Abstract
Maintenance of weight loss is often unsuccessful because of metabolic adaptations that conserve energy. Studies in rodents suggest that a reduction in leptin level during weight loss signals to the brain to increase feeding and decrease energy expenditure. In this issue of the JCI, Rosenbaum et al. examined this concept in obese patients who lost weight and were maintained at 10% below their initial weight (see the related article beginning on page 2583). Brain activity responses to visual food stimuli were visualized using functional MRI. Leptin levels fell during weight loss and increased brain activity in areas involved in emotional, cognitive, and sensory control of food intake. Restoration of leptin levels maintained weight loss and reversed the changes in brain activity. Thus, leptin is a critical factor linking reduced energy stores to eating behavior. Potentially, leptin therapy could sustain weight loss by overriding the tendency toward energy conservation.
Obesity results from an imbalance between food intake and energy expenditure, culminating in excessive accumulation of fat in adipose tissue, liver, muscle, pancreatic islets, and other organs involved in metabolism. Obesity increases the risk of diabetes, coronary artery disease, fatty liver, gall stones, sleep apnea, arthritis, and cancer and may shorten the lifespan (1). Our knowledge of the neurobiology of feeding and energy homeostasis has benefited from the discovery of fat- and gut-derived hormones and their targets in the hypothalamus (2, 3) (Figure 1). However, eating is a complex behavior that is determined not only by conscious decision-making, but is also subject to environmental factors such as the availability and properties of food and social and cultural norms. A greater understanding of how brain areas involved in reward, cognition, and executive control of feeding can override metabolic regulation will facilitate the prevention and treatment of obesity.
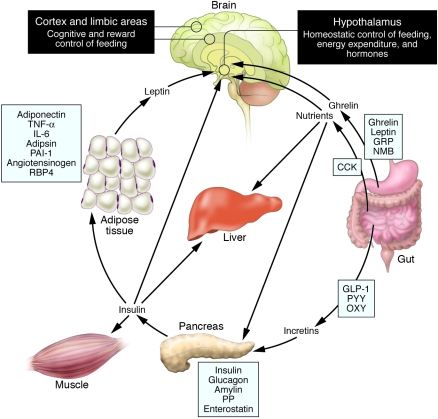
Figure 1. Schematic illustration of peptides secreted by the gut and adipose tissue (fat) that control energy balance.
Ghrelin is produced in the stomach and is a potent stimulator of appetite in the brain. In addition to increasing the uptake of nutrients by muscle, liver, and fat, insulin acts in the brain to suppress food intake. Gut-derived peptides such as GLP-1 augment insulin release from the pancreas. Leptin levels decline during weight loss and signal to the hypothalamus to stimulate feeding, reduce energy expenditure, and promote weight regain. As Rosenbaum et al. demonstrate in this issue of the JCI (4), low leptin levels during weight loss also increase the activity of brain areas involved in the decision-making and reward aspects of eating behavior. Thus, preventing the decline of leptin levels during weight loss by hormone replacement may be a means of overriding the homeostatic and behavioral tendencies toward energy conservation and weight regain during dieting. Image modified with permission from Gastroenterology (23). CCK, cholecystokinin; GLP-1, glucagon-like peptide–1; GRP, gastrin-releasing peptide; NMB, neuromedin B; OXY, oxyntomodulin; PAI-1, plasminogen activator inhibitor 1; PP, pancreatic polypeptide; PYY, peptide YY; RBP4, retinol-binding protein–4.
Caloric restriction is a logical strategy for weight reduction, but cannot be sustained in the long term partly because of increased hunger and reduction in metabolic rate, which promote energy conservation and regain of weight. In the current issue of the JCI, Rosenbaum et al. describe specific patterns of brain activity in the cerebral cortex, limbic areas, and hypothalamus when weight-reduced patients were presented with visual food stimuli (4). Interestingly, restoration of leptin to pre–weight loss levels reversed the changes in brain activity while preventing weight regain. Thus, in addition to controlling hypothalamic neuronal circuits, metabolic signals such as leptin may entrain the perception of food through unexpected effects on the processing of visual and other sensory information.
Leptin’s ups and downs
Leptin is secreted by fat cells (adipocytes), and was originally thought to signal to the brain to inhibit food intake and decrease weight (2, 5). This concept was partly driven by the observation that humans and rodents lacking a functional leptin protein or receptor manifested voracious feeding and obesity (2). As was predicted, leptin treatment, particularly direct injection of leptin into the cerebral ventricle or hypothalamus, profoundly inhibited food intake and decreased weight and fat in animals lacking leptin (2, 3, 6). However, the notion of leptin as an anti-obesity hormone was called into question because obesity is typically associated with high leptin levels and not leptin deficiency (6, 7). Moreover, rodents and humans that become obese on a high-fat (Western) diet do not respond to leptin (6, 7). In rodents, studies have demonstrated that leptin is transported into the brain, binds to its receptor in the hypothalamus, and activates JAK-STAT3, leading to suppression of “orexigenic peptides” (e.g., neuropeptide Y and agouti-related protein, which normally increase food intake), and increase in “anorexigenic peptides” (e.g., proopiomelanocortin and corticotrophin-releasing hormone, which normally decrease food intake) (3). Although there is no obvious defect of leptin receptors in diet-induced obese rodents, the transport of leptin across the blood-brain barrier is diminished and levels of SOCS3, an inhibitor of leptin signaling, is increased in the hypothalamus (3, 6). However, it is unknown whether these factors are involved in “common” (diet-induced; polygenic) obesity in humans.
Leptin levels fall rapidly in response to fasting and evoke profound changes in energy balance and hormone levels (5, 6). Low leptin levels induce overfeeding and suppress energy expenditure, thyroid and reproductive hormones, and immunity (5, 8–10). In rodents, low leptin levels increase levels of orexigenic peptides and reduce levels of anorexigenic peptides (3, 5). Leptin replacement reverses these alterations in metabolism, immunity, and levels of hormones and hypothalamic neuropeptides (5, 8–10). Moreover, restoration of leptin in patients lacking fat cells (lipodystrophy) improves reproductive function and reverses abnormal lipid and glucose metabolism (11, 12). Together, these studies demonstrate that the dominant role of leptin is to signal energy deficiency in the brain (6). Teleologically, the adaptations mediated by reduced leptin levels may have evolved as a protection against the threat of starvation by limiting energy use and enhancing energy storage in the form of fat (5, 6). In the modern environment, where food is plentiful and exercise is sparse, this metabolic efficiency predisposes toward obesity.
In earlier studies, Rosenbaum et al. examined the concept that leptin is a critical signal for metabolic alterations induced by caloric restriction in humans (13, 14). Obese subjects were fed a liquid diet to maintain their usual weight for several weeks, or were fed a reduced-calorie liquid diet to maintain a 10% reduction in weight. The hypothesis was that a decrease in leptin levels in the weight-reduced state would reduce energy expenditure and promote weight regain. Leptin hormone replacement prevented the decline in plasma leptin level and restored energy expenditure by increasing skeletal muscle work efficiency, sympathetic nervous system tone, and thyroid hormone to pre–weight loss levels (13, 14). These changes were associated with maintenance of weight reduction (13, 14).
Imaging leptin activity in human brain
Although studies in animals have unraveled circuits in the hypothalamus and brainstem that are influenced by peptides derived from the gut and fat, these studies do not address the cognitive and emotional aspects of eating behavior (3, 5, 6). Appetite is a subjective feeling of the motivation to eat. Hunger, satiety, and satiation can be overridden by desire. Our food preferences and eating patterns are molded by past experiences and also influenced by sight, smell, and taste sensations. Eating behavior involves a complex interaction between psychological and physiological processes that cannot be adequately explored in animal models. Humans provide the appropriate model for addressing how sensory and hormonal signals interact to produce subjective and physiological changes in energy metabolism.
The study by Rosenbaum et al. in this issue of the JCI is an important contribution in elucidating how the human brain processes food stimuli (4). The authors hypothesized that the weight-reduced state is perceived by the brain as a state of relative leptin deficiency, which evokes specific patterns of brain activity when individuals are presented with visual food stimuli. Leptin replacement to individuals in the weight-reduced state was predicted to reverse brain activity to patterns similar to the obese (pre–weight loss) state. By delineating these differences, the authors hoped to gain insights into how metabolic signals produced by fat affect eating behavior. The experimental design was identical to previous studies (13, 14). Obese subjects underwent caloric restriction, were maintained at 10% lower than their initial weight, and received replacement doses of leptin or placebo in a crossover design (research design in which subjects receive both treatments in sequence so that, in effect, each individual serves as his/her own control) for 5 weeks. Brain activity elicited by viewing food and non-food visual stimuli was monitored using functional MRI (fMRI). This technique measures blood oxygen level–dependent (BOLD) signals and is based on the principle that deoxyhemoglobin concentration in the blood supply decreases upon activation of a group of neurons. The decline in deoxyhemoglobin is visualized as a high signal intensity when a T2-weighted image is obtained under high magnetic field and speed. BOLD signal is not reflective of the electrical activity of neurons. Rather, BOLD is determined by the amount of deoxyhemoglobin, which in turn depends on regional blood volume, blood flow, and oxygen consumption.
Rosenbaum et al. present a thorough description of brain activity under different weight conditions and leptin levels (4). At the initial weight, visual food stimuli induced activity in brain areas involved in energy homeostasis, autonomic and hormonal regulation, as well as emotional and executive control of eating behavior. The hypothalamus, amygdala, hippocampus, parahippocampal and cingulate gyri, and frontal and parietal cortex all showed increased activity. In contrast, the weight-reduced state was associated with increased activity in the brainstem, parahippocampal gyrus, culmen, and globus pallidus, as well as areas in the frontal and temporal cortex involved in decision-making functions. As predicted, leptin replacement in the weight-reduced state reversed brain activity to the pattern observed at the initial weight. These findings could have been strengthened by the inclusion of subjective ratings of appetite and measurement of other circulating metabolic factors aside from leptin. Furthermore, BOLD signals do not establish whether the brain regions affected by leptin are targeted directly or indirectly.
Nonetheless, the study extends the recent mapping of brain responses in patients with congenital leptin deficiency (15, 16). Farooqi et al. determined that in the leptin-deficient state, images of well-liked food elicited a desire to eat even when the subjects had just eaten (15). In contrast, after leptin treatment, images of well-liked food elicited a desire to eat only during fasting (15). The latter was similar to normal subjects and related to leptin’s ability to suppress activity in the striatum, a region involved in the pleasure and reward responses to food (15). Baicy et al. observed that leptin replacement decreased food intake and weight, and this was associated with reduced brain activity in regions related to hunger (16). On the other hand, leptin increased brain activity in areas linked to satiety (16).
Implications for obesity therapy
The earlier studies of Rosenbaum et al. revealed that leptin deficiency promoted weight regain by stimulating appetite and reducing energy output (13, 14). In the current study, the decline in leptin served as a key metabolic signal to modulate the reward and executive control of visual food stimuli (4) (Figure 1). The ability of leptin replacement to reverse these changes suggests that leptin itself or drugs that stimulate leptin signaling may facilitate the maintenance of weight loss. Indeed, leptin replacement augmented the effect of a weight loss drug, sibutramine, in diet-induced obese rats by further decreasing food intake and stimulating fatty acid oxidation (17). Likewise, it is possible that preventing the decline in leptin level, via hormone replacement, could sustain the effects of dieting or drug treatment in obese patients.
Another area that deserves further investigation is whether obese individuals with disproportionately low leptin levels might benefit from leptin therapy. This strategy is akin to insulin treatment in patients with type 2 diabetes characterized by relative insulin deficiency resulting from pancreatic β cell failure. Population studies indicate that approximately 10% of obese individuals have low plasma leptin levels (6). Are these obese individuals truly leptin deficient? Does low leptin level portend obesity later on in life? Indeed, humans and rodents with heterozygous mutation of the gene encoding leptin manifest partial leptin deficiency, increased body fat, and abnormal glucose and lipid metabolism (18, 19). Reduced leptin levels may precede obesity and predict a poor outcome to weight loss (20, 21). Thus, screening for partial leptin deficiency could identify obese individuals in need of leptin replacement therapy. Mapping of the structure, chemistry, and electrical activity of the human brain may also offer insights into therapeutic strategies targeting the central processing of food stimuli.
Of mice and humans
These elegant and inherently difficult studies by Rosenbaum et al. and others highlight the potential of clinical investigation (4, 13, 14–16). More than a decade after the discovery of leptin (2), we now have studies exploring the activity of this metabolic hormone in the human brain. There is a tendency to label clinical studies as “descriptive” or “lacking in mechanism.” To the contrary, the experiments outlined above are based on well-thought-out hypotheses, ingenious methodology, and cogent interpretation of data. Above all, the results are applicable in humans. There is no doubt that animal models, in particular mice, have advanced our knowledge of molecular genetics and facilitated preclinical experimentation. But lest we forget, mice are not human! In fact, there are numerous instances in which animal models based on inappropriate conceptual paradigms have led to spectacular failures and actually hindered medical discovery. Given the recent clamoring for “bench-to-bedside” investigation, it is gratifying to see the well-deserved publication of clinical/patient-oriented research (4, 15, 16, 22). This trend will undoubtedly encourage further research on obesity and metabolic disorders.
Footnotes
Nonstandard abbreviations used: BOLD, blood oxygen level–dependent.
Conflict of interest: The author has received research support from Biomeasure/Ipsen Inc. and served on scientific advisory boards of Bristol-Myers Squibb Co., Biomeasure/Ipsen Inc., and Ethicon Endo-Surgery (Johnson & Johnson).
Citation for this article: J. Clin. Invest. 118:2380–2383 (2008). doi:10.1172/JCI36284.
See the related article beginning on page 2583.
References
- 1.Ogden C.L., Yanovski S.Z., Carroll, Flegal K.M. The epidemiology of obesity. Gastroenterology. 2007;132:2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y., et al. Positional cloning of the mouse obese gene and its human homologue [erratum 1995, 374: 479]. . Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 3.Morton G.J., et al. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 4.Rosenbaum M., Sy M., Pavlovich K., Leibel R.L., Hirsch J. Leptin reverses weight loss–induced changes in regional neural activity responses to visual food stimuli. J. Clin. Invest. 2008;118:2583–2591. doi: 10.1172/JCI35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahima R.S., et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 6.Flier J.S. Clinical review 94: what’s in a name? In search of leptin’s physiologic role. J. Clin. Endocrinol. Metab. 1998;83:1407–1413. doi: 10.1210/jcem.83.5.4779. [DOI] [PubMed] [Google Scholar]
- 7.Heymsfield S.B., et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 8.Welt C.K., et al. Recombinant human leptin in women with hypothalamic amenorrhea. N. Engl. J. Med. 2004;351:987–997. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 9.Chan J.L., et al. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. . J. Clin. Invest. 2003;111:1409–1421. doi: 10.1172/JCI17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farooqi I.S., et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J. Clin. Invest. 2002;110:1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oral E.A., et al. Leptin-replacement therapy for lipodystrophy. N. Engl. J. Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 12.Oral E.A., et al. Effect of leptin replacement on pituitary hormone regulation in patients with severe lipodystrophy. J. Clin. Endocrinol. Metab. 2002;87:3110–3117. doi: 10.1210/jc.87.7.3110. [DOI] [PubMed] [Google Scholar]
- 13.Rosenbaum M., et al. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J. Clin. Endocrinol. Metab. 2002;87:2391–2394. doi: 10.1210/jc.87.5.2391. [DOI] [PubMed] [Google Scholar]
- 14.Rosenbaum M., et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. . J. Clin. Invest. 2005;115:3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farooqi I.S., et al. Leptin regulates striatal regions and human eating behavior. Science. 2007;317:1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baicy K., et al. Leptin replacement alters brain response to food cues in genetically leptin-deficient adults. Proc. Natl. Acad. Sci. U. S. A. 2007;104:18276–18279. doi: 10.1073/pnas.0706481104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boozer C.N., et al. Synergy of sibutramine and low-dose leptin in treatment of diet-induced obesity in rats. Metabolism. 2001;50:889–893. doi: 10.1053/meta.2001.24917. [DOI] [PubMed] [Google Scholar]
- 18.Farooqi I.S., et al. Partial leptin deficiency and human adiposity. Nature. 2001;414:34–35. doi: 10.1038/35102112. [DOI] [PubMed] [Google Scholar]
- 19.Begriche K., et al. Partial leptin deficiency favors diet-induced obesity and related metabolic disorders in mice. Am. J. Physiol. Endocrinol. Metab. 2008;294:E939–E951. doi: 10.1152/ajpendo.00379.2007. [DOI] [PubMed] [Google Scholar]
- 20.Verdich C., et al. Leptin levels are associated with fat oxidation and dietary-induced weight loss in obesity. Obes. Res. 2001;9:452–461. doi: 10.1038/oby.2001.59. [DOI] [PubMed] [Google Scholar]
- 21.Filozof C.M., et al. Low plasma leptin concentration and low rates of fat oxidation in weight-stable post-obese subjects. Obes. Res. 2000;8:205–210. doi: 10.1038/oby.2000.23. [DOI] [PubMed] [Google Scholar]
- 22.Batterham R.L., et al. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. . 2007;450:106–109. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]
- 23.[No authors listed]. 2007. Gastroenterology. 132(Issue 6), cover figure [Google Scholar]