SYNOPSIS
Objective.
The present study examined the scope of rapid human immunodeficiency virus (HIV) testing in urban U.S. hospitals.
Methods.
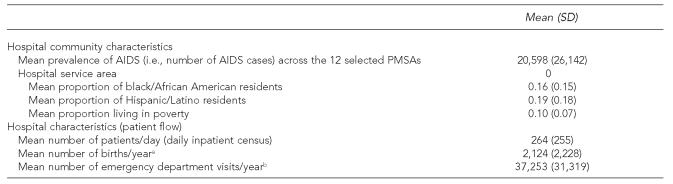
In a multistage national probability sample, 12 primary metropolitan statistical areas (three per region) were sampled randomly, with weights proportionate to acquired immunodeficiency syndrome (AIDS) populations. All 671 eligible hospitals within areas were selected. Laboratory staff from 584 hospitals (87%) were interviewed by telephone in 2005.
Results.
About 52% reported rapid HIV test availability (50% in occupational health, 29% in labor and delivery, and 13% in emergency department/urgent care), and 86% of hospitals offering rapid tests processed them in the laboratory. In multivariate models, rapid test availability was more likely in hospitals serving more patients, and located in high-poverty, high-AIDS prevalence areas, and in the South or Midwest vs. West. It was less likely in hospitals serving areas with large percentages of people who were black/African American or Hispanic/Latino (p<0.05).
Conclusions.
Rapid HIV testing is increasing across urban U.S. hospitals, primarily for occupational exposure and in hospitals with greater resources and need. To achieve routine HIV screening, policies should encourage greater breadth of diffusion of rapid testing at the point of care, especially in smaller facilities, the West, and communities with racial/ethnic diversity.
The Centers for Disease Control and Prevention (CDC) 2006 recommendations for human immunodeficiency virus (HIV) testing of adults, adolescents, and pregnant women encourage routine HIV screening in all public and health-care settings.1 Toward this goal, CDC recommends simplified counseling and testing procedures, including opt-out screening (in which patients can decline testing after notification that it will be performed) and optional prevention counseling; separate written informed consent is not recommended. CDC also recommends repeat screening of pregnant women in the third trimester in jurisdictions with high rates of HIV-infected pregnant women.
The complexity of traditional HIV testing has been a barrier to CDC efforts to expand HIV screening.2,3 Traditional testing requires that individuals return one to two weeks post-test for results receipt and prevention counseling, which can reduce risk behavior among those already infected.4 However, approximately a third of those tested do not return for results, representing a missed prevention opportunity.5
To increase HIV screening, CDC proposes use of same-day rapid HIV testing.1,5 Rapid tests allow HIV-negative individuals to learn their serostatus in less than an hour, eliminating the need to return for results. Six rapid HIV tests with high sensitivity (99.3% to 100%) and specificity (99.1% to 100%) have been approved by the U.S. Food and Drug Administration since 2002. Since November 2007, four have been approved for point-of-care use by trained staff in nonclinical settings under the 1988 Clinical Laboratory Improvement Amendments (CLIA).6–12 All reactive rapid test results require confirmatory testing.13
Rapid HIV testing in hospitals has been shown to be easy to use, to increase rates of results receipt, to be acceptable to patients, and to increase quality of care.6,14–22 Routine HIV screening can be cost-effective.23,24 Rapid testing may be especially useful to implement in emergency departments, where patients typically do not have continuing relationships with providers and may not return for results, and where substantially greater HIV prevalences have been found than in public health testing sites (2% to 17%).19,25 Rapid testing of source patients in occupational exposure situations facilitates employee decisions about post-exposure prophylaxis.17 Rapid testing during labor enables providers to take effective measures to prevent perinatal HIV transmission.26–29 Due to the need for expedience during labor, rapid testing may be more useful if test results are processed at the point of care vs. in hospital laboratories.6,19,27,30
The current scope of rapid HIV testing in U.S. hospitals is unknown. Research reviews suggest that HIV tests are not being performed routinely in health-care settings such as hospital emergency departments,31 but no nationally representative peer-reviewed survey has been conducted. Several barriers may inhibit nonrapid and rapid HIV test provision in hospitals,15,32,33 including policy-level barriers (e.g., consent and counseling requirements), logistical barriers (e.g., time pressures, insufficient resources), and educational barriers (e.g., low patient or provider acceptance, lack of provider training or knowledge).34 Regulatory environments for HIV testing, which vary by state,35 may impede HIV screening program implementation. Furthermore, general lack of public funds and insurance coverage for HIV screening is thought to be a major impediment to implementing screening programs.36
We examined the scope of rapid HIV testing in urban U.S. hospitals from 2002 to 2006, prior to release of CDC's 2006 recommendations encouraging routine HIV screening in health care. Diffusion of innovation (DOI) theory was used as a framework to describe rapid test availability over a several-year period.37 According to DOI, an innovation is likely to be adopted if it appears advantageous over existing methods; is compatible with existing infrastructure, resources, and norms; and is relatively easy to use. Institutions with greater resources and larger size are more likely to adopt innovations because they can more easily overcome obstacles.
We hypothesized that larger hospitals have greater resources to implement rapid testing, and would be more likely to offer rapid tests. We also hypothesized that hospitals located in high acquired immunodeficiency syndrome (AIDS) prevalence areas, and areas with high concentrations of subgroups in which HIV is increasing (i.e., black/African American, Hispanic/Latino, and high poverty), would perceive a greater need for rapid testing and would be more likely to offer rapid tests. Further, we predicted that rapid test provision would differ by geographic region, due to state variations in HIV test regulations.
METHODS
Sampling frame and procedures
We modeled our sampling design after the HIV Cost and Services Utilization Study,38,39 a national study of patients in care for HIV. We conducted multistage probability sampling by region to arrive at a nationally representative sample of hospitals in major U.S. metropolitan areas. In the first stage, we randomly sampled four geographic locations (primary metropolitan statistical sampling areas [PMSAs]) per census region (Northeast, Midwest, South, and West) from a comprehensive list of 104 PMSAs. We chose the highest-prevalence PMSA within each region with certainty (Los Angeles; Long Beach, California; New York; Miami; and Chicago) and eight other PMSAs (Atlanta; Boston; Indianapolis; Newark, New Jersey; Oakland, California; Riverside–San Bernardino, California; St. Louis; and Washington, D.C.) with probabilities proportionate to size of the number of AIDS cases reported to CDC as of December 2001.2
Sampling probabilities proportionate to size are widely used in multistage designs with sampling units that are heterogeneous in size, such as AIDS prevalence, to prevent selection of statistically inefficient or problematic samples in which few eligible respondents are present in the sampling unit.40 Selection of some sampling units with certainty is acceptable when the population distribution is highly skewed among units. Because most data were collected from PMSAs with moderate-to-high AIDS prevalences, precision was reduced for national estimates, but increased for estimates in areas of moderate-to-high AIDS prevalences.
In the second stage, we included all 666 eligible non-rehabilitation hospitals listed in the 2002 American Hospital Association (AHA) database within the selected PMSAs. Hospitals were ineligible if they were receiving CDC funds for rapid HIV test demonstration projects.1 Rehabilitation hospitals were ineligible because they were unlikely to be using HIV testing for prevention. Seventeen hospitals had closed since the AHA database was compiled. All remaining 649 hospitals were telephoned in random order. An additional 22 subsidiary hospitals were identified during interviews, bringing the total number of hospitals telephoned to 671. The RAND Corporation's institutional review board approved this study.
Hospital survey
The interview protocol and survey items were developed through extensive formative work, including qualitative interviews with laboratory and non-laboratory hospital providers, and consultation with laboratory experts on the study team and advisory board. Interviewers telephoned the hospital and asked to speak with laboratory directors or the person in charge of the laboratory's quality assurance and/or proficiency testing program. We chose to survey laboratory directors because they oversee all tests conducted in the hospital, including all test quality assurance procedures.
Interviewers asked respondents if rapid HIV tests were available in the hospital, with response options yes, no, and not yet, but have concrete plans to start in the near future (defined to respondents as “the next six months”). Respondents in hospitals using rapid tests were asked which hospital departments provided rapid tests (e.g., labor and delivery, emergency department) and the approximate time period when rapid testing was instituted (i.e., less than six months ago, between one and two years ago, in the past six months to one year, between one and two years ago, and more than two years ago). We calculated the probable year of rapid HIV test program implementation by subtracting the midpoint of the time period chosen (e.g., 1.5 years for the option “between one and two years ago”) from the interview date; we added three months to the interview date among respondents who indicated that they had concrete plans to start rapid testing “in the near future.” Respondents were also asked if the hospital had subsidiaries.
Hospital and hospital community characteristics
Patient flow.
Using AHA identification numbers, hospitals were linked to 2004 AHA data on average daily inpatient census, and number of births and emergency department visits annually.
AIDS cases.
The cumulative number of reported AIDS cases in 2001 for each PMSA was linked to each hospital.2
Hospital service area characteristics.
All zip codes in each hospital's service area were extracted from the zip code crosswalk of the Dartmouth Atlas of Healthcare and linked to census data on race/ethnicity and income. The Dartmouth Atlas of Healthcare defines hospital service areas as groupings of zip codes that contain residents who receive most of their hospitalizations from the hospitals in that area (operationalized as the zip codes where the greatest proportion of hospitals' Medicare residents are hospitalized). We calculated the mean percentages of residents who were black/African American, Hispanic/Latino, and living below the poverty level across zip codes in each service area.
Statistical analysis
Multivariate logistic regressions were used to examine the simultaneous contribution of the AHA, census, and AIDS prevalence variables in predicting rapid HIV test availability. To report the model results in terms of rate of HIV test availability, odds ratios were translated into adjusted percentages. Predicted probabilities show the probability of rapid HIV test availability given a particular value of the predictor, adjusting for other variables in the model.41 For example, the adjusted rate of HIV test usage in the Northeast region represents the mean of the predicted probability of testing in hospitals obtained from the multivariate model, if all hospitals sampled were in the Northeast. For a continuous predictor such as AIDS prevalence, the adjusted rate of rapid test availability in Chicago, for example, is the mean predicted probability from the multivariate model if all hospitals were in PMSAs with a prevalence of 22,703 (the number of AIDS cases in Chicago). Separate analyses were conducted for any rapid HIV testing in the hospital, in the emergency department/urgent care (of those hospitals with emergency departments/urgent care), in labor and delivery (of those hospitals with labor and delivery units), and in occupational health. Because hospital size and AIDS prevalence data were non-normally distributed, their logs were used.
Sampling weights taking into account the different selection probabilities for each hospital (based on the cumulative number of AIDS cases within each PMSA) were used to adjust the sample data to represent the target population of urban U.S. hospitals. Sampling weights ensure that weighted data from the sample are representative of the entire reference population and allow for calculations of nationally representative estimates. Nonresponse weights were not used because all hospitals listed were contacted, hospitals were called in a random order, and only three staff members refused participation.
RESULTS
Prevalence and diffusion of rapid HIV testing
Laboratory staff at 584 hospitals (87% completion rate) were interviewed by telephone from April to November 2005; 24% were located in the Midwest, 20% in the Northeast, 27% in the South, and 29% in the West; all hospitals in the West were located in California (Table 1). More than half (52%) of hospitals provided rapid HIV tests. Of hospitals not providing rapid tests, 21% had concrete plans to start offering them. Half of all hospitals provided rapid HIV tests for occupational health. Of hospitals with labor and delivery units, 29% provided rapid HIV tests during labor; of hospitals with emergency departments/urgent care units, 13% provided rapid HIV tests in the emergency department/urgent care. Smaller percentages were using rapid testing in other hospital inpatient settings, including in surgery (2%), on the ward (2%), in the blood bank (1%), in the HIV/infectious disease department (1%), in primary care (1%), for sexual assault cases (1%), and in admissions (0.3%). Most (86%) of the hospitals providing rapid tests ordered or performed the tests in the hospital laboratory. Overall, 14% of hospitals performed rapid tests at point of care (including 13% in occupational health, 2% in labor and delivery, and 1% in the emergency department/urgent care).
Table 1.
Characteristics of hospitals surveyed regarding rapid HIV test availability in 2002–2006 (n=584)
Among hospitals with labor and delivery units (unweighted n=360)
Among hospitals with emergency departments/urgent care units (unweighted n=498)
HIV = human immunodeficiency virus
SD = standard deviation
AIDS = acquired immunodeficiency syndrome
PMSA = primary metropolitan statistical sampling area
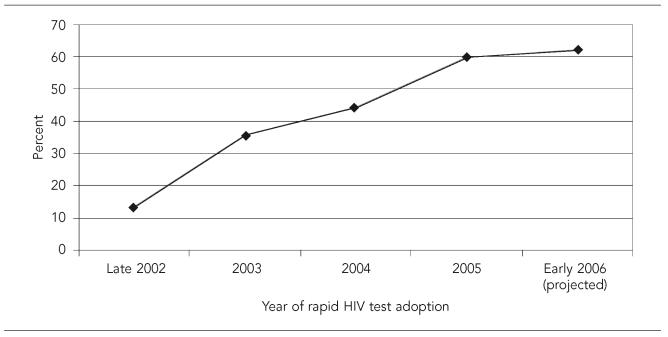
The proportion of the total sample providing rapid HIV tests steadily increased from 2002 to 2005; the projected proportion, based on those who planned to start, is expected to increase steadily (Figure).
Figure.
Cumulative percentage of U.S. hospitals offering rapid HIV tests from 2002 to 2006 (n=584)
Note: Data points are: 13.7% (late 2002), 35.8% (2003), 44.3% (2004), 59.9% (2005), and 62.2% (early 2006).
HIV = human immunodeficiency virus
Multivariate predictors of rapid HIV testing
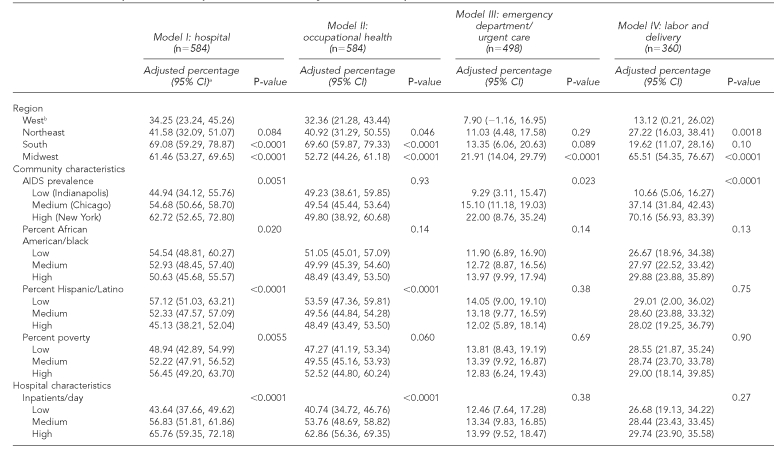
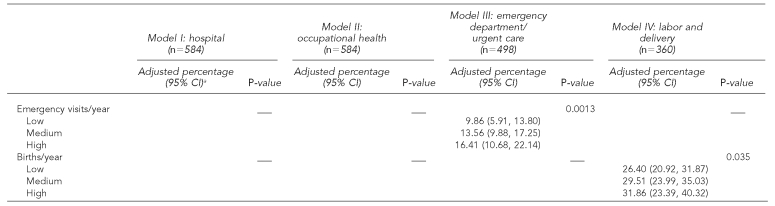
Table 2 shows the multivariate models. Hospitals in the South and Midwest vs. the West, in areas with a higher proportion of residents living in poverty, with a higher AIDS prevalence, and with a higher average daily census were more likely to provide rapid tests in any hospital department; hospitals in areas with a higher proportion of black/African American and Hispanic/Latino residents were less likely to provide rapid tests (Table 2, Model I). In occupational health, hospital location in the Northeast, South, or Midwest (vs. West) and a higher average daily census were related to a higher probability of rapid testing; location in areas with a higher proportion of Hispanic/Latino residents was associated with a lower probability of rapid testing (Table 2, Model II). In the emergency department/urgent care, the probability of rapid testing was higher in hospitals located in the Midwest vs. the West, in areas with a greater AIDS prevalence, and in areas with a higher number of emergency department visits per year (Table 2, Model III). In labor and delivery units, rapid testing was more likely in hospitals located in the Northeast or Midwest vs. the West, and in hospitals in areas with a greater AIDS prevalence and with a higher number of births annually (Table 2, Model IV).
Table 2.
Multivariate predictors of rapid HIV test availability in 584 U.S. hospitals
The percentages have been adjusted for all variables listed in the table. P-values were calculated from the multivariate model after sampling weights were applied. For illustrative purposes, adjusted percentages are presented at meaningful cut-points for continuous census variables (low = 25th percentile, medium = median, high = 75th percentile) and for AIDS prevalence (lowest, medium, and highest prevalence PMSAs, of the 12 selected PMSAs). However, these variables were included in the multivariate models as continuous. Adjusted percentages for all PMSAs are available by request to the first author.
This group was used as the comparison group in the multivariate analyses and the reference category for all calculated p-values.
CI = confidence interval
HIV = human immunodeficiency virus
AIDS = acquired immunodeficiency syndrome
PMSA = primary metropolitan statistical sampling area
DISCUSSION
Rapid HIV tests are increasingly available in urban U.S. hospitals. However, hospitals primarily provide the test for index patients in occupational health situations, to allow employees to make informed decisions about post-exposure prophylaxis. The numbers of patients screened for this purpose cannot have the larger public health impact of routine screening efforts recommended by CDC.
Rapid HIV testing availability was substantially lower in hospital venues other than occupational health. Less than 30% of hospitals offered rapid tests in labor and delivery. However, CDC recommends rapid HIV testing of all women in labor of undocumented HIV status, due to the relatively small window of time to administer antiretroviral therapy to prevent mother-child transmission. The CDC's Mother-Infant Rapid Intervention at Delivery (MIRIAD) study demonstrated that rapid HIV testing during labor is feasible, accurate, and acceptable to women with undocumented HIV status.6,42 Future research is critical for understanding the types of barriers, such as test cost and concerns about quality assurance procedures and staff training that may impede rapid testing in this setting.
Only 13% of hospitals provided rapid HIV tests in the emergency department/urgent care. Emergency departments routinely care for a large number of people across the socioeconomic spectrum, many of whom have limited contact with health care. Lack of rapid testing in the emergency department is said to be a missed opportunity to detect a high number of infections among individuals otherwise not intending to test.19,43–45 To overcome barriers to rapid testing, such as providers' time constraints, hospital emergency departments may need to employ dedicated staff for rapid HIV testing that are available to test patients and process test results.46 Streamlined, adjusted testing procedures, such as videotaped pre- and post-test counseling, have helped to decrease the burden of HIV testing in the emergency department.47
The finding that most rapid HIV testing (among those using the test) occurred in the hospital laboratory shows a possible inefficiency in testing protocols. Even with rapid testing, provision of results must be expedient enough to be of benefit. Although rapid test results may be available in less than an hour, test processing in the laboratory vs. at point of care may lead to results reporting delays.19,30,42 Of hospitals conducting rapid testing, only 1% conducted point-of-care tests in labor and delivery; however, research shows that point-of-care testing in labor and delivery can lead to faster provision of results.6,19 In addition, laboratory-based testing protocols that prioritize rapid HIV testing for labor and delivery ahead of other laboratory tests may help to shorten the length of time to receipt of results.
In accordance with DOI, hospital resources and need for testing predicted rapid HIV testing availability. Larger hospitals (with a higher inpatient caseload) were more likely to provide rapid HIV tests within and across departments. Larger hospitals tend to have greater resources, in terms of budgetary funds and staff members, with which to initiate and sustain testing programs. Further, hospitals, labor and delivery units, and emergency departments/urgent care centers in areas of higher AIDS prevalence were more likely to offer rapid HIV tests. As HIV testing becomes routine, one would expect observed differences in rapid testing across hospitals in different types of communities to decrease, and that hospitals will begin to offer rapid tests, regardless of community factors.
Geographic region was a robust predictor of rapid testing. Hospitals in the Western region (three PMSAs sampled in California) were less likely to be providing rapid HIV tests. The relatively strict regulatory environment of California for rapid HIV tests likely contributed to lower levels of availability in this region. In California, laboratories must apply in writing to the California Department of Health Services for permission to offer rapid HIV tests as waived tests; the backlog of applications in 2005 (the time of the present study) was high. Removing some of the bureaucratic barriers to testing may increase rapid testing rates.48
Hospital service area characteristics, including those associated with need for HIV screening (i.e., poverty and race/ethnicity), were significantly related to rapid HIV testing in the hospital as a whole. Hospitals in areas of higher poverty, representing those in greatest need, were more likely to be providing rapid tests. However, hospitals with higher percentages of African American and Latinos residents—those racial/ethnic groups most affected by HIV—were less likely to be providing rapid tests. This disparity is inconsistent with our findings for poverty. Hospitals with higher proportions of racial/ethnic minorities may have fewer resources with which to institute rapid testing programs, or they may prioritize resources for other health problems instead; institutional discrimination may also play a role. These inconsistent findings merit further investigation prior to making conclusions.
Limitations
Several limitations exist in our analysis. Rural hospitals and hospitals currently receiving funds from CDC for rapid HIV test demonstration projects (including MIRIAD) were not represented in our sampling frame.6 Geographic variation in testing could have been directly influenced by hospitals that participated in the MIRIAD study. However, analysis of the present study data with and without MIRIAD hospitals yielded similar results for region (data not shown), suggesting that geographic differences are due to another unmeasured variable.
We obtained census information for each hospital's service area as a proxy for the likely sociodemographic characteristics of patients; we therefore did not capture information about patients residing outside of the hospital service area. In addition, although we randomly selected PMSAs within region, all of the Western PMSAs were in California. Further, our results characterize the state of rapid testing relatively soon after approval; additional studies are needed to follow the scope of rapid testing over time, after more widespread dissemination of CDC's 2006 recommendations for HIV screening.
CONCLUSION
Our nationally representative survey found low rates of rapid HIV test provision in hospitals, even in clinical settings such as emergency departments and labor and delivery, where the test's short turnaround time would be most useful. Although rates of rapid testing in hospitals have been increasing, they remain lower than ideal to achieve CDC's recommendations, especially with respect to point-of-care use. Further research is needed to examine barriers to HIV testing in hospitals, including those related to cost, feasibility, training, staffing, regulatory environment, and hospital resources. Policies should be developed to encourage greater breadth of diffusion of rapid testing of patients within hospital settings, especially in smaller hospitals and at the point of care.
Adoption of streamlined counseling processes, instituting universal testing regardless of risk, and use of dedicated staff for HIV testing may further aid the acceptability of HIV testing to providers and patients.46,47,49–51 Revised counseling and testing procedures may be promising avenues for increasing HIV screening within hospitals. In the meantime, hospital-based rapid HIV testing is falling short of its potential to help identify the estimated one-in-four HIV-infected Americans unaware of their HIV status.52
Acknowledgments
The authors thank David Klein for database management, Andy Olds for data collection, and the following advisory committee members: Sandra Berry, MA, RAND Corporation; Lee Hilborne, PhD, RAND Corporation; Jonathan Fielding, MD, Los Angeles County Department of Health; Frank Galvan, PhD, Charles R. Drew University of Medicine and Science; Seth Kalichman, PhD, University of Connecticut; Allen Gifford, MD, Boston University and Edith Nourse Rogers Memorial Veteran's Hospital; Peter Kerndt, MD, Los Angeles County Department of Health; Steven Pinkerton, PhD, Medical College of Wisconsin; and Michael Stoto, PhD, Georgetown University.
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of CDC.
Footnotes
This research was funded by Grant #U65/CCU924523-01 from the Centers for Disease Control and Prevention (CDC). Manuscript preparation was partially supported by CDC Center Grant #U48/DP000056 and California HIV/AIDS Research Program Center Grant #CH05-618.
REFERENCES
- 1.Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55((RR-14)):1–17. [PubMed] [Google Scholar]
- 2.HIV and AIDS—United States, 1981–2001. MMWR Morb Mortal Wkly Rep. 2001;50(21):430–4. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (US) Advancing HIV prevention: interim technical guidance for selected interventions. 2003 [Google Scholar]
- 4.Weinhardt LS, Carey MP, Johnson BT, Bickham NL. Effects of HIV counseling and testing on sexual risk behavior: a meta-analytic review of published research, 1985–1997. Am J Public Health. 1999;89:1397–405. doi: 10.2105/ajph.89.9.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Revised guidelines for HIV counseling, testing, and referral. MMWR Recomm Rep. 2001;50(RR-19):1–58. [PubMed] [Google Scholar]
- 6.Bulterys M, Jamieson DJ, O'Sullivan MJ, Cohen MH, Maupin R, Nesheim S, et al. Rapid HIV-1 testing during labor: a multicenter study. JAMA. 2004;292:219–23. doi: 10.1001/jama.292.2.219. [DOI] [PubMed] [Google Scholar]
- 7.Delaney KP, Branson BM, Uniyal A, Kerndt PR, Keenan PA, Jafa K, et al. Performance of an oral fluid rapid HIV-1/2 test: experience from four CDC studies. AIDS. 2006;20:1655–60. doi: 10.1097/01.aids.0000238412.75324.82. [DOI] [PubMed] [Google Scholar]
- 8.Wesolowski LG, MacKellar DA, Facente SN, Dowling T, Ethridge SF, Zhu JH, et al. Post-marketing surveillance of OraQuick whole blood and oral fluid rapid HIV testing. AIDS. 2006;20:1661–6. doi: 10.1097/01.aids.0000238413.13442.ed. [DOI] [PubMed] [Google Scholar]
- 9.Greenwald JL, Burstein GR, Pincus J, Branson B. A rapid review of rapid HIV antibody tests. Curr Infect Dis Rep. 2006;8:125–31. doi: 10.1007/s11908-006-0008-6. [DOI] [PubMed] [Google Scholar]
- 10.Medford (NY): Chembio Diagnostic Systems Inc.; 2006. Chembio Diagnostic Systems Inc. HIV 1/2 STAT-PAK™ Assay. Package Insert. [Google Scholar]
- 11.OraSure Technologies Inc. OraQuick advance rapid HIV-1/2 antibody test. Package Insert. Bethlehem (PA): OraSure Technologies, Inc.; 2004. [Google Scholar]
- 12.Trinity Biotech PLC. Wicklow (Ireland): Trinity Biotech PLC; 2004. Uni-Gold™ Recombigen® HIV. Package Insert. [Google Scholar]
- 13.Notice to readers: protocols for confirmation of reactive rapid HIV tests. MMWR Morb Mortal Wkly Rep. 2004;53(10):221–2. [Google Scholar]
- 14.Kassler WJ, Dillon BA, Haley C, Jones WK, Goldman A. On-site, rapid HIV testing with same-day results and counseling. AIDS. 1997;11:1045–51. doi: 10.1097/00002030-199708000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Hutchinson AB, Corbie-Smith G, Thomas SB, Mohanan S, Del Rio C. Understanding the patient's perspective on rapid and routine HIV testing in an inner-city urgent care center. AIDS Educ Prev. 2004;16:101–14. doi: 10.1521/aeap.16.2.101.29394. [DOI] [PubMed] [Google Scholar]
- 16.Hutchinson AB, Branson BM, Kim A, Farnham PG. A meta-analysis of the effectiveness of alternative HIV counseling and testing methods to increase knowledge of HIV status. AIDS. 2006;20:1597–604. doi: 10.1097/01.aids.0000238405.93249.16. [DOI] [PubMed] [Google Scholar]
- 17.Landrum ML, Wilson CH, Perri LP, Hannibal SL, O'Connell RJ. Usefulness of a rapid human immunodeficiency virus-1 antibody test for the management of occupational exposure to blood and body fluid. Infect Control Hosp Epidemiol. 2005;26:768–74. doi: 10.1086/502615. [DOI] [PubMed] [Google Scholar]
- 18.Lubelchek R, Kroc K, Hota B, Sharief R, Muppudi U, Pulvirenti J, et al. The role of rapid vs. conventional human immunodeficiency virus testing for inpatients: effects on quality of care. Arch Intern Med. 2005;165:1956–60. doi: 10.1001/archinte.165.17.1956. [DOI] [PubMed] [Google Scholar]
- 19.Kelen GD, Shahan JB, Quinn TC. Emergency department-based HIV screening and counseling: experience with rapid and standard serologic testing. Ann Emerg Med. 1999;33:147–55. doi: 10.1016/s0196-0644(99)70387-2. [DOI] [PubMed] [Google Scholar]
- 20.Doyle NM, Levison JE, Gardner MO. Rapid HIV versus enzyme-linked immunosorbent assay screening in a low-risk Mexican American population presenting in labor: a cost-effectiveness analysis. Am J Obstet Gynecol. 2005;193(3 Pt2):1280–5. doi: 10.1016/j.ajog.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Wurcel A, Zaman T, Shen S, Stone D. Acceptance of HIV antibody testing among inpatients and outpatients at a public health hospital: a study of rapid versus standard testing. AIDS Patient Care STDS. 2005;19:499–505. doi: 10.1089/apc.2005.19.499. [DOI] [PubMed] [Google Scholar]
- 22.Roberts KJ, Grusky O, Swanson AN. Outcomes of blood and oral fluid rapid HIV testing: a literature review, 2000–2006. AIDS Patient Care STDS. 2007;21:621–37. doi: 10.1089/apc.2006.0196. [DOI] [PubMed] [Google Scholar]
- 23.Ekwueme DU, Pinkerton SD, Holtgrave DR, Branson BM. Cost comparison of three HIV counseling and testing technologies. Am J Prev Med. 2003;25:112–121. doi: 10.1016/s0749-3797(03)00115-6. [DOI] [PubMed] [Google Scholar]
- 24.Paltiel AD, Walensky RP, Schackman BR, Seage GR, 3rd, Mercincavage LM, Weinstein MC, et al. Expanded HIV screening in the United States: effect on clinical outcomes, HIV transmission, and costs. Ann Intern Med. 2006;145:797–806. doi: 10.7326/0003-4819-145-11-200612050-00004. [DOI] [PubMed] [Google Scholar]
- 25.Rothman RE, Ketlogetswe KS, Dolan T, Wyer PC, Kelen GD. Preventive care in the emergency department: should emergency departments conduct routine HIV screening? A systematic review. Acad Emerg Med. 2003;10:278–85. doi: 10.1111/j.1553-2712.2003.tb02004.x. [DOI] [PubMed] [Google Scholar]
- 26.ACOG Committee on Obstetric Practice. Prenatal and perinatal human immunodeficiency virus testing: expanded recommendations. Obstet Gynecol. 2004;104:1119–24. [PubMed] [Google Scholar]
- 27.Forsyth BW, Barringer SR, Walls TA, Landry ML, Ferguson D, Tinghitella TJ, et al. Rapid HIV testing of women in labor: too long a delay. J Acquir Immune Defic Syndr. 2004;35:151–4. doi: 10.1097/00126334-200402010-00008. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention (US) HIV counseling, testing and referral: standards and guidelines. 1994 [Google Scholar]
- 29.Guay LA, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 30.Rapid point-of-care testing for HIV-1 during labor and delivery—Chicago, Illinois, 2002. MMWR Morb Mortal Wkly Rep. 2003;52(36):866–8. [PubMed] [Google Scholar]
- 31.Branson B. Current HIV epidemiology and revised recommendations for HIV testing in health-care settings. J Med Virol. 2007;79(Suppl 1):S6–10. doi: 10.1002/jmv.20972. [DOI] [PubMed] [Google Scholar]
- 32.Sibbald B. New rapid HIV test opens Pandora's box of ethical concerns. CMAJ. 2000;162:1600. [PMC free article] [PubMed] [Google Scholar]
- 33.Galvan FH, Brooks RA, Leibowitz AA. Rapid HIV testing: issues in implementation. AIDS Patient Care STDS. 2004;18:15–8. doi: 10.1089/108729104322740875. [DOI] [PubMed] [Google Scholar]
- 34.Burke RC, Sepkowitz KA, Bernstein KT, Karpati AM, Myers JE, Tsoi BW, et al. Why don't physicians test for HIV? A review of the U.S. literature. AIDS. 2007;21:1617–24. doi: 10.1097/QAD.0b013e32823f91ff. [DOI] [PubMed] [Google Scholar]
- 35.Health Research and Educational Trust. State HIV testing laws—2007. [cited 2008 Feb 4]. Available from: URL: http://www.ucsf.edu/hivcntr/StateLaws/Index.html.
- 36.Levenson D. The challenges of universal HIV screening: why cost, logistics, and some state laws present hurdles to CDC recommendations. Clinical Laboratory News. 2007. [cited 2007 Nov 1]. Available from: URL: http://www.aacc.org/AACC/publications/cln/2007/oct/cover1_1007.htm.
- 37.Rogers EM. 5th ed. New York: Free Press; 2003. Diffusion of innovations. [Google Scholar]
- 38.Frankel MR, Shapiro MF, Duan N, Morton SC, Berry SH, Brown JA, et al. National probability samples in studies of low-prevalence diseases. Part II: designing and implementing the HIV cost and services utilization study sample. Health Serv Res. 1999;34(5 Pt 1):969–92. [PMC free article] [PubMed] [Google Scholar]
- 39.Shapiro MF, Berk ML, Berry SH, Emmons CA, Athey LA, Hsia DC, et al. National probability samples in studies of low-prevalence diseases. Part I: perspectives and lessons from the HIV cost and services utilization study. Health Serv Res. 1999;34(5 Pt 1):951–68. [PMC free article] [PubMed] [Google Scholar]
- 40.Kish L. Survey sampling. New York: John Wiley and Sons; 1965. [Google Scholar]
- 41.Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999;55:652–9. doi: 10.1111/j.0006-341x.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 42.Jamieson DJ, Cohen MH, Maupin R, Nesheim S, Danner SP, Lampe MA, et al. Rapid human immunodeficiency virus-1 testing on labor and delivery in 17 U.S. hospitals: the MIRIAD experience. Am J Obstet Gynecol. 2007;197(3) Suppl 1:S72–82. doi: 10.1016/j.ajog.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 43.Lyons MS, Lindsell CJ, Ledyard HK, Frame PT, Trott AT. Emergency department HIV testing and counseling: an ongoing experience in a low-prevalence area. Ann Emerg Med. 2005;46:22–8. doi: 10.1016/j.annemergmed.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 44.Lyons MS, Lindsell CJ, Ledyard HK, Frame PT, Trott AT. Health department collaboration with emergency departments as a model for public health programs among at-risk populations. Public Health Rep. 2005;120:259–65. doi: 10.1177/003335490512000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rothman RE. Current Centers for Disease Control and Prevention guidelines for HIV counseling, testing, and referral: critical role of and a call to action for emergency physicians. Ann Emerg Med. 2004;44:31–42. doi: 10.1016/j.annemergmed.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 46.Anderson J, Smith MJ, Kostman J. Implementation of a rapid HIV testing program in an inner city hospital emergency department. the XVIth International AIDS Conference; 2006 Oct 13–18; Toronto. Paper presented at. [Google Scholar]
- 47.Calderon Y, Leider J, Hailpern S, Haughey M, Chin R, Lombardi P, et al. Program B.R.I.E.F.: video assisted rapid HIV testing in an urban ED. the XVIth International AIDS Conference; 2006 Oct 13–18; Toronto. Paper presented at. [Google Scholar]
- 48.Rotheram-Borus MJ, Leibowitz AA, Etzel MA. Routine, rapid HIV testing. AIDS Educ Prev. 2006;18:273–80. doi: 10.1521/aeap.2006.18.3.273. [DOI] [PubMed] [Google Scholar]
- 49.Koo DJ, Begier EM, Henn MH, Sepkowitz KA, Kellerman SE. HIV counseling and testing: less targeting, more testing. Am J Public Health. 2006;96:962–4. doi: 10.2105/AJPH.2006.089235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spielberg F, Branson BM, Goldbaum GM, Lockhart D, Kurth A, Rossini A, et al. Choosing HIV counseling and testing strategies for outreach settings: a randomized trial. J Acquir Immune Defic Syndr. 2005;38:348–55. [PubMed] [Google Scholar]
- 51.Walensky RP, Losina E, Steger-Craven KA, Freedberg KA. Identifying undiagnosed human immunodeficiency virus: the yield of routine, voluntary inpatient testing. Arch Intern Med. 2002;162:887–92. doi: 10.1001/archinte.162.8.887. [DOI] [PubMed] [Google Scholar]
- 52.Glynn M, Rhodes P. Estimated HIV prevalence in the United States at the end of 2003. National HIV Prevention Conference; 2005 Jun 12–15; Atlanta. Paper presented at. [Google Scholar]