Abstract
In the kitten, as little as a week of monocular lid suture during early life causes a remarkable remodeling of the geniculocortical projections serving the deprived eye (Antonini & Stryker, 1993a, 1996). While the physiological effects of monocular deprivation have been shown to be due to competitive interactions between the projections serving the two eyes, it is not known whether these morphological changes are due to competitive interactions or to sensory disuse. We addressed this question by analyzing the morphology of geniculocortical arbors in kittens deprived of patterned vision by binocular lid suture for 1 week or 2 weeks ending at 6 weeks of age. Such deprivation would be expected to affect the afferents serving the two eyes equally, giving neither eye a competitive advantage. The arbors were anterogradely filled with Phaseolus lectin iontophoretically injected into lamina A of the lateral geniculate nucleus. The lectin was visualized immunohistochemically, and single geniculocortical arbors were serially reconstructed in three dimensions. Arbors reconstructed in binocularly deprived animals were compared with arbors serving the deprived and nondeprived eye in animals monocularly deprived by lid suture of one eye for a week and with arbors obtained in age-matched normal controls. Geniculocortical arbors in binocularly deprived animals did not suffer the drastic remodeling of the deprived arbors in monocularly deprived animals. Indeed, arbors in binocularly deprived animals were indistinguishable from arbors in normal kittens or nondeprived arbors in short-term monocularly deprived animals. These results support the notion that competitive mechanisms rather than sensory disuse are responsible for gross morphological remodeling of geniculocortical arbors.
Keywords: Lid suture, PHA-L, Visual cortex, Geniculocortical projections, Competitive interactions
Introduction
In mammals, during a critical period in early life, an imbalance of the visual information reaching the two eyes leads to dramatic anatomical and functional changes in the geniculocortical pathway. An example of incongruous activity between the two retinae is the classic experimental paradigm of monocular deprivation (MD—Hubel & Wiesel, 1970), in which the closed eye is deprived of patterned vision and receives no more than 10% of the amount of light that reaches the nondeprived eye (Crawford & Marc, 1976). Physiologically, MD is accompanied by a shift of cortical cell visual responses in favor of the open, nondeprived eye. Anatomically, monocular deprivation causes an overall reduction of the territory in layer IV innervated by the deprived geniculocortical terminals (Shatz & Stryker, 1978; LeVay et al., 1980), compared to normal conditions where layer IV is nearly equally shared by the geniculocortical afferents serving each eye. The contraction of the terminal field of the deprived pathway is also evident at a finer level, at which single geniculocortical afferents serving the deprived eye undergo a striking remodeling (Friedlander et al., 1991; Antonini & Stryker, 1993a, 1996). Indeed, previous work from our laboratory has shown that, in the cat, as little as a week of MD during the critical period causes a loss of about half of the total length and number of branch bifurcations of single geniculocortical arbors, as well as a reduction in arbor density (Antonini & Stryker, 1993a, 1996). Concurrent with the withdrawal of the deprived arbor terminals, cells in the laminae of the lateral geniculate nucleus (LGN) innervated by the deprived retina are reduced in size (Wiesel & Hubel, 1963; Guillery, 1972, 1973; Sherman et al., 1972) and show a lower level of cytochrome oxidase stain, indicating a decrease in neuronal activity (Wong-Riley, 1979; Kageyama & Wong-Riley, 1986).
The plastic phenomena produced by MD are thought to result from competitive interactions among the afferents serving the two eyes (Wiesel & Hubel, 1965). According to this view, neuronal connections are shaped and maintained by a mechanism of synapse consolidation relying on the coincidence of activity between the presynaptic pathway and the postsynaptic target cell (Miller et al., 1989; for a review: Rauschecker, 1991). If the deprived connections are less efficient than the nondeprived ones in driving cortical neurons, then their synapses might become destabilized and eliminated, while the nondeprived synapses become consolidated. Eventually, this process would lead to the retraction of synapse-bearing branches of the deprived arbors.
In support of this hypothesis is the evidence that in MD animals, shrinkage of LGN cells in the deprived laminae is restricted to the binocular portions of the nucleus, where interocular competition is present (Guillery & Stelzner, 1970; Guillery & Kaas, 1974; Sherman & Wilson, 1975; LeVay & Ferster, 1977). Indeed, the effects of MD were absent at sites where these binocular interactions were experimentally prevented (Guillery, 1972; Wilson & Sherman, 1977; Kageyama & Wong-Riley, 1986).
However, the simple reduction of visual stimulation per se, i.e. sensory disuse, could also, in principle, result in the activity-dependent elimination of synapses and withdrawal of terminals in the deprived pathway, independent of competitive interactions with the nondeprived pathway. Indeed, in monkeys (Von Noorden, 1973; Von Noorden & Middleditch, 1975; Von Noorden et al., 1976), but not in cats (Guillery, 1972; Guillery & Stelzner, 1970) and squirrels (Guillery & Kaas, 1974), MD has been reported to cause a substantial reduction of cell size in the monocular segment of the geniculate.
We have tested these two alternative hypotheses on the effects of deprivation, competition versus disuse, by analyzing the morphology of single geniculocortical arbors, serially reconstructed in binocularly deprived animals. Binocular deprivation (BD) reduces the visual input through the two eyes in an equivalent manner while eliminating any competitive differences between the pathways serving the two eyes as with MD. Arbors in BD animals, influenced solely by sensory disuse, are compared with arbors reconstructed in normal animals (Antonini & Stryker, 1993b) and with arbors serving the deprived eye in MD animals. These latter arbors, remodeled by disuse and/or by an unbalanced competition with the nondeprived arbors, have been the object of a previous study (Antonini & Stryker, 1993a, 1996).
Some of these results have been published as an abstract (Antonini & Stryker, 1995).
Materials and methods
Five kittens were used for this study. All were born in the University of California San Francisco cat colony and were kept with the mother throughout the experiment. Two animals (MUC 921 and MUC 956) were binocularly deprived for 1 week prior to sacrifice at 39/40 days of age (postnatal day 39/40, P39/40); two other animals (MUC 928 and MUC 957) were deprived for 2 weeks before P39/40. One kitten (K6) was raised with a normal visual experience and used as an age-matched control.
Binocular deprivation
In four animals, both eyes were closed by suturing the trimmed margins of the superior and inferior lids with sterile surgical #4 Vycril. The procedure was performed under halothane anesthesia (1−2%) in N2O/O2 (1:1). Topical antibiotic ointment was applied to the eye before suturing the eyelids. A small opening on the nasal end of the eyelid allowed lachrymal drainage. Antibiotics were administered systemically twice a day for 3 days, and the animals were checked once or twice a day to ensure that no openings along the eyelids were formed.
PHA-L injections
The anterograde tracer Phaseolus lectin, PHA-L (Vector Labs., Burlingame, CA; 2.5% solution in 0.1 M sodium phosphate buffered saline, pH 8; Gerfen & Sawchenko, 1984), was injected into the LGN in kittens between the ages of P25 and P28, in all cases prior to the deprivation in BD animals. The tracer was iontophoretically delivered for 4−5 min through a glass pipette (10−15 μm tip diameter) using a high-voltage current source device (Stoelting Midgard, Wood Dale, IL) with a positive current (8 μA, 7-s pulses), at LGN sites previously identified with a tungsten recording electrode. Usually, 2−4 lectin injections were made in one or both LGNs. Most of the PHA-L injections were confined to lamina A of the nucleus; thus, presumably, the vast majority of arbors reconstructed belong to the projection serving the contralateral eye.
The injection procedure has been described in detail in previous works (Antonini & Stryker, 1993b, 1996) and will be briefly summarized here. Atropine sulfate (0.4 mg/kg, sc.) and Dexamethasone (1 mg/kg, sc.) were also administered prior to the general anesthesia. The animal was initially anesthetized with a mixture of ketamine hydrochloride (0.2 mg/kg) and acepromazine (0.02 mg/kg). Anesthesia was maintained with halothane (1−2%) in N2O/O2 (1:1) throughout the experiment. The heart and respiration were continuously monitored. Pupils were dilated with topical atropine sulfate (2%) and the nictitating membranes were retracted with phenylephrine hydrochloride (10%). The corneae were protected with contact lenses. Tungsten microelectrode penetrations were made between the Horsley-Clarke stereotaxic coordinates AP 3.0−5.0 and ML 7.0−8.5. Once a clear response from lamina A was obtained, the microelectrode was withdrawn and substituted with a PHA-L filled glass pipette. The lectin was iontophoretically injected at the stereotaxic coordinates previously identified by the metal recording electrode, with verification of the depth by recording visual responses through the glass pipette. Not all current injections resulted in the delivery of PHA-L at the injection site. However, in the cases in which the deposit of lectin occurred, it always produced anterograde labeling in the visual cortex.
Perfusion and immunohistochemistry
At P39/40, the animals were sacrificed with an overdose of Nembutal (60 mg/kg) and perfused transcardially with, in succession, 300−500 ml of ice-cold 0.1 M phosphate buffer; 1000 ml of 4% paraformaldehyde + 0.5% glutaraldehyde in 0.1 M phosphate buffer; 1000 ml of 4% paraformaldehyde + 3.42 g/L L-Lysine (Sigma Chemical Co., St. Louis, MO) + 0.55 g/L sodium meta-periodate (Sigma Chemical Co., St. Louis, MO) in 0.1 M phosphate buffer. All solutions had a pH of 7.4. The brain was removed and post-fixed overnight. Blocks containing the LGN and the entire caudal pole of each hemisphere where the visual cortex is located were embedded in gelatin-albumin and cut (80 μm) at the vibratome in the frontal plane. All sections were collected in ice-cold potassium buffered saline (KPBS, pH 7.4) and processed for standard indirect immunohistochemistry as previously described (Antonini & Stryker, 1993b).
Axonal reconstructions
A total of 18 complete geniculocortical arbors were reconstructed: nine in animals deprived for 1 week and nine in animals deprived for 2 weeks. Another four arbors were reconstructed in one normal P40 kitten, adding to the sample published previously. PHA-L-filled arbors were reconstructed in three dimensions at 1000× from serial sections with the aid of a drawing tube and a computer graphics system, the Neurotrace system (Passera et al., 1988). Axonal reconstructions are shown in two views: in the coronal plane, as the arbors are reconstructed, and after rotation about the ventro-dorsal axis, providing in this case a view tangential to the pial surface.
For each arbor we made several measurements:
The total linear length of the arborization obtained by the addition of the lengths of all the branches constituting the terminal field of the arbor. Only the portion of the arbor located in layer IV and the adjacent part of layer III was considered for this analysis; the axonal trunk, and its bifurcations, were thus clipped just below layer IV. The position at which the axonal trunk has been clipped is shown as a gap in some of the arbors illustrated in Figs. 1 and 2.
The number of branch points of the terminal arborization clipped as described above.
The density of the terminal arborization in layer IV evaluated from the two-dimensional pial view of the arbor. The terminal arborization was considered to be compressed along an axis perpendicular to the pial surface and to lie in a single plane. The density at each 5 μm × 5 μm square within the territory covered by the arbor was calculated by summing the total lengths of the portions of all branches that lay within the area enclosed by a circle of 100-μm diameter. Both the maximal density and the mean density of the terminal arborization (expressed in μm/1000 μm2) were evaluated. In earlier publications, the clustering of terminal arbors in normal animals was characterized by a threshold density of 38 μm/1000 μm2. This density was used to define “standard patches” measured in arbors from binocularly deprived animals. The coverage area of each arbor was also calculated from the compressed pial view of the terminal arborization as the area over which the arbor had a minimum threshold density of >2 μm/1000 μm2.
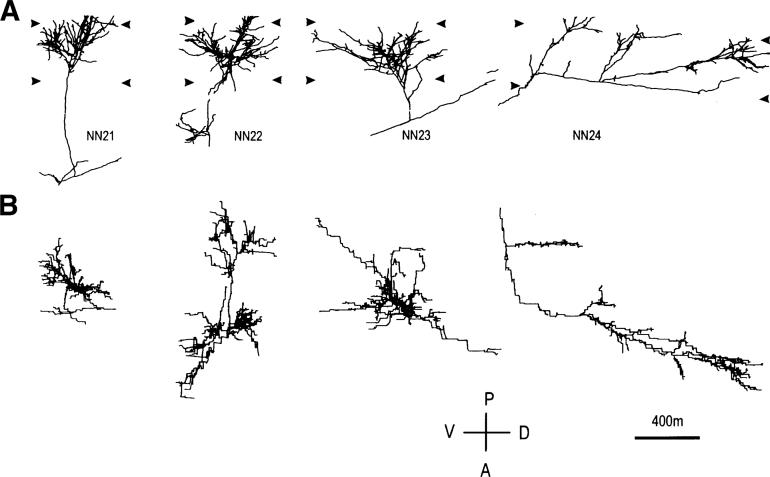
Fig. 1.
Computer reconstructions of PHA-L immunostained axonal arbors in area 17 in a normal P40 animal. Row A shows the arbors as originally reconstructed in the coronal plane, and row B shows the arbors from a surface view after a 90-deg rotation along the dorsoventral axis. The total length of the terminal arborization in layer IV has been computed distally to the small gap in the main axonal trunk, well visible in arbor NN22 (see Materials and methods). The arrowheads indicate the boundaries of the layer IV. VD and AP: ventrodorsal and anteroposterior direction along the lateral gyrus.
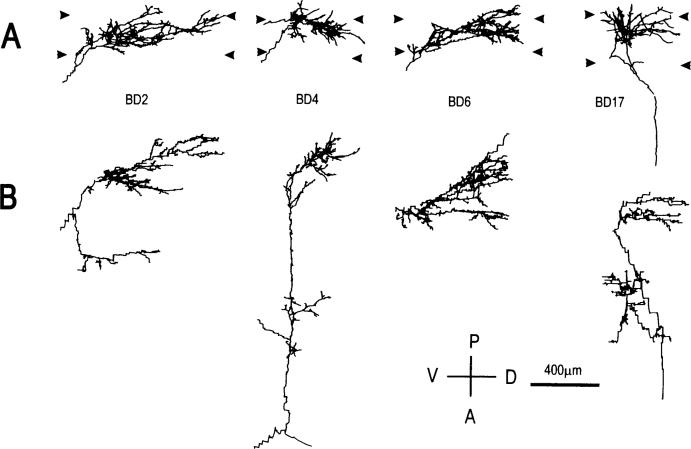
Fig. 2.
Computer reconstructions of PHA-L immunostained geniculocortical arbors reconstructed in animals binocularly deprived for 1 week. Arbors are shown in coronal view (A), and in surface view (B). Abbreviations and symbols are as in Fig. 1.
For all features measured, evaluation of the differences among groups has been obtained by comparing groups two at a time, using the Mann-Whitney U-test for nonparametric statistical analyses.
Results
Injection sites in the LGN
Injection sites were located in the central portion of the LGN, well within the binocular zone of the nucleus. Since all the injection sites were restricted to lamina A and only a few showed a slight involvement of lamina A1, it is reasonable to conclude that the vast majority of the reconstructed arbors belong to the geniculocortical projection serving the contralateral eye. This consideration is relevant in that geniculocortical arbors in BD animals will be compared with arbors serving the contralateral eye in MD animals (Antonini & Stryker, 1993a, 1996).
Morphology of geniculocortical arbors
All reconstructions were made from area 17, within the central portion of the medial bank of the lateral gyrus. This zone, distant from the cortical representation of the monocular segment of the visual field, receives afferents from both lamina A and A1 of the LGN and is the site of functional binocular interaction (Hubel &Wiesel, 1970; Shatz & Stryker, 1978). The terminal arborization in layer IV was well immunostained, and we are confident that the reconstructions from layer IV are complete. We did not analyze the layer VI ramifications of geniculocortical arbors due to inconsistent labeling, and the myelination obscured the paths of axons through the white matter preventing determination of whether the axonal trunks bifurcated there. For these reasons, we refer to our reconstructions as arbors in layer IV, and not as complete geniculocortical axons. Geniculocortical arbors were reconstructed from regions in which the overall axonal labeling in cortex was generally sparse (like that illustrated in Figs. 2 and 3 of Antonini & Stryker, 1993b), and, in cases, at the periphery of areas of dense labeling (like Fig. 9 of Antonini & Stryker, 1993b).
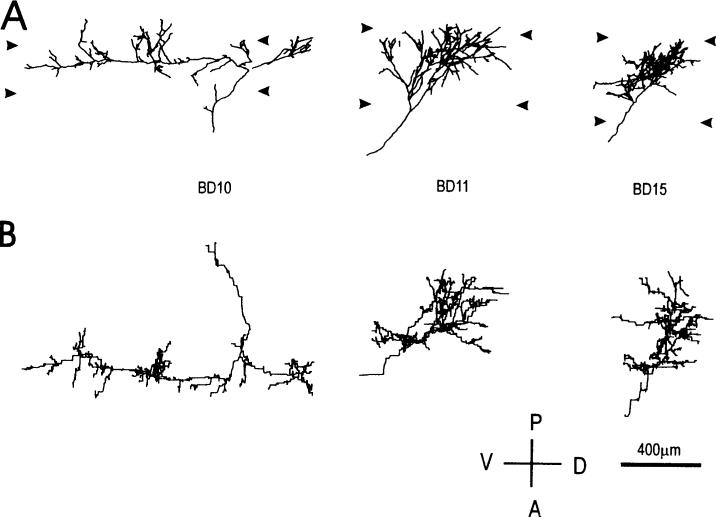
Fig. 3.
Computer reconstructions of PHA-L immunostained geniculocortical arbors reconstructed in animals binocularly deprived for 2 weeks. Arbors are shown in coronal view (A), and in surface view (B). Abbreviations and symbols are as in Fig. 1.
The general morphology of the terminal arborization in layer IV will be described for geniculocortical arbors reconstructed in animals binocularly deprived for 1 and 2 weeks and for a sample of arbors obtained in normal animals at P40. Additional arbors reconstructed in normal P40 animals have been described in a previous paper (Antonini & Stryker, 1993b).
Normal animal
Four arbors were reconstructed from one normal P40 kitten. They are shown in Fig. 1, in both coronal and pial views. The axon terminals covered mainly the upper portion of layer IV, straddling the layer III/IV border, suggesting that the arbors were of the Y-type. In pial view, the rich arborization revealed one (NN21, NN23) or two dense patches (NN22) of collaterals. NN24 was distinct from the other three arbors in that the terminal arborization was rather scanty and extended for more than 1.5 mm along the ventro-dorsal axis.
BD animals
The two populations of geniculocortical afferents reconstructed after 1 or 2 weeks of BD were qualitatively similar. Both groups presented the same degree of variability observed in normal animals, with the majority of arbors characterized by a rich and complex terminal field. Four arbors reconstructed in animals deprived for 1 week are shown in Fig. 2. From the localization of their terminals in the upper tier of layer IV, the majority of the arbors (6 out of 9) are likely to be of the Y-type (Humphrey et al., 1985). The remaining three arbors (BD6—Fig. 2, BD19 and BD26—not shown) ramified within the entire layer IV and were presumably X-type (Humphrey et al., 1985). As with arbors reconstructed in normal animals, BD arbors presented one or multiple clusters of terminals.
Three of the nine arbors obtained in animals deprived for 2 weeks are shown in Fig. 3. All but three arbors (BD11—Fig. 3, BD13, BD16—not shown) ramified in the upper portion of layer IV. Note the similarity in the innervation pattern between arbors BD10 and arbors NN24, reconstructed in normal controls (Fig. 1).
Quantitative analysis of geniculocortical arbors
As indicated in the Materials and methods section, several parameters have been chosen to quantify the terminal arborization in layer IV. These parameters have been selected to give a satisfactory estimate of the size of each geniculocortical arbor and its degree of complexity. Comparisons of the quantitative characteristics of arbors in BD animals are made with three other populations of geniculocortical arbors that have been described in previous publications (Antonini & Stryker, 1993a,b, 1996). The four arbors reconstructed at P40 described here, plus three more arbors previously obtained in kittens at the same age, are used as age-matched controls. To analyze whether BD results in an arrest of axonal growth, BD arbors at P40 will be compared with arbors from normal animals at P30/31, near the age at which 1 week BD was initiated. Finally, to examine the influence of competition and sensory disuse, BD arbors will be compared to arbors serving the nondeprived (ND) and the deprived (D) eye in animals subjected to 1 week of MD (Antonini & Stryker, 1993b, 1996). The qualitative and quantitative characteristics of this latter population of arbors show the dramatic plastic remodeling that can occur in only a week of MD. For all the parameters quantified, no differences were found between animals binocularly deprived for 1 or 2 weeks. Consequently, for statistical purposes the results obtained from these two groups of animals have been pooled. Statistical comparisons (see Materials and methods) were made between the pooled data from BD animals and all the other groups. Table 1 gives the median values of the quantified parameters for each experimental group.
Table 1.
Median values of the quantified parameters of geniculocortical arborsa
| Total length (μm) |
Branch points (N) |
Total area (μm2) |
Max Den (μm/1000 μm2) |
Sum SPA (μm/1000 μm2) |
|
|---|---|---|---|---|---|
| 1w BD | 15,242 | 129 | 292,356 | 71 | 20,268 |
| 2w BD | 14,731 | 154 | 260,892 | 82 | 18,540 |
| 6/7dND | 15,470 | 136 | 242,064 | 101* | 26,496 |
| 6/7dD | 6930* | 43* | 182,016* | 51* | 8928* |
| Normals P30/31 | 12,849 | 96 | 267,000 | 82 | 12,132 |
| Normals P40 | 17,686 | 140 | 364,068 | 114 | 22,608 |
Asterisks indicate a statistically significant difference (P < 0.02) between the pooled BD groups and each of the other experimental groups. Max Den: Maximal density. Sum SPA: Sum of Standard Patch Areas. 6/7dD and 6/7dND refer to deprived and nondeprived arbors studied after 6 to 7 days of MD.
Total length, number of branch points, and surface area of geniculocortical arbors
The total length of the each arborization was obtained by adding the individual collateral branches in layer IV. Similarly, the number of branch points was obtained from the arborization in layer IV. The scattergrams in Fig. 4 (A and B) show the distributions of both the total length and the number of branch points in all experimental groups. Arbors in BD animals did not appear to have been remodeled during the period of deprivation. Indeed, no statistical difference in the total length was found between the pooled data of the BD animals and data obtained in normal animals at P40 and P30/31. Similarly, the total length of BD geniculocortical arbors did not differ from that of ND arbors in MD animals. Only the deprived arbors in MD animals, which are known to have been affected by 1 week of deprivation, were found to significantly differ from BD arbors. Similarly, the number of branch points was significantly lower in the deprived arbors of MD animals than in arbors reconstructed in BD animals. BD arbors were otherwise comparable to normal arbors or ND arbors in MD animals.
Fig. 4.
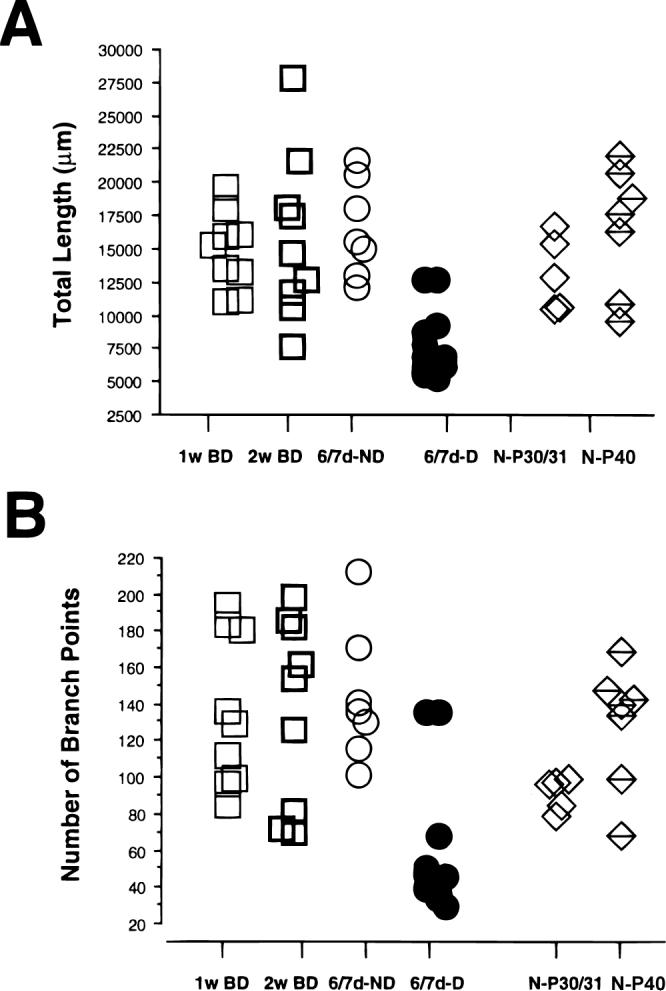
Scattergram of the total length (A) and number of branch points (B) of the terminal arborization in layer IV (see Materials and methods) for arbors reconstructed in animals binocularly deprived for 1 and 2 weeks. For comparison, data from arbors serving the deprived (D) and nondeprived (ND) eye in animals deprived for 6/7 days (6/7dD) and from arbors reconstructed in normal (N) animals at P30/31 and P40 are also plotted.
The surface area of geniculocortical arbors in BD animals was significantly larger than that of deprived arbors in MD animals (Table 1), and did not differ from the surface area of either nondeprived arbors in MD animals or arbors in normal P40 kittens.
Density of the terminal arborization
Fig. 5A illustrates the distribution of the maximal densities in all the experimental groups, and Table 1 gives the median values. The maximal density was significantly lower in the deprived arbors of MD animals than in BD animals. ND arbors in MD animals had on average a greater maximal density than BD arbors. Further, the maximal density in BD arbors was somewhat lower than that measured in normal animals at P40, although the difference between the two groups was barely statistically significant (P = 0.037).
Fig. 5.
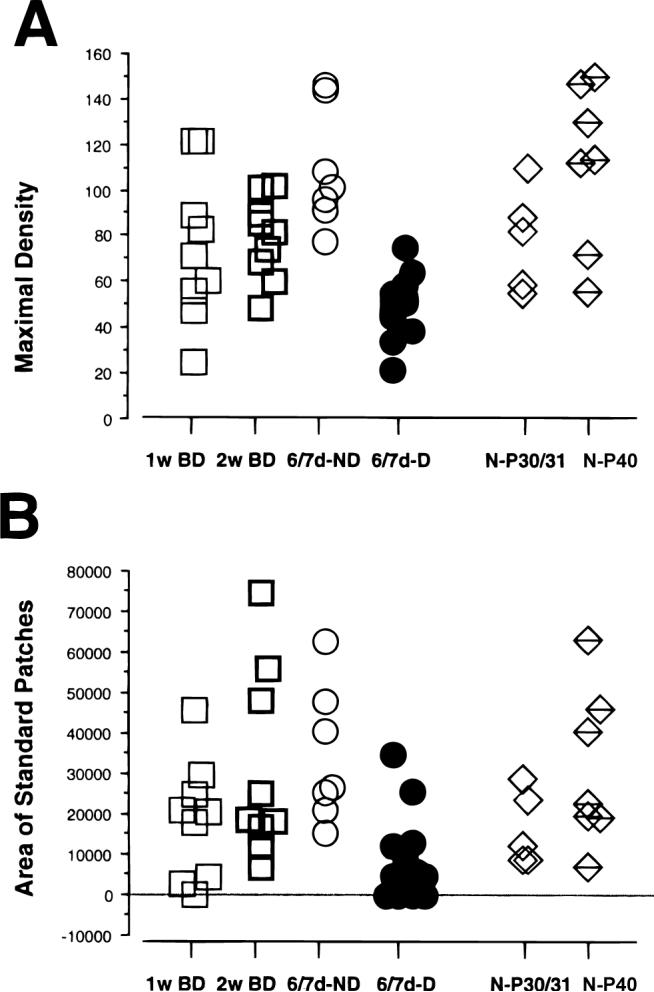
Density analysis of the terminal arborization in layer IV expressed as μm/1000 μm2. (A) Scattergram of the maximal density of arbors reconstructed from 1-week and 2-week binocularly deprived animals, of arbors serving the deprived (D) and nondeprived (ND) eye in 6/7dD animals, and of arbors reconstructed in normal animals at P30/31 and P40. (B) Scattergram of the cumulative area of the standard patches (see Results) of the terminal arborizations in all groups of animals. Note that the values for one arbor in 1-week BD group and four deprived arbors in 6/7dD are equal to 0, as they did not reach the threshold density of 38 μm/1000 μm2.
A characteristic of geniculocortical arbors in normal animals is the presence of distinct clusters of collaterals, most likely in register both with the physiologically defined ocular dominance columns, as well as with the patches of silver grains observed in layer IV when the entire geniculocortical projection is labeled transsynaptically after a monocular injection of tritiated proline (Shatz & Stryker, 1978). The number and extension of these clusters differ for the X- and Y-type of arbors. In a previous analysis of geniculocortical arbors reconstructed in normal animals at P30/31 and P40, we had defined the characteristics of these zones of high density of innervation, called “standard patches” (see Materials and methods; Antonini & Stryker, 1993b). The scattergram of Fig. 5B gives the distribution of the cumulative areas of the standard patches for arbors in each experimental group, and Table 1 gives the median values of these areas. BD arbors differ significantly only from deprived arbors in MD animals (Table 1). In contrast, they were not different from arbors in normal animals at P30/31 and P40, or from ND arbors in MD animals. Note, however, that a few arbors in BD animals had very small high-density areas. In one arbor the maximal density is below the threshold density for a standard patch, and in two other arbors the area of the standard patches is very small and far below the range for normal animals.
Discussion
We have previously reported the dramatic remodeling of deprived geniculocortical abors in MD kittens after only a week of deprivation and discussed the evidence that this effect is based on an activity-dependent competition between the pathways serving the two eyes (Antonini & Stryker, 1996). The competitive disadvantage of the deprived eye originates from the reduction of light and patterned visual stimulation (Crawford & Marc, 1976), a condition that most certainly results in anomalous activity in the deprived eye central projections and in a less efficient synaptic activation of the cortical target neurons. If correlated activity between the pre-synaptic and postsynaptic elements is the prerequisite for synaptic consolidation (Miller et al., 1989; Miller, 1994; Singer, 1994), then synapses serving the nondeprived eye will be more likely strengthened at the expense of those serving the deprived eye.
The alternative hypothesis for interpreting the plastic changes of the deprived afferents in MD animals considers that the decrease in activity along the deprived pathway per se could cause a retraction of the afferent terminals in layer IV, independent of the presence of activity in the terminals serving the nondeprived eye (Von Noorden et al., 1976).
The present results support the competition hypothesis by demonstrating that extensive plastic changes, comparable to those observed in MD animals, do not occur in deprived geniculocortical afferents if competition between the two eyes is eliminated by binocular deprivation. For nearly all of the parameters measured, geniculocortical arbors in BD animals are highly significantly different from deprived arbors reconstructed in MD animals. In nearly every respect examined, the BD arbors were indistinguishable from those in normal animals at P40 or from nondeprived arbors in MD animals. This failure to see significant differences between normal and BD arbors does not imply that binocular deprivation has no effect; it only puts an upper limit on the magnitude of that effect. We determined how abnormal the BD arbors would have to be for the comparison with normal arbors to be statistically significant, assuming the actual sample size and variability. We found that differences of 13% in total length and 25% in number of branch points would make the two groups significantly different at P <0.02. We cannot exclude the possibility that the smaller differences, which were actually observed, may be biologically significant, but they could equally have resulted from random sampling. Finally, it is also possible that some feature of the arbors that we did not measure might be abnormal in the BD animals.
The finding of lack of obvious abnormalities in the BD arbors would not be valid if our sample in BD animals was biased toward the least abnormal arbors. One may speculate, for example, that some of the BD arbors are so affected by the disruption of the normal activity in the geniculocortical pathway that transport of PHA-L lectin is impaired. This possibility is very unlikely because both deprived arbors in MD animals and arbors in animals treated with binocular injections of tetrodotoxin, which eliminates almost completely the activity in the geniculocortical pathway, are very well filled with the lectin and can easily be reconstructed (Antonini & Stryker, 1993a,b).
The relative importance of competition and sensory disuse
Evidence for competition
Numerous studies in the cat, dog, and squirrel have addressed the question of competition versus disuse in inducing the effects of MD in the visual pathways by analyzing the anatomical and physiological changes in the cells of origin of the geniculocortical projections (Wiesel & Hubel, 1963; Guillery & Stelzner, 1970; Guillery, 1972, 1973; Sherman et al., 1972, 1974; Guillery & Kaas, 1974; Sherman & Wilson, 1975; LeVay & Ferster, 1977). Altogether, these studies attribute the regressive phenomena of the deprived connections principally to the imbalanced interactions between the pathways originating from the two eyes, since the reduction of cell size occurs only in regions of the LGN that receive input from the binocular parts of the visual field, where interocular interaction is possible. Such cell shrinkage, and a concomitant reduction in cytochrome oxidase levels, do not occur in the monocular segment of the LGN, representing the periphery of the visual field seen only by the contralateral eye, or in an artificial monocular region of the LGN created by a small lesion in the homonymous portion of the nondeprived retina (Guillery & Stelzner, 1970; Guillery, 1972; Hickey et al., 1977; Wilson & Sherman, 1977; Kageyama & Wong-Riley, 1986). In agreement with these anatomical studies, both electrophysiological responses recorded from the visual cortex and visually evoked orienting behavior show that the deprived pathway in MD cats is impaired only within the representation of the binocular part of the visual field (Sherman, 1973; Sherman et al., 1974; Sherman & Guillery, 1976; Wilson & Sherman, 1977).
Evidence for noncompetitive effect of disuse
There is some evidence that sensory disuse can cause regressive phenomena in the visual pathway independent of the effects of competition. In BD cats, for example, a small shrinkage of LGN cells has been observed, although this is much less severe than in the binocular portion of LGN in MD cats (Sherman et al., 1972). As expected, this effect is homogeneous throughout the LGN, i.e. it is present in both the monocular and binocular portions of the nucleus, inasmuch as there are no regions in the visual field in which the two eyes, equally deprived, are competitively unbalanced. In addition, in the monkey, the monocular segment of the LGN shows a small degree of cell shrinkage even after a unilateral eye closure (Von Noorden et al., 1976; Crawford et al., 1991), suggesting a noncompetitive deprivation effect.
In general, the effects of BD on the responses of visual cortical neurons are also attributed to sensory disuse and not to abnormal binocular interactions (Wiesel & Hubel, 1965; Mower et al., 1981). Visual cortical responses after long-term BD, although less dramatically altered than after MD, are still highly abnormal. Numerous studies describe an unusually high proportion of both unresponsive and monocularly driven neurons. Furthermore, even when they are visually responsive, many neurons often are nonspecific in their response characteristics (Wiesel & Hubel, 1965; Blakemore & Van Sluyters, 1975; Sherk & Stryker, 1976; Watkins at al., 1978; Mower et al., 1981; for a review, see Fregnac & Imbert, 1984).
In our experiments, geniculocortical arbors in BD animals do not appear, as a population, to have been exposed to the same deleterious mechanisms that affect the deprived arbors in MD animals. Indeed, the total length, surface area, number of branch points, and the areas of high density in BD animals are not statistically different from those of normal arbors, suggesting that the primary cause of the arbor remodeling after short-term MD is abnormal binocular interactions. Only one aspect of BD arbors, the maximal density, appears to have been affected by deprivation, and this effect was only marginally significant. These data do not exclude the possibility that BD causes a more subtle plasticity, such as a minor loss of collateral branches or a reduction of the number of synapses, that would remain mostly undetectable at the level of analysis presented here. There is no question that the morphological effects of even 2 weeks of BD, if they exist, are very much less than those of 1 week of MD.
Finally, the greater effect of BD than MD on receptive fields of cortical neurons in the monocular segment of the visual cortex suggests that the dichotomy made here of competition versus disuse is not the whole story, at least after prolonged periods of deprivation (Watkins et al., 1978). In that case competition is absent and disuse is similar, so the difference between MD and BD requires some additional explanation.
Cortical effects of binocular deprivation and the geniculocortical connections
In our experiments, animals have been deprived at most for 2 weeks. Most of the earlier physiological studies have analyzed the effects of long-term binocular deprivation starting at eye opening, when the visual system is less mature, and these studies have generally disclosed profound loss of responsiveness, with lid suture more deleterious than dark rearing (Mower et al., 1981).
These effects in long-term lid-sutured animals may be due to an arrest of the normal developmental processes or to a later deterioration of cortical visual responsiveness. The evidence that an incipient orientation selectivity is already present in inexperienced kittens (Sherk & Stryker, 1976; Crair et al., 1998) favors the latter possibility. Further, Freeman et al. (1981) have shown that less than a week of dark rearing initiated after P30 can reduce responsiveness and selectivity, and single-unit recordings from the visual cortex of one of the BD kittens used in the present experiment (data not presented) also demonstrated a high percentage of poorly selective neurons. These findings indicate that binocular deprivation causes degenerative changes in cortical responsiveness, apparently through some intracortical mechanisms, or, possibly at the level of geniculocortical synapses without affecting the general morphological characteristics of geniculocortical afferents. However, it is possible that changes in the morphology of the afferents would be much greater after many months of binocular deprivation.
Acknowledgments
This work was supported by Grant NIH EY02874. We wish to thank Sheri Harris for her invaluable help during the experiments.
References
- Antonini A, Stryker MP. Rapid remodeling of axonal arbors in the visual cortex. Science. 1993a;260:1819–1821. doi: 10.1126/science.8511592. [DOI] [PubMed] [Google Scholar]
- Antonini A, Stryker MP. Development of individual geniculocortical arbors in cat striate cortex and effects of binocular impulse blockade. Journal of Neuroscience. 1993b;13:3549–3573. doi: 10.1523/JNEUROSCI.13-08-03549.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini A, Stryker MP. Geniculocortical afferent arbors in binocularly deprived kittens. Society for Neuroscience Abstracts. 1995;21:795.3, 2023. [Google Scholar]
- Antonini A, Stryker MP. Plasticity of geniculocortical afferents following brief or prolonged monocular occlusion in the cat. Journal of Comparative Neurology. 1996;369:64–82. doi: 10.1002/(SICI)1096-9861(19960520)369:1<64::AID-CNE5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Blakemore C, Van Sluyters RC. Innate and environmental factors in the development of the kitten's visual cortex. Journal of Physiology. 1975;248:663–716. doi: 10.1113/jphysiol.1975.sp010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC, Gillespie DG, Stryker MP. The role of visual experience in the development of columns in cat visual cortex. Science. 1998;279:566–570. doi: 10.1126/science.279.5350.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford MLJ, Marc RE. Light transmission of cat and monkey eyelids. Vision Research. 1976;16:323–324. doi: 10.1016/0042-6989(76)90118-8. [DOI] [PubMed] [Google Scholar]
- Crawford MLJ, Pesch TW, Von Noorden GK, Harwerth RS, Smith EL., III Bilateral form deprivation in monkeys, electrophysiological and anatomical consequences. Investigative Ophthalmology and Visual Science. 1991;32:2328–2336. [PubMed] [Google Scholar]
- Freeman RD, Mallach R, Hartley S. Responsivity of normal kitten striate cortex deteriorates after brief binocular deprivation. Journal of Neurophysiology. 1981;45:1074–1084. doi: 10.1152/jn.1981.45.6.1074. [DOI] [PubMed] [Google Scholar]
- Fregnac Y, Imbert M. Development of neuronal selectivity in primary visual cortex of cat. Physiological Reviews. 1984;64:325–434. doi: 10.1152/physrev.1984.64.1.325. [DOI] [PubMed] [Google Scholar]
- Friedlander MJ, Martin KAC, Wassenhove-McCarthy D. Effects of monocular visual deprivation of geniculocortical innervation of area 18 in the cat. Journal of Neuroscience. 1991;11:3268–3288. doi: 10.1523/JNEUROSCI.11-10-03268.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Sawchenko PE. An anterograde neuroanatomical tracing method that shows the detailed morphology of neurons, their axons and terminals: Immunohistochemical localization of an axonally transported plant lectin, Phaseolus vulgaris Leucoagglutinin (PHA-L). Brain Research. 1984;290:219–238. doi: 10.1016/0006-8993(84)90940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery RW. Binocular competition in the control of geniculate cell growth. Journal of Comparative Neurology. 1972;144:117–130. doi: 10.1002/cne.901440106. [DOI] [PubMed] [Google Scholar]
- Guillery RW. The effect of lid suture upon the growth of cells in the dorsal lateral geniculate nucleus of kittens. Journal of Comparative Neurology. 1973;148:417–422. doi: 10.1002/cne.901480402. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Kaas JH. The effects of monocular lid suture upon the development of the lateral geniculate nucleus in squirrels (Sciureus Carolinensis). Journal of Comparative Neurology. 1974;154:433–441. doi: 10.1002/cne.901540405. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Stelzner DJ. The differential effect of unilateral lid closure upon the monocular and binocular segments of the dorsal geniculate nucleus of the cat. Journal of Comparative Neurology. 1970;139:413–422. doi: 10.1002/cne.901390403. [DOI] [PubMed] [Google Scholar]
- Hickey TL, Spear PD, Kratz KE. Quantitative studies of cell size in the cat's dorsal lateral geniculate nucleus following visual deprivation. Journal of Comparative Neurology. 1977;172:265–281. doi: 10.1002/cne.901720206. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. Journal of Physiology. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey AL, Sur M, Ulrich DJ, Sherman SM. Projection patterns of individual X- and Y-cell axons from the lateral geniculate nucleus to cortical area 17 in the cat. Journal of Comparative Neurology. 1985;233:159–189. doi: 10.1002/cne.902330203. [DOI] [PubMed] [Google Scholar]
- Kageyama GH, Wong-Riley M. Differential effect of visual deprivation on cytochrome oxidase levels in major cell classes of the cat LGN. Journal of Comparative Neurology. 1986;246:212–237. doi: 10.1002/cne.902460207. [DOI] [PubMed] [Google Scholar]
- LeVay SM, Ferster D. Relay cell classes in the lateral geniculate nucleus of the cat and the effects of visual deprivation. Journal of Comparative Neurology. 1977;172:563–584. doi: 10.1002/cne.901720402. [DOI] [PubMed] [Google Scholar]
- LeVay SM, Wiesel TN, Hubel DH. The development of ocular dominance columns in normal and visually deprived monkeys. Journal of Comparative Neurology. 1980;191:1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- Miller KD, Keller JB, Stryker MP. Ocular dominance column development: Analysis and simulation. Science. 1989;245:605–615. doi: 10.1126/science.2762813. [DOI] [PubMed] [Google Scholar]
- Miller KD. Models of activity-dependent neural development. Progress in Brain Research. 1994;102:303–318. doi: 10.1016/S0079-6123(08)60548-8. [DOI] [PubMed] [Google Scholar]
- Mower GD, Berry D, Burchfiel JL, Duffy FH. Comparison of the effects of dark rearing and binocular suture on development and plasticity of cat visual cortex. Brain Research. 1981;220:255–267. doi: 10.1016/0006-8993(81)91216-6. [DOI] [PubMed] [Google Scholar]
- Passera A, Fulks S, Schneider GE, Ayres S, Jhaveri S, Erzurumlu RS. The M.I.T. “Neurotrace” system for microcomputer-aided microscopy. Society for Neuroscience Abstracts. 1988;14:550. [Google Scholar]
- Rauschecker JP. Mechanisms of visual plasticity: Hebb synapses, NMDA receptors, and beyond. Physiological Reviews. 1991;71:587–615. doi: 10.1152/physrev.1991.71.2.587. [DOI] [PubMed] [Google Scholar]
- Shatz CJ, Stryker MP. Ocular dominance in layer IV of the cat's visual cortex and the effects of monocular deprivation. Journal of Physiology. 1978;281:267–283. doi: 10.1113/jphysiol.1978.sp012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherk H, Stryker MP. Quantitative study of cortical orientation selectivity in visually unexperienced kitten. Journal of Neurophysiology. 1976;39:63–70. doi: 10.1152/jn.1976.39.1.63. [DOI] [PubMed] [Google Scholar]
- Sherman SM. Visual field defects in monocularly and binocularly deprived cats. Brain Research. 1973;49:25–45. [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Behavioral studies of binocular competition in cats. Vision Research. 1976;16:1479–1481. doi: 10.1016/0042-6989(76)90168-1. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW, Kaas JH, Sanderson KJ. Behavioral, electrophysiological and morphological studies of binocular competition in the development of the geniculo-cortical pathways of cats. Journal of Comparative Neurology. 1974;158:1–18. doi: 10.1002/cne.901580102. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Hoffmann K-P, Stone J. Loss of a specific cell type from dorsal lateral geniculate nucleus in visually deprived cats. Journal of Neurophysiology. 1972;35:532–541. doi: 10.1152/jn.1972.35.4.532. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Wilson JR. Behavioral and morphological evidence for binocular competition in the postnatal development of the dog's visual system. Journal of Comparative Neurology. 1975;15:183–195. doi: 10.1002/cne.901610204. [DOI] [PubMed] [Google Scholar]
- Singer W. Coherence as an organizing principle of cortical functions. International Review of Neurobiology. 1994;37:153–183. doi: 10.1016/s0074-7742(08)60245-7. [DOI] [PubMed] [Google Scholar]
- Von Noorden GK. Histological studies of the visual system in monkeys with experimental amblyopia. Investigative Ophthalmology and Visual Science. 1973;12:727–738. [PubMed] [Google Scholar]
- Von Noorden GK, Middleditch PR. Histological observation in the normal monkey lateral geniculate nucleus. Investigative Ophthalmology and Visual Science. 1975;14:55–58. [PubMed] [Google Scholar]
- Von Noorden GK, Crawford MLJ, Middleditch PR. The effects of monocular deprivation: Disuse or binocular interaction? Brain Research. 1976;111:277–285. doi: 10.1016/0006-8993(76)90772-1. [DOI] [PubMed] [Google Scholar]
- Watkins DW, Wilson JR, Sherman M. Receptive-fields properties of neurons in binocular and monocular segments of striate cortex in cats raised with binocular lid suture. Journal of Neurophysiology. 1978;41:322–337. doi: 10.1152/jn.1978.41.2.322. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Effects of visual deprivation on morphology and physiology of cells in the cat's lateral geniculate body. Journal of Neurophysiology. 1963;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. Journal of Neurophysiology. 1965;28:1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Sherman SM. Differential effects of early monocular deprivation on binocular and monocular segments of cat striate cortex. Journal of Neurophysiology. 1977;40:891–903. doi: 10.1152/jn.1977.40.4.891. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Research. 1979;171:11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]