Summary
Social hierarchies guide behavior in many species, including humans, where status also has an enormous impact on motivation and health. However, little is known about the underlying neural representation of social hierarchies in humans. In the present study, we identify dissociable neural responses to perceived social rank using functional magnetic resonance imaging (fMRI) in an interactive simulated social context. In both stable and unstable social hierarchies, viewing a superior individual differentially engaged perceptual-attentional, saliency, and cognitive systems, notably dorsolateral prefrontal cortex. In the unstable hierarchy setting, additional regions were recruited related to emotional processing (amygdala), social cognition (medial prefrontal cortex), and behavioral readiness. Furthermore, social hierarchical consequences of performance were neurally dissociable and of comparable salience to monetary reward, providing a neural basis for the high motivational value of status. Our results identify neural mechanisms that may mediate the enormous influence of social status on human behavior and health.
INTRODUCTION
Human social hierarchies are prominent in different domestic, work, and recreational settings, where they define implicit expectations and action dispositions that drive appropriate social behavior (Cummins, 2000). Furthermore, in humans, social status strongly predicts well-being, morbidity, and even survival (Boyce, 2004; Sapolsky, 2004, 2005). Festinger’s long-standing, prominent theory of social comparison processes (Festinger, 1954) suggests an important role for hierarchical rank in achieving accurate self-knowledge and self-improvement, particularly in the usage of upward social comparisons (Wheeler, 1966). Social hierarchies spontaneously and stably emerge in children as young as 2 years (Boyce, 2004; Cummins, 2000). Status within a social hierarchy is often made explicit (e.g., uniforms, honorifics, verbal assignment, or in some languages even through status-specific grammar (Pork, 1991)) but can also be inferred from cues such as facial features, height, gender, age, and dress (Karafin et al., 2004). In humans, dominance has been linked to heritable personality traits (Mehrabian, 1996); furthermore, superior status interacts with multiple neurotransmitter (Moskowitz et al., 2001) and neuroendocrine (Sapolsky, 2005) systems and can be automatically and efficiently inferred (Moors and De Houwer, 2005), indicating the existence of biological systems that process social rank information; yet virtually nothing is known about the neural representations of social hierarchies in humans.
We used functional magnetic resonance imaging (fMRI) to investigate the neural mechanisms that process social superiority and inferiority in humans. As human beings, social hierarchies can be established along various dimensions; we can be ranked according to ability or skill, as well as economic, physical, and professional standings. Here, in two experiments, we created an explicit and strongly reinforced social hierarchy based on incidental skill in the context of an interactive game (Figure 1). Participants performed a simple task for monetary reward simultaneously with one of two other players, alternatively, represented by photographs. Covertly, outcomes were fixed, and the two other players were simulated; behavioral measures (Figures S1 and S2), however, confirmed that participants strongly engaged in this virtual social interaction. Just prior to the scanning session, in an initial test run, a social hierarchy was created by identifying the performance of one other player as better (“three star player”) and one other player as worse (“one star player”) than the participant (“two star player”). The star system, inspired by military rank symbols, continually reinforced the hierarchy by being displayed throughout the session. Implicit cues related to social superiority (e.g., age, gender, race, facial expression) were controlled. Importantly, participants played simultaneously with the other [simulated] players, but they did not play against each other. As such, outcomes and reward did not depend on the other player, who remained entirely inconsequential to the performed task and could have been completely ignored by a “rational” participant. The explicitly non-competitive nature of the game ensured that the hierarchical status of the other player has no real or perceived impact on reward expectancy and task difficulty. Despite the game being non-competitive with the other players, participants were strongly engaged in the hierarchical context, as evident by post-session questionnaire data (Figures S1 and S2).
Figure 1.
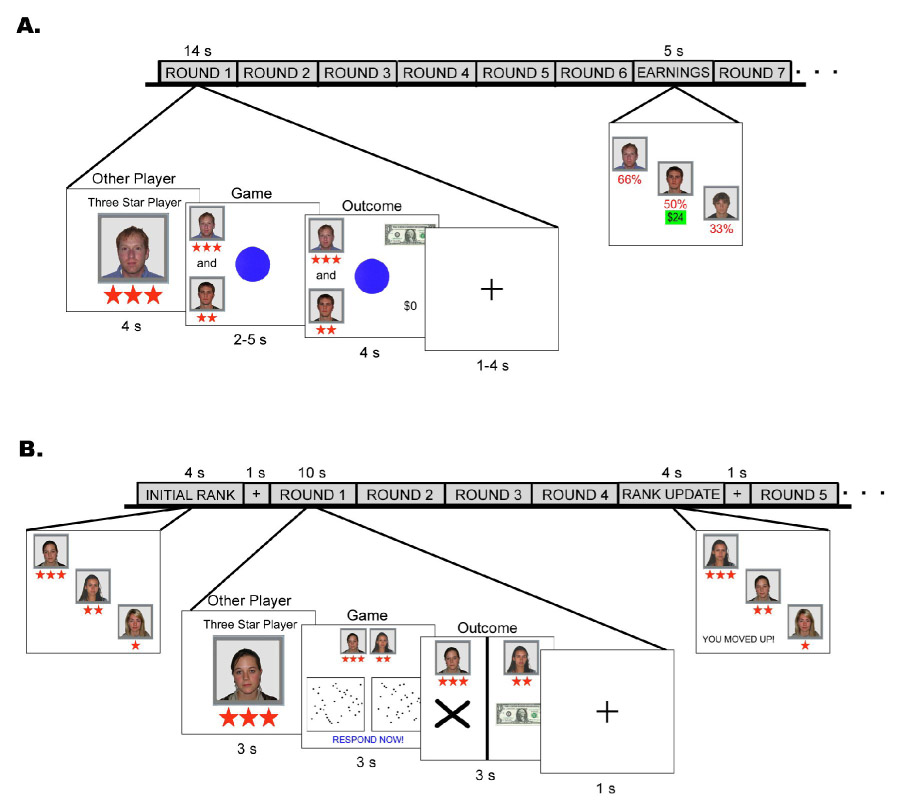
Schematic diagram of the experimental design. Each round in the tasks consisted of three phases: viewing the other player, playing the game, and viewing the outcomes. (A) In Experiment #1 (stable hierarchy), during the game phase participants pressed a button as soon as the blue circle changed to green. The initial hierarchical rankings did not change throughout the session. (B) In Experiment #2 (unstable hierarchy), during the game phase participants pressed a button to indicate which box contained more dots. The hierarchical rankings were updated throughout the session based on performance.
In the first experiment (Figure 1A), we established a stable hierarchy, i.e., social rank positions were explicitly fixed initially and did not change throughout the experiment. We predicted differential neural responses related to processing the relative status of the other players. In a second experiment (Figure 1B), we created an unstable hierarchy setting by occasionally updating players’ positions in the social hierarchy based on performance throughout the session. We expected to replicate our previous results from Experiment #1 regarding the neural representation of social status, but focused our primary interest on brain regions differentially active only in an unstable hierarchy setting. Moreover, we would now be able to examine the neural processing of outcomes that have a potential impact on relative social status. Finally, we examined the social specificity of our results through a nonsocial version of this experimental paradigm in which the [simulated] human players were replaced with computers.
RESULTS
Experiment #1: Stable hierarchy
In the first experiment, the fMRI analysis revealed several brain regions differential activated by viewing another individual of a particular relative status. Specifically, activity in the bilateral occipital/parietal cortex, ventral striatum, parahippocampal cortex, and dorsal lateral prefrontal cortex (DLPFC) was significantly (P < 0.005, FDR-corrected) greater when viewing the more superior player compared to viewing the more inferior player (“superior player > inferior player”) relative to the participant in the interactive game (Figure 2 and Table 1). No brain regions were significantly (P < 0.05; FDR-corrected) more activated by viewing the inferior player compared to viewing the superior player (“inferior player > superior player”); while the aforementioned brain regions were significantly activated by viewing an inferior player relative to the implicit baseline (i.e., that part of measured BOLD response not accounted for by the modeled task-related activity), this activation was less than that for superior players (Figure 2B).
Figure 2.
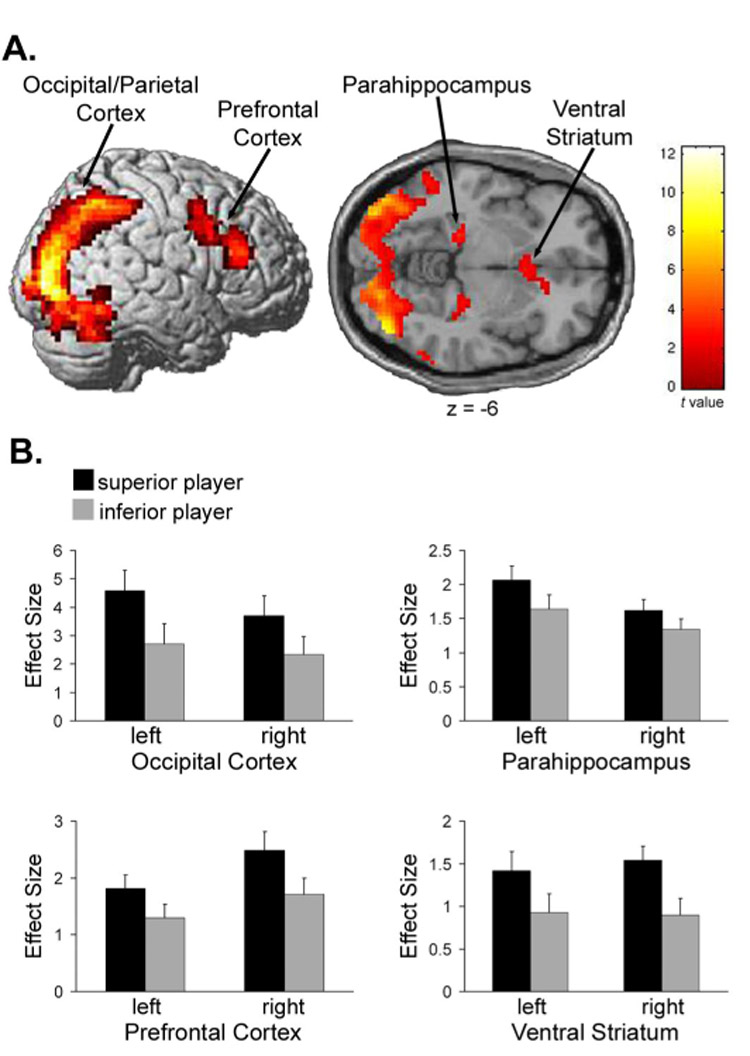
Significant activations for the contrast, “superior player > inferior player” in Experiment #1 (stable hierarchy). Displayed are (A) significant (P < 0.005, FDR-corrected) activations in occipital/parietal cortex [−24, −96, 9; 42, −81, −6], dorsal lateral prefrontal cortex [−36, 3, 42; 42, 30, 21], parahippocampal cortex [−21, −27, −9; 27, −24, −12], and ventral striatum [−3, 15, −6; 6, 18, −3], and (B) plots of the effect sizes (parameter estimates) when viewing the superior and inferior other player, extracted from the peak voxels in each activated region.
Table 1.
Significantly activated (P < 0.005, FDR corrected) brain regions for the contrast, “superior player > inferior player”
| Brain Regions | BA | Cluster Size (voxels) | Peak MNI Coordinates(x,y,z) | Peak Z Score | ||
|---|---|---|---|---|---|---|
| STABLE HIERARCHY: Experiment #1 | ||||||
| R inferior parietal gyrus, incl. | 7/40 | 1341‡ | 36, | −57, | 48 | 6.76 |
| R inferior/middle occipital gyrus | 18/19 | 42, | −81, | −6 | 6.16 | |
| L inferior/middle occipital gyrus | 18/19 | 709‡ | −24, | −96, | 9 | 6.31 |
| R precuneus | 7 | 59‡ | 6, | −57, | 39 | 4.54 |
| R inferior/middle frontal gyrus (DLPFC) | 9/46 | 465 | 42, | 30, | 21 | 4.50 |
| L inferior/middle frontal gyrus (DLPFC) | 9 | 133 | −36, | 3, | 42 | 4.48 |
| R parahippocampal gyrus | 70 | 27, | −24, | −12 | 3.71 | |
| L parahippocampal gyrus | 53 | −21, | −27, | −9 | 4.14 | |
| R ventral striatum, incl. | 93 | 6, | 18, | −3 | 3.99 | |
| L ventral striatum | −3, | 15, | −6 | 3.72 | ||
| L middle temporal gyrus | 21 | 52 | −57, | −51, | −6 | 3.95 |
| UNSTABLE HIERARCHY: Experiment #2 | ||||||
| R inferior/middle occipital gyrus, incl. | 18/19 | 847‡ | 36, | −93, | 3 | 7.03 |
| L inferior/middle occipital gyrus | 18/19 | −27, | −93, | 6 | 5.83 | |
| R inferior frontal gyrus (DLPFC) | 9 | 54‡ | 45, | 9, | 27 | 5.46 |
| R thalamus, incl. | 207‡ | 6, | −18, | 6 | 5.34 | |
| L thalamus | −6, | −18, | 15 | 4.68 | ||
| R parahippocampal gyrus | 81‡ | 27, | −21, | −15 | 5.23 | |
| L parahippocampal gyrus | 77‡ | −24, | −27, | −12 | 5.01 | |
| L precentral gyrus, incl. | 4/6 | 61‡ | −39, | −21, | 66 | 5.15 |
| L postcentral gyrus | 1/2/3 | −51, | −24, | 57 | 4.59 | |
| R ventral striatum | 53‡ | 9, | 9, | −3 | 4.78 | |
| L fusiform | 37 | 24‡ | −27, | −63, | −18 | 4.81 |
| R fusiform | 37 | 23‡ | 45, | −48, | −18 | 4.57 |
| R amygdala | 26† | 24, | −3, | −21 | 4.34 | |
| posterior cingulate | 23/29 | 31† | 3, | −42, | 21 | 4.25 |
| medial prefrontal cortex | 9/10 | 58, | 6, | 60 | 30 | 3.78 |
| R superior parietal lobule | 7/40 | 7 | 39, | −51, | 66 | 3.63 |
| supplementary motor area | 6 | 64 | 3, | −27, | 60 | 3.61 |
| L posterior insula | 13 | 61 | −45, | −18, | 12 | 3.57 |
| R precuneus | 7 | 26 | 9, | −60, | 66 | 3.50 |
cluster defined using P < 0.001, FDR corrected.
cluster defined using P < 0.0005, FDR corrected.
L = left hemisphere. R = right hemisphere. MNI = Montreal Neurological Institute. BA = Brodmann area. DLPFC = dorsolateral prefrontal cortex. Bold indicates regions uniquely activated in Experiment #2.
Experiment #2: Unstable hierarchy
The fMRI results from Experiment #1 were replicated in Experiment #2. As when the hierarchy was stable, no brain regions were significantly (P < 0.05; FDR-corrected) more activated by viewing the inferior compared to the superior player (“inferior player > superior player”) in the unstable hierarchy setting; however, brain activity when viewing a more superior player compared to viewing a more inferior player (“superior player > inferior player”) in the unstable hierarchy setting was again significantly greater in occipital/parietal cortex, ventral striatum, parahippocampal cortex, and DLPFC (Figure 3, Figure S3, and Table 1).
Figure 3.
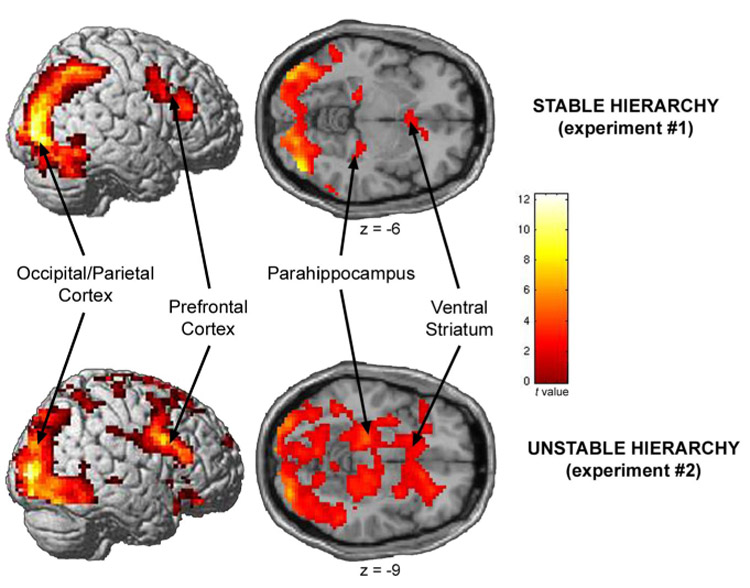
Significant activations for the contrast, “superior player > inferior player”, consistently observed in both Experiment #1 (top) and Experiment #2 (bottom). Significant (P < 0.005, FDR-corrected) activations were observed in occipital/parietal cortex (Experiment #1: [−24, −96, 9; 42, −81, −6]; Experiment #2: [−27, −93, 6; 36, −93, 3]), 35 dorsal lateral prefrontal cortex (Experiment #1: [−36, 3, 42; 42, 30, 21]; Experiment #2: [45, 9, 27]), parahippocampal cortex (Experiment #1: [−21, −27, −9; 27, −24, −12]; Experiment #2: [−24, −27, −12; 27, −21, −15]), and ventral striatum (Experiment #1: [−3, 15, −6; 6, 18, −3]; Experiment #2: [9, 9, −3]).
In addition, several brain areas were uniquely recruited in the unstable hierarchy setting (Table 1, bold text). When viewing a superior player compared to inferior, significant (P < 0.005; FDR-corrected) activations were also found in the bilateral thalamus, right amygdala, posterior cingulate, medial prefrontal cortex (MPFC), primary motor cortex, somatosensory cortex, and supplementary motor area (SMA). Furthermore, we observed significant positive correlations (P < 0.05; two-tailed; Pearson’s correlation) between the resultant activity in the thalamus (P = 0.011; r = 0.510), amygdala (P = 0.017; r = 0.481), and posterior cingulate (P = 0.018; r = 0.478) and the level of positive affect experienced by participants when in the top hierarchical position as assessed in post-session questionnaires (Figure 4).
Figure 4.
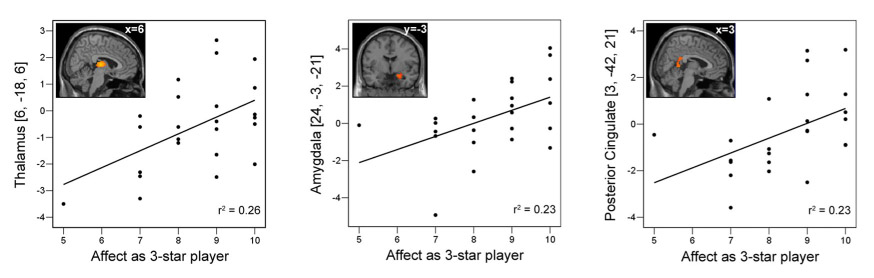
Correlations between brain activity and the level of positive affect experienced by the participant when in the top hierarchical position in areas uniquely activated in Experiment #2 for the contrast, “superior player > inferior player”. Displayed are significant (P < 0.05) correlations between the level of positive affect experienced by the participant as the three star player and parameter estimates at peak activations when viewing the superior player in the thalamus (P = 0.011; r = 0.51), amygdala (P = 0.017; r = 0.481) and posterior cingulate (P = 0.018; r = 0.478).
In Experiment #2, we also investigated the neural responses to various outcomes of interest (Table 2). Critically, we found that only outcomes with hierarchical value – that is, outcomes that potentially impact the participant’s status relative to the other players (Figure S4) – elicited significant brain responses after controlling for reward (subject won or lost) and the status of the other player in the round (superior or inferior). Specifically, in response to an outcome of negative hierarchical value associated with performing worse than an inferior individual compared to its control outcome (“subject lost/inferior won > subject lost/inferior lost”) (Figure 5A; Table 2), significantly greater (P < 0.05; FDR-corrected) brain activity was observed in the bilateral occipital/parietal cortex, ventral striatum, midbrain/thalamus, and anterior insula. Our data demonstrated a significant positive correlation (P < 0.05; two-tailed, Pearson’s correlation) between the level of positive affect experienced by the participant when in the top hierarchical position and the resultant activity in the insula (P = 0.030; r = 0.444) and ventral striatum (P = 0.008; r = 0.528) associated with performing worse than the inferior player (Figure 5A). Conversely, a number of regions were significantly differentially activated (P < 0.05; FDR-corrected) by an outcome of positive hierarchical value associated with performing better than the superior player compared to its control condition (“subject won/superior lost > subject won/superior won”) (Figure 5B and Table 2), notably in the dorsal striatum, midbrain/thalamus, MPFC, dorsal premotor cortex, and pre-SMA. We observed significant negative correlations (P < 0.05; two-tailed, Pearson’s correlation) between individual scores on the Trait Dominance-Submissiveness Scale (TDS) (Mehrabian, 1996), and activity in premotor cortex (P = 0.04; r = −0.453) associated with performing better than the superior player (Figure 5B). Non-hierarchical valuable outcome contrasts (“subject lost/superior won > subject lost/superior lost” and “subject won/inferior lost > subject won/inferior won”) did not reveal any significant (P < 0.05; FDR-corrected) activations.
Table 2.
Significantly activated (P < 0.05, FDR corrected) brain region during specific outcome phases in Experiment #2
| Brain Regions | BA | Cluster Size (voxels) | Peak MNI Coordinates(x,y,z) | Peak Z Score | |||
|---|---|---|---|---|---|---|---|
| sub lost/inf won > sub lost/inf lost | |||||||
| R inferior/middle occipital gyrus | 18/19 | 47 | 33, | −87, | 9 | 4.40 | |
| L inferior/middle occipital gyrus | 18/19 | 42 | −36, | −90, | −6 | 4.14 | |
| L ventral striatum, incl. | 127† | −6, | 6, | −3 | 4.11 | ||
| R ventral striatum | 9, | 9, | −3 | 4.01 | |||
| midbrain, incl. | 143† | −3, | −30, | −12 | 4.09 | ||
| thalamus | −6, | −24, | 9 | 3.67 | |||
| R inferior parietal lobule | 40 | 22† | 39, | −48, | 48 | 3.94 | |
| R fusiform | 37 | 49† | 48, | −60, | −12 | 3.88 | |
| L anterior insula | 13 | 65† | −42, | 15, | −6 | 3.77 | |
| R anterior insula | 13 | 52† | 36, | 24, | 6 | 3.33 | |
| sub lost/sup won > sub lost/sup lost | |||||||
| no significant activations (P < 0.05, FDR corrected) | |||||||
| sub won/sup lost > sub won/sup won | |||||||
| pre-supplementary motor area | 6 | 153‡ | 3, | 9, | 63 | 5.14 | |
| R precuneus | 7 | 172‡ | 21, | −87, | 42 | 5.10 | |
| L precuneus | 7 | 34‡ | −9, | −81, | 45 | 4.17 | |
| L precuneus | 7/19 | 38‡ | −24, | −78, | 33 | 3.95 | |
| R inferior/middle occipital gyrus | 18/19 | 54‡ | 45, | −84, | −6 | 4.58 | |
| L middle occipital gyrus | 19 | 24‡ | −48, | −72, | 0 | 4.07 | |
| R inferior frontal/orbitofrontal | 47 | 39‡ | 33, | 21, | −18 | 4.38 | |
| medial prefrontal cortex | 6 | 30‡ | 3, | 42, | 39 | 4.06 | |
| R middle frontal gyrus (DPMC) | 6 | 50‡ | 45, | 0, | 42 | 3.96 | |
| L middle frontal gyrus (DPMC) | 6 | 432 | −39, | −6, | 57 | 3.93 | |
| anterior cingulate | 32 | 29‡ | 9, | 42, | 18 | 3.84 | |
| L caudate | 26 | −6, | 6, | 9 | 3.98 | ||
| L fusiform | 37 | 22 | −36, | −63, | −18 | 3.88 | |
| R midbrain, incl. | 154 | 9, | −24, | −6 | 3.81 | ||
| R thalamus | 12, | −18, | 6 | 3.68 | |||
| L midbrain, incl. | 135 | −3, | −21, | −21 | 3.86 | ||
| L thalamus | −9, | −21, | 9 | 3.57 | |||
| sub won/inf lost > sub won/inf won | |||||||
| no significant activations (P < 0.05, FDR corrected) | |||||||
cluster defined using P < 0.001, uncorrected.
cluster defined using P < 0.02, FDR corrected.
L = left hemisphere. R = right hemisphere. MNI = Montreal Neurological Institute. BA = Brodmann area. DPMC = dorsal premotor cortex. sub = subject. inf = inferior player. sup = superior player.
Figure 5.
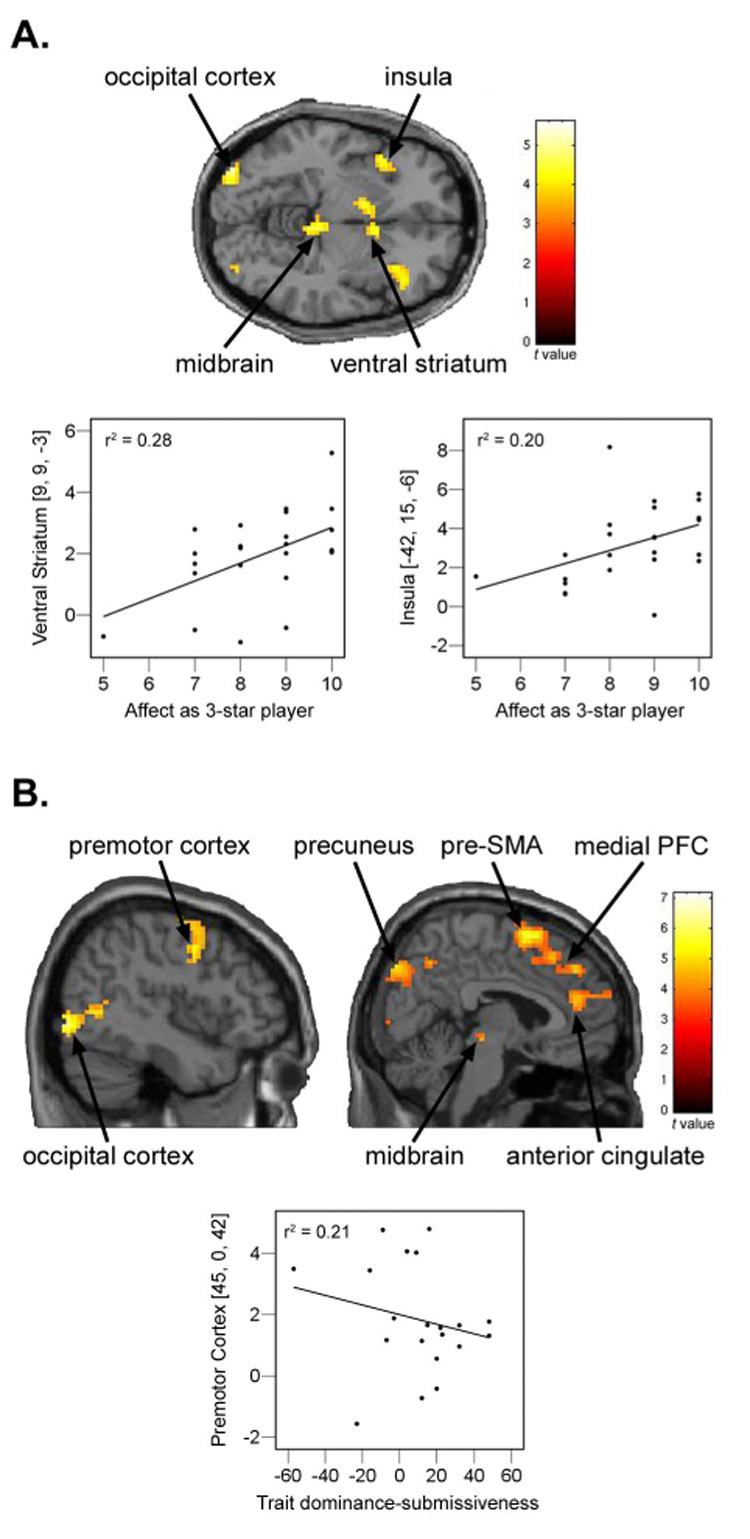
Significant activations to outcomes associated with hierarchical value in Experiment #2, displayed at P < 0.001, uncorrected. (A) Activations for the contrast, “subject lost/inferior won > subject lost/inferior lost”, observed in occipital cortex [−36, − 90, −6; 33, −87, 9], insula [−42, 15, −6; 36, 24, 6], midbrain [−3, −30, −12], and ventral straitum [−6, 6, −3; 9, 9, −3]. Also displayed are significant correlations between the level of positive affect experienced by the participant as the three star player and parameter estimates at peak activations when subject lost/inferior won in the ventral striatum (P = 0.008; r = 0.528) and insula (P = 0.030; r = 0.444). (B) Activations for the contrast, “subject won/superior lost > subject won/superior won”, observed in occipital cortex [− 48, −72, 0; 45, −84, −6], premotor cortex [−39, −6, 57; 45, 0, 42], precuneus [−9, −81, 45; −24, −78, 33; 21, −87, 42], midbrain [−3, −21, −21; 9, −24, −6], pre-supplementary motor area [3, 9, 63], medial prefrontal cortex [3, 42, 39], and anterior cingulate [9, 42, 18]. Not shown are activations in orbitofrontal cortex [33, 21, −18] and caudate [−6, 6, 9]. Also displayed are significant negative correlations between trait dominance/submissiveness scores and parameter estimates at peak activations when subject won/superior lost in the premotor cortex (P = 0.04; r = −0.453).
Assessment of the Social Specificity of the Results in Experiment #2
In order to assess the social specificity of the results from Experiment #2, we employed a non-social version of the experimental paradigm in which the human other players were replaced with two computers (Supplemental Methods) – a common method used in social cognition investigations (e.g. Spitzer et al., 2007). The fMRI results from the non-social control experiment are displayed in Table 3. In several regions, viewing a superior compared to an inferior other player in the non-social paradigm elicited significant activations (P < 0.05, FDR-corrected), which, although less extensive, were similar to some of those resulting in the social paradigm, namely in the occipital cortex, ventral striatum, parahippocampal cortex, sensorimotor cortex, and SMA. These common activations, therefore, could not been exclusively attributed to the social nature of the task (although we cannot exclude that anthropomorphizing of the computer players could have contributed to the overlap). However, several unique activations in other regions clearly distinguished the social paradigm from the non-social paradigm. Specifically, viewing a superior player compared to an inferior player activated the DLPFC, amygdala, thalamus, posterior cingulate, and MPFC in the social setting only; these regions were not significantly activated (P > 0.05, FDR-corrected) in the non-social task. Furthermore, all of the aforementioned activations following hierarchical valuable outcomes compared to their control outcome conditions were social specific, with the exception of activity in the occipital cortex in the negative hierarchical valuable outcome contrast, “subject lost/inferior won > subject lost/inferior lost”, which also resulted in the non-social control condition (P < 0.05, FDR-corrected).
Table 3.
Significantly activated (P < 0.05, FDR corrected) brain region in the non-social control paradigm (Experiment #3)
| Brain Regions | BA | Cluster Size (voxels) | Peak MNI Coordinates(x,y,z) | Peak ZScore | ||
|---|---|---|---|---|---|---|
| superior player > inferior player | ||||||
| L precentral gyrus, incl. | 4 | 838 | −36, | −30, | 69 | 4.35 |
| L postcentral gyrus | 1/2/3 | −48, | −30, | 60 | 4.25 | |
| L inferior/middle occipital gyrus | 18/19 | 476† | −27, | −96, | −15 | 4.02 |
| R inferior/middle occipital gyrus | 18/19 | 395† | 30, | −84, | 27 | 3.89 |
| supplementary motor area | 6 | 129† | 0, | −3, | 54 | 4.04 |
| L insula | 13 | 385 | −42, | 0, | 3 | 3.63 |
| R insula | 13 | 145 | 42, | 0, | 3 | 3.32 |
| R parahippocampal gyrus | 50† | 18, | −27, | −9 | 3.76 | |
| L ventral striatum | 160 | −9, | 12, | 0 | 3.52 | |
| R ventral striatum | 30† | 9, | 15, | −3 | 3.59 | |
| sub lost/inf won > sub lost/inf lost | ||||||
| L inferior occipital gyrus | 18/19 | 359‡ | −36, | −78, | −15 | 5.56 |
| L middle occipital gyrus | 18/19 | 79‡ | −33, | −90, | 12 | 4.54 |
| R middle occipital gyrus | 19 | 101‡ | 36, | −84, | 15 | 5.11 |
| R fusiform gyrus | 37 | 79‡ | 30, | −63, | −15 | 4.69 |
| R precuneus | 7 | 36‡ | 21, | −72 | 48 | 4.34 |
| sub won/sup lost > sub won/sup won | ||||||
| no significant activations | ||||||
cluster defined using P < 0.001, uncorrected.
cluster defined using P < 0.005, FDR corrected.
L = left hemisphere. R = right hemisphere. MNI = Montreal Neurological Institute. BA = Brodmann area. sub = subject. inf = inferior player. sup = superior player.
In addition to this specific neural signature, the social and non-social paradigms were dissociable behaviorally as well (Figure S5). In post-session questionnaires, participants reported being significantly more influenced/motivated by the other players in the social experiment compared to non-social experiment (P = 0.023; t(46) = 2.351; two-tailed; t test). Additionally, it was significantly (P = 0.007; t(45) = 2.823; two-tailed; t test) more important for participants to perform better than the superior player in the social compared to non-social paradigm.
DISCUSSION
In the present study, we identified pronounced differential neural responses based on status when viewing another individual, despite the fact that status was irrelevant for the game outcome. Hierarchical status can be either fixed or changeable, and this aspect of social stratification has pronounced implications for individuals. In non-human and human primates, the more subordinate position in stable social hierarchies are associated with greater stress, whereas in dynamic hierarchies, the dominant position experiences the most stressors due to increased competition and instability (Sapolsky, 2004, 2005) during times of reorganization, and may be at greater health risks (Sapolsky, 2004). To address neural differences in processing stable and unstable hierarchical information, we modulated hierarchy stability in two experiments. Importantly, in addition to hierarchy stability, we also investigated social specificity using a non-social control experiment, allowing for the separation between the neural processing of general hierarchical information (i.e. ranked relative to an inanimate entity) and social hierarchical information (i.e. ranked relative to other human beings).
In all hierarchical settings (stable, unstable, and non-social), brain activity when viewing a more superior player compared to viewing a more inferior player was significantly greater in occipital/parietal cortex, ventral striatum, and parahippocampal cortex, implicating these brain areas in the neural encoding of hierarchical rank, irrespective of the stability or specifically social nature of the hierarchy. Activity in the occipital/partietal cortex and ventral striatum indicates greater perceptual/attentional processing (Bradley et al., 2003) and salience (Zink et al., 2006) associated with the superior player, respectively, in excellent agreement with data on preferential attentional capture by high-rank individuals in monkeys (Deaner et al., 2005). Increased activity in the parahippocampal cortex, a region shown to play a central role in contextual associative processing (Aminoff et al., 2007), is suggestive of preferred contextual episodic encoding of the association between the superior rank status and the player’s picture.
While these regions did not appear to differentiate between social and non-social hierarchical information, the DLPFC activation to the superior versus inferior player was only seen in a social context, i.e. human other players, suggesting that the involvement of DLPFC in processing hierarchical information is specifically social. Our data support the notion that the DLPFC plays a role in making interpersonal judgments, including the assessment of social status (Mah et al., 2004). Furthermore, the DLPFC has been implicated in social norm compliance (Spitzer et al., 2007), a process that is strongly influenced by perceived social rank (Cummins, 2000). In accordance with the social specificity of DLPFC activity resulting here, the DLPFC’s role in social norm compliance was significantly more pronounced in a social compared to non-social context (Spitzer et al., 2007).
Interestingly, the social unstable hierarchical setting elicited multiple neural responses not produced with the other hierarchical settings (stable and non-social). Viewing a superior compared to inferior player in the social unstable hierarchy setting resulted in increases of activity in multiple areas linked with social emotional processing and social cognition. The amygdala, in particular, has been implicated in processing socially emotional stimuli (Adolphs, 2003), as well as social anxiety associated with hierarchical challenge (Rilling et al., 2004). Recently, Britton et al. (2006) demonstrated that activity in the thalamus, amygdala, and posterior cingulate was modulated by social emotional stimuli. We observed significant positive correlations between activity in these same regions and the level of positive affect experienced by participants when in the top hierarchical position. We conclude that activity in these regions represented an emotional arousal response to the superior player that only arises when the hierarchy is dynamic; that is, when relative performance, although irrelevant for the game outcome, can have social hierarchical consequences (e.g., a superior player, rather than the participant, has obtained the desired top hierarchical position).
The MPFC, an area known to play a pivotal role in social cognition, was also uniquely activated in the social unstable hierarchy setting when viewing the superior compared to inferior player. The MPFC is particularly associated with recognizing the intentions and motives of other people (mentalizing) and forming judgments of other people (person perception), including how others view us (reputation) (Amodio and Frith, 2006). Mitchell et al. (2005) have demonstrated that the MPFC is involved in forming impressions only in a social domain (i.e., judging people versus inanimate objects), a claim strongly supported by out data; the MPFC activation reported here was specific to a social context.
The data delineating brain regions uniquely activated by the superior player compared to the inferior player in the unstable hierarchical setting correspond well with the role of hierarchical rank in social comparison theory (Festinger, 1954). Wheeler has demonstrated that humans preferentially make upward comparisons, i.e., comparisons with superior others, which elicits negative affect, and that this propensity is stronger with higher levels of motivation to improve (Wheeler, 1966), as is possible only in the unstable hierarchical setting. A paradox thus follows: individuals with the greatest desire for success have the greatest tendency to make social comparisons with superior others, leading to negative feelings (Wheeler, 1966).
An important feature of the unstable hierarchy setting was that particular outcomes now acquired positive or negative hierarchical value based on their potential impact on the participant’s status relative to the other players. The fact that only outcome contrasts associated with hierarchical value elicited significant brain responses implicates social relevance as a primary determinant of how outcome was processed; furthermore, virtually all the resulting activations were social specific. The high salience of rank implications was confirmed by a general linear model analysis (Supplemental Methods) showing that hierarchical value of outcomes made a highly significant, unique contribution to ventral striatal activity of comparable magnitude to that of the primary monetary reward itself (Supplemental Results).
The occipital/parietal, midbrain, and ventral striatal activations associated with the negative hierarchically valuable outcome (i.e. performing worse than an inferior player) indicate increased perceptional/attentional processing (Bradley et al., 2003) and greater behavioral importance or saliency; (Horvitz, 2000; Zink et al., 2006), notably including key components of the dopaminergic system for saliency processing. The anterior insula activity is of particular interest given previous work implicating this region in processing emotional/affective pain (Eisenberger et al., 2003; Singer et al., 2004) and frustration (Abler et al., 2005). Intuitively, one may peg the ability to inflict pain (physical and emotional) on a superior individual, however, in unstable hierarchies it is only the superior individual who stands to lose something, meaning that it is the inferior participant who is capable of eliciting emotional pain by virtue of the threat to overtake the more superior position. Confirming this interpretation, our data demonstrate a significant positive correlation between the level of positive affect experienced by the participant when in the top hierarchical position and activity in the insula and ventral striatum, suggesting that losing to an inferior was more salient and emotionally painful for those who experience more positive affect from being in the top position of the social hierarchy. We suggest that this may be a neural system especially relevant for health risks associated specifically with superior status in unstable hierarchies and personality traits linked to dominance and competitiveness (Sapolsky, 2004). Importantly, the ventral striatal and insula activations reported here did not occur in the non-social paradigm.
The dorsal striatum, midbrain/thalamus, and medial prefrontal cortex activations found in response to positive hierarchical valuable outcomes (i.e. performing better than a superior player) have previously been implicated in rewarding, but antagonistic, social interactions such as altruistic punishment (de Quervain et al., 2004) and retaliation (Lotze et al., 2007), which can be associated with a position of superiority. In addition, significant activations were found in the dorsal premotor cortex and pre-SMA, regions previously associated with higher order action dispositions (Lotze et al., 1999; Picard and Strick, 1996), raising the intriguing possibility that acquiring a more superior position in the social hierarchy is associated with a bias towards an active state. If true, this system should be associated with personality traits related to dominance. Indeed we observed significant negative correlations between individual scores on the Trait Dominance-Submissiveness Scale (TDS) (Mehrabian, 1996), and activity in premotor cortex associated with performing better than the superior player. Higher scores on the TDS are associated with a more active state, i.e., “feelings of control and influence over everyday situations, events, and relationships” (Mehrabian, 1994), whereas lower scores are associated with a more passive state, i.e., “feelings of being controlled and influenced by circumstances and others” (Mehrabian, 1994). As such, the outcome associated with potentially achieving a more superior position elicited greater activity in association motor areas in individuals with a lower active state at baseline, perhaps as a compensatory response. Recruitment of these premotor areas is especially remarkable because the experimental social setting consists of a pure hierarchy with explicitly non-antagonistic interactions, i.e. the players did not have any options for action that were based on status.
In conclusion, the present study provides a characterization of the neural correlates associated with processing social hierarchies in humans. In this initial inquiry, we used incidental differences in skill and accompanying rank symbols to create a hierarchy; many other aspects governing social rank relationships in humans remain to be studied, including those related to power, physical, economic, and professional standings. Even so, our findings demonstrate that brain responses to superiority and inferiority are dissociable, even in the absence of explicit competition, both when encountering an individual of a particular status and when faced with an outcome that can affect one’s current position in the hierarchy. We hope that this research begins identifying neural mechanisms mediating the enormous impact of social status on decision-making, health, and survival in humans.
METHODS
Participants and Other Players
A total of seventy-two Caucasian, right-handed, healthy adults participated in the fMRI experiments: 24 participants (12 males, 12 females) in each of two social hierarchy experiments, as well as 24 participants (12 males, 12 females) in the non-social hierarchy control experiment, described in the Supplemental Methods. The mean ages in the two social hierarchy experiments were not significantly different (P = 0.174, two-tailed, independent-samples t test; Experiment #1: ages 22–43; mean = 27.6; S.D. = 5.1 and Experiment #2: ages 19–38; mean = 25.7; S.D. = 4.7). Participants had no history of any psychiatric or neurological disorders and gave written, informed consent for a protocol approved by the National Institute of Mental Health Institutional Review Board. The participants in both social experiments were told that they would perform the experimental task with two other people of comparable race, age, and gender. Unbeknownst to the participant, these other people were simulated and, like the participant, were represented in the task by their photograph. The 48 participants used in the social paradigms analysis did not give any indication that they believed the other players were indeed not real. Nine additional participants were scanned and not included in subsequent analysis due to technical issues during the scanning session or because they expressed doubts regarding whether the other players were real.
Training and Establishment of Social Hierarchy
Because the “other players” in the two experiments were simulated and believability was imperative to the study, the training period prior to scanning was an elaborate procedure to make the situation socially immersive and ensure that the participant did not doubt the presence of other players. Details regarding the training procedure can be found in the Supplemental Methods.
Just prior to the scanning session, participants performed ten trials of the task used in the experimental design (a reaction time task in Experiment #1 and a visual discrimination task in Experiment #2 – see below for details) to establish the explicit social hierarchy based on skill. They were instructed that the other two players were also performing ten trials of the task, and all players were ranked according to their performance (which was experimentally fixed). The social hierarchy was created by identifying one of the other players as faster/better (“three star player”) and one of the other players as slower/worse (“one star player”) than the participant (“two star player”). Within a given age range, the initial position in the hierarchy of the other players was counterbalanced across participants. In Experiment #1, the hierarchy did not change throughout the session; it was a stable hierarchy. In Experiment #2, the hierarchy was updated based on performance outcomes throughout the session; it was an unstable hierarchy. Subjects were explicitly informed about the nature of the hierarchy in each experiment.
Experimental Tasks
For all tasks, stimuli presentations and recording of reaction times were performed using the software, Presentation (Neurobehavioral Systems, Inc., San Francisco, CA).
Experiment #1: “Stable Hierarchy”
A schematic diagram of the experimental task is provided in Figure 1A. While in the scanner, participants performed multiple rounds of a simple reaction time task over three runs. Each run lasted ~9 minutes and consisted of 36 rounds of the game (108 rounds total). The participant performed rounds with the superior and inferior player, alternatively, for a total of 54 rounds with each (18 rounds with each per run). At the beginning of each round, the photograph and rank symbols (superior or inferior) of the other player participating in the upcoming round were displayed in the center of the screen for 4 s. The participant was told that while they were viewing this screen, the other player would be viewing the participant’s photograph and rank, and the third player not participating in the upcoming round would be viewing a blank screen throughout that round. Next, during the game phase of the task (2–5 s, average = 3.5 s), a blue circle appeared in the center of the screen. Participants were required to press a button, using their right thumb, as quickly as possible when the blue circle changed to green. The amount of time that the circle remained blue before changing to green varied from 0.5–3.5 s (average = 2 s). Participants were told that if they responded to the green circle quickly enough (i.e., within a fabricated, small critical time interval), then they would receive $1. If they did not respond at all or did not respond quickly enough, they would receive nothing. Participants never lost money. During the game phase, the picture and rank of the research participant and other player participating in the round were displayed on the left side of the screen, with the more superior of the two players positioned above the other. The purpose of these pictures was to reinforce that the participant was playing/viewing the same screen at the same time as another person and to reinforce the ranks. In the outcome phase (4 s), a dollar bill or a “0” appeared across (on the right side of the screen) from each person’s picture, depending if they had won (i.e., responded quickly enough) or lost (i.e., did not respond quickly enough). Importantly, the participants were playing the game at the same time as the other player, but not against; therefore, it was possible that both players won or lost within a given round, and perceived task difficulty did not differ in rounds with the inferior and superior other player. As such, the game was explicitly non-competitive. Eight different outcome situations were possible based on the result (win/lose) and the rank of the other player relative to the participant: subject won/superior player lost, subject won/superior player won, subject won/inferior player lost, subject won/inferior player won, subject lost/superior player won, subject lost/superior player lost, subject lost/inferior player won, subject lost/inferior player lost. All outcome situations were predetermined with the exception that if the participant did not respond to the green circle within 0.75 s, s/he automatically lost to ensure believability of a critical response window. If the participant did not respond within 0.75 s more than twice, then the experimental task was automatically terminated. Such a scenario never occurred with any participant. Each round ended with a fixation cross displayed for 1–4 s (average = 2.5 s). After every six rounds, the cumulative earnings screen was displayed (5 s) showing the picture and rank of the three players with their cumulative percent of wins displayed below their picture. The participant was also shown the exact amount of his/her cumulative monetary earnings and was told that each player was able to see their own exact amount, but only percent of wins were shown to everyone because the participant played in every round and the other players alternated. Throughout the scanning session the cumulative percent of wins converged on 66% for the superior other player, 50% for the participant, and 33% for the inferior other player. Therefore, although the game was non-competitive, the stable social hierarchy was reinforced by outcomes throughout the session.
FMRI Imaging Acquisition and Analysis
Scanning was performed on a 3 Tesla GE Signa scanner. For each participant, 276 whole-brain scans per run (three runs total) were acquired to measure the T2*-weighted blood oxygenation level dependent (BOLD) effect with the following parameters: gradient-recall echo-planar imaging; TR = 2000 ms; TE = 30 ms; flip angle = 90°; 64×64 matrix; FOV = 240 mm; 35 3.5 mm slices acquired with an interleaved order of slice acquisition. Four additional scans were acquired at the beginning of each run to allow for steady-state magnetization (discarded from analysis). Head movement during scanning was minimized with a vacuum pillow that conformed to the shape of the participant’s head and additional padding.
The data were pre-processed and analyzed using Statistical Parametric Mapping (SPM5) (Friston et al., 1994; http://www.fil.ion.ucl.ac.uk/spm/). Slice timing correction was used to adjust for time differences due to multi-slice imaging acquisition. Motion correction to the first functional scan was performed using a six-parameter rigid-body transformation. For each individual, the mean of the functional images was spatially normalized to the Montreal Neurological Institute (MNI) template conforming to the Talairach orientation system (Talairach and Tournoux, 1988) by applying a 12-parameter affine transformation followed by nonlinear warping using basis functions (Ashburner and Friston, 1999). The computed transformation parameters were applied to all of the functional images, interpolated to a final voxel size of 3 × 3 × 3 mm3. Images were subsequently spatially smoothed with an 8 mm Gaussian kernel.
A random-effects, event-related statistical analysis (Josephs et al., 1997) was performed with SPM5 in a two-level procedure. At the first level, a separate general linear model (GLM) was specified for each participant. BOLD responses to the other player (two separate regressors: superior, inferior), the game, the different outcomes (eight separate regressors), and the cumulative earnings screens were modeled separately, time-locked to event onset, by convolving the onset vectors with a synthetic hemodynamic response function as implemented by SPM5. At the model estimation stage, the data were high-pass filtered with a cut-off of 128 s to remove low-frequency drifts from the data and serial correlations were accounted for by an autoregressive model of the first order. Global scaling was not applied to the data. Contrast images were calculated for each participant to identify brain regions with greater activity following the presentation of the other player’s picture at the beginning of each round when the other player was superior compared to inferior (“superior player > inferior player”) and vice versa (“inferior player > superior player”). It should be noted that, although the entire paradigm was somewhat complex, these contrasts of interest were relatively simplistic to ensure that purely hierarchy-related activity was extracted (i.e. a face stimulus was being compared to another face stimulus, both with neutral expressions, with the only difference being the rank associated with the faces). The individual contrast images were then entered into a second level random-effects analysis, using a one-sample t test, to assess the group effect. The resulting summary statistical maps were thresholded at P < 0.005, FDR corrected for multiple corrections across the whole brain, voxel extent = 20.
Experiment #2: “Unstable Hierarchy”
A schematic diagram of the experimental task is provided in Figure 1B. While in the scanner, participants performed multiple rounds of a simple visual discrimination task over three runs. Run order was counterbalanced across participants. Each run lasted ~11 minutes and consisted of 56 rounds of the game (168 rounds total). The participant performed rounds with each of the other players, alternatively, for a total of 84 rounds with each (28 rounds with each per run). The runs began with a display of the initial rankings for 4 s followed by a 1 s fixation cross. At the beginning of each round, the photograph and rank (superior or inferior) of the other player participating in the upcoming round were displayed in the center of the screen for 3 s. The participant was told that while s/he was viewing this screen, the other player would be viewing the participant’s photograph and rank, and the third player not participating in the upcoming round would be viewing a blank screen throughout that round. Next, during the game phase of the task (3 s), two boxes were displayed side-by-side, each filled with a different (yet very similar) number of randomly distributed small, black dots. After 1 s, “RESPOND NOW!” appeared at the bottom of the screen, and participants were required to indicate which box contained more dots by pressing the corresponding button with their right thumb. Participants were told that they would receive $1 for correct responses and nothing for incorrect responses. Money was not withdrawn following incorrect responses. During the game phase, the picture and rank of the participant and other player participating in the round were displayed at the top of the screen, with the more superior of the two players positioned on the left. The purpose of these pictures was to reinforce that the participant was playing/viewing the same screen at the same time as another person and to reinforce the ranks. While the boxes did indeed contain different number of dots (34 or 36), the number of items exceeded visual processing capacity, making it impossible to reliably perceive the difference within the 1 sec allotted time period. It was therefore feasible to have fixed outcomes without knowledge of the participant. In the outcome phase (3 s), a dollar bill or an “X” appeared below each person’s picture, depending on whether they had won (i.e., correctly responded) or lost (i.e., incorrectly responded). Importantly, the participants were playing the game at the same time as the other player, but not against, and therefore, it was possible that both players won or lost within a given round, and perceived task difficulty did not differ in rounds with the inferior and superior other player. As such, the game was explicitly noncompetitive. Eight different outcome situations (Figure S4) were possible based on the result (win/lose) and the rank of the other player relative to the participant: subject won/superior player lost, subject won/superior player won, subject won/inferior player lost, subject won/inferior player won, subject lost/superior player won, subject lost/superior player lost, subject lost/inferior player won, subject lost/inferior player lost. Each outcome situation occurred 21 times throughout the session. All outcomes were predetermined with the exception that if the participant did not respond, s/he automatically lost to insure believability. If the participant did not respond more than twice, then the experimental task was automatically terminated. Such a scenario never occurred with any participant. Each round ended with a fixation cross displayed for 1 s. Unlike in Experiment #1, after every four rounds, the rank of the players within the social hierarchy was updated according to percent of correct responses over the preceding eight rounds played. Therefore, although the game was non-competitive, the unstable social hierarchy was reinforced and adapted by outcomes throughout the session. The new ranking was displayed for 4 s (followed by a 1s fixation cross) showing the pictures and new ranks of the three players. The direction of the adjustment for the participant was written at the bottom: “YOU MOVED UP!”, “YOU MOVED DOWN!”, or “YOU STAYED THE SAME!” The participant was told that each player was able to see the new rankings, but each individual received their own message regarding their particular movement within the hierarchy. Because the hierarchy was updated based on performance throughout the session, certain outcomes in Experiment #2 possessed positive or negative hierarchical value based on the impact of the outcome on the participant’s status relative to the other players. Specifically, performing worse than the inferior player, which occurred when the participant responded incorrectly in a round being performed at the same time as an inferior player who responded correctly (“subject lost/inferior won”), had a negative hierarchical value because such an outcome could allow the inferior player to move above the participant in the social hierarchy. On the other hand, performing better than the superior player, which occurred when the participant responded correctly in a round being performed at the same time as a superior player who responded incorrectly (“subject won/superior lost”), had a positive hierarchical value because such an outcome could allow the participant to overtake the superior position in the hierarchy. These hierarchical valuable outcomes were of particular interest in the subsequent fMRI analysis.
FMRI Imaging Acquisition and Analysis
Scanning was performed on a 3 Tesla GE Signa scanner (different scanner than that used in Experiment #1). For each participant, 265 whole-brain scans per run (three runs total) were acquired to measure the T2*- weighted blood oxygenation level dependent (BOLD) effect with the following parameters: gradient-recall echo-planar imaging; TR = 2500 ms; TE = 30 ms; flip angle = 90°; 64×64 matrix; FOV = 240 mm; 30 (36 for four subjects) 3.5 mm slices acquired with an interleaved order of slice acquisition. Four additional scans were acquired at the beginning of each run to allow for steady-state magnetization (discarded from analysis). Head movement during scanning was minimized with a vacuum pillow that conformed to the shape of the participant’s head and additional padding.
Image preprocessing was identical to Experiment #1. A random-effects, event-related statistical analysis (Josephs et al., 1997) was performed with SPM5 in a two-level procedure. At the first level, a separate general linear model (GLM) was specified for each participant. BOLD responses to the other player (two separate regressors: superior, inferior), the game, the different outcome situations (eight separate regressors), and the different rank change screens (3 separate regressors: up, down, same) were modeled separately, time-locked to event onset, by convolving the onset vectors with a synthetic hemodynamic response function as implemented by SPM5. At the model estimation stage, the data were high-pass filtered with a cut-off of 128 s to remove low-frequency drifts from the data and serial correlations were accounted for by an autoregressive model of the first order. Global scaling was not applied to the data. Contrast images were calculated for each participant to identify brain regions with greater activity following the presentation of the other player’s picture when the other player was superior compared to inferior (“superior player > inferior player”) and vice versa (“inferior player > superior player”). The resulting summary statistical maps were thresholded at P < 0.005, FDR corrected for multiple comparisons, voxel extent = 20. Unlike in Experiment #1, contrast images were also calculated for various outcomes of particular interest while controlling for reward (subject won or lost) and the status of the other player in the round (superior or inferior): “subject won/inferior lost > subject won/inferior won”, “subject lost/inferior won > subject lost/inferior lost”, “subject won/superior lost > subject won/superior won”, and “subject lost/superior won > subject lost/superior lost”. The resulting summary statistical maps were thresholded at P < 0.05, FDR corrected for multiple comparisons. It should be noted that while the inter-trial interval is fixed in Experiment #2, as a consequence of the repeated rank changes throughout the session, the order of the events of interest (e.g. superior player and inferior player) are jittered, thus ensuring an efficient task design for detecting differences between them.
Pre-Scan Temperament Assessment and Post-Scan Questionnaire
Prior to the scanning day, within a week before, participants completed the computer administered/scored version of the PAD Temperament Scale (Mehrabian, 1996). The software generates scores for Trait Pleasure (P), Trait Arousability (A), and Trait Dominance (D). For the purposes of our study, we had a particular interest in the Trait Dominance-Submissiveness Scale, which “deals with a person’s characteristic feelings of control and influence over everyday situations, events, and relationships versus feelings of being controlled and influenced by circumstances and others.” (Mehrabian, 1994).
Immediately following the scan, participants completed a questionnaire. Most of the questions consisted of a ten-point scale rating their thoughts and feelings during various aspects of the game. Specifically, we assessed the task difficulty, how much the other players made the participant anxious, happy, and motivated, how much the rank of the other player in a given round influenced the participant, how important it was for the participant to perform better than the other player when the other player was superior and inferior, how good it felt to be in the one, two, or three star position, and how much the participant liked rounds played with a superior and inferior player. We were particularly interested in the level of positive affect associated with being the three star player, to assess how much participants liked/desired being in the top hierarchical position in the dynamic hierarchy setting.
We employed a Pearson's correlation coefficient analysis (two-tailed, p < 0.05) between these behavioral scales (PAD Temperament and post-scan questionnaire) and parameter estimates from peak voxels of significantly activated brain regions in the group contrast maps to investigate whether neural responses may influence dominance-related behavior.
Supplementary Material
ACKNOWLEDGMENTS
We thank Lucas B. Kempf for medical coverage, Courtnea A. Rainey and Catherine K. Draper for research assistance, and Shabnam Hakimi and Heike Tost for thoughtful discussion. We also thank Chris Frith for his valuable comments on the manuscript. This research was supported by the Intramural Research Program of the National Institute of Mental Health, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abler B, Walter H, Erk S. Neural correlates of frustration. Neuroreport. 2005;16:669–672. doi: 10.1097/00001756-200505120-00003. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Is the human amygdala specialized for processing social information? Ann. N.Y. Acad. Sci. 2003;985:326–340. doi: 10.1111/j.1749-6632.2003.tb07091.x. [DOI] [PubMed] [Google Scholar]
- Aminoff E, Gronau N, Bar M. The parahippocampal cortex mediates spatial and nonspatial associations. Cereb. Cortex. 2007;17:1493–1503. doi: 10.1093/cercor/bhl078. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum. Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT. Social stratification, health, and violence in the very young. Ann. N.Y. Acad. Sci. 2004;1036:47–68. doi: 10.1196/annals.1330.003. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Sabatinelli D, Lang PJ, Fitzsimmons JR, King W, Desai P. Activation of the visual cortex in motivated attention. Beh. Neurosci. 2003;117:369–380. doi: 10.1037/0735-7044.117.2.369. [DOI] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Welsh RC, Berridge KC, Liberzon I. Neural correlates of social and nonsocial emotions: An fMRI study. Neuroimage. 2006;31:397–409. doi: 10.1016/j.neuroimage.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Cummins DD. How the social environment shaped the evolution of mind. Synthese. 2000;122:3–28. [Google Scholar]
- de Quervain DJ, Fischbacher U, Treyer V, Schellhammer M, Schnyder U, Buck A, Fehr E. The neural basis of altruistic punishment. Science. 2004;305:1254–1258. doi: 10.1126/science.1100735. [DOI] [PubMed] [Google Scholar]
- Deaner RO, Khera AV, Platt ML. Monkeys pay per view: adaptive valuation of social images by rhesus macaques. Curr. Biol. 2005;15:543–548. doi: 10.1016/j.cub.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Festinger L. A Theory of Social Comparison Processes. Human Relations. 1954;7:117–140. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear. Hum. Brain Mapp. 1994;2:189–210. [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Josephs O, Turner R, Friston KJ. Event-related fMRI. Hum. Brain Mapp. 1997;5:243–248. doi: 10.1002/(SICI)1097-0193(1997)5:4<243::AID-HBM7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Karafin MS, Tranel D, Adolphs R. Dominance attributions following damage to the ventromedial prefrontal cortex. J. Cognitive Neurosci. 2004;16:1796–1804. doi: 10.1162/0898929042947856. [DOI] [PubMed] [Google Scholar]
- Lotze M, Montoya P, Erb M, Hulsmann E, Flor H, Klose U, Birbaumer N, Grodd W. Activation of cortical and cerebellar motor areas during executed and imagined hand movements: an fMRI study. J. Cognitive Neurosci. 1999;11:491–501. doi: 10.1162/089892999563553. [DOI] [PubMed] [Google Scholar]
- Lotze M, Veit R, Anders S, Birbaumer N. Evidence for a different role of the ventral and dorsal medial prefrontal cortex for social reactive aggression: An interactive fMRI study. Neuroimage. 2007;34:470–478. doi: 10.1016/j.neuroimage.2006.09.028. [DOI] [PubMed] [Google Scholar]
- Mah L, Arnold MC, Grafman J. Impairment of social perception associated with lesions of the prefrontal cortex. Am. J. Psychiat. 2004;161:1247–1255. doi: 10.1176/appi.ajp.161.7.1247. [DOI] [PubMed] [Google Scholar]
- Mehrabian A. Manual for the Revised Trait Dominance-Submissiveness Scale. 1994 TDS. [Google Scholar]
- Mehrabian A. Pleasure-arousal-dominance: A general framework for describing and measuring individual differences in temperament. Curr. Psychol. 1996;14:261–292. [Google Scholar]
- Mitchell JP, Neil Macrae C, Banaji MR. Forming impressions of people versus inanimate objects: social-cognitive processing in the medial prefrontal cortex. Neuroimage. 2005;26:251–257. doi: 10.1016/j.neuroimage.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Moors A, De Houwer J. Automatic processing of dominance and submissiveness. Exp. Psychol. 2005;52:296–302. doi: 10.1027/1618-3169.52.4.296. [DOI] [PubMed] [Google Scholar]
- Moskowitz DS, Pinard G, Zuroff DC, Annable L, Young SN. The effect of tryptophan on social interaction in everyday life: a placebo-controlled study. Neuropsychopharmacol. 2001;25:277–289. doi: 10.1016/S0893-133X(01)00219-6. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb. Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- Pork M-R. Conflict Avoidance in Social Interaction: A Sociolinguistic Comparison of the Korean and Japanese Honorific Systems. In: Hoji H, editor. In Japanese/Korean Linguistics. Vol. 1. Stanford: CSLI Publications; [Google Scholar]
- Rilling JK, Winslow JT, Kilts CD. The neural correlates of mate competition in dominant male rhesus macaques. Biol. Psychiat. 2004;56:364–375. doi: 10.1016/j.biopsych.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Social status and health in humans and other animals. Annu. Rev. Anthropol. 2004;33:393–418. [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Spitzer M, Fischbacher U, Herrnberger B, Gron G, Fehr E. The neural signature of social norm compliance. Neuron. 2007;56:185–196. doi: 10.1016/j.neuron.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers, Inc.; 1988. [Google Scholar]
- Wheeler L. Motivation as a determinant of upward comparison. J. Exp. Soc. Psychol. 1966 SUPPLEMENT 1:27–31. [Google Scholar]
- Zink CF, Pagnoni G, Chappelow J, Martin-Skurski M, Berns GS. Human striatal activation reflects degree of stimulus saliency. Neuroimage. 2006;29:977–983. doi: 10.1016/j.neuroimage.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


