Abstract
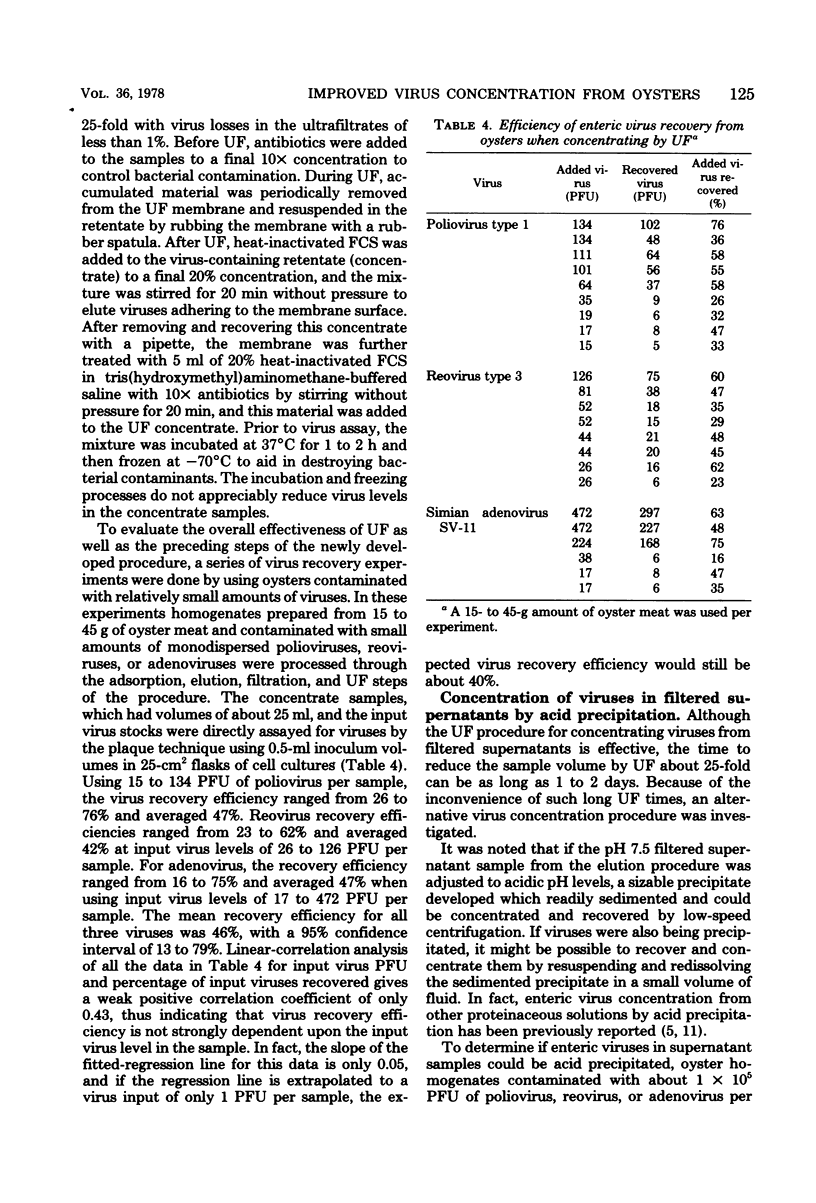
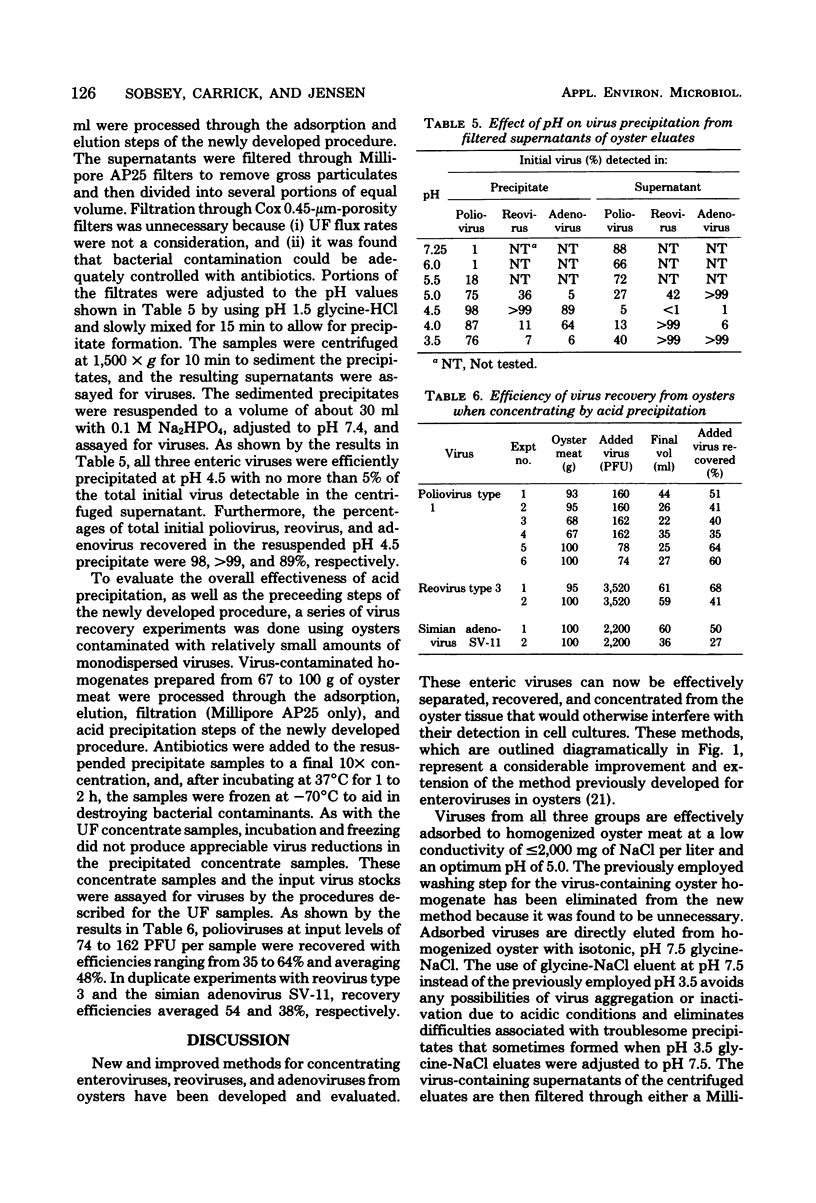
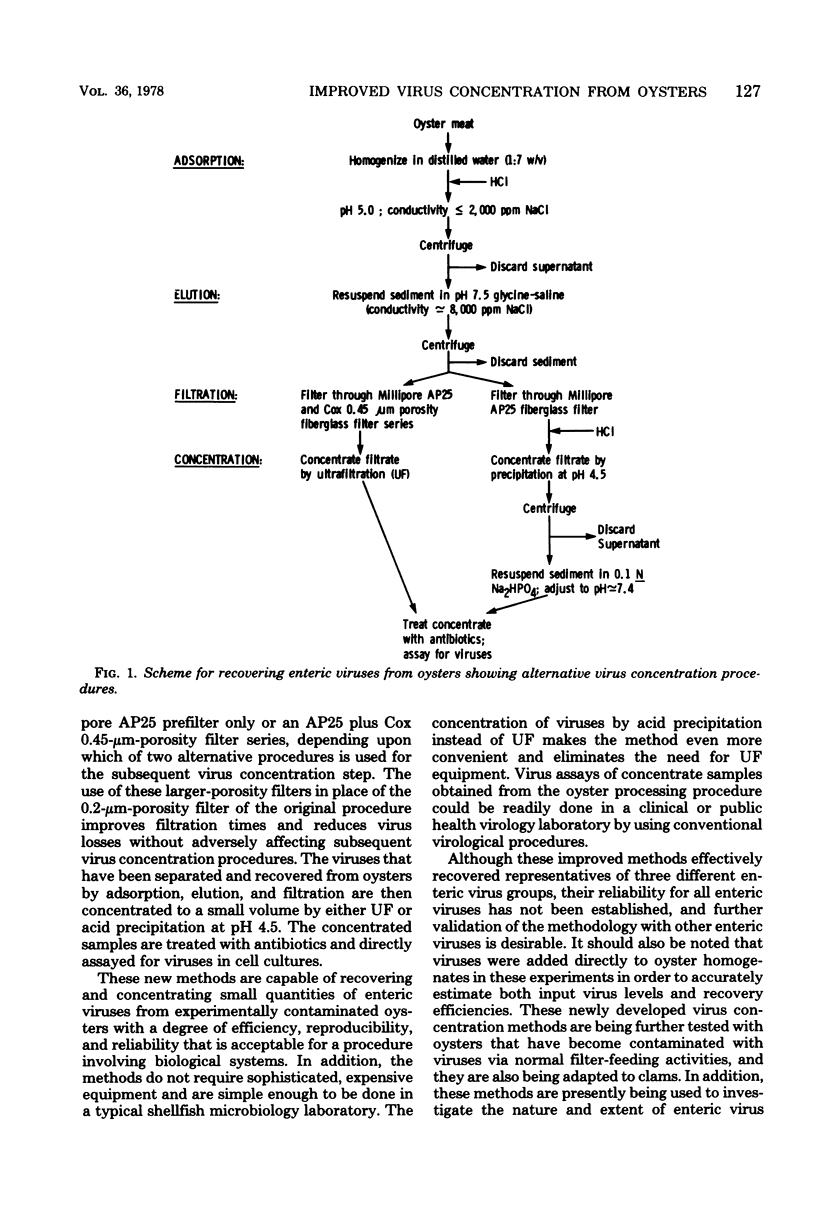
New and improved methods for concentrating enteroviruses, reoviruses, and adenoviruses from oysters have been developed and evaluated. Viruses are efficiently adsorbed to homogenized oyster meat by adjusting the homogenate to pH 5.0 and a conductivity of less than or equal to 2,000 mg of NaCl per liter. After low-speed centrifugation, the virus-free supernatant is discarded and the viruses are eluted from the sedimented oyster solids with pH 7.5 glycine-NaCl having a conductivity of 8,000 mg of NaCl per liter. The oyster solids are removed by low-speed centrifugation and filtration, and the viruses in the filtered supernatant are concentrated to a small volume by either ultrafiltration or acid precipitation at pH 4.5. The concentrate is treated with antibiotics and inoculated into cell cultures for virus isolation and quantitation. When these methods were tested with oysters experimentally contaminated with polioviruses, reoviruses, and adenoviruses, recovery efficiencies averaged about 46%. With the exception of virus assay and quantitation, these methods are simple and inexpensive enough to be done in typical shellfish microbiology laboratories.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleton H., Pereira M. S. A possible virus aetiology in outbreaks of food-poisoning from cockles. Lancet. 1977 Apr 9;1(8015):780–781. doi: 10.1016/s0140-6736(77)92960-9. [DOI] [PubMed] [Google Scholar]
- Cliver D. O. Food-associated viruses. Health Lab Sci. 1967 Oct;4(4):213–221. [PubMed] [Google Scholar]
- Dienstag J. L., Gust I. D., Lucas C. R., Wong D. C., Purcell R. H. Mussel-associated viral hepatitis, type A: serological confirmation. Lancet. 1976 Mar 13;1(7959):561–563. doi: 10.1016/s0140-6736(76)90358-5. [DOI] [PubMed] [Google Scholar]
- England B. Concentration of reovirus and adenovirus from sewage and effluents by protamine sulfate (salmine) treatment. Appl Microbiol. 1972 Sep;24(3):510–512. doi: 10.1128/am.24.3.510-512.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R., Sharp D. G. Aggregation of poliovirus and reovirus by dilution in water. Appl Environ Microbiol. 1977 Jan;33(1):159–167. doi: 10.1128/aem.33.1.159-167.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblet F. E., Hill W. F., Jr, Akin E. W., Benton W. H. Oysters and human viruses: effect of seawater turbidity on poliovirus uptake and elimination. Am J Epidemiol. 1969 May;89(5):562–571. doi: 10.1093/oxfordjournals.aje.a120969. [DOI] [PubMed] [Google Scholar]
- Herrmann J. E., Cliver D. O. Methods for detecting food-borne enteroviruses. Appl Microbiol. 1968 Oct;16(10):1564–1569. doi: 10.1128/am.16.10.1564-1569.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenelson E., Fattal B., Hostovesky T. Organic flocculation: an efficient second-step concentration method for the detection of viruses in tap water. Appl Environ Microbiol. 1976 Oct;32(4):638–639. doi: 10.1128/aem.32.4.638-639.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff R. S., Grady G. F., Chalmers T. C., Mosley J. W., Swartz B. L. Viral hepatitis in a group of Boston hospitals. 3. Importance of exposure to shellfish in a nonepidemic period. N Engl J Med. 1967 Mar 30;276(13):703–710. doi: 10.1056/NEJM196703302761301. [DOI] [PubMed] [Google Scholar]
- Konowalchuk J., Speirs J. I. Enterovirus recovery from laboratory-contaminated samples of shellfish. Can J Microbiol. 1972 Jul;18(7):1023–1029. doi: 10.1139/m72-159. [DOI] [PubMed] [Google Scholar]
- Kostenbader K. D., Jr, Cliver D. O. Polyelectrolyte flocculation as an aid to recovery of enteroviruses from oysters. Appl Microbiol. 1972 Oct;24(4):540–543. doi: 10.1128/am.24.4.540-543.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASON J. O., McLEAN W. R. Infectious hepatitis traced to the consumption of raw oysters. An epidemiologic study. Am J Hyg. 1962 Jan;75:90–111. doi: 10.1093/oxfordjournals.aje.a120238. [DOI] [PubMed] [Google Scholar]
- METCALF T. G., STILES W. C. THE ACCUMULATION OF ENTERIC VIRUSES BY THE OYSTER, CRASSOSTREA VIRGINICA. J Infect Dis. 1965 Feb;115:68–76. doi: 10.1093/infdis/115.1.68. [DOI] [PubMed] [Google Scholar]
- Mitchell J. R., Presnell M. W., Akin E. W., Cummins J. M., Liu O. C. Accumulation and elimination of poliovirus by the eastern oyster. Am J Epidemiol. 1966 Jul;84(1):40–50. doi: 10.1093/oxfordjournals.aje.a120626. [DOI] [PubMed] [Google Scholar]
- Portnoy B. L., Mackowiak P. A., Caraway C. T., Walker J. A., McKinley T. W., Klein C. A., Jr Oyster-associated hepatitis. Failure of shellfish certification programs to prevent outbreaks. JAMA. 1975 Sep 8;233(10):1065–1068. doi: 10.1001/jama.233.10.1065. [DOI] [PubMed] [Google Scholar]
- Sharp D. G., Floyd R., Johnson J. D. Initial fast reaction of bromine on reovirus in turbulent flowing water. Appl Environ Microbiol. 1976 Feb;31(2):173–181. doi: 10.1128/aem.31.2.173-181.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey M. D., Wallis C., Melnick J. L. Development of a simple method for concentrating enteroviruses from oysters. Appl Microbiol. 1975 Jan;29(1):21–26. doi: 10.1128/am.29.1.21-26.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


