Abstract
Previous work in rats and primates has shown that normal aging can be associated with a decline in cognitive flexibility mediated by prefrontal circuits. For example, aged rats are impaired in rapid reversal learning, which in young rats depends critically on the orbitofrontal cortex. To assess whether aging-related reversal impairments reflect orbitofrontal dysfunction, we identified aged rats with reversal learning deficits and then recorded single units as these rats, along with unimpaired aged cohorts and young control rats, learned and reversed a series of odor discrimination problems. We found that the flexibility of neural correlates in orbitofrontal cortex was markedly diminished in aged rats characterized as reversal-impaired in initial training. In particular, although many cue-selective neurons in young and aged-unimpaired rats reversed odor preference when the odor-outcome associations were reversed, cue-selective neurons in reversal-impaired aged rats did not. In addition, outcome-expectant neurons in aged-impaired rats failed to become active during cue sampling after learning. These altered features of neural encoding could provide a basis for cognitive inflexibility associated with normal aging.
INTRODUCTION
Cognitive flexibility, defined as the ability to rapidly adapt established patterns of behavior in the face of changing circumstances, depends critically on prefrontal cortex (Miller 2000). Normal aging is associated with a decline in cognitive flexibility mediated by these circuits. For example, subpopulations of aged monkeys and humans are unable to modify responding appropriately when the position or cue associated with reward changes or is made irrelevant. This inflexibility is evident in tasks that incorporate reversal learning and set-shifting procedures (Bartus et al. 1979; Denburg et al. 2005a,b; Hartman et al. 2001; Lamar and Resnick 2004; Rapp and Amaral 1989; Steere and Arnsten 1997; Tsuchida et al. 2002; Voytko 1999). Similarly, subsets of aged rats are slower than young rats at learning to modify responding in discrimination tasks when established cue-outcome associations are reversed or made irrelevant to task performance (Barense et al. 2002; Schoenbaum et al. 2002a). In such settings, impaired aged animals often perform normally during initial acquisition but are then unable to modify responding when the contingencies change. These results suggest an inability to modify or control established behavior, which is often thought to require prefrontal cortical systems.
Deficits in cognitive flexibility may reflect impaired processing within specific subdivisions of prefrontal cortex. For example, an inability to rapidly learn reversals is a prominent symptom of damage to the orbitofrontal cortex (OFC) in humans, non-human primates, and rats (Chudasama and Robbins 2003; Dias et al. 1997; Fellows and Farah 2003; Izquierdo et al. 2004; Jones and Mishkin 1972; Kim and Ragozzino 2005; Rolls et al. 1994; Schoenbaum et al. 2002b, 2003a). This deficit is associated with the finding that neurons in OFC provide a flexible representation of the associations between predictive cues and outcomes during discrimination learning (Rolls et al. 1996; Schoenbaum et al. 1999, 2003b; Thorpe et al. 1983). Such flexible representations would be particularly useful during reversal learning. Deficient formation or flexibility of such representations in aged animals could provide a basis for cognitive rigidity observed in normal aging. To test this hypothesis, we recorded neural activity from OFC in rats performing a go, no-go odor discrimination task. Electrodes were implanted in OFC in young rats and also in aged rats the performance of which on reversals in the task had been previously characterized as either impaired or unimpaired. Neural recordings were obtained from OFC neurons in these rats as they learned and reversed new discrimination problems. We found that reversal-impaired aged rats had fewer cue-selective neurons in the task and that the remaining cue-selective activity was markedly less flexible than that in either young or aged-unimpaired counterparts.
METHODS
Subjects
This research was conducted in accordance with National Institutes of Health guidelines for animal research. The subjects consisted of pathogen-free male Long-Evans rats. Aged rats were obtained as retired breeders at 8–9 mo of age from Charles River Laboratories (Wilmington, MA) and were 22 mo old at the start of odor-discrimination training. Young rats were obtained from the same source and were 4 mo old at the start of behavioral testing. Rats were housed individually on a 24-h light/dark cycle with ad libitum access to food and water except during odor-discrimination training. During odor-discrimination training, the rats continued to have ad libitum access to food but were only allowed access to water for 30–60 min at the end of the day after the training sessions were completed. The health of all subjects during this phase was monitored to ensure adequate hydration. All testing was performed during the light phase of the cycle.
Surgical methods
Surgeries were conducted after the prerecording phase of training (see Behavioral methods). Drivable microelectrode arrays composed of 10 25-μm-diam FeNiCr wires (Stablohm 675, California Fine Wire, Grover Beach, CA) in a 27-gauge thin-wall cannula (Small Parts, Miami Lakes, FL) were implanted in 16 rats dorsal to OFC in the left hemisphere at 3.0 mm anterior to bregma, 3.2 mm laterally, and 4.0 mm ventral to the surface of the brain. Immediately prior to implantation, the wires were freshly cut with surgical scissors to extend ~1 mm beyond the cannula and electroplated with platinum (H2PtCl6, Aldrich, Milwaukee, WI) to an impedance of ~300 kΩ. During recording, the electrode bundle was advanced in 40-μm increments to acquire activity from new neurons for the following day.
Histology
After testing, rats were given an overdose of pentobarbital and prepared for perfusion. Immediately prior to perfusion, the final electrode position was marked by passage of a 15-μA current through each microwire for ~10 s to create a small iron deposit. The rats were then perfused intra-cardially with 0.9% saline followed by 4% formaldehyde followed by 100 ml of 3% potassium ferrocyanide in perfusate to visualize the iron deposit. Brains were removed from the skulls and stored in a 30% sucrose/4% formaldehyde/3% potassium ferrocyanide solution for several days until sectioning. The brains were sectioned on a freezing microtome with coronal sections (40 μm) collected through OFC. Sections were mounted on glass slides, stained with thionin, and coverslipped with Permount. Electrode placements were verified under a light microscope and drawn onto plates adapted from the atlas of Swanson (1992).
Behavioral methods
Preoperative behavioral testing was conducted to familiarize the rats with the odor-discrimination task and to characterize the reversal performance of the aged subjects prior to recording. In this part of the study, the rats were trained on a standard series of odor-discrimination problems and reversals in a paradigm employed in past experiments (Saddoris et al. 2005; Schoenbaum and Setlow 2003; Schoenbaum et al. 2003a,b). A subset of these behaviorally characterized rats underwent surgery to implant recording electrodes. In subsequent recording sessions, neural data were obtained as the rats learned novel odor problems.
Prerecording training
Odor-discrimination training was conducted in aluminum chambers ~18-in on each side with sloping walls narrowing to an area of 12 × 12-in at the bottom. An odor port and fluid well were located on a panel (Fig. 1), which was located in the right wall of each chamber below two panel lights. Odor-discrimination problems were composed of odor pairs chosen from compounds obtained from International Flavors and Fragrances (New York). Discrimination problems were constructed from dissimilar odors, and the odor-discrimination sequence was arranged such that similar compounds were counterbalanced by valence and did not repeat across days. During training, rats were maintained on water restriction. After each session, the rats were given ad lib access to water for 10–30 min depending on the fluid intake of each rat during the session.
Fig. 1.
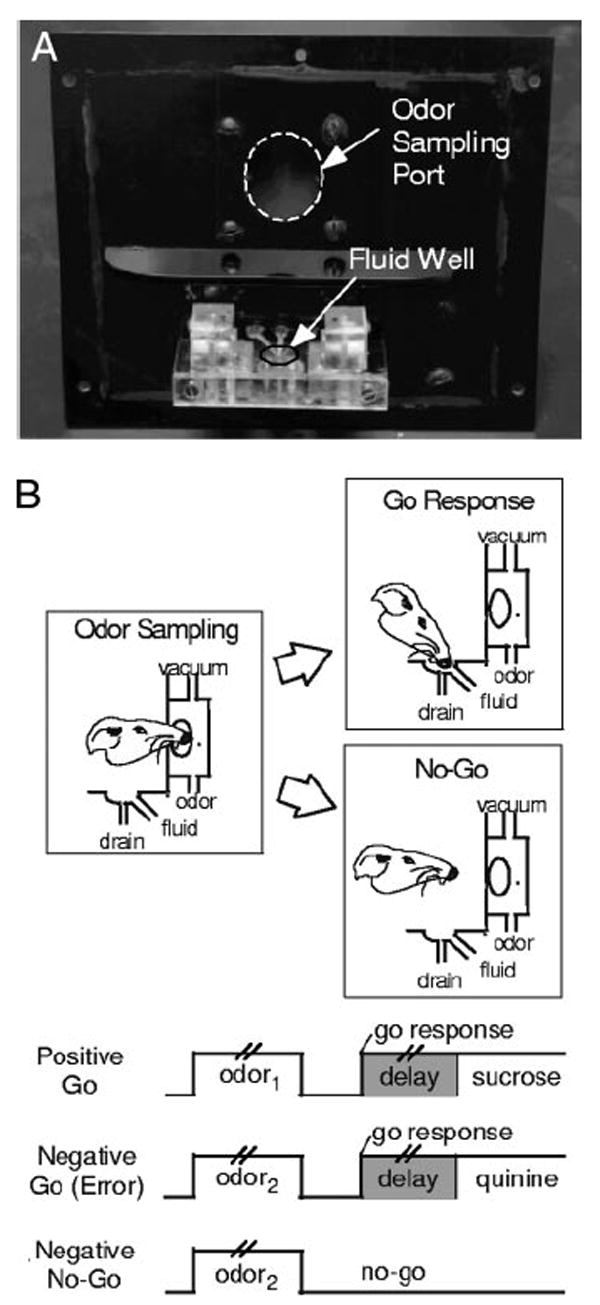
Illustration of training apparatus and behaviors in the task. A: photograph of the polycarbonate panel removed from the operant chamber to show the odor sampling port and the fluid delivery well. B: schematic illustrating behaviors in the task. Pairs of vertical lines during odor presentation and the delay between a go response and fluid delivery denote the variable duration of these events; odor sampling typically lasted 250–750 ms, and the delay was programmed to vary from 500 to 1,500 ms.
Trials were signaled by illumination of the panel lights inside the box. When these lights were on, nose-poke into the odor port (Fig. 1) resulted in delivery of the preselected odor cue to a small hemicylinder located behind this opening. The rat terminated odor sampling by leaving the odor port and then had 3 s to make a go response at the fluid well located below the port (Fig. 1). If a response was made after sampling a positive odor, then a 0.05-ml bolus of an appetitive 10% sucrose solution was delivered to the well after a variable delay (500–1,500 ms). If the same response was made after sampling a negative odor, then a 0.05-ml bolus of an aversive 0.02 M quinine solution was delivered after a similar delay. If the rat did not respond within 3 s, the trial was counted as a no-go (Fig. 1). A behavioral criterion was defined as 18 correct responses in a moving block of 20 trials.
Prerecording odor discrimination training was divided into two phases as in prior reports (Saddoris et al. 2005; Schoenbaum et al. 2003b; Setlow et al. 2003). In the first phase, rats were required to learn a series of four two-odor-discrimination problems (D1–D4). Note that the first odor problem (D1) served as a “shaping” problem in which the rats were introduced to odors and to the sucrose and quinine reinforcers for the first time in the context of the task. Training continued on this and each of the subsequent nonshaping odor problems until the rat met a criterion of 18 correct responses in a moving block of 20 trials. Rats were run for ~1 h each day or until this criterion was achieved. Once an odor problem was acquired, training was begun on the next problem the following day.
Once the first four odor problems (D1–D4) were acquired, the rats began the second phase of training. In the second training phase, the rats were required to learn a series of reversals in which the contingencies signaled by the odor cues in a single discrimination problem were altered. This phase began with presentation of the most recently acquired odor problem (D4) using the same contingencies that were employed in initial training (S1+/S2−). Once the rats demonstrated retention of this odor problem with the original contingencies by meeting the behavioral criterion of 18 correct responses in a moving block of 20 trials, the response contingencies were reversed, provided the rat had maintained 80% performance over a block of 60 trials preceding reversal. This secondary performance requirement ensured that all rats were equally proficient on the odor problem before reversal. Training on the reversed problem (S1−/S2+) continued until the behavioral criterion was met again.
After this first reversal was completed, the contingencies for the same odor cues were reversed a second time in the same manner. The reversed discrimination problem (S1−/S2+) was presented, and the rats were required to demonstrate retention of this problem with these contingencies by achieving the behavioral criterion. When these criteria were met, the problem was immediately reversed back to the original contingencies (S1+/S2−), provided the rat had maintained 80% performance over a block of 60 trials preceding reversal. Training on this re-reversal continued until each rat met the behavioral criterion again.
Recording
After completion of the prerecording training and recovery from surgery, rats with microelectrodes underwent training in a series of sessions in which neural data were collected as the rats acquired novel discrimination problems. Recording sessions were conducted in a single aluminum chamber identical in all respects to the set of chambers used for training prior to surgery. During recording, we introduced a diluted solution of condensed milk in place of sucrose and substituted a prolonged inter-trial interval in place of quinine. Previously we have found that rats will work more when rewarded with condensed milk rather than sucrose or when punished with a timeout rather than quinine. This change was made so that we could acquire acquisition and reversal data in single sessions more easily. Thus the rats were trained on a novel problem each day until they met the behavioral criterion and for an additional 60–100 trials after this criterion was achieved. In many sessions, the discrimination problem was also reversed, and neural data were obtained as the rats acquired the reversal problem.
Data acquisition and analysis
The recording chamber was mated to a commutator (Crist Instrument, Damascus, MD) and equipment from Datawave Technologies (Longmont, CO) for gathering neurophysiological data. For each recording session, the rat was placed in the training chamber, and the electrode wires were screened for neural activity while the rat explored the open chamber. If no activity was detected, the rat was removed, and the electrode assembly was advanced 40 or 80 μm. Otherwise, active wires were selected for recording, and a training session was begun.
Neural activity was recorded using a single Datawave Enhanced Discovery system, capable of recording neural waveforms on up to eight channels. Signals from active wires were passed through a unity-gain JFET headstage, band-pass filtered at 300–3,000 kHz, and amplified differentially (relative to a silent reference electrode) at 5,000 times (Neuralynx). Waveforms (>2.5:1 signal-to-noise) were digitized at 25 kHz and recorded to disk by the data-acquisition software along with timestamps indicating when significant events occurred (odor onset, responding, fluid delivery, etc).
These files were analyzed using software from Plexon (Dallas, TX). For this analysis, files were first imported into Off-line Sorter where waveforms on each channel were sorted using a template matching algorithm. These waveforms were compared with notes regarding the waveforms made during the session, and the inter-spike interval histograms were inspected to ensure that spike events were separated by >1 ms. Typically one to three waveforms could be isolated on an active channel.
Sorted files were then processed in Neuroexplorer to extract these unit time stamps and relevant event markers. These data were subsequently analyzed using statistical routines in Matlab (Natick, MA) to examine firing activity during odor sampling (from 50 ms after odor onset to 50 ms after odor offset), during the variable delay after a response at the fluid well (from 50 ms before the response until fluid delivery), and after fluid delivery (1st 500 ms). Single-unit firing activity (spikes/s) in each time window was compared on positive and negative trials during pre- and postcriterion trial blocks and after reversal using ANOVA (P < 0.05), and neurons with a significant difference in activity were categorized as “selective” in that time window and performance phase (we have also applied a Kruskal-Wallis test to these data, and obtained similar results; ANOVA’s are used here for consistency with prior reports). Performance phases were defined based on go, no-go performance to include a precriterion phase, before the rat met criterion on the discrimination, and a postcriterion phase, after the rat met criterion. The precriterion phase was further divided into an early period, including on average the first 13 trials, and late period. Reversal performance were analyzed as a separate phase. A Pearson χ2 test (P < 0.05) was used to compare the proportions of neurons with different firing properties in intact and lesioned rats. In addition, we computed a contrast for cue-selective neurons in each performance phase. This index of selectivity was calculated as: contrast = (frpod − frnpod)/(frpod + frnpod), where frpod was the firing rate on trials involving the preferred odor from the postcriterion period and frnpod was the firing rate on trials involving the nonpreferred odor. The average contrast for the cue-selective neurons in each group was compared across phases by ANOVA. Finally population histograms were constructed by averaging across histograms from each neuron.
RESULTS
Characterization of reversal abilities in aged rats prior to recording
The experiment began before recording with the characterization of reversal learning abilities in a small set of young (4 mo; n = 9) and aged (22 mo; n = 11) male Long-Evans rats. For this, we used a procedure we have used previously to examine the effects of brain lesions on reversal performance in rats (Schoenbaum and Setlow 2003; Schoenbaum et al. 2003a). This task is illustrated in Fig. 1. Briefly the rats were trained on a series of two-odor-discrimination problems, in which one of two odors was presented at an odor port on each trial; one odor predicted the availability of an appetitive outcome at a nearby fluid well and the other odor predicted the availability of an aversive outcome. The rats learned four such problems and then were confronted with two serial reversals of the final odor problem.
As expected, aged rats had no difficulty learning the initial four discrimination problems when compared with controls (Fig. 2A). A two-factor ANOVA (group × odor problem) revealed no effect of age on acquisition (F < 0.12, P > 0.74). There was a significant main effect of odor problem[F(3,54) = 37.7, P < 0.001], such that acquisition on the first shaping problem (D1) required more trials than subsequent nonshaping problems (D2–D4); this was true for both aged and young rats.
Fig. 2.
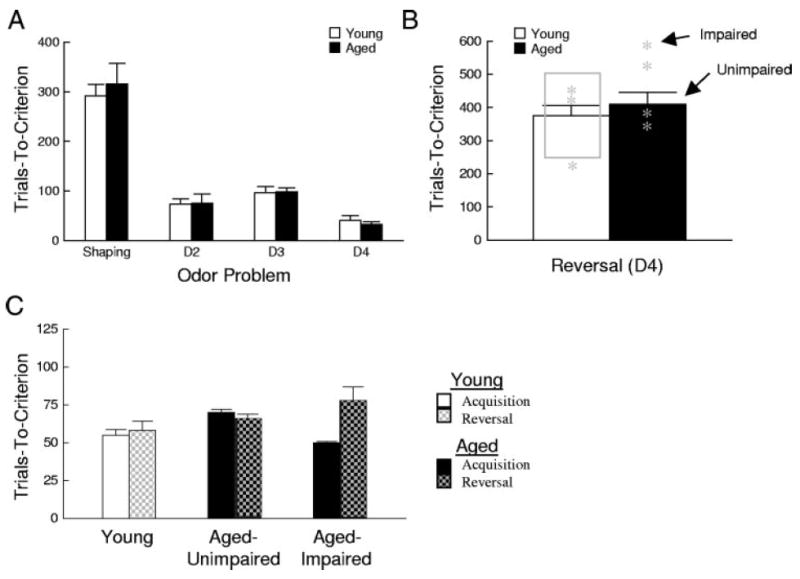
Comparison between performance of young and aged rats on a series of novel odor discrimination problems and serial reversals of the final odor problem. A: trials required by young and aged rats to meet a behavioral criterion of 18/20 correct responses on 4 2-odor, symmetrically reinforced go, no-go odor-discrimination problems. There was no difference between the groups. B: average trials required by young and aged rats to meet the behavioral criterion on 2 serial reversals of the final odor problem (D4). Boxed region, 99% confidence range for the performance of normal young rats, based on the variability of young rats’ reversal performance in this and 2 published reports (Schoenbaum and Setlow 2003; Schoenbaum et al. 2003b). Asterisk, performance of rats from which recording data were obtained. Although the means of the 2 groups did not differ, 4/11 aged rats fell outside the range of normal rats and were classified as impaired on the reversals, including 2 rats from whom neural recordings were subsequently obtained. C: average trials required by young and behaviorally-characterized aged rats to meet behavioral criterion on the discriminations and reversals during the recording sessions. Young and aged-unimpaired rats required similar numbers of trials for learning and reversing the problems; rats that had been characterized as reversal impaired (see B) required significantly more trials to learn the reversals.
After learning the four initial discriminations, the rats were then trained on two serial reversals of the final odor problem (D4). Figure 2B shows the average performance of the rats in this reversal learning phase. Consistent with prior reports in which we have employed this exact task (Schoenbaum and Setlow 2003; Schoenbaum et al. 2003a), young rats acquired the reversals in around 400 trials, on average. The gray box shown in Fig. 2B illustrates the 99% confidence interval for the performance of young rats based on the variance of rats in this and the prior datasets. Although the young and aged rats in this study did not differ significantly overall [F(1,18) = 0.26, P = 0.61], 4 of the 11 aged rats (vs. 0/9 young rats) exhibited performance that was worse than the upper range of this 99% confidence interval. The abnormal reversal performance on the part of these aged rats is consistent with our prior report of individual differences in reversal learning among a larger sample of aged rats (Schoenbaum et al. 2002a), in which slightly <50% of 2-yr-old male Long-Evans rats from this study population performed poorly on reversal problems when compared with young controls.
Description of the recording dataset
After completion of preoperative testing, a subset of the rats underwent surgery to implant a drivable bundle of microwires in OFC and recording sessions commenced. Rats that were implanted with electrodes were chosen on the basis of their performance on the reversals conducted prior to surgery. Thus electrodes were implanted in young controls (n = 3), in aged rats that performed like young controls in these reversals (aged-unimpaired, n = 2), and in aged rats that performed outside the 99% confidence interval for young controls (aged-impaired, n = 2). The prerecording performance of these rats is indicated by asterisks in Fig. 2B.
In the recording sessions, neural data were acquired as these rats learned new odor problems and subsequent reversals of those problems. Neural recordings were obtained from 321 neurons in 45 sessions in the young rats, including 261 neurons recorded in 31 reversal sessions, from 262 neurons in 26 sessions in the aged-impaired rats, including 157 neurons recorded in 12 reversal sessions, and from 225 neurons in 31 sessions in the aged-unimpaired rats, including 117 neurons recorded in 13 reversal sessions. Figure 3 illustrates the recording sites in these sessions; recordings were generally made in the lateral orbital areas or in ventral agranular insular regions. These areas are notable because they receive overlapping projections from olfactory regions and the basolateral amygdala complex (Kita and Kitai 1990; Price et al. 1991).
Fig. 3.
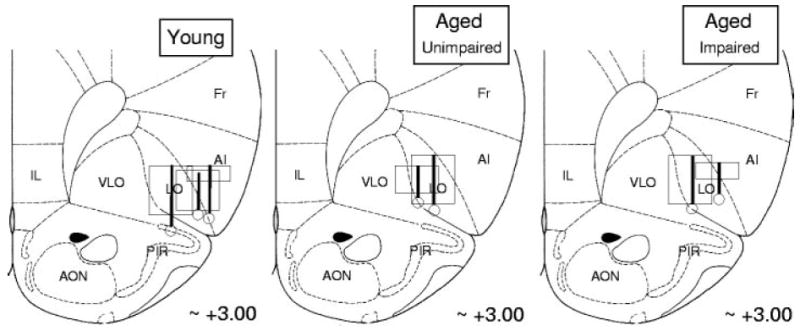
Drawings of electrode placements in orbitofrontal cortex (OFC) in young (left), aged-unimpaired (middle), and aged-impaired rats (right). Vertical bars on the drawing indicate the center of the electrode track in each rat; boxes indicate approximate extent of recording sessions vertically and give an estimate of lateral (and AP) spread of the wires (~1 mm). The recording sites within OFC were similar in young, aged-impaired, and aged-unimpaired rats and to those in earlier studies examining neural correlates in OFC during learning in this paradigm (Schoenbaum et al. 1998, 1999, 2003). The distribution and mean firing rates of the neurons were similar in young (5.30 spikes/s), aged-impaired (5.66 spikes/s), and aged-unimpaired rats (4.45 spikes/s).
As expected from prior studies of this type (Schoenbaum et al. 2002a,b), reversal performance improved with further training for young and aged rats alike. However, analysis of performance during the recording sessions revealed that the aged rats characterized as reversal-impaired prior to recording continued to show abnormal performance during the reversals in the recording sessions. These data, illustrated in Fig. 2C, show that whereas the young and aged-unimpaired rats learned the discriminations and reversals at the same rate during recording, the aged-impaired rats learned the reversals more slowly than the initial discriminations. Consistent with this interpretation, a two-factor ANOVA comparing the average trials-to-criterion for each rat (group × acquisition/reversal) showed a significant main effect of acquisition/reversal [F(1,4) = 9.20, P < 0.05] and a significant interaction between group and acquisition/reversal [F(2,4) = 9.64, P < 0.05]. Post hoc comparisons indicated that while the young and aged-unimpaired rats acquired the discriminations and reversals in the same number of trials (F < 0.6, P > 0.48), the aged-impaired rats required significantly more trials to acquire the reversals than the initial discriminations [F(1,4) = 26.04, P = 0.007]. Rats identified as reversal-impaired based on their initial reversal-learning performance also made more errors during reversal sessions overall than controls, responding correctly on only 68% of reversal trials on average compared with 79 and 74% for young and aged-unimpaired rats, respectively [F(2,53) = 6.58, P < 0.01]. Subsequent comparisons revealed a significant difference between young and aged-impaired rats (P < 0.001).
Cue-selective activity in reversal-impaired aged rats is diminished and less flexible
We analyzed neural activity during sampling of the predictive odor cues, focusing on firing during the postcriterion trial block. This trial block, which consisted on average of 79 trials in young rats, 82 trials in aged-unimpaired, and 83 trials in aged-impaired rats, included trials after the rats had met the behavioral criterion in each recording session but before reversal. Our analysis, the results of which are summarized in Table 1, examined how encoding in these neurons developed with learning, was affected by reversal, and related to firing activity in other periods of the trial.
Table 1.
Summary of neurophysiological results
| Young | Aged-Unimpaired | Aged-Impaired | |
|---|---|---|---|
| Neurons with differential activity during cue-sampling (cue-selective) | |||
| Postcriterion | 103/321 | 75/225 | 62/262* |
| Developed cue-selectivity with learning | 79/103 | 46/75 | 48/62 |
| Maintained cue-selectivity with reversal | 8/84 | 5/36 | 5/38 |
| Lost cue-selectivity with reversal | 53/84 | 20/36 | 32/38* |
| Reversed cue-selectivity with reversal | 23/84 | 11/36 | 1/38* |
| New cue-selectivity with reversal in nonselective neurons | 32/177 | 12/81 | 11/119* |
| Neurons with differential activity in delay after responding (outcome-expectant) | |||
| PreCriterion | 68/321 | 59/225* | 45/262 |
| Developed cue-selectivity for appropriate odor | 12/68 | 13/59 | 2/45* |
Statistically significant difference, please see text for details.
As we have reported previously, many OFC neurons in young rats exhibited differential activity in this trial block during odor sampling, firing more during sampling of one odor than the other. This population amounted to 103 neurons or 32% of the recorded neurons and included neurons that fired more to the positive cue or to the negative cue. Similar proportions of cue-selective neurons were found in the aged-unimpaired rats, where 75 neurons or 33% of the population responded differentially to the odor cues (χ2 = 0.09, NS). However, the same analysis of OFC neurons recorded in aged-impaired rats revealed that only 62 neurons or 24% of the population exhibited cue-selective firing after learning in the postcriterion block of trials. This represented a significant reduction in the proportion of neurons that fired selectively to one or the other of the predictive cues compared with both the young rats (χ2 = 5.04, P < 0.05) and aged-unimpaired counterparts (χ2 = 5.60, P < 0.05).
We next examined neural activity in the cue-selective neurons to see how selectivity changed both during learning in initial acquisition and after reversal. The results of this analysis for neurons from each group are shown in Fig. 4. Consistent with our previously published observations (Schoenbaum et al. 1999, 2003b), the majority of the cue-selective OFC neurons in the young rats showed significant associative encoding, developing a cue-preference during learning and/or reversal. For example, 77% of the neurons with a postcriterion odor preference were not selective for the preferred odor in the precriterion trials (79 of the 103 cue selective cells). This increase in selectivity is evident in the population responses and activity contrasts shown in Fig. 4, A and B, for the cue-selective neurons recorded in the young rats. Flexibility in cue-selectivity in the young rats was further evident after reversal, when 90% of the postcriterion selective neurons changed their postcriterion odor preference, including 27% that completely reversed cue preference in alignment with the new contingencies (Fig. 4C). In addition, a second population of cue-selective OFC neurons emerged after reversal; this population amounted to 32 of the 177 previously nonselective neurons. Only eight neurons in young rats maintained selectivity for the same odor cue across reversal training, and, of those, only three neurons were selective for the same odor cue throughout training. The influence of these flexible populations is evident in Fig. 4, A and B, which show a reversal in the population response to the odor cues in young rats between the postcriterion and reversal phases.
Fig. 4.
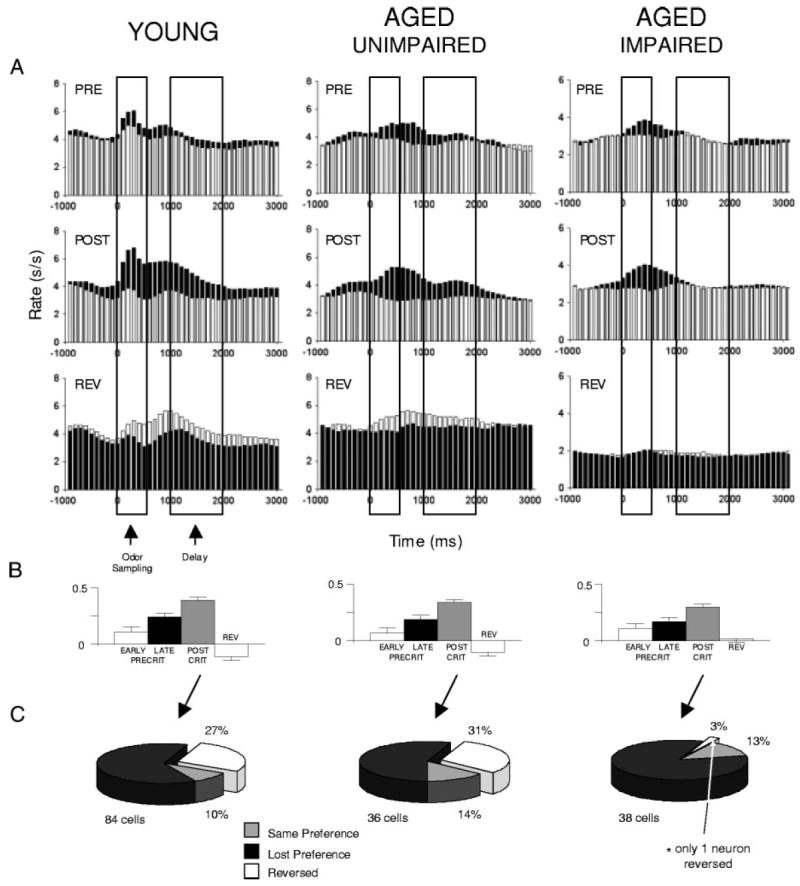
Effect of learning and reversal on cue-selective neurons in young, aged-unimpaired and aged-impaired rats. A: histograms showing the average response of the cue-selective neurons during learning and after reversal. Activity is shown separately for trials involving each odor cue during the precriterion (PRE), postcriterion (POST), and reversal trial blocks (REV). ■, response to the preferred odor cue in the postcriterion phase. □, response to the nonpreferred odor. Activity is synchronized to odor onset, displayed in 100-ms bins in spikes/s (s/s), and box overlays indicate the average duration of odor sampling and of the delay between responding and reinforcement when a response was made at the fluid well. B: contrast in activity to the preferred and nonpreferred odor cues for the neurons that were cue-selective in the postcriterion trial block. Calculation is described in the methods. C: effect of reversal on neurons that were cue-selective in the postcriterion trial block.
Aged-unimpaired rats also exhibited flexible associative encoding during odor sampling. The majority of neurons selective for an odor cue developed that preference in the postcriterion trial block (61% or 46 of the 75 cells), and 86% of these cue-selective neurons changed their preference after reversal, including 31% that reversed in alignment with the new contingencies (Fig. 4C). Again, many of the previously nonselective neurons (12/81) also became cue-selective after reversal. As a result, cue-selective activity in aged-unimpaired resembled that observed in the young rats, becoming more selective from pre- to postcriterion and then reversing after reversal of the odor-outcome associations (Fig. 4, A and B).
By contrast, cue-selective firing in aged-impaired rats was impoverished and substantially less flexible. This was evident first in the reduction in the proportion of cue-selective neurons in the aged-impaired rats noted previously. In addition, cue-selective neurons in aged-impaired rats also failed to reverse their odor preference during reversal training. Instead, as illustrated by Fig. 4C, these neurons typically lost their cue-selective firing. The lack of neurons that reversed selectivity during reversal training distinguished the aged impaired rats from each of the other groups (young: χ2 = 10.1, P < 0.01; aged-unimpaired: χ2 = 10.6, P < 0.01). Furthermore, after reversal, only 11 of the 119 nonselective neurons in aged-impaired rats became cue-selective. This proportion was smaller than that observed in young rats (χ2 = 4.47, P < 0.05). As a result of these differences, the population response in OFC in reversal-impaired aged rats became nonselective for the odor cues during reversal learning (Fig. 4, A and B).
Cue-selective activity in reversal-impaired aged rats is not integrated with outcome-expectant firing
One additional distinguishing feature was evident in the cue-selective firing activity observed in aged-impaired rats. This was a reduction in the proportion of the cue-selective population derived from neurons that also encoded outcome expectancies. Outcome-expectant neurons are identified early in training by differential firing on positive and negative trials during a delay after a response is made at the fluid well but prior to outcome delivery (Schoenbaum et al. 1998, 2003b); these neurons often go on to be come selective for the cue associated with their preferred outcome (Schoenbaum et al. 2003b).
In the current dataset, we found that many OFC neurons recorded in young rats (n = 68; 21%), aged-impaired (n = 45; 17%), and aged-unimpaired rats (n = 59; 26%) encoded outcome expectancy in the precriterion trials. Although young and aged-impaired rats had similar proportions of these neurons [χ2 = 1.48, NS; and aged-unimpaired rats actually had significantly more such neurons (χ2 = 5.90, P < 0.05)], there were significant differences in whether these outcome-expectant neurons developed selective activity for the odor corresponding to their preferred outcome after learning. Specifically, in reversal-impaired aged rats, these neurons were unlikely to become selective for the appropriate predictive odor cue, whereas in the other two groups, they were equally likely to do so. Thus in young rats, 18% of the outcome-expectant neurons (12 cells) became selective for the odor cue that predicted the neuron’s preferred outcome. This selectivity developed after learning in the postcriterion trial block. A similar population was found in aged-unimpaired rats where 22% of the outcome-expectant neurons (13 cells) became selective for the appropriate odor cue in the postcriterion phase (χ2 = 0.38, NS). These populations are illustrated in Fig. 5.
Fig. 5.
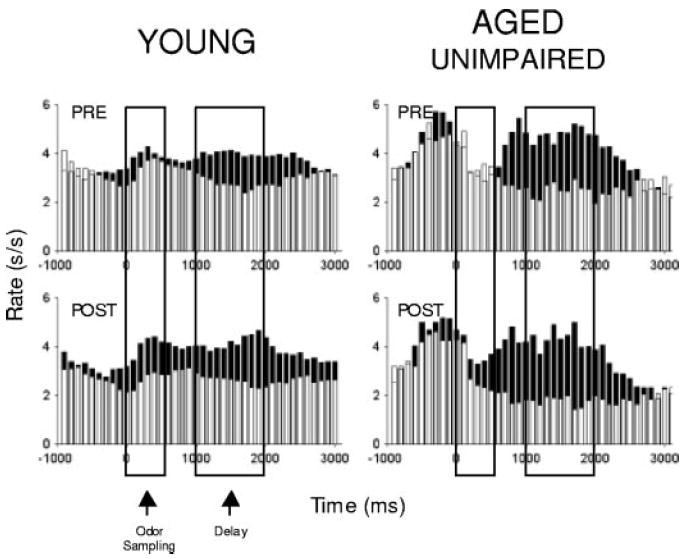
Cue-outcome encoding in OFC in young and aged-unimpaired rats. Histograms show the average response of neurons that fired selectively during the delay before delivery of 1 of the outcomes and also during sampling of the odor that predicted that outcome. ■, response on trials involving that preferred outcome. □, response to the nonpreferred outcome. Activity is synchronized to odor onset, displayed in 100-ms bins in spikes/s (s/s), and box overlays indicate the average duration of odor sampling and of the delay between responding and reinforcement when a response was made at the fluid well.
By contrast, outcome-expectant neurons recorded in the reversal-impaired aged rats did not become selective for the appropriate odor cue after learning; only two cells or 4% of the neurons with outcome-expectant activity developed selective firing to the corresponding odor cue postcriterion. This proportion was significantly lower than the proportion observed in young (χ2 = 4.35, P = 0.037) or aged-unimpaired rats (χ2 = 6.40, P = 0.011). This difference is evident in the cue-selective population histogram for the aged impaired rats shown in Fig. 4A, which is notable for the absence of selective firing during the delay period when compared with population histograms from young and aged-unimpaired rats.
DISCUSSION
OFC is critical to flexible behavior (Schoenbaum and Roesch 2005). This role is particularly evident when the contingencies for achieving specific goals or outcomes are uncertain or have been altered as during reversal learning (Chudasama and Robbins 2003; Dias et al. 1997; Fellows and Farah 2003; Izquierdo et al. 2004; Jones and Mishkin 1972; Kim and Ragozzino 2005; Rolls et al. 1994; Schoenbaum et al. 2002b, 2003a). The role of OFC in guiding such flexible behavior has been linked to the sensitivity of neural activity in OFC to stimulus-outcome associations. This sensitivity is particularly evident in “reversal” neurons, which switch selectivity between cues when the outcome associations are switched. These neurons were first described in monkeys by Rolls and colleagues (Thorpe et al. 1983), who speculated that they were likely the critical neural substrate for rapid reversal learning. Here we show that OFC neurons in reversal-impaired aged rats fail to show this normal sensitivity to stimulus-outcome associations. These rats exhibited impoverished encoding of the significance of the odor cues relative to young controls and aged-unimpaired cohorts during learning, and the neural correlates that were evident were less flexible, failing to reverse cue selectivity when the cue-outcome associations were switched. The finding that reversal-impaired aged rats exhibit fewer reversal neurons in OFC provides a possible neural substrate for their reversal impairment. In addition, the failure of OFC neurons to flexibly encode the outcomes predicted by discrete cues in the environment may underlie some of the changes in cognitive flexibility reported to occur in normal aging (Barense et al. 2002; Bartus et al. 1979; Denburg et al. 2005a,b; Hartman et al. 2001; Lamar and Resnick 2004; Rapp and Amaral 1989; Steere and Arnsten 1997; Tsuchida et al. 2002; Voytko 1999).
Importantly, deficient behavior and encoding during reversal learning was evident against a backdrop of substantial preserved function in the aged-impaired rats. For example, these rats learned the initial discriminations normally, which required the rats to inhibit the same go response that they were unable to inhibit during reversal learning. Thus deficient reversal performance was not due to a general inability to inhibit responding or to simple preservation (Kim and Ragozzino 2005; Schoenbaum et al. 2002b, 2003a).
The preserved performance of the aged-impaired rats on the initial discriminations parallels that of rats, monkeys, and humans after OFC damage, which typically does not cause deficits in simple discrimination learning (Chudasama and Robbins 2003; Dias et al. 1997; Fellows and Farah 2003; Izquierdo et al. 2004; Meunier et al. 1997; Rolls et al. 1994; Schoenbaum et al. 2002a). Such performance is thought to be mediated by stimulus-response learning in other neural structures (e.g., dorsal striatum). Stimulus-response associations or habits would, however, be somewhat less flexible during reversal learning when the animal must adapt performance after a change in the associations between stimuli and outcomes. The aged-impaired rats also improved their reversal performance with extended training, again much like OFC-lesioned animals (Schoenbaum et al. 2002a,b). This improved performance may also reflect encoding of other types of associative information in other neural structures. The preservation of these capabilities shows that the reversal-learning deficit is a selective impairment in cognitive flexibility and does not reflect a global deficit in learning functions.
Some neural correlates in OFC were relatively preserved in the reversal-impaired aged rats. For example, these rats had substantial populations of neurons that fired to the odor cues and in anticipation of and during presentation of the sucrose and quinine reinforcers. Moreover, many of these neural responses appeared to be acquired, reflecting learned information. Firing rates and average yields per wire were also similar in young and aged rats, including those with identified reversal impairment. Thus in many ways, the aged-impaired rats were quite similar to the other two groups. The difference was primarily in the flexibility of these representations in the face of changes in the stimulus-outcome associations. This suggests that aging does not result in a dramatic disruption of information processing by OFC, even in rats with behavioral impairments, and that cognitive decline and associated encoding changes in these rats likely do not reflect gross brain damage. Indeed, cognitive dysfunction in normal aging is not typically attributable to frank neurodegeneration or cortical cell loss in rats and monkeys (Rapp and Gallagher 1996; Rapp and Heindel 1994; Rasmussen et al. 1996).
The encoding deficits in aged rats may be attributable to more subtle changes in function within a circuit of structures that promote the flexible use of outcome-related information. Based on recent data in rats and monkeys, key parts of this system that could be implicated in our findings would include the interconnections of OFC with the basolateral complex of the amygdala (ABL) and the functional integrity of the ascending monoaminergic systems that innervate the forebrain. The OFC has strong reciprocal connections with the ABL. Input from ABL to OFC is important for the formation of neural correlates of predicted outcome (Schoenbaum et al. 2003b), including some of the correlates found lacking in reversal-impaired aged rats. For example, ABL-lesioned rats trained in this odor-discrimination task fail to form flexible representations of the odor cues, and they also fail to activate representations of the predicted outcomes during odor sampling. The impairment we observe in neural encoding in aging may be related to changes, either within ABL or OFC, that affect the processing of ABL input by OFC neurons.
Processing of ABL input within OFC could be altered by changes in ascending monoaminergic systems that occur in aging. These systems have been proposed to provide important learning signals (Daw et al. 2002) and diminished integrity of the major ascending monoamine systems, and a loss of signaling functions targeted by these systems in the cortex is a widely studied topic in the field of aging research. In the current context, a role in reversal learning has been documented by studies using neurochemical lesions and pharmacological manipulations in young animals, and amelioration of reversal learning impairment in aged subjects has been reported with pharmacological treatments. For example, cognitive inflexibility in the marmosets is observed after serotonin depletion in OFC (Clarke et al. 2004), and systemic administration of an alpha 2A noradrenergic agonist has been reported to restore performance in reversal-impaired aged monkeys (Steere and Arnsten 1997). Disruption of ascending input to OFC could cause a decline in plasticity underlying the abnormal encoding observed in aged-impaired rats, thereby causing the behavioral changes we have observed and the associated deficits in neuroplasticity in OFC. Such mechanisms could be explored by additional studies that attempt to model the present results using pharmacological manipulations in young rats combined with further studies to explore the basis of encoding abnormalities in the aged brain.
Acknowledgments
We thank Dr. Stephen Warrenburg at International Flavors and Fragrances for assistance.
GRANTS This work was supported by National Institutes of Health Grants P01-AG-09973 to M. Gallagher, K08-AG-00882 and R01-DA-015718 to G. Schoenbaum, and F32-MH-12699 to B. Setlow.
Footnotes
Publisher's Disclaimer: Journal of Neurophysiology publishes original articles on the function of the nervous system. It is published 12 times a year (monthly) by the American Physiological Society, 9650 Rockville Pike, Bethesda MD 20814-3991.
References
- Barense MD, Fox MT, Baxter MG. Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats. Learn Mem. 2002;9:191–201. doi: 10.1101/lm.48602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, Fleming DL. Aging in the rhesus monkey: effects on visual discrimination learning and reversal learning. J Gerontol. 1979;34:209–219. doi: 10.1093/geronj/34.2.209. [DOI] [PubMed] [Google Scholar]
- Cador M, Robbins TW, Everitt BJ. Involvement of the amygdala in stimulus-reward associations: interactions with the ventral striatum. Neuroscience. 1989;30:77–86. doi: 10.1016/0306-4522(89)90354-0. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Muir JL, Balleine BW. The role of the nucleus accumbens in instrumental conditioning: evidence of a functional dissociation between accumbens core and shell. J Neurosci. 2001;21:3251–3260. doi: 10.1523/JNEUROSCI.21-09-03251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, Kakade S, Dayan P. Opponent interactions between serotonin and dopamine. Neural Networks. 2002;15:603–616. doi: 10.1016/s0893-6080(02)00052-7. [DOI] [PubMed] [Google Scholar]
- Denburg NL, Recknor EC, Bechara A, Tranel D. Psychophysiological anticipation of positive outcomes promotes advantageous decision-making in normal older adults. Int J Psychophysiol. doi: 10.1016/j.ijpsycho.2005.10.021. In press. [DOI] [PubMed] [Google Scholar]
- Denburg NL, Tranel D, Bechara A. The ability to decide advantageously declines prematurely in some normal older persons. Neuropsychologia. 2005b;43:1099–1106. doi: 10.1016/j.neuropsychologia.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Deroche V, Le Moal M, Piazza PV. Cocaine self-administration increases the incentive motivational properties of the drug in rats. Eur J Neurosci. 1999;11:2731–2736. doi: 10.1046/j.1460-9568.1999.00696.x. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin card sort test: restriction to novel situations and independence from “on-line” processing. J Neurosci. 1997;17:9285–9297. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Hartman M, Bolton E, Fehnel SE. Accounting for age differences on the Wisconsin Card Sorting test: decreased working memory, not inflexibility. Psychol Aging. 2001;16:385–399. [PubMed] [Google Scholar]
- Izquierdo AD, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci. 2004;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B, Mishkin M. Limbic lesions and the problem of stimulus-reinforcement associations. Exp Neurol. 1972;36:362–377. doi: 10.1016/0014-4886(72)90030-1. [DOI] [PubMed] [Google Scholar]
- Kim J, Ragozzino KE. The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiol Learn Mem. 2005;83:125–133. doi: 10.1016/j.nlm.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Kitai ST. Amygdaloid projections to the frontal cortex and the striatum in the rat. J Comp Neurol. 1990;298:40–49. doi: 10.1002/cne.902980104. [DOI] [PubMed] [Google Scholar]
- Lamar M, Resnick SM. Aging and prefrontal functions: dissociating orbitofrontal and dorsolateral abilities. Neurobiol Aging. 2004;25:553–558. doi: 10.1016/j.neurobiolaging.2003.06.005. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald RJ, White NM. A triple dissociation of memory systems: hippocampus, amygdala, and dorsal striatum. Behav Neurosci. 1993;107:3–22. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M. Effects of orbital frontal and anterior cingulate lesions on object and spatial memory in rhesus monkeys. Neuropsychologia. 1997;35:999–1015. doi: 10.1016/s0028-3932(97)00027-4. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nat Neurosci Rev. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Price JL, Carmichael ST, Carnes KM, Clugnet M-C, Kuroda M, Ray JP. Olfactory input to the prefrontal cortex. In: Davis J, Eichenbaum H, editors. Olfaction: A Model System for Computational Neuroscience. Cambridge, MA: MIT Press; 1991. pp. 101–120. [Google Scholar]
- Rapp PR, Amaral DG. Evidence for task-dependent memory dysfunction in the aged monkey. J Neurosci. 1989;9:3568–3576. doi: 10.1523/JNEUROSCI.09-10-03568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc Natl Acad Sci USA. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Heindel WC. Memory systems in normal and pathological aging. Curr Opin Neurobiol. 1994;7:294–298. doi: 10.1097/00019052-199408000-00003. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Schleimann T, Sorensen JC, Zimmer J, West M. Memory impaired aged rats: no loss of principal hippocampal and subicular neurons. Neurobiol Aging. 1996;17:143–147. doi: 10.1016/0197-4580(95)02032-2. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JF, Sahakian BJ, Robbins TW. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Critchley HD, Mason R, Wakeman EA. Orbitofrontal cortex neurons: role in olfactory and visual association learning. J Neurophysiol. 1996;75:1970–1981. doi: 10.1152/jn.1996.75.5.1970. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddoris MP, Gallagher M, Schoenbaum G. Rapid associative encoding in basolateral amygdala depends on connections with orbitofrontal cortex. Neuron. 2005;46:321–331. doi: 10.1016/j.neuron.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J Neurosci. 1999;19:1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Rapid changes in functional connectivity in orbitofrontal cortex and basolateral amygdala during learning and reversal. J Neurosci. 2000;20:5179–5189. doi: 10.1523/JNEUROSCI.20-13-05179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Eichenbaum H. Information coding in the rodent prefrontal cortex. II. Ensemble activity in orbitofrontal cortex. J Neurophysiol. 1995;74:751–762. doi: 10.1152/jn.1995.74.2.751. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent S, Saddoris MP, Gallagher M. Teaching old rats new tricks: age-related impairments in olfactory reversal learning. Neurobiol Aging. 2002a;23:555–564. doi: 10.1016/s0197-4580(01)00343-8. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent S, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002b;13:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR. Orbitofrontal cortex, associative learning, and expectancies. Neuron. 2005;47:633–636. doi: 10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B. Lesions of nucleus accumbens disrupt learning about aversive outcomes. J Neurosci. 2003;23:9833–9841. doi: 10.1523/JNEUROSCI.23-30-09833.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Gallagher M. Orbitofrontal cortex: modeling prefrontal function in rats. In: Squire L, Schacter D, editors. The Neuropsychology of Memory. 3. New York: Guilford; 2002c. pp. 463–477. [Google Scholar]
- Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learn Mem. 2003a;10:129–140. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003b;39:855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- Setlow B, Schoenbaum G, Gallagher M. Neural encoding in ventral striatum during olfactory discrimination learning. Neuron. 2003;38:625–636. doi: 10.1016/s0896-6273(03)00264-2. [DOI] [PubMed] [Google Scholar]
- Steere JC, Arnsten AF. The alpha-2A noradrenergic receptor agonist guanfacine improves visual object discrimination reversal performance in aged rhesus monkeys. Behav Neurosci. 1997;11:883–891. doi: 10.1037//0735-7044.111.5.883. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Miller EK, Desimone R. Object and place memory in the Macaque entorhinal cortex. J Neurophysiol. 1997;78:1062–1081. doi: 10.1152/jn.1997.78.2.1062. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain. New York: Elsevier; 1992. [Google Scholar]
- Thorpe SJ, Rolls ET, Maddison S. The orbitofrontal cortex: neuronal activity in the behaving monkey. Exp Brain Res. 1983;49:93–115. doi: 10.1007/BF00235545. [DOI] [PubMed] [Google Scholar]
- Tsuchida J, Kubo N, Kojima S. Position reversal learning in aged Japanese macaques. Behav Brain Res. 2002;129:107–112. doi: 10.1016/s0166-4328(01)00336-9. [DOI] [PubMed] [Google Scholar]
- Voytko ML. Impairments in acquisition and reversal of two-choice discriminations by aged rhesus monkeys. Neurobiol Aging. 1999;20:617–627. doi: 10.1016/s0197-4580(99)00097-4. [DOI] [PubMed] [Google Scholar]


