Abstract
Improved methods to increase surface hardness of metallic biomedical implants are being developed in an effort to minimize the formation of wear debris particles that cause local pain and inflammation. However, for many implant surface treatments, there is a risk of film delamination due to the mismatch of mechanical properties between the hard surface and the softer underlying metal. In this article, we describe the surface modification of titanium alloy (Ti-6Al-4V), using microwave plasma chemical vapor deposition to induce titanium nitride formation by nitrogen diffusion. The result is a gradual transition from a titanium nitride surface to the bulk titanium alloy, without a sharp interface that could otherwise lead to delamination. We demonstrate that vitronectin adsorption, as well as the adhesion and spreading of human mesenchymal stem cells to plasma-nitrided titanium is equivalent to that of Ti-6Al-4V, while hardness is improved 3- to 4-fold. These in vitro results suggest that the plasma nitriding technique has the potential to reduce wear, and the resulting debris particle release, of biomedical implants without compromising osseointegration; thus, minimizing the possibility of implant loosening over time.
Keywords: titanium nitride, chemical vapor deposition, mesenchymal stem cells, osseointegration, vitronectin
INTRODUCTION
Commercially pure Ti, Ti-6Al-4V, and CoCrMo are metals currently approved by the Food and Drug Administration for use in biomedical implants. Titanium and its alloys are attractive because of their low density, low modulus of elasticity, excellent corrosion resistance, and biocompatibility. Pure Ti is the most biocompatible metal, but its low strength precludes its use in orthopedic implants. Ti-6Al-4V is more commonly used when osseointegration is desired, such as in the anchoring regions of artificial joints. This is because the alloy is nearly as biocompatible as pure Ti, but has higher strength than pure Ti. Still, Ti-6Al-4V’s low hardness and its tendency to seize when in sliding contact with itself or other metals does not make it attractive for metal-on-metal articulating components in artificial joint replacements. For this reason, CoCrMo (with improved wear-resistance over that of Ti-6Al-4V) is commonly used when high abrasion resistance is needed, despite the reduced biocompatibility compared to Ti and its alloys.
The low wear performance of titanium and its alloys in abrasive and adhesive wearing conditions has led to the efforts to try and surmount this weakness. Conventional ion implantation,1,2 plasma source nitrogen ion implantation,3 conventional nitriding,4 conventional plasma nitriding,5,6 intensified plasma ion nitriding,7 and PVD and CVD TiN films8–11 have been reported. Titanium nitride (TiN) is a ceramic material often used as a coating to improve wear resistance and to provide aesthetic appeal due to its characteristic gold color. However, as a discrete coating on metals, an abrupt metal/ nitride interface may lead to film delamination due to mismatch of mechanical properties across the interface. With plasma nitriding, it is possible to create a gradual transition in bonding and therefore minimize the mechanical instabilities associated with a sharp interface and associated mechanical property mismatch.
One common technique used for surface modification of biomaterials that can produce a composition gradient with a diffuse interface is ion implantation. Implantation of titanium and titanium-alloy with nitrogen ions is well-studied.12–18 Ion implantation is a process in which high-energy ions, typically 10–200 kiloelectron volts (KeV) in energy, are injected into the near-surface region of a substrate. The ions impinge on the substrate with kinetic energies 4–5 orders of magnitude greater than the binding energy of the solid substrate and form an alloy with the surface upon impact. For the case of nitrogen ions in titanium, typical peak concentration depths of 30–150 nm have been reported.12,15,18 The near-surface alloy produced by ion implantation is different from conventional coatings in that the implanted ion is surrounded by atoms of the original surface material. Alloying at the surface can be as high as 50 atomic percent of the implanted element. It produces no discrete coating, nor will delamination of the altered surface occur. In addition to the very shallow implantation depth, the disadvantages of this technique include high capital costs (in the range of $500,000+, depending on size and process type), line-of-sight limitations with most processes, high surface stress leading to partial deformations (if concentrations are high), and surface weakening by radiation effects.
In this study, we show that by using microwave plasma chemical vapor deposition (MPCVD), we can achieve deeper nitriding (greater than 1μm) with higher resulting hardness at a given depth. This can be done without the limitations mentioned earlier for ion implantation. As with conventional ion-implantation methods, a nitrogen gradient is produced in the Ti alloy, with no distinct boundary between the two phases, which we expect to minimize the risk of delamination. However, MPCVD allows sub-surface implantation of nitrogen combined with deep diffusion into the substrate to increase the reaction depth of nitrogen with titanium. The result is a hardness that is 3–4 times higher than untreated Ti-6Al-4V at 100 nm depth and twice as hard as untreated Ti-6Al-4V at 1 μm depth. As we will show, this is done without compromising biocompatibility. This MPCVD technique can have immediate application for improving abrasion resistance of commercial implants that are currently treated by ion implantation.
MATERIALS AND METHODS
Preparation of ti-6al-4v and plasma-nitrided ti-6al-4v substrates
Ti-6Al-4V sheets with 1 mm thickness were purchased from Robin Materials (Mountain View, CA). Seven millimeter diameter disks were punched from the sheet, and were then polished to a root-mean-square (RMS) roughness of <5 nm using a mechanical polisher with SiC paper, followed by a chemical–mechanical polish with a 0.06 μm colloidal silica solution containing 10% hydrogen peroxide. The polished disks were cleaned by ultrasonic agitation in a series of detergent solution, methanol, acetone, and finally deionized water.
Cleaned substrates were placed in a Wavemat MPCVD reactor, equipped with a 6 kW, 2.4 GHz microwave generator. Surfaces were nitrided using a gas mixture of 50 sccm N2 and 50 sccm H2 at 1.35 kW power and a chamber pressure of 40 Torr. The surface temperature was maintained at 775°C for 40 min before initiating a 10 min gradual cool down. We observed a gold color on the sample surface, characteristic of the TiN structure.
Surface characterization
The crystalline phase of the coating was investigated by glancing angle X-ray diffraction (XRD) with CuKα radiation from a 5° incident beam. Hardness was measured using a NanoIndenter XP having a Berkovich diamond tip with nominal radius of 50 nm. For nanoindentation, a fused silica standard was tested before and after each measurement to insure accurate calibration. Elemental composition and depth profiles of nitrided and bare Ti-6Al-4V substrates were determined from XPS measurements with a Kratos AXIS 165 Multitechnique Spectrometer. Depth profiles were measured using a 4 kV Ar+ ion-beam etching providing the etching rate of 5 nm/min (in terms of a SiO2 standard). Peak-fit analysis of the XPS spectra was performed after a 5-point Savistsky-Golay smooth and a Shirley background correction. RMS roughness was measured on 10 × 10 μm2 scan areas by atomic force microscopy (AFM). Surface wettability of the substrates was determined by the half angle method using a CAM-MICRO model contact angle meter (Tantec Inc., Schaumburg, IL), with deionized water as the probe liquid. Two spots were measured from each of the three samples of equal RMS roughness and averaged. Measurements of surface roughness before and after nitriding were between 4 and 5 nm.
Adsorption of vitronectin by ti-6al-4v versus plasma-nitrided ti-6al-4v
Vitronectin ELISA was performed by placing both plasma-nitrided and control-polished Ti-6Al-4V discs (7 mm diameter) into 48-well cell culture dishes. Discs were coated overnight at 4°C with either adult human serum (Hyclone, Logan, UT) or 10 μg/mL purified vitronectin (Chemicon, Temecula, CA) in tris-buffered saline (TBS). To reduce non-specific binding of ELISA reagents, the samples were subsequently overcoated with heat-denatured bovine serum albumin (dBSA) for 90 min at 4°C to cover the uncoated areas of the discs. As a negative control, duplicate samples were coated with dBSA. Following protein coating, discs were exposed to a primary antibody against vitronectin (Chemicon) and then to a horseradish peroxidase-conjugated secondary antibody (Amersham Life Science, Piscataway, NJ), each for 1 h at 4°C. A colorimetric substrate for the peroxidase (Chromagen, Biosource International, Camarillo, CA) was then added. After 20 min, an equal volume of 0.9M H2SO4 was added to stop the reaction, and enzyme activity was quantified by absorbance spectroscopy at 450 nm wavelength. Three independent experiments were performed, with each surface assayed in triplicate.
Cell culture
Human mesenchymal stem cells (hMSCs) were isolated from bone marrow donations, as previously described.19 Briefly, cells were pelleted by centrifugation, resuspended in Dulbecco’s Modified Eagle Medium (DMEM), and then applied to a Histopaque-1077 column (Sigma, St. Louis, MO). A density gradient was generated by centrifugation at 500g for 30 min. Cells from the DMEM/Histopaque interface were extracted with a syringe and seeded onto tissue culture dishes and cultured in DMEM containing 10% fetal bovine serum. Cells had a homogenous and fibroblast-like appearance, and no osteoclasts or adipocytes were present, as measured by Tartrate Resistant Acid Phosphatase (TRAP) and Oil-O-red staining, respectively. Bone marrow samples were obtained with prior approval from the University of Alabama Institutional Review Board.
Cell attachment assays
hMSCs from a 48-year-old female donor (passages 3–5) were used to perform the cell adhesion assays. Discs were coated overnight at 4°C with either dBSA, adult human serum, 20 μg/mL bovine dermal collagen I (Vitrogen, Cohesion, Palo Alto, CA), or 20 μg/mL vitronectin. Following protein coating, samples were washed with TBS to remove loosely-bound proteins. hMSCs were added to the discs in serum-free DMEM and allowed to adhere for 1 h at 37°C. Unattached cells were then removed by three washes with phosphate-buffered saline (PBS) on a mechanical shaker. Cells were fixed in 3.7% formaldehyde, followed by staining with a 1 mg/mL solution of crystal violet (Sigma, St. Louis, MO) in borate buffer for 20 min. Stained cells were washed 3 times with deionized water, and 1 mL of 10% acetic acid was then added to each disc to solubilize cells. Absorbance was read on a spectrometer at 540 nm. Three independent experiments were performed, with each surface tested in triplicate.
Microscopic analysis of cell morphology
To observe cell morphology, 6 ×104 hMSCs were added to plasma nitrided and control Ti-6Al-4V discs that had been precoated with human serum or left uncoated in 48-well cell culture dishes. Cells were seeded onto the discs and cultured in serum-free DMEM at 37°C for either 1 or 24 h. Unattached cells were then removed from the discs by three washes with PBS on a mechanical shaker. Digital images of the discs were taken following fixation in 3.7% formaldehyde, using reflected light microscopy (Fisher Micromaster light microscope equipped with top-mounted light source, objective lens, and digital camera). Cells could be visualized without staining on the highly polished surfaces. Images of 1 h morphology are of hMSCs isolated from a 21-year-old male donor (passage 6), and images of 24 h morphology are of hMSCs isolated from a 48-year-old female donor (passage 3).
RESULTS
Xrd analysis
Figure 1 shows the glancing-angle XRD pattern of the nitrided Ti-6Al-4V surface. The pattern was indexed as cubic TiN and hexagonal Ti. A least squares fit was used to obtain a lattice parameter of a = 4.2479 Å for cubic TiN, which is comparable to the literature value for bulk TiN of a = 4.244 Å. The fitted lattice constants for hexagonal titanium were a = 2.9412 Å and c = 4.7411 Å.
Figure 1.
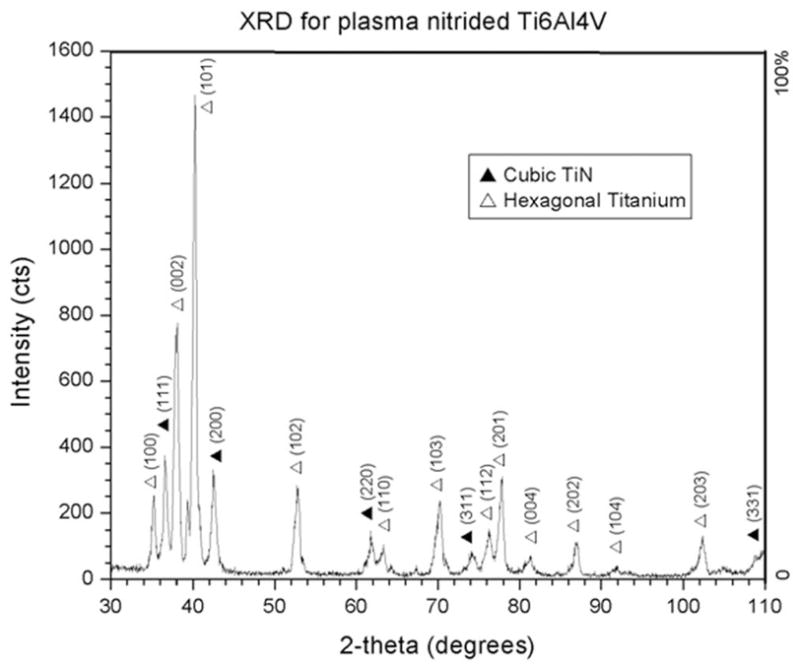
Glancing-angle XRD of plasma-nitrided Ti-6Al-4V. Cubic TiN and hexagonal titanium were detected using 5° incident beam.
Surface hardness gain is a factor of three to four
Figure 2 shows nanoindentation hardness as a function of indentation depth of the two most commonly used biomedical alloys, Ti-6Al-4V and CoCrMo, as well for the MPCVD nitrided Ti-6Al-4V. Each data point represents an average of seven indents and is plotted with a standard deviation error bar. The plasma nitriding technique produces a surface 3–4 times harder than untreated titanium alloy at a depth of 100 nm. The gradual drop in hardness of the plasma-nitrided Ti alloy demonstrates the gradual transition from TiN at the surface to bulk Ti alloy. However, there is sufficient nitrogen reaction with titanium to produce a hardness that is twice that of bulk alloy at 1 μm depth.
Figure 2.
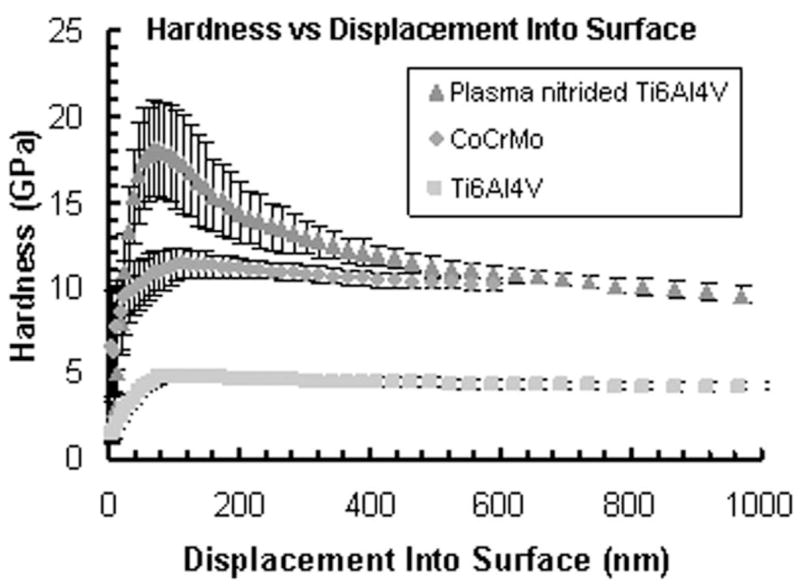
Hardness of microwave plasma-nitrided Ti-6Al-4V, CoCrMo, and bare Ti-6Al-4V as measured by nanoin-dentation. The gradual drop in hardness of the plasma-nitrided sample demonstrates the gradual transition from TiN at the surface to bulk Ti alloy. There is sufficient nitrogen reaction with titanium to produce a hardness that is twice that of bulk alloy at 1 μm depth.
Xps analysis
The atomic concentrations of a representative sample (with 12 GPa maximum hardness) were estimated from the areas under the individual spectra of elements. Depth profiles of Ti 2p, Al 2p, V 2p, N 1s, and O 1s elements are shown in Figure 3 as atomic concentrations of each element versus the calculated etching depth. The chemical states of Ti were determined from high resolution XPS spectra shown in Figure 4. The states of Ti were assigned for the Ti2p3/2 peaks as follows: Ti elemental (453.8 eV), TiN (454.8 eV), TiON (456.2–456.9 eV), and TiO2 (458.2 eV). Note, that the chemical state assigned as TiON is contentious and is possibly a mixture of TiOx and TiON states.20–23 Surface atomic composition of nitrided samples (bare substrate data are shown in parentheses) before etching was determined as 33.2% (52.3) of O, 22.9% (25.8) of C, 22.9% (18.0) of Ti, 1.0% (2.1) of Al, 18.8% (1.4) of N, 0.8% (0) of S, and 0.3% (0.4) of V. XPS lines from C, and S elements disappeared after the first 30 s of etching and most probably resulted from the surface contamination. The surface Ti is present in the form of TiO2 (~40%), TiON (~40%), and TiN (~20%). Figures 3 and 5 demonstrate that the thicknesses of TiO2, TiON, and TiN reach up to 40, 150, 700 nm, respectively. The concentration of alloying element, vanadium, is very low at the surface and within the surface oxide layer, and gradually increases to its bulk value of ~4%. Aluminum, another alloying element, is also depleted at the surface, then in the oxide layer it is present mainly in the oxide form, and then reaches its bulk value of ~6% away from the surface.
Figure 3.
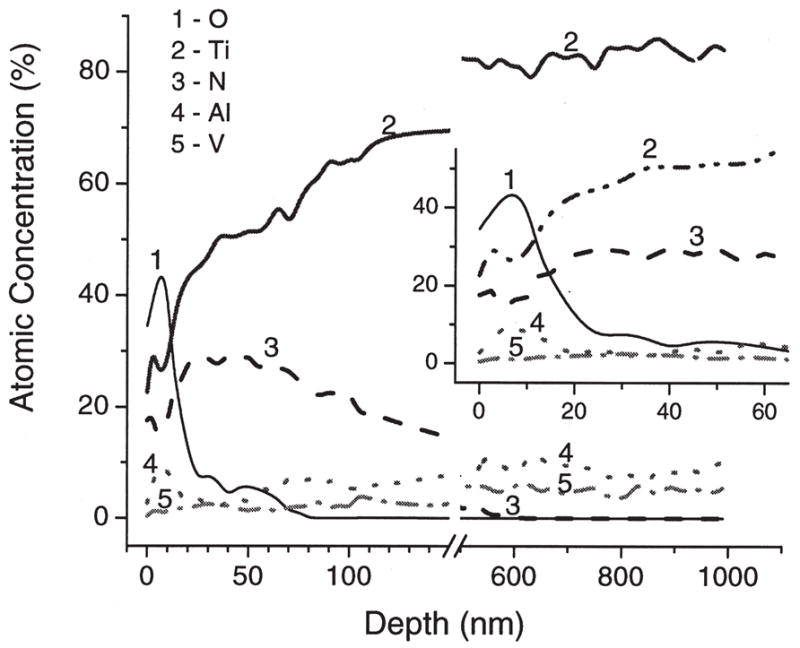
XPS depth profiles of a MPCVD-nitrided Ti-6Al-4V sample with 12 GPa maximum hardness. Depth was calculated from etching time using the etching rate for SiO2 standard of 5 nm/min. The insert shows zoomed initial part of the profile.
Figure 4.
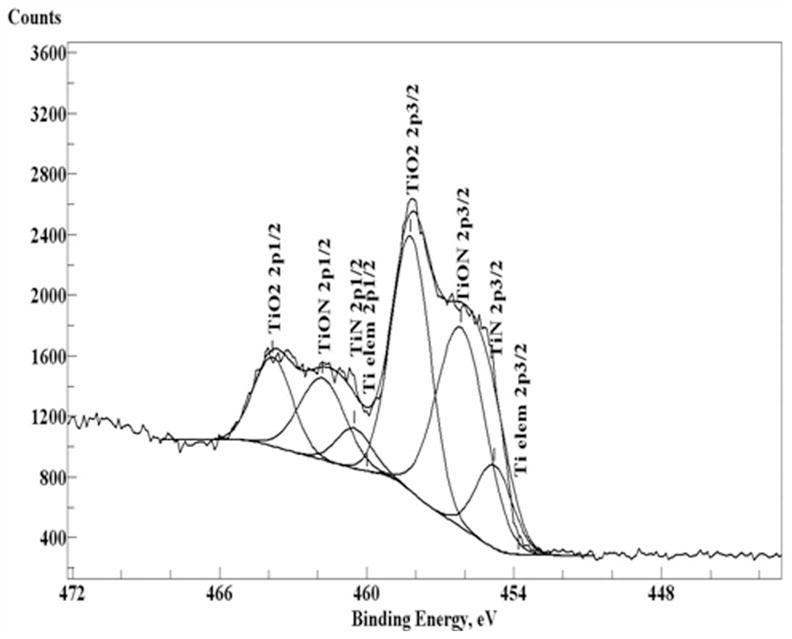
XPS high resolution spectra of Ti2p1/2 and 3/2 peaks before etching. Smooth curves were obtained from peak-fit analysis.
Figure 5.
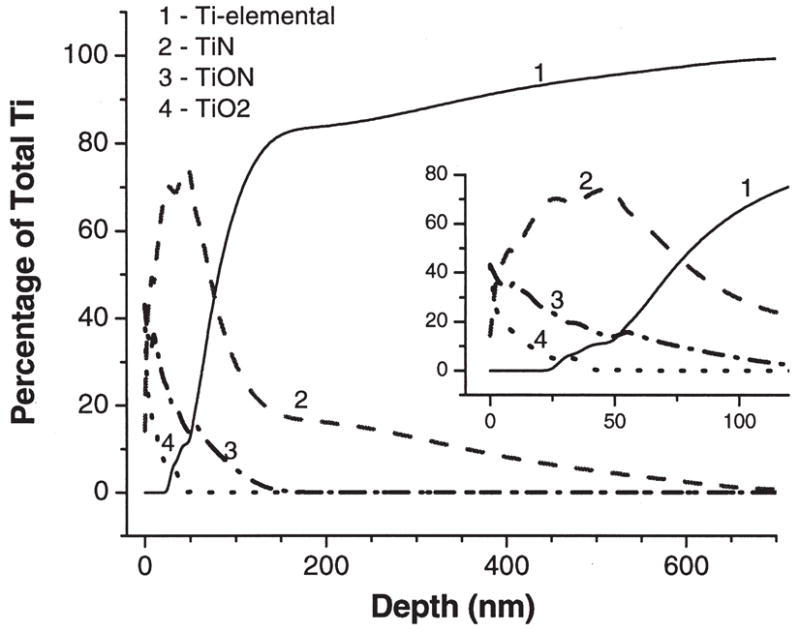
XPS depth profiles of the percentage of different chemical states of Ti to the total Ti content: Ti elemental (1), TiN (2), TiON (3), TiO2 (4).
Wettability is slightly increased
The water contact angle measurements are 43°± 7° and 31°± 4° for Ti-6Al-4V and plasma-nitrided Ti-6Al-4V, respectively. The plasma nitriding technique makes the surface slightly more hydrophilic.
The microwave plasma nitriding technique does not significantly affect vitronectin adsorption compared to untreated ti-6al-4v
The biocompatibility of implant materials depends, in part, upon the capacity of the material surface to adsorb endogenous proteins that regulate cell behavior. Previous studies suggest that the adsorption of vitronectin, which is abundant in blood, may play an important role in mediating cell/biomaterial interactions [reviewed in 24]. Accordingly, we coated bare and MPCVD-nitrided Ti-6Al-4V with either purified human vitronectin or whole human serum and then used an ELISA to compare the amount of vitronectin deposited on the material surface (Fig. 6). These studies showed that there was no statistical difference between the amount of vitronectin associated with plasma-nitrided versus control Ti-6Al-4V, regardless of whether samples were coated with purified vitronectin or whole serum.
Figure 6.
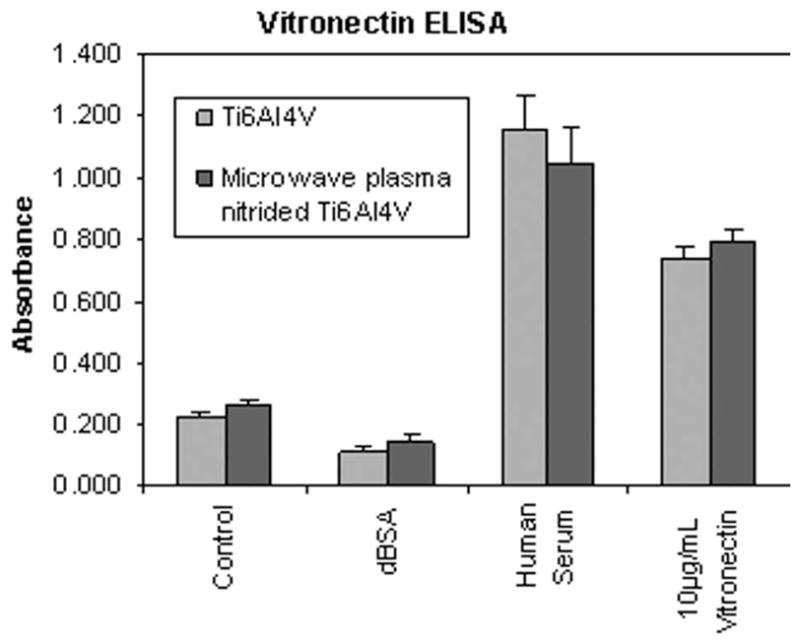
Vitronectin ELISA. Substrates were coated with human serum, purified human vitronectin, denatured BSA, or left uncoated (control). The amount of adsorbed vitronectin was then measured by ELISA. There was no statistical difference between the amount of vitronectin associated with plasma-nitrided versus control Ti-6Al-4V surfaces.
The microwave plasma nitriding technique does not significantly affect mesenchymal stem cell adhesion and spreading compared to untreated ti-6al-4v
We next evaluated whether the plasma nitriding technique affects the behavior of mesenchymal stem cells, a multipotent cell type that undergoes osteoblastic differentiation at the tissue/implant interface.25 To this end, we examined hMSC adhesion on uncoated material surfaces, or on substrates precoated with human serum, purified vitronectin, or collagen I (Fig. 7). Collagen I was included in these assays because, as the principal constituent of the organic bone matrix, this is a molecule that would normally be encountered by hMSCs in vivo.26 For all of the surface treatments tested, we found no statistical difference between the number of hMSCs that adhered to plasma-nitrided versus control Ti-6Al-4V. Interestingly, cell adhesion to the uncoated substrates was equivalent to that noted on surfaces coated with serum, suggesting that cells bind well to titanium alloy in the absence of adsorbed extracellular matrix molecules. Also of note, cell adhesion was maximal on substrates coated with collagen I.
Figure 7.
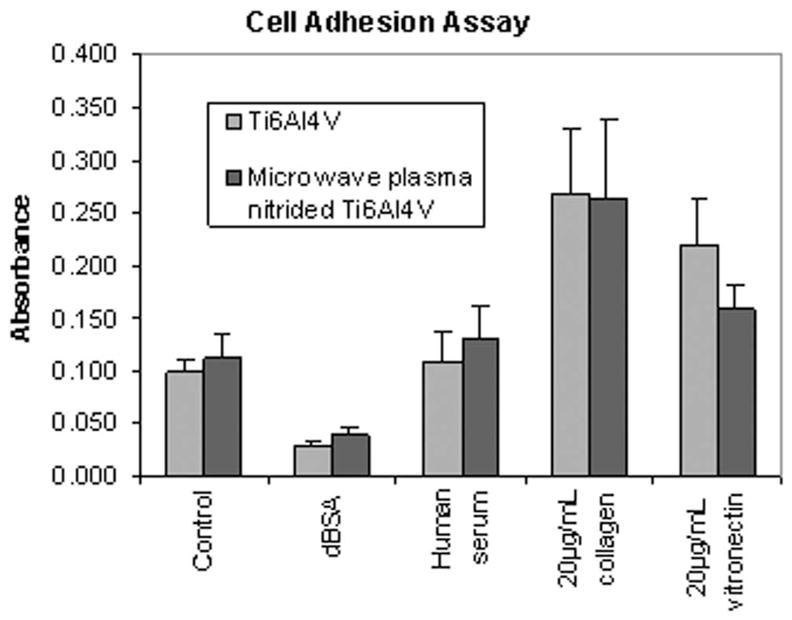
hMSC adhesion assay. Substrates were coated with human serum, vitronectin, collagen I, denatured BSA, or left uncoated (control). hMSCs were then seeded onto the substrates in serum-free media and allowed to adhere for 1 h. Adhesion was quantified using a standard colorimetric assay. These studies showed that there was no statistical difference between the number of hMSCs that adhere to plasma-nitrided versus control Ti-6Al-4V, regardless of the surface treatment.
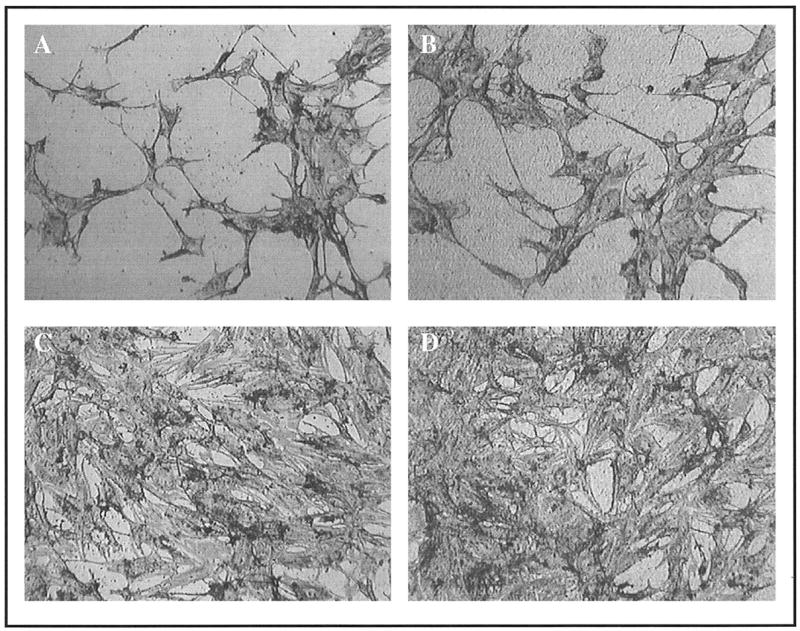
When cells attach to endogenous extracellular matrices, they reorganize their actin cytoskeleton, adopting a spread morphology. This event is necessary for the survival of most adherent cell types, and is also important for the osteoblastic differentiation of MSCs. As shown in Figure 8, cells were able to spread on both the plasma-nitrided surfaces and control Ti-6Al-4V. In fact, the morphology of the cells was indistinguishable for the two materials, suggesting that the plasma nitriding technique did not adversely affect cell behavior.
Figure 8.
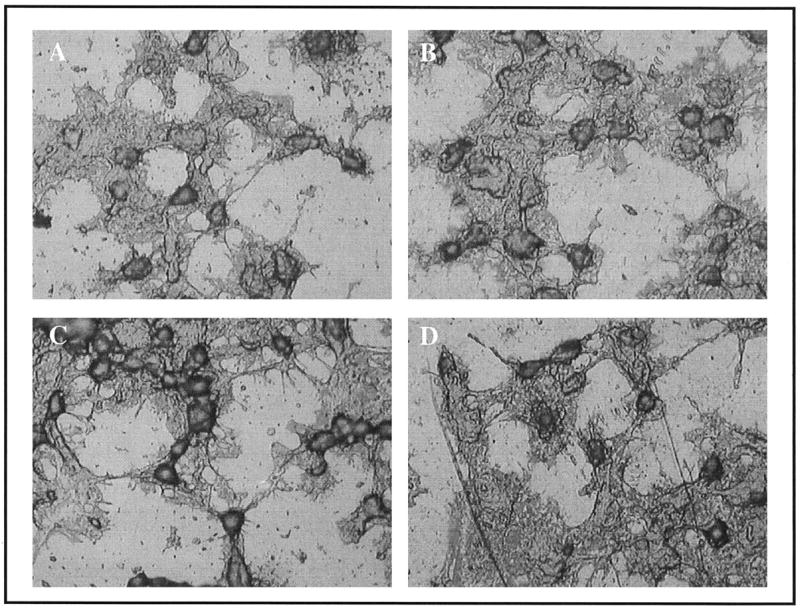
Light microscope images showing the indistinguishable morphology of hMSCs cultured for 1 h on uncoated control Ti-6Al-4V (A) versus Plasma-nitrided Ti-6Al-4V (B) surfaces. Similarly, no differences in morphology were apparent for cells adherent to serum-coated Ti-6Al-4V (C) or plasma-nitrided Ti-6Al-4V (D) substrates.
The experiments described in Figure 8 were performed at 1 h following cell seeding to minimize the possibility that cells secrete their own extracellular matrix molecules, thereby remodeling the adsorbed protein layer. However, to assess potential long-term effects of the plasma nitriding technique on cell behavior, we also examined the morphology of cells at 24 h following cell seeding (Fig. 9). These experiments revealed that, as before, there were no distinguishable differences in the morphology of cells adherent to the plasma-nitrided versus control Ti-6Al-4V samples, regardless of whether surfaces were uncoated or pre-coated with serum. Not surprisingly, however, substantially more cells were apparent on serum-coated substrates, which quite likely reflect increased cell proliferation and survival. Importantly, the collective data shown in Figures 7–9 indicate that cells respond equally well to plasma-nitrided and control Ti-6Al-4V surfaces.
Figure 9.
Morphology of cells 24 h after seeding on uncoated or serum-coated plasma-nitrided or control Ti-6Al-4V substrates. Panels represent: uncoated Ti-6Al-4V (A), uncoated plasma-nitrided Ti-6Al-4V (B), serum-coated Ti-6Al-4V (C), and serum-coated plasma-nitrided Ti-6Al-4V (D).
DISCUSSION
Conventional ion implantation methods achieve peak nitrogen penetration depths in titanium that reach only a fraction of a micron (typically 50–150 nm).12,15,18 Our MPCVD technique for surface nitriding results in significantly increased nitrogen penetration depth. In nanoindentation testing, we found that even at 1 μm depth, the hardness is still twice that of untreated Ti-6Al-4V and is comparable to that of CoCrMo.
Surface characterization and biological testing were performed on TiN samples prepared in the CVD reactor at 775°C using 1.35 kW power and pressure of 40 Torr. However, there is still room for optimization of the nitriding protocol to induce even deeper nitrogen diffusion. For example, an increase of only 2 mm in the sample stage height during nitriding resulted in a maximum hardness increase from 12 to 18 GPa. XPS analysis was not performed on the sample with 18 GPa hardness, but nitrogen penetration exceeding 1 μm depth would be expected for this sample. Variables such as temperature, sample stage geometry, heat sink design, gas pressure, gas concentration, and microwave power can all affect nitrogen depth and will be optimized in later studies.
For biomedical applications, characterization of the surface chemistry is important, since this chemistry regulates cell behavior. The plasma-nitrided surface Ti is present in the form of TiO2 (~40%), TiON (~40%), and TiN (~20%). We demonstrate in this study that hMSC adhesion is not affected by the microwave plasma nitriding technique. Previous studies have also shown clotting time and platelet adhesion to be statistically equivalent between TiO2, TiNOx, and TiN surfaces.27 Surface atomic composition consisted of 22.9% carbon. Carbon-containing compounds, such as CO2, are known to adsorb from air onto the surface and cannot be removed by the standard cleaning protocol of acetone, alcohols, or water.27 However, it has been demonstrated that oxide thickness and carbon contamination does not influence protein adsorption or platelet contact activation.28 Since cell biology could be influenced by these contaminating molecules, the samples were stored in air-tight containers prior to the biological assays.
Upon insertion into the body, any material surface in contact with blood or other body fluids becomes quickly coated with an adsorbed protein layer, and this protein film directly influences cell adhesion.24,29 Accordingly, characterizing the composition and properties of the adsorbed protein layer is important for understanding implant osseointegration. Many factors can influence protein adsorption, including surface chemistry, topography, and wettability.24,29 In our studies, we observed that the plasma nitriding method slightly increased the surface wettability of our substrates. Increased wettability could, in turn, affect the amount and ratio of different proteins that adsorb to the implant surface from the blood,30,31 or cause proteins to adsorb in different orientations or conformations.32–34 For example, fibronectin was reported to adopt a V-shaped orientation on a hydrophilic surface, whereas a thin, dense meshwork was formed on a hydrophobic surface.34 To investigate the effect of MPCVD nitriding on the protein layer, adsorption of a representative adhesive protein, vitronectin, was examined by ELISA. Vitronectin is a known ligand for the integrin family of cell adhesion receptors, and several studies have suggested that vitronectin plays an important role in regulating cell adhesion and reorganization of the actin cytoskeleton [reviewed in 26]. Vitronectin may be particularly important in regulating osteoblast attachment; prior depletion of vitronectin, but not fibronectin, from serum was found to significantly inhibit cell binding to serum-coated titanium and steel.35 Despite the slight increase in wettability of our nitrided surfaces, there was no significant difference in the amount of vitronectin adsorbed to plasma-nitrided versus control Ti-6Al-4V. This was true whether substrates were coated with purified vitronectin solution or whole human serum, which contains a relatively high concentration of vitronectin (in addition to other proteins that can compete for the material surface). We cannot currently exclude the possibility that vitronectin adsorbs in different conformations to the two materials; however, cell adhesion was equivalent on vitronectin-coated plasma-nitrided and Ti-6Al-4V surfaces. These data suggest that the vitronectin adsorbed to both Ti-6Al-4V and MPCVD-nitrided Ti-6Al-4V assumed a conformation that was recognized by cell surface integrin receptors.
To prevent the loosening of orthopedic implants over time, osseointegration at the anchoring region of the device is essential. This process of bone integration is thought to be dependent upon the attachment of hMSCs to the material surface, followed by osteoblastic differentiation of these cells, and finally, production of a mineralized matrix.36,37 In this study, we show that hMSCs bind equally well to plasma-nitrided and control Ti-6Al-4V, regardless of whether the surfaces are precoated with vitronectin, human serum, or collagen I. Additionally, no differences were noted in the morphology of cells adherent to the two types of substrates. These data suggest that the plasma nitriding method does not have any adverse affect on cell behavior.
One unexpected finding of this investigation is that the degree of initial cell attachment and spreading on uncoated materials was equivalent to that of cells on serum-coated surfaces. The source of ligands for cell adhesion receptors on uncoated substrates is not currently known; however, consistent with our results, the binding of cells to titanium in the absence of known integrin ligands has been previously reported.38,39 In contrast to these initial cell responses to the biomaterial surface, serum-coatings did influence cell behavior over a longer time frame; at 24 h following initial cell attachment, there were clearly more cells adherent to serum-coated substrates. Hence, adsorbed serum proteins quite likely played a prominent role in regulating cell survival or proliferation on the materials, although further experiments will be required to confirm this hypothesis. Another noteworthy finding is that cells adhered better to surfaces coated with collagen I, as compared with uncoated, serum-coated, or vitronectin-coated surfaces. These data imply that the bioactivity of titanium alloys could potentially be improved by surface coatings of collagen I, or collagen-derived bioactive peptides. Such studies are currently in progress by our group.
CONCLUSIONS
Plasma nitriding by MPCVD improves current state-of-the-art nitriding of titanium alloy by significantly increasing depth of nitrogen diffusion through biomedical implant surfaces compared to conventional ion implantation set-ups. There is a gradual transition from TiN at the surface to bulk titanium alloy, which reduces the stress in the film and is expected to minimize the possibility of film delamination. In vitro tests indicate that biocompatibility is equivalent to that of untreated Ti alloy, while hardness is improved 3- to 4-fold.
Acknowledgments
Contract grant sponsor: National Institute of Dental and Craniofacial Research (NIDCR)
Contract grant sponsor: National Institutes of Health (NIH); contract grant number: R01 DE013952
References
- 1.Krupa D, Basrzkiewicz J, Kozubowski JA, Barcz A, Sobczak IW, Bilinski A, Lewandowska-Szumiel M, Rajchel B. Effect of calcium-ion implantation on the corrosion resistance and bio-compatibility of titanium. Biomaterials. 2001;22:2139–2151. doi: 10.1016/s0142-9612(00)00405-1. [DOI] [PubMed] [Google Scholar]
- 2.Dong H, Shi W, Bell T. Potential of improving tribological performance of UHMWPE by engineering the Ti6Al4V counterfaces. Wear. 1999;225–229:146–153. [Google Scholar]
- 3.Qiu X, Conrad JR, Dodd RA, Worzala FJ. Plasma source nitrogen ion implantation of Ti–6Al–4V. Metall Trans. 1990;21A:1663–1667. [Google Scholar]
- 4.Venugopalan R, George MA, Weimer JJ, Lucas LC. Surface topography, corrosion and microhardness of nitrogen-diffusion-hardened titanium alloy. Biomaterials. 1999;20:1709–1716. doi: 10.1016/s0142-9612(99)00079-4. [DOI] [PubMed] [Google Scholar]
- 5.Wierzchon T, Fleszar A. Properties of surface layers on titanium alloy produced by thermo-chemical treatments under glow discharge conditions. Surf Coat Technol. 1997;96(2–3):205–209. [Google Scholar]
- 6.Salehi M, Bell T, Morton PH. The effect of surface topography on tribo-oxidation of titanium nitrided surfaces. J Phys D: Appl Phys. 1992;25:889–895. [Google Scholar]
- 7.Meletis EI. Intensified plasma-assisted processing: Science and engineering. Surf Coat Technol. 2002;149(2–3):95–113. [Google Scholar]
- 8.Shima M, Okado J, McColl IR, Waterhouse RB, Hasegawa T, Kasaya M. The influence of substrate material and hardness on the fretting behaviour of TiN. Wear. 1999;229:38–45. [Google Scholar]
- 9.Wu PQ, Drees D, Stals L, Celis JP. Comparison of wear and corrosion wear of TiN coatings under uni- and bidirectional sliding. Surf Coat Technol. 1999;113(3):251–258. [Google Scholar]
- 10.Vitchev RG, Blanpain B, Celis JP. Electronic spectroscopic study of the tribochemical modifications of TiN-corundum pairs after fretting wear. Wear. 1999;231(2):220–227. [Google Scholar]
- 11.Vancoille E, Banpain B, Xingpu Y, Celis JP, Roos JR. Tribo-oxidation of a TiN coating sliding against corundum. J Mater Res. 1994;9:992–998. [Google Scholar]
- 12.Ueda M, Silva MM, Otani C, Reuther H, Yatsuzuka M, Lepienski CM, Berni LA. Improvement of tribological properties of Ti6Al4V by nitrogen plasma immersion ion implantation. Surf Coat Technol. 2003;169:408–410. [Google Scholar]
- 13.Torrisi L. Ion implantation and thermal nitridation of biocompatible titanium. Biomed Mater Eng. 1996;6:379–388. [PubMed] [Google Scholar]
- 14.Kapczinski MP, Kinast EJ, dos Santos CA. Near-surface composition and tribological behaviour of plasma nitrided titanium. J Phys D: Appl Phys. 2003;36:1858–1863. [Google Scholar]
- 15.Tang BY, Fetherston RP, Shamim M, Breun RA, Chen A, Conrad JR. Measurement of ion species ratio in the plasma source ion implantation process. J Appl Phys. 1993;73:4176–4180. [Google Scholar]
- 16.Zhou X, Dong HK, Li HD, Liu BX. Reverse sequence of formation of titanium nitrides by nitrogen implantation. J Appl Phys. 1988;63:4942–4945. [Google Scholar]
- 17.Anttila A, Keinonen J, Uhrmacher M, Vahvaselkä S. Nitrogen implantation of metals. J Appl Phys. 1985;57:1423–1425. [Google Scholar]
- 18.Vardiman RG, Kant RA. The improvement of fatigue life in Ti-6Al-4V by ion implantation. J Appl Phys. 1982;53:690–694. [Google Scholar]
- 19.Kilpadi KL, Sawyer AA, Prince CW, Chang PL, Bellis SL. Primary human marrow stromal cells and Saos-2 osteosarcoma cells use different mechanisms to adhere to hydroxylapatite. J Biomed Mater Res. 2004;68A:273–285. doi: 10.1002/jbm.a.20043. [DOI] [PubMed] [Google Scholar]
- 20.Jiang N, Zhang HJ, Bao SN, Shen YG, Zhou ZF. XPS study for reactively sputtered titanium nitride thin films deposited under different substrate bias. Physica B. 2004;352(1–4):118–126. [Google Scholar]
- 21.Lee YK, Kim JY, Lee YK, Lee MS, Kim DK, Jin DY, Nam TH, Ahn HJ, Park DK. Surface chemistry of non-stoichiometric TiNx films grown on (100)Si substrate by DC reactive magnetron sputtering. J Crystal Growth. 2002;234(2–3):498–504. [Google Scholar]
- 22.Bertoti I, Mohai M, Sullivan JL, Saied SO. Surface characterization of plasma-nitrided titanium—An XPS study. Appl Surface Sci. 1995;84:357–371. [Google Scholar]
- 23.Kuznetsov MV, Zhuravlev JF, Gubanov VA. XPS analysis of adsorption of oxygen molecules on the surface of Ti and TiNx films in vacuum. J Electron Spectrosc and Relat Phenom. 1992;58(3):169–176. [Google Scholar]
- 24.Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ. Mediation of biomaterial-cell interactions by adsorbed proteins: A review. Tissue Eng. 2005;11(1–2):1–18. doi: 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- 25.Caplan AI. The mesengenic process. Clin Plast Surg. 1994;21:429–435. [PubMed] [Google Scholar]
- 26.Salgado AJ, Coutinho OP, Reis RL. Bone tissue engineering: State of the art and future trends. Macromol Biosci. 2004;4:743–765. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 27.Tsyganov I, Maitz MF, Wieser E. Blood compatibility of titanium-based coatings prepared by metal plasma immersion ion implantation and deposition. Appl Surface Sci. 2004;235(1–2):156–163. [Google Scholar]
- 28.Wälivaara B, Aronsson B, Rodahl M, Lausmaa J, Tengvall P. Titanium surfaces with different surface oxides: In vitro studies of protein adsorption and contact activation. Biomaterials. 1994;15:827–834. doi: 10.1016/0142-9612(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 29.Siebers MC, ter Brugge PJ, Walboomers XF, Jansen JA. Integrins as linker proteins between osteoblasts and bone replacing materials. A critical review. Biomaterials. 2005;26:137–146. doi: 10.1016/j.biomaterials.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 30.Yang YZ, Glover R, Ong JL. Fibronectin adsorption on titanium surfaces and its effect on osteoblast precursor cell attachment. Colloids Surf B. 2003;30(4):291–297. [Google Scholar]
- 31.MacDonald DE, Markovic B, Boskey AL, Somasundaran P. Physico-chemical properties of human plasma fibronectin binding to well characterized titanium dioxide. Colloids Surf B. 1998;11(3):131–139. [Google Scholar]
- 32.Grinnell F, Feld MK. Fibronectin adsorption on hydrophilic and hydrophobic surfaces detected by antibody binding and analyzed during cell adhesion in serum-containing medium. J Biol Chem. 1982;257:4888–4893. [PubMed] [Google Scholar]
- 33.Grinnell F, Feld MK. Adsorption properties of fibronectin in relationship to biological activity. J Biomed Mater Res. 1981;15:363–81. doi: 10.1002/jbm.820150308. [DOI] [PubMed] [Google Scholar]
- 34.Emch R, Zenhausern F, Jobin M, Taborelli M, Descouts P. Morphological difference between fibronectin sprayed on mica and on PMMA. Ultramicroscopy. 1992;42–44:1155–1160. doi: 10.1016/0304-3991(92)90417-i. [DOI] [PubMed] [Google Scholar]
- 35.Howlett CR, Evans MDM, Walsh WR, Johnson G, Steele JG. Mechanisms of initial attachment of cells derived from human bone to commonly used prosthetic materials during cell culture. Biomaterials. 1994;15:213–222. doi: 10.1016/0142-9612(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 36.Puleo DA, Nanci A. Understanding and controlling the bone-implant interface. Biomaterials. 1999;20:2311–2321. doi: 10.1016/s0142-9612(99)00160-x. [DOI] [PubMed] [Google Scholar]
- 37.Anselme K. Osteoblast adhesion on biomaterials. Biomaterials. 2000;21:667–681. doi: 10.1016/s0142-9612(99)00242-2. [DOI] [PubMed] [Google Scholar]
- 38.Schneider G, Burridge K. Formation of focal adhesions by osteoblasts adhering to different substrata. Exp Cell Res. 1994;214:264–269. doi: 10.1006/excr.1994.1257. [DOI] [PubMed] [Google Scholar]
- 39.Gronowicz G, McCarthy MB. Response of human osteoblasts to implant materials: Integrin-mediated adhesion. J Orthop Res. 1996;14:878–887. doi: 10.1002/jor.1100140606. [DOI] [PubMed] [Google Scholar]


