Abstract
A number of behavioral studies have emphasized the importance of interactions between the pontine-wave (P-wave) generator and the dorsal hippocampus (DH) in two-way active avoidance (TWAA) memory processing; however, the direct involvement of the P-wave generator in the TWAA training trial-induced molecular events in the DH and amygdala has not been systematically evaluated. Here we demonstrate that the TWAA learning training trials activate P-wave generator, and increase phosphorylation of CREB (pCREB) and expression of activity-regulated cytoskeletal-associated (Arc) protein, as well as messenger ribonucleic acid (mRNAs) of Arc, brain-derived nerve growth factor (BDNF) and early growth response-1 (Egr-1) in the DH and amygdala. Selective elimination of P-wave-generating cells abolished P-wave activity and suppressed TWAA learning training trial-induced expression of pCREB and Arc proteins and Arc, BDNF and Egr-1 mRNAs in the DH and amygdala. Following a session of TWAA training, all rats were equal in terms of time spent in wakefulness, slow-wave sleep and rapid eye movement (REM) sleep irrespective of P-wave lesions. The second set of experiments demonstrated that localized cholinergic stimulation of the P-wave generator increased expression of Arc, BDNF and Egr-1 mRNAs in the DH. Together, these findings provide the first direct evidence that activation of P-wave-generating cells is critically involved in the TWAA training trial-induced expression of plasticity-related genes in the DH and amygdala. These findings are discussed in relation to the role of P-wave generator activation for the REM sleep-dependent development and cognitive functions of the brain.
Keywords: Arc, avoidance learning, BDNF, Egr-1, pCREB, REM sleep
Introduction
The pontine-wave (P-wave) is one of the most prominent phasic events of rapid eye movement (REM) sleep (Bizzi & Brooks, 1963; Jouvet, 1965; Gottesmann, 1969; Marks et al., 1980; Datta, 1997, 2000). Generated by the phasic activation of a group of glutamatergic cells in the pons (Datta et al., 1992, 1998; Datta & Hobson, 1994, 1995; Mavanji et al., 2004; Datta, 2006), the P-wave occurs during REM sleep as a singlet and in clusters containing a variable number of waves (3–5 waves/burst) at a frequency range of 30–60 spikes/min (Datta et al., 1998; Datta, 2000; Pace-Schott & Hobson, 2002; Datta & MacLean, 2007). The P-wave is proposed to be involved in several important brain functions, such as sensorimotor integration (Morrison & Bowker, 1975), memory consolidation (Datta, 2006; Stickgold & Walker, 2007), and development of the hippocampus, amygdala and visual system (Davenne & Adrien, 1984; Dang-Vu et al., 2006; Guzman-Marin & McGinty, 2006).
Various cellular and molecular studies have demonstrated that glutamate-induced neuronal activation-dependent gene expression and protein synthesis in the dorsal hippocampus (DH) and amygdala are necessary for memory processing and behavioral plasticity (Smith et al., 1991; Bailey et al., 1999; Hall et al., 2001; Kandel, 2001; Lonze & Ginty, 2002; Ribeiro et al., 2002; Scharf et al., 2002; Pavlides & Rebeiro, 2003; Lee et al., 2004). Although the precise role of individual genes and proteins continues to be debated, recent studies have shown that the activation of cAMP response element-binding protein (CREB) and expression of immediate-early genes, early growth response 1 (also called Egr-1, Zif 268, NGFI-A, Krox 24 and TIS8; Tischmeyer & Grimm, 1999; Malkani et al., 2004), activity-regulated cytoskeletal-associated protein (Arc; Guzowski et al. 2000, 2001; Huff et al., 2006) and brain-derived nerve growth factor (BDNF; Alonso et al. 2005; Pollak et al., 2005; Agassandian et al., 2006; Castillo et al., 2006) in the hippocampus and amygdala are involved in the memory processing.
Recent studies have indicated that learning-induced activation of the P-wave generator during the subsequent REM sleep period contributes to memory processing of two-way active avoidance (TWAA; reviewed in Datta, 2006). More recent studies have shown that the TWAA training trials increase expression of phosphorylated CREB (pCREB), Arc and BDNF proteins in the DH and amygdala (Saha & Datta, 2005; Ulloor & Datta, 2005). It has also been demonstrated that the DH and amygdala receive direct anatomical projections from these P-wave-generating cells, and activation of the P-wave generator increases glutamate release in the DH and increases the frequency of hippocampal theta wave activity (Datta et al., 1998; Karashima et al., 2002, 2004; Datta, 2006). Therefore, in this study we hypothesized that the TWAA training trial-induced expression of plasticity-related genes and proteins in the DH and amygdala is mediated by the activation of the P-wave generator. To test this hypothesis, in the present study, we measured TWAA training trial-induced pCREB and Arc proteins, as well as Egr-1, Arc and BDNF messenger ribonucleic acid (mRNA) expressions in the DH and amygdala of rats with and without P-wave generator activation. Additionally, we measured P-wave generator activation-induced Egr-1, Arc and BDNF mRNA expressions in the DH.
Materials and methods
Subjects and housing
Experiments were performed on adult male Sprague–Dawley rats (Charles River, Wilmington, MA, USA) weighing between 250 and 300 g at the time of surgery. Rats were housed individually at 24 °C with free access to food and water. Lights were on from 07.00 h to 19.00 h (light phase) and off from 19.00 h to 07.00 h (dark phase). To minimize the possible stress that could be imposed by the final experimental protocol, animals were routinely handled every day before surgery (15–20 min/day, between 09.00 h and 10.00 h) for about 2 weeks. Principles for the care and use of laboratory animals in research, as outlined by the National Institute of Health (1996) were strictly followed. This study was approved by the Boston University Animal Care and Use Committee.
Surgical procedures for the implantation of electrodes and guide tube
All surgical procedures were performed stereotaxically under aseptic conditions, and were in accordance with the guidelines approved by the Institutional Animal Care and Use Committee (Protocol: AN-14085). A total of 96 rats were anesthetized with pentobarbital (40 mg/kg, i.p.; Abbott Laboratories, Chicago, IL, USA), placed in the stereotaxic apparatus and secured using blunt rodent ear bars as described elsewhere (Paxinos & Watson, 1997; Datta, 2000). To record the behavioral states of vigilance, cortical electroencephalogram (EEG), dorsal neck muscle electromyogram (EMG), hippocampal EEG (to record theta wave) and pontine EEG (to record P-wave) recording electrodes were chronically implanted, as described elsewhere (Datta, 2000; Mavanji et al., 2004). In addition to these electrodes, bilateral stainless steel guide tubes (26 gage) with equal length stylets inside were stereotaxically implanted 2 mm above the P-wave-recording electrodes, for the microinjection of vehicle control (saline), ibotenic acid or carbachol solution into the P-wave generator (in relation to sterotaxic ‘0’: posterior, 0.80 mm; lateral, 1.3 mm; horizontal/dorso-ventral, 2.0 mm; dorsal part of the subcoeruleus nucleus) of the freely moving rat as described previously (Mavanji & Datta, 2003; Datta et al., 2004). All electrodes and guide tubes were secured to the skull with dental acrylic. Electrodes were crimped to mini-connector pins and brought together in a plastic connector. To avoid tissue damage by passing through the locus coeruleus and part of the P-wave generator, all guide tubes approached the P-wave generator latero-medially at an angle of 30 °. Following completion of the surgical procedure, animals were administered saline (5 cc, s.c.) to prevent dehydration, and ampicillin (50 mg/rat, s.c.; Bristol-Myers Squibb Company, Princeton, NJ, USA) to control any potential post-surgical infection. Potential postoperative pain was controlled with buprenorphine (0.05 mg/kg, s.c.; Abbott Laboratories).
Habituation and baseline recordings
After a post-surgical recovery period of 7–10 days, rats were habituated to the sound-attenuated recording cage, shuttle box, free-moving recording conditions and experimenter for 7 days, as described in our earlier publications (Datta et al., 2004; Ulloor & Datta, 2005). During their recovery, habituation and free-moving recording conditions, all rats were housed under the same 12 h light : dark cycle with free access to food and water. These habituation sessions were considered to be the adaptation recording sessions for this study. After the adaptation recording sessions, all rats underwent two sessions of baseline recording for electrode testing and additional habituation with the recording setup. During these two baseline recording sessions, pontine EEG was studied carefully to identify rats with good quality P-wave activity during REM sleep. Of the original 96 rats, 72 rats exhibited good quality P-waves during REM sleep recording. In another 24 of those 96 rats, very few to no P-waves have been expressed during REM sleep periods. In these rats, P-wave-recording electrodes may have been unintentionally placed outside of the P-wave generator; thus, these rats were not included in this study.
TWAA learning paradigm
The apparatus was an automated two-way shuttle scan shock-avoidance box (45.7 × 20.3 × 30.5 cm) with sides made of high-grade acrylic (Shuttle-flex test chamber; Model: SF II, AccuScan Instruments, Columbus, OH, USA). This apparatus was described in detail in our earlier publications (Datta, 2000; Datta et al., 2004). The procedure involved placing the rat in one compartment of the apparatus. After 15 min of acclimatization, training trials were begun. During acclimatization and the training trials, the rats could move freely from one compartment to the other within the shuttle box. Rats were trained on a massed 30-trial shuttle box TWAA task. The procedures for the conditioned stimulus (CS) and unconditioned stimulus (UCS) were detailed in an earlier publication (Datta, 2000). In brief, a tone (3600 Hz, 65 db) and a pulsatile light (2.5 Hz) were presented as a CS in the compartment with the animal. A 0.5-mA scrambled foot shock (UCS) was delivered 5 s later through the floor grid. To avoid receiving a foot shock the rat had 5 s to move to the opposite compartment. If the animal did not move to the other compartment, UCS was delivered for a maximum of 5 s and CS ended with UCS. While receiving UCS, if the animal moved to the other compartment, both CS and UCS ended immediately. The intertrial interval was variable with a mean of 60 s.
Microinjection of ibotenic acid for the elimination of P-wave-generating cells
After the post-surgical recovery and adaptation recording sessions, 42 of those 72 rats were randomly selected for the bilateral microinjections of ibotenic acid solution or control saline into the P-wave generator, as described in our earlier publication (Mavanji et al., 2004). Briefly, the microinjection system consisted of a 32-gage stainless steel injector cannula with a 26-gage collar that extended 2.0 mm beyond the implanted guide tube. The collar was connected to a 1.0-µL motor-driven microsyringe with PE 20 tubing. After filling the injection system with control vehicle (0.9% saline) or ibotenic acid solution (10 mg/mL), the stylet was removed and a control vehicle-filled or ibotenic acid (Sigma-Aldrich, St Louis, MO, USA) solution-filled injector was introduced through the guide tube for the first injection. One minute after the insertion of the injector cannula, 0.05 µL of control saline or ibotenic acid (0.5 µg in 0.05 µL) was microinjected over a 60-s period. The cannula was gently withdrawn 2 min after the injection, and the stylet was reintroduced inside the guide tube. Immediately after the first microinjection, using the same injector and solution, a second microinjection was administered in the contralateral P-wave generator. Of those 42 rats, 18 rats were randomly selected for bilateral microinjections of control saline and the remaining 24 rats received bilateral microinjections of ibotenic acid solution. Detailed procedures for microinjection into the P-wave generator of the freely moving rat have been described in our earlier publications (Datta et al., 2004; Mavanji et al., 2004). Rats with control saline microinjections were used as P-wave generator sham-lesioned group. From here onward, this sham-lesioned group will be referred to as the ‘S-L’ group. Rats that received ibotenic acid solution microinjections were used as P-wave generator-lesioned rats. Hereafter, this ibotenic acid microinjection-induced P-wave generator-lesioned group of rats will be referred to as the ‘PWG-L’ group. In this study, we have used ibotenic acid to destroy cells in the P-wave generator because it is well established that local microinjection of ibotenic acid at this dose into the brain destroys nerve cell bodies with minimal or no damage to passing nerve fibers (Moser et al., 1995; Galani et al., 2002; Mavanji et al., 2004; Datta et al., 2005). Importantly, the dose and volume of ibotenic acid solution used in this study were carefully standardized and shown to be very effective for the elimination of P-wave-generating cells without destroying cells of the neighboring areas (Mavanji et al., 2004; Datta et al., 2005). Microinjection of normal saline has been shown to cause no damage to brain cells (Mavanji & Datta, 2003; Mavanji et al., 2004). Thus, microinjection of normal saline was used as a sham lesion for the ibotenic acid lesion procedure. Following these microinjections, rats were further habituated with the experimental setup for another 7–10 days. After this habituation period, all rats underwent two consecutive sessions of baseline recordings for the post-injection electrode testing. During these electrode-testing days, all rats exhibited good quality polygraphic recording (Polygraph, Grass Instrument, Quincy, MA, USA), except P-waves were absent in the ibotenic acid-microinjected rats.
Experimental design and tissue collection
Experiment 1: the effects of the P-wave generator lesion on the acquisition of TWAA learning, sleep–wake behavior, and expression of genes and proteins in the DH and amygdala
These experiments were performed on three groups of rats. The first group of rats was PWG-L (n = 18), the second group of rats was S-L (n = 18), and the third group of rats did not receive any injections into the P-wave generator (control; n = 18). After the adaptation recordings, all 54 rats underwent one session of final baseline and another session of experimental recording (on the day after the final baseline recording session). During baseline recordings, rats were placed in the shuttle box for 45 min (09.14 h–09.59 h) and transferred to a recording cage for 6 h of undisturbed polygraphic recordings (between 10.00 h and 16.00 h). On the experimental day, the S-L, PWG-L and control groups of rats were placed in the shuttle box at 09.14 h, and after 15 min of acclimatization S-L and PWG-L groups, but not the control group, were subjected to 30 CS trials (TWAA training trials; between 09.29 h and 09.59 h). The control group of rats simply remained in the shuttle box for a 45-min period without any CS–UCS trials (shuttle box control). After this session, rats were transferred to the polygraphic recording cage (at about 10.00 h). While in the polygraphic recording cage, rats were recorded for sleep–wake signs. At this point, rats were randomly divided into three subgroups, which determined at what time they would be killed. S-L-1 h (n = 6 rats), PWG-L-1 h (n = 6 rats) and control-1 h (n = 6 rats) were killed 1 h after the end of TWAA training or control trials. S-L-2 h, PWG-L-2 h and control-2 h rats were killed 2 h after the end of TWAA training or control trials. S-L-3 h, PWG-L-3 h and control-3 h rats were killed 3 h after the end of TWAA training or control trials. In order to minimize possible variations due to such differences in the sleep–wake state, at the time of death all animals were awakened by shaking their cage and kept awake for 1 min before they were killed with CO2.
Experiment 2: the effects of P-wave generator stimulation on the sleep–wake behavior, and expression of Arc, BDNF and Egr-1 genes in the DH
These experiments were conducted on another 18 rats. Of those 18 rats, 12 rats exhibited good quality P-waves during REM sleep and six rats whose P-wave generator was lesioned by local microinjection of ibotenic acid into the P-wave generators. After surgical recovery and adaptation recording sessions, these rats underwent two consecutive sessions of sleep–wake recordings: (1) baseline; and (2) experimental. For baseline recordings, rats were connected to the polygraphic recording system at 09.55 h for 3 h of undisturbed sleep–wake recordings (from 10.00 h to 13.00 h). The duration of the experimental recording session of this experiment is about half of Experiment 1; thus, the duration of the baseline recording session (3 h) of this experiment is also half of the baseline recording session of Experiment 1 (6 h). On the day following the baseline recording session, rats were connected to the polygraphic recording system at 09.55 h. While these rats were connected to the polygraphic recording system, 100 nL of saline (vehicle control; SP group; n = 6 rats) or a single dose of carbachol solution (50 ng in 100 nL; CP group; n = 6 rats) was microinjected unilaterally (P-wave-recording side) into the P-wave generator as described in our earlier publications (Mavanji & Datta, 2003; Datta et al., 2004). Selection of these rats for saline or carbachol microinjections was random. A third group of rats (PWG-L group; n = 6 rats) was treated like the SP and CP group, except a dummy injector was introduced through the guide tube. These rats did not receive saline or carbachol microinjection. While in the polygraphic recording cage, rats were recorded for sleep–wake signs and killed 1 h after the end of microinjection or introduction of dummy injector. Immediately prior to death with CO2, all animals were awakened by shaking their cage and then kept awake for 1 min.
Tissue collection
After death, the rat brains were rapidly removed. The DH (between −1.80 and −3.80, in relation to bregma) and amygdala were then dissected under a dissecting microscope on an ice-chilled Petri dish as described earlier (Ulloor & Datta, 2005). For Experiment 1, tissues were collected from both sides of the brain. For Experiment 2, in order to focus on the effects of P-wave generator activation, only the DH tissue was collected from the hemisphere ipsilateral to the P-wave generator treated with saline or carbachol. The tissue levels of pCREB, CREB and Arc were determined by a standard Western blot technique, as described below. The mRNA levels of Arc, BDNF and Egr-1 were determined by a standard reverse transcriptase-polymerase chain reaction (RT-PCR) technique, as described below.
Western blotting
The frozen brain tissues were thawed and homogenized in ice-chilled lysis buffer (CelLytic™ MT Mammalian Tissue Lysis/Extraction Reagent, Sigma) at 4 °C. The homogenate was centrifuged for 10 min at 18 000 g and the soluble fraction was used for the analysis. The amount of protein in the supernatant (soluble fraction) was measured using Bradford’s method (1976). Suitable aliquots (30–40 µg) of protein were boiled for 5 min in sample buffer with reducing agent, and resolved on 12% sodium dodecyl sulfate (SDS) –polyacrylamide gel. The proteins were transferred to Immun-Blot polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA, USA) by electroblotting. Membranes were pretreated with Tris-buffered saline with 5% milk and 0.05% Tween 20 (TBST: 50 mm Tris-HCl, pH 7.4 and 150 mm NaCl, 0.05% Tween 20) and then incubated with anti-pCREB (1 : 1000; Upstate Biotechnology, Charlottesville, VA, USA) or Arc (1 : 1000; rabbit polyclonal antibody raised against a recombinant protein corresponding to amino acids 1300 mapping at the amino terminals of Arc; Santa Cruz Biotechnology, Santa Cruz, CA, USA). After overnight incubation, the membranes were washed with TBST three times for 10 min each. Then membranes were incubated with a 1 : 5000 dilution of horseradish peroxidase-conjugated anti-rabbit IgG for 2 h, and washed with TBST three times for 10 min each. The immunocomplexes were detected with a chemiluminescent substrate (SuperSignal® West Femto Maximum Sensitivity Substrate, Pierce, Rockford, IL, USA). The quantification of the immunoreactive band was carried out utilizing a Kodak imaging densitometer (PerkinElmer, Shelton, CT, USA). To calculate the phosphorylated vs non-phosphorylated form of CREB, the same membrane was stripped with stripping buffer (62.5 mm Tris-HCl, pH 6.7, 2% SDS, 100 mm 2-mercaptoethanol) at 70 °C for 30 min and incubated with anti-CREB (1 : 1000; Upstate Biotechnology) and detected as described above.
Real-time quantitative PCR
Total RNA from brain tissues was extracted by ‘SV total RNA isolation system’ (Promega, Madison, WI, USA), as recommended by the manufacturer. The quality of extracted RNA was excellent, as identified by the A260/A280 ratio of 1.8 or higher. The total RNA specimen from each sample was subjected to RT using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA; Product 4322171) following the manufacturer’s suggestions. Briefly, 1 µg of total RNA sample in 50 µL of water was mixed with 50 µL of 2 × RT mix containing RT buffer, dNTPs, random primers and Multiscribe™ Reverse Transcriptase. The RT reaction was performed in a thermal cycler for 10 min at 25 °C, followed by 120 min at 37 °C. To control for DNA contamination, RT was also carried out in parallel tubes, where reverse transcriptase was omitted. The reaction products were stored at −20 °C.
The BDNF, Arc, Egr-1 (Zif 268) and β-actin mRNA quantification was performed using ABI Prism 7500 Sequence Detection System (Applied Biosystems), which uses the 5′ nuclease activity of Taq DNA polymerase to generate a real-time quantitative DNA analysis assay. Briefly, gene-specific oligonucleotide probes with 5′ fluorescent and 3′ quencher dye (TaqMan probes) and primers were designed using Primer Express software (Applied Biosystems). An oligonucleotide probe specific for the rat actin gene was used as a housekeeping gene. Each reaction contained 25 µL of TaqMan Universal PCR master Mix, 2.5 µL of assays on demand primer (BDNF = Rn00560868; Arc = Rn00571208_g1; Egr-1 = Rn00561138_m1; β-actin = 4352340E), 5 µL of cDNA sample and 17.5 µL of nuclease-free water in 96-well optical reaction plates. The following PCR conditions were used: 50 °C for 2 min, then 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min.
To analyse RT-PCR results, CT values for each reaction were determined using TaqMan Sequence Detection System analysis software (Applied Biosystems). For each amount of RNA tested, the triplicate CT values were averaged. Because CT values vary linearly with the logarithm of the amount of RNA, this average represents a geometric mean. The average CT value for actin was subtracted from the average CT values of Arc, BDNF and Egr-1 to yield the ΔCT value. The average ΔCT value for the positive control was then subtracted from the average ΔCT value of respective target genes to get the ΔΔCT. Then, 2−ΔΔCT was calculated for each sample, and values between treated and untreated samples were compared. The statistical significance was determined by one-factor anovas followed by a post hoc F-test.
Data analysis for the sleep–wake states, P-wave density and TWAA training
For the purpose of determining possible effects on sleep and wakefulness (W), polygraphic data were captured on-line in a computer using ‘Gamma’ software (Grass product group, Astro-Medical, West Warwick, RI, USA). From these captured data, three behavioral states were distinguished and scored visually using ‘Rodent Sleep Stager’ software (Grass product group, Astro-Medical), as described earlier (Datta, 2000; Datta et al., 2004; Mavanji et al., 2004). Because the microinjections of ibotenic acid into the P-wave generator eliminated P-waves by destroying P-wave-generating cells, after microinjections of ibotenic acid, REM sleep episodes were identified based on the signs in the cortical EEG, EMG and hippocampal EEG. W, slow-wave sleep (SWS) and REM sleep were scored in successive 10-s epochs. The polygraphic measures provided the following dependent variables that were quantified for each recording session: (1) percentage of recording time spent in W; (2) percentage of recording time spent in SWS; (3) percentage of recording time spent in REM sleep; and (4) REM sleep P-wave density. To calculate P-wave density, P-wave spikes were visually identified and isolated from the background EEG activities as described earlier (Datta et al., 1998; Datta, 2000). P-waves from one side of the brain were then counted during all REM sleep periods. Next, the mean P-wave density (number of P-waves per minute) of each rat was calculated for each recording session, as described in our earlier publications (Datta & Hobson, 1994; Datta, 2000; Mavanji & Datta, 2003; Datta et al., 2004). For the purpose of determining possible effects of the P-wave generator lesion on the performance of TWAA training and also the possible effects of foot shock in the expression of genes and proteins in the DH and amygdala, three variables were quantified for each training session. The first variable is the number of successful avoidance per session. A trial is considered as ‘avoidance’ when the rat moves to the other compartment of the shuttle box within 5 s from the beginning of the CS. In this individual trial, the rat does not receive any foot shock (Datta, 2000). The second variable is the number of ‘escape’ per session. A trial is considered an escape when the rat moves to the other compartment of the shuttle box between the beginning and end of the UCS. In this trial the rat receives foot shock, but the total shock time is always less than 5 s. The rat could receive foot shock for a maximum of 5 s per trial, if they do not avoid or escape. This trial is called a no-go trial. The third variable is the total shock time per session. The total shock time per session is calculated by adding times that the rat received UCS in the escape trials and 5 s for each no-go trial.
For statistical analyses, anova procedures and post hoc Scheffe F-tests were performed using Stat View statistical software (Abacus Concepts, Berkeley, CA, USA). Specific statistics for specific comparisons are described in the Results.
Results
Effects of elimination of cells in the P-wave generator on W, SWS, REM sleep, and REM sleep P-wave density
In the first baseline polygraphic recording session, before microinjections of the control saline (S-L) or ibotenic acid (PWG-L), all 36 rats exhibited good quality P-waves during REM sleep episodes. Good quality P-wave recording indicated that our P-wave-recording electrodes were within the P-wave generator zone. In this 6-h baseline recording session, total percentages of time spent in W, SWS and REM sleep, and REM sleep P-wave density were not significantly different (one-factor anova) between the control, S-L and PWG-L (one-factor anova; Table 1). Thus, these results indicate that, before P-wave-generating cells were eliminated, the groups were initially equal in terms of time spent in W, SWS and REM sleep and P-wave density for the final 6-h baseline recording session.
Table 1.
The total percentages (mean ± SEM) of time spent in wakefulness, slow-wave (S-W) sleep and REM sleep, and REM sleep P-wave density during baseline recording sessions
| Group | Wakefulness | S-W sleep | REM sleep | P-wave density (waves/min) |
|---|---|---|---|---|
| Experiment 1 | ||||
| Baseline 1 (6 h) | ||||
| Control (n = 18) | 36.50 ± 1.8 | 53.0 ± 2.9 | 10.2 ± 0.9 | 38.2 ± 2.2 |
| S-L (n = 18) | 34.1 ± 2.2 | 52.6 ± 3.5 | 11.3 ± 0.7 | 38.6 ± 1.8 |
| PWG-L (n = 18) | 35.63 ± 1.8 | 54.4 ± 3.1 | 9.8 ± 1.2 | 38.0 ± 2.0 |
| Baseline 2 (6 h) | ||||
| Control (n = 18) | 35.8 ± 1.3 | 52.6 ± 2.6 | 11.5 ± 0.8 | 40.3 ± 1.7 |
| S-L (n = 18) | 35.0 ± 2.1 | 54.7 ± 3.3 | 10.4 ± 1.1 | 39.4 ± 1.5 |
| PWG-L (n = 18) | 36.2 ± 1.9 | 53.1 ± 3.8 | 10.7 ± 0.9 | 4.9 ± 1.8*** |
| Experiment 2 | ||||
| Baseline (3 h) | ||||
| SP (n = 6) | 38.0 ± 1.9 | 54.9 ± 2.4 | 7.1 ± 1.3 | 39.6 ± 3.8 |
| CP (n = 6) | 40.2 ± 2.2 | 53.0 ± 3.1 | 6.8 ± 0.9 | 40.4 ± 2.8 |
| PWG-L (n = 6) | 39.9 ± 2.4 | 52.5 ± 2.9 | 7.5 ± 1.4 | 2.2 ± 1.5*** |
W, S-W sleep, REM sleep and P-wave density values in Experiment 1 were analysed from a 6-h period of undisturbed sleep recording. In baseline 1, values were from the baseline recordings prior to any microinjections into the P-wave generator. In baseline 2, values were from the final baseline recording after recovery from saline (S-L) and ibotenic acid (PWG-L) microinjections into the P-wave generator. The control values were from the comparable time periods of S-L and PWG-L groups of baseline recordings. The control group of rats did not receive any microinjections. In Experiment 2, W, S-W sleep, REM sleep and P-wave density values were analysed from 3 h of undisturbed sleep recording. Final baseline recordings were taken from the day prior to the experimental recording day. In Experiment 2, during the experimental recording day, the SP group of rats was microinjected with control saline and the CP group of rats was microinjected with carbachol into the P-wave generator. The PWG-L group of rats did not receive any injections. Note that there were no significant differences in W, S-W sleep and REM sleep. Also note that the P-wave density in the PWG-L group of rats was significantly lower in the second baseline of Experiment 1 and baseline of Experiment 2, indicating that the microinjections of ibotenic acid into the P-wave generator did not change the total percentages of W, S-W sleep and REM sleep, but did almost eliminate the expression of P-waves. Post hoc tests (Scheffe F-test)
P < 0.001 (in Experiment 1 compared with control and in Experiment 2 compared with SP).
During the final day of the 6-h baseline recording, 7–10 days after microinjections of control saline or ibotenic acid into the P-wave generator, the total percentages of time spent in W, SWS and REM sleep remained comparable between the control, S-L and PWG-L groups (one-factor anova). Individual comparisons (post hoc Scheffe F-test) between the control and S-L, control and PWG-L, and S-L and PWG-L were also not significantly different for the total percentages of time spent in W, SWS and REM sleep (Table 1). These results indicated that the elimination of P-wave-generating cells did not change the total percentages of time spent in W, SWS and REM sleep. These results agree with previous P-wave generator lesion studies in both cats and rats, that have shown that an excitotoxic lesion of the P-wave generator has no effect on the total percentages of time spent in W, SWS and REM sleep (Datta & Hobson, 1995; Mavanji et al., 2004). During the 6-h baseline recording session, however, REM sleep P-wave density between the three different groups (control, S-L and PWG-L) was significantly different (one-factor anova, F2,33 = 25.62, P < 0.001). A comparison between the control and S-L groups revealed no significant difference (post hoc Scheffe F-test) in REM sleep P-wave densities, indicating that in the S-L rats P-wave-generating cells remained intact. A similar comparison revealed that the REM sleep P-wave density in the PWG-L group was significantly less (87.5% less F = 32.27, P < 0.001) compared with the S-L group, indicating that the microinjection of ibotenic acid into the P-wave generator successfully suppressed the expression of P-wave activity in the PWG-L group (Table 1). Suppression of P-wave activity after local microinjection of ibotenic acid is a typical sign of cell loss within the P-wave generator (Datta & Hobson, 1995; Mavanji et al., 2004).
Effects of P-wave generator lesion on the TWAA training performance
To determine the effects of a P-wave generator lesion on TWAA training performance, 18 S-L and 18 PWG-L rats were subjected to a session of TWAA training, a learning task that involves the P-wave generator, DH and amygdala. Figure 1 illustrates the effects of P-wave generator lesion on the number of avoidance, number of escape and total foot shock time. During this TWAA training trials session, the number of avoidance, number of escape and total foot shock time were not significantly different (one-factor anova) between S-L and PWG-L groups (Fig. 1). These variables were then further analysed to make sure that there are no differences between different subgroups. One-factor anovas between six subgroups (S-L-1 h, S-L-2 h, S-L-3 h, PWG-1 h, PWG-L-2 h and PWG-L-3 h) revealed no significant group effects in the number of avoidance (F5,30 = 0.15, P = 0.97), number of escape (F5,30 = 0.76, P = 0.58) and total shock time (F5,30 = 0.62, P = 0.68). These results indicate that the elimination of P-wave-generating cells did not alter the normal acquisition process for the TWAA training. These results also indicate that the elimination of P-wave-generating cells has no negative effect on the short-term working memory involved in TWAA learning. This finding is in agreement with an earlier study that demonstrated that the elimination of P-wave-generating cells does not affect the normal acquisition process (Mavanji et al., 2004). Thus, in the present study it is reasonable to suggest that the differences in the molecular changes observed between the S-L and PWG-L groups of rats are not simply due to the differences in foot shock and/or avoidance performance.
FIG. 1.
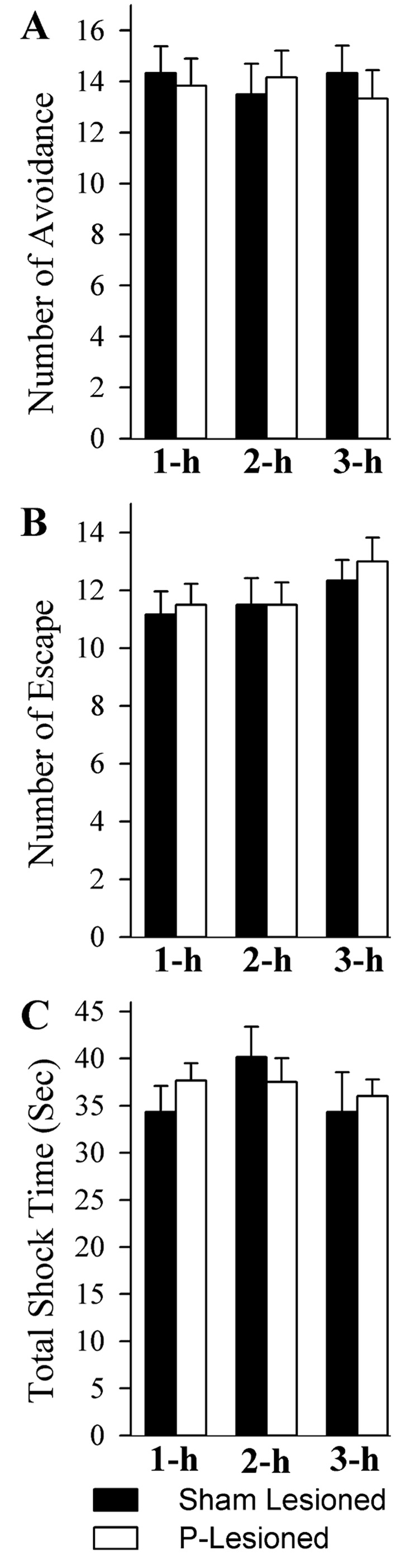
Effects of pontine-wave (P-wave) generator lesion on the TWAA learning performance. Each bar represents mean and SE of number of avoidance (A), number of escape (B) and total shock time (C). On the X-axis: 1-h, group of animals killed 1 h after the end of TWAA training trials; 2-h, group of animals killed 2 h after the end of TWAA training trials; 3-h, group of animals killed 3 h after the end of TWAA training trials. Note that the number of avoidance, number of escape and total shock time are comparable (one-factor anova) between the S-L (black bars) and PWG-L (white bars) groups of animals. Also note that these variables are comparable between three subgroups (1 h, 2 h and 3 h; n = 6 rats / subgroup) of each group (S-L and PWG-L), indicating that all six subgroups (three subgroups of S-L and three subgroups of PWG-L) of rats were equal in terms of their ability to learn this TWAA training task.
Effects of TWAA training and P-wave generator lesion on the sleep–wake states and P-wave density
After a session of TWAA training trials, S-L, PWG-L and control groups of rats were recorded for a 1-h, 2-h or 3-h session of undisturbed sleep–wake. Typical polygraphic appearances of REM sleep in the control, PWG-L and S-L groups of rats are shown in Fig. 2. During the 1-h, 2-h or 3-h recording sessions, the total percentages of Wand SWS were not significantly different (one-factor anova) between control, S-L and PWG-L groups of rats (Fig. 3A and B). These results agree with previous sleep learning studies using both similar and other types of learning paradigms that have also shown that learning trials do not have an effect on the amount of post-trial W and SWS (Fishbein et al., 1974; Smith & Rose, 1997; Datta, 2000). Contrary to the total percentages of W and SWS, the total percentages of REM sleep were significantly different (one-factor anova) between groups during the 1-h (F2,15 = 13.78, P < 0.001), 2-h (F2,15 = 19.00, P < 0.001) and 3-h (F2,15 = 17.81, P < 0.001) recording sessions. Individual post hoc tests (Scheffe F-test) indicated that, after TWAA training trials, S-L-1 h (216.86% more than control-1 h; F = 9.40, P < 0.001), S-L-2 h (88.56% more than control-2 h; F = 16.19, P < 0.001) and S-L-3 h (98.70% more than control-3 h; F = 13.01, P < 0.001) groups of animals spent significantly more time in REM sleep (Fig. 3C). These post hoc tests also revealed that after TWAA training trials, PWG-L-1 h (236.75% more than control-1 h; F = 11.2, P < 0.001), PWG-L-2 h (76.76% more than control-2 h; F = 12.11, P < 0.001) and PWG-L-3 h (101.25% more than control-3 h; F = 13.70, P < 0.001) groups of animals spent significantly more time in REM sleep (Fig. 3C). However, the total percentages of time spent in REM sleep after TWAA training trials were not significantly different between the S-L and PWG-L subgroups of 1 h (F = 0.08, NS), 2 h (F = 0.29, NS) or 3 h (F = 0.01, NS). These results indicate that the S-L and PWG-L groups of rats responded equally in terms of time spent in W, SWS and REM sleep following a session of TWAA learning training trials. These results exclude the possibility that the observed differences in the molecular changes between S-L and PWG-L groups of rats might have been due to the differences in post-training sleep–wake changes. The finding that TWAA learning training trials is followed by an increase in REM sleep agrees with previous animal and human studies that, using a variety of protocols and test paradigms, have consistently shown that learning training increases subsequent REM sleep (Fishbein, 1971; Leconte & Hennevin, 1981; Hennevin et al., 1995; Smith, 1995; Stickgold, 1998; Maquet, 2001; Datta, 2006). Because the P-wave is critical for the memory processing, and this wave is expressed only during REM sleep, the increased amount of REM sleep following learning training in animals with or without P-wave generator has been suggested to be a homeostatic response that facilitates P-wave generator activation processes to express more P-waves (Datta, 2006).
FIG. 2.
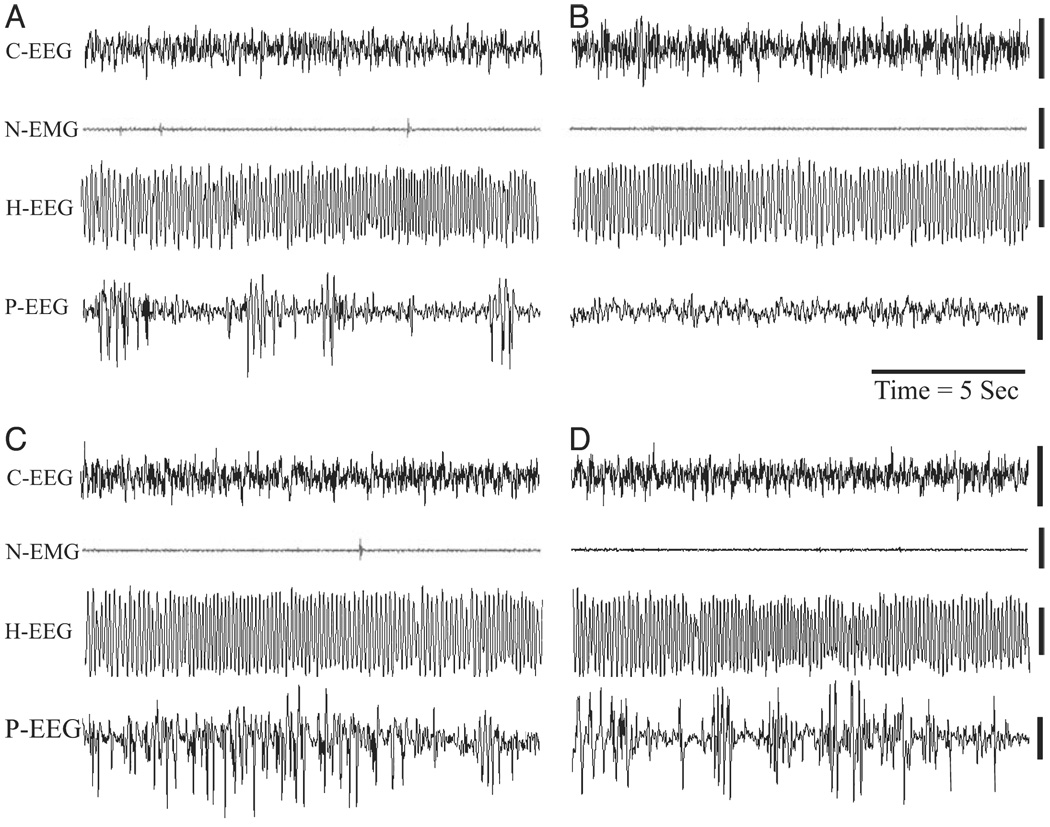
Sample polygraphic appearance of REM sleep episode recorded in four different rats. (A) A REM sleep episode of a control rat recorded about 1.5 h after a session of control condition. (B) A REM sleep episode of a PWG-L rat recorded about 1.5 h after a session of TWAA training trials. (C) A REM sleep episode of a S-L rat recorded about 1.5 h after a session of TWAA training trials. (D) A REM sleep episode of a rat recorded about 45 min after a single microinjection of carbachol into the P-wave generator (CP group of rat, used in Experiment 2). Abbreviations: EEG, cortical electroencephalogram; H-EEG, hippocampal EEG; N-EMG, neck electromyogram; P-EEG, pontine EEG.
FIG. 3.
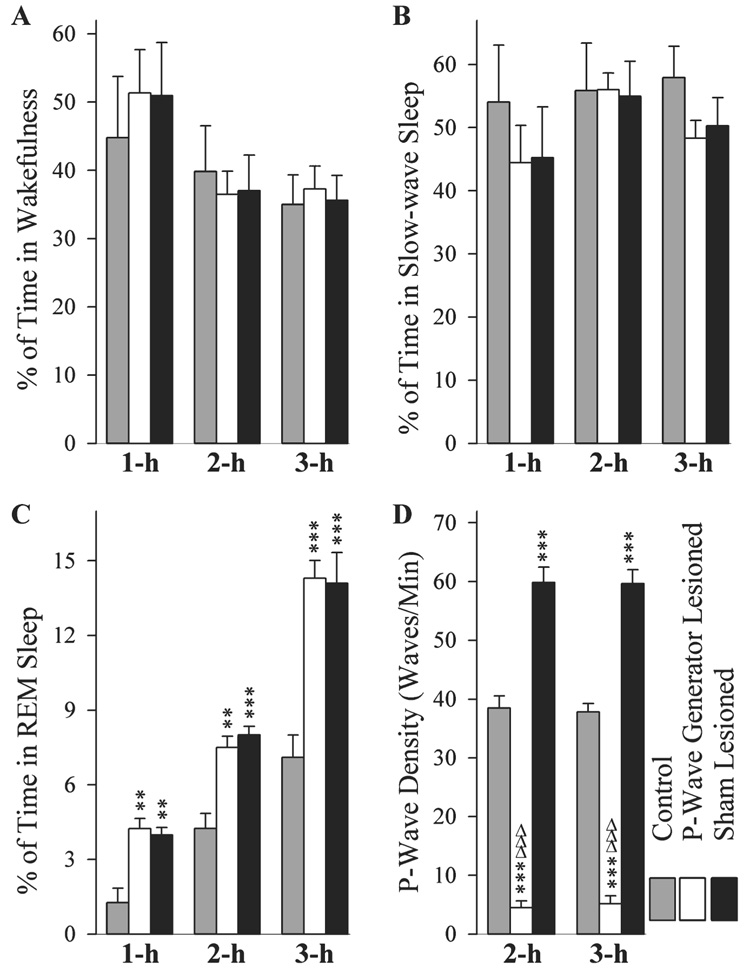
Effects of P-wave generator lesion and a session of TWAA training on the total percentages of W (A), SWS (B), rapid eye movement (REM) sleep (C), and REM sleep P-wave density (D). Bars represent the total percentages (mean and SE) of time spent in W, SWS, REM sleep, and REM sleep P-wave density of three different subgroups of rats (1 h, 2 h and 3 h) of control group after cage control (gray bars) and S-L (black bars) and PWG-L (white bars) groups after TWAA training trials. Note, compared with controls, a significant increase in REM sleep was present in both S-L and PWG-L groups. Also note that, compared with controls, P-wave density was higher in the S-L group. On the contrary, in the PWG-L group, P-wave density was significantly less compared with both control and S-L groups. Note that the P-wave density data are missing at the 1 h period (D) because during the first hour of recordings very few or no REM sleep episodes were present in the control group. Post hoc tests (Scheffe F-test): **P < 0.01 and ***P < 0.001, compared with control; ΔΔΔP < 0.001, compared with S-L.
Similar to time spent in REM sleep, REM sleep P-wave density was significantly different (one-factor anova) between experimental groups during sleep recording sessions of 2 h (F2,15 = 191.60, P < 0.001) and 3 h (F2,15 = 242.69, P < 0.001; Fig. 3D). Individual comparisons (Scheffe F-test) revealed that the REM sleep P-wave density after TWAA training trials was significantly higher in the S-L-2 h (55.41% higher than control-2 h; F = 27.99, P < 0.001) and S-L-3 h (57.71% higher than control-3 h; F = 38.44, P < 0.001) groups of animals (Fig. 3D). Conversely, REM sleep P-wave density after TWAA training trials was significantly less in the PWG-L-2 h (755.56% less than control-2 h; F = 27.99, P < 0.001) and PWG-L-3 h (632.20% less than control-3 h; F = 38.44, P < 0.001) groups of animals (Fig. 3D). To determine the combined effects of the P-wave generator lesion and TWAA training trials, REM sleep P-wave density was compared between S-L and PWG-L groups. This comparison revealed that the REM sleep P-wave density was much higher in the S-L-2 h (1229.62% higher than PWG-L-2 h; F = 188.31, P < 0.001) and S-L-3 h (1054.77% higher than PWG-L-3 h; F = 239.54 P < 0.001) groups of animals (Fig. 3D). These findings demonstrate that animals with a bilateral lesion to the P-wave generator fail to increase P-wave activity even during the increased REM sleep periods present after TWAA training trials (Fig 2 and Fig 3D). This low and unchanged P-wave density in the PWG-L group of rats after avoidance learning training also provides additional physiological confirmation of our earlier anatomical and physiological observations that the microinjection of ibotenic acid was localized to cells within the P-wave generator (Mavanji et al., 2004).
Effects of P-wave generator lesion and TWAA learning training trials on the expressions of CREB, pCREB and Arc proteins, and Arc, BDNF and Egr-1 mRNAs in the DH and amygdala
Figure 4 illustrates the representative Western blots and densitometric results of CREB in the DH and amygdala in the control, PWG-L and S-L groups of animals during all three different recording sessions (1 h, 2 h and 3 h). In the DH, the levels of CREB expression in the three different groups (control, PWG-L and S-L) were significantly different (one-factor anova) at intervals 1 h (F2,15 = 4.24, P < 0.05), 2 h (F2,15 = 4.53, P < 0.05), but not at 3 h (F2,15 = 0.001, NS). Individual comparisons (Scheffe F-test) demonstrated significance only in the PWG-L group at the 1-h interval where the CREB level was 17.65% less than in the control group (Fig. 4A). In the amygdala, the levels of CREB expression in the three different groups (control, PWG-L and S-L) were not significantly different (one-factor anova) at intervals 1 h (F2,15 = 0.78, P = 0.47), 2 h (F2,15 = 1.94, P = 0.17) and 3 h (F2,15 = 0.96, P = 0.40; Fig. 4B). These results indicate that the elimination of P-wave generator and/or TWAA training has very little or no effect on the expression of CREB protein in the DH and amygdala.
FIG. 4.
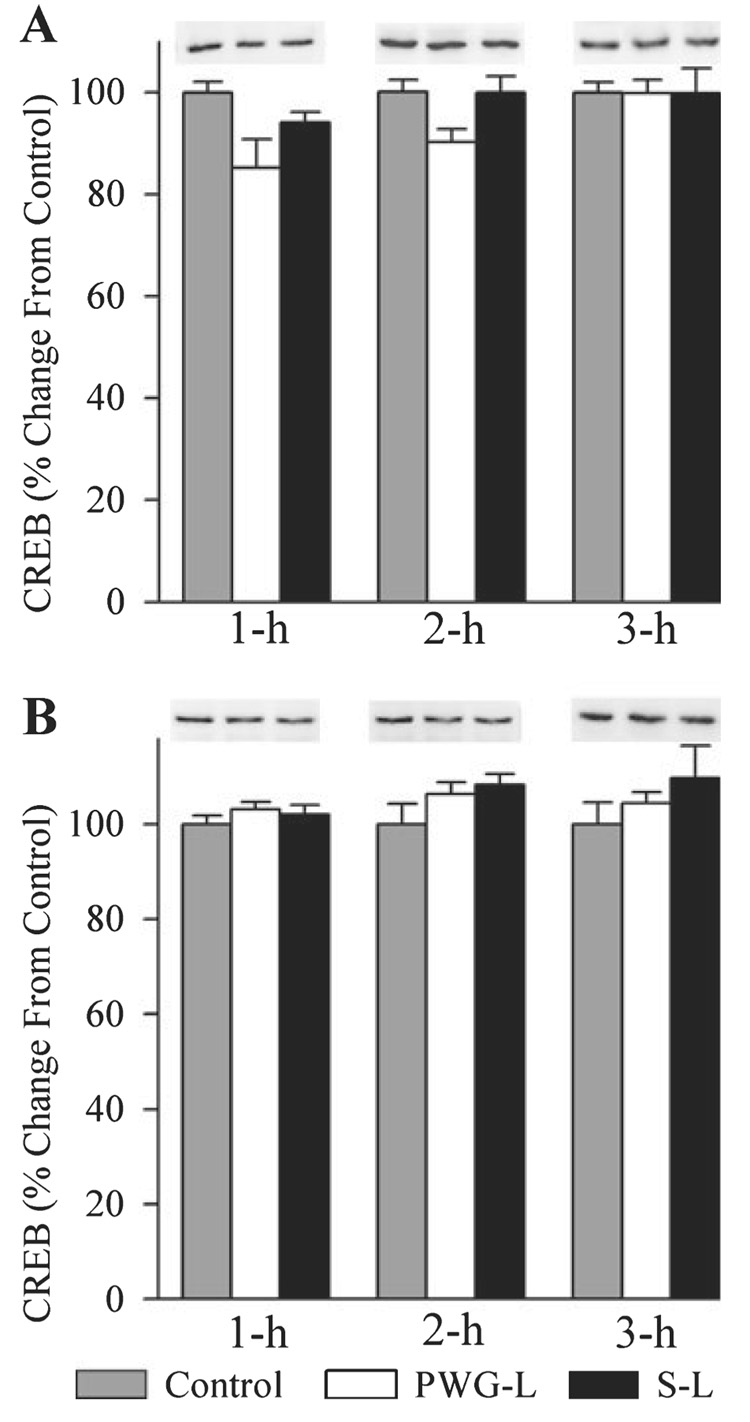
Effects of P-wave generator lesion and a session of TWAA training on the cAMP response element-binding protein (CREB) expression in the DH (A) and amygdala (B). In Western blots, the first, second and third lanes represent levels of CREB in the brain extracts from control (gray bars), P-wave generator-lesioned (PWG-L; white bars) and sham-lesioned (S-L; black bars) animals, respectively. Representative blots of 1 h, 2 h and 3 h subgroups of control, PWG-L and S-L groups are placed directly above their mean densitometric bars. Data from densitometric analysis of Western blots are expressed as a percentage of control. Each bar represents the mean + SE of 1 h, 2 h, 3 h subgroups of control, PWG-L and S-L groups of animals (n = 6 rats / subgroup). Post hoc tests (Scheffe F-test): *P < 0.05, compared with control.
Figure 5 illustrates the representative Western blots and densitometric results of pCREB in the DH and amygdala in the control, PWG-L and S-L groups of animals during all three recording sessions (1 h, 2 h and 3 h). In the DH, the levels of pCREB expression in all three experimental groups (control, PWG-L and S-L) were significantly different (one-factor anova) at intervals 1 h (F2,15 = 147.34, P < 0.001), 2 h (F2,15 = 187.47, P < 0.001) and 3 h (F2,15 = 72.28, P < 0.001). To determine the changes in the levels of pCREB expression after TWAA training, pCREB levels of PWG-L and S-L groups at the three different time intervals were compared (Scheffe F-test) with the corresponding time intervals of the control group. These tests revealed that in the PWG-L group, the levels of pCREB were significantly less at 1 h (60.65% less; F = 41.58, P < 0.001), 2 h (39.54% less; F = 20.12, P < 0.001) and 3 h (50.42% less; F = 15.56, P < 0.001; Fig. 5A). On the contrary, similar comparisons revealed that the pCREB levels in the S-L group were significantly higher at 1 h (33.27% higher; F = 32.29, P < 0.001), 2 h (56.61% higher; F = 80.29, P < 0.001) and 3 h (53.53% higher; F = 26.85, P < 0.001; Fig. 5A). To determine the effects of the P-wave generator lesion, the levels of pCREB were then compared between PWG-L and S-L. These comparisons revealed that, compared with the PWG-L group, pCREB levels in S-L rats were significantly more at 1-h (114.12% more; F = 147.15, P < 0.001), 2-h (118.54% more; F = 180.79, P < 0.001) and 3-h (130.93% more; F = 71.03, P < 0.001) intervals (Fig. 5A). These results demonstrate that TWAA training trials increase pCREB expression in the DH of animals with an intact P-wave generator but decrease in the PWG-L animals.
FIG. 5.
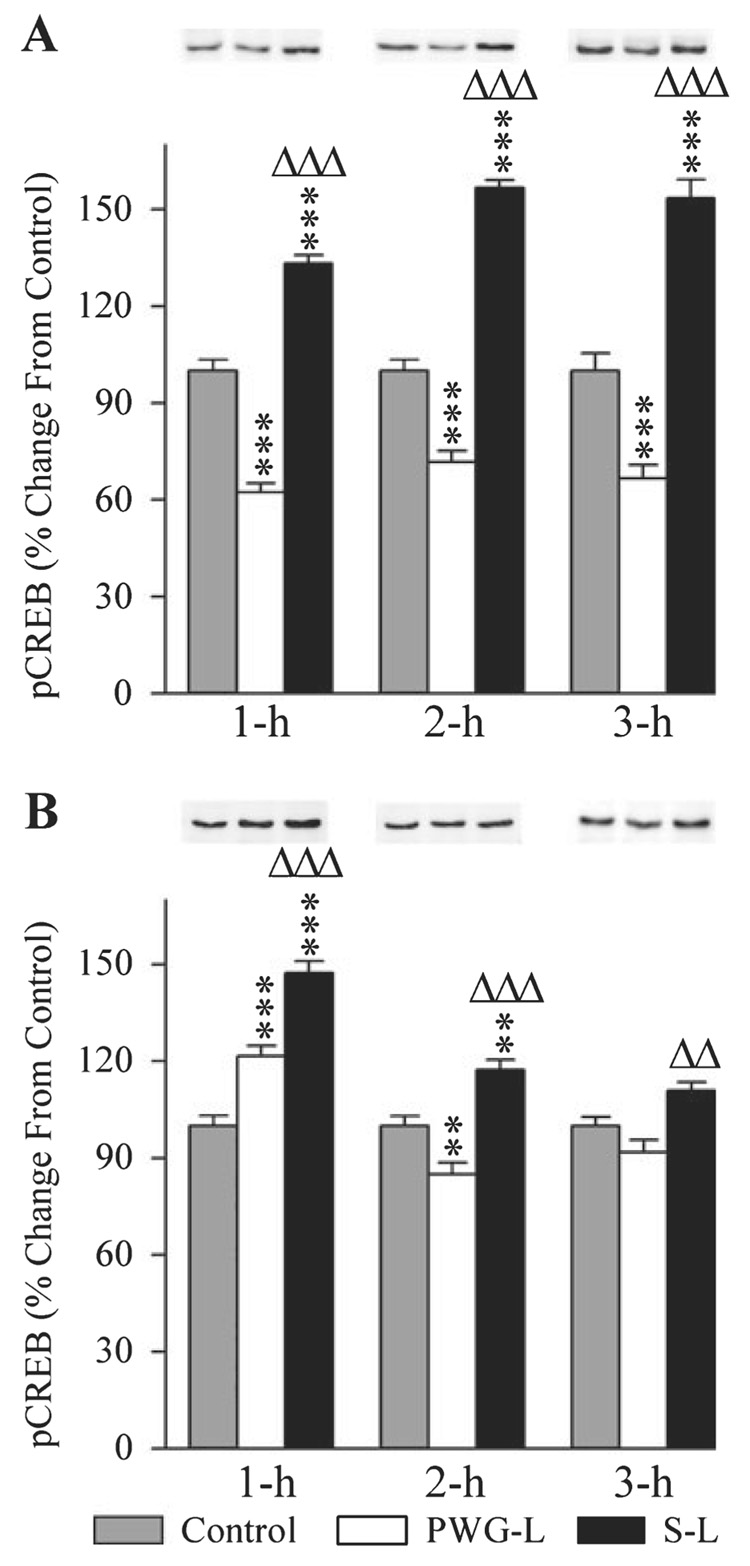
Effects of P-wave generator lesion and a session of TWAA training on the expression of phosphorylated CREB (pCREB) in the DH (A) and amygdala (B). In Western blots, the first, second and third lanes represent levels of pCREB in the brain extracts from control (gray bars), P-wave generator-lesioned (PWG-L; white bars) and sham-lesioned (S-L; black bars) animals, respectively. Representative blots of 1 h, 2 h and 3 h subgroups of control, PWG-L and S-L groups are placed directly above their mean densitometric bars. Data from densitometric analysis of Western blots are expressed as a percentage of control. Each bar represents the mean + SE of 1 h, 2 h, 3 h subgroups of control, PWG-L and S-L groups of animals (n = 6 rats/subgroup). Post hoc tests (Scheffe F-test): **P < 0.01 and ***P < 0.001, compared with control; ΔΔP < 0.01 and ΔΔΔP < 0.001, compared with S-L.
In the amygdala, as in the DH, the levels of pCREB expression in the three different groups were also significantly (one-factor anova) different at intervals 1 h (F2,15 = 49.84, P < 0.001), 2 h (F2,15 = 25.25, P < 0.001) and 3 h (F2,15 = 10.24, P < 0.01). Compared with the control (Scheffe F-test), levels of pCREB in the amygdala of S-L rats were significantly more at 1-h (47.32% more; F = 49.69, P < 0.001) and 2-h (17.33% more; F = 7.43, P < 0.01) intervals (Fig. 5B). In the PWG-L group, compared with the control, levels of pCREB were significantly more at 1-h (21.46% more; F = 10.22, P < 0.001) but less at 2-h (14.58% less; F = 5.26, P < 0.01) intervals (Fig. 5B). Similar comparisons between PWG-L and S-L groups revealed that pCREB levels in S-L rats were significantly higher at 1 h (21.29% more; F = 14.85, P < 0.001), 2 h (37.37% more; F = 25.18, P < 0.001) and 3 h (20.73% more; F = 10.17, P < 0.01; Fig. 5B). These results demonstrated that the TWAA training trials increase pCREB expression in the amygdala. The increased pCREB expression effect lasted for about 2 h in the animals with an intact P-wave generator and 1 h in PWG-L animals. These results also demonstrated that the level of pCREB expression in animals with an intact P-wave generator is more than the PWG-L animals. Another interesting aspect of these results is that 2 h after the TWAA training trials, the pCREB level in the PWG-L animals is significantly less than the control level.
Figure 6 illustrates the representative Western blots and densitometric results of Arc in both the DH and amygdala of control (1 h, 2 h and 3 h), PWG-L (1 h, 2 h and 3 h) and S-L (1 h, 2 h and 3 h) groups of rats. The levels of Arc protein expression in the DH of three different groups were significantly different (one-factor anova) at intervals of 1 h (F2,15 = 133.15, P < 0.001), 2 h (F2,15 = 85.44, P < 0.001) and 3 h (F2,15 = 109.32, P < 0.001). To determine the changes in the levels of Arc expression after TWAA training trials, Arc levels of PWG-L and S-L rats at all three different time intervals were compared (Scheffe F-test) with respect to corresponding time intervals of the control group. These individual comparisons revealed that the Arc levels in the S-L group were significantly more at 1 h (38.90% more; F = 43.93, P < 0.001), 2 h (59.12% more; F = 39.52, P < 0.001) and 3 h (37.91% more; F = 26.38, P < 0.001; Fig. 6A). On the contrary, the levels of Arc in the PWG-L rats were significantly less at 1 h (39.98% less; F = 23.68, P < 0.001), 2 h (34.46% less; F = 7.43, P < 0.01) and 3 h (61.10% less; F = 28.29, P < 0.001; Fig. 6A). Individual comparisons between the PWG-L and S-L rats revealed that the Arc levels in S-L rats were significantly more at 1 h (94.43% more; F = 132.11, P < 0.001), 2 h (113.95% more; F = 81.21, P < 0.001) and 3 h (122.10% more; F = 109.31, P < 0.001; Fig. 6A). These results demonstrated that Arc expression after TWAA training trials increased in the DH of animals with an intact P-wave generator, but decreased in the PWG-L animals.
FIG. 6.
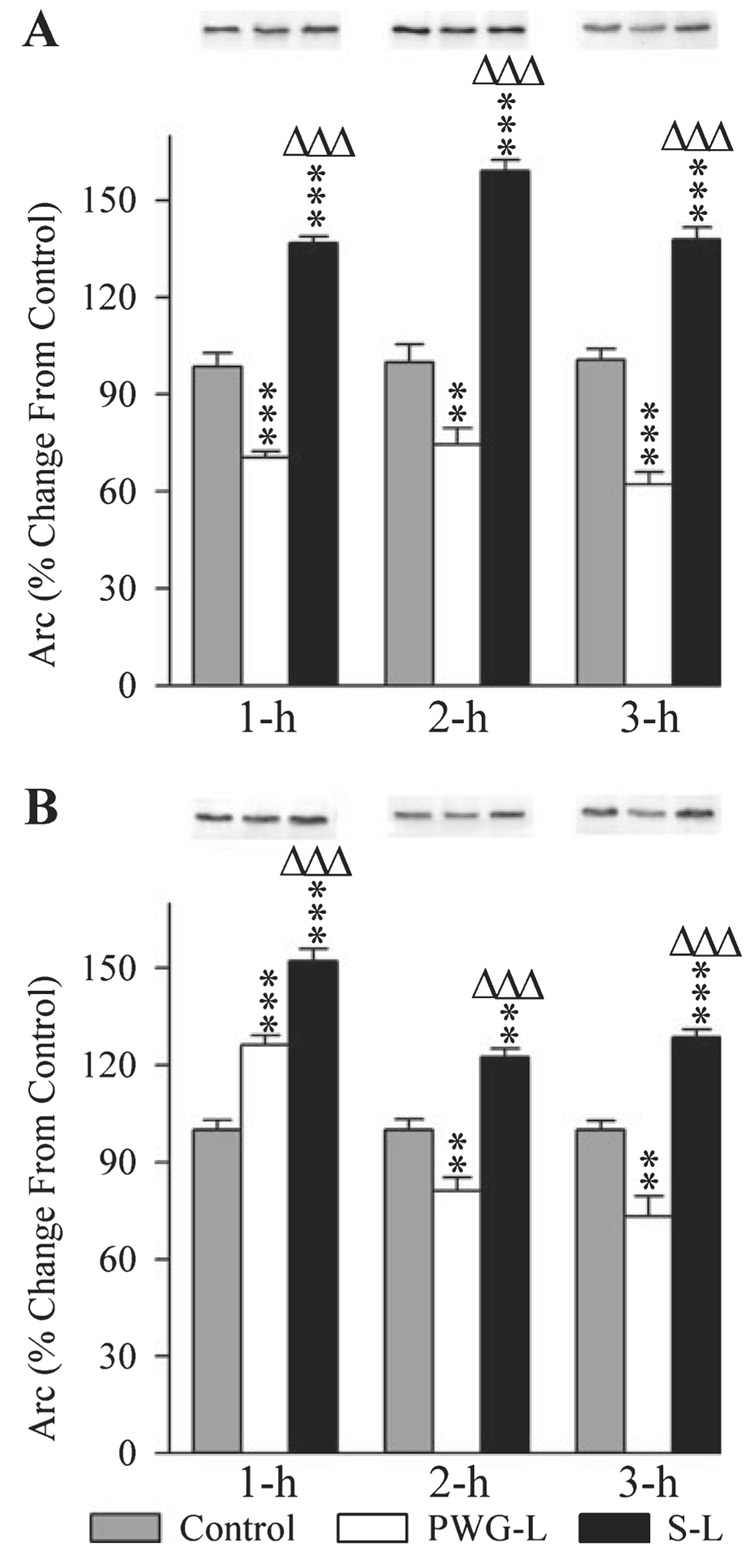
Effects of P-wave generator lesion and a session of TWAA training on the expression of activity-regulated cytoskeletal-associated protein (Arc) in the DH (A) and amygdala (B). In the Western blots, the first, second and third lanes represent levels of Arc in the brain extracts from control (gray bars), P-wave generator-lesioned (PWG-L; white bars) and sham-lesioned (S-L; black bars) animals, respectively. Representative blots of 1 h, 2 h and 3 h subgroups of control, PWG-L and S-L groups are placed exactly on the top of their mean densitometric bars. Data from densitometric analysis of Western blots are expressed as a percentage of control. Each bar represents the mean + SE of 1 h, 2 h, 3 h subgroups of control, PWG-L and S-L groups of animals (n = 6 rats/subgroup). Post hoc tests (Scheffe F-test): **P < 0.01 and ***P < 0.001, compared with control; ΔΔΔP < 0.001, compared with S-L.
In the amygdala, the levels of Arc expression in the three experimental groups were also significantly different (one-factor anova) at intervals 1 h (F2,15 = 63.50, P < 0.001), 2 h (F2,15 = 38.1, P < 0.001) and 3 h (F2,15 = 43.58, P < 0.01). Compared with the control group (Scheffe F-test), Arc levels in the S-L group were significantly higher at 1 h (52.14% higher; F = 63.49, P < 0.001), 2 h (22.53% higher; F = 11.25, P < 0.01) and 3 h (28.64% higher; F = 11.63, P < 0.001; Fig. 6B). Compared with the control group, the level of Arc in the PWG-L group was significantly higher at the 1-h (26.2% higher; F = 16.04, P < 0.001) interval; but significantly less at 2-h (18.88% less; F = 7.90, P < 0.01) and 3-h (26.79% less; F = 10.17, P < 0.01) intervals (Fig. 6B). Compared with the PWG-L rats, Arc levels in the S-L group of rats were significantly more at 1 h (20.55% more; F = 15.71, P < 0.001), 2 h (51.04% more; F = 38.0, P < 0.001) and 3 h (75.70% more; F = 43.56, P < 0.01; Fig. 6B). These results demonstrated that the TWAA training trials increases Arc expression in the amygdala of both the P-wave generator intact and PWG-L animals. This increase in Arc expression is much less and also lasts for only a short period of time in the PWG-L animals. These results also demonstrated that, in the PWG-L animals, Arc expression levels in the amygdala after 2 and 3 h after the TWAA training trials are significantly less than the control levels.
The changes in the levels of Arc mRNA expression in the DH and amygdala of three different groups (control, PWG-L and S-L) of rats at three different post-training time intervals (1 h, 2 h and 3 h) are illustrated in Fig. 7. Similar to Arc protein, the levels of Arc mRNA expression in the DH of all three experimental groups were significantly different (one-factor anova) at 1 h (F2,15 = 69.66, P < 0.001), 2 h (F2,15 = 173.15, P < 0.001) and 3 h (F2,15 = 93.70, P < 0.001). Individual comparisons show that compared with the control group, the Arc mRNA expression levels in the S-L group were significantly higher (Scheffe F-test) at 1 h (16.33% higher; F = 22.97, P < 0.01), 2 h (22.5% higher; F = 54.50, P < 0.001) and 3 h (44.33% higher; F = 48.16, P < 0.001; Fig. 7A). However, similar comparisons revealed that the level of Arc mRNA was significantly less in the PWG-L group at 1 h (12% less; F = 12.40, P < 0.01), 2 h (17.5% less; F = 32.97, P < 0.001) and 3 h (15.17% less; F = 5.64, P < 0.05; Fig. 7A). Compared with PWG-L rats, the level of Arc mRNA expression in the S-L group was also significantly more at 1 h (32.20% more; F = 69.12, P < 0.001), 2 h (48.48% more; F = 172.25, P < 0.001) and 3 h (70.14% more; F = 86.76, P < 0.001; Fig. 7A). These results indicated that Arc gene expression after TWAA training trials increased in the DH of rats with the P-wave generator intact, but decreased in the PWG-L rats.
FIG. 7.
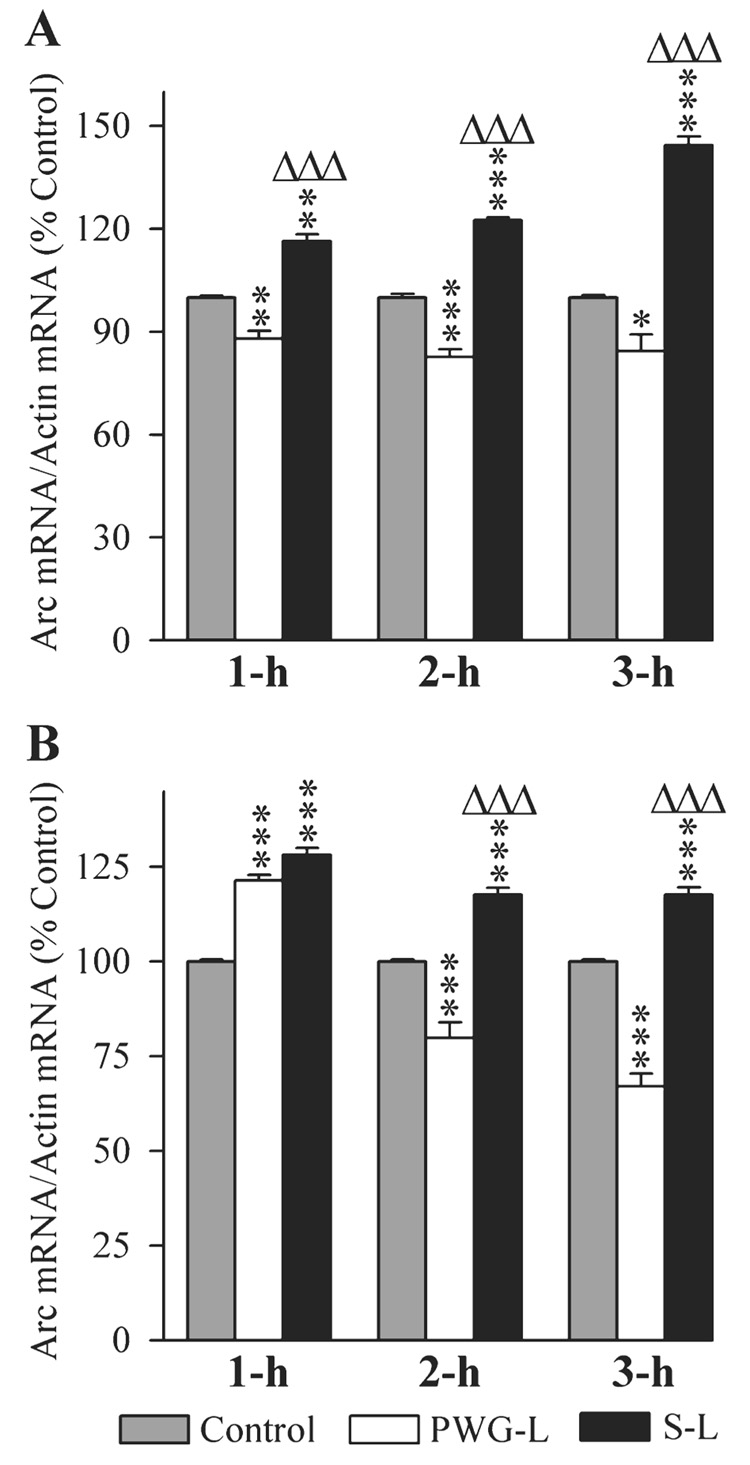
Effects of P-wave generator lesion and a session of TWAA training on the expression of activity-regulated cytoskeletal-associated (Arc) mRNA in the DH (A) and amygdala (B). The levels of Arc and β-actin were measured by RT-PCR. The target gene mRNA level was normalized with the β-actin mRNA level. All data are expressed as percentage of control. Each bar represents the mean + SE of 1 h, 2 h, 3 h subgroups of control (gray bars), P-wave generator-lesioned (PWG-L; white bars) and sham-lesioned (S-L; black bars) groups of animals (n = 6 rats/subgroup). Post hoc tests (Scheffe F-test): *P < 0.05, **P < 0.01 and ***P < 0.001, compared with control; ΔΔΔP < 0.001, compared with S-L.
In the amygdala, there was a significant group difference (one-factor anova) in the levels of Arc mRNA expression at 1-h (F2,15 = 123.16, P < 0.001), 2-h (F2,15 = 53.38, P < 0.001) and 3-h (F2,15 = 132.47, P < 0.001) intervals. Individual comparisons between the control and S-L groups show that the levels of Arc mRNA were significantly more in the S-L group at 1 h (28.17% more; F = 112.74, P < 0.001), 2 h (17.667% more; F = 11.64, P < 0.001) and 3 h (17.67% more; F = 15.63, P < 0.001; Fig. 7B). However, compared with the control, the level of Arc mRNA in the PWG-L group of rats was significantly more only at 1 h (21.5% more; F = 65.69, P < 0.001), and significantly less at the 2 h (20.17% less; F = 15.15, P < 0.001) and 3 h (33.0% less; F = 54.53, P < 0.001) intervals (Fig. 7B). Compared with PWG-L rats, the levels of Arc mRNA expression in the S-L group of rats was significantly higher at the 2-h (47.39% higher; F = 53.31, P < 0.001) and 3-h (75.62% higher; F = 128.55, P < 0.001) intervals, but not at the 1-h interval (Fig. 7B). These results demonstrated that, in P-wave generator intact animals, TWAA training trials increase Arc gene expression in the amygdala, and this increased expression level remains high even at the 3-h post-training interval. In the PWG-L rats, Arc gene expression increases for a short period of time (1 h) after TWAA training trials, and then decreases and remains low even at the 3-h interval.
The changes in the expression of BDNF mRNA in the DH and amygdala of the three experimental groups of rats at three different post-training time intervals are illustrated in Fig. 8. One-factor anovas revealed a significant group difference in the levels of BDNF mRNA in the DH at 1-h (F2,15 = 126.13, P < 0.001), 2-h (F2,15 = 133.02, P < 0.001) and 3-h (F2,15 = 255.10, P < 0.001) intervals. Individual comparisons (Scheffe F-test) show that compared with the control group, the BDNF mRNA level in the S-L group was significantly higher at 1-h (25.5% higher; F = 24.26, P < 0.001), 2-h (36.17% higher; F = 41.21, P < 0.001) and 3-h (53.17% higher; F = 95.38, P < 0.001) intervals. Similar comparisons revealed that, compared with the control, the BDNF mRNA level in the PWG-L group of rats was significantly less at 1-h (48.15% less; F = 39.41, P < 0.001), 2-h (40.19% less; F = 25.89, P < 0.001) and 3-h (49.25% less; F = 36.75, P < 0.001) intervals. Compared with the PWG-L rats, the level of BDNF mRNA expression in the S-L group of rats was also significantly more at 1 h (85.93% more; F = 125.52, P < 0.001), 2 h (90.89% more; F = 132.43, P < 0.001) and 3 h (128.61% more; F = 250.52, P < 0.001; Fig. 8A). These results demonstrated that the BDNF gene in the DH after TWAA training trials increased in animals with intact P-wave generator and decreased in the PWG-L animals.
FIG. 8.
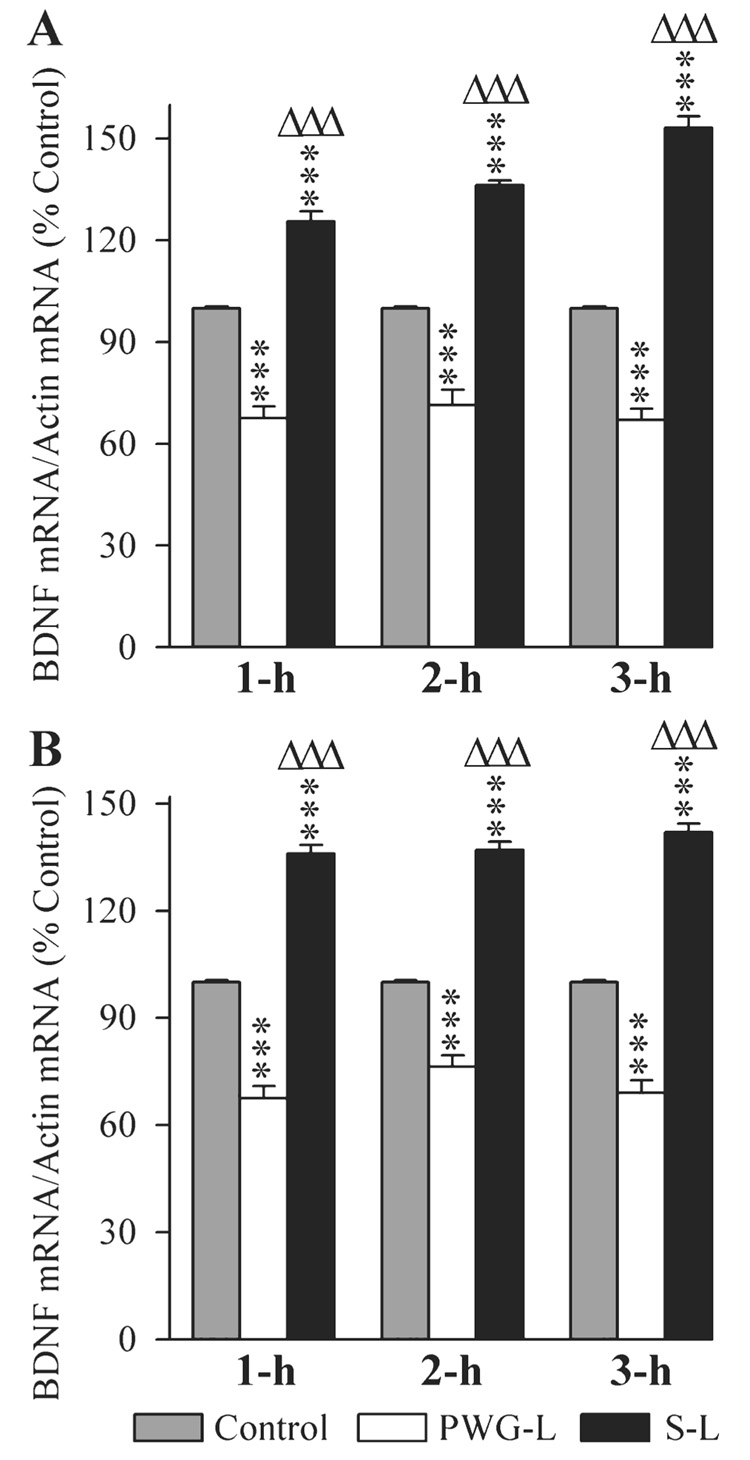
Effects of P-wave generator lesion and a session of TWAA training on the expression of brain-derived nerve growth factor (BDNF) mRNA in the DH (A) and amygdala (B). The levels of BDNF and β-actin were measured by RT-PCR. The target gene mRNA level was normalized with the β-actin mRNA level. All data are expressed as percentage of control. Each bar represents the mean + SE of 1 h, 2 h, 3 h subgroups of control (gray bars), P-wave generator-lesioned (PWG-L; white bars) and sham-lesioned (S-L; black bars) groups of animals (n = 6 rats/subgroup). Post hoc tests (Scheffe F-test): ***P < 0.001, compared with control; ΔΔΔP < 0.001, compared with S-L.
In the amygdala, the BDNF mRNA levels were significantly different (one-factor anova) in the three different groups at 1-h (F2,15 = 201.85, P < 0.001), 2-h (F2,15 = 192.40, P < 0.001) and 3-h (F2,15 = 220.46, P < 0.001) intervals. Individual comparisons (Scheffe F-test) show that compared with the control, in the S-L group, the BDNF mRNA level in the amygdala was significantly more at 1 h (36.0% more; F = 55.70, P < 0.001), 2 h (37.0% more; F = 70.43, P < 0.001) and 3 h (42.0% more; F = 72.43, P < 0.001; Fig. 8B). Conversely, in the PWG-L group, the BDNF mRNA level in the amygdala was significantly less at 1 h (48.15% less; F = 45.40, P < 0.001), 2 h (31.0% less; F = 28.82, P < 0.001) and 3 h (44.93% less; F = 39.46, P < 0.001; Fig. 8B). Compared with the PWG-L group, the level of BDNF mRNA in the S-L group of rats was significantly higher at 1 h (101.48% more; F = 201.67, P < 0.001), 2 h (79.48% more; F = 189.35, P < 0.001) and 3 h (105.80% more; F = 218.8, P < 0.001; Fig. 8B). These results demonstrated that after TWAA training trials, the BDNF gene in the amygdala increases in the P-wave generator intact but decreases in the PWG-L rats.
The changes in the expression of Egr-1 mRNA in the DH and amygdala of three different groups of rats at three different post-training time intervals are illustrated in Fig. 9. One-factor anovas revealed a significant group difference in the levels of Egr-1 mRNA in the DH at 1-h (F2,15 = 250.33, P < 0.001), 2-h (F2,15 = 125.39, P < 0.001) and 3-h (F2,15 = 29.21, P < 0.001) intervals. Individual comparisons (Scheffe F-test) show that compared with the control, the Egr-1 mRNA level in S-L rats was significantly higher at 1-h (38.67% higher; F = 106.23, P < 0.001) and 2-h (28.17% higher; F = 40.6, P < 0.001) intervals (Fig. 9A). On the contrary, the Egr-1 gene level in the PWG-L group was significantly less at 1 h (19.67% less; F = 27.48, P < 0.001), 2 h (26.85% less; F = 22.93, P < 0.001) and 3 h (13.21% less; F = 7.85, P < 0.05). Compared with the PWG-L group, the level of Egr-1 mRNA in the S-L group of rats was also significantly more at 1 h (72.62% more; F = 241.78, P < 0.001), 2 h (62.58% more; F = 124.55, P < 0.001) and 3 h (25.47% more; F = 15.19, P < 0.01; Fig. 9A). These results indicated that Egr-1 mRNA expression in the DH after TWAA training trials increases in rats with an intact P-wave generator, but decreases in the PWG-L rats.
FIG. 9.
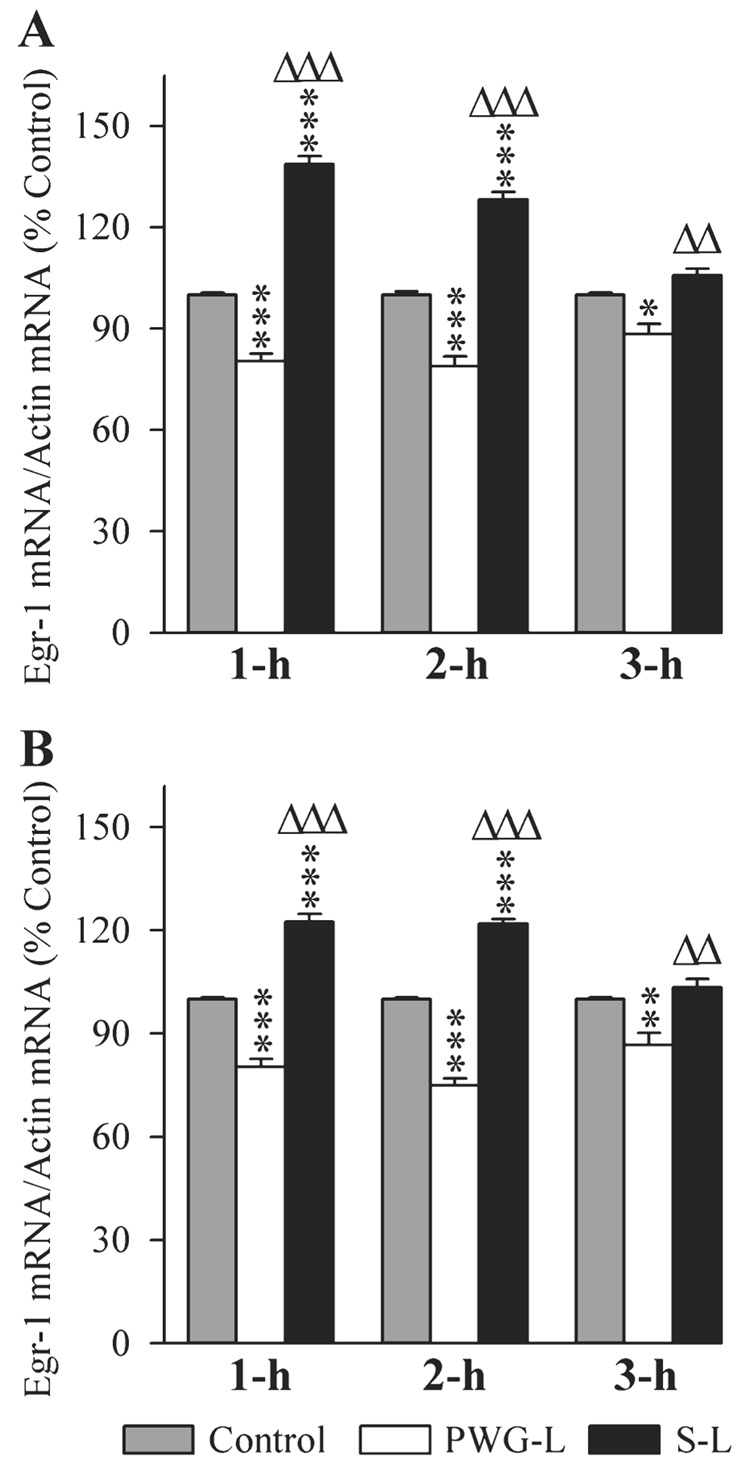
Effects of P-wave generator lesion and a session of TWAA training on the expression of early growth response 1 (Egr-1) mRNA in the DH (A) and amygdala (B). The levels of Egr-1 and β-actin were measured by RT-PCR. The target gene mRNA level was normalized with the β-actin mRNA level. All data are expressed as percentage of control. Each bar represents the mean + SE of 1 h, 2 h, 3 h subgroups of control (gray bars), P-wave generator-lesioned (PWG-L; white bars) and sham-lesioned (S-L; black bars) groups of animals (n = 6 rats/subgroup). Post hoc tests (Scheffe F-test): *P < 0.05, **P < 0.01, ***P < 0.001, compared with control; ΔΔP < 0.01, ΔΔΔP < 0.001, compared with S-L.
The Egr-1 mRNA levels in the amygdala were also significantly different (one-factor anova) in all three experimental groups at 1-h (F2,15 = 132.30, P < 0.001), 2-h (F2,15 = 282.70, P < 0.001) and 3-h (F2,15 = 13.63, P < 0.001) intervals. In the S-L group of rats, compared with the control group (Scheffe F-test), the Egr-1 mRNA level in the amygdala was significantly more at 1 h (22.5% more; F = 37.61, P < 0.001) and 2 h (21.83% more; F = 61.35, P < 0.001; Fig. 8B). Conversely, the Egr-1 mRNA level in PWG-L rats was significantly less at 1 h (24.48% less; F = 28.74, P < 0.001), 2 h (33.33% less; F = 80.43, P < 0.001) and 3 h (15.38% less; F = 7.68, P < 0.01; Fig. 8B). Compared with the PWG-L group, the level of Egr-1 mRNA in the S-L group of rats was significantly higher at 1-h (52.49% higher; F = 132.10, P < 0.001), 2-h (62.44% higher; F = 282.27, P < 0.001) and 3-h (19.42% higher; F = 12.24, P < 0.01) intervals (Fig. 9B). These results indicated that the TWAA training trials increase Egr-1 gene in the amygdala of animals with an intact P-wave generator, but decrease in the PWG-L rats.
The results of this study already demonstrated that S-L and PWG-L rats had comparable sleep–wake time during the baseline recording days before and after the P-wave generator lesion in addition to responding equally to the TWAA with respect to sleep–wake time. These results also demonstrated that both groups of rats had received a comparable amount of foot shock, and subsequent TWAA learning training performance was comparable. Thus, the differences in molecular changes, discussed above, are mainly due to the presence and absence of P-wave-generating cells.
Effects of P-wave generator stimulation and lesion on the expressions of Arc, BDNF and Egr-1 mRNAs in the DH
Earlier studies have shown that TWAA training trials increase P-wave generator activity during subsequent REM sleep periods (Datta, 2000; Mavanji & Datta, 2003; Datta et al., 2004; Ulloor & Datta, 2005). The results of Experiment 1 of the present study have documented that the TWAA training trial-induced Arc, BDNF and Egr-1 gene expression in the DH increases in animals with an intact P-wave generator and decreases in the PWG-L animals. These results suggest that the activation of the P-wave generator might be involved in the TWAA training trial-induced expression of these immediate-early genes in the DH. Thus, Experiment 2 was designed to see whether the direct activation of the P-wave generator with local microinjection of carbachol could increase Arc, BDNF and Egr-1 gene expression in the DH. Based on the results of our Experiment 1, in this experiment, we have also included a group of PWG-L animals to test a possibility that the PWG-L animals used in Experiment 1 might have lower baseline level of gene expression.
In the final baseline polygraphic recording session, the total percentages of W, SWS and REM sleep were not significantly different (one-factor anova) between the SP, CP and PWG-L groups (Table 1). During this baseline recording session, the REM sleep P-wave density was comparable between the SP (39.6 ± 3.8) and CP (40.4 ± 2.8) groups. In the PWG-L group of rats, REM sleep P-waves were almost absent (2.2 ± 1.5). On the experimental day, microinjection of carbachol into the P-wave generator (CP groups of rats) significantly increased the REM sleep P-wave density (80.4% increase from the baseline) in all six animals (Fig. 2D). But the microinjection of saline and introduction of dummy injector into the PWG-L animals did not change the REM sleep P-wave density compared with their baseline P-wave densities. During the experimental recording session, the total percentages of W, SWS and REM sleep were not significantly different (one-factor anova) between the SP, CP and PWG-L groups. These results indicate that the microinjections of carbachol or saline did not change their total percentages of time spent in W, SWS and REM sleep.
The changes in the expression of Arc, BDNF and Egr-1 mRNAs in the DH of three different groups (SP, CP and PWG-L) of rats are illustrated in Fig. 10. One-factor anovas revealed a significant group effect in the levels of Arc (F2,15 = 369.10, P < 0.001), BDNF (F2,15 = 431.76, P < 0.001) and Egr-1 (F2,15 = 63.72, P < 0.001) mRNA expressions in the DH. Individual comparisons show that compared with SP (saline control) group, the Arc, BDNF and Egr-1 mRNA expression levels in the PWG-L group were not significantly different (post hoc Scheffe F-test; Fig. 10). However, similar comparisons revealed that the Arc (47.17% higher; F = 276.81, P < 0.001), BDNF (59.10% higher; F = 327.48, P < 0.001) and Egr-1 (20.40% higher; F = 47.79, P < 0.001) mRNA expression levels in the CP group were significantly higher than controls (Fig. 10). Similarly, compared with the PWG-L group, Arc (47.17% higher; F = 276.81, P < 0.001), BDNF (58.04% higher; F = 320.12, P < 0.001) and Egr-1 (20.40% higher; F = 47.79, P < 0.001) mRNA expression levels in the CP group were significantly higher (Fig. 10). The results of this experiment show that the P-wave generator lesion alone does not change the baseline levels of these genes expressions in the DH. These results also showed that the application of carbachol into the P-wave generator increases expressions of the Arc, BDNF and Egr-1 genes in the DH.
FIG. 10.
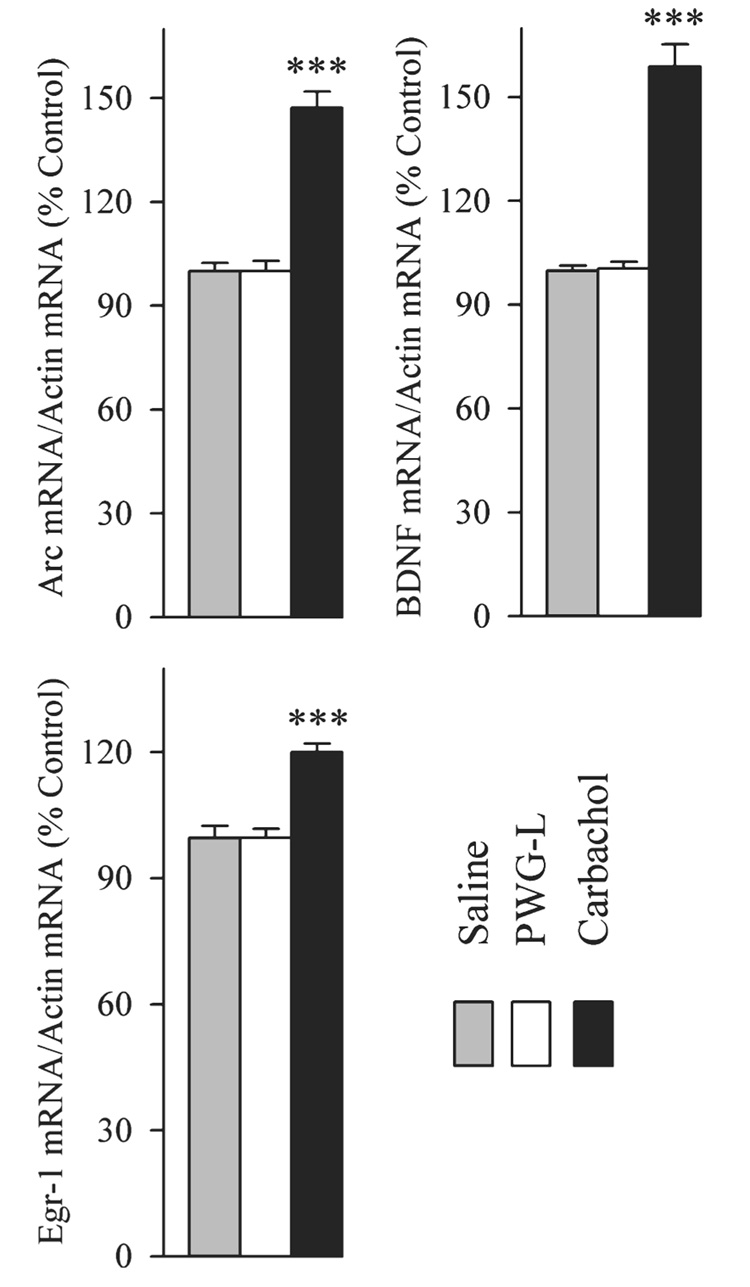
Effects of carbachol microinjection into the P-wave generator on the expression of activity-regulated cytoskeletal-associated (Arc), brain-derived nerve growth factor (BDNF) and early growth response 1 (Egr-1) mRNAs in the DH. The levels of Arc, BDNF, Egr-1 and β-actin were measured by RT-PCR. The target gene mRNA level was normalized with the β-actin mRNA level. All data are expressed as percentage of control (‘Saline’, control saline microinjection into the P-wave generator). Each bar represents the mean + SE of control (gray bars), P-wave generator-lesioned (PWG-L, introduction of microinjector into the guide tube but no injection; white bars) and carbachol-microinjected (Carbachol; black bars) groups of animals (n = 6 rats/group). Post hoc Scheffe F-test (compared with control, saline), ***P < 0.001.
Discussion
In the present study, we demonstrate that the elimination of P-wave generator cells suppressed TWAA learning training trial-induced expression of pCREB and Arc proteins, and Arc, BDNF and Egr-1 mRNAs in the DH and amygdala. Additionally, localized chemical stimulation of P-wave generator increased expression of Arc, BDNF and Egr-1 mRNAs in the DH. These findings are important because, for the first time, they provide direct evidence indicating that the involvement of P-wave-generating cells is critical for the TWAA training trial-induced expression of genes in the DH and amygdala. Because these genes in the DH and amygdala are known to participate in the memory processing and/or brain development, the following discussion is organized around the premise that the P-wave generator activation is an important physiological process for the molecular events of memory processing and/or brain development in the DH and amygdala. However, at the outset, it is important to acknowledge that molecular events induced by P-wave generator activation need to be confirmed by further research. Future studies would need to demonstrate that blocking these genes expression could also block P-wave generator activation-induced memory processing and / or brain development.
Using a variety of learning paradigms, a number of studies have suggested that the neuronal activation-induced phosphorylation of CREB is involved in the increased expression of a variety of genes and proteins in the DH and amygdala for memory consolidation (Davis & Squire, 1984; Nguyen et al., 1994; Bailey et al., 1999; Saha & Datta, 2005; Ulloor & Datta, 2005). Depending on the type of learning paradigm, CREB phosphorylation occurred in the DH, amygdala and / or frontal cortex (Moore et al., 1996; Bernabeu et al., 1997; Josselyn et al., 2001; Izquierdo et al., 2002). The results of the present study demonstrated that the exposure to TWAA training trials increased REM sleep P-wave activity and expression of pCREB and Arc proteins in the DH and amygdala. Elimination of P-waves by lesioning the P-wave generator, however, suppressed TWAA training trial-induced expression of pCREB and Arc proteins in the DH and amygdala. These results provided direct evidence that the P-wave-generating cells are involved in the expression of pCREB and Arc proteins in the DH and amygdala. We also suggest that the TWAA training trial-induced P-wave generator activation may have released glutamate in the DH and amygdala, causing the observed increase in pCREB and Arc proteins. Indeed, anatomical and physiological studies have shown that these P-wave-generating glutamatergic cells heavily project to the DH and amygdala, and activation of these cells increases glutamate release in the DH (Datta et al., 1998; Datta, 2006). Studies have also shown that the pCREB and Arc expression in the DH depends on the activation of postsynaptic N-methyl-d-aspartate (NMDA) receptors by the endogenous release of glutamate (Link et al., 1995; Lyford et al., 1995; Vianna et al., 2000; Guzowski et al., 2001; Steward & Worley, 2001a,b; Athos et al., 2002; Kelly & Deadwyler, 2003; Frankland et al., 2004; Vazdarjanova & Guzowski, 2004). Studies have shown that P-wave generator activation induced by TWAA training trials peaks at the 3-h interval, and this TWAA avoidance memory processing occurs between 1 h and 6 h (Datta, 2000; Datta et al., 2005); thus, it is also reasonable to suggest that this TWAA training trial-induced expression of pCREB and Arc in the DH and amygdala might have been caused by the homeostatic demand for TWAA memory processing. To our surprise, the results also show that 1 h after TWAA training trials, the expression of pCREB and Arc increased in the amygdala of PWG-L animals. This transient increase in the expression of pCREB and Arc in the amygdala after TWAA training trials may have been caused by a mechanism(s) independent of P-wave generator activation.
In addition to pCREB and Arc protein synthesis, our results demonstrated, for the first time, that TWAA training trials increased expression of Arc, BDNF and Egr-1 mRNAs in the DH and amygdala. Our results also demonstrated that the elimination of P-wave-generating cells suppressed TWAA training trial-induced mRNAs expression in the DH and amygdala. Additionally, the results of the present study also demonstrated, for the first time, that the activation of P-wave generator via cholinergic stimulation increased expression of Arc, BDNF and Egr-1 mRNAs in the DH. Thus, it is reasonable to suggest that this TWAA training trial-induced expression of Arc, BDNF and Egr-1 mRNAs in the DH and amygdala is mediated by the activation of the P-wave generator. Based on the results of our present study and earlier reports, it is also reasonable to suggest that the activation of brainstem P-wave generator is involved in memory processing by increasing Arc, BDNF and Egr-1 gene expression in the DH and amygdala. In accordance with our interpretation, a number of studies have already indicated that immediate-early genes, BDNF, Arc and Egr-1 in the DH and amygdala are involved in the modulation of memory processing (Kesslak et al., 1998; Tischmeyer & Grimm, 1999; Guzowski et al., 2000, 2001; Hall et al., 2000, 2001; Ribeiro et al., 2002; Mizuno et al., 2003; Malkani et al., 2004; Alonso et al., 2005; Pollak et al., 2005; Agassandian et al., 2006; Castillo et al., 2006; Huff et al., 2006). In situ hybridization revealed a rapid and selective induction of BDNF expression in the CA1 subfield of the hippocampus during hippocampus-dependent contextual learning in rats (Hall et al., 2000). Intracerebroventricular infusion of anti-BDNF antibodies resulted in an impairment of spatial learning in a water maze test in rats (Mu et al., 1999). Injection of Egr-1 antisense oligonucleotides into the hippocampus impaired reconsolidation of contextual fear conditioning memory in the rat (Lee et al., 2004).
It has been reported that after intense neuronal activation, Arc mRNA is rapidly distributed throughout the dendritic arbor where the protein is locally translated and enriched in the somatodendritic cytoplasm in response to synaptic stimulation (Link et al., 1995; Lyford et al., 1995; Steward & Worley, 2001a). It is also reported that the Arc protein mediates cytoskeletal changes underlying stimulus-induced synaptic plasticity and the stabilization of synaptic efficacy (Steward et al., 1998; Steward & Worley, 2001b; Waltereit et al., 2001; Kelly & Deadwyler, 2003). Some studies have also suggested that the development and maturation of the brain depends on the synthesis of BDNF (Pencea et al., 2001; Lee et al., 2002). A number of publications have suggested that the phasic activities occurring during REM sleep may play a critical role in the development and maturation of the brain (Roffwarg et al., 1966; Davenne & Adrien, 1984; Smith, 1995; Katz & Shatz, 1996; Datta, 1997, 2006; Garcia-Rill, 1997; Kobayashi et al., 2004; Dang-Vu et al., 2006; Guzman-Marin & McGinty, 2006). In the present study we have demonstrated that the activation of P-wave generator by exposing animals to TWAA training trials increased Arc and BDNF genes expression in the DH and amygdala. We have also demonstrated that the localized chemical stimulation of the P-wave generator increased Arc and BDNF genes expression in the DH. Thus, it is possible that the activation of the P-wave generator during REM sleep may also participate in the maturation and development of dendritic spines in the DH and amygdala. We would again acknowledge that further studies are necessary to confirm these speculative functions.
In this study, the TWAA training trials increased pCREB expression, but the levels of total CREB remain unchanged. It is known that the activation of different neurotransmitter’s receptors, including glutamate receptors, phosphorylate CREB protein and increase Arc synthesis by activating intracellular protein kinase A (PKA), Ca2+ / calmodulin-dependent protein kinase II (CaMKII) and mitogen-activated protein kinase (MAPK; Borrelli et al., 1992; Nestler & Greengard, 1994; Duman, 1995; Impey et al., 1999; Soderling, 1999; Sabban & Kvetnansky, 2001; Waltereit et al., 2001; Pandey et al., 2006). Therefore, it is possible that the P-wave generator activation-induced increase in pCREB expression in the DH and amygdala may be due to glutamate receptor-mediated activation of intracellular PKA, CaMKII and/or MAPK. A number of studies have suggested that CREB could be phosphorylated (pCREB) by various physiological stimuli to regulate the expression of several downstream cAMP-inducible genes, including Arc, BDNF and Egr-1 (Shieh et al., 1998; Mayr & Montminy, 2001; Lonze & Ginty, 2002; Ying et al., 2002; McClung & Nester, 2003; Morinobo et al., 2003; Duman, 2004; Johannessen et al., 2004; Kreibich & Blendy, 2004; Pandey et al., 2006; Han et al., 2006; Sou et al., 2006). Thus, it is possible that the expression of the Arc, BDNF and Egr-1 genes may be due to the P-wave generator activation-induced phosphorylation of CREB (pCREB). We again acknowledge, however, that a future study is needed to confirm this tentative interpretation.
In conclusion, our findings provide direct evidence to indicate that the activation of the P-wave-generating cells is capable of triggering a series of cellular and molecular events in the DH and amygdala. These findings also support our hypothesis that the activation of P-wave-generating cells may provide a glutamatergic-activating stimulus to the hippocampus and amygdala for the neuronal activation-dependent gene expression and protein synthesis that are necessary for the cognitive and neurogenesis functions of the hippocampus and amygdala.
Acknowledgements
We thank Jan K. Blusztajn, Barbara E. Slack and Tiffany Mellott for their help and advice on the Western blotting, John N. Flanagan for his technical help and advice on the RT-PCR. We thank R. R. MacLean, S. Saha and Jessica L. Shea for their technical assistance. This study was supported by NIH research grants NS 34004 and MH 59839.
Abbreviations
- Arc
activity-regulated cytoskeletal-associated protein
- BDNF
brain-derived nerve growth factor
- CaMKII
Ca2+ / calmodulin-dependent protein kinase II
- CP
animals received carbachol microinjection into the P-wave generator
- CREB
cAMP response element-binding protein
- CS
conditioned stimulus
- DH
dorsal hippocampus
- EEG
electroencephalogram; Egr-1, early growth response 1
- EMG
electromyogram
- MAPK
mitogen-activated protein kinase
- mRNA
messenger ribonucleic acid
- PCR
polymerase chain reaction
- pCREB
phosphorylated CREB
- PKA
protein kinase A
- P-wave
pontine-wave
- PWG-L
P-wave generator-lesioned animals
- REM
rapid eye movement
- RT
reverse transcription
- SDS
sodium dodecyl sulfate
- S-L
sham-lesioned animals
- SP
animals received saline microinjection into the P-wave generator
- SWS
slow-wave sleep
- TWAA
two-way active avoidance
- UCS
unconditioned stimulus
- W
wakefulness
References
- Agassandian K, Gedney M, Cassel MD. Neurotrophic factors in the central nucleus of amygdala may be organized to provide substrates for associative learning. Brain Res. 2006;1076:780–786. doi: 10.1016/j.brainres.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Alonso M, Bekinschtein P, Cammarota M, Vianna MR, Izquierdo I, Medina JH. Endogenous BDNF is required for long-term memory formation in the rat parietal cortex. Learn. Mem. 2005;12:504–510. doi: 10.1101/lm.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athos J, Impey S, Pineda VV, Chen X, Storm DR. Hippocampal CRE-mediated gene expression is required for contextual memory formation. Nat. Neurosci. 2002;5:1119–1120. doi: 10.1038/nn951. [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Kim JJ, Sun W, Thompson RF, Helmstetter FJ. Acquisition of fear conditioning in rats requires the synthesis of mRNA in the amygdala. Behav. Neurosci. 1999;11:276–282. doi: 10.1037//0735-7044.113.2.276. [DOI] [PubMed] [Google Scholar]
- Bernabeu R, Bevilaqua L, Ardenghi P, Bromberg E, Schmitz P, Bianchin M, Izquierdo I, Medina JH. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc. Natl Acad. Sci. USA. 1997;94:7041–7046. doi: 10.1073/pnas.94.13.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzi E, Brooks DC. Pontine reticular formation: relation to lateral geniculate nucleus during deep sleep. Science. 1963;141:270–272. doi: 10.1126/science.141.3577.270. [DOI] [PubMed] [Google Scholar]
- Borrelli E, Montmayeur JP, Foulkes NS, Sassone-Corsi P. Signal transduction and gene control: the cAMP pathway. Crit. Rev. Oncog. 1992;3:321–338. [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive methods for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Castillo DV, Figueroa-Guzman Y, Escobar ML. Brain-derived neurotrophic factor enhances conditioned taste aversion retention. Brain Res. 2006;1067:250–255. doi: 10.1016/j.brainres.2005.10.085. [DOI] [PubMed] [Google Scholar]
- Dang-Vu TT, Desseilles M, Peigneux P, Maquet P. A role for sleep in brain plasticity. Pediatr. Rehabil. 2006;9:98–118. doi: 10.1080/13638490500138702. [DOI] [PubMed] [Google Scholar]
- Datta S. Cellular basis of pontine ponto-geniculo-occipital wave generation and modulation. Cell Mol. Neurobiol. 1997;17:341–365. doi: 10.1023/a:1026398402985. [DOI] [PubMed] [Google Scholar]
- Datta S. Avoidance task training potentiates phasic pontine-wave density in the rat: a mechanism for sleep-dependent plasticity. J. Neurosci. 2000;20:8607–8613. doi: 10.1523/JNEUROSCI.20-22-08607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S. Activation of phasic pontine-wave generator: a mechanism for sleep-dependent memory processing. Sleep Biol. Rhyth. 2006;4:16–26. [Google Scholar]
- Datta S, Calvo JM, Quattrochi J, Hobson JA. Cholinergic microinjection of the peribrachial nucleus in the cat. I. Immediate and prolonged increases in ponto-geniculo-occipital waves. Arch. Ital. Biol. 1992;130:263–284. [PubMed] [Google Scholar]
- Datta S, Hobson JA. Neuronal activity in the caudolateral peribrachial pons: relationship to PGO waves and rapid eye movements. J. Neurophysiol. 1994;71:95–109. doi: 10.1152/jn.1994.71.1.95. [DOI] [PubMed] [Google Scholar]
- Datta S, Hobson JA. Suppression of ponto-geniculo-occipital waves by neurotoxic lesions of pontine caudo-lateral peribrachial cells. Neuroscience. 1995;67:703–712. doi: 10.1016/0306-4522(95)00081-s. [DOI] [PubMed] [Google Scholar]
- Datta S, MacLean RR. Neurobiological mechanisms for the regulation of mammalian sleep-wake behavior: reinterpretation of historical evidence and inclusion of contemporary cellular and molecular evidence. Neurosci. Biobehav. Rev. 2007;31:775–824. doi: 10.1016/j.neubiorev.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Mavanji V, Ulloor J, Patterson EH. Activation of phasic pontine-wave generator prevents rapid eye movement sleep deprivation-induced learning impairment in the rat: a mechanism for sleep-dependent plasticity. J. Neurosci. 2004;24:1416–1427. doi: 10.1523/JNEUROSCI.4111-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Saha S, Prutzman SL, Mullins OJ, Mavanji V. Pontine-wave generator activation-dependent memory processing of avoidance learning involves the dorsal hippocampus in the rat. J. Neurosci. Res. 2005;80:727–737. doi: 10.1002/jnr.20501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Siwek DF, Patterson EH, Cipolloni PB. Localization of pontine PGO wave generation sites and their anatomical projections in the rat. Synapse. 1998;30:409–423. doi: 10.1002/(SICI)1098-2396(199812)30:4<409::AID-SYN8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Davenne D, Adrien J. Suppression of PGO waves in the kitten: anatomical effects on the lateral geniculate nucleus. Neurosci. Lett. 1984;45:33–38. doi: 10.1016/0304-3940(84)90325-2. [DOI] [PubMed] [Google Scholar]
- Davis HP, Squire LR. Protein synthesis and memory: a review. Psycho. Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- Duman RS. Regulation of intracellular signal transduction and gene expression by stress. In: Friedman MJ, Charney DS, Deutch AY, editors. Neurobiology and Clinical Consequences of Stress. Philadelphia: Lippencott-Raven; 1995. pp. 27–43. [Google Scholar]
- Duman RS. Role of neurotropic factors in the etiology and treatment of mood disorders. Neuromol. Med. 2004;5:11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- Fishbein W. Disruptive effects of rapid eye movement sleep deprivation on long-term memory. Physiol. Behav. 1971;6:270–282. doi: 10.1016/0031-9384(71)90155-7. [DOI] [PubMed] [Google Scholar]
- Fishbein W, Kastaniotis C, Chattman D. Paradoxical sleep: prolonged augmentation following learning. Brain Res. 1974;79:61–75. doi: 10.1016/0006-8993(74)90566-6. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Josselyn SA, Anagnostaras SG, Kogan JH, Takahashi E, Silva AJ. Consolidation of CS and US representations in associative fear conditioning. Hippocampus. 2004;14:557–569. doi: 10.1002/hipo.10208. [DOI] [PubMed] [Google Scholar]
- Galani R, Obis S, Coutureau E, Jarrard L, Cassel JCA. Comparison of the effects of fimbria-fornix, hippocampal, or entorhinal cortex lesions on spatial reference and working memory in rats: short versus long postsurgical recovery period. Neurobiol. Learn. Mem. 2002;77:1–16. doi: 10.1006/nlme.2000.3998. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E. Disorders of the reticular activating system. Med. Hypoth. 1997;49:379–387. doi: 10.1016/s0306-9877(97)90083-9. [DOI] [PubMed] [Google Scholar]
- Gottesmann C. Etude sur les activités électrophysiologiques phasiques chezle Rat. Physiol. Behav. 1969;4:495–504. [Google Scholar]
- Guzman-Marin R, McGinty D. Sleep deprivation suppresses adult neurogenesis: clues to the role of sleep in brain plasticity. Sleep Biol. Rhyth. 2006;4:27–34. [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J. Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J. Neurosci. 2001;21:5089–5098. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat. Neurosci. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Cellular imaging of zif 268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: selective activation of hippocampal CA1 neurons during the recall of contextual memories. J. Neurosci. 2001;21:2186–2193. doi: 10.1523/JNEUROSCI.21-06-02186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M, Bolanos C, Green T, Olson V, Neve R, Liu R, Aghajanian G, Nestler E. Role of cAMP response element-binding protein in the rat locus ceruleus: regulation of neuronal activity and opiate withdrawal behaviors. J. Neurosci. 2006;26:4624–4629. doi: 10.1523/JNEUROSCI.4701-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennevin E, Hars B, Maho C, Bloch V. Processing of learned information in paradoxical sleep: relevance for memory. Behav. Brain Res. 1995;69:125–135. doi: 10.1016/0166-4328(95)00013-j. [DOI] [PubMed] [Google Scholar]
- Huff NC, Frank M, Wright-Hardesty K, Sprunger D, Matus-Amat P, Higgins E, Rudy JW. Amygdala regulation of immediate-early gene expression in the hippocampus induced by contextual fear conditioning. J. Neurosci. 2006;26:1616–1623. doi: 10.1523/JNEUROSCI.4964-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Storm D. Making new connections: role of ERK/MAP kinase signaling in neuronal plasticity. Neuron. 1999;23:11–14. doi: 10.1016/s0896-6273(00)80747-3. [DOI] [PubMed] [Google Scholar]
- Izquierdo LA, Barros DM, Vianna MR, Coitinho A, deDavid eSilva T, Choi H, Moletta B, Medina JH, Izquierdo I. Molecular pharmacological dissection of short- and long-term memory. Cell. Mol. Neurobiol. 2002;22:269–287. doi: 10.1023/a:1020715800956. [DOI] [PubMed] [Google Scholar]
- Johannessen M, Delghandi MP, Moens U. What turns CREB on? Cell Signal. 2004;16:1211–1227. doi: 10.1016/j.cellsig.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Josselyn SA, Shi C, Carlezon WA, Neve RL, Nestler EJ, Davis M. Long-term memory is facilitated by cAMP response element-binding protein overexpression in the amygdala. J. Neurosci. 2001;21:2404–2412. doi: 10.1523/JNEUROSCI.21-07-02404.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvet M. Paradoxical sleep - a study of its nature and mechanisms. Prog. Brain Res. 1965;18:20–62. doi: 10.1016/s0079-6123(08)63582-7. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Karashima A, Nakamura K, Sato N, Nakao M, Katayama N, Yamamoto M. Phase-locking of spontaneous and elicited ponto-geniculo-occipital waves is associated with acceleration of hippocampal theta waves during rapid eye movement sleep in cats. Brain Res. 2002;958:347–358. doi: 10.1016/s0006-8993(02)03673-9. [DOI] [PubMed] [Google Scholar]
- Karashima A, Nakao M, Honda K, Iwasaki N, Katayama N, Yamamoto M. Theta wave amplitude and frequency are differentially correlated with pontine waves and rapid eye movements during REM sleep in rats. Neurosci. Res. 2004;50:283–289. doi: 10.1016/j.neures.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kelly MP, Deadwyler SA. Experience-dependent regulation of the immediate-early gene Arc differs across brain regions. J. Neurosci. 2003;23:6443–6451. doi: 10.1523/JNEUROSCI.23-16-06443.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesslak JP, So V, Choi J, Cotman CW, Gomez-Pinilla F. Learning upregulates brain-derived neurotrophic factor messenger ribonucleic acid: a mechanism to facilitate encoding and circuit maintenance? Behav. Neurosci. 1998;112:1012–1019. doi: 10.1037//0735-7044.112.4.1012. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Good C, Biedermann J, Barnes C, Skinner RD, Garcia-Rill E. Developmental changes in pedunculopontine nucleus neurons. J. Neurophysiol. 2004;91:1470–1481. doi: 10.1152/jn.01024.2003. [DOI] [PubMed] [Google Scholar]
- Kreibich A, Blendy J. cAMP response element-binding protein is required for stress but not cocaine-induced reinstatement. J. Neurosci. 2004;24:6686–6692. doi: 10.1523/JNEUROSCI.1706-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leconte P, Hennevin E. Post-learning paradoxical sleep, reticular activation, and noradrenergic activity. Physiol. Behav. 1981;26:587–594. doi: 10.1016/0031-9384(81)90129-3. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J. Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Lee JL, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc. Natl Acad. Sci. USA. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- Malkani S, Wallace KJ, Donley MP, Rosen JB. An egr-1 (zif268) antisense oligodeoxynucleotide infused into the amygdale disrupts fear conditioning. Learn. Mem. 2004;11:617–624. doi: 10.1101/lm.73104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–1051. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- Marks GA, Farber J, Rubinstein M, Roffwarg HP. Demonstration of ponto-geniculo-occipital waves in the albino rat. Exp. Neurol. 1980;69:648–666. doi: 10.1016/0014-4886(80)90058-8. [DOI] [PubMed] [Google Scholar]
- Mavanji V, Datta S. Activation of the phasic pontine-wave generator enhances improvement of learning performance: a mechanism for sleep-dependent plasticity. Eur. J. Neurosci. 2003;17:359–730. doi: 10.1046/j.1460-9568.2003.02460.x. [DOI] [PubMed] [Google Scholar]
- Mavanji V, Ulloor J, Saha S, Datta S. Neurotoxic lesions of phasic pontine-wave generator cells impair retention of 2-way active avoidance memory. Sleep. 2004;27:1282–1292. doi: 10.1093/sleep/27.7.1282. [DOI] [PubMed] [Google Scholar]
- Mayr B, Montminy M. Transcription regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell. Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- McClung CA, Nester EJ. Regulation of gene expression and cocaine reward by CREB and Delta FosB. Neuroscience. 2003;6:1208–1215. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, He J, Nakajima A, Nabeshima T. Involvement of BDNF receptor TrkB in spatial memory formation. Learn. Mem. 2003;10:108–115. doi: 10.1101/lm.56003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AN, Waxham MN, Dash PK. Neuronal activity increases the phosphorylation of the transcription factor cAMP response element-binding protein (CREB) in rat hippocampus and cortex. J. Biol. Chem. 1996;271:14214–14220. doi: 10.1074/jbc.271.24.14214. [DOI] [PubMed] [Google Scholar]
- Morinobo S, Fujimaki K, Kawano K, Tanaka K, Takahashi J, Ohkawa M, Yamawaki S, Kata N. Influence of immobilization stress on the expression and phosphatase activity of protein phosphatase 2A in the rat brain. Biol. Pyschiat. 2003;54:1060–1066. doi: 10.1016/s0006-3223(03)00417-7. [DOI] [PubMed] [Google Scholar]
- Morrison AR, Bowker RM. The biological significance of PGO spikes in the sleeping cat. Acta Neurobiol. Exp. (Wars.) 1975;35:821–840. [PubMed] [Google Scholar]
- Moser MB, Moser EI, Forrest E, Andersen P, Morris RGM. Spatial learning with a minislab in the dorsal hippocampus. Proc. Natl Acad. Sci. USA. 1995;92:9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu JS, Li WP, Yao ZB, Zhou XF. Deprivation of endogenous brain-derived neurotrophic factor results in impairment of spatial learning and memory in adult rats. Brain Res. 1999;835:259–265. doi: 10.1016/s0006-8993(99)01592-9. [DOI] [PubMed] [Google Scholar]
- National Institute of Health. Guide for the Care and Use of Laboratory Animals. Washington D.C.: National Academy Press; 1996. http://www.nap.edu/readingroom/books/labrats/ [Google Scholar]
- Nestler EJ, Greengard P. Protein phosphorylation and the regulation of neuronal function. In: Siegel GJ, Agranoff BW, Albers RW, Molinoff PB, editors. Basic Neurochemistry: Molecular, Cellular, and Medical Aspects. Boston: Little Brown; 1994. pp. 449–474. [Google Scholar]
- Nguyen PV, Abel T, Kandel ER. Requirement of a critical period of transcription for induction of a late phase of LTP. Science. 1994;265:1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Hobson JA. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat. Rev. Neurosci. 2002;3:591–605. doi: 10.1038/nrn895. [DOI] [PubMed] [Google Scholar]
- Pandey S, Zhang H, Roy A, Misra K. Central and medial amygdaloid brain derived neurotrophic factor signaling plays a critical role in alcohol-drinking and anxiety-like behaviors. J. Neurosci. 2006;6:8320–8331. doi: 10.1523/JNEUROSCI.4988-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides C, Rebeiro S. Recent evidence of memory processing in sleep. In: Maquet P, Smith C, Stickgold R, editors. Sleep and Brain Plasticity. Oxford: Oxford University Press; 2003. pp. 327–362. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Pencea V, Bingaman KD, Wiegand SL, Lushkin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J. Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak DD, Herkner K, Hoeger H, Lubec G. Behavioral testing upregulates pCaMKII, BDNF, PSD-95 and egr-1 in hippocampus of FVB/N mice. Behav. Brain Res. 2005;163:128–135. doi: 10.1016/j.bbr.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Ribeiro S, Mello CV, Velho T, Gardner TJ, Jarvis ED, Pavlides C. Induction of hippocampal long-term potentiation during waking leads to increased extrahippocampal zif-268 expression during ensuing rapid-eye-movement sleep. J. Neurosci. 2002;22:10914–10923. doi: 10.1523/JNEUROSCI.22-24-10914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152:604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- Sabban EL, Kvetnansky R. Stress-triggered activation of gene expression in catecholaminergic systems: dynamics of transcriptional events. Trends Neurosci. 2001;24:91–98. doi: 10.1016/s0166-2236(00)01687-8. [DOI] [PubMed] [Google Scholar]
- Saha S, Datta S. Two-way active avoidance training-specific increases in phosphorylated cAMP response element-binding protein in the dorsal hippocampus, amygdale, and hypothalamus. Eur. J. Neurosci. 2005;21:3403–3414. doi: 10.1111/j.1460-9568.2005.04166.x. [DOI] [PubMed] [Google Scholar]
- Scharf MT, Woo NH, Lattal KM, Young JZ, Nguyen PV, Abel T. Protein synthesis is required for the enhancement of long-term potentiation and long-term memory by spaced training. J. Neurophysiol. 2002;87:2770–2777. doi: 10.1152/jn.2002.87.6.2770. [DOI] [PubMed] [Google Scholar]
- Shieh PB, Hu SC, Bobb K, Timmusk T, Ghosh A. Identification of signaling pathway involved in calcium regulation of BDNF expression. Neuron. 1998;20:727–740. doi: 10.1016/s0896-6273(00)81011-9. [DOI] [PubMed] [Google Scholar]
- Smith C. Sleep states and memory processes. Behav. Brain Res. 1995;69:137–145. doi: 10.1016/0166-4328(95)00024-n. [DOI] [PubMed] [Google Scholar]
- Smith C, Rose GM. Posttraining paradoxical sleep in rats is increased after spatial learning in the Morris water maze. Behav. Neurosci. 1997;111:1197–1204. doi: 10.1037//0735-7044.111.6.1197. [DOI] [PubMed] [Google Scholar]
- Smith C, Tenn C, Annett R. Some biochemical and behavioral aspects of the paradoxical sleep window. Can. J. Psychol. 1991;45:115–124. doi: 10.1037/h0084279. [DOI] [PubMed] [Google Scholar]
- Soderling TR. The Ca2+-calmodulin-dependent protein kinase cascade. Trends Biochem. Sci. 1999;24:232–236. doi: 10.1016/s0968-0004(99)01383-3. [DOI] [PubMed] [Google Scholar]
- Sou S, Kimura Y, VanTol H. Starvation induces cAMP response element-binding protein-dependent gene expression through octopamine-Gq signaling in Caenorhabditis elegans. J. Neurosci. 2006;26:10082–10090. doi: 10.1523/JNEUROSCI.0819-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Steward O, Worley PF. A cellular mechanism for targeting newly synthesized mRNAs to synaptic sites on dendrites. Proc. Natl Acad. Sci. USA. 2001a;98:7062–7068. doi: 10.1073/pnas.131146398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Worley PF. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron. 2001b;30:227–240. doi: 10.1016/s0896-6273(01)00275-6. [DOI] [PubMed] [Google Scholar]
- Stickgold R. Sleep: off-line memory processing. Trends Cogn. Sci. 1998;2:484–492. doi: 10.1016/s1364-6613(98)01258-3. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Walker MP. Sleep-dependent memory consolidation and reconsolidation. Sleep Med. 2007;8:331–343. doi: 10.1016/j.sleep.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischmeyer W, Grimm R. Activation of immediate early genes and memory formation. Cell. Mol. Life Sci. 1999;55:564–574. doi: 10.1007/s000180050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloor J, Datta S. Spatio-temporal activation of cyclic AMP response element-binding protein, activity-regulated cytoskeletal-associated protein and brain-derived nerve growth factor: a mechanism for pontine-wave generator activation-dependent two-way active-avoidance memory processing in the rat. J. Neurochem. 2005;95:418–428. doi: 10.1111/j.1471-4159.2005.03378.x. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, Guzowski JF. Difference in hippocampal neuronal population responses to modifications of an environmental context: evidence for distinct, yet complementary, functions of CA and CA1 ensembles. J. Neurosci. 2004;24:6489–6496. doi: 10.1523/JNEUROSCI.0350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna MR, Alonso M, Viola H, Quevedo J, de Paris F, Furman M, de Stein ML, Medina JH, Izquierdo I. Role of hippocampal signaling pathways in long-term memory formation of a nonassociative learning task in the rat. Learn. Mem. 2000;7:333–340. doi: 10.1101/lm.34600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltereit R, Dammermann B, Wulff P, Scafidi J, Staubli U, Kauselmann G, Bundman M, Kuhl D. Arg3.1/Arc mRNA induction by Ca2+ and cAMP requires protein kinase A and mitogen-activated protein kinase/extracellular regulated kinase activation. J. Neurosci. 2001;21:5484–5493. doi: 10.1523/JNEUROSCI.21-15-05484.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying S, Futter M, Rosenblum K, Webber M, Hunt S, Bliss T, Bramham C. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J. Neurosci. 2002;22:1532–1540. doi: 10.1523/JNEUROSCI.22-05-01532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


