davidson LA, Callaway ES, Fan YY, Lupton JR, Chapkin RS. n-3 PUFA alter caveolae lipid composition and resident protein localization in mouse colon. Faseb Journal Express Article. 2004a;18:1040–1042. doi: 10.1096/fj.03-1430fje. April 15, 2004. 10.1096/fj.o3-0604fje. [DOI] [PubMed] [Google Scholar]
- Ma DW, Seo J, Switzer KC, Fan YY, McMurray DN, Lupton JR, Chapkin RS. n-3 PUFA and membrane microdomains: a new frontier in bioactive lipid research. Journal of Nutritional Biochemistry. 2004b;15:700–706. doi: 10.1016/j.jnutbio.2004.08.002. [DOI] [PubMed] [Google Scholar]
- MacLean CH, Newberry SJ, Mojica WA, et al. Effects of omega-3 fatty acids on cancer risk. JAMA. 2006;295:403–415. doi: 10.1001/jama.295.4.403. [DOI] [PubMed] [Google Scholar]
- Mangues R, Seidman I, Gordon JW, Pellicer A. Overexpression of the N-ras proto-oncogene, not somatic mutational activation, associated with malignant tumors in transgenic mice. Oncogene. 1992;7:2073–2076. [PubMed] [Google Scholar]
- Mariadason JM, Corner GA, Augenlicht LH. Genetic reprogramming in pathways of colonic cell maturation induced by short chain fatty acids: comparison with Trichostatin A, Sulindac, and Curcumin and implications for chemoprevention of colon cancer. Cancer Res. 2000;60:4561–4572. [PubMed] [Google Scholar]
- Meers P, Hong K, Papahadjopoulos D. Free fatty acid enhancement of cation-induced fusion of liposomes: synergism with synexin and other promoters of vesicle aggregation. Biochemistry. 1988;27:6784–6794. doi: 10.1021/bi00418a021. [DOI] [PubMed] [Google Scholar]
- Mitchell DC, Litman BJ. Effect of cholesterol on molecular order and dynamics in highly polyunsaturated phospholipid bilayers. Biophys. J. 1998a;75:896–908. doi: 10.1016/S0006-3495(98)77578-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DC, Litman BJ. Molecular order and dynamics in bilayers consisting of highly polyunsaturated phospholipids. Biophys. J. 1998b;74:879–891. doi: 10.1016/S0006-3495(98)74011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DA, Vasudevan A, Linder ME, Deschenes RJ. Protein palmitoylation by a family of DHHC protein S-acyltransferases. J. Lipid Res. 2006;47:1118–1127. doi: 10.1194/jlr.R600007-JLR200. [DOI] [PubMed] [Google Scholar]
- Muszbek L, Laposata M. Covalent modification of proteins by arachidonate and eicosapentaenoate in platelets. J. Biol. Chem. 1993;268:18243–18248. [PubMed] [Google Scholar]
- Muszbek L, Haramura G, Cluette-Brown JE, Van Cott EM, Laposata M. The pool of fatty acids covalently bound to platelet proteins by thioester linkages can be altered by exogenously supplied fatty acids. Lipids. 1999;34 Suppl:S331–S337. doi: 10.1007/BF02562334. [DOI] [PubMed] [Google Scholar]
- Ng Y, Barhoumi R, Tjalkens RB, Fan YY, Kolar S, Wang N, Lupton JR, Chapkin RS. The role of docosahexaenoic acid mediating mitochondrial membrane lipid oxidation and apoptosis in colonocytes. Carcinogenesis. 2005;26:1914–1921. doi: 10.1093/carcin/bgi163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura K, Imai H, Koumura T, Arai M, Nakagawa Y. Mitochondrial phospholipid hydroperoxide glutathione peroxidase suppresses apoptosis mediated by a mitochondrial death pathway. J. Biol. Chem. 1999;274:29294–29302. doi: 10.1074/jbc.274.41.29294. [DOI] [PubMed] [Google Scholar]
- O'Brien PJ, St Jules RS, Reddy TS, Bazan NG, Zatz M. Acylation of disc membrane rhodopsin may be nonenzymatic. J. Biol. Chem. 1987;262:5210–5215. [PubMed] [Google Scholar]
- Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu. Rev. Pharmacol. Toxicol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- Park Y, Hunter DJ, Spiegelman D, et al. Dietary fiber intake and risk of colorectal cancer. A pooled analysis of prospective cohort studies. JAMA. 2005;294:2849–2857. doi: 10.1001/jama.294.22.2849. [DOI] [PubMed] [Google Scholar]
- Peli J, Schroter M, Rudaz C, Hahne M, Meyer C, Reichmann E, Tschopp J. Oncogenic Ras inhibits Fas ligand-mediated apoptosis by downregulating the expression of Fas. EMBO J. 1999;18:1824–1831. doi: 10.1093/emboj/18.7.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters U, Sinha R, Chatterjee N, Subar AF, Ziegler RG, Kulldorff M, Bresalier R, Weissfeld JL, Flood A, Schatzkin A, Hayes RB. Dietary fibre and colorectal ademoma in a colorectal cancer early detection programme. Lancet. 2003;361:1491–1495. doi: 10.1016/S0140-6736(03)13173-X. [DOI] [PubMed] [Google Scholar]
- Phillips MN, Chavarro J, Stampfer M, Willet W, Ma J. A prospective study of fish, n-3 fatty acid intake, and colorectal cancer risk in men. Boston, MA: AACR International Conference on Frontiers in Cancer Prevention Research; 2006. Nov 12, [Google Scholar]
- Poulos A, Voglmayr JK, White IG. Phospholipid changes in spermatozoa during passage through the genital tract of the bull. Biochim. Biophys. Acta. 1973;306:194–202. doi: 10.1016/0005-2760(73)90225-7. [DOI] [PubMed] [Google Scholar]
- Rao CV, Hirose Y, Indranie C, Reddy BS. Modulation of Experimental Colon Tumorigenesis by Types and Amounts of dietary Fatty Acids. Cancer Res. 2001;61:1927–1933. [PubMed] [Google Scholar]
- Reddy BS, Patlolla JM, Simi B, Wang SH, Rao CV. Prevention of colon cancer by low doses of Celecoxib, a cyclooxygenase inhibitor, administered in a diet rich in ω-3 polyunsaturated fatty acids. Cancer Res. 2005;65:8022–8027. doi: 10.1158/0008-5472.CAN-05-0212. [DOI] [PubMed] [Google Scholar]
- Resh MD. Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci. Stke. 2006a;359:re14. doi: 10.1126/stke.3592006re14. [DOI] [PubMed] [Google Scholar]
- Resh MD. Trafficking and signaling by fatty-acylated and prenylated proteins. Nat. Chem. Biol. 2006b;2:584–590. doi: 10.1038/nchembio834. [DOI] [PubMed] [Google Scholar]
- Resh MD. Use of analogs and inhibitors to study the functional significance of protein palmitoylation. Methods. 2006c;40:191–197. doi: 10.1016/j.ymeth.2006.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocks O, Peyker A, Kahms M, Verveer PJ, Koerner C, Lumbierres M, Kuhlmann J, Waldmann H, Wittinghofer A, Bastiaens PIH. An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science. 2005;307:1746–1752. doi: 10.1126/science.1105654. [DOI] [PubMed] [Google Scholar]
- Rocks O, Peyker A, Bastianens PIH. Spatio-temporal segregation of Ras signals: one ship, three anchors, many harbors. Curr. Opin. Cell Biol. 2006;18:351–357. doi: 10.1016/j.ceb.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Turco EB, Deretic D, Bazan NG, Papermaster DS. Post-Golgi vesicles cotransport docosahexaenoyl-phospholipids and rhodopsin during frog photoreceptor membrane biogenesis. J. Biol. Chem. 1997;272:10491–10497. doi: 10.1074/jbc.272.16.10491. [DOI] [PubMed] [Google Scholar]
- Roth MG, Bi K, Ktistakis NT, Yu S. Phospholipase D as an effector for ADP-ribosylation factor in the regulation of vesicular traffic. Chem. Phys. Lipids. 1999;98:141–152. doi: 10.1016/s0009-3084(99)00026-2. [DOI] [PubMed] [Google Scholar]
- Sanders LM, Henderson CE, Hong MY, Barhoumi R, Burghardt RC, Wang N, Spinka CM, Carroll RJ, Turner ND, Chapkin RS, Lupton JR. Enhancement of reactive oxygen species by dietary fish oil and attenuation of antioxidant defenses by dietary pectin coordinately heightens apoptosis in rat. J. Nutr. 2004;134:3233–3238. doi: 10.1093/jn/134.12.3233. [DOI] [PubMed] [Google Scholar]
- Sears RC, Nevins JR. Signaling networds that link cell proliferation and cell fate. J. Biol. Chem. 2002;277:11617–11620. doi: 10.1074/jbc.R100063200. [DOI] [PubMed] [Google Scholar]
- Sebolt-Leopold JS, Dudley DT, Herrera R, Becelaere KV, Wiland A, Gopwan RC, Tecle H, Barrett SD, Bridges A, Przybranowski S, Leoplold WR, Saltiel AR. Blockade of MAP kinase pathway suppresses growth of colon tumors in vivo. Nature Med. 1999;5:810–816. doi: 10.1038/10533. [DOI] [PubMed] [Google Scholar]
- Seo J, Barhoumi R, Johnson AE, Lupton JR, Chapkin RS. Docosahexaenoic acid selectively inhibits plasma membrane targeting of lipidated proteins. FASEB J. 2006;20:770–772. doi: 10.1096/fj.05-4683fje. 10.1096/fj.05-4683fje. [DOI] [PubMed] [Google Scholar]
- Shaikh SR, Caffrey CV, Stillwell W, et al. Interaction of cholesterol with docosa-hexaenoic acid-containing phosphatidylethanolamine: trigger for microdomain/raft formation? Biochemistry. 2003;42:12028–12037. doi: 10.1021/bi034931+. [DOI] [PubMed] [Google Scholar]
- Shaikh SR, Dumaual AC, Castillo A, et al. Oleic and docosahexaenoic acid differentially phase separate from lipid raft molecules: A comparative NMR, DSC, AFM, and detergent extraction study. Biophys. J. 2004;87:1752–1766. doi: 10.1529/biophysj.104.044552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh SR, Edidin M. Immunosuppressive effects of polyunsaturated fatty acids on antigen presentation by human leukocyte antigen class I molecules. J. Lipid Res. 2007;48:127–138. doi: 10.1194/jlr.M600365-JLR200. [DOI] [PubMed] [Google Scholar]
- Sheng H, Shao J, Morrow JD, Beauchamp RD, Dubois RN. Modulation of apoptosis and bcl-2 by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362–366. [PubMed] [Google Scholar]
- Siniscrope FA, Ruan SB, Cleary KR, Stephens LC, Lee JJ, Levin B. bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res. 1995;55:237–241. [PubMed] [Google Scholar]
- Smaby JM, Momsen MM, Brockman HL, Brown RE. Phosphatidylcholine acyl unsaturation modulates the decrease in interfacial elasticity induced by cholesterol. Biophys. J. 1997;73:1492–1505. doi: 10.1016/S0006-3495(97)78181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JG, Yokoyama W, German JB. Butyric acid form the diet: actions at the level of gene expression. Crit. Rev. Food Sci. 1998;38:259–297. doi: 10.1080/10408699891274200. [DOI] [PubMed] [Google Scholar]
- Smith WL. Cyclooxygenases, peroxide tone and the allure of fish oil. Curr. Opin. Cell Biol. 2005;17:174–182. doi: 10.1016/j.ceb.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Stillwell W, Jenski LJ, Zerouga M, Dumaual AC. Detection of lipid domains in docasahexaenoic acid-rich bilayers by acyl chain-specific FRET probes. Chem. Phys. Lipids. 2000;104:113–132. doi: 10.1016/s0009-3084(99)00122-x. [DOI] [PubMed] [Google Scholar]
- Stillwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem. Phys. Lipids. 2003;126:1–27. doi: 10.1016/s0009-3084(03)00101-4. [DOI] [PubMed] [Google Scholar]
- Stillwell W, Shaikh SR, LoCascio D, Siddiqui RA, Seo J, Chapkin RS, Wassall SR. Frontiers in Nutrition Research. Hauppauge, NY: Nova Science Publishers; 2006. Docosahexaenoic acid. An influential membrane-altering omega-3 fatty acid; pp. 249–271. Chapter 8. [Google Scholar]
- Stone WL, Farnsworth CC, Dratz EA. A reinvestigation of the fatty acid content of bovine, rat and frog retinal rod outer segments. Exp. Eye. Res. 1979;28:387–397. doi: 10.1016/0014-4835(79)90114-3. [DOI] [PubMed] [Google Scholar]
- Storz P, Doppler H, Toker A. Protein kinase D mediates mitochondrion-tonucleus signaling and detoxification from mitochondrial reactive oxygen species. Mol. Cell. Biol. 2005;25:8520–8530. doi: 10.1128/MCB.25.19.8520-8530.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stulnig TM, Berger M, Sigmund T, Raederstorff D, Stockinger H, Waldhausl W. Polyunsaturated fatty acids inhibit T cell signal transduction by modification of detergent-insoluble membrane domains. J. Cell Biol. 1998;143:637–644. doi: 10.1083/jcb.143.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stulnig TM, Huber J, Leitinger N, Imre EM, Angelisova P, Nowotny P, Waldhausl W. Polyunsaturated eicosapentaenoic acid displaces proteins from membrane rafts by altering raft lipid composition. J. Biol. Chem. 2001;276:37335–37340. doi: 10.1074/jbc.M106193200. [DOI] [PubMed] [Google Scholar]
- Stulnig TM. Immunomodulation by polyunsaturated fatty acids: mechanisms and effects. Int. Arch. Allergy Immunol. 2003;132:310–321. doi: 10.1159/000074898. [DOI] [PubMed] [Google Scholar]
- Talbot WA, Zheng LX, Lentz BR. Acyl chain unsaturation and vesicle curvature alter outer leaflet packing and promote poly(ethylene glycol)-mediated membrane fusion. Biochemistry. 1997;36:5827–5836. doi: 10.1021/bi962437i. [DOI] [PubMed] [Google Scholar]
- Tavani A, Pelucchi C, Parpinel P, Negri M, Franeschi E, Levi S, F. & La Vecchia C. n-3 polyunsaturated fatty acid intake and cancer risk in Italy and Switzerland. Int. J. Cancer. 2003;105:113–116. doi: 10.1002/ijc.11018. [DOI] [PubMed] [Google Scholar]
- Taylor DD, Taylor CG, Jiang CG, Black PH. Characterization of plasma membrane shedding from murine melanoma cells. Int. J. Cancer. 1988;41:629–635. doi: 10.1002/ijc.2910410425. [DOI] [PubMed] [Google Scholar]
- van Blitterswijk WJ, Emmelot P, Hilkmann HA, Hilgers J, Feltkamp CA. Rigid plasma-membrane-derived vesicles, enriched in tumour-associated surface antigens (MLr), occurring in the ascites fluid of a murine leukaemia (GRSL) Int. J. Cancer. 1979;23:62–70. doi: 10.1002/ijc.2910230112. [DOI] [PubMed] [Google Scholar]
- Wang TG, Gotoh Y, Jennings MH, Rhoads CA, Aw TY. Lipid hydroperoxide-induced apoptosis in human CaCo-2 cells is associated with an early loss of cellular redox balance. Faseb J. 2000;14:1567–1576. doi: 10.1096/fj.14.11.1567. [DOI] [PubMed] [Google Scholar]
- Wang TY, Leventis R, Silvius JR. Partitioning of lipidated peptide sequences into liquid-ordered lipid domains in model and biological membranes. Biochemistry. 2001;40:13031–13040. doi: 10.1021/bi0112311. [DOI] [PubMed] [Google Scholar]
- Webb Y, Hermida-Matsumoto L, Resh MD. Inhibition of protein palmitoylation, raft localization, and T cell signaling by 2-bromopalmitate and polyunsaturated fatty acids. J. Biol. Chem. 2000;275:261–270. doi: 10.1074/jbc.275.1.261. [DOI] [PubMed] [Google Scholar]
- Weitzel F, Ursini F, Wendel A. Phospholipid hydroperoxide glutathione peroxidase in various mouse organs during selenium deficiency and repletion. Biochim. Biophys. Acta. 1990;1036:88–94. doi: 10.1016/0304-4165(90)90018-r. [DOI] [PubMed] [Google Scholar]
- Williams EE, Jenski LJ, Stillwell W. Docosahexaenoic acid (DHA) alters the structure and composition of membranous vesicles exfoliated from the surface of a murine leukemia cell line. Biochim. Biophys. Acta. 1998;1371:351–362. doi: 10.1016/s0005-2736(98)00039-x. [DOI] [PubMed] [Google Scholar]
- Williams EE, May BD, Stillwell W, Jenski LJ. Docosahexaenoic acid (DHA) alters the phospholipid molecular species composition of membranous vesicles exfoliated from the surface of a murine leukemia cell line. Biochim. Biophys. Acta. 1999;1418:185–196. doi: 10.1016/s0005-2736(99)00032-2. [DOI] [PubMed] [Google Scholar]
- Zeyda M, Staffler G, Horejsi V, Waldhausl W, Stulnig TM. LAT displacement from lipid rafts as a molecular mechanism for the inhibition of T cell signaling by polyunsaturated fatty acids. J. Biol. Chem. 2002;277:28418–28423. doi: 10.1074/jbc.M203343200. [DOI] [PubMed] [Google Scholar]
- Zeyda M, Saemann MD, Stuhlmeir KM, Mascher DG, Nowotny PN, Zlabinger GJ, Waldhausl W, Stulnig TM. Polyunsaturated fatty acids block dendritic cell activation and function independently of NF-kB activation. J. Biol. Chem. 2005;280:14293–14301. doi: 10.1074/jbc.M410000200. [DOI] [PubMed] [Google Scholar]


