Summary
Exposure to stress during prenatal or early postnatal life can dramatically impact adult behavior and neuroendocrine function. We recently began to selectively breed Sprague-Dawley rats for high (high responder, HR) and low (low responder, LR) novelty-seeking behavior, a trait that predicts a variety of differences in emotional reactivity, including differences in neuroendocrine stress response, fear- and anxiety-like behavior, aggression, and propensity to self-administer drugs of abuse. We evaluated genetic-early environment interactions by exposing HR- and LR-bred animals to prenatal stress (PS) from pregnancy day 3 to 20, hypothesizing that PS exposure would differentially impact HR versus LR behavior and neuroendocrine reactivity. We evaluated novelty-induced locomotion, anxiety-like behavior, and corticosterone stress response in weanling (25-day-old) and adult HR-LR stressed and control males. Exposure to PS did not alter HR-LR differences in locomotion, but did impact anxiety-like behavior, specifically in LR animals. Surprisingly, LR animals exposed to PS exhibited less anxiety than LR controls. HR rats were not affected by PS, with both stress and control groups showing low levels of anxiety. PS differentially impacted neuroendocrine stress reactivity in young versus adult HR-LR animals, leading to an exaggerated corticosterone response in LR pups compared to LR controls, while HRs pups were unaffected. In contrast, exposure to PS produced an exaggerated stress response in HR adults, compared to HR controls, while LR animals were not significantly affected. These findings highlight how genetic predisposition may shape individual's response to early life stressors, and furthermore, show that a history of early life stress may differentially impact an organism at different points in life. Future work will explore neural mechanisms which underlie the different behavioral and neuroendocrine consequences of prenatal stress in HR versus LR animals.
Keywords: high responder (HR), low responder (LR), corticosterone, anxiety, open field, elevated-plus-maze
1. Introduction
Inborn differences in personality and emotional reactivity strongly shape how individuals respond to stress, and may predispose certain people to develop psychiatric disorders such as depression, anxiety, and drug addiction. Exposure to early-life stress is known to increase the risk for developing emotional disorders (Weinstock, 1997, Johnson et al., 2001, Mueser et al., 2002), but this risk is greatly magnified if an individual also carries a genetic predisposition for psychiatric illness (Caspi et al., 2002, Caspi et al., 2003). While such observations in humans are compelling, the neural mechanisms that underlie this interactive pathway to disease remain poorly understood. Therefore, rodent models offer a powerful tool to examine how such gene-environment interactions shape brain development, stress-sensitivity, and emotional behavior.
Numerous rodent studies demonstrate that stress during pregnancy produces offspring with a variety of long-term neurobiological (Maccari et al., 1995, Barbazanges et al., 1996, Lemaire et al., 2000, Morley-Fletcher et al., 2004, Van den Hove et al., 2006), behavioral (Ward and Stehm, 1991, Szuran et al., 1994, Vallee et al., 1996, Vallee et al., 1997, Morley-Fletcher et al., 2003, Patin et al., 2005), and endocrine (Fride et al., 1986, Takahashi et al., 1990, Takahashi and Kalin, 1991, Henry et al., 1994, Maccari et al., 2003, Morley-Fletcher et al., 2003) abnormalities. While prenatal stress (PS) has clear deleterious effects on offspring, the specific types and severity of stress-induced alterations vary from study to study. Such inconsistent results are likely due to a host of technical factors, including use of different stressors (e.g. restraint, footshock, forced swimming, predator odor, noise), and application of stressors at different points in pregnancy, such as throughout the entire pregnancy (Rojo et al., 1985, Fride et al., 1986), during the first (Suchecki and Palermo Neto, 1991, Tazumi et al., 2005, Bosch et al., 2006) or second half of pregnancy (Maccari et al., 1995, Vallee et al., 1996, Lehmann et al., 2000, Lemaire et al., 2000, Morley-Fletcher et al., 2003, Poltyrev et al., 2005, Van den Hove et al., 2006). Different rat strains are frequently used across experiments, which is important since each strain may be differentially vulnerable to early life stress (Stohr et al., 1998). Furthermore, besides inter-strain differences, it is also important to consider that rats within a given strain may exhibit individual differences in stress-reactivity, making them more or less sensitive to early life stress (Neumann et al., 2005, Bosch et al., 2006).
We recently began to selectively breed Sprague-Dawley rats based on their innate differences in “novelty-seeking” -- a trait in rodents which predicts several key facets of emotional reactivity, including fear- and anxiety-like behavior (Kabbaj et al., 2000, Stead et al., 2006), and propensity to self-administer drugs of abuse (Piazza et al., 1989, Kabbaj et al., 2001). Outbred rats, like humans and all other organisms, show a wide range of emotional response to environmental challenges. When exposed to a novelty, some rats (High Responders, HR) vigorously explore the new environment, while others (Low Responders, LR), are inhibited and exhibit very little activity. HR-LR rats exhibit a variety of other interesting behavioral differences, including differences in anxiety-like behavior (Kabbaj et al., 2000, Stead et al., 2006), aggression (Abraham et al., 2006), stress responsiveness (Piazza et al., 1989, Piazza et al., 1991a, Piazza et al., 1993, Kabbaj et al., 2000), and willingness to self-administer psychostimulants (Piazza et al., 1989, Kabbaj et al., 2001). For example, HR animals show reduced anxiety-like behavior across multiple tests, including the elevated plus maze, light dark box, and open field test, compared to LRs (Kabbaj et al., 2000, Stead et al., 2006, Mallo et al., 2007, White et al., 2007), although prior benzodiazepine treatment eliminates these differences (Stead et al., 2006). HR males also show enhanced aggressive behavior (Abraham et al., 2006) and greater behavioral response to cocaine and amphetamine (Deminiere et al., 1989, Piazza et al., 1989, Kabbaj et al., 2001, Alttoa et al., 2007) compared to LRs. Taken together, these data suggest that HR and LR animals exhibit fundamental differences in emotional reactivity, interacting differently with their environment across numerous conditions.
Neurochemical and neural gene expression differences contribute, at least in part, to the HR-LR behavioral phenotypes (Piazza et al., 1991b, Hooks et al., 1994a, Hooks et al., 1994b, Kabbaj et al., 2000, Kabbaj, 2004). For example, HR rats exhibit decreased hippocampal glucocorticoid receptor (GR) mRNA expression compared to LR rats, and local infusion of the GR receptor antagonist RU38486 into the hippocampus equalizes HR-LR behavioral differences, leading LRs to explore more and show decreased anxiety-like behavior (Kabbaj et al., 2000). Another study using Affymetrix microarrays identified numerous putative gene expression differences in the hippocampus of HR-LR rats both basally and following psychosocial stress, highlighting differences in a range of molecules involved in intracellular signal transduction pathways and neurogenesis (Kabbaj et al., 2004).
The first goal of the present study was to confirm whether our Selectively Bred HR-LR rats show hypothalamic-pituitary-adrenal (HPA) axis differences similar to those reported in commercially purchased HR-LR animals. Specifically, we used in situ hybridization to measure mRNA expression of GR and the mineralocorticoid receptor (MR) in the hippocampus of Selectively-Bred HR-LR males, and evaluated their patterns of corticosterone secretion following novelty stress. Our results show that Selectively-Bred HR rats, like purchased HRs, express lower levels of hippocampal GR mRNA, and also exhibit exaggerated stress-induced corticosterone secretion compared to LRs.
Since HR-LR animals show clear baseline differences in HPA axis reactivity, we hypothesized that they would differentially react to PS. Moreover, because the HR-LR traits appear to involve a strong genetic component (Stead et al., 2006), we also wanted to explore possible gene-environment interactions which may differentially impact behavioral and/or neuroendocrine aspects of the HR-LR phenotypes. Therefore, the second major goal of the present study was to expose Selectively-Bred HR-LR animals to PS and assess its impact on behavior and neuroendocrine reactivity in both weanling and adult offspring. We chose to evaluate the impact of PS in both young and adult animals to determine a) whether the HR-LR phenotype is present in early life, and b) whether PS differentially impacted behavior and stress-reactivity in young versus adult animals. Our results show that while PS only subtly influences the HR-LR behavioral phenotypes, it exerts greater influence on neuroendocrine stress reactivity, with differential effects in young versus adult HR-LR offspring.
2. Materials & Methods
2.1. Animals
Selectively-Bred HR and LR animals were acquired from our in-house breeding colony where we have maintained the HR and LR lines for several generations. We recently published a description of our breeding strategy and initial behavioral characterization of the Selectively-Bred HR-LR lines (Stead et al., 2006). Male and female rats were housed in separate rooms on a 12:12 and 14:10 light-dark cycle, respectively (lights on at 6 a.m.). Female rats (as well as male-female mating pairs) were kept on the 14:10 light-dark cycle as this has been shown to promote regular estrous cycles and fertility (Everett and Sawyer, 1949, Ying et al., 1973). Litters were weaned from their mothers on postnatal day 21, but remained in the female housing room (under the same 14:10 light-dark cycle) until postnatal day 30, when male and female animals were separated. Animals were housed 3-4 per cage, with female cages remaining in the female housing room, and male cages transferred to the male housing room. Only male rats were used for the current set of experiments. Food and water were always available ad libitum, and all experiments were conducted in accordance with the National Institute of Health (NIH) guidelines on laboratory animal use and care (National Research Council, 1996).
2.2. In situ hybridization to assess hippocampal GR and MR mRNA expression
Adult male rats (N=10 HR, N=10 LR, approximately 75 days old) from the 6th generation of our Selectively-Bred HR-LR Lines were sacrificed by rapid decapitation, and their brains were removed, snap frozen, and stored at -80 °C. Brains were cryostat sectioned at 12μm, and sections were mounted on Fisherbrand Superfrost®/Plus Microscope Slides. Sections were taken at 240μm intervals through the hippocampus, then prepared for in situ hybridization as previously decribed (Kabbaj et al., 2000)
Briefly, sections were fixed in 4% parafomaldehyde at room temperature for 1 hr. The slides were then washed three times in room temperature 2× SSC (300mM NaCl/30mM sodium citrate, pH 7.2), 5 min each wash. Next, the slides were placed in a solution containing acetic anhydride (0.25%) in triethanolamine (0.1 M), pH 8.0, for 10 min at room temperature, rinsed in distilled water, and dehydrated through graded ethanol washes (50, 75, 85, 95, and 100%). After air-drying, the sections were hybridized with a 35S-labeled cRNA probe for GR or MR. The GR probe was a 451 nucleotide fragment directed against the rat GR mRNA coding region (nucleotides 2364-2815). The MR probe was a 400 nucleotide fragment directed against the 3′ untranslated region of MR mRNA. The probes were labeled in a reaction mixture consisting of 1 μg of linearized plasmid, 1 × transcription buffer (Epicenter Technologies, Madison, WI), 125 μCi of 35S-labeled-UTP, 125 μCi of 35S-CTP, 150 μM ATP and GTP, 12.5 mM dithiothreitol, 0.5 μl of RNase inhibitor, and 1.5 μl of T3 RNA polymerase. The reactions were incubated for 120 min at 37°C, and then 1 μl of DNAse (RNAse free) was added to the reaction to incubate for another 15 min at room temperature. The labeled probes were purified using Micro Bio-Spin P-30 Tris Spin Columns (Bio-Rad Laboratories), then diluted in hybridization buffer (containing 50% formamide, 10% dextran sulfate, 3×SSC, 50 mM sodium phosphate buffer, pH 7.4, 1×Denhardt's solution, 0.1 mg/ml yeast tRNA, and 10 mM dithiothreitol) to yield 106 dpm/70 μl. A cover slip with 70 μl of diluted riboprobe was placed on each slide. Slides were placed in a humidified box with filter paper saturated with 50% formamide buffer, and incubated overnight at 55°C. Following hybridization, coverslips were removed and the slides were washed in room temperature 2× SSC twice for 5 min each, and then incubated for 1 h in RNaseA (200 μg/ml in 10 mM Tris buffer containing 0.5 M NaCl, pH 8) at 37° C. The slides were then washed in increasingly stringent SSC solutions: 2×, 1×, and 0.5× for 5 min each at room temperature, followed by incubation for 1 h in 0.1× SSC at 65°C. Finally, slides were rinsed in distilled water and dehydrated through graded ethanol washes, air-dried, and apposed to Kodak XAR film (Eastman Kodak, Rochester, NY) for 7 d.
Autoradiograms were digitized using a ScanMaker 1000XL Pro (Microtek, Carson, CA) with LaserSoft Imaging software (AG, Kiel, Germany). Digitized images were analyzed using Scion Image Beta 3b for PC. Optical density measurements were taken for 4 subregions of the hippocampus (Cornu Ammonis fields CA1-CA3, and the dentate gyrus) from the left and right sides of the brain. For each animal, we analyzed 8-10 sections spaced 240 μm apart across the rostro-caudal extent of the hippocampus. Optical density measurements were corrected for background, and then multiplied by the area sampled to produce an integrated density measurement. These data were averaged to produce one data point for each hippocampal subregion per animal, and then group averages were calculated and compared statistically.
2.3. Chronic unpredictable stress during pregnancy
Male and female rats (N=10 HR male/female pairs, and N=10 LR male/female pairs) from the 10th generation of our Selectively-Bred HR-LR Lines were housed together for up to 10 days, with the timing of mating determined by detection of sperm plugs. Once sperm plugs were detected (gestational day 0, G0), males were removed. Pregnant females were randomly assigned to either the stress or non-stress experimental group (N=5 HR-LR females per stress and non-stress group) and housed 2 per cage until G20 (the conclusion of the chronic stress regimen). Pregnant rats were single-housed on G20. Litters were reduced to 12 pups (6 males plus 6 females) on postnatal day 1 then left undisturbed with their mothers until weaning on postnatal day 21 except for weekly change of bedding. Weaned males were housed up to 4 animals per cage with water and food available ad libitum until testing at either postnatal day 25, or adulthood (postnatal day 60). Each experimental group included male offspring from at least 3-4 different litters.
Between G2 and G19, pregnant HR females (N=5) and LR females (N=5) in the stress group were subjected to a chronic unpredictable stress paradigm based on a protocol originally developed by Katz (Katz et al., 1981). Stressed animals received an 18-day-long variable and non-habituating stress regimen consisting of six randomly assigned stressors described below. Stress duration ranged from 15 min to 2 h, and the time of stress exposure was counterbalanced between morning and afternoon. Non-stressed control females (N=5 HR and N=5 LR) were handled daily during this period of time. Pregnant rats were weighed after 6, 12, and 18 days of the chronic stress regimen or daily handling control procedure.
The stressors used as part of this paradigm included the following. (1) Forced Swim: rats were forced to swim for 15 min in a water tank (70 × 50 × 40 cm) filled with 37° C water. After swimming, animals were dried with towels before returning to the home colony. (2) Cage-mate switch: a female's cage-mate was removed and replaced by another unknown female rat for 2 h. At the conclusion of the 2 h period, original cage-mates were returned to the home cage. (3) Novel environment: females were individually placed in novel environments consisting of boxes with different shapes (e.g. circular or rectangular) or different floor texture (e.g. mesh floor or smooth floor). One environment was used per day and the order of different environments was randomized across rats. Animals were exposed to the novel environment for 2 h in a room separate from the home colony. (4) Isolation: animals were transferred to a room adjacent to the home colony and individually placed into cages similar to home cages for 2 h with free access to food and water. At the conclusion of the 2-h isolation period, all rats were returned to their home cages in the colony room. (5) Crowding: several females (in this case 5) were placed in one cage for 2 h with free access to food and water. After 2 h, all females were returned to their home cages. (6) Loud Noise: rats were transferred to an adjacent room in their home cages and placed into an acoustically insulated box (1.2 × 1.0 × 1.0 m). Rats were subjected to 30 min of white noise at an intensity of 110 dB (A scale), then returned to their home colony. Previous work in our laboratory has established that all of these stressors, when administered acutely, effectively increase plasma corticosterone levels 30 min after the onset of stress (Isgor et al., 2004).
2.4. Behavior testing and blood collection in pregnant females
On G20, approximately 24 h after the final stressor, all female rats were tested on the Elevated Plus Maze to assess anxiety-like behavior. The apparatus was constructed of black Plexiglas, with four elevated arms (70 cm from the floor, 45 cm long, and 12 cm wide) arranged in a cross. Two opposite arms were enclosed by 45-cm-high walls, and the other two other arms were open. A central square platform at the intersection of the open and closed arms provided access to all arms. The test room was dimly lit (approximately 30 lux), and behavior was monitored using a computerized videotracking system (Noldus Ethovision, Leesburg, VA). At the beginning of the 5 min test, each rat was placed in the central square facing a closed arm. The computerized tracking system recorded the latency to first enter the open arm, the amount of time spent in the open arm, closed arm, or center square, and the total distance traveled over the course of the 5 min test. Behavior testing was performed between 8:00-11:30 a.m.
On G21 (between 10:00-11:00 a.m.), blood samples (100 μl) were collected in EDTA-coated tubes vial tail vein nick to assess basal corticosterone levels as a neuroendocrine sign of chronic stress in the dams exposed to the chronic stress paradigm. Dams were removed from their home cage, immobilized lightly, and a lateral tail vein was punctured with the corner of a razor blade to collect blood. Following blood collection, rats were released and returned to their home cages. Blood samples were separated by centrifugation (1000 × g, 10 min at 4 °C), and plasma was removed, frozen and stored at −80 °C until assay. Plasma corticosterone was measured using commercially available radioimmunoassay kits (MP Biomedicals, Solon, OH) according to package instructions. The sensitivity of the assay was 12.5 ng/ml, and intra- and inter-assay coefficients of variation were less than 5%.
2.5. Impact of prenatal stress on HR- and LR-offspring
We evaluated the impact of PS on behavior and neuroendocrine stress response of HR-LR male offspring at two developmental timepoints: shortly after weaning (postnatal day 25) and adulthood (postnatal day 60).
2.5.1. Behavior & neuroendocrine stress response in 25-day-old HR-LR offspring
HR-LR offspring with a history of PS and controls not exposed to PS (N=10-12 per group) were tested in the Open Field test to assess both novelty-induced locomotor activity and anxiety-like behavior. The Open field apparatus was a 100 × 100 × 50 cm white Plexiglas box with black Plexiglas floor. Testing was conducted under dim light (30 lux) between 8:00-11:30 a.m. Behavior was recorded using a computerized videotracking system (Noldus Ethovision, Leesburg, VA). The experiment was started by placing the rat into one corner of the open field. The computerized tracking system recorded the latency to first enter the center of the open field, the amount of time spent in the center, periphery, or corner of the test apparatus, and the total distance traveled over the course of the 5 min test. Rats were transferred back to their home cage at the conclusion of the test.
To assess neuroendocrine stress response following the open field test, blood was collected via tail nick as described above. Baseline blood samples were collected 24 h before testing (between 8:00-11:30 a.m.), and the other blood samples were collected 20- and 60-min after the beginning of the test. Blood samples were processed, stored, and assayed as described above. Animals were sacrificed via rapid decapitation after the final blood collection; brain tissue was harvested for other studies.
2.5.2. Behavior & neuroendocrine stress response in adult HR-LR offspring
At postnatal day 60, HR-LR adult offspring with a history of PS and non-stressed controls (N=15 per group) were screened for novelty-induced locomotor activity by placing them in a standard size (43 × 21.5 × 24.5 cm) clear acrylic cage in a different room from the rats' housing colony. Locomotor activity was monitored every 5 min for 1 hour by two panels of photocells connected to a computer. The first panel of three photocells was placed at ground level to record horizontal locomotion, with the second panel of 5 photocells located near the top of the cage to determine rearing behavior. The locomotion testing rig and motion recording software were created in-house at the University of Michigan. Locomotion activity was tested between 9:00-11:30am, and final locomotion scores were determined by summing horizontal and rearing activities.
One week later all rats were tested on the Elevated Plus Maze as described above. Neuroendocrine stress response following the elevated plus maze test was assessed by collecting tail blood as described above. Baseline blood samples were collected 24 h day before testing (between 8:00-11:30 a.m.). Additional blood samples were collected 20-, 30- and 60-min after the beginning of the test. Blood samples were processed, stored, and assayed as described above. Animals were sacrificed via rapid decapitation after the final blood collection; brain tissue was harvested for other studies.
2.6. Data Analysis
In situ hybridization data were analyzed with a two-way ANOVA (HR-LR phenotype × hippocampal subregion). Weight data for stressed and non-stressed pregnant females (collected at three timepoints during the stress period) were assessed via repeated measures ANOVA with timepoint, HR-LR phenotype, and stress condition as independent variables. Data from the behavioral experiments and data regarding litter-size and baseline corticosterone levels in HR-LR dams were analyzed by two-way ANOVA (HR-LR phenotype × PS condition). Data from the corticosterone radioimmunoassay were analyzed in two ways. We first performed a repeated measures ANOVA (HR-LR phenotype × blood collection timepoint), separately analyzing PS and non-stressed control groups in order to contrast HR-LR rats under each condition; this allowed us to confirm baseline HR-LR neuroendocrine differences which were previously reported in commercially purchased animals (e.g. those described in (Kabbaj et al., 2000)). Next, we performed repeated measures ANOVAs (blood collection timepoint × PS group), separately analyzing HR-LR animals in order to examine how PS impacted the pattern of stress-induced corticosterone secretion in either group. Finally, we calculated area under the curve for corticosterone data collected over multiple time points. These data were analyzed by 2-way ANOVA (HR-LR phenotype × PS condition). ANOVAs were followed by Fisher's post-hoc comparisons when necessary. Data were analyzed using Statview 5.0.1 for Windows, and for all tests α=0.05.
3. Results
3.1. Hippocampal GR and MR receptor mRNA in HR versus LR males
3.1.1. GR mRNA expression
HR rats exhibited significantly lower levels of hippocampal GR mRNA compared to LR rats (Fig. 1). Two-way ANOVA revealed main effects for HR-LR phenotype (F=3.85, df 1,1, p<0.05) and hippocampal subregion (F=437.34, df 1,3, p<0.0001), as well as a significant phenotype × subregion interaction (F=4.62, df 1,3, p<0.01). Post-hoc analysis showed that HR rats exhibited significantly less GR mRNA levels in the CA1 region of the hippocampus compared to LR animals (F=18.16, df 1,1, p<0.001).
Figure 1.
Baseline expression of glucocorticoid receptor (GR) and mineralicorticoid receptor (MR) mRNAs in hippocampus of HR versus LR males (N=10 per group). Top left and middle panels show autoradiograms from representative X-ray films exposed for 7 d after in situ hybridization with antisense riboprobes against rat GR and MR mRNAs. The schematic in the top right panel indicates the 4 subregions of the hippocampus (Cornu Ammonis fields CA1-CA3, and the dentate gyrus) that were analyzed. Lower panels show graphs depicting relative GR and MR expression in HR versus LR animals. HR rats exhibited significantly less GR mRNA levels in the CA1 region of the hippocampus compared to LR animals. There were no differences in MR mRNA expression between HR-LR rats in any hippocampal subfield. **indicates p<0.01
3.1.2. MR mRNA expression
HR-LR rats expressed similar levels of hippocampal MR mRNA across all hippocampal subfields analyzed – CA1, CA2, CA3, and dentate gyrus (Fig. 1). Two-way ANOVA revealed a significant main effect for hippocampal subregion (F=88.28, df 1,3, p<0.0001), but there was no main effect for HR-LR phenotype, and no significant phenotype × subregion interaction.
3.2. Impact of chronic unpredictable stress on pregnant HR-LR females
3.2.1. Weight during pregnancy and litter-size
Stressed and non-stressed HR-LR pregnant females were weighed three times during the 18-day stress procedure – day 6, day 12, and the final day 18. There was a main effect of time (F=52.38, df, 1, 2, p<0.0001) as all females gained weight during pregnancy. At the first weighing (stress day 6, corresponding to G8), average female weight was 304 ± 5.5 g (standard error); at the second weighing (stress day 12, corresponding to G14), females averaged 325 ± 5.3 g; and at the final weighing (stress day 18, corresponding to G20), females averaged 360 ± 5.4 g. There was no effect of stress condition or HR-LR phenotype on weight across this time period, and there was no significant stress condition × phenotype interaction. The average size of all litters across experimental groups was 15 pups, with no main effect of stress or HR-LR phenotype, and no significant stress × phenotype interaction for litter size.
3.2.2. Anxiety-like behavior in pregnant dams
All pregnant females were tested on the Elevated Plus Maze 24 h after the final stressor in the chronic stress regimen. There were no main effects of stress or HR-LR phenotype on the percentage of time spent in the anxiogenic open arm of the Elevated Plus Maze, or the frequency of visits to the open arms, but there were significant stress × phenotype interactions for both time spent in the open arm (F=13.03, df 1, 16, p < 0.01; Fig. 2A), and open arm entries (F=9.38, df 1, 16, p < 0.01; Fig. 2C). Post-hoc analysis revealed that HR control females spent greater time in the open arm compared to LR control females (p<0.01), and also made more frequent visits to the open arm (p<0.05). Chronic stress severely inhibited HR females, with stressed HRs spending significantly less time in the open arm (p<0.01) and making fewer entries into the open arms (p<0.05) compared to HR controls. However, chronic stress appeared to exert the opposite effect on LR females. Stressed LR females spent greater time in the open arm and made more frequent visits to the open arms relative to LR controls (p<0.05) and stressed HR animals (p<0.05), making them essentially indistinguishable from HR controls. There were no main effects of HR-LR phenotype or stress on the latency to initially enter the open arms, and no phenotype × stress interactions on this variable (Fig. 2B). As a measure of overall activity, we also assessed the number of closed arm entries made by each group (Fig. 2D). There was a main effect of phenotype (F=6.49, df 1,16, p<0.01), and a significant stress × phenotype interaction (F=9.02, df 1,16, p<0.01) for closed arm entries. Post-hoc analysis showed that HR control animals were significantly more active than stressed HRs and both LR groups (p<0.01, Fig. 2D). There was no main effect of stress on overall activity.
Figure 2.
Chronic unpredictable stress differentially impacts anxiety-like behavior in pregnant HR-LR females. Pregnant HR-LR females (N=5 per experimental group) were tested on the Elevated Plus Maze 24 h after exposure to the last stressor in the chronic unpredictable stress paradigm. Exposure to chronic stress reversed the normal pattern of HR-LR anxiety behavior differences. Non-stressed animals showed typical HR-LR differences, with HR rats spending greater time (A) and making more frequent entries (C) in the anxiogenic open arm compared to LRs. Chronic stress reversed this pattern, with stressed LRs spending greater time and making more entries in the open arm, while stressed HR females spent very little time and made few entries in the open arm (A, C), and showed an overall reduction in exploratory activity compared to non-stressed HRs (D). There were no group differences in the latency of pregnant females to initially enter the open arm (B). * indicates p<0.05; **indicates p<0.01
3.2.3. Baseline corticosterone levels in pregnant dams
On the morning after the Elevated Plus Maze test, tail blood samples were collected to assess baseline corticosterone levels in stressed and non-stressed HR-LR females. There was a main effect of stress on baseline corticosterone levels (F=10.77, df 1, 16, p<0.01). Baseline corticosterone levels were elevated in both HR and LR females exposed to chronic unpredictable stress (HR=58.3 ± 28.1 ng/ml; LR=60.9 ± 21.2 ng/ml) compared to handled control groups (HR=2.0 ± 0.4 ng/ml; LR=1.6 ± 0.1 ng/ml). There was no main effect of HR-LR phenotype, and no significant stress × phenotype interaction.
3.3. Impact of prenatal stress on HR vs. LR behavior
3.3.1. Impact of prenatal stress on novelty-induced locomotor activity in HR vs. LR weanlings and adults
Both weanling and adult HR offspring showed markedly higher locomotor response to novelty compared to LR offspring (Fig. 3A-B). Locomotor response to novelty was measured in the pups via the open field test. There was a strong main effect for HR-LR phenotype on pups' novelty-induced locomotor activity, assessed via total distance moved in the open field during the 5 min test (F=42.95, df 1, 36, p<0.0001). HR pups, regardless of PS condition, were much more active in the open field compared to LR animals (Fig. 3A). There was no main effect of PS, and no significant PS × phenotype interaction for overall activity.
Figure 3.
Prenatal stress does not impact HR-LR differences in locomotor response to novelty. Both weanling (A) and adult (B) HR males showed exaggerated locomotor response to novelty compared to their LR counterparts (N=10-12 per group). HR pups, regardless of previous exposure to prenatal stress, were much more active and moved a significantly greater distance during the 5-min open field test compared to LR pups (A). Similarly, HR adults, regardless of a history of prenatal stress, were substantially more active in a novel environment compared to LR adults. Prenatal stress exposure did produce a slight but significant decrease in locomotor response to novelty in adult offspring; however, this effect was apparent in both HR-LR animals (B). ** indicates p<0.01; *** indicates p<0.0001.
Locomotor response to novelty was assessed in adult animals in our traditional locomotor test where rats were placed into a novel cage with a wire mesh floor to monitor both horizontal and rearing movements over a 1 hour test period. Similar to our findings with weanling animals, there was a main effect of HR-LR phenotype on adult animals' novelty-induced locomotor activity (F=219.04, df 1,56, p<0.0001) since HR adult males, regardless of PS condition, were much more active in response to novelty compared to LR adults (Fig. 3B). There was also a main effect of PS on total locomotor activity in adult offspring (F=7.20, df 1,56, p<0.01), since a history of PS produced a slight but significant decrease in locomotor activity for both HR and LR adults (Fig. 3B). There was no significant PS × phenotype interaction for this variable. Rats' total locomotor scores were derived by summing their total rearing and horizontal movements over the 1 hour test period, so in a separate analysis, we examined the effect of HR-LR phenotype and PS on either rearing or horizontal movement. This analysis showed similar results, with a main effect of phenotype (F=214.53, df 1,56, p<0.0001) and PS (F=6.80, df 1,56, p<0.01) on rearing, as well as main effects of phenotype (F=142.10, df 1,56, p<0.0001) and PS (F=5.10, df 1,56, p<0.05) on horizontal movement. HRs overall showed greater rearing and horizontal movement compared to LRs, and animals exposed to PS had a slight but significant reduction of both rearing and horizontal movement compared to non-PS rats (data not shown). There was no significant PS × phenotype interaction for either rearing or horizontal movement.
3.3.2. Impact of prenatal stress on anxiety-like behavior in HR vs. LR pups
Anxiety-like behavior was evaluated in weanling HR and LR pups in the open field test by assessing multiple behavioral measures, including the time spent in the anxiogenic center region of the open field, the number of visits made to the center, and the latency to initially enter the center. HR rats, regardless of PS condition, were more willing to explore the anxiogenic center portion of the open field compared to both LR groups, evident by the main effect of phenotype on both the percentage of time spent in the center of the open field (F=11.37, df 1, 36, p<0.001; Fig. 4A) and the frequency of visits to the center of the open field (F=20.15, df 1, 36, p<0.001; Fig. 4C). There was no main effect of PS, and no significant PS × phenotype interaction for time spent in the center; however, there was a significant PS × phenotype interaction on number of visits to the center. Post hoc analysis showed that HR control pups made more frequent trips to the center compared to HR-PS pups and both LR groups (Fig. 4C). There was also a main effect of phenotype (F=8.51, df 1, 36, p<0.01) and a significant phenotype × PS interaction (F=4.63, df 1, 36, p<0.05) on the latency to initially enter the center of the open field. Post-hoc analysis showed that LR control rats took much longer to initially enter the center of the open field compared to HR control rats (p<0.001, Fig. 4B). Interestingly, LRs with a history of PS behaved more like HR rats and showed reduced latency to enter the center relative to LR control animals (p<0.01), while a history of PS did not alter HR 's latency to enter the center (Fig. 4B). Finally, there was a main effect of phenotype on number of entries into the periphery of the open field (F=34.30, df 1, 36, p<0.0001), with HR pups making more frequent entries to the periphery compared to LR pups (Fig. 4D). There was no main effect of PS and no significant PS × phenotype interaction on this variable.
Figure 4.
Prenatal stress subtly decreases anxiety-like behavior in LR weanling offspring, but does not affect HR pups' behavior. HR rats, regardless of a history of prenatal stress, spend more time exploring the anxiogenic center portion of the open field (A) and make more frequent entries into the center (C) compared to both LR groups (N=10-12 per group). LR control pups displayed the longest latency to initially enter the center of the open field compared to all other groups (B). Interestingly, LR pups exposed to prenatal stress behaved more like HR rats and exhibited reduced latency to enter the center of the open field compared to LR control pups (B). Overall, HR rats made more frequent entries into the periphery of the open field compared to LRs, another measure indicating that they were more active in the novel environment compared to LRs (D). * indicates p<0.05; ** indicates p<0.01; ***indicates p<0.0001
3.3.3. Impact of prenatal stress on anxiety-like behavior HR vs. LR adults
Anxiety-like behavior was evaluated in adult HR and LR offspring in the Elevated Plus Maze by assessing multiple behavioral measures, including the time spent in the anxiogenic open arm of the maze, the number of visits made to the open arms, and the latency to initially enter the open arms. HR adults, regardless of PS condition, were more willing to explore the anxiogenic open arms of the Elevated Plus Maze compared to LR adults, evident by a main effect of phenotype on percent time spent in the open arms (F=5.67, df 1, 56, p<0.05; Fig. 5A), and frequency of visits to the open arms of the Elevated Plus Maze (F=4.11, df 1, 56, p<0.05; Fig. 5C). There was no main effect of PS, and no significant PS × phenotype interaction for these variables. As with the pups, there was no main effect of PS or phenotype on the latency to initially enter the open arm, but there was a significant phenotype × PS interaction (F=5.46, df 1, 56, p<0.05). Post-hoc analysis showed that LR control rats showed greater latency to initially enter the open arm compared to LR rats with a history of PS, as well as both HR groups (p<0.05, Fig. 5B). Thus, LR rats with a history of PS behaved more like HR rats and showed reduced latency to enter the anxiogenic open arms compared to LR control animals, while HR animals' performance was unaffected by a history of PS. Lastly, there was no main effect of phenotype or PS, and no significant PS × phenotype interaction on the number of closed arm entries (Fig. 4D).
Figure 5.
Prenatal stress subtly decreases anxiety-like behavior in adult LR offspring, while HR behavior remains unaffected. Adult HR offspring, regardless of previous exposure to prenatal stress, were more willing to explore the anxiogenic open arms of the elevated plus maze, spending greater time in the open arms (A) and making more open arm entries (C) compared to both LR groups (N=10-12 per group). Adult LR control animals displayed the greatest latency to initially enter the open arms compared to all other groups (B). Interestingly, LR animals previously exposed to prenatal stress behaved more like HR rats and exhibited reduced latency to enter the open arms compared to LR controls (B). Overall, HR and LR adults did not show differences in activity on the elevated plus maze, assessed via the number of closed arm entries made during the 5-min test (D). * indicates p<0.05.
3.4. Impact of prenatal stress on HR vs. LR neuroendocrine stress responsivity
3.4.1. Stress-evoked corticosterone response in weanling offspring
Our neuroendocrine study in weanling HR-LR pups examined corticosterone secretion following a 5 min exposure to the novel open field. Basal blood samples were collected 24 h prior to the open field test, and additional blood samples were taken 20- and 60-min after the beginning of the test.
Neuroendocrine data were evaluated in two ways. We first sought to contrast the acute stress response of HR versus LR control rats that were not exposed to PS, and then separately compared HR-LR rats with a history of PS. In HR-LR control rats with no history of PS, there was a main effect of blood collection timepoint (F=53.07, df 2,34, p<0.0001), a main effect of HR-LR phenotype (F=13.95, df 1, 34, p<0.01), and a significant phenotype × timepoint (F=6.11, df 2 34, p<0.01). Corticosterone levels sharply increased after exposure to the novel open field, with HR controls showing an exaggerated and prolonged response compared to LRs (Fig. 6A). Post-hoc analysis revealed that non-PS control HR rats exhibited higher stress-evoked corticosterone levels at 60-min post stress compared to non-PS control LRs (p<0.01, Fig. 6A). Exposure to PS eliminated these HR-LR differences, as there was no main effect of HR-LR phenotype, and no phenotype × timepoint interaction in animals which were exposed to PS (Fig. 6D).
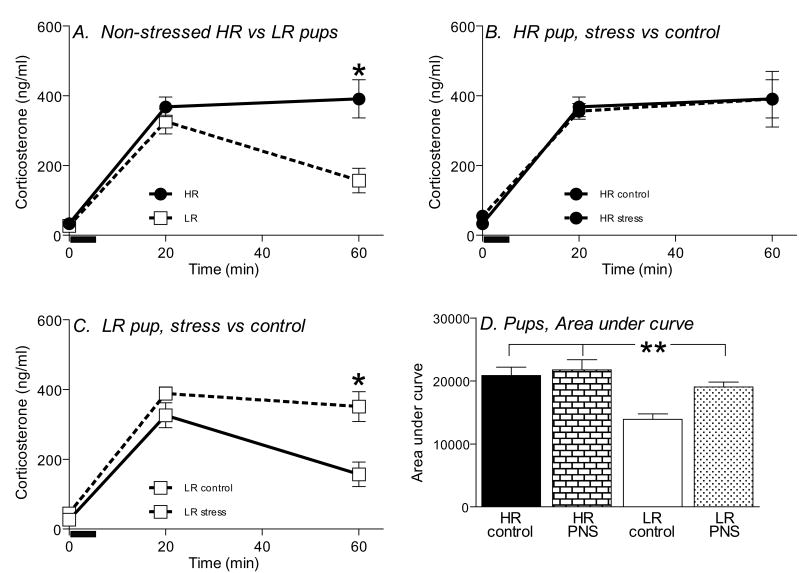
Figure 6.
Prenatal stress leads to exaggerated stress-induced corticosterone secretion specifically in LR weanling offspring. Basal blood samples (t=0) were collected 24 h prior to the open field test, and additional blood samples were taken 20 min, and 60 min after the beginning of the test (N=10-12 animals per group). Black bar indicates the 5 min open field (OF) test period. HR control pups showed a prolonged stress-induced corticosterone secretion following the open field test, compared to LR control animals (A). HR pups, regardless of their history of prenatal stress, showed identical patterns of stress-induced corticosterone secretion (B). LR pups that were exposed to PS exhibited an “HR-like” stress response, with PS-LR pups showing high levels of corticosterone levels 60-min after the open field test, compared to LR controls (C). Analysis of area under the curve for each group revealed that the LR control group had reduced area under the curve compared to both HR groups, as well as the LR group exposed to PS. * indicates p<0.05; ** indicates p<0.01.
In our second analysis, we separately examined the impact of PS on HR versus LR rats. Exposure to PS did not impact the pattern of stress-evoked corticosterone secretion in HR animals, as there was no main effect of PS, and no timepoint × PS interaction (Fig. 6B). For LR rats, however, there was a main effect of PS (F=35.24, df 1, 34, p<0.0001), and also a significant timepoint × PS interaction (F=10.40, df 2,34, p<0.001). LR rats with a history of PS showed exaggerated corticosterone release compared to LR controls, with the post hoc analysis revealing that LR rats with a history of PS showed higher corticosterone levels 60-min after the open field test compared to LR control rats (p<0.01, Fig. 6C). Our area under the curve analysis showed a significant HR-LR phenotype × PS interaction (F=9.18, df 1, 34, p<0.01), but no main effect for HR-LR phenotype or PS condition. Post hoc analysis showed HR control pups showed an exaggerated stress response compared to LR control pups, and that PS selectively altered the corticosterone stress response in LR rats (p<0.01), making them appear more “HR-like”, while HR animals showed similar stress responses, regardless of PS condition (Fig. 6D).
3.4.2. Stress-evoked corticosterone response in HR vs. LR adults
Our neuroendocrine study in adult HR-LR animals examined corticosterone secretion following a 5 min exposure to the Elevated Plus Maze. Basal blood samples were collected 24 h prior to the open field test, and additional blood samples were taken 20-, 30-, and 60-min after the beginning of the test.
We analyzed the neuroendocrine data in two ways. First, we sought to contrast the stress response of HR versus LR control rats that were not exposed to PS, and then separately compared HR-LR rats with a history of PS. In HR-LR control rats there was a main effect of blood collection timepoint (F=10.88, df 3,24, p<0.0001), and a main effect of HR-LR phenotype (F=5.98, df 1, 24, p<0.05), but no significant phenotype × timepoint interaction. Corticosterone levels sharply increased after exposure to the Elevated Plus Maze, with HR control rats exhibiting an exaggerated stress-evoked corticosterone response compared to LR control animals. Although there was not a significant phenotype × timepoint interaction, these HR-LR differences are most obvious at 15- and 30-min post stress (Fig. 7A). In HR-LR animals with a history of PS, there was a main effect of blood collection timepoint (F=16.94, df 3,24, p<0.0001), but no main effect of HR-LR phenotype, and no phenotype × timepoint interaction (Fig. 7D).
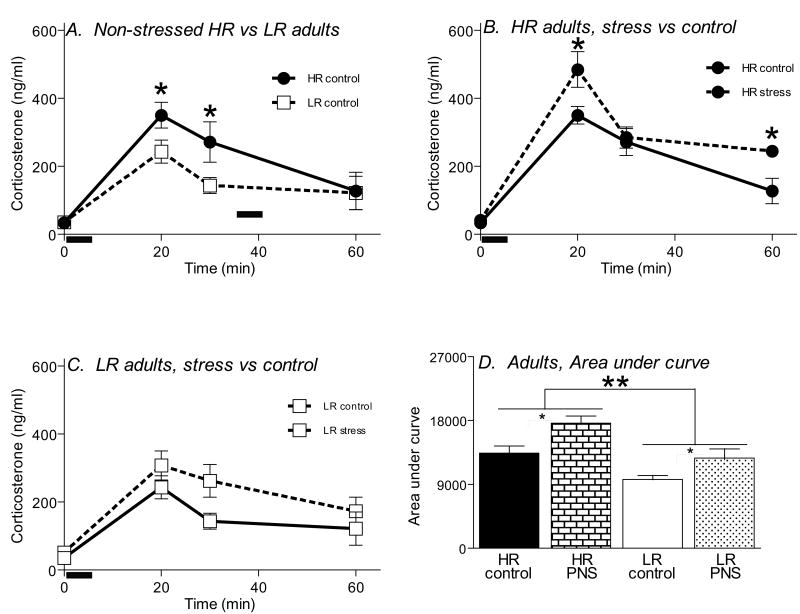
Figure 7.
Impact of PS on stress-induced corticosterone secretion in adult HR-LR offspring. Basal blood samples were collected 24 h prior to the open field test, and additional blood samples were taken 20-, 30-, and 60-in after the beginning of the test (N=10-12 animals per group). Black bar indicates the 5 min elevated plus maze (EPM) test period. HR adult controls exhibited an exaggerated stress-induced corticosterone response (most obvious at 20- and 30-min post stress) following the Elevated Plus Maze test, compared to LR controls (A). HR adults with a history of PS showed an even greater stress response, compared to HR controls (B). PS appeared to slightly increase the stress response in LR adult males, although this effect did not achieve statistical significance (C). Analysis of area under the curve for each group revealed a main effect for HR-LR phenotype, with LR animals generally showing reduced area under the curve compared to HR groups. This analysis also revealed a main effect for PS, with both stressed groups showing greater area under the curve relative to non-stressed groups (D). * indicates p<0.05; ** indicates p<0.01.
In our second analysis, we separately examined the impact of PS on HR versus LR rats. Exposure to PS did affect corticosterone secretion in HR animals, as there was a main effect of PS (F=4.76, df 1,24, p<0.05), but no timepoint × PS interaction. HR rats which were exposed to PS exhibited an exaggerated and prolonged corticosterone response (particularly evident both 20- and 60-min post stress) compared to HR control rats (Fig. 7B). Adult LR rats were not significantly affected by the history of PS, since there was no main effect of PS, and no significant timepoint × PS interaction (Fig. 7C). Our area under the curve analysis revealed a main effect of HR-LR phenotype (F=10.69, df 1, 24, p<0.01), and main effect of PS (F=4.45, df 1, 24, p<0.05), but no significant HR-LR phenotype × PS condition interaction. HR rats generally had greater area under the curve compared to LR animals, and similarly, animals that were exposed to PS generally exhibited greater area under the curve compared to unstressed controls (Fig. 7D).
4. Discussion
The present study demonstrates that Selectively-Bred HR-LR males show HPA axis differences which are consistent with previous studies in commercially purchased HR-LR animals (e.g. (Piazza et al., 1989, Kabbaj et al., 2000)). Selectively-Bred HR rats, like purchased HR rats, express lower levels of hippocampal GR mRNA compared to their LR counterparts (Fig.1), and also exhibit an exaggerated corticosterone response to novelty stress compared to LR rats (Fig. 7A). Since GR is thought be important for recovery and adaptation of the stress response (De Kloet and Derijk, 2004), the reduction of hippocampal GR in HR versus LR is likely related to their differences in stress-induced corticosterone secretion. Moreover, previous studies have demonstrated that GR differences also contribute to divergent HR-LR behavior, since infusing the GR antagonist RU38486 into the hippocampus of LR rats reduced anxiety-like behavior, making them behavior more “HR-like” (Kabbaj et al., 2000). It seems paradoxical that HR rats vigorously explore new environments and exhibit reduced anxiety-like behavior compared to LRs, yet, they simultaneously show enhanced stress-induced glucocorticoid secretion, a biological sign that they are “more stressed”. Nevertheless, prior studies suggest that HR rats actually find the elevated glucocorticoid levels rewarding since they will self-administer corticosterone more readily than LRs (Piazza et al., 1993).
Considering baseline HR-LR differences in HPA axis reactivity, we hypothesized that HR and LR animals would differentially react to PS since this manipulation occurs at a critical point in HPA axis development, and has been shown to potently alter HPA function in other animal models (Fride et al., 1986, Takahashi et al., 1990, Takahashi and Kalin, 1991, Takahashi et al., 1992, Henry et al., 1994, Maccari et al., 2003, Morley-Fletcher et al., 2003). Furthermore, because the HR-LR traits appear to involve a strong genetic component (Stead et al., 2006), we also wanted to explore possible interactions between genetic endowment and environmental factors (i.e. gestational stress), which may influence behavioral and/or neuroendocrine aspects of the HR-LR phenotypes. Results from these experiments are discussed in turn below and summarized in Table 1.
Table 1.
Summary of findings
| Novelty-induced activity | % Time in Anxiogenic Area (Open Arm of EPM or Center of OF) | Latency to enter Anxiogenic Area (Open arm of EPM or Center of OF) | Baseline Corticosterone Levels | Pattern of acute stress-induced Corticosterone Secretion | |
|---|---|---|---|---|---|
| Chronic unpredictable stress (dams) | |||||
| HR pregnant females | ↓ | ↓ | ― | ↑ | n.s. |
| LR pregnant females | ― | ↑ | ― | ↑ | n.s. |
| Prenatal stress (25 day-old offspring) | |||||
| HR male pups | ― | ― | ― | ― | ― |
| LR male pups | ― | ― | ↓ | ― | ↑ |
| Prenatal stress (adult offspring) | |||||
| HR male adults | ↓ | ― | ― | ― | ↑ |
| LR male adults | ↓ | ― | ↓ | ― | ― |
― no change relative to non-stressed control group
↓ decreased relative to non-stressed control group
↑ increased relative to non-stressed control group
Abbreviations: EPM=elevated plus maze; OF=open field; n.s.=not studied
4.1 Chronic unpredictable stress differentially impacts anxiety-like behavior in pregnant HR vs. LR dams
We previously showed that both commercially purchased and Selectively-Bred HR-LR females exhibit behavior differences similar to those observed in HR-LR males (Stead et al., 2006, Clinton et al., 2007). However, the present results show that exposure to chronic unpredictable stress reverses the pattern of HR-LR anxiety-like behavior differences. While non-stressed HR controls showed less anxiety-like behavior compared to LR controls, chronic stress completely reversed the HR-LR difference. Chronically stressed HRs became very inhibited, spending little to no time in the open arm of the Elevated Plus Maze, while chronic stress seemed to activate LRs, making them more willing to explore the open arm than LR controls and stressed HR animals (Fig. 2A and 2C). These results are consistent with previous findings showing that a) prolonged social isolation abolished HR-LR anxiety differences by increasing anxiety-like behavior in HRs (Kabbaj et al., 2000); and b) that social defeat stress dramatically affected LR animals, leading them resemble HRs and readily self-administer psychostimulants (Kabbaj et al., 2001). Other studies suggest that an optimal level of glucocorticoids may promote certain behaviors, including locomotor activity (Galina et al., 1983) and psychostimulant self-administration (Goeders and Guerin, 1996). Work by Piazza (Piazza et al., 1989, Piazza et al., 1991a) as well as our group (Kabbaj et al., 2000) clearly demonstrates that HR-LR animals exhibit distinct neuroendocrine reactivity, and such differences may set the stage for these animals to react differently when confronted with chronic stress. While non-stressed HRs may exhibit optimal glucocorticoid levels that favor exploration in a novel environment, chronic stress elevates basal corticosterone levels, potentially exceeding this optimal range and thereby inhibiting HRs' behavior. By contrast, while non-stressed LR animals may exhibit suboptimal glucocorticoid levels, chronic stress may stimulate their HPA axis, making them more willing to explore.
Several groups have demonstrated that chronic unpredictable stress produces to increased depressive-like behavior (Bielajew et al., 2003), and anhedonia (Willner et al., 1987, Papp et al., 1991, D'Aquila et al., 1994, Konkle et al., 2003, Grippo et al., 2005, Ushijima et al., 2006), but there are mixed reports on whether it impacts anxiety-like behavior (D'Aquila et al., 1994, Vyas and Chattarji, 2004, Mitra et al., 2005). Our data suggest that individual differences in emotional reactivity may predispose individuals to react differently to chronic stress, with some animals becoming quite anxious and inhibited (in our hands, the stressed HR females), while other animals become more activated and exploratory (in our case, the stressed LR females). Thus, individual differences within experimental groups in these earlier studies may have increased variability in their data, thus contributing to inconsistent findings in the impact of chronic stress on anxiety-like behavior. Future studies with the HR-LR model should move beyond tests of anxiety-like behavior to also examine the effect of chronic unpredictable stress on depressive and anhedonic behavior. Future work should also evaluate how male and female HR/LR rats react to chronic stress since each gender may be differentially susceptible to stress, and should examine how pregnancy hormones may have interacted with stress exposure, thus influencing behavior of HR vs. LR dams in the present study.
4.2. HR-LR behavioral and neuroendocrine phenotypes are present in early-life
Until now, the wealth of studies on the HR-LR model have exclusively focused on adult animals, primarily because the behavior screen to determine whether rats showed high or low novelty-induced locomotor activity could only be performed in adult animals (postnatal day 60+). Thus, a major goal of our selective breeding for the HR-LR traits was to generate phenotypic predictability, namely the ability to predict whether an animal will have an HR or LR phenotype at very early stages of development, prior to the age when behavioral testing has traditionally been performed. This predictability has already been largely accomplished, as over 99% of our selectively-bred HR animals come from HR parents, and 99% of selectively-bred LR animals come from LR parents (Stead et al., 2006). The present behavioral and neuroendocrine data from weanling HR-LR pups are the first demonstration that the HR-LR phenotypes are present in early life. HR control pups, like their adult counterparts, were significantly more active in a novel environment compared to LR control pups (Fig. 3A, 4D), showed lower levels of anxiety-like behavior in the open field test (Fig. 4A-C), and exhibited a prolonged novelty-induced corticosterone secretion (Fig. 6A) compared to LRs. Ongoing work is examining the neural underpinnings that may occur during early life that may give rise to these marked behavioral and neuroendocrine HR-LR differences (Clinton, 2006).
4.3. Prenatal stress does not affect HR-LR locomotor activity differences, but mildly reduces anxiety-like behavior in LR offspring
Exposure to PS did not dramatically impact basic HR-LR behavioral differences in either weanling or adult rats. Both weanling and adult HR animals, regardless of PS history, were significantly more active in a novelty environment (Fig. 3) and expressed low levels of anxiety-like behavior compared to the LR groups (Figs. 4-5). Although PS and control LR weanling and adult offspring spent similarly short periods of time in the anxiogenic portions of the test apparatus (center of the open field for pups and the open arms of the elevated plus maze for adults), in each case, the LR animals with a history of PS displayed a shorter latency to initially enter the anxiogenic region, suggesting that a history of PS actually reduced anxiety-like behavior in LR animals (Figs. 4B and 5B). These data may at first glance appear to contradict previous reports which have found that exposure to PS generally increases animals' anxiety-like behavior (Vallee et al., 1997, Zimmerberg and Blaskey, 1998, Rimondini et al., 2003, Estanislau and Morato, 2005, Richardson et al., 2006). However, our findings are consistent with a report by Bosch and colleagues which examined the impact of PS on Wistar rats which were selectively-bred for either high- or low-levels of anxiety on the elevated plus maze; they found that PS reversed the animals' high/low anxiety phenotypes, making the high-anxiety rats (which resemble our LR animals) explore more and reduce their levels of anxiety-like behavior, while the low-anxiety rats (similar to our HR rats) became quite inhibited (Bosch et al., 2006).
4.4. Prenatal stress differentially affects adrenal hormone secretion in HR vs. LR offspring depending on age
Exposure to PS differentially impacted HPA axis reactivity in HR and LR animals, although this effect varied depending on age (weanling vs. adulthood) of the animals. In the weanling animals, PS selectively affected novelty-induced corticosterone secretion in LRs, while HRs were unaffected. Corticosterone levels in LR control pups rose steeply 20-min after the open field test, but then dropped back near baseline levels 60-min after the stressor; however, in LRs with a history of PS, corticosterone levels remained high at this 60-min timepoint. Previous work by Takahashi and colleagues showed that PS lead to exaggerated footshock-induced adrenocorticotropic hormone and corticosterone secretion in preweanling pups (postnatal day 14), but found that these effects disappeared by 21 days of age (Takahashi et al., 1988, Takahashi et al., 1990, Takahashi and Kalin, 1991). Other studies have reported persistent HPA axis hyperactivity in adult rats with a history of PS (Fride et al., 1986, Maccari et al., 2003). Such effects are likely related to a host of neuroanatomical changes and gene expression differences related to PS exposure. For example, multiple studies reported that PS reduced GR density in the adult hippocampus (Henry et al., 1994, Maccari et al., 1995), altered neurogenesis in the dentate gyrus (Lemaire et al., 2000), reduced hippocampal weight (Szuran et al., 1994, Maccari et al., 2003), and induced widespread gene expression changes that reflect possible PS-induced alterations in hippocampal synaptic function (Kinnunen et al., 2003). Our findings suggest that certain individuals (LR pups in our hands) may be particularly susceptible to developing these effects.
Several elegant studies have described the ontogeny of the rodent HPA axis, and specifically demonstrated that glucocorticoid suppression of the HPA axis remains immature and continues to develop during the postweaning period (21-25 days old) (Goldman et al., 1973, Sapolsky and Meaney, 1986, Vazquez et al., 1996). For instance, two studies showed that ether stress elicited a prolonged corticosterone response in 25 day-old pups, with near peak corticosterone levels persisting 60-min after stress exposure, unlike adult animals that showed a rapid inhibition of corticosterone secretion (Goldman et al., 1973, Vazquez et al., 1996). Our present results with HR weanlings (both PS and control groups), as well as the PS-LR weanlings resemble these findings, since all three groups show high, near peak corticosterone levels 60-min after exposure to the open field. If such effects are indeed due to immature glucocorticoid suppression mechanisms, it suggests that a) the HPA axis of LR weanlings may mature faster than HR, since 25-day-old LR controls more efficiently suppress circulating corticosterone levels compared to HR control animals (Fig. 6A), and b) that PS may interfere with LR's normal pattern of HPA axis development, since PS leads to an exaggerated stress response in LR pups (Fig. 6C). Future studies will examine potential differences in the ontogeny of the HPA axis in HR versus LR rats, and will also investigate possible mechanisms whereby PS may modify development of HR versus LR stress circuits.
Exposure to PS also influenced HPA axis reactivity in adult animals (Fig 7D), although, unlike the findings with weanling rats, PS predominantly affected neuroendocrine response of HR animals (Fig. 7B). Adult HR controls showed enhanced stress-induced glucocorticoid secretion compared to LR controls (Fig. 7A); however, HR rats with a history of PS showed an even greater corticosterone stress response relative to HR controls (Fig. 7B). PS appeared to slightly increase the stress response in adult LRs, although this effect did not achieve statistical significance (Fig. 7C). Interestingly, analysis of the area under the curve for each group revealed a main effect for PS, indicating that animals exposed to PS exhibited an increased corticosterone stress response compared to non-stressed control groups (Fig. 7D). Our data are generally compatible with earlier reports showing that PS lead to impaired feedback inhibition of the HPA axis in adult animals (Fride et al., 1986, Maccari et al., 2003). However, our findings suggest that HR adults may be especially sensitive to the influence of PS, an effect that may be due, in part, to their reduced levels of hippocampal GR compared to LR. Previous studies showed that PS leads to reduced GR density in the hippocampus (Henry et al., 1994, Maccari et al., 1995). Since HR rats basally express lower levels of hippocampal GR relative to LR rats, the additional loss of GR as a result of PS exposure may therefore exert a greater effect on HPA axis function on HRs. Thus, our results from weanling and adult animals suggest that PS has different consequences not just according to individual differences in stress-reactivity (i.e. HR versus LR), but also due to different stages of life (i.e. weanling versus adulthood). Future studies will address these issues by comparing and contrasting HPA axis structure and function in developing versus adult HR-LR animals, and furthermore, examine neural changes that may modify these circuits in response to PS.
4.5. Technical considerations
An important limitation of the present study was that the effect of PS was not directly compared between Selectively-Bred HR/LR rats and a commercially purchased control group. As discussed above, a substantial body of previous work has powerfully demonstrated the variety of deleterious consequences of PS, including PS-induced neuroendocrine (Fride et al., 1986, Takahashi et al., 1990, Takahashi and Kalin, 1991, Henry et al., 1994, Maccari et al., 2003, Morley-Fletcher et al., 2003) and behavioral (Ward and Stehm, 1991, Szuran et al., 1994, Vallee et al., 1996, Vallee et al., 1997, Morley-Fletcher et al., 2003, Patin et al., 2005) abnormalities. On the whole, these studies generally suggest that a history of PS yields animals that exhibit exaggerated HPA axis reactivity and enhanced anxiety-like behavior. The present findings suggest that individual differences in stress responsivity may further color how certain animals respond to PS.
Our results generally agree that PS leads to enhanced HPA axis reactivity; however, baseline individual differences in the structure and function of the HPA axis (e.g. baseline differences in hippocampal GR expression) may dictate how PS affects an animal. Our behavioral data show that PS slightly reduces anxiety-like behavior specifically in LR animals, and does not affect HRs -- results that appear to contradict previous findings in commercially purchased rats where PS increased anxiety-like behavior. After many generations of breeding, the HR/LR behavioral phenotypes have dramatically diverged and become rather extreme. The Selectively-Bred HR/LR lines obviously act quite differently from each other, but also behave differently from commercially purchased animals, which tend to exhibit intermediate levels of activity and anxiety-like behavior compared to the HR/LR extremes (Stead et al., 2006; unpublished observations). Thus, while PS may potently influence animals that exhibit a more “intermediate phenotype” to begin with (i.e. a commercially purchased animal), it may have only limited effects on animals that naturally exhibit more extreme behavioral phenotypes (i.e. Selectively-Bred HR/LR rats).
4.6. Conclusions
The present set of behavioral and neuroendocrine studies in HR-LR animals have uncovered several novel findings. 1) First, we confirmed baseline differences in HPA-reactivity in Selective-Bred HR-LR males (decreased hippocampal GR mRNA expression and exaggerated novelty-induced corticosterone release in HR versus LR), which is consistent with previous work, and 2) also demonstrated that the HR-LR behavioral and neuroendocrine phenotypes are present in early life, at least as early as postnatal day 25. 3) We found that exposure to PS did not fundamentally alter the HR-LR behavioral phenotype, although it did subtly decrease anxiety-like behavior in both adult and weanling LR animals. 4) Finally, we show that PS differentially impacted neuroendocrine reactivity in young versus adult HR-LR rats. PS selectively affected the stress response in LR pups, leading to an exaggerated and prolonged corticosterone response to novelty, although this effect largely disappeared by adulthood. On the other hand, while PS did not alter the stress response of HR pups, it produced an enhanced stress-induced corticosterone response in adult HRs. Taken together, these findings highlight how genetic predisposition may shape individual's response to early life stressors, and furthermore, show that a history of early life stress may differentially impact an organism at different points in life. Future work will explore neural mechanisms which underlie the different behavioral and neuroendocrine consequences of prenatal stress in HR versus LR animals, and will also evaluate whether PS has similar consequences on female HR-LR rats.
Acknowledgments
We are extremely grateful to Antony Abraham and Tracy Bedrosian for excellent technical assistance. This study was funded by the Office of Naval Research, grant N00014-02-1-0879 to HA, NIDA RO1 DA13386 to HA, NIMH PO1 MH42251 to SJW, L'Oréal USA Fellowship for Women in Science (SMC).
The authors are extremely grateful to Antony Abraham and Tracy Bedrosian for excellent technical assistance. This study was funded by the Office of Naval Research, grant N00014-02-1-0879 to HA, NIDA R01 DA13386 to HA and NIMH P01 MH42251 to SJW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham A, Clinton SM, Watson SJ, et al. Individual Differences in Novelty-Seeking Correlate with Differences in Aggressive Behavior. Society for Neuroscience Abstract, 36th Annual Meeting; Atlanta, GA. 2006. [Google Scholar]
- Alttoa A, Eller M, Herm L, et al. Amphetamine-induced locomotion, behavioral sensitization to amphetamine, and striatal D2 receptor function in rats with high or low spontaneous exploratory activity: differences in the role of locus coeruleus. Brain Research. 2007;1131:138–148. doi: 10.1016/j.brainres.2006.10.075. [DOI] [PubMed] [Google Scholar]
- Barbazanges A, Piazza PV, Le Moal M, et al. Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. Journal of Neuroscience. 1996;16:3943–3949. doi: 10.1523/JNEUROSCI.16-12-03943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielajew C, Konkle AT, Kentner AC, et al. Strain and gender specific effects in the forced swim test: effects of previous stress exposure. Stress. 2003;6:269–280. doi: 10.1080/10253890310001602829. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Kromer SA, Neumann ID. Prenatal stress: opposite effects on anxiety and hypothalamic expression of vasopressin and corticotropin-releasing hormone in rats selectively bred for high and low anxiety. European Journal of Neuroscience. 2006;23:541–551. doi: 10.1111/j.1460-9568.2005.04576.x. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Clinton SM, Stead JDH, Watson SJ, Akil H. Developmental Emergence of Individual Differences in Emotional Reactivity; Society for Neuroscience Annual Meeting; Atlanta, GA. 2006. [Google Scholar]
- Clinton SM, Vázquez DM, Kabbaj M, et al. Individual differences in novelty-seeking and emotional reactivity correlate with variation in maternal behavior. Hormones and Behavior. 2007 doi: 10.1016/j.yhbeh.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aquila PS, Brain P, Willner P. Effects of chronic mild stress on performance in behavioural tests relevant to anxiety and depression. Physiol Behav. 1994;56:861–867. doi: 10.1016/0031-9384(94)90316-6. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Derijk R. Signaling pathways in brain involved in predisposition and pathogenesis of stress-related disease: genetic and kinetic factors affecting the MR/GR balance. Ann N Y Acad Sci. 2004;1032:14–34. doi: 10.1196/annals.1314.003. [DOI] [PubMed] [Google Scholar]
- Deminiere JM, Piazza PV, Le Moal M, et al. Experimental approach to individual vulnerability to psychostimulant addiction. Neuroscience and Biobehavioral Reviews. 1989;13:141–147. doi: 10.1016/s0149-7634(89)80023-5. [DOI] [PubMed] [Google Scholar]
- Estanislau C, Morato S. Prenatal stress produces more behavioral alterations than maternal separation in the elevated plus-maze and in the elevated T-maze. Behavioral Brain Research. 2005;163:70–77. doi: 10.1016/j.bbr.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Everett JW, Sawyer CH. A neural timing factor in the mechanism by which progesterone advances ovulation in the cyclic rat. Endocrinology. 1949;45:581–595. doi: 10.1210/endo-45-6-581. illust. [DOI] [PubMed] [Google Scholar]
- Fride E, Dan Y, Feldon J, et al. Effects of prenatal stress on vulnerability to stress in prepubertal and adult rats. Physiol Behav. 1986;37:681–687. doi: 10.1016/0031-9384(86)90172-1. [DOI] [PubMed] [Google Scholar]
- Galina ZH, Sutherland CJ, Amit Z. Effects of heat-stress on behavior and the pituitary adrenal axis in rats. Pharmacol Biochem Behav. 1983;19:251–256. doi: 10.1016/0091-3057(83)90048-5. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. Role of corticosterone in intravenous cocaine self-administration in rats. Neuroendocrinology. 1996;64:337–348. doi: 10.1159/000127137. [DOI] [PubMed] [Google Scholar]
- Goldman L, Winget C, Hollingshead GW, et al. Postweaning development of negative feedback in the pituitary-adrenal system of the rat. Neuroendocrinology. 1973;12:199–211. doi: 10.1159/000122169. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Sullivan NR, Damjanoska KJ, et al. Chronic mild stress induces behavioral and physiological changes, and may alter serotonin 1A receptor function, in male and cycling female rats. Psychopharmacology (Berl) 2005;179:769–780. doi: 10.1007/s00213-004-2103-4. [DOI] [PubMed] [Google Scholar]
- Henry C, Kabbaj M, Simon H, et al. Prenatal stress increases the hypothalamo-pituitary-adrenal axis response in young and adult rats. Journal of Neuroendocrinology. 1994;6:341–345. doi: 10.1111/j.1365-2826.1994.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Juncos JL, Justice JB, Jr, et al. Individual locomotor response to novelty predicts selective alterations in D1 and D2 receptors and mRNAs. Journal of Neuroscience. 1994a;14:6144–6152. doi: 10.1523/JNEUROSCI.14-10-06144.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks MS, Sorg BA, Kalivas PW. The relationship between MRNA levels and the locomotor response to novelty. Brain Research. 1994b;663:312–316. doi: 10.1016/0006-8993(94)91278-5. [DOI] [PubMed] [Google Scholar]
- Isgor C, Kabbaj M, Akil H, et al. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14:636–648. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Kasen S, et al. Association of maladaptive parental behavior with psychiatric disorder among parents and their offspring. Archives of General Psychiatry. 2001;58:453–460. doi: 10.1001/archpsyc.58.5.453. [DOI] [PubMed] [Google Scholar]
- Kabbaj M. Neurobiological bases of individual differences in emotional and stress responsiveness: high responders-low responders model. Archives of Neurology. 2004;61:1009–1012. doi: 10.1001/archneur.61.7.1009. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR, et al. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. Journal of Neuroscience. 2000;20:6983–6988. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbaj M, Evans S, Watson SJ, et al. The search for the neurobiological basis of vulnerability to drug abuse: using microarrays to investigate the role of stress and individual differences. Neuropharmacology. 2004;47 1:111–122. doi: 10.1016/j.neuropharm.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Norton CS, Kollack-Walker S, et al. Social defeat alters the acquisition of cocaine self-administration in rats: role of individual differences in cocaine-taking behavior. Psychopharmacology (Berl) 2001;158:382–387. doi: 10.1007/s002130100918. [DOI] [PubMed] [Google Scholar]
- Katz RJ, Roth KA, Carroll BJ. Acute and chronic stress effects on open field activity in the rat: implications for a model of depression. Neuroscience and Biobehavioral Reviews. 1981;5:247–251. doi: 10.1016/0149-7634(81)90005-1. [DOI] [PubMed] [Google Scholar]
- Kinnunen AK, Koenig JI, Bilbe G. Repeated variable prenatal stress alters pre- and postsynaptic gene expression in the rat frontal pole. Journal of Neurochemistry. 2003;86:736–748. doi: 10.1046/j.1471-4159.2003.01873.x. [DOI] [PubMed] [Google Scholar]
- Konkle AT, Baker SL, Kentner AC, et al. Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: sex and strain compared. Brain Res. 2003;992:227–238. doi: 10.1016/j.brainres.2003.08.047. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Stohr T, Feldon J. Long-term effects of prenatal stress experiences and postnatal maternal separation on emotionality and attentional processes. Behavioral Brain Research. 2000;107:133–144. doi: 10.1016/s0166-4328(99)00122-9. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, et al. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci U S A. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccari S, Darnaudery M, Morley-Fletcher S, et al. Prenatal stress and long-term consequences: implications of glucocorticoid hormones. Neuroscience and Biobehavioral Reviews. 2003;27:119–127. doi: 10.1016/s0149-7634(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Maccari S, Piazza PV, Kabbaj M, et al. Adoption reverses the long-term impairment in glucocorticoid feedback induced by prenatal stress. Journal of Neuroscience. 1995;15:110–116. doi: 10.1523/JNEUROSCI.15-01-00110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallo T, Alttoa A, Koiv K, et al. Rats with persistently low or high exploratory activity: behaviour in tests of anxiety and depression, and extracellular levels of dopamine. Behavioral Brain Research. 2007;177:269–281. doi: 10.1016/j.bbr.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Mitra R, Vyas A, Chatterjee G, et al. Chronic-stress induced modulation of different states of anxiety-like behavior in female rats. Neurosci Lett. 2005;383:278–283. doi: 10.1016/j.neulet.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Morley-Fletcher S, Darnaudery M, Mocaer E, et al. Chronic treatment with imipramine reverses immobility behaviour, hippocampal corticosteroid receptors and cortical 5-HT(1A) receptor mRNA in prenatally stressed rats. Neuropharmacology. 2004;47:841–847. doi: 10.1016/j.neuropharm.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Morley-Fletcher S, Rea M, Maccari S, et al. Environmental enrichment during adolescence reverses the effects of prenatal stress on play behaviour and HPA axis reactivity in rats. European Journal of Neuroscience. 2003;18:3367–3374. doi: 10.1111/j.1460-9568.2003.03070.x. [DOI] [PubMed] [Google Scholar]
- Mueser KT, Rosenberg SD, Goodman LA, et al. Trauma, PTSD, and the course of severe mental illness: an interactive model. Schizophr Res. 2002;53:123–143. doi: 10.1016/s0920-9964(01)00173-6. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Wigger A, Kromer S, et al. Differential effects of periodic maternal separation on adult stress coping in a rat model of extremes in trait anxiety. Neuroscience. 2005;132:867–877. doi: 10.1016/j.neuroscience.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Papp M, Willner P, Muscat R. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl) 1991;104:255–259. doi: 10.1007/BF02244188. [DOI] [PubMed] [Google Scholar]
- Patin V, Lordi B, Vincent A, et al. Effects of prenatal stress on anxiety and social interactions in adult rats. Brain Research Developmental Brain Research. 2005;160:265–274. doi: 10.1016/j.devbrainres.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, et al. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche V, Deminiere JM, et al. Corticosterone in the range of stress-induced levels possesses reinforcing properties: implications for sensation-seeking behaviors. Proceedings of the National Academy of Science of the USA. 1993;90:11738–11742. doi: 10.1073/pnas.90.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Maccari S, Deminiere JM, et al. Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proceedings of the National Academy of Sciences of the USA. 1991a;88:2088–2092. doi: 10.1073/pnas.88.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Rouge-Pont F, Deminiere JM, et al. Dopaminergic activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Research. 1991b;567:169–174. doi: 10.1016/0006-8993(91)91452-7. [DOI] [PubMed] [Google Scholar]
- Poltyrev T, Gorodetsky E, Bejar C, et al. Effect of chronic treatment with ladostigil (TV-3326) on anxiogenic and depressive-like behaviour and on activity of the hypothalamic-pituitary-adrenal axis in male and female prenatally stressed rats. Psychopharmacology (Berl) 2005;181:118–125. doi: 10.1007/s00213-005-2229-z. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Zorrilla EP, Mandyam CD, et al. Exposure to repetitive versus varied stress during prenatal development generates two distinct anxiogenic and neuroendocrine profiles in adulthood. Endocrinology. 2006;147:2506–2517. doi: 10.1210/en.2005-1054. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Agren G, Borjesson S, et al. Persistent behavioral and autonomic supersensitivity to stress following prenatal stress exposure in rats. Behav Brain Res. 2003;140:75–80. doi: 10.1016/s0166-4328(02)00273-5. [DOI] [PubMed] [Google Scholar]
- Rojo M, Marin B, Menendez-Patterson A. Effects of low stress during pregnancy on certain parameters of the offspring. Physiol Behav. 1985;34:895–899. doi: 10.1016/0031-9384(85)90010-1. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Research. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Stead JD, Clinton SM, Neal C, et al. Selective Breeding for Divergence in Novelty-seeking Traits: Heritability and Enrichment in Spontaneous Anxiety-related Behaviors. Behavioral Genetics. 2006;36:697–712. doi: 10.1007/s10519-006-9058-7. [DOI] [PubMed] [Google Scholar]
- Stohr T, Schulte Wermeling D, Szuran T, et al. Differential effects of prenatal stress in two inbred strains of rats. Pharmacology, Biochemistry, and Behavior. 1998;59:799–805. doi: 10.1016/s0091-3057(97)00541-8. [DOI] [PubMed] [Google Scholar]
- Suchecki D, Palermo Neto J. Prenatal stress and emotional response of adult offspring. Physiol Behav. 1991;49:423–426. doi: 10.1016/0031-9384(91)90259-q. [DOI] [PubMed] [Google Scholar]
- Szuran T, Zimmermann E, Welzl H. Water maze performance and hippocampal weight of prenatally stressed rats. Behavioral Brain Research. 1994;65:153–155. doi: 10.1016/0166-4328(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Baker EW, Kalin NH. Ontogeny of behavioral and hormonal responses to stress in prenatally stressed male rat pups. Physiology and Behavior. 1990;47:357–364. doi: 10.1016/0031-9384(90)90154-v. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Kalin NH. Early developmental and temporal characteristics of stress-induced secretion of pituitary-adrenal hormones in prenatally stressed rat pups. Brain Res. 1991;558:75–78. doi: 10.1016/0006-8993(91)90715-8. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Kalin NH, Barksdale CM, et al. Stressor controllability during pregnancy influences pituitary-adrenal hormone concentrations and analgesic responsiveness in offspring. Physiol Behav. 1988;42:323–329. doi: 10.1016/0031-9384(88)90273-9. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Turner JG, Kalin NH. Prenatal stress alters brain catecholaminergic activity and potentiates stress-induced behavior in adult rats. Brain Res. 1992;574:131–137. doi: 10.1016/0006-8993(92)90809-n. [DOI] [PubMed] [Google Scholar]
- Tazumi T, Hori E, Uwano T, et al. Effects of prenatal maternal stress by repeated cold environment on behavioral and emotional development in the rat offspring. Behav Brain Res. 2005;162:153–160. doi: 10.1016/j.bbr.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Ushijima K, Morikawa T, To H, et al. Chronobiological disturbances with hyperthermia and hypercortisolism induced by chronic mild stress in rats. Behav Brain Res. 2006;173:326–330. doi: 10.1016/j.bbr.2006.06.038. [DOI] [PubMed] [Google Scholar]
- Vallee M, Mayo W, Dellu F, et al. Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: correlation with stress-induced corticosterone secretion. Journal of Neuroscience. 1997;17:2626–2636. doi: 10.1523/JNEUROSCI.17-07-02626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee M, Mayo W, Maccari S, et al. Long-term effects of prenatal stress and handling on metabolic parameters: relationship to corticosterone secretion response. Brain Research. 1996;712:287–292. doi: 10.1016/0006-8993(95)01459-4. [DOI] [PubMed] [Google Scholar]
- Van den Hove DL, Lauder JM, Scheepens A, et al. Prenatal stress in the rat alters 5-HT1A receptor binding in the ventral hippocampus. Brain Research. 2006;1090:29–34. doi: 10.1016/j.brainres.2006.03.057. [DOI] [PubMed] [Google Scholar]
- Vazquez DM, Van Oers H, Levine S, et al. Regulation of glucocorticoid and mineralocorticoid receptor mRNAs in the hippocampus of the maternally deprived infant rat. Brain Res. 1996;731:79–90. doi: 10.1016/0006-8993(96)00465-9. [DOI] [PubMed] [Google Scholar]
- Vyas A, Chattarji S. Modulation of different states of anxiety-like behavior by chronic stress. Behav Neurosci. 2004;118:1450–1454. doi: 10.1037/0735-7044.118.6.1450. [DOI] [PubMed] [Google Scholar]
- Ward IL, Stehm KE. Prenatal stress feminizes juvenile play patterns in male rats. Physiol Behav. 1991;50:601–605. doi: 10.1016/0031-9384(91)90552-y. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Does prenatal stress impair coping and regulation of hypothalamic-pituitary-adrenal axis? Neuroscience and Biobehavioral Reviews. 1997;21:1–10. doi: 10.1016/s0149-7634(96)00014-0. [DOI] [PubMed] [Google Scholar]
- White DA, Kalinichev M, Holtzman SG. Locomotor response to novelty as a predictor of reactivity to aversive stimuli in the rat. Brain Research. 2007;1149:141–148. doi: 10.1016/j.brainres.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, et al. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Ying SY, Gove S, Fang VS, et al. Ovulation in postpartum rats. Endocrinology. 1973;92:108–116. doi: 10.1210/endo-92-1-108. [DOI] [PubMed] [Google Scholar]
- Zimmerberg B, Blaskey LG. Prenatal stress effects are partially ameliorated by prenatal administration of the neurosteroid allopregnanolone. Pharmacol Biochem Behav. 1998;59:819–827. doi: 10.1016/s0091-3057(97)00540-6. [DOI] [PubMed] [Google Scholar]