Abstract
Muscarinic acetylcholine receptors are members of the G protein-coupled receptor superfamily expressed in neurons, cardiomyocytes, smooth muscle, and a variety of epithelia. Five subtypes of muscarinic acetylcholine receptors have been discovered by molecular cloning, but their pharmacological similarities and frequent colocalization make it difficult to assign functional roles for individual subtypes in specific neuronal responses. We have used gene targeting by homologous recombination in embryonic stem cells to produce mice lacking the m1 receptor. These mice show no obvious behavioral or histological defects, and the m2, m3, and m4 receptors continue to be expressed in brain with no evidence of compensatory induction. However, the robust suppression of the M-current potassium channel activity evoked by muscarinic agonists in sympathetic ganglion neurons is completely lost in m1 mutant mice. In addition, both homozygous and heterozygous mutant mice are highly resistant to the seizures produced by systemic administration of the muscarinic agonist pilocarpine. Thus, the m1 receptor subtype mediates M current modulation in sympathetic neurons and induction of seizure activity in the pilocarpine model of epilepsy.
Muscarinic acetylcholine receptors (mAChRs) are the predominant cholinergic receptors in the central nervous system where they play a major role in learning and memory (1, 2), control of movement, rapid eye movement sleep (3), central nociception (4), and the generation of epileptic seizures (5). Many of the mAChRs in the central nervous system exhibit high affinity for the muscarinic antagonist pirenzepine, whereas mAChRs in the heart and smooth muscles have a low affinity for this antagonist (6). These two pharmacological subtypes were initially designated M1 and M2, respectively. The subsequent development of additional nonclassical mAChR antagonists permitted the distinction of pharmacologically defined M3 and M4 receptors as well. Molecular cloning has now identified five distinct subtypes of mAChR, called m1–m5, which are encoded by separate genes (7–9).
mAChRs belong to the family of seven putative transmembrane-spanning receptors that transduce signals via coupling to G proteins (10–12). The m1, m3, and m5 subtypes preferentially couple to the Gq/G11 family of G proteins, stimulating phospholipase C. The m2 and m4 subtypes usually interact with the Go/Gi family to inhibit adenylyl cyclase. Although the pharmacologically defined M1, M2, M3, and M4 receptors frequently correspond to the genetically defined m1, m2, m3, and m4 receptors, respectively, the unequivocal identification of the subtype mediating a specific functional response is complicated by (i) lack of strongly selective agonists or antagonists, (ii) presence of multiple subtypes in most tissues, and (iii) variations in antagonist affinities caused by differences in ligand binding assay conditions or membrane environment. The inability to determine the bioavailabilty of administered drugs can also make it difficult to achieve subtype specificity in vivo. Subtype-specific antibodies have increased our knowledge of the anatomical distribution of these receptors, allowing some predictions regarding functional roles. However, production of mice with null mutations in the mAChR genes will prove invaluable in elucidating the individual roles of these receptors. Toward achieving this goal, we have used homologous recombination in mouse embryonic stem cells to generate mice lacking the m1 receptor.
In this study we describe the analysis of two responses involving neuronal excitability, purported to be mediated by the m1 mAChR subtype. At the cellular level, we have analyzed mAChR-mediated M current modulation, and at the level of the whole animal, we have measured seizure activity evoked by muscarinic agonists. The M current is a voltage-dependent K+ current found in sympathetic ganglion neurons (13) and in hippocampal pyramidal neurons (14). Suppression of this tonically active current by muscarinic agonists leads to membrane depolarization, rendering the cell more susceptible to firing action potentials (15). The M1 receptor has been implicated pharmacologically as the mAChR responsible for modulating the M current in sympathetic ganglion neurons (16, 17). However, immunoprecipitation has shown that m1, m2, and m4 mAChR subtypes are all present (18).
Chronic seizures induced in mice by systemic injection of pilocarpine, a non-subtype-specific partial muscarinic agonist, show similarities to human temporal lobe epileptic seizures. For example, there is hippocampal cell loss, dentate granule cell dispersion, supra- and intragranular mossy fiber sprouting (19), and alterations in the electroencephalogram, starting in the hippocampus and spreading to the cortical regions (20). Light microscopy shows damage to several brain regions, including the hippocampus, amygdala, thalamus, olfactory cortex, neocortex, and substantia nigra. Simultaneous injection of the muscarinic antagonists atropine or pirenzepine with pilocarpine prevents the onset of seizures (20), but muscarinic antagonists have no effect on established seizures (21–23), indicating that mAChRs are involved in the initiation but not the maintenance of epileptic seizures. The m1 and m4 receptor subtypes are both found in the cerebral cortex, hippocampus, and thalamus and have high affinity for pirenzepine (12), so the identity of the mAChR subtype responsible for agonist-induced seizures has been ambiguous.
The induction of seizures involves the interaction of multiple neurotransmitter pathways (24, 25). Stimulation of mAChRs initiates seizures that progressively involve noncholinergic systems and become resistant to muscarinic receptor antagonists. These seizures cause an excessive release of glutamate from glutamatergic neurons that stimulates continuous release of acetylcholine. This “self-propagating” seizure activity results in neuronal damage mediated by the N-methyl-d-aspartate receptor. Blocking N-methyl-d-aspartate receptors attenuates seizures induced by the glutamate receptor agonist kainic acid and by the muscarinic agonist carbachol. However, blocking muscarinic transmission with the general muscarinic antagonist atropine has no effect on seizures induced by glutamate release (24). Thus, in addition to rating the behavior of m1-deficient mice injected with pilocarpine, we also rate the behavior of these mice when exposed to kainic acid.
MATERIALS AND METHODS
Construction of the m1 Targeting Vector.
An 8-kb HindIII–SstI fragment containing the entire coding region of m1 (1.4 kb on a single exon) was isolated from a mouse genomic library (26). A 0.3-kb KpnI–SstI fragment containing 5′ noncoding sequences and 165 nucleotides of the m1 N terminus was replaced with the neomycin-resistance gene to use as a positive selectable marker. This deletes the start site of translation, the extracellular N-terminal domain, the first putative transmembrane domain, and a portion of the first cytoplasmic loop in the m1 receptor. In addition, the m1 clone was truncated by 0.2 kb at the 3′ end (and a SalI site was added at this location by PCR) to provide sequences outside the construct appropriate for probes to use for PCR and Southern blot analyses. The herpes simplex virus thymidine kinase gene was inserted outside the m1 genomic DNA as a negative selection marker for homologous recombination.
Generation of m1 Mutant Mice.
Embryonic stem cell culture and electroporation techniques are described in Brandon et al. (27). The m1 targeting vector was linearized with NotI and used at a concentration of 22 μg/ml for electroporation. REK 3 ES cells (27) were used at a concentration of 1.2 × 107 cells per ml. Colonies that survived 10 days in G418 (0.3 mg/ml; 66% active; GIBCO) and 2 μM ganciclovir were screened by PCR. Of the six recombinant cell lines injected into C57BL/6 blastocysts to generate chimeric mice, one contributed to the germ line. All mice used in the studies reported here were F2 hybrids (from C57BL/6 × 129SvJ parents) derived from this line.
mAChR Subtype-Specific Antisera.
Affinity-purified rabbit polyclonal antibodies used for immunohistochemistry were raised against the third cytoplasmic loops of m1 through m4 and purified as described (28). Additional affinity-purified antibodies to the third cytoplasmic loops of mouse m1 (amino acids 233–332) and mouse m3 (amino acids 314–434) were generated for immunoprecipitation assays as described by McKinnon and Nathanson (29). An anti-porcine m2 monoclonal antibody (30) and the above mentioned anti-m4 polyclonal antisera (28) were also used in the immunoprecipitation assays.
Immunohistochemistry.
Five female mice (two wild type and three mutants) were deeply anesthetized with sodium pentobarbital and perfused transcardially with 0.9% saline/0.005% sodium nitroprusside for 1 min followed by 4% paraformaldehyde in 0.1 M sodium phosphate buffer for 10 min and 10% sucrose in 0.1 M sodium phosphate buffer for 10 min. Brains were immediately removed, immersed in 30% sucrose at 4°C until they sank, frozen on dry ice, and sectioned at 50 μm on a sliding microtome. Tissue sections were processed for immunohistochemistry by using subtype-specific anti-mAChR antibodies and peroxidase anti-peroxidase (Sternberger Monoclonals, Baltimore, MD) or avidin-biotin-peroxidase (Vectastain Elite, Vector Laboratories) methods as described (28). Sections to be immunostained with m3 or m4 antibodies were pretreated with 1.0% NaOH/0.9% H2O2 for 10 min to enhance immunoreactivity. The antibodies to m1, m3, and m4 were used at 0.75 μg/ml and those to m2 were used at 0.5 μg/ml. For control experiments, primary antibody was omitted.
Ligand Binding Assays.
Mouse forebrains and cerebellums were frozen immediately in liquid nitrogen upon dissection and stored at −70°C until use. Tissues were homogenized by hand with 20 strokes of a ground-glass homogenizer in phosphate-buffered saline containing 0.4 mM phenylmethylsulfonyl fluoride and leupeptin at 10 μg/ml. Membranes were isolated as described in Luetje et al. (30). Ligand binding experiments using the muscarinic antagonist [3H]quinuclidinyl benzilate (QNB) were performed as described by Nathanson (31). Means are reported as significantly different when P < 0.05, by Student’s t test.
Immunoprecipitation Assays.
Tissue membranes were prepared as described above. Solubilized [3H]QNB-labeled mAChRs were immunoprecipitated as described by McKinnon and Nathanson (29). The efficiencies of the antisera at immunoprecipitating their respective labeled mAChRs from transfected cells were as follows: anti-m1, 84%; anti-m2, 80%; anti-m3, 58%; and anti-m4, 85%. Cross-reactivity of each of these antisera with noncognate subtypes was less than 5%. Binding values were corrected to account for the variation in efficiencies of immunoprecipitation of each antisera. When P < 0.05 for means compared by Student’s t test, they are considered significantly different.
M Current Recording.
Neurons were obtained from superior cervical ganglion (SCG) of adult female mice (60–110 days of age) with genotype unknown to the experimenter. The mice were anesthetized with CO2 and decapitated. Neurons were dissociated and cultured for 16–24 hr prior to recording as described for rat SCG cells (32, 33) with the following minor modifications. Cells were incubated overnight in DMEM supplemented with 5% fetal bovine serum and nerve growth factor (20 ng/ml) (all from GIBCO) in poly-(l-lysine)-coated 35-mm dishes (Nunc) in 5% CO2.
Currents were recorded with the whole-cell configuration of the patch-clamp technique by using a List EPC-7 amplifier and fire-polished pipettes (1–2 MΩ) pulled from hematocrit glass (VWR). The pipette solution contained 175 mM KCl, 5 mM MgCl2, 5 mM Hepes, 0.1 mM bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate, 3 mM Na2ATP, 0.1 mM NaGTP, and 0.08 mM leupeptin (pH 7.4 with KOH). Whole-cell current was sampled at 2 kHz and low-pass-filtered at 1 kHz. The access resistance was less than 10 MΩ and partial series-resistance compensation was sometimes used. Membrane potentials have been corrected for a −2-mV junction potential. The M current was operationally defined as the difference between the currents at 9 ms and 500 ms after a 30-mV hyperpolarizing voltage step from the holding potential of −30 mV. In some cells rundown of M current was approximated by a single exponential based on the control period before drug (time constant = 552 ± 68 s; n = 15) and corrected for during the off-line analysis. One cell from each culture dish was used. After a gigaohm seal was obtained, the area containing the cell was perfused continuously at 1.5–2 ml/min with a Ringer’s solution containing 160 mM NaCl, 2.5 mM KCl, 5 mM CaCl2, 1 mM MgCl2, 10 mM Hepes, and 8 mM glucose (pH 7.4 with NaOH). Tetrodotoxin (500 nM) was present in the Ringer’s solution to block voltage-gated sodium currents. Where appropriate, data are expressed as the mean ± SEM. Means, compared by using Student’s t test, were considered significantly different when P < 0.05.
Seizure Studies.
Age-matched (2–8 months) wild-type and mutant mice were used, weighing 17–36 g. Animals were weighed and injected with pilocarpine (200 mg/kg or 300 mg/kg, i.p.) or kainic acid (25 mg/kg or 35 mg/kg, s.c.) in 100–200 μl of saline. Animal behavior was recorded for 45 min by an observer who did not know the genotypes of the animals, and the severity of seizures was rated by using published criteria (22, 34–36). Mice experiencing seizures induced by pilocarpine were assigned a rating for the entire 45-min period according to the following scale: 1, head bobbing; 2, single body or head jerks; 3, multiple clonic jerks; 4, one tonic-clonic seizure; 5, two tonic-clonic seizures; 6, three seizures. Animals that died as a result of seizure activity within the observation period were also assigned a rating of 6. Kainic acid produces less discrete seizures often involving predominantly clonic movements. Mice injected with kainic acid were assigned a rating for each 5-min period of the 45-min observation period according to the following scale: 1, rigid or slowed walking, tail stiffening; 2, neck arch/forebody contractions, isolated body jerks; 3, repetitive scratching, head bobbing; 4, forelimb clonus, rearing and falling, clonic body jerks; 5, mouse on its side with clonic fore and hind limbs, body rolls, jumping like a kangaroo. Animals that died as a result of seizure activity were assigned a rating of 6 for each of the remaining 5-min periods. The mean of the nine 5-min ratings was determined for each mouse.
RESULTS
Mice Lacking the m1 mAChR Demonstrate No Overt Abnormalities.
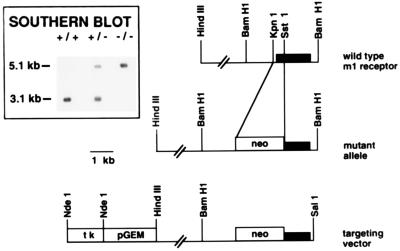
Mice deficient for m1 were generated by homologous recombination in mouse embryonic stem cells. The targeting construct (Fig. 1) was created from an 8-kb genomic fragment in which DNA corresponding to the first 55 amino acids of the receptor was deleted and replaced with a gene encoding neomycin resistance. One of six homologous recombinant clones gave rise to germ-line-competent chimeras that were bred with C57BL/6 mice to generate mice heterozygous for the deficiency in m1. These heterozygotes were crossed to obtain homozygotes. The Southern blot (Fig. 1, Inset) shows that the wild-type allele digested with BamHI and probed with the KpnI–BamHI fragment containing the coding region yields a 3.1-kb fragment. The same digest of the mutant allele gives rise to a 5.1-kb band.
Figure 1.
Targeting the m1 gene by homologous recombination. Restriction endonuclease map of the wild-type m1 mAChR gene (Top), the targeting vector (Bottom), and the mutant allele (Middle) resulting from homologous recombination at the m1 locus. (Inset) Example of genomic Southern blot used to determine mouse genotypes. The 1.8-kb KpnI–BamHI fragment, which includes the entire coding region of m1, was used as a probe. BamHI digests of mouse tail DNA yield a 3.1-kb band in the wild type (+/+) and a 5.1-kb band in the m1 mutant (−/−). Both bands are present in the heterozygote (+/−). tk, Herpes simplex virus thymidine kinase gene cassette; neo, gene cassette for neomycin resistance; pGEM, intervening plasmid sequences.
Homozygous mice deficient for m1 arose from heterozygote matings at expected Mendelian frequencies (28%, +/+; 47%, +/−; 25%, −/−; n = 164) indicating no detrimental effects on embryonic development. No differences in longevity (up to 2 years), fertility, and body weight were noted (data not shown).
m1-Deficient Mouse Brains Are Histologically Normal.
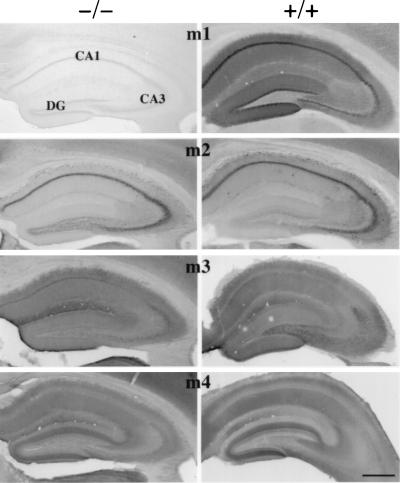
Immunohistochemistry was performed with antibodies against m1–m4. Fig. 2 compares hippocampal immunoreactivity in m1-deficient and wild-type mice and shows the complete absence of m1 receptor in the hippocampal CA1 and CA3 cell layers of Ammon’s horn and in the dentate gyrus of the m1-deficient mouse. No obvious histological abnormalities were apparent in the brains of mutant mice. The immunoreactivity seen with antibodies against m2–m4 is similar in the mutant and wild-type mice, indicating no gross changes in levels and distributions of these receptors have occurred to compensate for the absence of m1.
Figure 2.
Light microscopic localization of m1–m4 in the hippocampus of m1 knockout (−/−, Left) and wild-type mice (+/+, Right). Note the absence of m1 immunoreactivity in the hippocampus and overlying cortex from the mutant mice when compared with the dark staining evident in the wild-type mice. Minimal background staining in the m1-deficient mice was similar to controls in which the primary antibody was omitted for mutant and wild-type brain sections. CA1 and CA3, regions of Ammon’s horn; DG, dentate gyrus. (Bar = 500 μm.)
Mutant Mouse Forebrains Contain Half the Normal Number of mAChRs.
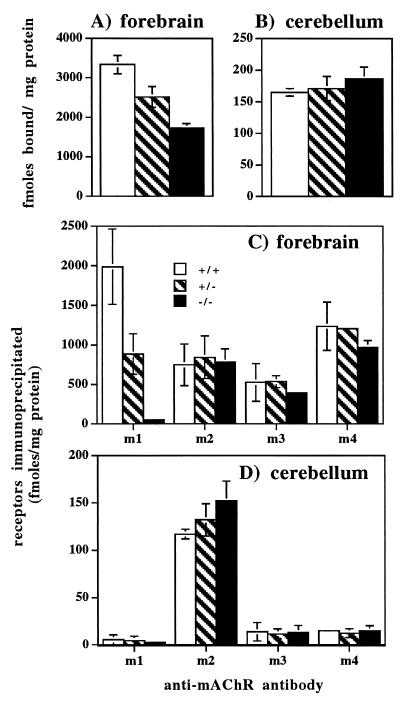
Total mAChR abundance in mouse brain was determined by measuring the binding of [3H]QNB, a high-affinity non-subtype-specific muscarinic antagonist, in mouse forebrain (Fig. 3A) and cerebellum (Fig. 3B) homogenates. In the mutant forebrain [3H]QNB binding was 48% lower than in wild-type forebrain, where m1 is estimated to represent 50% of the total mAChRs (11). Heterozygotes showed an intermediate value, corresponding to a 24% loss in total [3H]QNB binding. In the cerebellum, where m1 contributes less than 5% to the total mAChR number (11), there was no significant difference in [3H]QNB binding among the wild-type, heterozygote, and homozygote mutant mice.
Figure 3.
Concentration of total mAChRs and specific m1–m4 subtypes in mouse forebrain and cerebellum. Total mAChR numbers were determined by [3H]QNB binding assays and protein concentrations were measured by the Lowry assay on tissue homogenates from the forebrain (A) and cerebellum (B). [3H]QNB-labeled receptors were solubilized and immunoprecipitated with subtype-specific antisera from mouse forebrain (C) and cerebellum (D). In all cases values are the mean ± SD, n = 4.
mAChR Subtypes m2, m3, and m4 Do Not Compensate for the Absence of m1.
Immunoprecipitation was used to quantitate the abundance of the m1, m2, m3, and m4 mAChR subtypes in the forebrain (Fig. 3C) and the cerebellum (Fig. 3D). The m1 mutant mice showed a total loss of m1 receptor subtype in forebrain. Again, the heterozygotes showed an intermediate value corresponding to a 55% decrease. There were no significant differences in the abundances of m2, m3, and m4 in the forebrains of wild-type vs. homozygote mutant mice. In the cerebellum, where approximately 80% of the mAChRs belong to the m2 subtype, no significant differences in subtype distributions were observed. Hence, there is no measurable increase in the levels of m2, m3, and m4 protein expression in the brain to compensate for the loss of the m1 receptor in the mutant mice.
M Current in Sympathetic Neurons of m1-Deficient Mice Does Not Respond to Muscarinic Agonists.
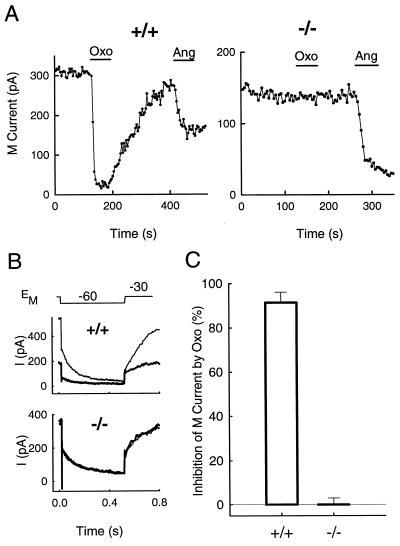
In mouse SCG neurons, hyperpolarizing voltage steps from a holding potential of −30 mV to a test potential of −60 mV elicited a slowly decaying outward tail current characteristic of M currents (37). The size of the M current varied considerably from cell to cell (range, 50 pA to 1000 pA) and was correlated with the size of the neuron (capacitance range, 14 pF to 86 pF). Neurons from m1-deficient mice had a larger M current density than cells from wild-type mice (9 ± 1 pA/pF vs. 5 ± 1 pA/pF; P < 0.05).
In SCG neurons from rats, muscarinic agonists reduce M currents by a pathway that has the pharmacological properties of M1 receptors (17). With neurons from wild-type mice, we found that oxotremorine M (Oxo), a non-subtype-specific muscarinic agonist, inhibited M currents. At a test concentration of 10 μM, the maximal inhibition of M current was reached in 20–30 s, and this inhibition partially reversed after return to control Ringer’s solution (Fig. 4A). In contrast, there was no effect on any whole-cell current at either −30 mV or −60 mV (Fig. 4B) at any time during a 1-min application of Oxo to SCG neurons from m1-deficient mice. As expected, inhibition of M current in wild-type cells is reflected in a large decrease in the holding current at −30 mV where M current is tonically activated. Quantitatively, inhibition of M currents by Oxo (Fig. 4C) in wild-type mouse SCG neurons was robust (91 ± 5%) and observed in all cells, whereas there was no effect in neurons from the m1-deficient mice (−0.1 ± 3%, n = 9). Angiotensin II (500 nM) was tested in most of the same mutant cells in which the muscarinic agonist was ineffective (Fig. 4A). Angiotensin II moderately inhibited the M current in all tested cells (47 ± 9%, n = 8). In wild-type cells already partially inhibited by Oxo, angiotensin II further inhibited the M current (22 ± 9%, n = 4). Thus, the muscarinic pathway that normally inhibits M current seems to be entirely nonfunctional in mutant mice, whereas the angiotensin II pathway remains functional.
Figure 4.
Modulation of M current by a muscarinic agonist is missing in the −/− mouse. (A) Time course of action of Oxo and angiotensin II. Oxo (10 μM) had a large and partially reversible inhibitory effect on the M current in a SCG cell from a +/+ mouse but had no effect on M current in a SCG neuron from a −/− mouse. The inhibitory action of angiotensin II (500 nM) was observed in both cells. (B) Current traces from the cells in A 4 s before (thin traces) and after 1 min of Oxo application (thick traces). The holding current and the relaxing tail current are Oxo-sensitive only in the cell from the +/+ mouse. (C) Oxo strongly inhibited M current in all cells from +/+ mice (n = 7) but had no effect in cells from −/− mice (n = 9).
m1-Deficient Mice Are Resistant to Pilocarpine-Induced Seizures.
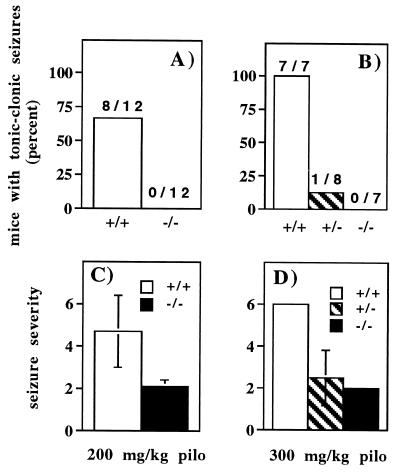
At a low dose of pilocarpine (200 mg/kg, i.p.), none of the m1 mutant mice experienced seizures (n = 12), whereas eight of 12 wild-type mice experienced seizures (Fig. 5A). At a high dose of pilocarpine (300 mg/kg, i.p.), every wild-type mouse experienced multiple tonic-clonic seizures (n = 7), whereas none of the m1 null mice experienced seizures (n = 7; Fig. 5B). One heterozygote of eight experienced tonic-clonic seizures. At the low dose of pilocarpine, the severity of seizures in wild-type mice ranged from 2 to 6 with a mean of 4.7 ± 1.7 (Fig. 5C). One mutant mouse was assigned a rating of 3, and the remaining 11 were rated at 2, yielding a mean of 2.1 ± 0.3. All wild-type mice injected with the high dose of pilocarpine received a rating of 6 (Fig. 5D). Heterozygote ratings ranged from 2 (7 mice) to 6 (1 mouse), averaging 2.5 ± 1.3. The m1-deficient mice all received a rating of 2. Administration of pilocarpine to both wild-type and mutant mice causes a variety of peripheral and central effects such as salivation, eye watering, myoclonic jerks, and tremors, which thus do not require the m1 subtype. When seizure severities are analyzed nonparametrically by the Mann–Whitney U test, both the heterozygote and homozygote mutant mouse responses are significantly different from that of the wild-type mice (P < 0.005); the heterozygote and homozygote mutant mouse responses are not significantly different from each other (P > 0.2). Thus the m1-deficient mice exhibit virtually no seizure activity in response to agonist when our data are analyzed by (i) the percent of mice experiencing seizures or (ii) the severity of seizure behavior.
Figure 5.
Absence of pilocarpine-induced seizures in m1 mutant mice. Mice were injected with 200 mg/kg (A and C) or 300 mg/kg (B and D) pilocarpine (i.p.) and observed for 45 min. The percentage of mice experiencing at least one tonic-clonic seizure is shown in A and B. Seizure severities and behavior ratings assigned for the entire 45-min period are presented for these mice in C and D. Values are expressed as the mean ± SD for each genotype.
m1-Deficient Mice Are Susceptible to Seizures Caused by Kainic Acid.
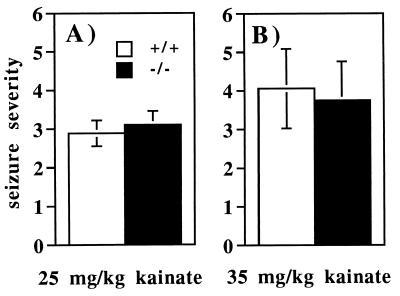
Administration of the glutamate receptor agonist kainic acid induces seizures in the limbic system that often involve automatisms, rearing and falling, and bilateral upper extremity clonic movements (38). To determine whether mice missing the m1 receptor are still susceptible to seizures induced by stimulating this alternative receptor pathway, we injected mice at low (25 mg/kg, s.c.) and high (35 mg/kg, s.c.) doses of kainic acid. At the low dose (Fig. 6A), seizure severities in wild-type and mutant mice were indistinguishable from each other (Mann–Whitney U test, P > 0.1). The higher dose also failed to distinguish any significant behavioral differences between the wild-type and mutant mice (Fig. 6B). Thus, the m1 mutant mice, although resistant to pilocarpine-induced seizures, are susceptible to those caused by stimulating the kainate receptor pathway.
Figure 6.
Assessment of kainic acid-induced seizures in wild-type and m1 mutant mice. Mice were injected with kainic acid (s.c.) at 25 mg/kg (A) (n = 9) or 35 mg/kg (B) (n = 9) and observed for 45 min. Mice were assigned ratings for their behavior during each 5-min period. Values represent the mean ± SEM for each genotype.
DISCUSSION
In the forebrain the M1 pharmacological class of mAChR, as defined by pirenzepine antagonism, is linked to phosphatidylinositol turnover (39), the inhibition of the M current (40), and seizure induction by muscarinic agonists (22, 23). Because both m1 and m4 subtypes are recovered when [3H]pirenzepine-labeled receptors are immunoprecipitated (28), the involvement of multiple muscarinic receptor subtypes has not been ruled out.
Our data show that the m1 mAChR subtype is required for the initiation of epileptic seizures induced by muscarinic agonists. At low (200 mg/kg) and high (300 mg/kg) doses of pilocarpine, the m1-deficient mice never experience tonic-clonic seizures. Interestingly, only one of eight heterozygotes had seizures at the high dose. Although these heterozygotes still have 45% of their forebrain m1 receptors, they are virtually resistant to seizures induced by muscarinic agonists. The level of m1 receptors in these animals is thus below the threshold needed for seizure induction via the m1 pathway. Our results also indicate that m1 receptors are not necessary for the initiation and maintenance of seizures induced by kainic acid. This is consistent with the proposed interaction of neurotransmitter pathways for induction of seizures summarized in Gale (24) and Solberg and Belkin (25).
We have shown that mice lacking the m1 receptor show no M current modulation in their sympathetic ganglion neurons in response to muscarinic agonists although the response to angiotensin II is intact. Because muscarinic suppression of the M current increases the rate of firing of sympathetic neurons (14), the loss of M current modulation in m1-deficient mice would be predicted to depress excitability in response to increased preganglionic stimulation compared with wild-type mice. However, the physiological consequence of this abolished muscarinic modulation in SCG neurons is not known. It will be of great interest to determine whether hippocampal and other central neurons from m1-deficient mice also exhibit the loss of muscarinic M current suppression observed in sympathetic neurons. The loss of muscarinic suppression of the hippocampal M current would be expected to decrease the excitability of these neurons, which might contribute to the resistance of m1-deficient mice to pilocarpine-induced seizures.
The inability of pilocarpine to evoke seizures in both homozygous and heterozygous m1 mutant mice indicates that the m1 receptor plays a crucial role in the initiation of seizures in the pilocarpine model of epilepsy. These mutant mice will be useful in the identification of the cellular pathways and molecular mechanisms involved in seizure initiation by muscarinic agonists. For example, activation by muscarinic agonists potentiates the response of hippocampal neurons to N-methyl-d-aspartate (41), providing another potential m1-related mechanism for seizure induction. In addition, these results raise the possibility that mutations in the m1 receptor that cause constitutive activity or mutations in other components of the signal transduction pathway that increase the physiological sensitivity of the m1 receptor could result in the initiation of seizure activity.
Pharmacological studies have implicated M1-like receptors in many processes in the central nervous system, including memory and learning (1, 2), regulation of circadian rhythm (42), establishment of ocular dominance columns in the visual cortex (43), and control of drinking (44). The m1 mutant mice should be of great use in evaluating the role of the m1 receptor in mediating these processes. In addition, it should be of interest to determine the effects of disrupting the genes encoding other mAChR subtypes to define the functions of each of the muscarinic receptors in the nervous system.
Acknowledgments
We thank Kirstin A. Gerhold, Dr. Kimberly A. Burton, and Mona Belyamani for their technical expertise, advice, and encouragement regarding all aspects of the mouse work. We also thank Dr. Howard Rees for technical expertise at immunohistochemistry, Kristen Souweine for maintaining the m1 mouse colonies, and Drs. Richard D. Palmiter and Charles Chavkin for their suggestions regarding this manuscript. This work was supported by National Institutes of Health Grants NS26920 (to N.M.N.), 5T32HL07312 (to S.E.H.), NS30454 (to A.I.L.), NS08174 (to B.H.), and HL44948 (to R.L.I. and G.S.M.).
ABBREVIATIONS
- mAChR
muscarinic acetylcholine receptor
- QNB
quinuclidinyl benzilate
- SCG
superior cervical ganglion
- Oxo
oxotremorine M
References
- 1.Messer W S, Bohnett M, Stibbe J. Neurosci Lett. 1990;116:184–189. doi: 10.1016/0304-3940(90)90407-z. [DOI] [PubMed] [Google Scholar]
- 2.Messer W S, Stibbe J R, Bohnett M. Brain Res. 1991;564:66–72. doi: 10.1016/0006-8993(91)91352-2. [DOI] [PubMed] [Google Scholar]
- 3.Velaquez-Moctezuma J, Gillin J C, Shiromani P J. Brain Res. 1989;503:128–131. doi: 10.1016/0006-8993(89)91712-5. [DOI] [PubMed] [Google Scholar]
- 4.Bartolini A, Ghelardini C, Fantetti L, Malcangio M, Malmberg-Aiello P, Giotti A. Br J Pharmacol. 1992;105:77–82. doi: 10.1111/j.1476-5381.1992.tb14213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turski L, Ikonomidou C, Turski W A, Bortolotto A A, Cavalheiro E A. Synapse. 1989;3:154–171. doi: 10.1002/syn.890030207. [DOI] [PubMed] [Google Scholar]
- 6.Hammer R, Giachetti A. Life Sci. 1982;31:2991–2999. doi: 10.1016/0024-3205(82)90066-2. [DOI] [PubMed] [Google Scholar]
- 7.Bonner T I, Buckley N J, Young A C, Brann M R. Science. 1987;237:527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- 8.Bonner T I, Young A C, Brann M R, Buckley N J. Neuron. 1988;1:403–410. doi: 10.1016/0896-6273(88)90190-0. [DOI] [PubMed] [Google Scholar]
- 9.Hulme E C, Birdsall N J M, Buckley N J. Annu Rev Pharmacol Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- 10.Nathanson N M. Annu Rev Neurosci. 1987;10:195–236. doi: 10.1146/annurev.ne.10.030187.001211. [DOI] [PubMed] [Google Scholar]
- 11.Caulfield M P. Pharmacol Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- 12.Wess J. Crit Rev Neurobiol. 1996;10:69–99. doi: 10.1615/critrevneurobiol.v10.i1.40. [DOI] [PubMed] [Google Scholar]
- 13.Adams P R, Brown D A, Constanti A. J Physiol. 1982;332:223–262. doi: 10.1113/jphysiol.1982.sp014411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole A E, Nicoll R A. Brain Res. 1984;305:283–290. doi: 10.1016/0006-8993(84)90434-7. [DOI] [PubMed] [Google Scholar]
- 15.Marrion N V. Annu Rev Physiol. 1997;59:483–504. doi: 10.1146/annurev.physiol.59.1.483. [DOI] [PubMed] [Google Scholar]
- 16.Marrion N V, Smart T G, Marsh S J, Brown D A. Br J Pharmacol. 1989;98:557–573. doi: 10.1111/j.1476-5381.1989.tb12630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernheim L, Mathie A, Hille B. Proc Natl Acad Sci USA. 1992;89:9544–9548. doi: 10.1073/pnas.89.20.9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorje F, Levey A I, Brann M R. Mol Pharmacol. 1991;40:459–462. [PubMed] [Google Scholar]
- 19.Mello L M, Cavalheiro E A, Tan A M, Kupfer W R, Pretorius J K, Babb T L, Finch D M. Epilepsia. 1993;34:985–995. doi: 10.1111/j.1528-1157.1993.tb02123.x. [DOI] [PubMed] [Google Scholar]
- 20.Turski W A, Cavalheiro E A, Bortolotto Z A, Mello L M, Schwarz M, Turski L. Brain Res. 1984;321:237–253. doi: 10.1016/0006-8993(84)90177-x. [DOI] [PubMed] [Google Scholar]
- 21.Wasterlain C G, Jonec V. Brain Res. 1983;271:311–323. doi: 10.1016/0006-8993(83)90293-7. [DOI] [PubMed] [Google Scholar]
- 22.Cruickshank J W, Brudzynski S M, McLachlan R S. Brain Res. 1994;643:125–129. doi: 10.1016/0006-8993(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 23.Maslanski J A, Powelt R, Deirmengiant C, Patelt J. Neurosci Lett. 1994;168:225–228. doi: 10.1016/0304-3940(94)90456-1. [DOI] [PubMed] [Google Scholar]
- 24.Gale K. In: Epilepsy: Models, Mechanisms, and Concepts. Schwartzkroin P A, editor. Cambridge: Cambridge Univ. Press; 1993. pp. 48–93. [Google Scholar]
- 25.Solberg Y, Belkin M. Trends Pharmacol. 1997;18:183–185. doi: 10.1016/s0165-6147(97)89540-5. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro R A, Scherer N M, Habecker B A, Subers E M, Nathanson N M. J Biol Chem. 1988;263:18397–18403. [PubMed] [Google Scholar]
- 27.Brandon E P, Gerhold K A, Qi M, McKnight G S, Idzerda R L. Recent Prog Horm Res. 1995;50:403–408. doi: 10.1016/b978-0-12-571150-0.50028-7. [DOI] [PubMed] [Google Scholar]
- 28.Levey A I, Kitt C A, Simonds W F, Price D L, Brann M R. J Neurosci. 1991;11:3218–3226. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKinnon L A, Nathanson N M. J Biol Chem. 1995;270:20636–20642. doi: 10.1074/jbc.270.35.20636. [DOI] [PubMed] [Google Scholar]
- 30.Luetje C W, Brumwell C, Morman M G, Peterson G L, Schimerlik M I, Nathanson N M. Biochemistry. 1987;26:6892–6896. doi: 10.1021/bi00396a003. [DOI] [PubMed] [Google Scholar]
- 31.Nathanson N M. J Neurochem. 1983;41:1545–1549. doi: 10.1111/j.1471-4159.1983.tb00862.x. [DOI] [PubMed] [Google Scholar]
- 32.Bernheim L, Beech D J, Hille B. Neuron. 1991;6:859–867. doi: 10.1016/0896-6273(91)90226-p. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro M S, Hille B. Neuron. 1993;10:11–20. doi: 10.1016/0896-6273(93)90237-l. [DOI] [PubMed] [Google Scholar]
- 34.Starr M S, Starr B S. Pharmol Biochem Behav. 1994;47:127–131. doi: 10.1016/0091-3057(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 35.Hosford D A, McNamara J O. Brain Res. 1988;462:205–210. doi: 10.1016/0006-8993(88)90548-3. [DOI] [PubMed] [Google Scholar]
- 36.Morrison R S, Wenzel H J, Kinoshita Y, Robbins C A, Donehower L A, Schwarzkroin P A. J Neurosci. 1996;16:1337–1345. doi: 10.1523/JNEUROSCI.16-04-01337.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Constanti A, Brown D A. Neurosci Lett. 1981;24:289–294. doi: 10.1016/0304-3940(81)90173-7. [DOI] [PubMed] [Google Scholar]
- 38.Lothman E W, Collins R C. Brain Res. 1981;218:299–318. doi: 10.1016/0006-8993(81)91308-1. [DOI] [PubMed] [Google Scholar]
- 39.Brown E, Kendall D A, Nakorski S R. J Neurochem. 1984;42:1379–1387. doi: 10.1111/j.1471-4159.1984.tb02798.x. [DOI] [PubMed] [Google Scholar]
- 40.McCormick D A, Prince D A. Proc Natl Acad Sci USA. 1985;82:6344–6348. doi: 10.1073/pnas.82.18.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harvey J, Balasubramaniam R, Collingridge G L. Neurosci Lett. 1993;162:165–168. doi: 10.1016/0304-3940(93)90586-a. [DOI] [PubMed] [Google Scholar]
- 42.Liu C, Gillette M U. J Neurosci. 1996;16:744–751. doi: 10.1523/JNEUROSCI.16-02-00744.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gu Q, Singer W. Eur J Neurosci. 1993;5:475–485. doi: 10.1111/j.1460-9568.1993.tb00514.x. [DOI] [PubMed] [Google Scholar]
- 44.Polidori C, Massi M, Lambrecht G, Mutschler E, Tacke R, Melchiorre C. Eur J Pharmacol. 1990;179:159–165. doi: 10.1016/0014-2999(90)90413-z. [DOI] [PubMed] [Google Scholar]