Abstract
The rapid entry of drugs into the brain is thought to increase the propensity for addiction. The mechanisms that underlie this effect are not known, but variation in the rate of intravenous cocaine delivery does influence its ability to induce immediate early gene expression (IEG) in the striatum, and to produce psychomotor sensitization. Both IEG induction and psychomotor sensitization are dependent upon dopamine and glutamate neurotransmission within the striatum. We hypothesized, therefore, that varying the rate of intravenous cocaine delivery might influence dopamine and/or glutamate overflow in the striatum. To test this we used microdialysis coupled to on-line capillary electrophoresis and laser-induced fluorescence, which allows for very rapid sampling, to compare the effects of a rapid (5 sec) versus a slow (100 sec) intravenous cocaine infusion on extracellular dopamine and glutamate levels in the striatum of freely moving rats. An acute injection of cocaine had no effect on extracellular glutamate, at either rate tested. In contrast, although peak levels of dopamine were unaffected by infusion rate, dopamine levels increased more rapidly when cocaine was administered over 5 versus 100 seconds. Moreover, c-fos mRNA expression in the region of the striatum sampled was greater when cocaine was administered rapidly than when given slowly. These data suggest that small differences in the temporal dynamics of dopamine neurotransmission may have a large effect on the subsequent induction of intracellular signalling cascades that lead to immediate early gene expression, and in this way influence the ability of cocaine to produce long-lasting changes in brain and behaviour.
Keywords: cocaine, microdialysis, rate, infusion, addiction, dopamine, glutamate
Introduction
It is widely accepted that the rapid delivery of drugs to the brain increases susceptibility to addiction. Little is known, however, about how rate of drug delivery influences the behavioral or neurobiological actions of addictive substances. It was recently reported that the rapid (5 sec) intravenous (i.v.) infusion of cocaine or nicotine more readily induces sensitization to their psychomotor activating effects (Samaha et al., 2002), and in the case of cocaine, to its incentive motivational effects as well (Liu et al., 2005). The rapid infusion of cocaine or nicotine also produces more robust mRNA expression for the immediate early genes (IEGs) c-fos and arc in mesocorticolimbic structures, relative to even moderately slower infusions (25-100 sec; Samaha et al., 2004; Samaha et al., 2005). The induction of IEGs is thought to represent an initial step in producing forms of neurobehavioral plasticity involved in addiction (Hyman and Malenka, 2001; Nestler, 2001), and therefore, how rate of drug delivery alters drug-induced IEG expression is of interest.
The ability of cocaine to induce IEG expression in the striatum is thought to be primarily due to its effects on dopamine and glutamate neurotransmission (Young et al., 1991; Berretta et al., 1992; Torres and Rivier, 1993; Fosnaugh et al., 1995). We hypothesized, therefore, that variation in the rate of intravenous cocaine delivery may influence the amount of dopamine and/or glutamate overflow in the striatum, and this may contribute to the effect of rate of cocaine delivery on IEG expression (Samaha and Robinson, 2005). As a first step towards addressing this question we examined the influence of a rapid (5 sec) vs. slower (100 sec) i.v. infusion of cocaine on dopamine and glutamate overflow in the striatum of awake freely moving rats, using microdialysis coupled to on-line capillary electrophoresis and laser-induced fluorescence (CE-LIF; Bowser and Kennedy, 2001; Shou et al., 2006). This method provides the temporal resolution necessary to compare the effects of variation in infusion rate over this time scale (5-100 sec) on changes in the extracellular concentrations dopamine and glutamate.
Results
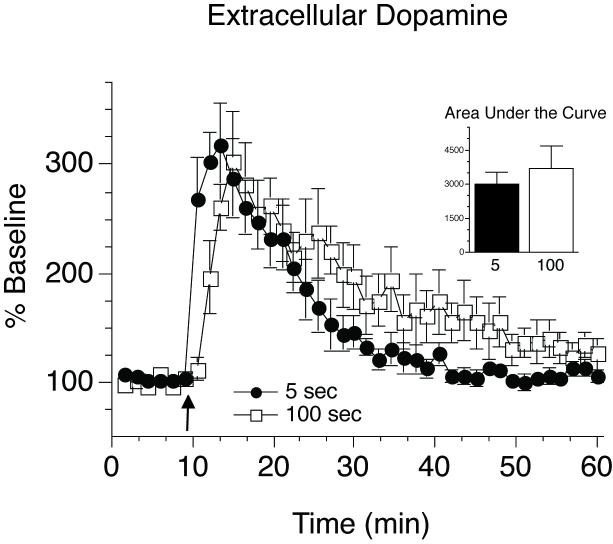
Figure 1 shows the influence of rate of intravenous cocaine delivery on c-fos mRNA expression in the portion of the striatum we targeted for microdialysis sampling. As expected, the rapid (5 sec) intravenous administration of cocaine produced significantly greater c-fos mRNA expression than a slower (100 sec) infusion within the microdialysis sampling region. The effect of varying the rate of intravenous cocaine delivery on extracellular glutamate is shown in Figure 2. Cocaine had no effect on extracellular glutamate concentrations in the striatum under either condition. This is consistent with data obtained in a previous study in which we used the same methods to sample specifically in the ventral striatum (Venton et al., 2006a). In contrast, the same dose of cocaine produced a large increase in dopamine when delivered over either 5 or 100 seconds and there was no effect of infusion rate on the “total” amount of dopamine detected, as indicated by comparison of the area under the curves (Fig. 3 inset). Furthermore, peak dopamine levels did not differ between the two rates tested. However, the time to achieve peak dopamine overflow was less when cocaine was administered rapidly relative to when it was administered more slowly (Fig. 3). In addition, an i.v. infusion of saline (delivered over 5 or 100 sec) produced no change in either extracellular dopamine or glutamate (data not shown). See figure captions for all statistics.
Figure 1.
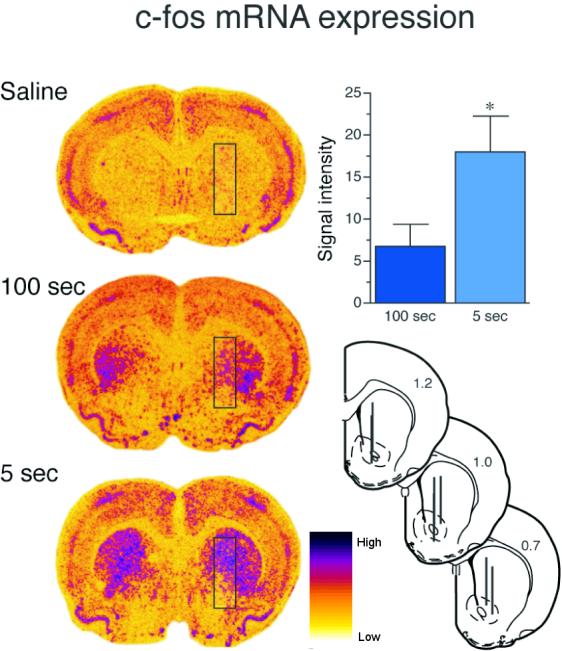
Intensity of c-fos mRNA expression as a function of rate of i.v. cocaine infusion. The left panel shows representative densitograms of c-fos mRNA expression in the brains of animals given cocaine delivered over 5 or 100 seconds (2.0 mg/kg) or saline delivered over 5 seconds. The black box overlay on the densitograms shows the area in which c-fos mRNA expression was quantified. This region was chosen because it corresponds to the central location of the microdialysis sampling region for dopamine detection. Individual microdialysis probe placements are shown at the bottom right. Each line represents the active area (3 mm) of the microdialysis probe (40 μm inner and 200 μm outer diameter) for one animal and distance from bregma is indicated on each section. Animals in the saline group showed low levels of c-fos mRNA expression in the striatum, possibly due to the mild stress of handling. However, there was no effect of rate of saline infusion (5 versus 100 seconds) on c-fos mRNA expression. In order to account for c-fos induction in the control group, the average response to saline was subtracted from each individual value obtained for rats receiving cocaine. The bar graph in the upper right hand corner shows average (+SEM) c-fos mRNA signal intensity for animals receiving cocaine over 5 or 100 seconds. Consistent with a previous report (Samaha et al., 2004), cocaine-induced c-fos mRNA expression within the mircodialysis sampling region was significantly greater when the drug was administered over 5 versus 100 seconds (unpaired t-test t (1, 20) = 2.36, p < .05). These data were obtained by analysis of autoradiographs obtained in a study by Samaha et al. (2004).
Figure 2.
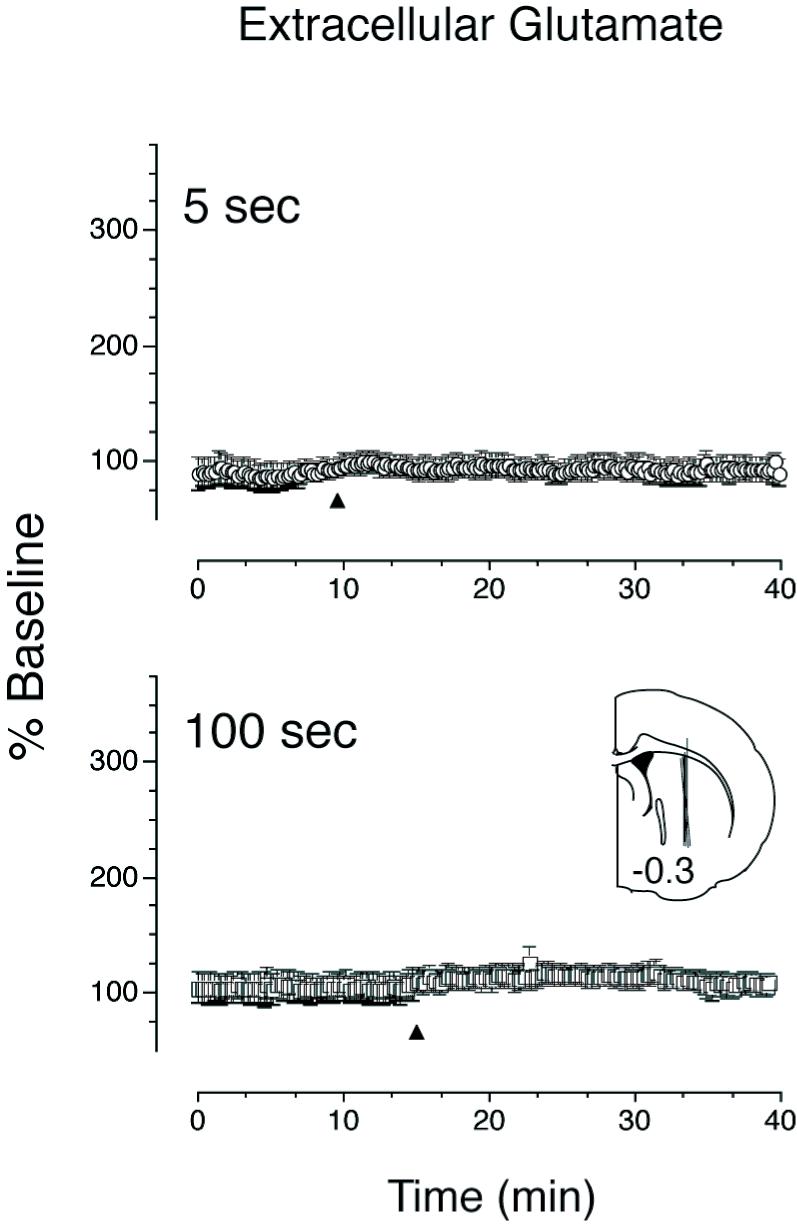
Mean (±SEM) percent change from baseline of extracellular glutamate in response to cocaine (2.0 mg/kg) administered over 5 (top panel) or 100 seconds (bottom panel). The arrowhead indicates the start of cocaine infusion. Microdialysis samples were taken every 12 seconds and baseline was calculated as average extracellular glutamate detected over 15 samples taken prior to cocaine infusion. Cocaine delivered at either rate did not significantly alter extracellular glutamate levels in the dorsal striatum. The inset shows the microdialysis probe placements (guide cannula coordinates: 0.3 mm posterior and 2.5 mm lateral to bregma, and 3 mm ventral to bregma); each line represents the active area (3 mm) of the microdialysis probe for one animal (40 μm inner and 200 μm outer diameter; the distance from bregma is indicated on the section). Similar results were obtained when samples were taken from the nucleus accumbens (Venton et al., 2006a).
Figure 3.
Mean (±SEM) percent change from baseline of extracellular dopamine in response to cocaine (2.0 mg/kg) administered over 5 or 100 seconds (guide cannula coordinates: 1mm posterior and 2 mm lateral to bregma, and 4 mm ventral to dura). The arrow indicates the start of cocaine infusion. Microdialysis samples were taken every 90 seconds, and baseline was calculated as average extracellular dopamine detected over 15 samples taken prior to cocaine infusion. Cocaine infusion significantly increased extracellular dopamine above baseline, and this effect varied with the rate of intravenous drug delivery (mixed model ANOVA effect of time F (35, 1) = 18.5, p < 0.001, effect of rate F (1, 1) = 7.5, p < 0.01, time × rate interaction F (35, 1) = 2.13, p = 0.02). The peak level of extracellular dopamine induced by cocaine delivery did not differ with infusion rate (planned paired t-test, p > 0.05). In addition, there was no difference in total dopamine detected between the two rates (area under the curve analysis from start of infusion to 50 min post infusion: p > 0.05). However, the rise of extracellular dopamine in response to cocaine administered over 5 seconds was steeper than that following delivery of cocaine over 100 seconds (mixed model ANOVA from start of infusion to peak: effect of time F (3, 1) = 45.8, p < 0.001, effect of rate F (1, 1) = 16.1, p < 0.001, time × rate interaction F (3, 1) = 6.5, p < 0.01) and the subsequent return to baseline was faster when cocaine was given over 5 versus 100 seconds (mixed model ANOVA from peak through end of data collection: effect of time F (31, 1) = 9.8, p < 0.001, effect of rate F (1, 1) = 19.2, p < 0.001, time × rate interaction F (31, 1) = 0.4, p > 0.05).
Discussion
The present findings demonstrate that: (1) A rapid (5 sec) intravenous infusion of cocaine was more effective at inducing c-fos mRNA expression in the striatum than a slower infusion (100 sec), consistent with previous reports (Samaha et al., 2004). (2) This was associated with a more rapid cocaine-induced rise in extracellular dopamine, but there was no effect of rate of infusion on either “peak” extracellular dopamine, or total dopamine (area under the curves). (3) An acute injection of cocaine had no effect on extracellular glutamate at either infusion speed tested. We have previously reported that a rapid infusion of cocaine is also more effective at inducing one form of behavioral plasticity that has been associated with the development of addiction-like behavior, psychomotor sensitization (Samaha et al 2005). Given that alterations in IEG expression are thought to represent an initial step in a cascade of molecular events leading to long-term changes in brain responsible for the development of sensitization, it is tempting to conclude that the influence of rate of cocaine infusion on the temporal dynamics of dopamine overflow and subsequent c-fos induction may contribute to such effects. Of course, the data reported here do not allow us to draw such strong causal inferences, but it is worth noting that in mice in which c-fos induction in dopamine D1 receptor-expressing neurons is disrupted the ability of repeated cocaine to induce both psychomotor sensitization and associated structural plasticity is attenuated (Zhang et al., 2006).
It is known that the ability of cocaine to induce IEG expression in the striatum is modulated by NMDA receptor activation by glutamate (Torres and Rivier, 1993; Konradi et al., 1996). However, in the present study extracellular glutamate levels in the dorsal striatum did not change for up to 40 min after an i.v. infusion of 2.0 mg/kg cocaine, regardless of infusion rate, although this dose of cocaine elicited a locomotor response in the current study and was effective in inducing c-fos mRNA. This suggests that glutamate does not contribute to the ability of cocaine to induce c-fos mRNA expression under the conditions of this study. The failure to detect any effect of cocaine on glutamate overflow was probably not because the method was insufficiently sensitive. Using this same method we were able to demonstrate that stress or a fearful stimulus produces a rapid, transient and very large increase in extracellular glutamate (Venton et al., 2006a; 2006b). Indeed, the absence of a glutamate response is consistent with a number of studies reporting that an initial acute injection of cocaine, or other psychostimulants, does not increase extracellular glutamate in the dorsal striatum (Stephans and Yamamoto, 1994; Reid et al., 1997; Robinson et al., 1997; Zhang et al., 2001). In contrast, there are inconsistent results from microdialysis studies of cocaine-induced glutamate overflow in the ventral striatum. For example, using the same dose of cocaine as in the current study, Venton et al. (2006) found that an acute i.v. infusion of cocaine did not alter glutamate levels in the ventral striatum, and Pierce et al. (1996) also reported no effect of acute cocaine administration on glutamate overflow. In contrast, Reid et al. (1997) did report acute cocaine increased glutamate overflow. However, the increase in glutamate seen by Reid et al., “... had a gradual onset with the peak increase occurring 50-60 minutes after injection” (p 103). It is possible, therefore, that cocaine administration under our conditions might eventually influence striatal glutamate levels but that we did not sample long enough after the infusion to detect this. Whatever the case, the c-fos response to cocaine was measured 30 min after drug administration. Therefore, the effects of infusion rate on c-fos mRNA expression are unlikely to be due to changes in dorsal striatum glutamate occurring more than 50 minutes after cocaine administration.
Cocaine binds to and blocks the dopamine transporter resulting in the increased availability of dopamine (Ritz et al., 1990; Pierce and Kumaresan, 2006). The fact that varying the rate of cocaine delivery altered the rate of rise of dopamine, but not peak dopamine overflow, is consistent with the pharmacokinetic model developed by Pan et al. (1991), which predicts that varying the rate of an intravenous infusion between 5 and 100 seconds would not influence the peak brain concentrations of cocaine (see Fig.1 in Samaha et al., 2002). The more rapid rate of rise of extracellular dopamine following a faster cocaine infusion is also consistent with a previous report showing that peak dopamine uptake inhibition occurs more quickly after rapid versus slow intravenous administration of cocaine (Samaha et al., 2004). However, Samaha et al. (2004) also found that the peak level of dopamine reuptake inhibition was greater following faster infusions of cocaine. Several methodological differences between these two studies may account for the different findings. Perhaps most importantly, Samaha et al. (2004) did not directly measure the effect of cocaine on extracellular dopamine, as here, but instead, measured the effect of cocaine on the clearance of electrically-evoked dopamine release. In addition, the present study was conducted using awake, freely moving rats, whereas Samaha et al. (2004) used anesthetized rats.
Neither the peak amount of dopamine nor the total amount of dopamine detected varied as a function of cocaine infusion rate (see Fig 3 inset). Thus, if the effect of cocaine infusion rate on c-fos mRNA expression is due to its effects on dopamine neurotransmission, then it would seem that the temporal dynamics of dopamine overflow rather than the peak or total amount of dopamine represents the critical factor. We do not know the mechanism responsible for this effect. It is possible that a rapid versus slow rise in extracellular dopamine could produce different patterns of D1 versus D2 receptor activation that would result in differential effects on c-fos mRNA expression in the striatum (Missale et al., 1998, Paul et al., 1992), and, of course, non-dopaminergic mechanisms may also play a role. For example, cocaine-induced changes in striatal IEG expression are also influenced by serotonergic and cholinergic transmitter systems (Rouillard et al., 1996; Wirtshafter, 2004), although there is evidence to suggest that these are indirect effects mediated by glutamate and/or dopamine (Scruggs et al., 2000; Wirtshafter, 2004). Although we do not know the mechanism by which variation in rate of cocaine delivery produces the effects described here, the idea that small differences in the temporal pattern of synaptic activity can influence subsequent plasticity is not new. For example, the induction of long-term potentiation is known to be sensitive to the temporal pattern of synaptic activation (Larson et al., 1986; Greenstein et al., 1988; Tsukada et al., 1994). Thus, it would seem that this is an area ripe for further study.
In summary, investigations of the neurobehavioral impact of rapid versus slow cocaine infusions have shown that rapidly administered cocaine produces: (1) faster and greater reuptake inhibition of electrically-evoked dopamine (Samaha et al., 2004); (2) a faster rise in extracellular dopamine in the striatum (present report); (3) greater IEG expression in mesocorticolimbic regions (Samaha et al., 2004); (4) no effect on extracellular glutamate levels; and (5) an increased susceptibility to sensitization to both the psychomotor and motivational effects of the drug (Samaha et al., 2002; Liu et al., 2005). We suggest, therefore, that small differences in the temporal pattern of dopamine neurotransmission due to variation in the rate of drug delivery may have large effects on the resulting cascade of cellular events that eventually produce long-lasting changes in brain and behavior associated with the use of potentially addictive drugs - changes that may contribute to the development of addiction.
Experimental Procedure
c-fos mRNA data were obtained by analysis of autoradiographs from a study by Samaha et al. (2004) in which animals received an intravenous infusion of either saline or cocaine (2mg/kg) at one of two infusion rates: 5 seconds (N=10) or 100 seconds (N=12). In situ hybridization was used to detect c-fos mRNA expression. Coronal brains sections (16 μm) were cut at 200 μm intervals from +3.8 to -0.8 mm relative to bregma. Sections were thaw-mounted onto Superfrost/Plus slides (Fisher Scientific, Pittsburgh, PA) and stored at 80°C until processed. Adjacent brain sections were processed for in situ hybridization of c-fos mRNA using 35S-UTP- and -CTP-labeled riboprobes complementary to c-fos (680-mer, linearized from c-fos plasmid donated by Dr. T. Curran, St. Jude Children’s Research Hospital,Memphis, TN). Labeled sections were then exposed to x-ray film (Kodak Biomax-MR; Eastman Kodak, Rochester, NY) for 3-4 days. Autoradiographs of brain sections were captured digitally and one section per rat was analyzed using NIH Image (version 1.61 for Macintosh computer). c-fos mRNA density was determined in a region which included both the medial caudate-putamen and the nucleus accumbens, (approximately 1.0 mm AP relative to bregma) as illustrated in Fig. 1. This region was selected for analysis because it corresponds to the area covered by the microdialysis probe in the dopamine study described below. A background value was obtained from the corpus callosum in each section, and only pixels with a mean gray value exceeding the background value by 3.5 SD were included in the analysis (see Samaha et al., 2004 for detailed methods).
The influence of rate of cocaine delivery on the extracellular levels of glutamate or dopamine was assessed in two independent experiments using microdialysis coupled to CE-LIF. Microdialysate samples were derivatized “on-line” and then electrokinetically injected into a separation capillary (16 cm long, 10 μm inner and 360 μm outer diameter). Fluorescence was then excited using a 413 nm line 14 mW CL-2000 diode pumped crystal laser (CrystaLaser, Reno, NV). The emitted fluorescence was collected by the objective of an Axioskop 20 fluorescence microscope (Carl Zeiss, Hanover, MD, USA) and filtered. Electropherograms were collected and analyzed using in-house software (Shackman et al. 2004) written in LabVIEW (National Instruments, Austin, TX, USA). Conditions for dopamine and glutamate derivatization are not identical and detailed methods can be found in Bowser and Kennedy (2001) and Shout et al. (2006).
In the “glutamate experiment” we were able to obtain a microdialysis sample every 12 seconds. In the “dopamine experiment” the fastest sampling rate we achieved was one sample every 90 seconds (see Figure 4 and Shou et al., 2006 for sample electropherograms of dopamine separation). Dopamine data was obtained from 7 rats and glutamate data was obtained from 6 rats. In both experiments a catheter was secured in the right jugular vein and a unilateral guide cannula was placed above the striatum of all animals approximately 7 days prior to testing, using procedures described previously (Weeks, 1972; Venton et al., 2006a).
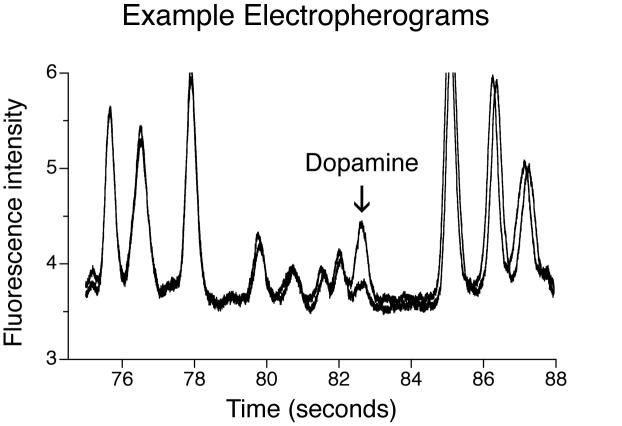
Figure 4.
Overlaid electropherograms taken before (lower trace) and after (upper trace) a single intravenous cocaine infusion (2.0 mg/kg). Microdialysis samples were taken and analyzed on line every 90 seconds. Each peak in the above traces corresponds to a compound in the sample, and the arrow indicates the peak corresponding to dopamine. The increase in the height of the dopamine peak (increase in intensity of fluorescence) indicates an increase in extracellular levels of the transmitter. Standards of known dopamine concentration were used to determine the average basal dopamine concentration in the sample: 18.1 ± 1.53 nM (n = 12, Mean ± S.E.M). For complete methods see (Bowser and Kennedy, 2001; Shou et al., 2006).
Coordinates for guide cannula placement for dopamine probes were 1mm posterior and 2 mm lateral to bregma, and 4 mm ventral to dura, whereas coordinates for glutamate probe cannula placements were -0.3 mm posterior and 2.5 mm lateral to bregma, and 3 mm ventral to bregma. Previous work using an infusion procedure and a glutamate detection method identical to those of the current study found that cocaine did not alter extracellular glutamate levels in rostral regions of the striatum containing portions of the nucleus accumbens and caudate-putamen (Venton et al., 2006). Therefore, in the current study, we chose to examine a more caudal portion of the striatum comprised of caudate-putamen. Infusion-rate dependent alterations in extracellular dopamine have not previously been measured in any striatal regions. Given that dopamine-mediated neuroadaptations in both the nucleus accumbens and caudate-putamen play an important role in mediating the long term behavioral effects of repeated drug exposure, we chose to measure dopamine in a rostral portion of the striatum that contains portions of both the nucleus accumbens and caudate putamen.
For the “dopamine experiment” a microdialysis probe was inserted through the guide cannula the evening prior to testing, and for the “glutamate experiment” the probe was inserted 1 hour and 20 minutes prior to testing (Venton et al., 2006a). In both experiments microdialysis samples were first obtained over a 20 min period while the animals were undisturbed in order to determine the basal levels of dopamine or glutamate. The animals then received an intravenous infusion of saline (10 μl given over 5 or 100 sec) and sampling continued for an additional 20 min. Following this, each animal received two intravenous infusions of cocaine (2.0 mg/kg/infusion in 10 μl of saline) given 1 hour apart. One infusion of cocaine was delivered over 5 seconds and the other over 100 seconds, and the order was counterbalanced such that half of the rats received a 5 second infusion first, whereas the other half received a 100 second infusion first.
Acknowledgements
We would like to thank Dr. B.J. Venton & MEJ Newman for helpful comments. Supported by grants from NIDA (R37 DA04294) to T.E.R. and NIBIB (R01 EB003320) to R.T.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berretta S, Robertson HA, Graybiel AM. Dopamine and glutamate agonists stimulate neuron-specific expression of Fos-like protein in the striatum. J Neurophysiol. 1992;68:767–777. doi: 10.1152/jn.1992.68.3.767. [DOI] [PubMed] [Google Scholar]
- Bowser MT, Kennedy RT. In vivo monitoring of amine neurotransmitters using microdialysis with on-line capillary electrophoresis. Electrophoresis. 2001;22:3668–3676. doi: 10.1002/1522-2683(200109)22:17<3668::AID-ELPS3668>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Fosnaugh JS, Bhat RV, Yamagata K, Worley PF, Baraban JM. Activation of arc, a putative “effector” immediate early gene, by cocaine in rat brain. J Neurochem. 1995;64:2377–2380. doi: 10.1046/j.1471-4159.1995.64052377.x. [DOI] [PubMed] [Google Scholar]
- Greenstein YJ, Pavlides C, Winson J. Long-term potentiation in the dentate gyrus is preferentially induced at theta rhythm periodicity. Brain Res. 1988;438:331–334. doi: 10.1016/0006-8993(88)91358-3. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:605. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Konradi C, Leveque JC, Hyman SE. Amphetamine and dopamine-induced immediate early gene expression in striatal neurons depends on postsynaptic NMDA receptors and calcium. J Neurosci. 1996;16:4231–4239. doi: 10.1523/JNEUROSCI.16-13-04231.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986;368:347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- Liu Y, Roberts DC, Morgan D. Sensitization of the reinforcing effects of self-administered cocaine in rats: effects of dose and intravenous injection speed. Eur J Neurosci. 2005;22:195–200. doi: 10.1111/j.1460-9568.2005.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: From structure to function. Pharm Revs. 1998;78:189–226. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular neurobiology of addiction. Am J Addict. 2001;10:201–217. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- Pan HT, Menacherry S, Justice JB., Jr. Differences in the pharmacokinetics of cocaine in naive and cocaine-experienced rats. J Neurochem. 1991;56:1299–1306. doi: 10.1111/j.1471-4159.1991.tb11425.x. [DOI] [PubMed] [Google Scholar]
- Paul ML, Graybiel AM, David JF, Robertson HA. D1-like and D2-like dopamine receptors synergistically activate rotation and c-fos expression in the dopamine depleted striatum in a rat model of Parkinson’s disease. J. Neurosci. 1992;12:3729–3742. doi: 10.1523/JNEUROSCI.12-10-03729.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J. Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Reid MS, Hsu K, Jr., Berger SP. Cocaine and amphetamine preferentially stimulate glutamate release in the limbic system: studies on the involvement of dopamine. Synapse. 1997;27:95–105. doi: 10.1002/(SICI)1098-2396(199710)27:2<95::AID-SYN1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Cone EJ, Kuhar MJ. Cocaine inhibition of ligand binding at dopamine, norepinephrine and serotonin transporters: a structure-activity study. Life Sci. 1990;46:635–645. doi: 10.1016/0024-3205(90)90132-b. [DOI] [PubMed] [Google Scholar]
- Robinson SE, Kunko PM, Smith JA, Wallace MJ, Mo Q, Maher JR. Extracellular aspartate concentration increases in nucleus accumbens after cocaine sensitization. Eur J Pharmacol. 1997;319:31–36. doi: 10.1016/s0014-2999(96)00923-5. [DOI] [PubMed] [Google Scholar]
- Rouillard C, Bovetto S, Gervais J, Richard D. Fenfluramine-induced activation of the immediate-early gene c-fos in the striatum: possible interaction between serotonin and dopamine. Brain Res Mol Brain Res. 1996;37:105–115. doi: 10.1016/0169-328x(95)00284-y. [DOI] [PubMed] [Google Scholar]
- Samaha AN, Robinson TE. Why does the rapid delivery of drugs to the brain promote addiction? Trends Pharmacol Sci. 2005;26:82–87. doi: 10.1016/j.tips.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Samaha AN, Li Y, Robinson TE. The rate of intravenous cocaine administration determines susceptibility to sensitization. J Neurosci. 2002;22:3244–3250. doi: 10.1523/JNEUROSCI.22-08-03244.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha AN, Yau WY, Yang P, Robinson TE. Rapid delivery of nicotine promotes behavioral sensitization and alters its neurobiological impact. Biol Psychiatry. 2005;57:351–360. doi: 10.1016/j.biopsych.2004.11.040. [DOI] [PubMed] [Google Scholar]
- Samaha AN, Mallet N, Ferguson SM, Gonon F, Robinson TE. The rate of cocaine administration alters gene regulation and behavioral plasticity: implications for addiction. J Neurosci. 2004;24:6362–6370. doi: 10.1523/JNEUROSCI.1205-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scruggs JL, Patel S, Bubser M, Deutch AY. DOI-Induced activation of the cortex: dependence on 5-HT2A heteroceptors on thalamocortical glutamatergic neurons. J Neurosci. 2000;20:8846–8852. doi: 10.1523/JNEUROSCI.20-23-08846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou M, Ferrario CR, Schultz KN, Robinson TE, Kennedy RT. Monitoring Dopamine in Vivo by Microdialysis Sampling and On-Line CE-Laser-Induced Fluorescence. Anal Chem. 2006;78:6717–6725. doi: 10.1021/ac0608218. [DOI] [PubMed] [Google Scholar]
- Stephans SE, Yamamoto BK. Methamphetamine-induced neurotoxicity: roles for glutamate and dopamine efflux. Synapse. 1994;17:203–209. doi: 10.1002/syn.890170310. [DOI] [PubMed] [Google Scholar]
- Torres G, Rivier C. Cocaine-induced expression of striatal c-fos in the rat is inhibited by NMDA receptor antagonists. Brain Res Bull. 1993;30:173–176. doi: 10.1016/0361-9230(93)90055-g. [DOI] [PubMed] [Google Scholar]
- Tsukada M, Aihara T, Mizuno M, Kato H, Ito K. Temporal pattern sensitivity of long-term potentiation in hippocampal CA1 neurons. Biol Cybern. 1994;70:495–503. doi: 10.1007/BF00198802. [DOI] [PubMed] [Google Scholar]
- Venton BJ, Robinson TE, Kennedy RT. Transient changes in nucleus accumbens amino acid concentrations correlate with individual responsivity to the predator fox odor 2,5-dihydro-2,4,5-trimethylthiazoline. J Neurochem. 2006a;96:236–246. doi: 10.1111/j.1471-4159.2005.03549.x. [DOI] [PubMed] [Google Scholar]
- Venton BJ, Robinson TE, Kennedy RT, Maren S. Dynamic amino acid increases in the basolateral amygdala during acquisition and expression of conditioned fear. Eur J Neurosci. 2006b;23:3391–3398. doi: 10.1111/j.1460-9568.2006.04841.x. [DOI] [PubMed] [Google Scholar]
- Weeks J. Long-term intravenous infusions. Methods in psychobiology. 1972;2:155–168. [Google Scholar]
- Wirtshafter D. Role of dopamine D1 receptors in the striatal and cortical fos expression induced by the muscarinic agonist pilocarpine. Eur J Pharmacol. 2004;488:85–90. doi: 10.1016/j.ejphar.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Young ST, Porrino LJ, Iadarola MJ. Cocaine induces striatal c-fos-immunoreactive proteins via dopaminergic D1 receptors. Proc Natl Acad Sci U S A. 1991;88:1291–1295. doi: 10.1073/pnas.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang L, Jiao H, Zhang Q, Zhang D, Lou D, Katz JL, Xu M. c-Fos facilitates the acquisition and extinction of cocaine-induced persistent changes. J Neurosci. 2006;51:13287–96. doi: 10.1523/JNEUROSCI.3795-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Loonam TM, Noailles PA, Angulo JA. Comparison of cocaine- and methamphetamine-evoked dopamine and glutamate overflow in somatodendritic and terminal field regions of the rat brain during acute, chronic, and early withdrawal conditions. Ann N Y Acad Sci. 2001;937:93–120. doi: 10.1111/j.1749-6632.2001.tb03560.x. [DOI] [PubMed] [Google Scholar]