Abstract
In recent studies, green tea components have been shown to induce cell growth arrest and apoptosis in head and neck squamous cell carcinoma (HNSCC) cells. In this report, we have investigated the effects of epicatechin gallate (ECG), one of the catechins in green tea, on anti-cancer activity in vitro. We found that cyclin D1 was highly expressed in HNSCC cells, and ECG suppressed 90% of cyclin D1 expression in SCC7 cells. We have also evaluated the effect of ECG on cell growth and apoptosis, showing that ECG (50 μM) exhibited a significant inhibition (50%) on the growth of SCC7 cells via G1 cell cycle arrest. ECG suppressed cyclin D1 in SCC7 cells in a dose- and time-dependent manner, and the suppression of the β-catenin pathway by ECG is one of the mechanism to facilitate ECG-induced cell growth arrest. These results suggest that ECG has a potential usage as a chemopreventive agent in HNSCC.
Keywords: ECG, Cyclin D1, Apoptosis, HNSCC, β-Catenin, Chemoprevention
1. Introduction
Head and neck squamous cell carcinoma (HNSCC) includes epithelial malignancies that originate in the mucosal lining of the oral cavity, pharynx, nose and paranasal sinuses, and larynx. In the United States, 37,200 patients are newly diagnosed with HNSCC each year.1 Although some patients achieve long-term survival, particularly those diagnosed with early-stage disease, most patients with this type of cancer have advanced disease at the time of diagnosis. These patients run a risk of recurrent disease, distant metastases, or second primary tumours, and have a median survival of only 6–8 months.2 Over the last 20 years, though, diagnosis and management have improved through combined efforts in surgery, radiotherapy, and chemotherapy, but long-term survival rates have improved only marginally, as the overall 5-year survival rate for patients with HNSCC is among the lowest of the major cancers. Moreover, curative therapy for HNSCC may result in the expense of debilitating post-operative changes in appearance, speech, swallowing, and breathing. Therefore, preventive strategies are desirable, and much research is currently devoted to chemoprevention in order to prevent HNSCC progression at an early stage.
Epidemiological studies have suggested that nutrition plays an important role in carcinogenesis, and a diet high in vegetables and fruits could prevent at least 20% of all cancers. Many leafy plants, either fruits or vegetables, contain high phenolic compounds (also referred to as polyphenols), which have been found to inhibit tumour formation in experimental animals. One of the richest sources for polyphenols is from the tea leaves of Camellia sinensis. The anti-tumour effects of green tea have been studied at the cellular level, and apoptosis induction and cell cycle arrest are the major mechanisms induced by the compounds.3–5 The major polyphenols in green tea are epigallocatechin gallate (EGCG), epicatechin gallate (ECG), epigallocatechin (EGC), and epicatechin (EC). Of these, EGCG has been studied more extensively for its biological action because it is the most abundant catechin in this beverage. We have recently reported that EGCG and ECG induce apoptosis in human colorectal cancer, but not EGC or EC.6 Thus, both EGCG and ECG may play an important role in green tea-induced anti-tumorigenesis. However, the precise molecular mechanisms by which ECG induces apoptotic cell death are still largely unknown.
It has been shown that cyclin D1 is a key cell cycle regulatory protein for the mammalian G1-S phase transition and is involved in the regulation of proliferation and differentiation. 7 The proper balance of proliferation and differentiation is crucial for the maintenance of tissue homeostasis. Therefore, deregulated cyclin D1 expression promotes genetic instability in vitro and tumorigenesis in vivo.8 Amplification of the cyclin D1 gene was reported in 34% of HNSCC and is associated with a poor prognosis.9 In addition, the deregulation of cyclin D1 expression has been shown to occur early during the tumorigenic process in HNSCC and enables subsequent cyclin D1 gene amplification.10 As a result, cyclin D1 may be an important molecular target of oral cancer prevention.
In this study, we investigated the anti-tumorigenic effect of several dietary compounds and found that ECG treatment significantly suppresses cyclin D1 expression in HNSCC cell lines. In addition, we have found that cell growth and apoptotic cell death were induced in the presence of ECG in HNSCC cells. As a result, we suggest that ECG could be a potential agent for the chemoprevention of HNSCC.
2. Materials and methods
2.1. Cell lines and reagents
Human HNSCC cell lines Tu212, Tu177, Spccy1, and 38 were obtained from Dr. Dong M. Shin (Emory University, Atlanta, GA). The mouse oral SCC cell line, SCC7, was provided by Dr. Jatin P. Shah (Memorial Sloan Cancer Center, NY). Cell lines were maintained in a 5% CO2 atmosphere at 37 °C in DMEM supplemented with 10% Fetal Bovine Serum, penicillin (100 IU/mL), and streptomycin (100 μg/mL). Catechins (EGCG and ECG) and curcumin were purchased from Sigma (St. Louis, MO), and apigenin was obtained from Tocris (Ellisville, MO). All the chemicals were dissolved in DMSO. The NAG-1 antibody was described previously.11 and cyclooxygenase (COX)-1, COX-2, and cyclin D1 antibodies were purchased from Cayman (Ann Arbor, MI), Oxford Biomedical Research (Oxford, MI), and Cell Signaling Inc. (Beverly, MA), respectively.
2.2. Western blot analysis
The level of protein expression was evaluated using Western blot analysis with specific antibodies. Cells were grown to 60–80% confluency in 60-mm plates and treated with the indicated compounds for 24 h. Total cell lysates were isolated using RIPA buffer (50 mM Tris–HCl pH 7.4, 150 mM NaCl, 1mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS 1 mM phenylmethylsulphonyl fluoride, 1 μg/mL aprotinin, and 1 μg/mL leupeptin) and centrifuged at 12,000 rpm for 5 min at 4 °C. Total proteins (30 μg) were separated by SDS–PAGE and transferred for 1 h to a nitrocellulose membrane (Osmonics, Minnetonka, MN). The blots were blocked for 1 h with 5% skim milk in TBS/Tween 0.05% (TBS-T) and probed overnight with each antibody at 4 °C. After washing with TBS-T, the blots were treated with a horseradish peroxidase- conjugated secondary antibody for 1 h and washed four times. Protein bands were detected by the enhanced chemiluminescence system (Amersham Bioscience, Piscataway, NJ).
2.3. Transient transfections
Transient transfections were performed using the Lipofectamine™2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instruction. SCC7 cells were plated in 12-well plates at the concentration of 1 × 105 cells/well. After 18 h of growth, plasmid mixtures containing 1.6 μg of promoter linked to luciferase, 0.1 μg of pRL-null vector, and 4 μL of Lipofectamine™2000 reagent were transfected for 24 h. The transfected cells were cultured in the absence or presence of 50 μM ECG for 24 h. The cells were harvested in 1× luciferase lysis buffer, and luciferase activity was normalised to the pRL-null luciferase activity using a dual luciferase assay kit (Promega, Madison, WI).
2.4. Cell proliferation assay
The effect of ECG on cell proliferation was investigated using CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI). The assay was carried out as described in the manufacturer’s protocol. Briefly, on day 0, cells were plated in a 96-well tissue culture plate at a density of 1000 cells/well. Cells were incubated for 3 days with different concentrations of ECG. Twenty microlitres of freshly prepared MTS (3-[4,5-dimethylthiazol-2-yl-5]-[3-carboxymethoxyphenyl]-2-[4-sulphophenyl]-2H tetrazolium) mixture was added to each well, and after 1 h, absorbance was measured at 490 nm using an ELISA plate reader (Bio-Tek Instruments, Inc. Winooski, VT).
2.5. Measurement of DNA content and apoptosis by flow cytometry
In a 6-well plate, SCC7 cells were plated at a density of 1 × 105 cells/well in 2 mL of medium, incubated for 16 h, and then treated with either the vehicle or 50 μM of ECG in the presence of serum. After treatment, the cells were harvested, washed with PBS, fixed by the slow addition of cold 70% ethanol to a total of 1 mL, and stored at −20 °C overnight. The fixed cells were pelleted, washed once with PBS, and stained in a solution containing 1 mL of 20 μg/mL of propidium iodide solution and 1 mg/mL RNase for 20 min. Flow cytometry was used to examine 7500 cells with Becton Dickinson FACSort equipped with CellQuest software. Cells were gated on an area versus width dot plot to exclude cell debris and cell aggregates. Each cell cycle distribution was analyzed by the McCycle program using one cell cycle parameter. Sub-G1 cells were considered as an apoptotic cell population.
2.6. Statistical analysis
Statistical analysis was performed with a Student’s t test, with statistical significance set at *P < 0.05; **P < 0.01; and ***P < 0.001 compared to vehicle treatment.
3. Results
3.1. Expression levels of NAG-1, COX-1, COX-2, and cyclin D1 in HNSCC cell lines
Various proteins, such as COX-1,12 COX-2,13 cyclin D1,14 and NAG-115 have been known to be associated with tumorigenesis in HNSCC. Therefore, we performed Western blot analysis to determine whether any difference existed between expressions of these genes in the various HNSCC cell lines. As shown in Fig. 1, Western blot analysis showed different cyclin D1 expression levels among various HNSCC cell lines. The expression level of cyclin D1 was lowest in Spccy1; intermediate in Tu177, Tu212, and 38; and highest in SCC7 cells. COX-1 and COX-2 expression was also abundant in SCC7 cells, whereas other cells showed various patterns of COX expression with low levels. NAG-1 was marginally expressed in most of the cells tested here.
Fig. 1.
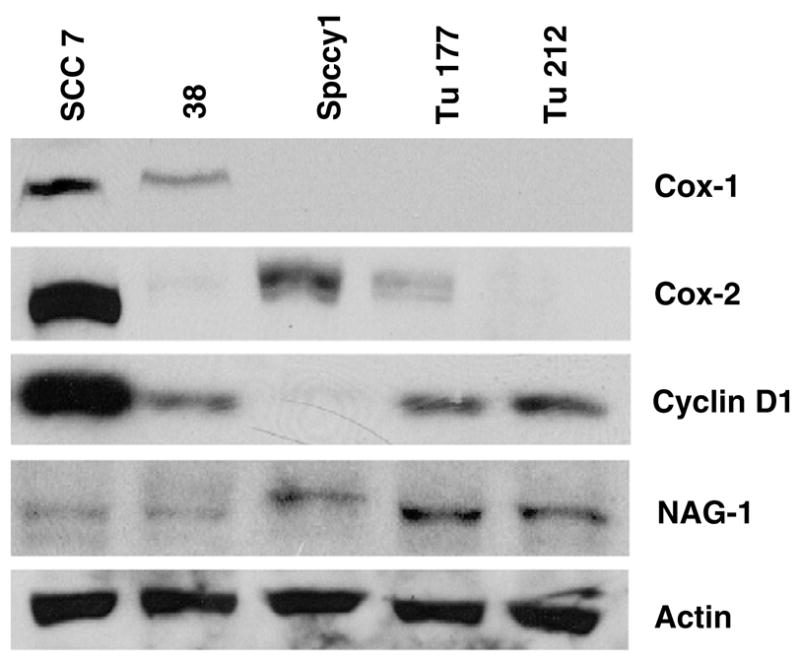
Expression of COX-1, COX-2, cyclin D1, and NAG-1 in HNSCC cell lines. Total proteins were prepared from cell lines SCC7, 38, Spccy1, Tu177, and Tu212, and were subjected to Western blot analysis using NAG-1, COX-1, COX-2, and cyclin D1 antibodies as described in Section 2. Actin antibody was used for loading control. The data represent two independent experiments.
3.2. Effect of various dietary polyphenols on the expression of cyclin D1 and COX-2 in the SCC7 cell line
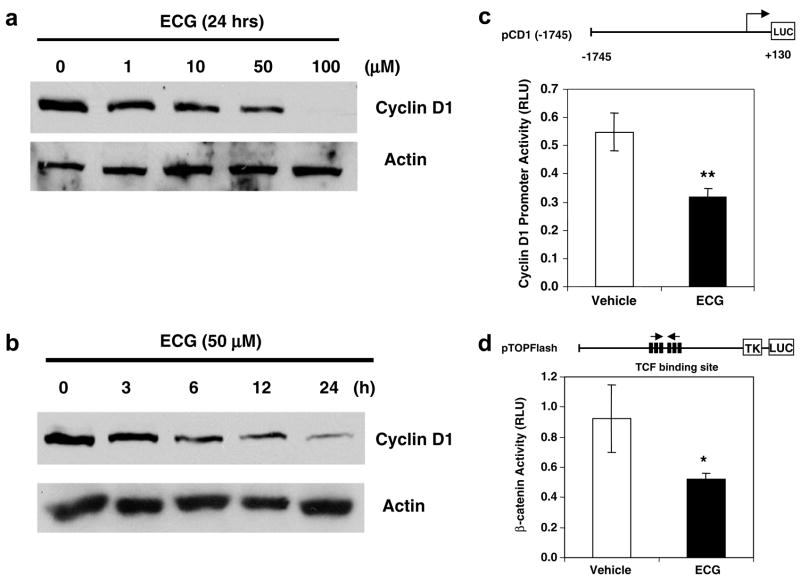
Since SCC7 cells highly expressed cyclin D1 and COX-2 proteins, we investigated whether dietary polyphenols may repress their expression. We selected and treated SCC7 cells with the following known anticancer compounds: catechins, 4 apigenin,16 and curcumin.17 These compounds have been known to induce apoptosis in head and neck cancer. Catechins are flavonoid phytochemical compounds that appear predominantly in green tea. Apigenin is one of the polyphenols that belongs to the flavones, and it can be found in parsley, thyme, celery, and sweet red pepper. Curcumin is also a polyphenol derived from the herbal remedy and dietary spice turmeric. As shown in Fig. 2, apigenin and curcumin marginally suppressed cyclin D1 expression, whereas ECG dramatically reduced cyclin D1 expression, compared to other polyphenols (~90% reduction, P < 0.01). Because COX-2 expression was not affected by the polyphenols tested here, and ECG dramatically suppressed cyclin D1 expression, we have selected ECG for further investigation. SCC7 cells were treated with ECG at different concentrations or different time points. As shown in Fig. 3a and b, cyclin D1 expression was repressed in time- and concentration-dependent manners. The most significant reduction of cyclin D1 was seen at the 24 h point and the 100 μM concentration. To confirm cyclin D1 regulation by ECG, we examined the cyclin D1 promoter linked to the luciferase reporter gene. SCC7 cells were transfected and treated with vehicle or ECG. As shown in Fig. 3c, ECG significantly down-regulated the cyclin D1 promoter activity as assessed by the luciferase reporter gene (P < 0.01). Cyclin D1 is up-regulated by the β-catenin signaling pathway and indeed β-catenin signaling is activated in oral cancer cells.18 Thus, we investigated whether β-catenin signaling is affected by ECG treatment. The TOP Flash constructs containing 6 copies of TCF/LEF transcription factor binding sites were transfected into SCC7 cells, and luciferase activity was measured. As shown in Fig. 3d, β-catenin signaling was decreased in the presence of ECG treatment (P < 0.05), indicating that ECG affects β-catenin signaling, resulting in the decrease of cyclin D1 expression.
Fig. 2.
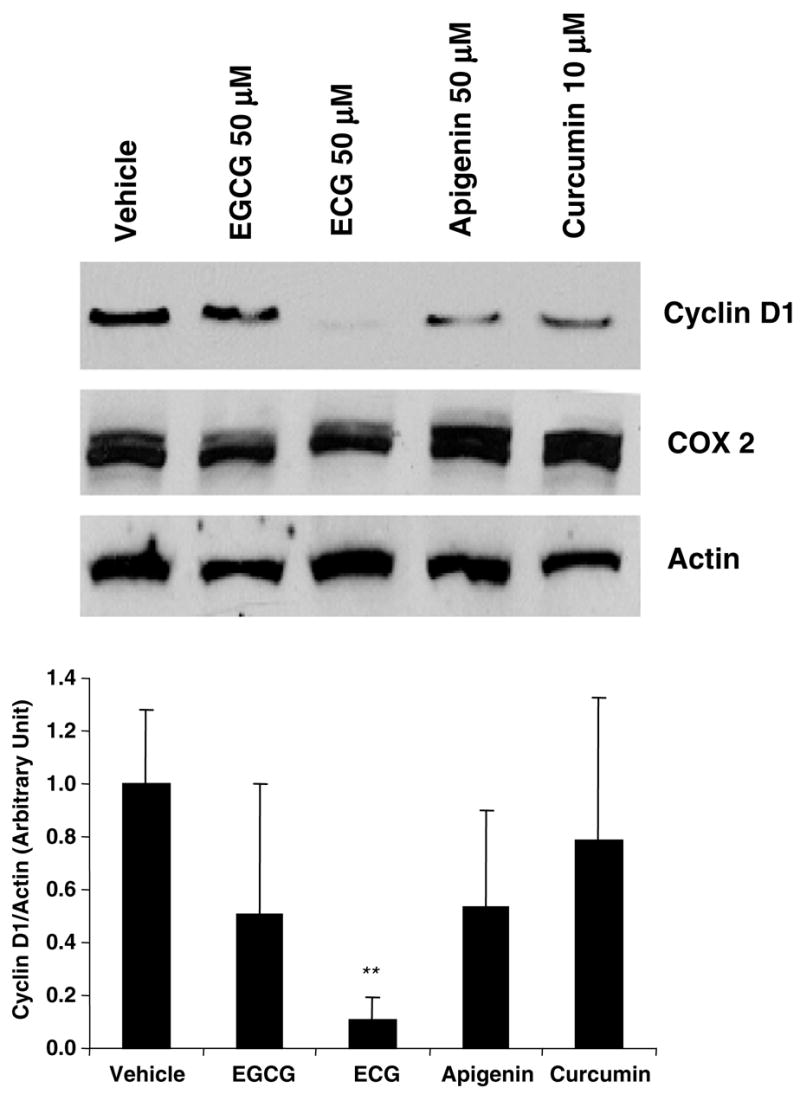
Expression of COX-2 and cyclin D1 by several polyphenols in SCC7 cells. SCC7 cells were treated with EGCG, ECG, or apigenin at 50 μM for 24 h, and curcumin at 10 μM for 24 h. Proteins were extracted from the treated cells and separated through SDS–PAGE. Western blot analysis was performed using cyclin D1, COX-2, or actin. DMSO (0.1%) was used for the vehicle treatment. The band intensities were quantified using Scion Image software, and values for the cyclin D1 were normalized to actin levels. The fold differences are shown in the bottom panel. The blots are representative of three independent experiments.
Fig. 3.
Inhibition of cyclin D1 expression and β-catenin signaling in ECG-treated SCC7 cells. (a) Dose response of cyclin D1 expression. SCC7 cells were grown in different concentrations of ECG for 24 h, and Western blot analysis was performed. Thirty microgram of total proteins was loaded in each lane and transferred onto a nitrocellulose membrane. The blot was hybridized with cyclin D1 antibody and re-probed with actin antibody. (b) Western blot analysis of cyclin D1 expression by ECG. SCC7 cells were treated with 50 μM ECG for various time points. Western analysis was performed as described above. (c) Cyclin D1 promoter construct (1.6 μg) containing −1745 to +130 of human cyclin D1 promoter region29 was co-transfected into SCC7 cells with pRL-null control vector (0.1 μg) using Lipofectamine™2000, and luciferase activity was measured. Values are expressed as means ± SD of 3 replicates. **P < 0.01 versus vehicle treated samples. (d) TOP Flash constructs containing six copies of TCF binding site (1.6 μg) were co-transfected into SCC7 cells with pRL-null control vector (0.1 μg) using Lipofectamine™2000, and luciferase activity was measured. Values are expressed as means ± SD of 3 replicates. *P < 0.05 versus vehicle treated samples.
3.3. Effect of ECG on cell growth and apoptosis
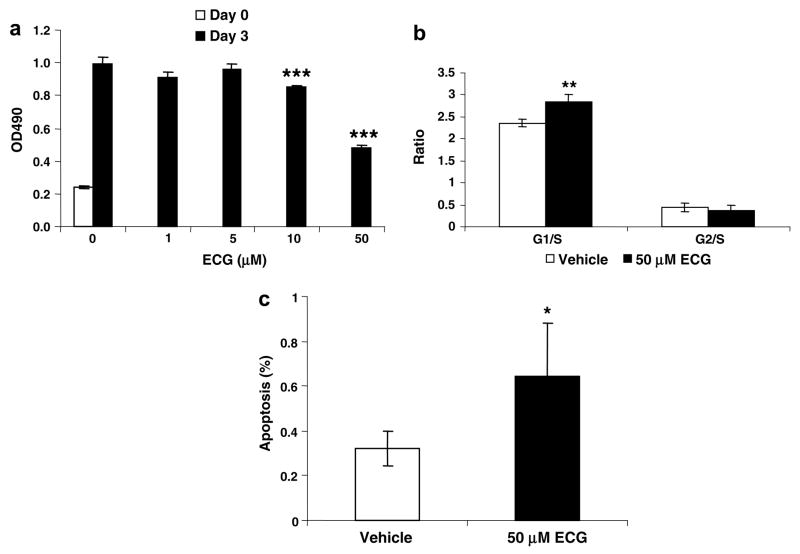
Next, we examined the ability of ECG to inhibit cell growth. SCC7 cells were treated with ECG for 3 days at various concentrations, and the overall cell growth was measured. Our results showed that ECG treatment resulted in the significant inhibition of cell growth at 10 and 50 μM (P < 0.001) (Fig. 4a). We also investigated whether ECG affected the cell cycle and apoptosis. As shown in Fig. 4b and c, a significant induction of G1 cell cycle arrest and apoptosis was observed in the presence of 50 μM ECG. These results suggest that the inhibition of cell proliferation by ECG is due to enhanced apoptosis and G1 cell cycle arrest.
Fig. 4.
Growth retardation and apoptosis of SCC7 cells treated with ECG. (a) Effect of ECG on SCC7 cell growth. SCC7 cells were plated at 1000 cells/well in a 96-well plate and incubated with the vehicle (DMSO) or various concentrations of ECG for 72 h. Cell growth was measured using CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI). Values are expressed as means ± SD of 6 replicates. (b and c) Cell cycle and apoptosis of ECG-treated SCC7 cells. Cells were plated at 1 × 105 cells/well in 6-well plates, incubated with the vehicle or ECG for 72 h, and analyzed for (b) cell cycle analysis and (c) apoptosis as described in Section 2.
4. Discussion
Green tea is the most widely consumed beverage in the world and is especially popular in the Far East (e.g. Korea, Japan, and China). Subsequent epidemiological studies conducted on the effect of green tea intake on populations revealed that in China and Japan, where green tea consumption is high, incidence of breast, colon, and head and neck cancers was lower than those in Western nations.19 Of the major catechins in green tea, EGCG shows a demonstrable chemopreventive activity and has been studied more often than other catechins. In a variety of animal models and organ systems, including the skin, lungs, gastrointestinal tract, breasts, and the head and neck, EGCG inhibited the progression of cancer. Potential mechanisms have been suggested to include anti-oxidative activity, inhibition of enzymes such as COX and lipoxygenase related to tumour promotion, inhibition of activator protein-1, inhibition of angiogenesis, activation of p53 tumour suppressor protein, and inhibition of telomerase activity.
Although ECG is the third major catechin in green tea,20 it shows a strong biological activity in several aspects, including apoptosis, transport, and growth inhibition of cells.6,21,22 However, ECG has not been studied in detail regarding molecular mechanisms. Several studies have shown that EGCG induces apoptosis in cancer cells, but little input has been directed to evaluating the induction of apoptosis by ECG. EGCG is the only known catechin present in plasma in large proportions (77–90%) and in a free form.23 The other catechins are highly conjugated with glucuronic acid and/or sulphate groups. Since it is always issued to use higher concentration of catechins in vitro, the rather poor bioavailability of ECG needs to be considered when we extrapolate results obtained in vitro to situations in vivo. In our previous study, we found that EGCG was not always the most potent chemopreventive agent among the green tea catechins. ECG, compared to EGCG, showed stronger anti-cancer gene expression in human colorectal cancer cells, including thrombospondin and NAG-1.6 Although EGCG marginally decreased cyclin D1 expression in SCC-7 cells (Fig. 2) and increased cell growth arrest in other HNSCC cells,24 ECG showed more potent cyclin D1 suppression in SCC-7 cells. This is supported by a recent report, which shows that both EGCG and ECG are the cell growth suppressors, but ECG seems to better modulate cell growth arrest in several cancer cells.25
Cyclin D1 is a key regulator of the mammalian cell-cycle at the restriction point in late G1, and its overexpression has been reported in several human malignancies, including HNSCC. Several studies have suggested that cyclin D1 overexpression may provide an independent prognostic value for clinical outcomes. For example, cyclin D1 protein expression was assessed in 149 cases of primary laryngeal carcinomas treated surgically, with or without post-operative radiotherapy. 26 Its overexpression was associated with an adverse effect of disease-free survival, independent of tumour stage, and was of borderline significance for overall survival. Moreover, early deregulation of cyclin D1 expression provides an important role in the tumorigenesis process. Izzo and colleagues7 have evaluated cyclin D1 gene copy status and protein expression during the multistep process of head and neck tumorigenesis, using a combination of fluorescence in situ hybridization and immunohistochemistry. Of the 16 amplified cases, nine demonstrated a continuous progression from premalignant to development of invasive carcinoma, and seven (77.7%) of these nine cases showed cyclin D1 gene amplification in premalignant lesions prior to development of invasive carcinoma. Thus, cyclin D1 probably plays a role in tumorigenesis, and its repression by any compounds will be likely beneficial.
Our results indicate that 4 out of the 5 HNSCC cell lines (SCC7, Tu177, Tu212, and 38) expressed detectable cyclin D1. These results are consistent with those of Kalish and colleagues,27 who reported that cyclin D1 is expressed in other HNSCC cell lines such as SCC25, Detroit 562, and FaDu. Of the cell lines expressing cyclin D1, SCC7 expressed the highest levels of cyclin D1. Interestingly, it is notable that ECG, but not EGCG, down-regulated the expression of cyclin D1, although EGCG has been known to be the most abundant catechin compound with anticancer activity in green tea. The reason for this finding remains unclear; however, EGCG may affect anti-tumorigenic activity in a cyclin D1-independent manner. This observation may lead to the conclusion that HNSCCs have marked heterogenesity in their biological behavior, and tumours of the same stage will often respond differently to the same treatment. In fact, it has been reported by several groups that ECG has better anti-cancer effects.6,28
In summary, our results demonstrate that ECG can inhibit β-catenin signaling and cyclin D1 expression, leading to suppression of cell growth and induction of apoptosis in HNSCC. Therefore, ECG has potential use as a chemopreventive agent in HNSCC, and the future study of its clinical use may be worthwhile.
Acknowledgments
We thank Dr. Crawford (State University of New York at Stony Brook) for providing the TOP Flash construct. We also thank Nancy Neilsen for her technical assistance and Misty Bailey for her critical reading. This work was supported by NIH Grant R21 CA109423.
Footnotes
Conflict of interest statement
None declared.
Publisher's Disclaimer: This article was originally published in a journal published by Elsevier, and the attached copy is provided by Elsevier for the author's benefit and for the benefit of the author's institution, for non-commercial research and educational use including without limitation use in instruction at your institution, sending it to specific colleagues that you know, and providing a copy to your institution's administrator. All other uses, reproduction and distribution, including without limitation commercial reprints, selling or licensing copies or access, or posting on open internet sites, your personal or institution's website or repository, are prohibited. For exceptions, permission may be sought for such use through Elsevier's permissions site at: http://www.elsevier.com/locate/permissionusematerial
References
- 1.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Spitz MR. Epidemiology and risk factors for head and neck cancer. Semin Oncol. 1994;21:281–8. [PubMed] [Google Scholar]
- 3.Hsu S, Lewis J, Singh B, et al. Green tea polyphenol targets the mitochondria in tumor cells inducing caspase 3-dependent apoptosis. Anticancer Res. 2003;23:1533–9. [PubMed] [Google Scholar]
- 4.Hsu S, Farrey K, Wataha J, et al. Role of p21WAF1 in green tea polyphenol-induced growth arrest and apoptosis of oral carcinoma cells. Anticancer Res. 2005;25:63–7. [PubMed] [Google Scholar]
- 5.Li N, Han C, Chen J. Tea preparations protect against DMBA-induced oral carcinogenesis in hamsters. Nutr Cancer. 1999;35:73–9. doi: 10.1207/S1532791473-79. [DOI] [PubMed] [Google Scholar]
- 6.Baek SJ, Kim JS, Jackson FR, Eling TE, McEntee MF, Lee SH. Epicatechin gallate-induced expression of NAG-1 is associated with growth inhibition and apoptosis in colon cancer cells. Carcinogenesis. 2004;25:2425–32. doi: 10.1093/carcin/bgh255. [DOI] [PubMed] [Google Scholar]
- 7.Izzo JG, Papadimitrakopoulou VA, et al. Cyclin D1 genotype, response to biochemoprevention, and progression rate to upper aerodigestive tract cancer. J Natl Cancer Inst. 2003;95:198–205. doi: 10.1093/jnci/95.3.198. [DOI] [PubMed] [Google Scholar]
- 8.Zhou P, Jiang W, Weghorst CM, Weinstein IB. Overexpression of cyclin D1 enhances gene amplification. Cancer Res. 1996;56:36–9. [PubMed] [Google Scholar]
- 9.Namazie A, Alavi S, Olopade OI, et al. Cyclin D1 amplification and p16(MTS1/CDK4I) deletion correlate with poor prognosis in head and neck tumors. Laryngoscope. 2002;112:472–81. doi: 10.1097/00005537-200203000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Izzo JG, Papadimitrakopoulou VA, Li XQ, et al. Dysregulated cyclin D1 expression early in head and neck tumorigenesis: in vivo evidence for an association with subsequent gene amplification. Oncogene. 1998;17:2313–22. doi: 10.1038/sj.onc.1202153. [DOI] [PubMed] [Google Scholar]
- 11.Baek SJ, Horowitz JM, Eling TE. Molecular cloning and characterization of human nonsteroidal anti-inflammatory drug-activated gene promoter. Basal transcription is mediated by Sp1 and Sp3. J Biol Chem. 2001;276:33384–92. doi: 10.1074/jbc.M101814200. [DOI] [PubMed] [Google Scholar]
- 12.Tiano HF, Loftin CD, Akunda J, et al. Deficiency of either cyclooxygenase (COX)-1 or COX-2 alters epidermal differentiation and reduces mouse skin tumorigenesis. Cancer Res. 2002;62:3395–401. [PubMed] [Google Scholar]
- 13.Lin DT, Subbaramaiah K, Shah JP, Dannenberg AJ, Boyle JO. Cyclooxygenase-2: a novel molecular target for the prevention and treatment of head and neck cancer. Head Neck. 2002;24:792–9. doi: 10.1002/hed.10108. [DOI] [PubMed] [Google Scholar]
- 14.Papadimitrakopoulou VA, Izzo J, Mao L, et al. Cyclin D1 and p16 alterations in advanced premalignant lesions of the upper aerodigestive tract: role in response to chemoprevention and cancer development. Clin Cancer Res. 2001;7:3127–34. [PubMed] [Google Scholar]
- 15.Kim KS, Yoon JH, Kim JK, et al. Cyclooxygenase inhibitors induce apoptosis in oral cavity cancer cells by increased expression of nonsteroidal anti-inflammatory drug-activated gene. Biochem Biophys Res Commun. 2004;325:1298–303. doi: 10.1016/j.bbrc.2004.10.176. [DOI] [PubMed] [Google Scholar]
- 16.Yin F, Giuliano AE, Van Herle AJ. Signal pathways involved in apigenin inhibition of growth and induction of apoptosis of human anaplastic thyroid cancer cells (ARO) Anticancer Res. 1999;19:4297–303. [PubMed] [Google Scholar]
- 17.Aggarwal S, Takada Y, Singh S, Myers JN, Aggarwal BB. Inhibition of growth and survival of human head and neck squamous cell carcinoma cells by curcumin via modulation of nuclear factor-kappaB signaling. Int J Cancer. 2004;111:679–92. doi: 10.1002/ijc.20333. [DOI] [PubMed] [Google Scholar]
- 18.Uraguchi M, Morikawa M, Shirakawa M, Sanada K, Imai K. Activation of WNT family expression and signaling in squamous cell carcinomas of the oral cavity. J Dent Res. 2004;83:327–32. doi: 10.1177/154405910408300411. [DOI] [PubMed] [Google Scholar]
- 19.Wynder EL, Fujita Y, Harris RE, Hirayama T, Hiyama T. Comparative epidemiology of cancer between the United States and Japan. A second look. Cancer. 1991;67:746–63. doi: 10.1002/1097-0142(19910201)67:3<746::aid-cncr2820670336>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 2002;42:25–54. doi: 10.1146/annurev.pharmtox.42.082101.154309. [DOI] [PubMed] [Google Scholar]
- 21.Okabe S, Suganuma M, Hayashi M, Sueoka E, Komori A, Fujiki H. Mechanisms of growth inhibition of human lung cancer cell line, PC-9, by tea polyphenols. Jpn J Cancer Res. 1997;88:639–43. doi: 10.1111/j.1349-7006.1997.tb00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaidyanathan JB, Walle T. Cellular uptake and efflux of the tea flavonoid (−)epicatechin-3-gallate in the human intestinal cell line Caco-2. J Pharmacol Exp Ther. 2003;307:745–52. doi: 10.1124/jpet.103.054296. [DOI] [PubMed] [Google Scholar]
- 23.Lee MJ, Maliakal P, Chen L, et al. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol Biomarkers Prev. 2002;11:1025–32. [PubMed] [Google Scholar]
- 24.Elattar TM, Virji AS. Effect of tea polyphenols on growth of oral squamous carcinoma cells in vitro. Anticancer Res. 2000;20:3459–65. [PubMed] [Google Scholar]
- 25.Ravindranath MH, Saravanan TS, Monteclaro CC, et al. Epicatechins purified from green tea (Camellia sinensis) differentially suppress growth of gender-dependent human cancer cell lines. Evid Based Complement Alternat Med. 2006;3:237–47. doi: 10.1093/ecam/nel003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pignataro L, Pruneri G, Carboni N, et al. Clinical relevance of cyclin D1 protein overexpression in laryngeal squamous cell carcinoma. J Clin Oncol. 1998;16:3069–77. doi: 10.1200/JCO.1998.16.9.3069. [DOI] [PubMed] [Google Scholar]
- 27.Kalish LH, Kwong RA, Cole IE, Gallagher RM, Sutherland RL, Musgrove EA. Deregulated cyclin D1 expression is associated with decreased efficacy of the selective epidermal growth factor receptor tyrosine kinase inhibitor gefitinib in head and neck squamous cell carcinoma cell lines. Clin Cancer Res. 2004;10:7764–74. doi: 10.1158/1078-0432.CCR-04-0012. [DOI] [PubMed] [Google Scholar]
- 28.Babich H, Krupka ME, Nissim HA, Zuckerbraun HL. Differential in vitro cytotoxicity of (−)-epicatechin gallate (ECG) to cancer and normal cells from the human oral cavity. Toxicol In Vitro. 2005;19:231–42. doi: 10.1016/j.tiv.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Lee SH, Yamaguchi K, Kim JS, et al. Conjugated linoleic acid stimulates an anti-tumorigenic protein NAG-1 in an isomer specific manner. Carcinogenesis. 2006;27:972–81. doi: 10.1093/carcin/bgi268. [DOI] [PubMed] [Google Scholar]