Abstract
Purpose
As human β-defensins (hBD) are important antimicrobial peptides at epithelial surfaces, including the ocular surface, we tested the effect of hyperosmolar conditions on the expression of these peptides by human corneal epithelial cells (HCECs).
Methods
Simian virus 40–transformed HCECs (n = 5) or primary cultured HCECs (n = 5) were treated with serum-free media or serum-free hyperosmolar (400–500 mOsm/kg) media for 24 hours or serum-free 500 mOsm/kg media for 12 to 48 hours. The effect of hyperosmolality on interleukin-1β (IL-1β)–induced hBD-2 expression was also tested. IL-6 expression was studied as a marker of IL-1β function. Expression of hBD-1, -2, and -3 and IL-6 mRNA was detected by reverse transcription–polymerase chain reaction (RT-PCR). The levels of active IL-1β (culture supernatants and cell lysates) and pro–IL-1β (cell lysates) were detected by enzyme-linked immunosorbent assay.
Results
HCECs constitutively expressed hBD-1 and -3 but not hBD-2. Hyperosmolar media had no effect on the basal expression of hBD-1 or -3 and did not induce the expression of hBD-2. Treatment with 500 mOsm/kg media for 24 hours decreased the ability of IL-1β to upregulate hBD-2 and IL-6 expression. Active or pro–IL-1β was not detected in any cell culture sample.
Conclusion
Our results suggest that the hyperosmolar environment observed in diseases such as dry eye does not alter defensin expression. However, a hyperosmolar environment may influence cytokine function in ocular surface cells and thus affect their response to injury and inflammation.
Keywords: hyperosmolality, human β-defensins, corneal epithelium, cytokines
Hyperosmolality at the ocular surface is a characteristic of diseases such as dry eye and diabetes mellitus. The normal osmolality of the tear fluid is approximately 300 mOsm/kg,1 whereas the suggested “gold standard” diagnostic of dry eye is 312 mOsm/kg or greater.2 It has been proposed that tear fluid hyperosmolality occurs because of either normal tear evaporation associated with reduced tear flow or excessive tear evaporation with a normal tear flow.3 The hyperosmolar tear film is thought to affect ocular surface epithelial function and differentiation in dry eye disease, with osmolalities as high as 417 mOsm/kg being reported.1,4,5 Also, Aragona et al6 showed that type 1 diabetic patients had a significantly higher mean tear osmolality (332.2 ± 18.3 mOsm/L), poor tear stability, altered tear function, and symptoms of ocular irritation akin to patients with dry eye than normal patients. Hyperosmolality in diabetes mellitus is probably attributable to increased levels of tear glucose seen in patients with both types 1 and 2.7
In human corneal epithelial cells (HCECs) in culture, chronic (up to 48 hours) hypertonic (350–600 mOsm/kg) stress was found to decrease proliferation rates and reduced the regulatory volume response (a mechanism by which cells recover their original volume in a hypertonic environment) by altering Na-K-2Cl cotransporter expression.8 Rabbit corneal epithelial cells exposed to a hyperosmolar media (330–407 mOsm/kg) showed decreased intercellular connections, increased desquamation, and loss of microplicae.9 Furthermore, HCECs cultured in high-osmolality media (412–512 mOsm/kg) secreted greater amounts of proinflammatory cytokines such as interleukin (IL)-1β and tumor necrosis factor (TNF)-α.10 A recent study showed that the hyperosmolality (350–500 mOsm/kg)-induced production of the matrix metalloproteinases (MMP)-1, -3, and -9 was partially mediated by activation of stress-activated protein kinase pathways (c-Jun N-terminal kinase pathway).11 These findings are consistent with observations in other tissues that hyperosmolality can cause a variety of effects on cellular function. For example, hyperosmolality reduces proliferation rates and induces apoptosis in murine renal medullary cells,12 increases hepatocyte growth factor secretion in human mesangial cells,13 increases platelet-derived growth factor secretion in human umbilical vein endothelial cells,14 activates various serine/threonine kinases,15 and alters calcium mobilization patterns in rat vascular smooth muscle cells.16
Recent studies have shown that the ocular surface epithelia produce antimicrobial peptides called β-defensins.17,18 Human β-defensin-1 (hBD-1) and hBD-3 are constitutively expressed by the corneal and conjunctival epithelia,18–20 whereas the expression of hBD-2 is inducible by proinflammatory cytokines such as IL-1α, IL-1β, TNF-α, bacterial by-products such as lipopolysaccharide and heat-killed Pseudomonas aeruginosa, and injury.19–22 We have also observed that hBD-2 is expressed by conjunctival epithelial cells from patients with moderate dry eye but not in cells from normal subjects.20 Because it has been reported that proinflammatory cytokines are secreted in greater amounts by HCECs in hyperosmolar media,10 we reasoned that a differential expression of β-defensins by HCECs may be observed when the cells are in a hyperosmolar environment. Such a response may underlie our observation of conjunctival hBD-2 expression in dry eye patients but not normal subjects. Altered β-defensin expression associated with hyperosmolality may be important in conditions such as dry eye. Although it is known that the antimicrobial activity of some defensins is compromised by high salt concentrations,23 which is in keeping with the observation that the compromised ocular surface of patients with dry eye caused by Sjogren syndrome is at risk for microbial infection.24 However, in addition to their antimicrobial effects, defensins are known to modulate the behavior of mammalian cells; thus, even if hyperosmolality-induced defensin expression cannot contribute to antimicrobial protection, the defensins may have other effects on ocular surface epithelial cells such as promoting migration and proliferation.25 Therefore, the goal of this study was to investigate if differential expression of β-defensins by HCECs in culture was observed under hyperosmolar growth conditions.
Materials and Methods
Cell Culture
Simian virus 40–transformed HCECs26 (SV40-HCECs) were a gift of Dr Araki Sasaki (Tane Memorial Hospital, Kumamoto, Japan). SV40-HCECs were cultured with Ham's F12 and Dulbecco minimal essential medium (1:1 vol/vol; supplemented hormone epithelial medium [SHEM]) containing 0.5% vol/vol dimethylsulphoxide, 10% fetal bovine serum, and gentamicin (30 μg/mL). Human corneas unsuitable for transplant were obtained from Heartlands Eye Bank (Columbia, MO) within 3 to 5 days of death of the donor. Primary cultured HCECs (P-HCECs) were derived from corneas of 5 donors (mean age, 66.1 ± 5.2 [SD] years) by using a method described previously.19 P-HCECs were grown in EpiLife media with Human Corneal Growth Supplement (Cascade Biologics, Portland, OR). All cells were maintained in a humidified atmosphere of 95% air/5% CO2 at 37°C. When confluent, cells were passed using standard trypsin–EDTA methods. P-HCECs of passages 1 to 2 and SV40-HCECs of passages 18, 19, 22, 26, and 31 were used in the experiments.
Hyperosmolality Experiments
On reaching approximately 80% confluency, HCECs were washed twice in phosphate-buffered saline (PBS). Fresh serum-free SHEM (308 ± 1.0 [SD] mOsm/kg) or supplement-free EpiLife (295.5 ± 2.1 mOsm/kg) media were added, and the cells were left to incubate overnight. On the basis of a previous study,10 serum-free media with osmolalities ranging from 400 to 500 mOsm/kg were made by adding appropriate amounts of 1 mol/L NaCl. Osmolality of the media was measured using a Vapro 5520 vapor pressure osmometer (Wescor, Logan, UT). The cells were exposed to growth media of different osmolalities (400–500 mOsm/kg; n = 4) for 24 hours or to 500 mOsm/kg media for various times (12–48 hours; n = 4) of incubation. In each experiment, media of normal osmolality containing IL-1β (10 ng/mL; 24-hour duration) was added to some cells as a positive control to induce hBD-2 expression.19
To test the effect of osmolality on the ability of IL-1β to stimulate the expression of hBD-2, SV40-HCECs and P-HCECs were incubated in media of normal osmolality or 400 to 500 mOsm/kg (n = 1–4 depending on osmolality) hyperosmolar media for 24 hours. At the end of the incubation, cells were treated with 10 ng/mL IL-1β for 6 hours. The above experiment was repeated with cells cultured in hyperosmolar media (500 mOsm/kg) made by the addition of 225 mmol/L mannitol. Because IL-6 induction is a marker of IL-1β function, expression of IL-6 mRNA in SV40-HCECs (n = 2) and P-HCECs (n = 2) cultured in 500 mOsm/kg (NaCl) media with or without IL-1β stimulation was studied. On completion of each experiment, cells and culture supernatants were collected and stored at −80°C until they were processed for RNA extraction (cells) or to detect active or pro–IL-1β protein secretion (cells and culture supernatants). To test the effect of osmolality on the ability of IL-1β to stimulate hBD-2 protein secretion, SV40-HCECs (n = 1) and P-HCECs (n = 2) were incubated in media of normal osmolality or 500 mOsm/kg hyperosmolar media for 24 hours. At the end of the incubation, cells were treated with 10 ng/mL IL-1β for 12 hours, and the culture supernatants were collected to detect hBD-2 protein secretion by immunoblotting. A 12-hour treatment duration was chosen based on earlier results20 that showed that IL-1β–induced hBD-2 protein secretion is detectable only after this period.
Reverse Transcription–Polymerase Chain Reaction
Total RNA was extracted from the cell samples by using an RNeasy kit (Qiagen, Valencia, CA). One-step reverse transcription–polymerase chain reaction (RT-PCR) was performed using a Superscript 1 kit (Invitrogen, Carlsbad, CA) using 250 ng of total RNA and 25 pmol of gene-specific primers to detect mRNA expression of the constitutively expressed GAPDH gene and the hBD-1, -2, and -3 genes. The primer sequences used and expected product sizes were as follows: GAPDH,21 forward 5′-GTGAAGGTCGGAGTCAACGGATTT-3′, reverse 5′-CACAGTCTTCTGGGTGGCAGTGAT-3′, 555 bp; hBD-1,18 forward 5′-CCCAGTTCCTGAAATCCTGA-3′, reverse 5′-CAGGTGCCTTGAATTTTGGT-3′, 215 bp; hBD-2,21 forward 5′-CCAGCCATCAGCCATGAGGGT-3′, reverse 5′-GGAGCCCTTTCTGAATCCGCA-3′, 257 bp; hBD-3,27 forward 5′-AGCCTAGCAGCTATGAGGATC-3′, reverse 5′-CTTCGGCAGCATTTTCGGCCA-3′, 206 bp; IL-6, forward 5′-CCTTCTCCACAAGCGCCTTC-3′, reverse 5′-GGCAAGTCTCCTCATTGAATC-3′, 327 bp.
Reverse transcription was performed at 50°C for 1 hour to generate the cDNA, followed by 5 minutes at 94°C to denature the enzyme. PCR amplification was performed for 35 cycles of denaturation at 94°C (50 seconds), annealing 60°C (1 minute), and primer extension at 72°C (1 minute). Initial RT-PCR experiments were performed (data not shown) to determine that 35 cycles of PCR amplification was still within the linear range of the reaction. Ethidium bromide–stained 1.3% agarose gels were used to analyze the PCR products. An Alpha-imager (Alpha Innotech, San Leandro, CA) gel documentation system was used to obtain digital images and analyze them semiquantitatively. hBD-2 expression levels were normalized with GAPDH expression and used in statistical analysis. A nonparametric 2-way analysis of variance by ranks (Friedman test) was performed on some of the hBD-2 expression data to compare the effect of various treatments on SV40-HCECs. Controls in which either the nucleic acid or reverse transcriptase was omitted were also performed, in which case no product was obtained (data not shown). PCR products were sequenced (Seqwright, Houston, TX) to confirm their identities.
Immunoblotting
Immunoblotting was performed as described previously20 to detect hBD-2 protein secretion in P-HCECs (n = 2) and SV40-HCECs (n = 1). Two hundred microliters of culture supernatant was applied by gravity to a nitrocellulose membrane by using a dot-blot apparatus. Nonspecific binding sites were blocked by incubating in Tris-buffered saline (TBS) containing 5% nonfat powdered milk for 30 minutes at room temperature. The membrane was incubated overnight with rabbit anti-human hBD-2 (donated by Dr T. Ganz, UCLA), diluted 1:2000 in TBS containing 5% nonfat powdered milk, 5% goat serum, 0.05% Tween 20, and 0.02% sodium azide. After incubation, the membrane was washed (3 × 10 minutes; TBS) and incubated for 1 hour at room temperature with a horseradish peroxidase–conjugated goat anti-rabbit antibody (Jackson Laboratories, West Grove, PA), diluted 1:10,000 in TBS containing 5% nonfat powdered milk. Immunoreactivity was visualized by enhanced chemiluminescence (Amersham, Piscataway, NJ). The membrane was scanned using a desktop scanner to document the results.
ELISA
Cell lysates (cell samples that were dissolved in 100 μL of PBS with 1% proteinase A and sonicated; 0.5 μg total protein) and cell culture supernatants (dilutions tested, 1:40, 1:20, 1:10, 1:5, 1:2, and undiluted supernatant) were used in an enzyme-linked immunosorbent assay (ELISA) to quantify the amount of active IL-1β produced by HCECs (n = 2, SV-40 HCECs; n = 2, P-HCECs) cultured in normal or hyperosmolar (500 mOsm/kg; NaCl or mannitol) media for 6 or 24 hours. Cell lysates (0.5 μg total protein) were also used in an ELISA to quantify the amount of pro–IL-1β in SV40-HCECs (n = 1) and P-HCECs (n = 2) cultured in the same conditions as for the active IL-1β ELISA. Culture supernatant or cell lysates from primary cultured corneal fibroblasts treated with IL-1α (24-hour duration) were used as a positive control for the active and pro–IL-1β assays, respectively. The assays were performed following the manufacturer's instructions (R&D Systems, Minneapolis, MN).
Results
Effect of Hyperosmolar Media on hBD-1 and hBD-3 Expression by HCECs
As seen previously,19 hBD-1 and hBD-3 were constitutively expressed by both SV40-HCECs (n = 4) and P-HCECs (n = 2). As shown in Figure 1, exposure to hyperosmolar media (500 mOsm/kg; NaCl) did not alter the baseline expression of hBD-1 and hBD-3 in SV40-HCECs (n = 4) and P-HCECs (n = 2; data not shown).
Figure 1.
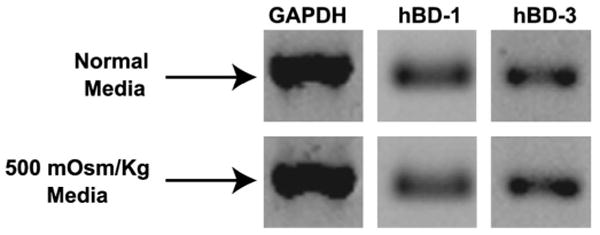
Hyperosmolar media does not alter hBD-1 and hBD-3 expression. SV40-HCECs (n = 4) were cultured for 24 hours in serum-free normal osmolality media or 500 mOsm/kg (NaCl) media. The figure shows RT-PCR products for GAPDH, hBD-1, and hBD-3 from 1 representative experiment. Similar results were obtained with P-HCECs (n = 2; data not shown).
Effect of Hyperosmolar Media on hBD-2 Expression by HCECs
hBD-2 was not expressed by SV40-HCECs but was weakly expressed by P-HCECs at baseline. As shown in Figure 2A, a 24-hour exposure of SV40-HCECs (n = 4) to a range of hyperosmolar media (400–500 mOsm/kg) did not stimulate hBD-2 expression, whereas the addition of the proinflammatory cytokine IL-1β as a positive control did induce hBD-2 in these cells. Incubation of SV40-HCECs (n = 4) in 500 mOsm/kg media for various periods (12–48 hours; Fig. 2B) did not induce hBD-2, whereas IL-1β (24-hour incubation) was able to induce this defensin in these cells. These results were confirmed in P-HCECs (n = 2; data not shown).
Figure 2.
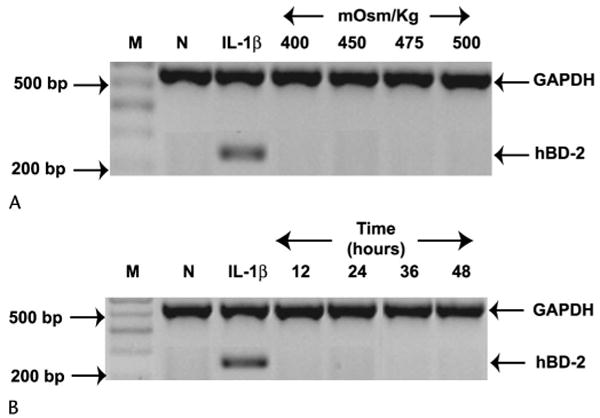
Hyperosmolar medium does not induce hBD-2 expression in HCECs. A, SV40-HCECs (n = 4) were cultured for 24 hours in serum-free normal osmolality media only (N) or with the addition of IL-1β as a positive control (IL-1β) or serum-free hyperosmolar media. B, The cells were exposed to 500 mOsm/kg (NaCl) media for various lengths of time or to IL-1β (positive control) or normal media for 24 hours. The figure shows RT-PCR products for GAPDH and hBD-2. Similar results were obtained when the experiment was repeated with P-HCECs (n = 2; data not shown). M, size marker.
Effect of Hyperosmolar Media on the Ability of IL-1β to Induce hBD-2 and IL-6 in HCECs
Exposure to 500 mOsm/kg hyperosmolar media (NaCl) decreased the ability of IL-1β to induce hBD-2 in SV40-HCECs (n = 4; Fig. 3A, lanes N+I vs. H+I). However, exposure to hyperosmolar media for 24 hours, followed by a brief exposure (6 hours) to normal-osmolality media, restored the ability of IL-1β to induce hBD-2 in SV40-HCECs (Fig. 3A, lane H+N+I). A Friedman test of the normalized hBD-2 expression data revealed the media-only treatment (Fig. 3A, lane N) to have the lowest rank (1.00), followed by the 500 mOsm/kg + IL-1β treatment (rank 2.25; Fig. 3A, lane H+I), the media + IL-1β treatment (rank 2.75; Fig. 3A, lane N+I), and the 500 mOsm/kg + exposure to normal-osmolality media + IL-1β treatment (rank 4.00; Fig. 3A, lane H+N+I). This ranking was statistically significant (P = 0.01). IL-1β function was affected in a similar manner in SV40-HCECs cultured in media of 400 or 450 mOsm/kg (NaCl; data not shown), in P-HCECs cultured in 500 mOsm/kg hyperosmolar media (NaCl; data not shown), and in SV40-HCECs cultured in 500 mOsm/kg media (mannitol; data not shown). Also, immunoblotting revealed that exposure to 500 mOsm/kg hyperosmolar media (NaCl) decreased the ability of IL-1β to induce hBD-2 protein secretion in P-HCECs (n = 2; Fig. 3B, lanes N+I vs. H+I). However, exposure to hyperosmolar media for 24 hours, followed by a brief exposure (6 hours) to normal-osmolality media, restored the ability of IL-1β to induce hBD-2 protein secretion in P-HCECs (Fig. 3B, lane H+N+I). Furthermore (Fig. 3C, lanes N+I vs. H+I), hyperosmolar media (NaCl) also decreased the ability of IL-1β to induce IL-6 expression in SV40-HCECs and P-HCECs.
Figure 3.
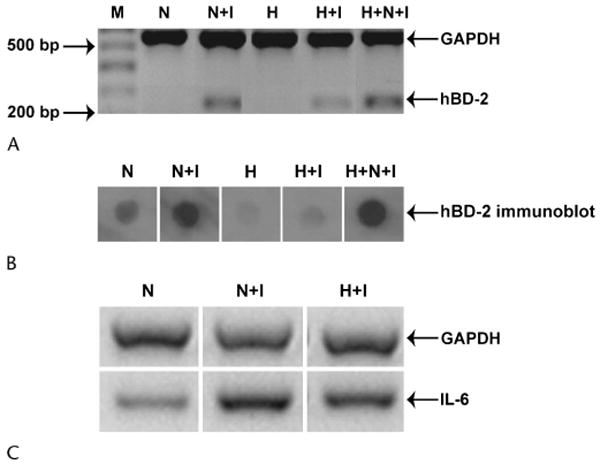
The ability of IL-1β to stimulate hBD-2 and IL-6 expression in HCECs is reduced by 500 mOsm/kg hyperosmolar media. A, SV40-HCECs (n = 4) were cultured for 24 hours in serum-free normal osmolality media (N) or 500 mOsm/kg (NaCl) hyperosmolar media (H). At the end of the incubation, either (1) 10 ng/mL IL-1β was directly added to the culture media of cells in the normal-osmolality media (N+I) and to those in hyperosmolar media (H+I) for 6 hours or (2) the hyperosmolar media was aspirated and substituted with normal-osmolality media containing 10 ng/mL IL-1β for 6 hours (H+N+I). The figure shows representative RT-PCR products for GAPDH and hBD-2 from 1 experiment. M, marker lane. Similar results were obtained with P-HCECs (n = 2, data not shown). B, P-HCECs (n = 2) were cultured for 24 hours in extract-free normal-osmolality media (N) or 500 mOsm/kg (NaCl) hyperosmolar media (H). Experimental conditions were similar to those described for panel A. The figure shows changes in hBD-2 protein secretion by P-HCECs for the various conditions. Similar results were obtained with SV40-HCECs (n = 1, data not shown). C, The experimental conditions were similar to those in panel A. The figure shows RT-PCR products for GAPDH and IL-6. The data are representative of 1 experiment repeated twice with SV40-HCECs. Similar results were obtained with P-HCECs (n = 2, data not shown).
IL-1β Protein Secretion by HCECs
Active IL-1β was not detected in culture supernatants and cell pellets of SV40-HCECs (n = 2) and P-HCECs (n = 2) cultured in normal or hyperosmolar (500 mOsm/kg; NaCl or mannitol) media for 6 or 24 hours. Pro–IL-1β was not detected in cell pellets of SV40-HCECs (n = 1) and P-HCECs (n = 2) cultured in similar conditions as for the active IL-1β assay. However, active (7.9 ng/mg of total protein) and pro–IL-1β (31 ng/mg of total protein) were detected in primary cultured fibroblasts treated with IL-1α, which were the positive controls for the ELISA.
Discussion
The results of this study indicate that hyperosmolality per se does not affect the baseline expression of β-defensins. Thus, at the ocular surface, hyperosmolality would not be expected to modify the risk of microbial infections because of alteration in defensin expression. However, a significant finding of our study was that hyperosmolality reduced the ability of the proinflammatory cytokine IL-1β to upregulate hBD-2 and IL-6 mRNA expression and hBD-2 protein secretion by HCECs. Therefore, a hyperosmolar environment may affect the ocular surface inflammatory response by modulating cytokine function and thereby may also attenuate other roles played by defensins in cell migration and proliferation.
We expected to find an increased expression of hBD-2 in HCECs cultured in hyperosmolar media from the evidence that a hyperosmolar environment increases proinflammatory cytokine secretion by HCECs10,11 and that these cytokines can induce hBD-2 expression by HCECs.19 However, as evident from the results, we did not observe an induction of hBD-2 in HCECs after culture in hyperosmolar media. We attributed this finding to insufficient amounts of IL-1β in the culture media. Therefore, we tested for the presence of active IL-1β in culture supernatants and cell pellets and pro–IL-1β in cell pellet samples obtained from SV40-HCECs and P-HCECs cultured in hyperosmolar media. We were unable to detect active or pro–IL-1β in any of the samples tested, which is in contradiction to Li et al,10 who showed that hyperosmolar media increases proinflammatory cytokine secretion in P-HCECs. However, we were able to detect the expression of active and pro–IL-1β in primary-cultured corneal fibroblasts treated with IL-1α, which were the positive controls for the ELISA. The fact that we did not detect a change in IL-1β levels is compatible with the lack of hBD-2 upregulation observed in our study. We hypothesize that the lack of change in IL-1β levels could be attributed to differences in composition of cell cultures, because Kim et al28 used the limbus as their main source for cells and thus may have a mixed population of limbal and corneal epithelial cells.
Our results regarding IL-1β–induced IL-6 expression are similar to a previous study that showed that peritoneal dialysis fluids, which are often hyperosmolar, reduce the ability of IL-1β to stimulate IL-6 secretion in human peritoneal mesothelial cells.29 An inhibition of IL-1β activity was also shown in human endothelial cells cultured in hyperosmolar media.30 We speculate that hyperosmolar media could affect IL-1β function in 1 or more ways. For instance, hyperosmolality could downregulate the expression of the IL-1 receptor-1 (IL-1R1) or upregulate the IL-1 receptor antagonist, either of which would result in a decrease in the ability of IL-1β to affect cellular gene expression. Hyperosmolality could also affect the intracellular signaling pathways, destabilize IL-1β peptide, or prevent it from binding to IL-1R1. A reduction in IL-1β function in hyperosmolar conditions has important implications for the ocular surface. Because IL-1 is considered to be a major modulator of the ocular surface wound healing and immune response,31 decreased function of this cytokine in patients with a hyperosmolar tear film could affect these processes. This supposition is supported by the fact that preexisting dry eye is a risk factor for severe ocular surface epithelial damage after refractive corneal surgeries.32 It must be noted that a hyperosmolar environment could conceivably affect the functions of other cytokines that are also involved in corneal wound healing and immune response processes. Recent research33 suggests that an increased activity of inflammatory cytokines such as IL-1β is responsible for the ocular surface epithelial damage observed in patients with dry eye. Our results, however, imply that increased levels of IL-1 in patients with dry eye, who show hyperosmolality at the ocular surface, may not necessarily mean an increased activity of this cytokine at the ocular surface.
In summary, hyperosmolality does not alter baseline β-defensin expression by human corneal epithelial cells but may affect the functional ability of proinflammatory cytokines to stimulate the expression of other genes in the inflammatory cascade.
Acknowledgments
Supported by Grants NEI EY 13175 (A.M.M.), NIH P30 EY 07551 (University of Houston, College of Optometry), NIH T35 EYO 7088 (J.M.), and William C. Ezell Fellowship (S.N.).
References
- 1.Gilbard JP, Farris RL, Santamaria J., 2nd Osmolarity of tear microvolumes in keratoconjunctivitis sicca. Arch Ophthalmol. 1978;96:677–681. doi: 10.1001/archopht.1978.03910050373015. [DOI] [PubMed] [Google Scholar]
- 2.Farris RL. Tear osmolarity—a new gold standard? Adv Exp Med Biol. 1994;350:495–503. doi: 10.1007/978-1-4615-2417-5_83. [DOI] [PubMed] [Google Scholar]
- 3.Bron AJ, Tiffany JM, Yokoi N, et al. Using osmolarity to diagnose dry eye: a compartmental hypothesis and review of our assumptions. Adv Exp Med Biol. 2002;506:1087–1095. doi: 10.1007/978-1-4615-0717-8_153. [DOI] [PubMed] [Google Scholar]
- 4.Anagnoste SR, Hall LS, Lisman RD. Vapor pressure osmolarity of tears as a clinical tool for evaluation of keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. 1996;37:S852. [Google Scholar]
- 5.Gilbard JP, Rossi SR, Heyda KG. Ophthalmic solutions, the ocular surface, and a unique therapeutic artificial tear formulation. Am J Ophthalmol. 1989;107:348–355. doi: 10.1016/0002-9394(89)90657-0. [DOI] [PubMed] [Google Scholar]
- 6.Aragona P, DiStefano G, Ferreri G. Role of tear osmolarity in the pathogenesis of ocular discomfort in type 1 diabetic patients. Invest Ophthalmol Vis Sci. 1999;40:S542. [Google Scholar]
- 7.Barearoli M, Del Beato P, Tanzilli P. Diabetes mellitus and dry eye syndrome: tear film glucose level in diabetic patients. Invest Ophthalmol Vis Sci. 1997;38:S150. [Google Scholar]
- 8.Bildin VN, Yang H, Crook RB, et al. Adaptation by corneal epithelial cells to chronic hypertonic stress depends on upregulation of Na:K:2Cl cotransporter gene and protein expression and ion transport activity. J Membr Biol. 2000;177:41–50. doi: 10.1007/s002320001098. [DOI] [PubMed] [Google Scholar]
- 9.Gilbard JP, Carter JB, Sang DN, et al. Morphologic effect of hyperosmolarity on rabbit corneal epithelium. Ophthalmology. 1984;91:1205–1212. doi: 10.1016/s0161-6420(84)34163-x. [DOI] [PubMed] [Google Scholar]
- 10.Li DQ, Chen Z, Song XJ. Hyperosmolarity stimulates production of MMP-9, IL-1beta and TNF-alpha; by human corneal epithelial cells via a c-Jun NH2-terminal kinase pathway. ARVO e-abstract. 1981:2002. [Google Scholar]
- 11.Li DQ, Chen Z, Song XJ, et al. Stimulation of matrix metalloproteinases by hyperosmolarity via a JNK pathway in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:4302–4311. doi: 10.1167/iovs.04-0299. [DOI] [PubMed] [Google Scholar]
- 12.Michea L, Ferguson DR, Peters EM, et al. Cell cycle delay and apoptosis are induced by high salt and urea in renal medullary cells. Am J Physiol Renal Physiol. 2000;278:F209–F218. doi: 10.1152/ajprenal.2000.278.2.F209. [DOI] [PubMed] [Google Scholar]
- 13.Couper JJ, Littleford KD, Couper RT, et al. High glucose and hyperosmolality stimulate hepatocyte growth factor secretion from cultured human mesangial cells. Diabetologia. 1994;37:533–535. doi: 10.1007/s001250050143. [DOI] [PubMed] [Google Scholar]
- 14.Mizutani M, Okuda Y, Yamaoka T, et al. High glucose and hyperosmolarity increase platelet-derived growth factor mRNA levels in cultured human vascular endothelial cells. Biochem Biophys Res Commun. 1992;187:664–669. doi: 10.1016/0006-291x(92)91246-m. [DOI] [PubMed] [Google Scholar]
- 15.Terada Y, Tomita K, Homma MK, et al. Sequential activation of Raf-1 kinase, mitogen-activated protein (MAP) kinase kinase, MAP kinase, and S6 kinase by hyperosmolality in renal cells. J Biol Chem. 1994;269:31296–31301. [PubMed] [Google Scholar]
- 16.Wang R, Liu Y, Sauve R, et al. Hyperosmolality-induced abnormal patterns of calcium mobilization in smooth muscle cells from non-diabetic and diabetic rats. Mol Cell Biochem. 1998;183:79–85. doi: 10.1023/a:1006813223216. [DOI] [PubMed] [Google Scholar]
- 17.Hattenbach LO, Gumbel H, Kippenberger S. Identification of beta-defensins in human conjunctiva. Antimicrob Agents Chemother. 1998;42:3332. doi: 10.1128/aac.42.12.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haynes RJ, Tighe PJ, Dua HS. Antimicrobial defensin peptides of the human ocular surface. Br J Ophthalmol. 1999;83:737–741. doi: 10.1136/bjo.83.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDermott AM, Redfern RL, Zhang B, et al. Defensin expression by the cornea: multiple signalling pathways mediate IL-1β stimulation of hBD-2 expression by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:1859–1865. doi: 10.1167/iovs.02-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narayanan S, Miller WL, McDermott AM. Expression of human β-defensins in conjunctival epithelium: relevance to dry eye disease. Invest Ophthalmol Vis Sci. 2003;44:3795–3801. doi: 10.1167/iovs.02-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNamara NA, Van R, Tuchin OS, et al. Ocular surface epithelia express mRNA for human β-defensin-2. Exp Eye Res. 1999;69:483–490. doi: 10.1006/exer.1999.0722. [DOI] [PubMed] [Google Scholar]
- 22.McDermott AM, Redfern RL, Zhang B. Human β-defensin 2 is up-regulated during re-epithelialization of the cornea. Curr Eye Res. 2001;22:64–67. doi: 10.1076/ceyr.22.1.64.6978. [DOI] [PubMed] [Google Scholar]
- 23.Ganz T, Lehrer RI. Defensins. Pharmacol Ther. 1995;66:191–205. doi: 10.1016/0163-7258(94)00076-f. [DOI] [PubMed] [Google Scholar]
- 24.Musch DC, Sugar A, Meyer RF. Demographic and predisposing factors in corneal ulceration. Arch Ophthalmol. 1983;101:1545–1548. doi: 10.1001/archopht.1983.01040020547007. [DOI] [PubMed] [Google Scholar]
- 25.McDermott AM. Defensins and other antimicrobial peptides at the ocular surface. Ocul Surf. 2004;2:229–247. doi: 10.1016/s1542-0124(12)70111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Araki-Sasaki K, Ohashi Y, Sasabe T, et al. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci. 1995;36:614–621. [PubMed] [Google Scholar]
- 27.Harder J, Bartels J, Christophers E, et al. Isolation and characterization of human β -defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–5713. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 28.Kim HS, Jun Song X, de Paiva CS, et al. Phenotypic characterization of human corneal epithelial cells expanded ex vivo from limbal explant and single cell cultures. Exp Eye Res. 2004;79:41–49. doi: 10.1016/j.exer.2004.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jorres A, Bender TO, Finn A, et al. Biocompatibility and buffers: effect of bicarbonate-buffered peritoneal dialysis fluids on peritoneal cell function. Kidney Int. 1998;54:2184–2193. doi: 10.1046/j.1523-1755.1998.00178.x. [DOI] [PubMed] [Google Scholar]
- 30.Ochi H, Masuda J, Gimbrone MA. Hyperosmotic stimuli inhibit VCAM-1 expression in cultured endothelial cells via effects on interferon regulatory factor-1 expression and activity. Eur J Immunol. 2002;32:1821–1831. doi: 10.1002/1521-4141(200207)32:7<1821::AID-IMMU1821>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 31.Wilson SE, Liu JJ, Mohan RR. Stromal-epithelial interactions in the cornea. Prog Retin Eye Res. 1999;18:293–309. doi: 10.1016/s1350-9462(98)00017-2. [DOI] [PubMed] [Google Scholar]
- 32.Toda I, Asano-Kato N, Hori-Komai Y, et al. Laser-assisted in situ keratomileusis for patients with dry eye. Arch Ophthalmol. 2002;120:1024–1028. doi: 10.1001/archopht.120.8.1024. [DOI] [PubMed] [Google Scholar]
- 33.Solomon A, Dursun D, Liu Z, et al. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42:2283–2292. [PubMed] [Google Scholar]


