Abstract
Purpose
Human ocular surface epithelia express four antimicrobial peptides (APs): β-defensin (hBD) 1-3 and LL-37. Here the expression of additional APs (hBD 4-6, HE2β1; histatin-1, -3; liver expressed antimicrobial peptide-1, -2; macrophage inflammatory protein (MIP)-3α, and thymosin (T)β-4) was sought and activity against common ocular pathogens studied.
Methods
AP expression was determined in human corneal and conjunctival epithelial cells (HCEC, HCjEC) by RT-PCR and in corneal sections by immunostaining. Antimicrobial assays were performed to assess peptide (hBD 1-3, LL-37, MIP-3α, and Tβ4) activity against Pseudomonas aeruginosa (PA), Staphylococcus aureus (SA), and Staphylococcus epidermidis (SE) in the presence of NaCl or tears.
Results
HCEC and HCjEC expressed MIP-3α and Tβ4. hBD 1-3, MIP-3α, and Tβ4 showed activity against PA. hBD-3 had potent activity against SA and SE, whereas hBD-2, MIP-3α and Tβ4 had moderate activity and hBD-1 had none. NaCl markedly attenuated, and tears almost completely inhibited the activity of hBD 1-2 and Tβ4, but not that of hBD-3.
Conclusions
The ocular surface epithelia additionally express MIP-3α and Tβ4 which have moderate antimicrobial activity. The current data support a role for hBD-3 as an antimicrobial peptide in vivo, but call in to question the effectiveness of some other APs. However, further study is required to conclusively elucidate the physiological role of each AP.
Keywords: Antimicrobial peptides, thymosin β-4, MIP-3α/CCL20, β-defensins, cathelicidin, corneal epithelium, ocular surface, Pseudomonas aeruginosa, Staphylococcus aureus
Introduction
Despite constant threat from pathogenic microbes in the air and foreign objects such as contact lenses and occasionally unclean fingertips, the incidence of ocular surface infection is relatively low. This is mainly attributable to a very dynamic innate immune system at the ocular surface preventing the development of serious eye infections such as bacterial keratitis or conjunctivitis. Several components contribute to this important host defense, including intact corneal and conjunctival epithelia that form the initial barrier between the eye and the external environment and enzymes and other proteins in the tear film that have potent antimicrobial activity.1 Previous studies indicate that ocular surface epithelial cells are capable of producing small antimicrobial peptides which may help protect the eye against a wide range of micro-organisms such as bacteria, fungi, and some viruses.2 There is much evidence suggesting that these peptides are multifunctional; that is, in addition to their direct antimicrobial functions, these molecules may also participate in numerous non-antimicrobial activities including immune cell recruitment and activation, providing a link to adaptive immunity.3 Antimicrobial peptides have also been implicated as mediators of inflammation with impact on epithelial and inflammatory cell behaviors such as cytokine production, cell migration, proliferation, and wound healing.2,4
While some elements of the ocular immune response such as lysozyme, lactoferrin, lipocalin-1, phospholipase A2, and mucin have been studied, the expression and activity of antimicrobial peptides and their functional contributions at the ocular surface remain to be investigated in depth.5–8 To date, studies have focused on two classes of antimicrobial peptides known as the β-defensins and cathelicidins. We reported previously that corneal and conjunctival epithelial cells express three β-defensins (hBD-1, -2, -3) and LL-37, the only cathelicidin described in humans.9 hBD-1, hBD-3, and LL-37 are constitutively expressed by both corneal and conjunctival epithelia whereas hBD-2 expression is inducible by conditions mimicking injury, inflammation, and in response to bacterial products.10–14 These peptides are believed to exert their antimicrobial effects by creating pores or otherwise disrupting the cell membrane of target organisms through electrostatic interactions leading to leakage of cellular contents and death.15 It has been shown that the antimicrobial activity of some defensins and LL-37 may be reduced in the presence of high salt content (a major component of the tear fluid), and this brings into question the effectiveness of these peptides at the ocular surface in vivo. However, it is now apparent that synergy between host defense proteins (such as lactoferrin and lysozyme) and antimicrobial peptides as well as between these peptides themselves may help overcome the effect of salt.16 Thus, it is very possible that the spectrum of antimicrobial peptide expression at the ocular surface encompasses many different molecules.
In addition to defensins and cathelicidin, recent studies have identified several different classes of antimicrobial peptides in humans that are expressed by epithelial cells of various tissues, immune cells, and cells at different mucosal sites. hBD-4, -5, -6, and HE2β1 are β-defensin isoforms found in the epididymis.17 Histatins (Hist-1, -3) are antifungal agents present in saliva.18 Liver expressed antimicrobial peptides (LEAP1-2, also known as hepcidins) are antibacterial peptides expressed in the liver.19,20 Macrophage inflammatory protein (MIP)-3α, also known as CC chemokine ligand (CCL)20, is constitutively expressed in lung and intestine and has recently been detected in human tears.21–23 Yang et al. have identified twenty-one chemokines with antimicrobial activity including MIP-3α.24 Notably, MIP-3α shares structural and functional properties with β-defensins which include a charge distribution critical for antimicrobial activity and the ability to signal selectively via the same receptor, CC-chemokine receptor-6 (CCR6).25 Thymosin β-4 (Tβ4), a G-actin sequestering peptide, has also been shown to have antimicrobial activity.26 With the exception of Histatins which are primarily candidacidal, all of these peptides have significant activity against Gram-positive and Gram-negative bacteria.
Antimicrobial peptides are potential candidates for future development as innovative therapeutic agents, particularly in the light of recent data suggesting that, in addition to their antimicrobial activity, some of these peptides may also help stimulate epithelial healing processes and participate in the regulation of the adaptive immune response. To better understand the role of the various antimicrobial peptides in ocular surface defense, the primary goal of this study was to explore the spectrum of antimicrobial peptides expressed by human corneal and conjunctival epithelia and study the antimicrobial activity of the peptides in vitro against common ocular pathogens. Activity of these peptides under conditions that better mimic the physiological environment (presence of sodium chloride (150 mM) or human tears) was also investigated.
Materials and Methods
Peptides and Antibodies
Recombinant peptides hBD-1, -2, and -3, MIP-3α/CCL20, and a polyclonal anti-human MIP-3α/CCL20 antibody were purchased from Peprotech Inc. (Rocky Hill, NJ). Secondary antibodies for immunostaining and Western blots conjugated to horseradish peroxidase or Cy3 were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA). Custom produced synthetic Tβ4 was acquired from RegeneRx (Rockville, MD).
Tear Sample Collection from Human Subjects
All procedures involving human subjects were performed with approval of the University of Houston Institutional Review Board and in accordance with the tenets of the Declaration of Helsinki regarding research involving human subjects. All subjects had a complete optometric examination at the University Eye Institute (University of Houston) and were found to be free of any ocular surface disease. Six subjects (2 males, 4 females; age range 28–47 years) took part in this study. Unstimulated tears were collected from the inferior tear meniscus using 5-μl microcapillary tubes (Drummond Scientific, Broomall, PA). Anesthetizing the ocular surface can lead to a reduction in tear production; therefore, tears were collected without the use of anesthetics.27 80–100 μl of tears were collected from each subject over a total of three visits spaced 2–3 days apart. Tear samples were stored at −80°C until analysis.
Human Corneal Epithelium
Human corneas were obtained from Lions Eye Banks (Central Florida and Heartlands) and utilized in accordance with the tenets of the Declaration of Helsinki regarding the use of human tissue for research. Epithelial cells were cultured as described below or the epithelium was scraped off using a scalpel blade then immediately placed in RNA lysis buffer (Qiagen, Valencia, CA) for RT-PCR assays or snap frozen in liquid nitrogen and stored at −80°C for immunoblot assays.
Cell Culture
Primary cultures of human corneal epithelial cells (HCEC) were prepared from single or pairs of normal eye bank corneas based on the method described previously.12 Following incubation in Dispase II (1.2 U/mL) for 4–5 hours at 37°C, the epithelial layer was scraped free from the underlying stroma with a #15 scalpel blade and transferred to a tube containing Dulbecco's Modified Eagle's Medium (DMEM) and 10% fetal bovine serum (FBS) and centrifuged. The cell pellet was re-suspended in EpiLife medium (Cascade Biologics, Portland, OR), and a single cell suspension was obtained by triturating through a syringe fitted with a 22G needle. The cells were transferred to a culture flask coated with a mixture of fibronectin and collagen (FNC; AthenaES, Baltimore, MD) containing 5 mL of serum-free EpiLife media with human corneal growth supplement (HCGS, Cascade Biologics). Primary cultured HCEC of passages1 to 2 were used in the experiments. Human conjunctival tissue from two donors was obtained from the National Disease Research Interchange (NDRI, Philadelphia, PA). Primary conjunctival epithelial cells were cultured as described previously.14 Briefly, conjunctival tissue was incubated overnight at 4°C in a 1:1 (v/v) solution of EpiLife medium and dispase (20 U/mL). Using a scalpel blade, the epithelial cells were then scraped free and seeded into a FNC coated flask with EpiLife media and HCGS. Primary-cultured conjunctival epithelial cells of passages 1 to 3 were used for the experiments. Some experiments were performed using SV40-transformed human corneal epithelial cells (SV40-HCEC)28 or a normal human conjunctival epithelial cell line (IOBA-NHC).29 SV40-HCEC were cultured in media (DMEM; Ham's F12 1:1 v/v) supplemented with 10% FBS, 1% dimethyl sulphoxide (DMSO, Sigma-Aldrich, St. Louis, MO), and 50 μg/ml gentamicin. IOBA-NHC were cultured in DMEM-F12 (1:1 v/v), containing 10% FBS, 2 ng/mL mouse epidermal growth factor (EGF; Sigma-Aldrich), 1 μg/mL bovine insulin (Sigma-Aldrich), 0.1 μg/mL cholera toxin (Sigma-Aldrich), 5 μg/mL hydrocortisone (Sigma-Aldrich), 2.5 μg/mL amphotericin B, and a penicillin streptomycin mixture (5000 U/mL and 5000 μg/mL, respectively). In some experiments, cells were treated with 10 ng/ml human recombinant IL-1β or TNF-α (R&D Systems, Minneapolis, MN) diluted in serum-free culture media for 3, 6, and 24 hours or with media alone as the control. Collected cells were immediately placed in RNA lysis buffer (Qiagen) for RT-PCR assays or snap frozen in liquid nitrogen and stored at −80°C for immunoblot analysis.
Reverse Transcription–Polymerase Chain Reaction
Total RNA from all cell samples was extracted using an RNeasy kit (Qiagen). 250 ng of total RNA were used per RT-PCR reaction using a Superscript II kit (Invitrogen, Carlsbad, CA). Reactions containing normal human testis, salivary gland, liver or thymus RNA (Clontech Laboratories, Palo Alto, CA), or RNAse free water in place of the RNA served as positive controls and a negative control, respectively. Reverse transcription was performed at 50°C for 60 minutes. In some reactions, the reverse transcriptase was omitted (–RT control). After denaturation of the enzyme (94°C, 5 minutes), amplification of the cDNA was performed for 35–40 cycles: denaturation, 94°C for 50 seconds; annealing, 56–62°C for 30 seconds; extension 72°C for 1 minute. The specific primers used for β-actin,30β-defensins (hBD-4, hBD-5, hBD-6, HE2β1);17 His-1, -3; Tβ4; MIP-3α/CCL20, and LEAP 1-2 are summarized in Table 1. Products generated with these primers were sequenced (Seqwright, Houston, TX) to confirm their identities. RT-PCR products were visualized on agarose gels using an Alpha Imager gel documentation system (Alpha Innotec, San Leandro, CA).
Table 1. Antimicrobial Peptide PCR Primer Sequences and Size.
| Gene | Primer sequences (5′-3′) | Size (bp) |
|---|---|---|
| β-Actin | CCTCGCCTTTGCCGATCC
GGATCTTCATGAGGTAGTCAGTC |
626 |
| hBD-4 | CCAGTGAGAAGCGAATTTGA
CTGAGGTCCTACTTCCAGCG |
220 |
| hBD-5 | TTGGTTCAACTGCCATCAGG
CCAGGTCTGCTTCTAAGGCC |
250 |
| hBD-6 | CAGTCATGAGGACTTTCCTC
AGAAGCTAGGTTATGTATGC |
249 |
| HE2β1 | TCTGGCTTGCAGTGCTCTTG
CTTGGGATACTTCAACATCC |
550, 470 |
| Hist-1 | ACCGCATCACACTACCACTG
TTGCTTTTGGAGAGGAATGAA |
201 |
| Hist-3 | TCTTGGCTCTCATGCTTTCC
CACGAGTCCAAAGCGAATTT |
194 |
| LEAP-1 | CAGACAGACGGCACGATG
GCAGCTCTGCAAGTTGTCC |
136 |
| LEAP-2 | TCCCTCAGGCCTATTGGAG
GGAGGTGACTGCTGTCCTTT |
158 |
| MIP-3α | TTGCTCCTGGCTGCTTTG
ACCCTCCATGATGTGCAAG |
363 |
| Tβ4 | ACAAACCCGATATGGCTGAG
GAAGGCAATGCTTGTGGAAT |
178 |
To clarify the effects of cytokine treatment on MIP-3α and Tβ4 expression, selected samples were subjected to real-time RT-PCR analysis. Isolated RNA (2 μg) was reversed-transcribed to cDNA using the Thermoscript RT-PCR System (Invitrogen, Carlsbad, CA). Real-time PCR was performed to quantitatively evaluate the expression of MIP-3α and Tβ4 in the cell samples using an Mx3005P Quantitative PCR System (Stratagene, La Jolla, CA). The specific primer sequences used for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were (forward) 5′-GAAGGTGAAGGTCGGAGTC-3′, (reverse) 5′-GAAGATGGTGATGGGATTTC-3′, and for MIP-3α/CCL20 were (forward) 5′-TGTCAGTGCTGCTACTCCACCT-3′, (reverse) 5′-CTGTGTATCCAAGACAGCAGTCAA-3,31 and for Tβ4 were as listed in Table 1. All real-time PCR amplifications were performed using the SYBR Green QPCR Mastermix Kit (Stratagene). Briefly, 2 μl of each cDNA sample was combined with 2x Mastermix and reference dye, ROX, according to the manufacturer's recommendations. Triplicate reactions were performed for each sample. No template and no reverse transcriptase controls were included. PCR cycling conditions were as follows: 95°C for 10 minutes, followed by 40 cycles of amplification at 95°C for 1 minute, and 60°C for 1 minute to allow for denaturing and annealing-extension. The PCR products were examined by melting curve analysis on the Mx3005 with only one peak being detected in each curve and by agarose gel electrophoresis to confirm that primers yielded predicted size products. Data analysis was performed using the Mx3005 software. Amplified gene products were normalized to GAPDH and calibrated to non-treated samples. The relative fold change of cytokine-treated versus media-treated samples was then determined with the normalized value of media-treated samples being set to one. The data were analyzed by Student's t-test with values of P < 0.05 being considered significant.
Immunoblot Analysis for MIP-3α and Tβ4
Epithelial samples that had been snap frozen were used in immunoblots to detect MIP-3α and Tβ4. Each sample was homogenized in 100 μl of ice cold tris buffered saline (TBS, 150 mM NaCl, 20 mM Tris-HCl, pH 7.5). Cell lysate (25 μg of total protein) was blotted directly onto a nitrocellulose membrane using a Bio-Dot Microfiltraion apparatus (Life Science, Hercules, CA). Recombinant MIP-3α (5 ng) or synthetic Tβ4 (10 ng) peptide were also blotted onto the membrane as positive controls. Nonspecific binding sites were blocked by incubation in 5% blotto and then the membrane was incubated with a rabbit anti-MIP-3α or a rabbit anti-Tβ4 polyclonal antibody (a gift of Dr. Livaniou, NCSR Demokritos) diluted 1 in 5000 in 3% blotto. After an overnight incubation the membranes were then incubated with a horseradish peroxidase linked second antibody diluted 1 in 10,000 in 3% blotto. Immunoreactivity was visualized by enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ).
Immunostaining
Human corneas were obtained from Lions Eye Banks (Central Florida and Heartlands) and utilized in accordance with the tenets of the Declaration of Helsinki regarding the use of human tissue for research. The maximum elapsed time between donor death and receipt of the tissue was 5 days. The mean age of the donors was 68 ± 2 years. The corneas were embedded in OCT (Optimal Cutting Temperature compound), frozen, then sectioned on a cryostat. The sections were fixed in 4% paraformaldehyde, then incubated with blocking solution (0.1% goat serum, 0.05% gelatin, and 0.05% Tween-20 diluted in PBS). After blocking, the sections were incubated with a rabbit anti-MIP-3α (1 in 500) or anti-Tβ4 (1 in 300) polyclonal antibody at 4°C overnight and then with a cy3-conjugated second antibody diluted 1 in 300 in blocking solution. In selected blocking experiments, the anti-Tβ4 polyclonal antibody was pre-incubated with Tβ4 peptide at 4°C overnight prior to being utilized. Sections in which the peptide specific polyclonal antibody was omitted served as background controls. The slides were viewed under a microscope equipped for fluorescence and digital imaging.
Antibacterial Assay
Pseudomonas aeruginosa PA, ATCC 19660 and ATCC 27853, were tested in this study. ATCC 27853 is known to invade the cornea while ATCC 19660 has been characterized as a cytotoxic strain. Both strains are capable of inducing severe ocular infection in experimentally infected animal models of bacterial keratitis.32–34 The majority of our studies were carried out using ATCC 27853 strain and selected experiments were repeated with ATCC 19660 and two PA clinical isolates from corneal scrapings of subjects with bacterial keratitis.
One single isolated PA colony was used to inoculate 5 ml of nutrient broth (NB) overnight at 37°C. Fifty microliters of this bacterial suspension were used to inoculate 50 ml of fresh NB, which was then incubated for 2.5 hours with vigorous shaking at 37°C to achieve mid-log phase growth. Twenty-five milliliters of the warm PA culture were centrifuged at 3100 g for 10 minutes, and the bacterial cell pellet was resuspended in phosphate buffer (PB, 8.2 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4). Optical density of the suspension was adjusted to 0.2 at 620 nm (approximately 107 cfu/ml) by adding an appropriate volume of PB. The antimicrobial assay procedure was performed as previously described.35 Briefly, reaction mixtures (final volume 50 μl) containing 10 μl of 107 cfu/ml PA diluted in PB and 5 μl peptide (final concentration 0.05, 0.1, 0.5, 1, 10, 25, 50, and 100 μg/ml, additional concentrations of 250, 500 μg/ml, and 1 mg/ml were tested for Tβ4) were incubated at 37°C for 2 hours with vigorous shaking. In each experiment, reaction mixtures containing 5 μl of 0.01% acetic acid, the vehicle for diluting the peptides, acted as a control. At the end of the incubation, serial dilutions of each reaction mixture were used to inoculate NB agar plates. Samples (10 μl) were spread evenly over the surface of the plates using sterile glass spreaders. After incubation at 37°C for 24 hours, the plates were placed on a light-board and a digital image captured using an Alpha Imager documentation system. The number of colonies was counted and EC50 values (the effective concentration that resulted in 50% killing of the bacteria) were calculated using GraphPad Prism4 software (GraphPad Software, San Diego, CA). Experiments were also performed to test the antimicrobial activity of the peptides (EC50 and minimum concentrations achieving 100% killing) against PA in the presence of physiological NaCl solution (150 mM NaCl) and human tears. Tears were diluted in PB to give final reaction mixtures containing 70% v/v tears. Due to other constituents of the reaction mixture, 70% v/v was the maximum tear concentration obtainable in these experiments. Additional experiments were also performed to test the antimicrobial activity of each peptide against Staphylococcus aureus (SA, ATCC 29213) and Staphylococcus epidermidis (SE, ATCC 155). Testing conditions in these experiments were identical to those described for PA with the exception of replacing NB with trypticase soy broth (TSB).
Results
Human Ocular Surface Epithelia Express MIP-3α and Tβ4
RT-PCR was performed to study the expression of various human peptides with known antimicrobial activity (hBD 4, -5, -6, HE2β1, MIP-3α, Hist-1, -3, LEAP 1, -2, and Tβ4) in human corneal and conjunctival epithelial cells (Fig. 1). Figure 1A shows representative data from scraped corneal epithelium (n = 3) and primary cultured conjunctival cells (n = 2). Two peptides, MIP-3α and Tβ4, were detected in the epithelial samples while all of the others were only detected in the appropriate positive control RNA. Identical results were obtained with primary cultured HCEC (n = 3), SV40-transformed HCEC (n = 3), and IOBA-NHC (n = 3), data not shown. Immunoblotting was performed to study MIP-3α and Tβ4 peptide expression by human corneal epithelium. As shown in Figure 1B, MIP-3α and Tβ4 peptide were present in both scraped (n = 2) and primary cultured (n = 3) corneal epithelial cells. Immunostaining was performed to localize MIP-3α and Tβ4 peptide in normal human corneal sections. Strong MIP-3α and Tβ4 immunoreactivity was detectable throughout the corneal epithelial layer as shown in representative images (Fig. 2) from one of three corneas. Pre-incubation of anti-Tβ4 antibody with Tβ4 peptide completely eliminated the Tβ4 immunoreactivity in the corneal sections (data now shown). Immunoreactivity was not present in background control samples in the absence of the primary antibody. Of note, faint, yet apparent positive staining of MIP-3α and Tβ4 was also observed in some parts of the corneal stroma (data not shown).
Figure 1.
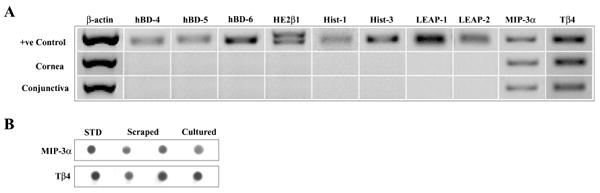
Expression of MIP-3α and Tβ4 mRNA and protein by ocular surface epithelia. (A) RT-PCR. The figure shows representative results for cornea = scraped human corneal epithelium (n = 3); conjunctiva = primary cultured human conjunctival epithelial cells (n = 2); + ve controls = positive controls: testis (hBD 4–6, HE2β1, MIP-3α), salivary gland (Hist-1, -3), liver (LEAP 1-2), and thymus (Tβ4). (B) Immunoblotting. The figure shows representative results for: STD (standard) = 5 ng human rMIP-3α or 10 ng Tβ4 synthetic peptide; scraped = 25 μg cellular protein from two scraped human corneal epithelial samples (n = 2); cultured = 25 μg cellular protein from primary cultured human corneal epithelial cells (n = 3).
Figure 2.
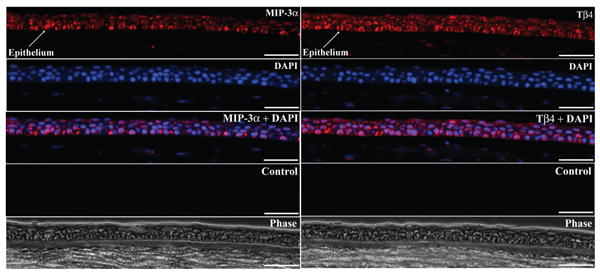
Immunostaining of MIP-3α and Tβ4 in the cornea. The figure shows representative images for MIP-3α (left) and Tβ4 (right) expression in normal human corneal epithelium (sections from central cornea). Specific immunolabeling for MIP-3α or Tβ4 is shown in red; DAPI: nuclear labeling (blue); MIP-3α or Tβ4+ DAPI: merged image; control: background labeling in the absence of primary antibody; phase: phase contrast image. Identical findings were seen in corneas from three different donors. (Scale bars: 40 μm).
IL-1β and TNF-α Modulate MIP-3α But Not Tβ4 Expression in HCEC and IOBA-NHC
To determine if conditions mimicking inflammation induced or upregulated expression of antimicrobial peptides, the expression of MIP-3α and Tβ4, hBD 4-6, HE2β1, Hist-1, -3, and LEAP 1-2 mRNA was studied by RT-PCR in cultured human corneal and conjunctival epithelial cells treated with proinflammatory cytokines IL-1β and TNF-α for 24 hours. MIP-3α and Tβ4 were expressed by both untreated and treated epithelial cells. Figure 3 shows representative data from primary cultured HCEC; all media-treated epithelial samples expressed a low level of MIP-3α mRNA (n = 3 for primary cultured HCEC; n = 2 for primary cultured conjunctival epithelial cells; n = 3 for SV-40 transformed HCEC, and n = 4 for IOBA-NHC). Nine of the twelve cytokine-treated epithelial samples collected 24 hours post treatment showed that MIP-3α mRNA expression was significantly upregulated compared to the untreated samples (p < 0.05). This was confirmed using real-time RT-PCR (Fig. 3C and 3D) on selected epithelial samples (n = 3 for primary cultured HCEC, 13.6–21.1 fold; n = 2 for SV-40 transformed HCEC, 9.4–18.1 fold; and n = 4 for IOBA-NHC, 2.5–6.7 fold). Expression of MIP-3α as upregulated by IL-1β as early as 3 hours at the level comparable to that observed at 24-hour post-stimulation (data not shown). Treatment with IL-1β or TNF-α (Fig. 3B–D) did not alter the expression of Tβ4 (identical results were observed with SV40-transformed HCEC and IOBA-NHC and confirmed with real-time RT-PCR). Expression of the other antimicrobial peptides was not detected in either untreated or cytokine-treated cells and thus was not modulated by IL-1β or TNF-α (data not shown).
Figure 3.
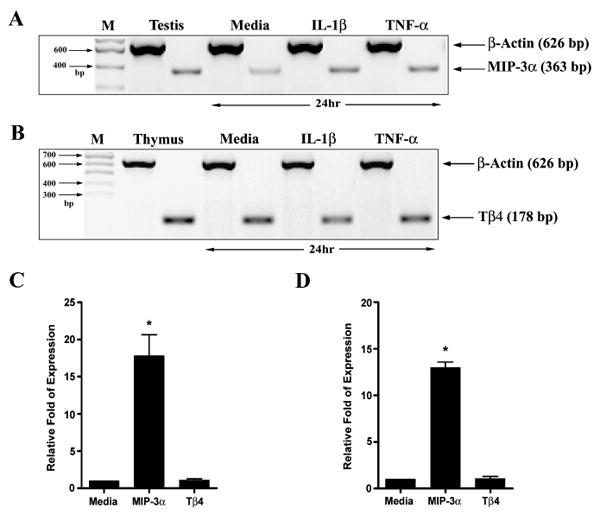
Effect of pro-inflammatory cytokines on MIP-3α and Tβ4 expression in ocular surface epithelial cells. MIP-3α (A) and Tβ4 (B) mRNA expression by corneal epithelial cells treated with IL-1β(10 ng/ml) or TNF-α (10 ng/ml) for 24 hours. M = base pair marker. Real-time PCR showing relative levels of mRNA expression for MIP-3α and Tβ4 in IL-1β (C) or TNF-α (D) treated epithelial cell samples. The figure shows representative results for primary cultured HCEC (n = 3, * p < 0.05, compared to the untreated control). Media = normalized expression in media-treated samples set to one.
Antibacterial Activity of Antimicrobial Peptides against Common Ocular Pathogens
Antibacterial assays were performed to study the activity of hBD1–3 and Tβ4 against PA (ATCC 27853 and ATCC 19660), SA (ATCC 29213), and SE (ATCC 155). As shown in Figure 4A–C, hBD-1, -2, and -3 inhibited growth of PA in a concentration dependent manner. The EC50 values for killing ATCC 27853 were 21.4 ± 1.5 μg/ml (n = 3), 1.2 ± 0.2 μg/ml (n = 3), and 3.4 ± 1.3 μg/ml (n = 3) for hBD-1, -2 and -3, respectively. At 100 μg/ml, these three β-defensins completely killed PA. hBD-2 was only weakly effective against staphylococcal strains while hBD-1 showed no activity against SA or SE (data not shown). In contrast, as shown in Figure 4C, hBD-3 was strongly effective against Staphylococcal strains with EC50 values of 5.3 ± 1.5 (n = 3) and 0.9 ± 0.2 (n = 3) μg/ml for killing SA and SE, respectively. Tβ4 also exhibited activity against PA (Fig. 4D) with EC50 values of 18.4 ± 1.5 and 28.7 ± 1.6 μg/ml for each of the two laboratory PA strains, ATCC 27853 and ATCC 19660 respectively. Notably, a very high Tβ4 concentration (1 mg/ml) was required to achieve 100% killing as compared to the three β-defensins (100 μg/ml) tested. While hBD1-3 and Tβ4 killed PA in a dose-dependent manner, hBD-2 and hBD-3 were 6- to 18-fold more potent than hBD-1 or Tβ4. A complete concentration-dependent response was not determined for MIP-3α (against PA, SA, and SE) or Tβ4 (against SA and SE). MIP-3α (25 μg/ml) appeared to be much more effective than Tβ4 (200 μg/ml) against PA. Although both MIP-3α and Tβ4 were only weakly effective against SA and SE compared to LL-37 (50 μg/ml), the positive control, MIP-3α exerted stronger antibacterial effect than Tβ4 toward Staphylococcal strains (Fig. 4E).
Figure 4.
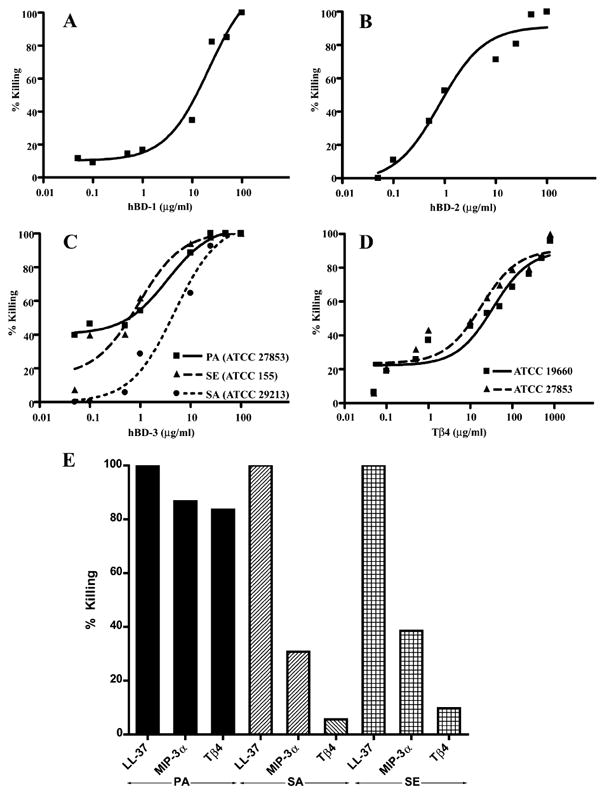
Antibacterial effect of hBD 1-3 and Tβ4 against common ocular pathogens. (A-B) 107 cfu/ml PA (ATCC 27853) were incubated with hBD-1 (A) or hBD-2 (B) for 2 hours. The graphs show the average data of three experiments. The EC50values were 21.4 ± 1.5 and 1.2 ± 0.2 μg/ml for hBD-1 and hBD-2, respectively. (C) 107 cfu/ml PA (ATCC 27853), SA (ATCC 29213), and SE (ATCC 155) were incubated with hBD-3 for 2 hours. The graph shows the average data of three experiments against each strain. The EC50values were 3.4 ± 1.3 (PA), 5.3 ± 1.5 (SA), and 0.9 ± 0.2 μg/ml (SE). (D) 107 cfu/ml PA (ATCC 27853 and ATCC 19660) were incubated with Tβ4 for 2 hours. The graph shows the average data of three experiments against each strain. The EC50 values were 18.4 ± 1.5 μg/ml (PA, ATCC 27853) and 28.7 ± 1.6 μg/ml (PA, ATCC 19660). (E) 107 cfu/ml PA (ATCC 27853), SA (ATCC 29213), and SE (ATCC 155) were incubated with LL-37 (50 μg/ml), MIP-3α (25 μg/ml), and Tβ4 (200 μg/ml) at 37°C for 2 hours. The graph shows representative data from two experiments.
Effects of Antimicrobial Peptides against Pseudomonas aeruginosa in the Presence of NaCl and Human Tear Fluid
As shown in Figure 5, the effectiveness against PA of hBD1-2 (Fig. 5A and 5B) and Tβ4 (Fig. 5D), used at their established EC50 concentrations, was almost completely lost while that of hBD-3 (Fig. 5C) was only moderately reduced at physiological salt concentration (150 mM NaCl). When tested at a high concentration (minimum concentration achieving 100% killing of PA), the activity of hBD1-3 and Tβ4 was only slightly reduced in the presence of NaCl, with that of hBD-3 being the least affected. Human tears almost completely inhibited the antimicrobial effect against PA of hBD1-2 and Tβ4 regardless of peptide concentrations, but not that of hBD-3. When tested in the presence of tears (Fig. 5C), activity of 3 μg/ml hBD-3 (mean EC50 of the peptide established previously) was moderately reduced similar to the extent found in the presence of NaCl, whereas the activity of a high concentration of hBD-3 (100 μg/ml) was not altered in the presence of human tear fluid. Identical results were obtained when experiments were repeated with the three other PA strains.
Figure 5.
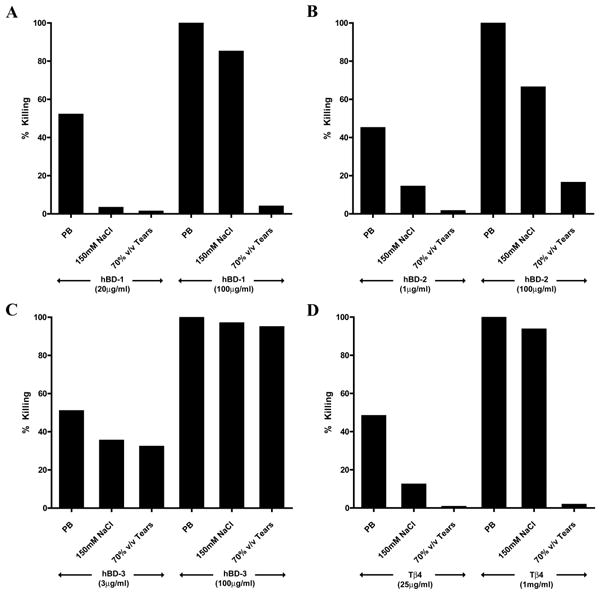
Antibacterial effect of hBD 1–3 and Tβ4 against Pseudomonas aeruginosa (ATCC 27853) in the presence of NaCl and Tears. 107 cfu/ml PA were incubated with (A) hBD-1 (20 μg/ml or 100 μg/ml), (B) hBD-2 (1 μg/ml or 100 μg/ml), (C) hBD-3 (3 μg/ml or 100 μg/ml), or (D) Tβ4 (25 μg/ml or 1 mg/ml) in the presence or absence of 150 mM NaCl or tears (70% v/v) at 37°C for 2 hours. The graph shows representative data from one of two or three experiments. PB = phosphate buffer, control.
Discussion
We have previously shown that human ocular surface epithelia express a number of antimicrobial peptides including β-defensins (hBD 1-3) and cathelicidin (LL-37) as part of their innate immune response in preventing microbial invasion.10–12,14 The present study complements these earlier findings in an effort to better define the spectrum of antimicrobial peptide expression at the ocular surface and we have now characterized the expression of two additional peptides with antimicrobial activity, MIP-3α and Tβ4, by human corneal and conjunctival epithelial cells.
In a previous study McIntosh et al.36 reported detection of relatively low levels of expression of hBD-4 and LEAP 1-2 by RT-PCR in corneal and conjunctival epithelial cell samples collected from patients by impression cytology and in cells in culture. This is in contrast to findings in the present study, as we did not detect expression of these peptides in any of our cell samples. McIntosh et al. did note that hBD-4 was chiefly expressed by cultured cells and was found in only a small number of patient samples, leading them to conclude that hBD-4 is not typically expressed at the ocular surface. In contrast, LEAP-1 and -2 were detected in the majority of their samples. One notable difference between these two studies, which may contribute to the contrasting observations, is the manner in which the samples were obtained, including the fact that the HCEC cultures in the study by McIntosh et al. were prepared from limbal explants and therefore likely contained a mixed population of limbal and corneal epithelial cells. A preliminary study by Steele and Jumblatt37 has shown that human corneal and conjunctival tissues also express statherin, an antimicrobial phosphoprotein. Previously, Yang et al.24 identified 21 human chemokines that exhibit antimicrobial activity. Of these antimicrobial chemokines, mRNA expression of CCL28 has been detected in corneal and conjunctival epithelia.37 Also as we confirmed here, Shirane et al. recently demonstrated that HCEC express MIP-3α.38 Additionally, Spandau et al.39 have shown that epithelial cells from inflamed corneas strongly express mRNA for the antimicrobial chemokine CXCL-1. Therefore, in addition to β-defensins and LL-37, corneal and conjunctival epithelial cells express Tβ4, MIP-3α and, as discussed above, several other antimicrobial chemokines, which may all contribute to the host-defense functions of the ocular surface.
We investigated the antimicrobial activity of hBD1-3, MIP-3α, and Tβ4 against Staphylococci (the most commonly occurring organisms in bacterial conjunctivitis) and PA (the infectious organism most frequently encountered in keratitis associated with extended contact lens wear). Of the three β-defensins, hBD-2 and hBD-3 (with EC50 values of approximately 1–5 μg/ml) showed the strongest antibacterial effect against PA. Among the five antimicrobials, hBD-3 was the only peptide which exerted potent antibacterial activity against all strains of bacteria tested. In our study, hBD-1 exhibited moderate activity against PA and no activity against SA or SE strains tested. Similar to results previously described, hBD-2 was more effective than hBD-1 against PA by greater than 10-fold.40 Our data compare favorably to previously published data: Harder et al. reported a LD90 for hBD-2 of 10 μg/ml in killing PA and of 2.5–4 μg/ml against SA for hBD-3 purified from psoriatic scale extract,41,42 Singh et al. found an EC50 for recombinant hBD-2 against PA to be 100 ng/ml,40 and our previously determined EC50 for synthetic hBD-2 peptide was 2.5 μg/ml (unpublished observation). In addition, we have observed that MIP-3α is also effective against PA but exhibits poor antimicrobial activity against SA. This is in agreement with studies by Hoover et al. and Starner et al. in which they determined the MIC of MIP-3α to be approximately 5–19 μg/ml against PA (PA01 and another mucoid strain), but >79–250 μg/ml for SA strains.25,43 On the contrary, Yang et al. have reported previously that the anti-SA activity of MIP-3α (LD50 of 10 μg/ml) appears to be greater than that of hBD-2.24 The reason for these discrepancies is not fully understood but is most likely due to the use of different antimicrobial assays (radial diffusion assay vs. colony formation assay) and different strains of bacteria. Interestingly in Yang et al.'s study, MIP-3α also showed potent antibacterial effect against Streptococcus pyogenes, another ocular pathogen known to instigate acute bacterial conjunctivitis. In addition to hBD1–3 and MIP-3α, we have demonstrated that Tβ4 exhibits some antimicrobial activity in vitro, although it is not as effective as the cationic peptides. Unlike most other antimicrobial peptides, Tβ4 is an acidic and anionic antimicrobial peptide and therefore must exert its antibacterial effect through mechanisms distinct from that of the cationic peptides.44 We have shown for the first time that PA is susceptible to the antimicrobial activity of Tβ4 although higher concentrations of Tβ4 were required as compared to that of hBD-2 and hBD-3. Tβ4 was minimally effective against the Staphylococcal strains tested. These findings are comparable to a study by Tang et al. in which they determined that a high concentration (50–100 μg/ml) of the peptide was needed to cause a reduction of 2–3 log10 cfu/ml in SA and Escherichia coli (EC), although Tβ4 had considerably greater activity against EC than SA.26
Cationic antimicrobial peptides achieve their antibacterial effects by permeabilizing the anionic microbial cell membrane through electrostatic interaction leading to cell lysis and death.15 Of note, the killing activity of many cationic peptides, including that of MIP-3α, exhibits salt sensitivity.45–48 Previous studies have also shown that these peptides are easily inactivated by alterations in ionic strength at mucosal surfaces or of biological fluids and that they lose their antibacterial effects as they change structural conformation in extracellular environments.45,47,49,50 As these findings call into question whether such peptides may be active at the ocular surface due to the salt content of the tear film, we studied the activity of selected antimicrobial peptides under physiological salt conditions (150 mM NaCl, comparable to that in the tear fluid) and in the presence of human tears. When tested in NaCl, lower concentrations (EC50) of hBD 1–2 or Tβ4 did not have significant activity against PA while high concentrations of hBD 1–2 (100 μg/ml) or Tβ4 (1 mg/ml) exerted potent antimicrobial activity. This is expected as increased concentrations of some antimicrobial peptides have been shown to counteract the inhibitory effect of salt.40,51 The actual concentrations of the various antimicrobial peptides at the ocular surface have not been determined, although previously we have estimated the level of β-defensin secretion by corneal epithelial cells in vivo to be approximately 1.4 μg and 0.5 μg per cornea for hBD-1 and hBD-2, respectively, in conditions mimicking inflammation.52 In addition, we and others have also reported that hBD-2 expression by the ocular surface epithelia is significantly increased during epithelial wound healing and in response to bacterial products.11–14 Notably, we have shown in the current study that expression of MIP-3α by both human corneal and conjunctival epithelial cells to be significantly up-regulated by IL-1β and TNF-α pro-inflammatory cytokines known to be increased during inflammation, infection, and after injury. This is in agreement with a previous study published by Shriane et al. in which they determined the level of MIP-3α secretion by corneal epithelial cells in vitro to be approximately 3–5 ng/ml (an effective concentration range for chemotactic activity, but a significantly lower amount than required for antimicrobial activity in vitro) upon stimulation by proinflammatory cytokines.38,53–56 However, our data indicated that Tβ4 expression was not up-regulated by IL-1β and TNF-α although we cannot exclude the possibility that other cytokines may modulate its expression. Recent studies have also suggested that local concentrating effects in vivo can raise the concentration of antimicrobial peptides to levels adequate for antibacterial activity.57–59 We speculate that increased production and local concentrating effects of certain antimicrobial peptides may allow these molecules to overcome the effects of physiological salt concentration at the ocular surface in vivo. Furthermore, synergistic effects between antimicrobial peptides and other host defense proteins have been demonstrated in terms of antimicrobial activity, and these have been shown to help overcome salt sensitivity.16,60,61
It is noteworthy that when tested at high concentrations, activity of hBD-1, -2, and Tβ4 was markedly more reduced in tear fluid compared to the reduction observed in NaCl, therefore implying that additional factors (other than the salt content) present in the tears are capable of impairing activity. One previous study has shown that tears differentially attenuated the microbicidal activity of rabbit defensins against PA.62 The exact mechanism of how human tears may interfere with the antimicrobial activity of certain antimicrobial peptides is unclear. Evidence from other studies indicates that some β-defensins are susceptible to degradation and inactivation by proteases;63 also certain proteins notably known be to present in the tear film may compromise the expected antimicrobial activity of defensins by complex formation.64,65 As already stated, Tβ4 is anionic peptide rather than cationic, therefore the reduction in its activity may occur through a mechanism distinct from that of the cationic peptides. In contrast to hBD-1, -2, Tβ4, and MIP-3α, the activity of a low concentration (EC50) of hBD-3 was only moderately reduced in the presence of NaCl, and high concentration of hBD-3 retained strong activity against PA. This is in agreement with previous studies showing that of the three β-defensins, hBD-3 is the least sensitive to the effects of salt.42,48,66 This characteristic and the increased activity of hBD-3 have been attributed to the increased ability of the peptide to form dimers and the higher net positive surface charge compared with hBD-1 and hBD-2.67 Of even greater significance is our observation that the activity of hBD-3 was not reduced in the presence of tears. We have previously identified another antimicrobial peptide at the ocular surface, LL-37, with a comparable spectrum of activity as that of hBD-3, which is also not affected by tears.35 This lack of tear sensitivity implies that hBD-3 and LL-37 are particularly important for antimicrobial protection of the ocular surface.
It is now well recognized that many antimicrobial peptides subserve dual roles in that they are microbicidal and are able to modulate mammalian cell functions, such as migration, proliferation, and cytokine production.4 Although findings of the current study indicated that some of these peptides are not effective in terms of their antimicrobial activity in the presence of salt or tear fluid, they may be capable of inducing other effects at the ocular surface. In keeping with this hypothesis, MIP-3α and Tβ4 are known to exert a variety of non-microbicidal effects. MIP-3α is one of the many chemokines known to be actively involved in innate and acquired immunity due to its ability to promote directed migration of various types of leukocytes, such as immature dendritic cells and effector T cells.68 Sosne et al. have recently demonstrated that Tβ4 modulated matrix metalloproteinase expression and prevented neutrophil infiltration in the mouse cornea following alkali injury69 and, in a separate study, found the peptide-stimulated corneal epithelial healing and cytokine production after ethanol debridement.70 Interestingly, they have recently shown an additional protective effect of Tβ4 as the peptide-inhibited benzalkonium chloride-mediated apoptosis in human corneal and conjunctival epithelial cells in vitro.71 Previously, Sosne et al. have reported that the level of Tβ4 expression was increased in murine corneas during re-epithelialization72 and therefore speculated the peptide may play important roles promoting wound healing, as they have previously shown it to stimulate human corneal and conjunctival epithelial cell migration.70,73 In support of this, Goldstein et al. have recently reported that Tβ4 has key roles in the repair and remodeling of ulcerated tissues and solid organs including the cornea.74 Katz et al. have reported that Tβ4 in normal human tear fluid ranges in concentration from 1–7 μg/ml,72 which is significantly lower than the concentration required for the peptide alone to exert significant antibacterial activity. Based on data from the current study, we do not expect this peptide to have significant independent antimicrobial activity in vivo, although the concentration of Tβ4 in tears is considered sufficient for other non-antimicrobial functions as discussed.
In summary, our data show that human corneal and conjunctival epithelial cells express MIP-3α and Tβ4. It is also evident that the antibacterial activity of MIP-3α and Tβ4 is comparatively less than that of previously described antimicrobial peptides (β-defensins and cathelicidin) expressed at the ocular surface. Although the peptides tested in this study exerted some antimicrobial effects against various known ocular pathogens, activity of all peptides tested, except hBD-3, is reduced in physiological NaCl concentrations and eliminated in the presence of human tears. This casts some doubt on their roles as primary components of the innate immune system, although it is possible that some of the peptides are capable of synergizing with each other and other antimicrobial factors to achieve significant antimicrobial effect under inflammatory conditions during microbial infection or epithelial wound healing. The fact that the antibacterial activity of some antimicrobial peptides is sensitive to salt and tears may also imply that the principal roles of these particular peptides are more likely to be as regulatory mediators of immune responses or modulators of wound healing. Current data does support an antimicrobial role for hBD-3, indicating it is an important component in the epithelial defense at the ocular surface. Future studies are warranted to further delineate the functional roles and activities of the peptides at the ocular surface in vivo and to examine the potential interactions among these peptides.
Acknowledgments
The authors thank Lions Eye Banks (Arizona and Heartlands) for providing the human corneas, Dr. Livaniou (NCSR Demokritos) for her generous donation of Tβ4 antibodies, and RegeneRx for supplying the synthetic Tβ4 peptide. This research was supported by a Vision Research Support Grant (LCH) from the University of Houston, College of Optometry (UHCO), a GEAR grant from UH (AMM), EY007551 (UHCO CORE Grant), #T35 EY007088 (DJ), and EY13175 (AMM). LCH was partially supported by a William C. Ezell Fellowship from the American Optometric Foundation.
References
- 1.Sack RA, Nunes I, Beaton A, et al. Host-defense mechanism of the ocular surfaces. Biosci Rep. 2001;21:463–480. doi: 10.1023/a:1017943826684. [DOI] [PubMed] [Google Scholar]
- 2.McDermott AM. Defensins and other antimicrobial peptides at the ocular surface. The Ocular Surface. 2004;2:229–247. doi: 10.1016/s1542-0124(12)70111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patrzykat A, Douglas SE. Antimicrobial peptides: cooperative approaches to protection. Protein Pept Lett. 2005;12:19–25. doi: 10.2174/0929866053406057. [DOI] [PubMed] [Google Scholar]
- 4.Beisswenger C, Bals R. Functions of antimicrobial peptides in host defense and immunity. Curr Protein Pept Sci. 2005;6:255–264. doi: 10.2174/1389203054065428. [DOI] [PubMed] [Google Scholar]
- 5.McClellan KA. Mucosal defense of the outer eye. Surv Ophthalmol. 1997;42:233–246. doi: 10.1016/s0039-6257(97)00090-8. [DOI] [PubMed] [Google Scholar]
- 6.Fluckinger M, Haas H, Merschak P, et al. Human tear lipocalin exhibits antimicrobial activity by scavenging microbial siderophores. Antimicrob Agents Chemother. 2004;48:3367–3372. doi: 10.1128/AAC.48.9.3367-3372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qu XD, Lehrer RI. Secretory phospholipase A2 is the principal bactericide for staphylococci and other Gram-positive bacteria in human tears. Infect Immun. 1998;66:2791–2797. doi: 10.1128/iai.66.6.2791-2797.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNamara NA. Innate defense of the ocular surface. Eye Contact Lens. 2003;29:S10–S12. doi: 10.1097/00140068-200301001-00004. [DOI] [PubMed] [Google Scholar]
- 9.Lehrer RI, Ganz T. Cathelicidins: a family of endogenous antimicrobial peptides. Curr Opin Hematol. 2002;9:18–22. doi: 10.1097/00062752-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Gordon YJ, Huang LC, Romanowski EG, et al. Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr Eye Res. 2005;30:385–394. doi: 10.1080/02713680590934111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDermott AM, Redfern RL, Zhang B. Human β-defensin 2 is up-regulated during re-epithelialization of the cornea. Curr Eye Res. 2001;22:64–67. doi: 10.1076/ceyr.22.1.64.6978. [DOI] [PubMed] [Google Scholar]
- 12.McDermott AM, Redfern RL, Zhang B, et al. Defensin expression by the cornea: multiple signaling pathways mediate IL-1 beta stimulation of hBD-2 expression by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:1859–1865. doi: 10.1167/iovs.02-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNamara NA, Van R, Tuchin OS, et al. Ocular surface epithelia express mRNA for human beta defensin-2. Exp Eye Res. 1999;69:483–490. doi: 10.1006/exer.1999.0722. [DOI] [PubMed] [Google Scholar]
- 14.Narayanan S, Miller WL, McDermott AM. Expression of human beta-defensins in conjunctival epithelium: relevance to dry eye disease. Invest Ophthalmol Vis Sci. 2003;44:3795–3801. doi: 10.1167/iovs.02-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van 't Hof W, Veerman EC, Helmerhorst EJ, et al. Antimicrobial peptides: properties and applicability. Biol Chem. 2001;382:597–619. doi: 10.1515/BC.2001.072. [DOI] [PubMed] [Google Scholar]
- 16.Singh PK, Tack BF, McCray PB, Jr, et al. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am J Physiol Lung Cell Mol Physiol. 2000;279:L799–L805. doi: 10.1152/ajplung.2000.279.5.L799. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi Y, Nagase T, Makita R, et al. Identification of multiple novel epididymis-specific beta-defensin isoforms in humans and mice. J Immunol. 2002;169:2516–2523. doi: 10.4049/jimmunol.169.5.2516. [DOI] [PubMed] [Google Scholar]
- 18.Oppenheim FG, Xu T, McMillian FM, et al. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J Biol Chem. 1988;263:7472–7477. [PubMed] [Google Scholar]
- 19.Krause A, Neitz S, Magert HJ, et al. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480:147–150. doi: 10.1016/s0014-5793(00)01920-7. [DOI] [PubMed] [Google Scholar]
- 20.Krause A, Sillard R, Kleemeier B, et al. Isolation and biochemical characterization of LEAP-2, a novel blood peptide expressed in the liver. Protein Sci. 2003;12:143–152. doi: 10.1110/ps.0213603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reibman J, Hsu Y, Chen LC, et al. Airway epithelial cells release MIP-3alpha/CCL20 in response to cytokines and ambient particulate matter. Am J Respir Cell Mol Biol. 2003;28:648–654. doi: 10.1165/rcmb.2002-0095OC. [DOI] [PubMed] [Google Scholar]
- 22.Izadpanah A, Dwinell MB, Eckmann L, et al. Regulated MIP-3alpha/CCL20 production by human intestinal epithelium: mechanism for modulating mucosal immunity. Am J Physiol Gastrointest Liver Physiol. 2001;280:G710–G719. doi: 10.1152/ajpgi.2001.280.4.G710. [DOI] [PubMed] [Google Scholar]
- 23.Sack RA, Conradi L, Krumholz D, et al. Membrane array characterization of 80 chemokines, cytokines, and growth factors in open- and closed-eye tears: angiogenin and other defense system constituents. Invest Ophthalmol Vis Sci. 2005;46:1228–1238. doi: 10.1167/iovs.04-0760. [DOI] [PubMed] [Google Scholar]
- 24.Yang D, Chen Q, Hoover DM, et al. Many chemokines including CCL20/MIP-3alpha display antimicrobial activity. J Leukoc Biol. 2003;74:448–455. doi: 10.1189/jlb.0103024. [DOI] [PubMed] [Google Scholar]
- 25.Hoover DM, Boulegue C, Yang D, et al. The structure of human macrophage inflammatory protein-3alpha /CCL20. Linking antimicrobial and CC chemokine receptor-6-binding activities with human beta-defensins. J Biol Chem. 2002;277:37647–37654. doi: 10.1074/jbc.M203907200. [DOI] [PubMed] [Google Scholar]
- 26.Tang YQ, Yeaman MR, Selsted ME. Antimicrobial peptides from human platelets. Infect Immun. 2002;70:6524–6533. doi: 10.1128/IAI.70.12.6524-6533.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta A, Heigle T, Pflugfelder SC. Nasolacrimal stimulation of aqueous tear production. Cornea. 1997;16:645–648. [PubMed] [Google Scholar]
- 28.Araki-Sasaki K, Ohashi Y, Sasabe T, et al. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci. 1995;36:614–621. [PubMed] [Google Scholar]
- 29.Diebold Y, Calonge M, Enriquez de Salamanca A, et al. Characterization of a spontaneously immortalized cell line (IOBA-NHC) from normal human conjunctiva. Invest Ophthalmol Vis Sci. 2003;44:4263–4274. doi: 10.1167/iovs.03-0560. [DOI] [PubMed] [Google Scholar]
- 30.Raff T, van der Giet M, Endemann D, et al. Design and testing of beta-actin primers for RT-PCR that do not co-amplify processed pseudogenes. Biotechniques. 1997;23:456–460. doi: 10.2144/97233st02. [DOI] [PubMed] [Google Scholar]
- 31.Ruth JH, Shahrara S, Park CC, et al. Role of macrophage inflammatory protein-3alpha and its ligand CCR6 in rheumatoid arthritis. Lab Invest. 2003;83:579–588. doi: 10.1097/01.lab.0000062854.30195.52. [DOI] [PubMed] [Google Scholar]
- 32.Hazlett LD, Moon MM, Strejc M, et al. Evidence for N-acetylmannosamine as an ocular receptor for P. aeruginosa adherence to scarified cornea. Invest Ophthalmol Vis Sci. 1987;28:1978–1985. [PubMed] [Google Scholar]
- 33.O'Callaghan RJ, Engel LS, Hobden JA, et al. Pseudomonas keratitis. The role of an uncharacterized exoprotein, protease IV, in corneal virulence. Invest Ophthalmol Vis Sci. 1996;37:534–543. [PubMed] [Google Scholar]
- 34.Fleiszig SM, Kwong MS, Evans DJ. Modification of Pseudomonas aeruginosa interactions with corneal epithelial cells by human tear fluid. Infect Immun. 2003;71:3866–3874. doi: 10.1128/IAI.71.7.3866-3874.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang LC, Petkova TD, Reins RY, et al. Multifunctional roles of human cathelicidin (LL-37) at the ocular surface. Invest Ophthalmol Vis Sci. 2006;47:2369–2380. doi: 10.1167/iovs.05-1649. [DOI] [PubMed] [Google Scholar]
- 36.McIntosh RS, Cade JE, Al-Abed M, et al. The spectrum of antimicrobial peptide expression at the ocular surface. Invest Ophthalmol Vis Sci. 2005;46:1379–1385. doi: 10.1167/iovs.04-0607. [DOI] [PubMed] [Google Scholar]
- 37.Steele PS, Jumblatt MM. Defense proteins of the ocular surface. Invest Ophthalmol Vis Sci. 2004;45 ARVO e-abstract 3792. [Google Scholar]
- 38.Shirane J, Nakayama T, Nagakubo D, et al. Corneal epithelial cells and stromal keratocytes efficiently produce CC chemokine-ligand 20 (CCL20) and attract cells expressing its receptor CCR6 in mouse herpetic stromal keratitis. Curr Eye Res. 2004;28:297–306. doi: 10.1076/ceyr.28.5.297.28682. [DOI] [PubMed] [Google Scholar]
- 39.Spandau UH, Toksoy A, Verhaart S, et al. High expression of chemokines Gro-alpha (CXCL-1), IL-8 (CXCL-8), and MCP-1 (CCL-2) in inflamed human corneas in vivo. Arch Ophthalmol. 2003;121:825–831. doi: 10.1001/archopht.121.6.825. [DOI] [PubMed] [Google Scholar]
- 40.Singh PK, Jia HP, Wiles K, et al. Production of beta-defensins by human airway epithelia. Proc Natl Acad Sci USA. 1998;95:14961–14966. doi: 10.1073/pnas.95.25.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harder J, Bartels J, Christophers E, et al. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 42.Harder J, Bartels J, Christophers E, et al. Isolation and characterization of human beta-defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–5713. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 43.Starner TD, Barker CK, Jia HP, et al. CCL20 is an inducible product of human airway epithelia with innate immune properties. Am J Respir Cell Mol Biol. 2003;29:627–633. doi: 10.1165/rcmb.2002-0272OC. [DOI] [PubMed] [Google Scholar]
- 44.Huff T, Muller CS, Otto AM, et al. beta-Thymosins, small acidic peptides with multiple functions. Int J Biochem Cell Biol. 2001;33:205–220. doi: 10.1016/s1357-2725(00)00087-x. [DOI] [PubMed] [Google Scholar]
- 45.Goldman MJ, Anderson GM, Stolzenberg ED, et al. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell. 1997;88:553–560. doi: 10.1016/s0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 46.Bals R, Wang X, Zasloff M, et al. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci USA. 1998;95:9541–9546. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bals R, Wang X, Wu Z, et al. Human beta-defensin-2 is a salt-sensitive peptide antibiotic expressed in human lung. J Clin Invest. 1998;102:874–880. doi: 10.1172/JCI2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Starner TD, Agerberth B, Gudmundsson GH, et al. Expression and activity of beta-defensins and LL-37 in the developing human lung. J Immunol. 2005;174:1608–1615. doi: 10.4049/jimmunol.174.3.1608. [DOI] [PubMed] [Google Scholar]
- 49.Johansson J, Gudmundsson GH, Rottenberg ME, et al. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J Biol Chem. 1998;273:3718–3724. doi: 10.1074/jbc.273.6.3718. [DOI] [PubMed] [Google Scholar]
- 50.Cole AM, Tahk S, Oren A, et al. Determinants of Staphylococcus aureus nasal carriage. Clin Diagn Lab Immunol. 2001;8:1064–1069. doi: 10.1128/CDLI.8.6.1064-1069.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turner J, Cho Y, Dinh NN, et al. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chemother. 1998;42:2206–2214. doi: 10.1128/aac.42.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McDermott AM, Redfern RL, Zhang B, et al. Defensin expression by the cornea: multiple signaling pathways mediate IL-1beta stimulation of hBD-2 expression by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:1859–1865. doi: 10.1167/iovs.02-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Charbonnier AS, Kohrgruber N, Kriehuber E, et al. Macrophage inflammatory protein 3alpha is involved in the constitutive trafficking of epidermal Langerhans cells. J Exp Med. 1999;190:1755–1768. doi: 10.1084/jem.190.12.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dieu-Nosjean MC, Massacrier C, Homey B, et al. Macrophage inflammatory protein 3alpha is expressed at inflamed epithelial surfaces and is the most potent chemokine known in attracting Langerhans cell precursors. J Exp Med. 2000;192:705–718. doi: 10.1084/jem.192.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scapini P, Laudanna C, Pinardi C, et al. Neutrophils produce biologically active macrophage inflammatory protein-3alpha (MIP-3alpha)/CCL20 and MIP-3beta/CCL19. Eur J Immunol. 2001;31:1981–1988. doi: 10.1002/1521-4141(200107)31:7<1981::aid-immu1981>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 56.Dieu MC, Vanbervliet B, Vicari A, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Braff MH, Di Nardo A, Gallo RL. Keratinocytes store the antimicrobial peptide cathelicidin in lamellar bodies. J Invest Dermatol. 2005;124:394–400. doi: 10.1111/j.0022-202X.2004.23443.x. [DOI] [PubMed] [Google Scholar]
- 58.Oren A, Ganz T, Liu L, et al. In human epidermis, beta-defensin-2 is packaged in lamellar bodies. Exp Mol Pathol. 2003;74:180–182. doi: 10.1016/s0014-4800(02)00023-0. [DOI] [PubMed] [Google Scholar]
- 59.Akinbi HT, Narendran V, Pass AK, et al. Host defense proteins in vernix caseosa and amniotic fluid. Am J Obstet Gynecol. 2004;191:2090–2096. doi: 10.1016/j.ajog.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 60.Nagaoka I, Hirota S, Yomogida S, et al. Synergistic actions of antibacterial neutrophil defensins and cathelicidins. Inflamm Res. 2000;49:73–79. doi: 10.1007/s000110050561. [DOI] [PubMed] [Google Scholar]
- 61.Chen X, Niyonsaba F, Ushio H, et al. Synergistic effect of antibacterial agents human beta-defensins, cathelicidin LL-37 and lysozyme against Staphylococcus aureus and Escherichia coli. J Dermatol Sci. 2005;40:123–132. doi: 10.1016/j.jdermsci.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 62.McDermott AM, Rich D, Cullor J, et al. The in vitro activity of selected defensins against an isolate of Pseudomonas in the presence of human tears. Br J Ophthalmol. 2006;90:609–611. doi: 10.1136/bjo.2005.083428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taggart CC, Greene CM, Smith SG, et al. Inactivation of human beta-defensins 2 and 3 by elastolytic cathepsins. J Immunol. 2003;171:931–937. doi: 10.4049/jimmunol.171.2.931. [DOI] [PubMed] [Google Scholar]
- 64.Panyutich AV, Hiemstra PS, van Wetering S, et al. Human neutrophil defensin and serpins form complexes and inactivate each other. Am J Respir Cell Mol Biol. 1995;12:351–357. doi: 10.1165/ajrcmb.12.3.7873202. [DOI] [PubMed] [Google Scholar]
- 65.Sathe S, Sakata M, Beaton AR, et al. Identification, origins and the diurnal role of the principal serine protease inhibitors in human tear fluid. Curr Eye Res. 1998;17:348–362. doi: 10.1080/02713689808951215. [DOI] [PubMed] [Google Scholar]
- 66.Ishimoto H, Mukae H, Date Y, et al. Identification of hBD-3 in respiratory tract and serum: the increase in pneumonia. Eur Respir J. 2006;27:253–260. doi: 10.1183/09031936.06.00105904. [DOI] [PubMed] [Google Scholar]
- 67.Schibli DJ, Hunter HN, Aseyev V, et al. The solution structures of the human beta-defensins lead to a better understanding of the potent bactericidal activity of HBD3 against Staphylococcus aureus. J Biol Chem. 2002;277:8279–8289. doi: 10.1074/jbc.M108830200. [DOI] [PubMed] [Google Scholar]
- 68.Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14:409–426. doi: 10.1016/s1359-6101(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 69.Sosne G, Christopherson PL, Barrett RP, et al. Thymosin-beta4 modulates corneal matrix metalloproteinase levels and polymorphonuclear cell infiltration after alkali injury. Invest Ophthalmol Vis Sci. 2005;46:2388–2395. doi: 10.1167/iovs.04-1368. [DOI] [PubMed] [Google Scholar]
- 70.Sosne G, Szliter EA, Barrett R, et al. Thymosin beta4 promotes corneal wound healing and decreases inflammation in vivo following alkali injury. Exp Eye Res. 2002;74:293–299. doi: 10.1006/exer.2001.1125. [DOI] [PubMed] [Google Scholar]
- 71.Sosne G, Albeiruti AR, Hollis B, et al. Thymosin beta4 inhibits benzalkonium chloride-mediated apoptosis in corneal and conjunctival epithelial cells in vitro. Exp Eye Res. 2006;83:502–507. doi: 10.1016/j.exer.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 72.Sosne G, Goldstein AL, Badamchian M, et al. Thymosin beta4 expression in corneal wound healing. Invest Ophthalmol Vis Sci. 2004;45 ARVO e-abstract 1425. [Google Scholar]
- 73.Sosne G, Hafeez S, Greenberry AL, 2nd et al. Thymosin beta4 promotes human conjunctival epithelial cell migration. Curr Eye Res. 2002;24:268–273. doi: 10.1076/ceyr.24.4.268.8414. [DOI] [PubMed] [Google Scholar]
- 74.Goldstein AL, Hannappel E, Kleinman HK. Thymosin beta4: actin-sequestering protein moonlights to repair injured tissues. Trends Mol Med. 2005;11:421–429. doi: 10.1016/j.molmed.2005.07.004. [DOI] [PubMed] [Google Scholar]


