Abstract
BACKGROUND
Alcoholism is characterized by impaired decision-making (i.e., choosing intoxication in the face of mounting negative consequences). This impairment may involve a reduced brain response to the negative consequences of behavior, which supports an inclination to engage in risky behaviors. The feedback error-related negativity (F-ERN) is hypothesized to reflect the valence attached to the negative consequences of behavior. Performance on the Balloon Analogue Risk Task (BART) measures risk-taking propensity. We recorded F-ERNs during the BART and during a BART simulation, where individuals observed the rewards and consequences of (someone else’s) BART performance.
METHODS
EEGs were recorded on 22 actively drinking, treatment-naïve alcoholics during the BART and BART simulation. F-ERNs were measured and their association with psychological and alcohol use measures was examined.
RESULTS
F-ERNs over fronto-central electrode sites were observed to balloon pops in the BART and BART simulation. F-ERNs during the BART were more than twice the amplitude of F-ERNs during the BART simulation. Smaller F-ERN amplitudes from the BART (but not the BART simulation) were associated with a greater family history density of alcohol problems.
CONCLUSION
The results suggest a possible link between the genetic vulnerability toward developing alcoholism and the brain’s response to the negative consequences of behavior.
Keywords: feedback, error-related negativity, alcoholism, decision-making, BART
1. INTRODUCTION
A hallmark of substance abuse is impaired decision-making that favors short-term rewards (e.g., intoxication) in the face of mounting negative long-term consequences in one’s personal, emotional, professional and social life (Rahman et al., 2001). These decision-making impairments are associated with a number of factors, including impulsivity, a reduced emotional valence attached to the negative consequences of behavior, impaired contingency learning, and an excessive dominance of the effects on behavior of immediate vs. delayed rewards (Clark and Robbins, 2002). Ultimately, impaired decision-making can lead to abuse of other substances (or other risky behaviors) and can hamper the ability to cease substance abuse and resist relapse. Thus, understanding the mechanisms behind decision-making impairments is an important aspect to promoting effective treatment strategies and positive recovery outcomes.
Pairing decision-making laboratory experiments with EEG recordings enables simultaneous investigation of the behavioral and electrophysiological correlates of impaired decision-making. In particular, event-related potentials generated by the human error processing system could elucidate how neural responses to negative events relate to poor decision-making in alcoholics. The primary electrophysiological marker of the error processing system is the error-related negativity (ERN) – a negative potential that occurs in response to errors, detectable over fronto-central regions of the scalp. In tasks that involve prior knowledge of correct stimulus-response mappings (e.g. a Stroop or flanker task), the participant does not necessarily require feedback to know that he has made a mistake. In this case, an ERN occurs about 50-150ms after the erroneous response and is called a response-locked error-related negativity (R-ERN) [for example: (Dikman and Allen, 2000; Falkenstein et al., 2001; Hajcak and Simons, 2002)]. The R-ERN reflects low-level error recognition, as it is present even when individuals are not consciously aware of their errors (Nieuwenhuis et al., 2001). Another type of ERN is observed during tasks with unexpected outcomes (e.g. pseudorandom gambling games) that require participants to use positive and negative feedback to evaluate their response as correct or incorrect. These tasks elicit an ERN that occurs about 200-300ms after negative feedback, now commonly referred to as the feedback error-related negativity (F-ERN) (Hajcak et al., 2006; Holroyd et al., 2004a; Nieuwenhuis et al., 2002; Nieuwenhuis et al., 2004; van Meel et al., 2005). F-ERN amplitude appears to be sensitive to changes along an abstract good-bad dimension, which depends on the most salient task objective (Nieuwenhuis et al., 2004). Consistent with a general good-bad evaluation, Hajcak and others demonstrated that F-ERNs reflected reward valence rather than reward magnitude (Hajcak et al., 2006). Recent studies have shown that the F-ERN is also present during error observation, suggesting that error monitoring is active even when errors are caused by someone else (van Schie et al., 2004; Yu and Zhou, 2006).
According to the reinforcement learning theory and error-processing model proposed by Holroyd and Coles, the R-ERN and F-ERN reflect the disinhibition of apical dendrites in the anterior cingulate cortex (ACC), which is caused by phasic decreases in mesencephalic dopaminergic activity from the basal ganglia to the ACC. This negative reinforcement signal occurs when the system realizes that ongoing events are worse than expected. Acting as a motor control filter, the ACC uses the reinforcement signals to determine the most suitable behavior for the task at hand (Holroyd and Coles, 2002). An alternative explanation is that the R-ERN represents the activation of a conflict-monitoring system after an error is committed. When conflict is present, centers for cognitive control are notified that attention needs to be redirected in order to avoid negative outcomes. The negative feedback that elicits the F-ERN also activates the conflict-monitoring system, and in turn, calls for behavioral changes (Botvinick et al., 2001). In either case, evidence suggests that normal F-ERN responses are crucial in the processing of negative events and consequently, central to effective decision making.
Evidence for the impact of alcoholism on the ERN is currently limited. Two studies found acute reductions in R-ERN amplitude with moderate doses of alcohol (Easdon et al., 2005; Ridderinkhof et al., 2002). Impulsivity, which is strongly related to alcoholism, was shown to be associated with weakened R-ERNs in a flanker task (Ruchsow et al., 2005). When rewards and punishments are added to the flanker task, impulsive individuals have reduced R-ERNs only when errors result in punishment, which indicates hyposensitivity specific to punishment rather than a generalized desensitization to reward and punishment (Potts et al., 2006). R-ERN deficits were also reported in patients with cocaine dependence (Franken et al., 2006). These studies open the possibility that compromised error processing and/or reduced sensitivity to punishment is related to substance abuse; however, we have yet to find a study that specifically examines the relationship between the ERN and chronic alcoholism.
In addition to understanding the F-ERN, observing risk-taking behavior on the Balloon Analogue Risk Task (BART) can provide insights into the underlying mechanisms of poor decision-making (Lejuez et al., 2002). In the experiment, an individual is presented with an empty balloon that can be inflated incrementally with money. After each balloon pump, the individual can choose to stop and collect the money inside the balloon or he can choose to add more money by pumping again. If he causes the balloon to pop, the money inside is lost and experiment continues with another empty balloon. The probability of the balloon bursting increases with each pump. Thus, each pump is a risky decision that can result in a gain in temporary earnings or a loss of all balloon earnings up to that point. This approach models real world risk-taking and provides a novel measure of an individual’s tendency to engage in risky behaviors (i.e. make risky decisions). BART performance has been associated with self-report measures of safety and health risk behaviors (e.g. drug use, gambling, stealing, unprotected sexual intercourse), which are real-world manifestations of poor decision making (Lejuez et al., 2002). The same study also found BART performance to be associated with deficiencies in behavioral constraint in adults (Lejuez et al., 2002). In a sample of young adults, antisocial behavior measures were positively correlated with risk-taking on the BART (Hunt et al., 2005). In a study differentiating smokers from nonsmokers, the BART was more effective than the Iowa Gambling Task (Lejuez et al., 2003a). Performance on the BART is related to real-world risk-taking in adolescent samples as well (Aklin et al., 2005; Lejuez et al., 2003b). Increased risk-taking on the BART was observed in adolescents with serious conduct and substance problems, including alcohol dependence and abuse (Crowley et al., 2006). Lejuez and colleagues have now developed a youth version of the BART to further investigate risk-taking propensity in adolescents (Lejuez et al., 2007). In the initial evaluation, BART performance was associated with measures of impulsivity but no associations were found in follow up studies (Aklin et al., 2005; Hunt et al., 2005; Lejuez et al., 2003a; Lejuez et al., 2003b). Lejuez and others suggest that the lack of association indicates that the BART measures a form of behavioral disinhibition that is distinct from self-report measures of impulsivity. Therefore, the BART is said to tap into a unique aspect of risk-taking, unaccounted for by self-report measures (Lejuez et al., 2003a; Lejuez et al., 2003b). This property offers a different look into the proclivity for risk-taking in samples with alcohol problems that is not captured by written questionnaires. We are unaware of any studies on electrophysiological activity during administration of the BART. F-ERN recording could help elucidate the brain mechanisms involved in processing the negative consequences of behavior during the BART. Furthermore, we have not encountered any implementations of a BART simulation that could replicate the ERN findings during error observation (van Schie et al., 2004; Yu and Zhou, 2006).
In this study, we examined decision-making on the BART and F-ERNs during the BART and BART simulation in actively drinking, treatment-naïve alcoholics (TxNA). Evidence for the relationship between substance abuse and poor decision-making suggests that our sample would make risky, disadvantageous decisions during the BART. We hypothesized that poor decision-making would be driven by hyposensitivity to negative feedback. The degree of hyposensitivity could be measured by the F-ERN, with weaker F-ERN responses indicating decreased sensitivity and stronger F-ERN responses indicating increased sensitivity. Consequently, we expected risk-taking during the BART to be negatively related to the F-ERN. Furthermore, we also expected to find that individuals with severe alcoholism (as measured by alcohol consumption, family history and personality profiles) would exhibit an even lesser sensitivity to negative feedback than those with less severe alcoholism.
2. METHODS
2.1 Subjects
The participants consisted of 22 TxNA individuals (9 females, 13 males) between the ages of 20 and 45 (mean = 29.35, SD = 7.96). All participants were recruited from respondents to flyer postings, mailings, newspaper advertisements, internet postings and referrals from other research participants. TxNA participants were accepted into the study if they met DSM-IV (American Psychiatric Association, 1994) criteria for alcohol dependence, were currently drinking, and never sought treatment for alcoholism. Participants were excluded if they met any of the following criteria: (1) significant history of head trauma or cranial surgery; (2) clinical or laboratory evidence of active hepatic disease; (3) Wernicke-Korsakoff syndrome; or (4) history of drug abuse or dependence (other then caffeine or nicotine). All participants provided written informed consent and were paid for their time and reimbursed for their travel in compliance with the guidelines set forth by our local institutional review board. None of the participants received any medication known to affect the EEG. All participants were instructed not to drink on the day of any assessments, and all took a Breathalyzer test before each assessment. If they did not have a 0.000 breath alcohol level, the assessment was rescheduled (this did not occur on the EEG/ERP assessment day for any of the subjects presented here).
2.2 Alcohol Use and Clinical Assessments
Participants underwent a series of alcohol use and clinical assessments during a single 1.5 to 2-hour visit. Alcohol use history was assessed using the timeline follow-back methodology of the Lifetime Drinking History questionnaire (Skinner and Allen, 1982; Sobell and Sobell, 1990). The questionnaire quantified the total alcohol burden on an individual in units of standard drinks. The density of a family history of alcohol problems was assessed using the family history drinking questionnaire (Mann et al., 1985). The family history density (FHD) measure was the proportion of first-degree relatives who had alcohol problems. FHD measures were collected for 21 out of the 22 subjects. One of the participants was adopted and could not report on alcohol problems in his biological relatives.
Psychiatric diagnoses and symptoms were assessed using the computerized diagnostic interview schedule (cDIS) (Robins et al., 1998). Symptom counts provide a continuous measure of psychiatric comorbidity, even when below the threshold of diagnosis. We have demonstrated the efficacy of these measures with long-term abstinent alcoholics (Fein et al., 2007). The total number of symptoms for antisocial personality disorder was recorded for comparison with the F-ERN.
2.3 Balloon Analogue Risk Task (BART) Implementation
The BART was implemented in our laboratory on E-prime 1.1 (Psychology Software Tools, Inc., Pittsburgh, PA). The general structure of the task was as follows: the participant had the opportunity to earn money by incrementally inflating a balloon, while the probability of the balloon bursting increased with each successive pump. There were two possible outcomes for each balloon pump. The balloon increased in size and value, or the balloon popped and all money inside was lost. The visual stimuli resulting from these outcomes were defined as positive and negative feedback respectively (Figure 1).
Figure 1.
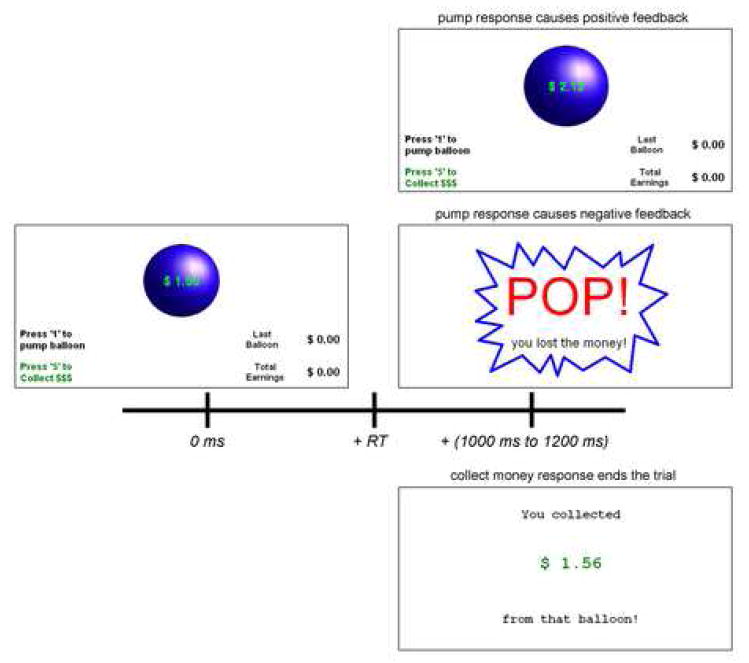
Example stimulus-response-feedback timeline for the BART. Upon balloon presentation, the participant can choose to end the trial and collect money from the balloon (bottom right) or he can choose to pump the balloon again. If he chooses to pump the balloon, negative feedback may appear in the form of a balloon burst (middle right) or positive feedback may appear in the form of a larger balloon with more money (top right). All feedback stimuli are presented after a random delay (1 to 1.2 ms) after a response is made.
Modifications to the task were implemented for ERP analysis. The task consisted of 60 trials (balloons) to increase the number of negative feedback epochs available for averaging. In the efforts to keep the experiment within a reasonable time length, the maximum breaking point for each balloon was shortened to 20 pumps. Experience from working with individuals in this population has shown that experiments lasting longer than 25 minutes or so often result in participant restlessness or tiredness, which in turn causes movement artifacts or alpha activity unrelated to the task at hand. The probability of the balloon bursting after the second pump was 1/19, after the third pump was 1/18, and so on, until the twentieth pump, where a balloon burst was certain. Pops resulting from the first pump of an empty balloon were disabled (preventing the presentation of negative feedback after this non-risky decision). Additionally, to increase the allure of rewards from risky behavior, rewards were incremented by two cents at each pump. The first pump added 50 cents to the balloon, the second added 52, the third 54 and so on. The balloon stimuli were blue spheres with radii that increased proportionally to the amount of money added to the balloon. The initial empty balloon subtended a visual angle of 4.5 degrees, which increased by 0.3 to 0.7 degrees (depending on the amount of money added to the balloon). A random delay of 1-1.2 seconds between the subject’s response and feedback (the balloon popping, or money in the balloon increasing) was added to separate those events. Noises were removed from the pop stimuli in our implementation as it could have induced unwanted startle responses and muscle artifact. Instead, the pop stimulus was changed to a silent balloon burst graphic with text indicating the loss of money. The balloon burst stimulus subtended a vertical visual angle of 11.5 degrees and a horizontal visual angle of 16.5 degrees. It is important to note that the modeling of real-world risk-taking from the original BART remained the same in this implementation by following two principles: a) on each successive balloon pump, the amount that will be lost if the balloon pops increases and b) the relative gain from each pump decreases after the first two pumps (as initially described by (Lejuez et al., 2002)).
Before administering the BART, subjects were given instructions and shown a non-interactive, 17-minute simulation of the task to test the hypothesis that the F-ERN is elicited by error observation. Participants knew that the objective of the task was to earn money so they understood the balloon burst as a negative event. However, they were also clearly instructed that although the simulation was an accurate depiction of the task, it did not affect their performance or possible earnings in any way. After the simulation, subjects were offered a short break and then the actual task was administered. A monetary bonus in the amount of 2.5% of the total earnings in the game was given to each participant at the end of the testing session (approximately $2 to $6, mean = $3.91, in addition to payments for time and travel). Participants were informed of the bonus (but were not told the exact percentage) ahead of time to encourage realistic decision-making that would have an effect on real-world rewards. Following the convention set by Lejuez and colleagues, the primary behavioral measure for the BART was mean adjusted pumps: the average number of pumps on balloons where money was collected before an explosion. This is preferred to other possible measures such as unadjusted average pumps, number of balloon bursts and total earnings, as it is the only measure that is not constrained by the pseudo-random popping threshold of the balloon (Lejuez et al., 2007).
2.4 EEG Acquisition and Analysis
EEG data were recorded during the BART and the BART simulation. Data were acquired with a SynAmps2 amplifier, using a 64-channel Quik-Cap and Scan 4.3 Acquisition Software (Compumedics Neuroscan, Inc., El Paso, TX). The SynAmps2 amplifier had a fixed range of ±333μV sampled with a 24-bit A/D converter where the least significant bit was 0.019μV. Data were recorded for approximately 35-40 minutes for both the BART and the BART simulation combined (with a sampling rate of 250Hz). The amplifier filtered the data online with a high-pass cut off at 0.3Hz and a low-pass cut off at 70Hz. The reference was the right ear for all recordings and ground was 8 cm above the nasion. Electrode impedances were maintained below 10kΩ. Vertical eye movements were recorded by electrodes above and below the left eye for later reduction of ocular artifact.
EEG recordings were processed offline using the Edit program in Scan 4.3 (Compumedics Neuroscan, Inc., El Paso, TX). Artifacts from eye movements were removed using an ocular artifact reduction algorithm implemented in Edit (Semlitsch et al., 1986). A low-pass filter was applied to the data at 30Hz using a zero phase lag filter at 48dB/octave. Feedback-locked epochs were created from positive (money in balloon increasing) and negative (balloon popping and all money in balloon lost) feedback and were baseline corrected using the 100ms pre-stimulus interval. Any epochs with out of range voltages (±100μV) were rejected as artifacts and excluded from further processing. Epochs locked to positive and negative feedback were averaged separately, producing one average waveform per feedback condition per subject. Negative feedback-locked waveforms were based on no less than 19 epochs per subject (mean = 27 epochs). Difference waves were then created by subtracting the positive feedback-locked waveform from the negative feedback-locked waveform. Minimum voltages were identified 200-300ms after the onset of feedback in the difference waves. Each difference waveform was visually inspected to ensure that the automated peak detection actually identified a negative potential at approximately 250ms post-stimulus. The maximum amplitude of the negative waveforms at fronto-central electrodes Fz, FCz and Cz were used for statistical analysis. P300 amplitude measures were also statistically analyzed and were defined as the maximum amplitude 300-600ms post-stimulus from difference waveforms at electrodes Fz, FCz and Cz.
2.5 Statistical Analysis
Statistical analyses were performed using SPSS 14.0 (SPSS Inc., 2006). Initially, F-ERN presence was tested using repeated measures ANOVA on amplitude measures from waveforms locked to positive and negative feedback with feedback condition as the within-subjects factor. However, this method was problematic because there was no semblance of any F-ERN-like component following positive feedback. Instead, positive feedback induced a positive potential during the F-ERN latency window (see Figure 2). Peak detection on positive feedback waveforms consequently understated the differences between the two conditions by choosing voltages at the upper and lower bounds of the latency window that were not representative of the actual ERP. In contrast, difference waves maintained the temporal accuracy of the potentials while highlighting the differences between the two conditions. Thus, a one-sample t-test on amplitude measures from difference waves (test value = 0) was the most appropriate method for detecting the F-ERN. A repeated measures ANOVA on difference wave amplitude measures was used to examine differences between the F-ERN from the BART and BART simulation (task was the within-subjects factor).
Figure 2.
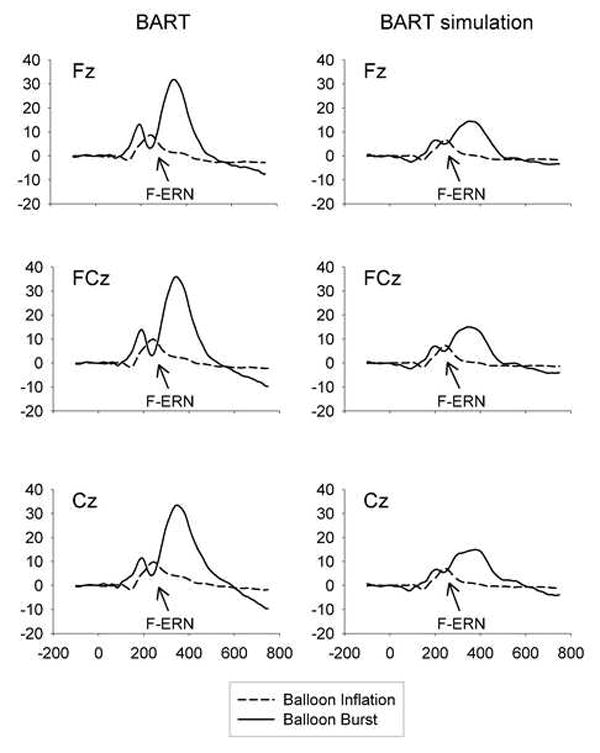
Average waveforms after positive and negative feedback by task and channel. The feedback ERN is visible approximately 240ms after the onset of negative feedback (balloon burst stimuli). There is no such negative deflection after the onset of positive feedback (balloon inflation stimuli). The feedback ERN and surrounding positive potentials are more pronounced in the BART than in the BART simulation.
Spearman correlations were used to find associations between the F-ERN and the following variables: performance on the BART (average adjusted pumps), FHD, total alcohol burden (drinks taken over the individual’s lifetime, with age partialled out) and symptoms of antisocial personality disorder. For all correlation analyses, F-ERN amplitude was taken from the difference waveforms (defined as the maximum amplitude of the negative waveform 200-300ms post-stimulus). Using Bonferroni correction for multiple comparisons, the significance threshold was 0.0125.
3. RESULTS
The F-ERN was present for both the BART and the BART simulation (Table 1), and was strongest at FCz. F-ERN amplitudes were more than twice as large during the BART compared to the BART simulation. Repeated-measures ANOVA revealed that the within-subject task effect accounted for 50.4% of the variance in F-ERN amplitude (F(1,19) = 6.435, p = 0.003).
Table 1.
One Sample T-Test Results (test value = 0)
| Channel | t | p | Mean Difference |
|---|---|---|---|
| BART | |||
| Fz | 5.513 | 0.000 | 9.66 |
| FCz | 5.744 | 0.000 | 10.73 |
| Cz | 4.561 | 0.000 | 8.01 |
| BART simulation | |||
| Fz | 3.969 | 0.001 | 3.46 |
| FCz | 3.909 | 0.001 | 3.99 |
| Cz | 2.920 | 0.008 | 2.62 |
These results are illustrated by average waveforms following positive and negative feedback by channel and task (Figure 2). Waveforms following balloon bursts at the three midline fronto-central electrode sites are characterized by an early positive potential about 180ms post-stimulus followed by the F-ERN, which reaches maximum amplitude roughly 240ms after presentation of negative feedback. The F-ERN was followed by a very large P300 peaking about 350ms after negative feedback. Balloon burst waveforms for the BART simulation have the same general morphology, with less pronounced positive and negative potentials. Topographic maps of the difference waves (based on the 10-20 international system) suggest similar morphology over much of the scalp, indicating that ERP differences between the two tasks are primarily amplitude and not topography differences (Figure 3). Spearman correlations also indicated that there is inter-individual consistency between the strength of the F-ERN in the BART and the BART simulation (r = 0.564, p = 0.006, Table 2).
Figure 3.
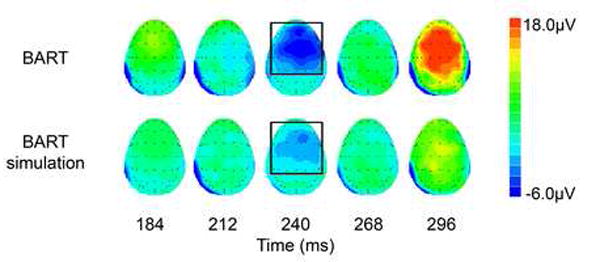
Scalp topography of difference waves (negative feedback minus positive feedback) by task at 28ms snapshots. The feedback ERN (denoted by bounding boxes) is visible 240ms after the onset of negative feedback. Spatial distribution of the feedback ERN is similar in both tasks, but stronger and more widespread during the BART.
Table 2.
Spearman Correlations between ERPs
| BART F-ERNa | BART P300a | |
|---|---|---|
| BART simulation F-ERNa | 0.564 ** | 0.174 |
| BART P300a | 0.107 | 1.000 |
| Visual Oddball Task P3a | -0.060 | 0.383 p=0.078 |
| Visual Oddball Task P3b | -0.043 | 0.497 * |
Amplitude from difference waveforms at maximal electrode FCz
p ≤ 0.05
p ≤ 0.01
p ≤ 0.001
The presence of a large P300 after negative feedback is to be expected because the balloon explosion is an extremely salient (and often unexpected) task event. However, the amplitude of the F-ERN was not correlated with BART P300 amplitude, providing evidence that the F-ERN is not part of the BART P300 response (Table 2). We also compared F-ERN amplitudes to P3a and P3b measures from a visual oddball experiment (in the same 22 individuals). We obtained similar non-significant results. In contrast, the BART P300 was significantly correlated with the visual P3b (and almost significantly correlated with the P3a). Moreover, the F-ERN findings discussed below are not extended to the P300 in the BART, which further supports the dissociation of the F-ERN and P300 elicited during the BART.
The F-ERN during the BART was significantly correlated with family history density (FHD) of alcohol problems (Table 3, Figure 4). Individuals with greater FHDs had weaker F-ERNs (r = -0.567, p = 0.007).
Table 3.
Spearman Correlations with Bonferroni Correction
| BART F-ERNa | BART simulation F-ERNa | |
|---|---|---|
| BART (avg. adjusted pumps) | 0.225 | 0.142 |
| Total Alcohol Burden (standard drinks) | -0.263 | -0.022 |
| Family History Density | -0.567* | -0.247 |
| Antisocial Personality Disorder (Sx) | -0.027 | -0.278 |
Amplitude from difference waveforms at maximal electrode FCz
p ≤ 0.0125
Figure 4.
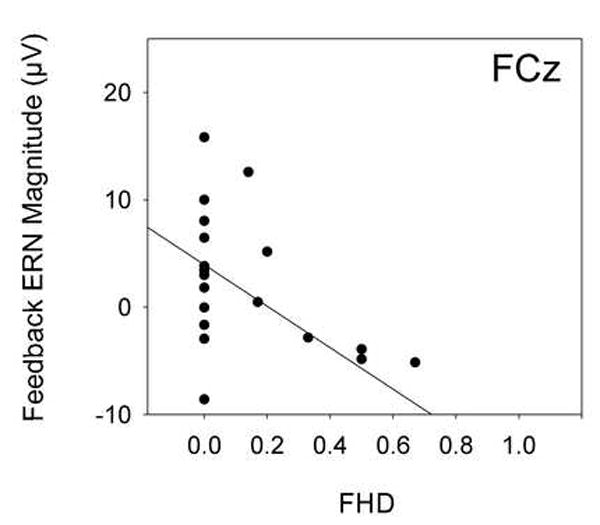
Family history density (FHD) of alcohol problems versus feedback ERN magnitude at electrode FCz. Reduced amplitude was significantly associated with greater FHDs of alcohol problems.
The F-ERN was not significantly correlated to average adjusted pumps from the BART. To test the hypothesis that larger losses cause larger F-ERNs, F-ERNs resulting from pops after less than 8 pumps were compared to F-ERNs resulting from pops after 8 pumps or more (8 was the median average adjusted pump across all subjects). Repeated measures ANOVA with F-ERN group (early versus late) as the within-subjects factor indicated that differences between early pop F-ERNs and late pop F-ERNs were not significant (effect size = 0.6%, p = 0.733).
The F-ERN was also unrelated to total alcohol burden (in standard drinks with age partialled out) and symptoms for antisocial personality disorder.
For the BART simulation, the F-ERN was not significantly correlated with FHD. The observation F-ERN was also unrelated to BART performance, total alcohol burden and symptoms for antisocial personality disorder.
4. DISCUSSION
We have clearly demonstrated that the F-ERN is present in response to popped balloons during the BART. In agreement with previous studies, the F-ERN was present at fronto-central electrodes after feedback indicated an error. Also, in agreement with previous studies, the F-ERN was detected when individuals observed negative feedback that was not caused by their own actions. If the ERN reflects the reinforcement signals utilized in reinforcement learning (Holroyd and Coles, 2002), then this finding is a reasonable extension since humans learn from the actions of others in addition to their own. The significant differences between the observation F-ERN and execution F-ERN may demonstrate that less importance is given to negative consequences that are not a direct result of one’s own actions, consistent with the work by Yeung and colleagues (Yeung et al., 2005). In other words, this suggests that learning by doing is more salient than learning by observing.
Interestingly, F-ERN amplitude was not associated with performance on the BART and it was not modulated by the amount of money lost by balloon bursts. Although findings have supported the idea that F-ERN amplitude is predictive of ongoing shifts in decision-making (Yasuda et al., 2004; Yeung and Sanfey, 2004), a relationship between overall risk-taking and amplitude have been recently observed only for R-ERNs (Hewig et al., 2006). It is possible that F-ERN amplitudes positively correlate with cautious behavior (as Hewig’s R-ERN results may suggest) but that this is not observed in alcoholic samples. Another important possibility is that the null associations with BART performance are due to uninteresting effects from balloons with lower popping thresholds. Originally, the BART was developed with balloons with a 128-pump capacity. Due to time constraints in our study, we shortened this capacity to 20 pumps, which may have had excessive limitations on the between-subject variance in mean adjusted pumps. However, this does not diminish the utility of the central finding that F-ERNs are detectable during the BART. This limitation essentially shortens the time length of each trial. Increasing that length should not have any impact on the observed F-ERNs.
The most intriguing finding is the negative relationship between FHD and F-ERN amplitudes, especially in the absence of any relationship between the F-ERN and alcohol use. This raises the possibility that reduced F-ERN amplitudes are a characteristic of the inherited vulnerability to alcoholism. More specifically, it suggests a reduced sensitivity to negative feedback in individuals with family history of alcohol abuse. Alcoholism is believed to be one manifestation of a heritable, externalizing “liability” that also predisposes individuals to antisocial behaviors and other substance abuse disorders (Krueger et al., 2002). In light of that theory, the degree to which the F-ERN is related to externalizing disorders is important. In the current study, F-ERN amplitude was not associated with symptoms of antisocial personality disorder. However, reduced R-ERNs have been reported in low-socialized individuals compared to high-socialized individuals (Dikman and Allen, 2000).
If the associations between FHD and F-ERN hold in future studies, it would indicate that areas of the brain involved with error processing are involved in the inherited vulnerability toward developing alcoholism. Conflict monitoring and reinforcement learning theories implicate the anterior cingulate cortex (ACC) as the neural generator of the ERN. Source localization techniques and fMRI studies support this notion (Holroyd et al., 2004b; Ruchsow et al., 2002; Ullsperger and von Cramon, 2001). Although the ERN is most strongly detected over the ACC (i.e. fronto-central scalp sites), lesion studies have shown that intact frontostriatal circuits are necessary for normal R-ERN responses. Patients with gray matter lesions in the lateral prefrontal cortex have greatly reduced R-ERNs, as well as patients with basal ganglia lesions (Ullsperger and von Cramon, 2006). Frontal white matter lesions between the lateral prefrontal cortex and the medial prefrontal cortex (assumed to include the neural pathway between the two regions) also diminish the R-ERN (Hogan et al., 2006). Given the overlap between these areas and the areas involved in decision-making (i.e. prefrontal cortex), impairments in decision-making and electrophysiological error responding may be caused by common anatomical deficiencies involved in alcoholism. From the other perspective, our recent work in long-term abstinent alcoholics suggests that reduced amygdala volumes are associated with decision-making impairments that persist into long-term abstinence (Fein et al., 2006). Bechara has shown that individuals with amygdala lesions show impaired decision-making, hypothesizing that such individuals fail to attach appropriate negative emotional valence to the negative consequences of behavior (Bechara et al., 1999). That is very similar to our hypotheses regarding reduced F-ERN amplitude. Based on this similarity, we would suggest that individuals with reduced amygdala volumes would have reduced F-ERNs to balloon pops during the BART.
Given the recent studies on the acute effect whereby alcohol reduces R-ERN amplitude (Easdon et al., 2005; Ridderinkhof et al., 2002), the non-association between alcohol use measures and F-ERN amplitude was surprising. As noted in the Methods, all subjects had a 0.00 breath alcohol level at the beginning of the EEG/ERP session. The alcohol effect on the ERN may only be an acute effect, and may not persist after alcohol is out of one’s system. Moreover, all of our subjects were alcohol dependent, and there may be a ceiling effect of alcohol intake. Without a light/non-drinking control group (the major limitation of the current study, along with the relatively small sample size), our data cannot speak to this issue. Future studies comparing F-ERNs from samples of actively drinking alcohol dependent individuals, abstinent alcohol dependent individuals, and light/non-drinking controls can help resolve these issues.
In summary, our findings demonstrate that the negative events occurring in the BART activate the error processing system responsible for the F-ERN. Our preliminary findings are that F-ERN amplitude is modulated by family history density of alcohol problems, a finding that could have important implications for the study of decision-making in alcoholics. The F-ERN is also elicited by negative feedback stimuli in the BART simulation, although amplitude from this component is not significantly related to family history density of alcohol problems. We conclude that the relationship between the inherited vulnerability toward developing alcoholism and electrophysiological signatures of error detection deserve further exploration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aklin WM, Lejuez CW, Zvolensky MJ, Kahler CW, Gwadz M. Evaluation of behavioral measures of risk taking propensity with inner city adolescents. Behav Res Ther. 2005;43(2):215–28. doi: 10.1016/j.brat.2003.12.007. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: 4th edition (DSM-IV) American Psychiatric Association Press; Washington, DC: 1994. [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19(13):5473–81. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108(3):624–52. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Clark L, Robbins T. Decision-making deficits in drug addiction. Trends Cogn Sci. 2002;6(9):361. doi: 10.1016/s1364-6613(02)01960-5. [DOI] [PubMed] [Google Scholar]
- Crowley TJ, Raymond KM, Mikulich-Gilbertson SK, Thompson LL, Lejuez CW. A risk-taking “set” in a novel task among adolescents with serious conduct and substance problems. J Am Acad Child Adolesc Psychiatry. 2006;45(2):175–83. doi: 10.1097/01.chi.0000188893.60551.31. [DOI] [PubMed] [Google Scholar]
- Dikman ZV, Allen JJ. Error monitoring during reward and avoidance learning in high- and low-socialized individuals. Psychophysiology. 2000;37(1):43–54. [PubMed] [Google Scholar]
- Easdon C, Izenberg A, Armilio ML, Yu H, Alain C. Alcohol consumption impairs stimulus- and error-related processing during a Go/No-Go Task. Brain Res Cogn Brain Res. 2005;25(3):873–83. doi: 10.1016/j.cogbrainres.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. Changes of error-related ERPs with age. Exp Brain Res. 2001;138(2):258–62. doi: 10.1007/s002210100712. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Finn P, Scheiner DL. Sub-diagnostic psychiatric comorbidity in alcoholics. Drug Alcohol Depend. 2007;87(23):139–45. doi: 10.1016/j.drugalcdep.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Landman B, Tran H, McGillivray S, Finn P, Barakos J, Moon K. Brain atrophy in long-term abstinent alcoholics who demonstrate impairment on a simulated gambling task. Neuroimage. 2006;32(3):1465–71. doi: 10.1016/j.neuroimage.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken IH, van Strien JW, Franzek EJ, van de Wetering BJ. Error-processing deficits in patients with cocaine dependence. Biol Psychol. 2006 doi: 10.1016/j.biopsycho.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF. The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biol Psychol. 2006;71(2):148–54. doi: 10.1016/j.biopsycho.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Simons RF. Error-related brain activity in obsessive-compulsive undergraduates. Psychiatry Res. 2002;110(1):63–72. doi: 10.1016/s0165-1781(02)00034-3. [DOI] [PubMed] [Google Scholar]
- Hewig J, Trippe R, Hecht H, Coles MG, Holroyd CB, Miltner WH. Decision-Making in Blackjack: An Electrophysiological Analysis. Cereb Cortex. 2006 doi: 10.1093/cercor/bhk040. [DOI] [PubMed] [Google Scholar]
- Hogan AM, Vargha-Khadem F, Saunders DE, Kirkham FJ, Baldeweg T. Impact of frontal white matter lesions on performance monitoring: ERP evidence for cortical disconnection. Brain. 2006;129(Pt 8):2177–88. doi: 10.1093/brain/awl160. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109(4):679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Larsen JT, Cohen JD. Context dependence of the event-related brain potential associated with reward and punishment. Psychophysiology. 2004a;41(2):245–53. doi: 10.1111/j.1469-8986.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Nieuwenhuis S, Yeung N, Nystrom L, Mars RB, Coles MG, Cohen JD. Dorsal anterior cingulate cortex shows fMRI response to internal and external error signals. Nat Neurosci. 2004b;7(5):497–8. doi: 10.1038/nn1238. [DOI] [PubMed] [Google Scholar]
- Hunt MK, Hopko DR, Bare R, Lejuez CW, Robinson EV. Construct validity of the Balloon Analog Risk Task (BART): associations with psychopathy and impulsivity. Assessment. 2005;12(4):416–28. doi: 10.1177/1073191105278740. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. J Abnorm Psychol. 2002;111(3):411–24. [PubMed] [Google Scholar]
- Lejuez CW, Aklin W, Daughters S, Zvolensky M, Kahler C, Gwadz M. Reliability and Validity of the Youth Version of the Balloon Analogue Risk Task (BART-Y) in the Assessment of Risk-Taking Behavior Among Inner-City Adolescents. J Clin Child Adolesc Psychol. 2007;36(1):106–11. doi: 10.1080/15374410709336573. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Jones HA, Richards JB, Strong DR, Kahler CW, Read JP. The Balloon Analogue Risk Task (BART) differentiates smokers and nonsmokers. Exp Clin Psychopharmacol. 2003a;11(1):26–33. doi: 10.1037//1064-1297.11.1.26. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Zvolensky MJ, Pedulla CM. Evaluation of the Balloon Analogue Risk Task (BART) as a predictor of adolescent real-world risk-taking behaviours. J Adolesc. 2003b;26(4):475–9. doi: 10.1016/s0140-1971(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Strong DR, Brown RA. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) J Exp Psychol Appl. 2002;8(2):75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Mann RE, Sobell LC, Sobell MB, Pavan D. Reliability of a family tree questionnaire for assessing family history of alcohol problems. Drug Alcohol Depend. 1985;15(12):61–7. doi: 10.1016/0376-8716(85)90030-4. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, Kok A. Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology. 2001;38(5):752–60. [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Talsma D, Coles MG, Holroyd CB, Kok A, van der Molen MW. A computational account of altered error processing in older age: dopamine and the error-related negativity. Cogn Affect Behav Neurosci. 2002;2(1):19–36. doi: 10.3758/cabn.2.1.19. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, Holroyd CB, Schurger A, Cohen JD. Sensitivity of electrophysiological activity from medial frontal cortex to utilitarian and performance feedback. Cereb Cortex. 2004;14(7):741–7. doi: 10.1093/cercor/bhh034. [DOI] [PubMed] [Google Scholar]
- Potts GF, George MR, Martin LE, Barratt ES. Reduced punishment sensitivity in neural systems of behavior monitoring in impulsive individuals. Neurosci Lett. 2006;397(12):130–4. doi: 10.1016/j.neulet.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Rahman S, B JS, R NC, Rogers R, Robbins T. Decision making and neuropsychiatry. Trends Cogn Sci. 2001;5(6):271–277. doi: 10.1016/s1364-6613(00)01650-8. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, de Vlugt Y, Bramlage A, Spaan M, Elton M, Snel J, Band GP. Alcohol consumption impairs detection of performance errors in mediofrontal cortex. Science. 2002;298(5601):2209–11. doi: 10.1126/science.1076929. [DOI] [PubMed] [Google Scholar]
- Robins LN, Cottler L, Buckholz K, Compton W. The Diagnostic Interview Schedule for DSM-IV. Washington University School of Medicine; St Louis, MO: 1998. [Google Scholar]
- Ruchsow M, Grothe J, Spitzer M, Kiefer M. Human anterior cingulate cortex is activated by negative feedback: evidence from event-related potentials in a guessing task. Neurosci Lett. 2002;325(3):203–6. doi: 10.1016/s0304-3940(02)00288-4. [DOI] [PubMed] [Google Scholar]
- Ruchsow M, Spitzer M, Gron G, Grothe J, Kiefer M. Error processing and impulsiveness in normals: evidence from event-related potentials. Brain Res Cogn Brain Res. 2005;24(2):317–25. doi: 10.1016/j.cogbrainres.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23(6):695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol. 1982;91(3):199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Self-reports issues in alcohol abuse: State of the art and future directions. Behaviorial Assessment. 1990;12:77–90. [Google Scholar]
- SPSS Inc. SPSS 14.0 for Windows. 14.0. SPSS Inc.; Chicago IL: 2006. [Google Scholar]
- Ullsperger M, von Cramon DY. Subprocesses of performance monitoring: a dissociation of error processing and response competition revealed by event-related fMRI and ERPs. Neuroimage. 2001;14(6):1387–401. doi: 10.1006/nimg.2001.0935. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. The role of intact frontostriatal circuits in error processing. J Cogn Neurosci. 2006;18(4):651–64. doi: 10.1162/jocn.2006.18.4.651. [DOI] [PubMed] [Google Scholar]
- van Meel CS, Oosterlaan J, Heslenfeld DJ, Sergeant JA. Telling good from bad news: ADHD differentially affects processing of positive and negative feedback during guessing. Neuropsychologia. 2005;43(13):1946–54. doi: 10.1016/j.neuropsychologia.2005.03.018. [DOI] [PubMed] [Google Scholar]
- van Schie HT, Mars RB, Coles MG, Bekkering H. Modulation of activity in medial frontal and motor cortices during error observation. Nat Neurosci. 2004;7(5):549–54. doi: 10.1038/nn1239. [DOI] [PubMed] [Google Scholar]
- Yasuda A, Sato A, Miyawaki K, Kumano H, Kuboki T. Error-related negativity reflects detection of negative reward prediction error. Neuroreport. 2004;15(16):2561–5. doi: 10.1097/00001756-200411150-00027. [DOI] [PubMed] [Google Scholar]
- Yeung N, Holroyd CB, Cohen JD. ERP correlates of feedback and reward processing in the presence and absence of response choice. Cereb Cortex. 2005;15(5):535–44. doi: 10.1093/cercor/bhh153. [DOI] [PubMed] [Google Scholar]
- Yeung N, Sanfey AG. Independent coding of reward magnitude and valence in the human brain. J Neurosci. 2004;24(28):6258–64. doi: 10.1523/JNEUROSCI.4537-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Zhou X. Brain responses to outcomes of one’s own and other’s performance in a gambling task. Neuroreport. 2006;17(16):1747–51. doi: 10.1097/01.wnr.0000239960.98813.50. [DOI] [PubMed] [Google Scholar]


